Effects of High Salinity on Nitrogen Removal Efficiency and Microbial Community Structure in a Three-Stage AO System
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactor Setup and Operating Conditions
2.2. Sludge and Experimental Water Quality
2.3. Analytical Methods
2.3.1. Conventional Water Quality Analysis Methods
2.3.2. Extraction and Detection of EPS
2.3.3. Three-Dimensional Excitation–Emission Matrix (3D-EEM) Fluorescence Spectroscopy
2.3.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.5. High-Throughput Sequencing
3. Results
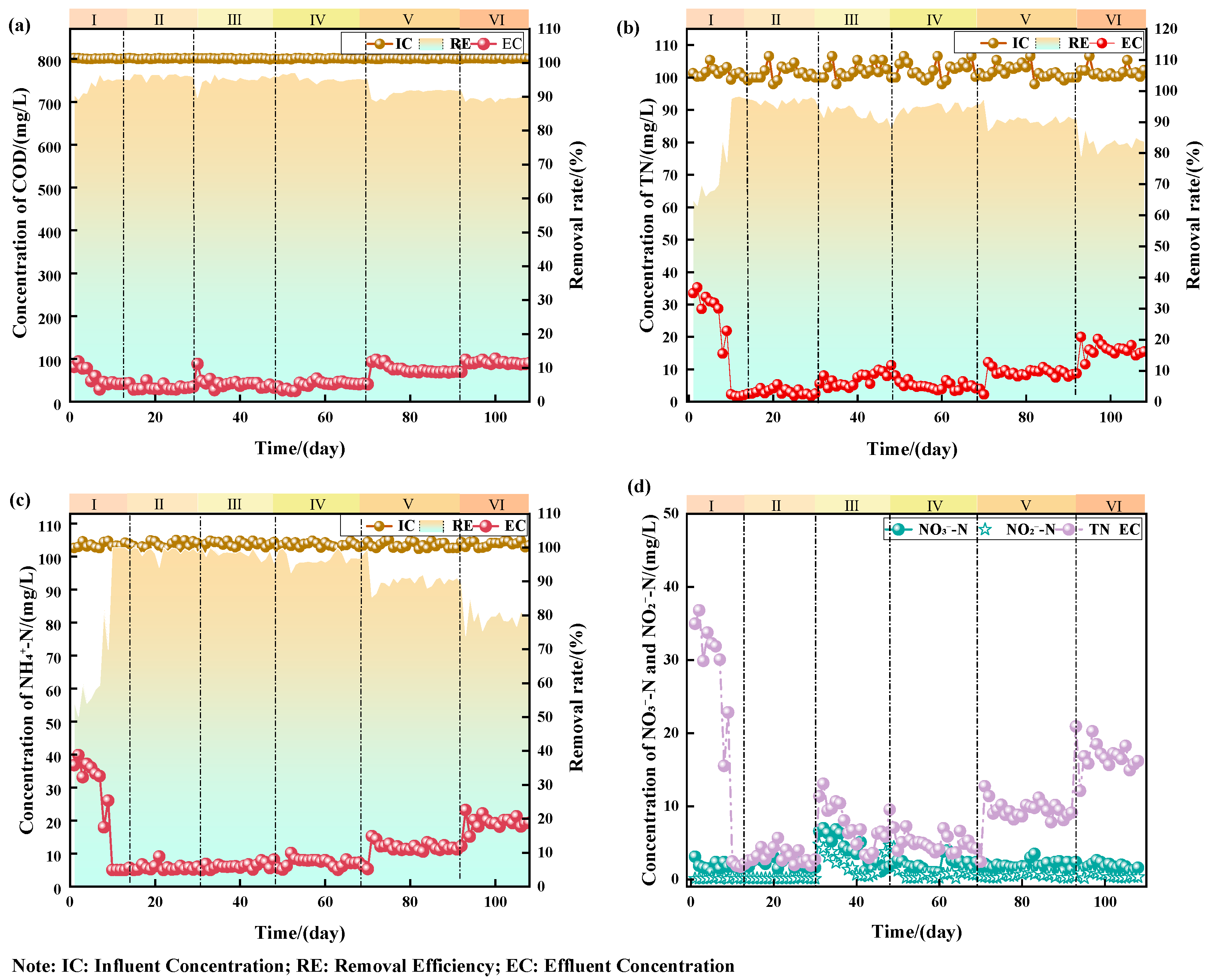
3.1. Effect of Salinity on Pollutant Removal Performance of the Reactor
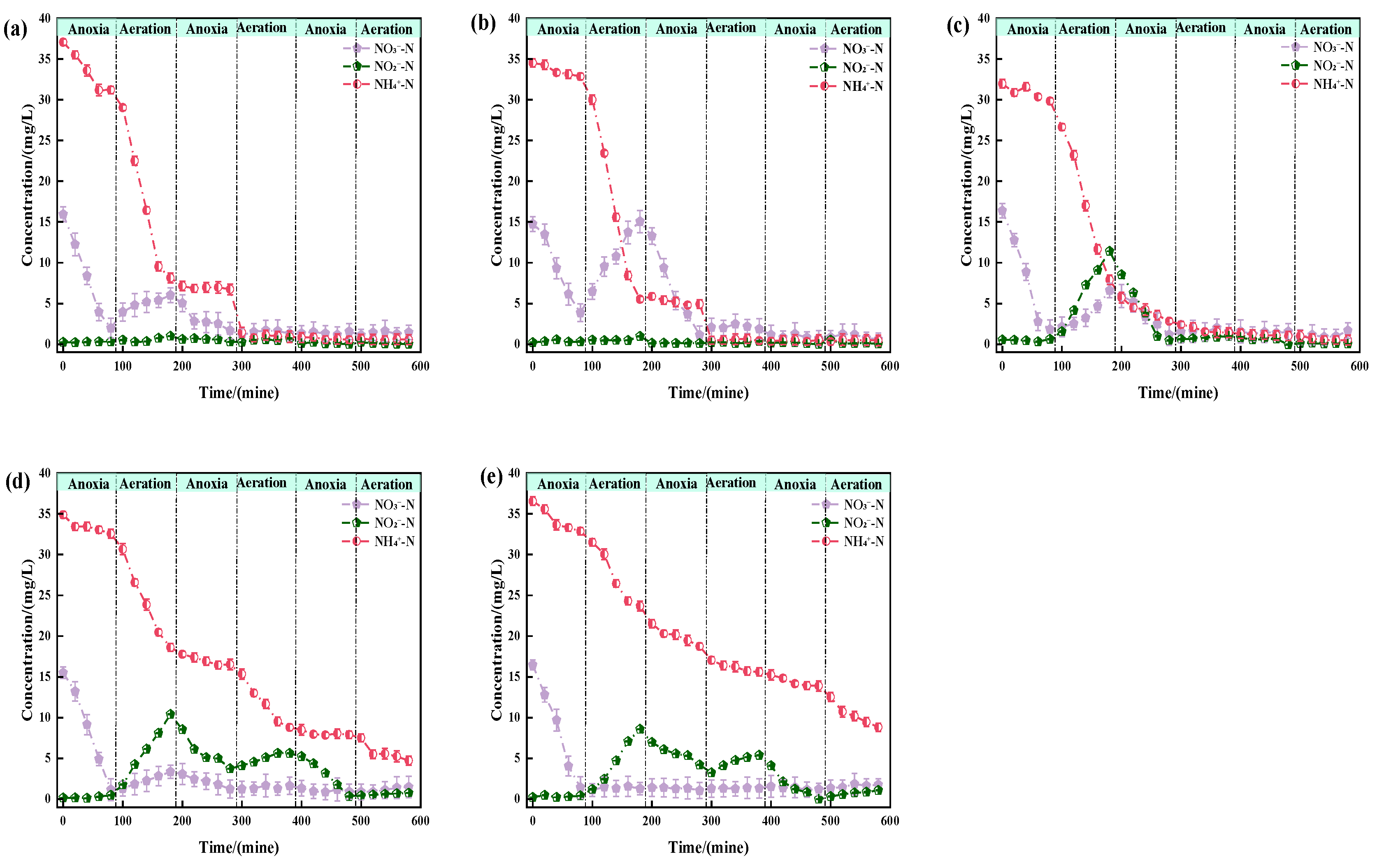
3.2. Effect of Salinity on Nitrogen Removal Pathways
3.3. Effect of Salinity on Activated Sludge Performance
3.3.1. Effect of Salinity on Activated Sludge Settling Performance
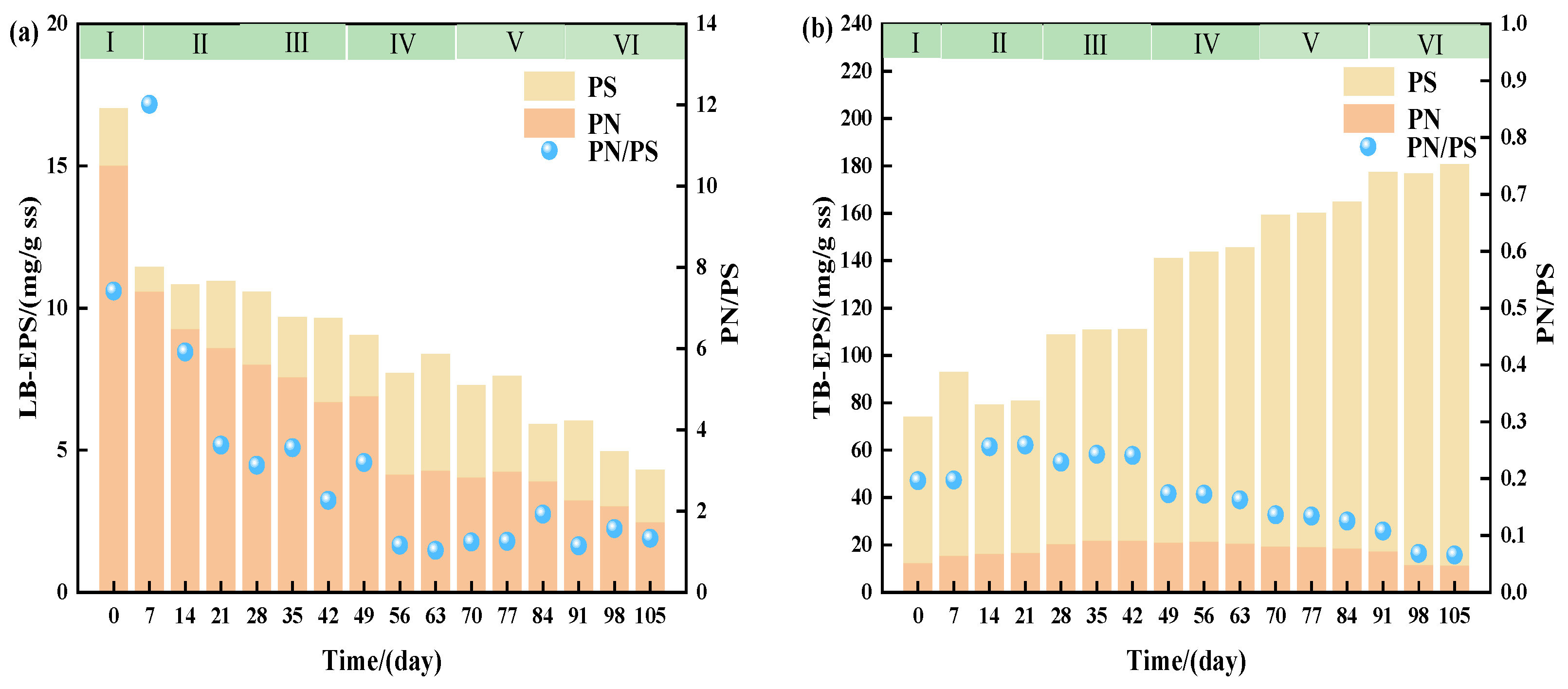
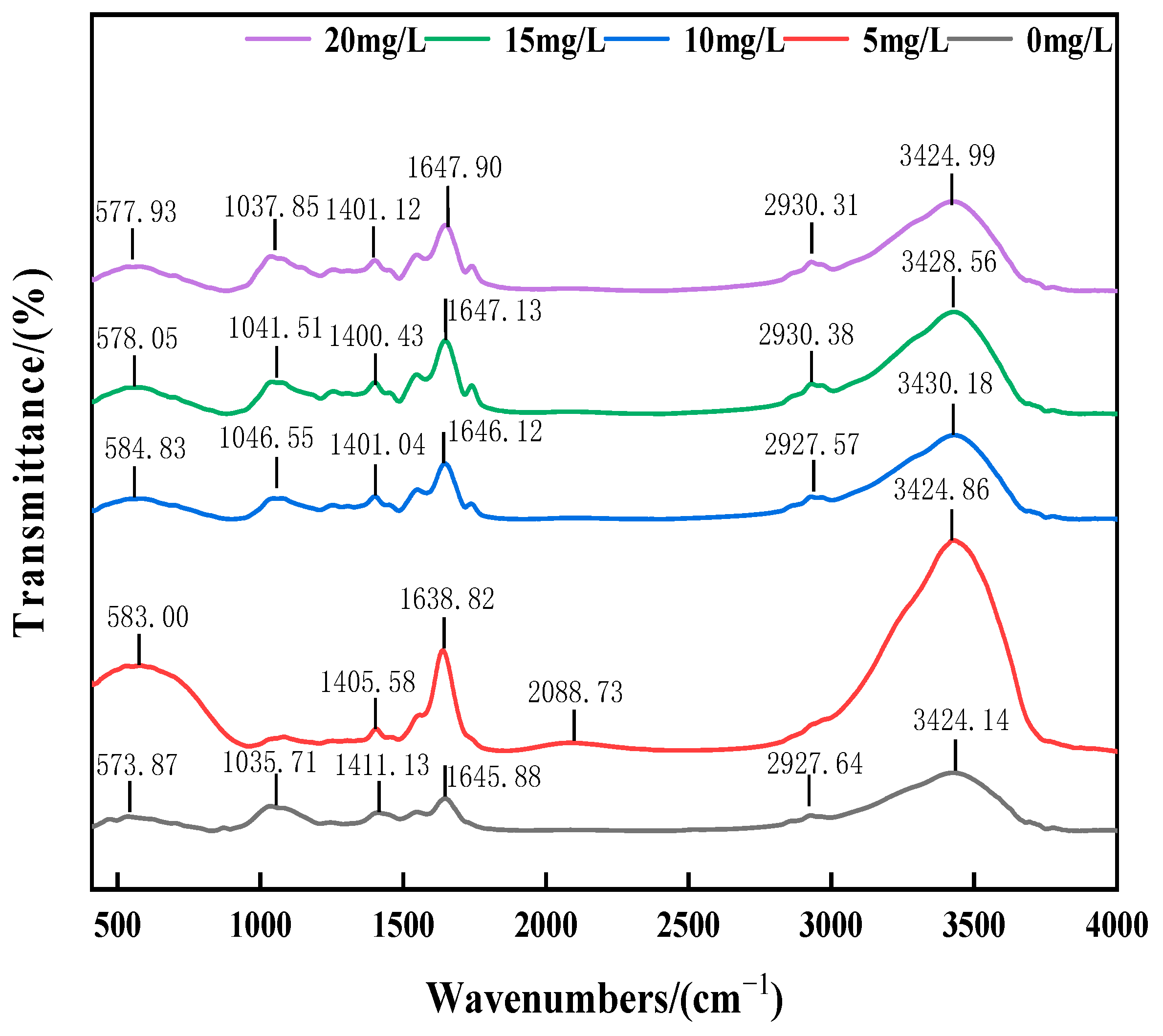
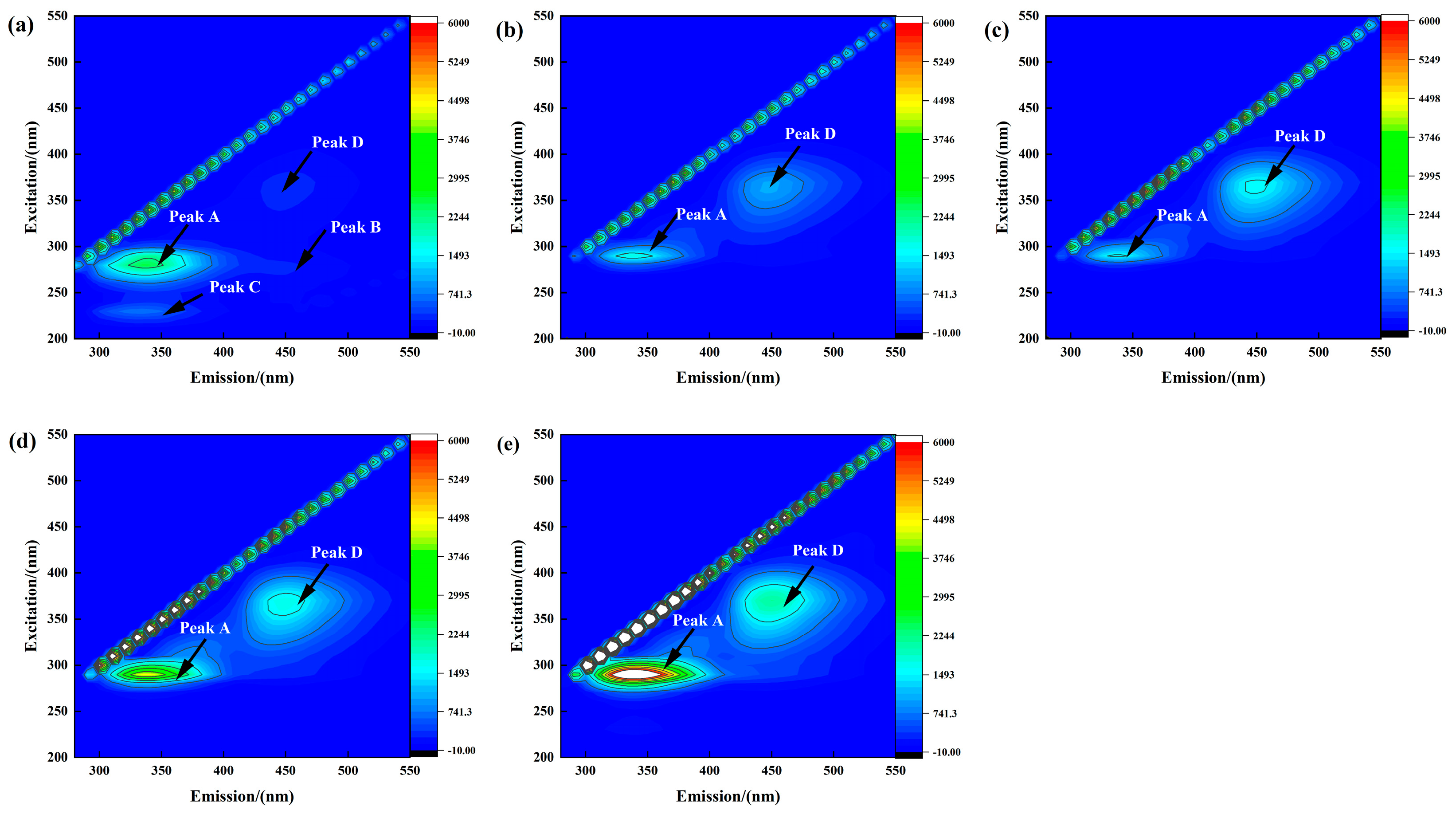
3.3.2. Effect of Salinity on EPS
3.4. Effect of Salinity on Microbial Community Structure
4. Conclusions
- (1)
- The nitrogen removal efficiency of the three-stage AO process and microbial adaptation mechanisms exhibit significant threshold effects related to the salinity. A critical salinity threshold of 10 g/L was identified: Below this level, the system stably maintained CODcr, TN, and NH4+-N removal rates > 98%, meeting Class 1A standards. Above 10 g/L, stronger inhibition of NOB compared to AOB resulted in nitrite accumulation, triggering a shift in the nitrogen removal pathways from traditional nitrification–denitrification (ND) to partial nitrification (PN) and denitrification. This caused a sharp decline in the TN removal efficiency with the increasing salinity (dropping to 83% at 20 g/L).
- (2)
- Microorganisms adapted to high-salt stress through dual strategies: (1) Enhanced resistance via extracellular polymeric substances (EPSs): Increased polysaccharide (PS) production dominated EPS secretion, while elevated TB-EPS proportions optimized sludge settleability. (2) Functional microbial community restructuring: Despite reduced Proteobacteria abundance, synergistic coupling with Planctomycetota established novel nitrogen removal metabolic pathways. High-salt-tolerant denitrifying bacteria like Thauera became dominant nitrogen removal contributors in later stages.
5. Additional Requirements
- At sodium chloride concentrations of 0–10 g/L, the three-stage AO system achieves stable CODcr, TN, and NH4+-N removal efficiencies above 98%, meeting the first-grade A standard in China’s discharge standards (GB 18918-2002). At a sodium chloride concentration above 20 g/L, removal efficiency significantly declines.
- When sodium chloride concentrations exceed 10 g/L, AOB and NOB are inhibited, shifting from the conventional ND pathway to PN and denitrification pathways, causing NO2−-N accumulation and reduced nitrogen removal efficiency (NAR).
- The EPS content rises with the salinity, with TB-EPS exceeding LB-EPS; PS increases more than PN, enhancing sludge salt tolerance and settling properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alattabi, A.W.; Harris, C.B.; Alkhaddar, R.M.; Ortoneda-Pedrola, M.; Alzeyadi, A.T. An investigation into the effect of MLSS on the effluent quality and sludge settleability in an aerobic-anoxic sequencing batch reactor (AASBR). J. Water Process Eng. 2019, 30, 100479. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Q.; Li, J.; Gao, R.; Kao, C.; Li, X.; Peng, Y. Nitrogen removal from high-saline municipal wastewater via anammox-based process driven by both nitritation and denitratation. Chem. Eng. J. 2023, 462, 142269. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, H.; Zhou, L.; Yang, J.; Zhang, K.; Yuan, X.; Ma, J.; Qian, Y. Effect of the rapid increase of salinity on anoxic-oxic biofilm reactor for treatment of high-salt and high-ammonia–nitrogen wastewater. Bioresour. Technol. 2021, 337, 125363. [Google Scholar] [CrossRef]
- Cataldo, S.; Iannì, A.; Loddo, V.; Mirenda, E.; Palmisano, L.; Parrino, F.; Piazzese, D. Combination of advanced oxidation processes and active carbons adsorption for the treatment of simulated saline wastewater. Sep. Purif. Technol. 2016, 171, 101–111. [Google Scholar] [CrossRef]
- Dahmardeh, H.; Akhlaghi Amiri, H.A.; Nowee, S.M. Evaluation of mechanical vapor recompression crystallization process for treatment of high salinity wastewater. Chem. Eng. Process.-Process Intensif. 2019, 145, 107682. [Google Scholar] [CrossRef]
- Song, Q.; Chen, X.; Hua, Y.; Chen, S.; Ren, L.; Dai, X. Biological treatment processes for saline organic wastewater and related inhibition mechanisms and facilitation techniques: A comprehensive review. Environ. Res. 2023, 239, 117404. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.; Ning, F.; Zhang, S.; Hou, Y.; Gao, M.; Wang, J.; Zhang, A.; Liu, Y. Resistance and adaptation of mature algal-bacterial granular sludge under salinity stress. Sci. Total Environ. 2023, 861, 160558. [Google Scholar] [CrossRef]
- Cao, T.N.-D.; Bui, X.-T.; Le, L.-T.; Dang, B.-T.; Tran, D.P.-H.; Vo, T.-K.-Q.; Tran, H.-T.; Nguyen, T.-B.; Mukhtar, H.; Pan, S.-Y.; et al. An overview of deploying membrane bioreactors in saline wastewater treatment from perspectives of microbial and treatment performance. Bioresour. Technol. 2022, 363, 127831. [Google Scholar] [CrossRef]
- Lin, L.; Pratt, S.; Li, Z.; Ye, L. Adaptation and evolution of freshwater Anammox communities treating saline/brackish wastewater. Water Res. 2021, 207, 117815. [Google Scholar] [CrossRef]
- Gomes, M.B.; Gonzales-Limache, E.E.; Sousa, S.T.P.; Dellagnezze, B.M.; Sartoratto, A.; Silva, L.C.F.; Gieg, L.M.; Valoni, E.; Souza, R.S.; Torres, A.P.R.; et al. Exploring the potential of halophilic bacteria from oil terminal environments for biosurfactant production and hydrocarbon degradation under high-salinity conditions. Int. Biodeterior. Biodegrad. 2018, 126, 231–242. [Google Scholar] [CrossRef]
- Chhetri, R.K.; Karvelas, S.; Sanchez, D.F.; Droumpali, A.; Kokkoli, A.; Andersen, H.R. A modified nitrification inhibition test for high-salinity wastewater. Chem. Eng. J. 2022, 429, 132460. [Google Scholar] [CrossRef]
- Ma, T.; Sun, S.; Fu, G.T.; Hall, J.W.; Ni, Y.; He, L.H.; Yi, J.W.; Zhao, N.; Du, Y.Y.; Pei, T.; et al. Pollution exacerbates China’s water scarcity and its regional inequality. Nat. Commun. 2020, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chu, G.; Wang, Q.; Zhang, Z.; Lu, S.; She, Z.; Zhao, Y.; Jin, C.; Guo, L.; Ji, J.; et al. Metagenomic analysis and nitrogen removal performance evaluation of activated sludge from a sequencing batch reactor under different salinities. J. Environ. Manag. 2022, 323, 116213. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Liu, J.; Huang, Y.; Yang, H.; Deng, L. How to combine SBR and A/O process for pollutant removal in treating digested effluent of swine wastewater. J. Environ. Manag. 2025, 375, 124189. [Google Scholar] [CrossRef]
- Lou, T.; Peng, Z.; Jiang, K.; Niu, N.; Wang, J.; Liu, A. Nitrogen removal characteristics of biofilms in each area of a full-scale AAO oxidation ditch process. Chemosphere 2022, 302, 134871. [Google Scholar] [CrossRef]
- Jijingi, H.E.; Yazdi, S.K.; Abakar, Y.A.; Etim, E. Evaluation of membrane bioreactor (MBR) technology for industrial wastewater treatment and its application in developing countries: A review. Case Stud. Chem. Environ. Eng. 2024, 10, 100886. [Google Scholar] [CrossRef]
- Yan, Z.; Han, X.; Wang, H.; Jin, Y.; Song, X. Influence of aeration modes and DO on simultaneous nitrification and denitrification in treatment of hypersaline high-strength nitrogen wastewater using sequencing batch biofilm reactor (SBBR). J. Environ. Manag. 2024, 359, 121075. [Google Scholar] [CrossRef]
- Zhao, H. A new technology for the effluent of the municipal sewage treatment plant to reach the first-class A standard. Water Supply Drain. Dyn. 2011, 2. [Google Scholar]
- Salama, Y.; Chennaoui, M.; Sylla, A.; Mountadar, M.; Rihani, M.; Assobhei, O. Characterization, structure, and function of extracellular polymeric substances (EPS) of microbial biofilm in biological wastewater treatment systems: A review. Desalination Water Treat. 2016, 57, 16220–16237. [Google Scholar] [CrossRef]
- She, Z.L.; Zhao, L.T.; Zhang, X.L.; Jin, C.J.; Guo, L.; Yang, S.Y.; Zhao, Y.G.; Gao, M.C. Partial nitrification and denitrification in a sequencing batch reactor treating high-salinity wastewater. Chem. Eng. J. 2016, 288, 207–215. [Google Scholar] [CrossRef]
- Zhang, M.; Jiao, T.; Chen, S.; Zhou, W. A review of microbial nitrogen transformations and microbiome engineering for biological nitrogen removal under salinity stress. Chemosphere 2023, 341, 139949. [Google Scholar] [CrossRef] [PubMed]
- Corsino, S.F.; Capodici, M.; Di Pippo, F.; Tandoi, V.; Torregrossa, M. Comparison between kinetics of autochthonous marine bacteria in activated sludge and granular sludge systems at different salinity and SRTs. Water Res. 2019, 148, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, Y.; Li, L.; Pen, Y.; Wang, S. Simultaneous nitritation and denitritation of domestic wastewater without addition of external carbon sources at limited aeration and normal temperatures. Desalination Water Treat. 2010, 21, 210–219. [Google Scholar] [CrossRef]
- Lin, C.; Liu, Y.; Li, Y.-Y.; Liu, J. Achieving rapid start-up of mainstream partial nitritation by sludge treatment using high salinity. Chem. Eng. J. 2024, 483, 149290. [Google Scholar] [CrossRef]
- Wang, Z.; van Loosdrecht, M.C.M.; Saikaly, P.E. Gradual adaptation to salt and dissolved oxygen: Strategies to minimize adverse effect of salinity on aerobic granular sludge. Water Res. 2017, 124, 702–712. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Lv, J.; Wu, D.; Jiang, L.; Lv, W. Short-time aerobic digestion treatment of waste activated sludge to enhance EPS production and sludge dewatering performance by changing microbial communities: The impact of temperature. Water Cycle 2024, 5, 146–155. [Google Scholar] [CrossRef]
- Moussa, M.S.; Sumanasekera, D.U.; Ibrahim, S.H.; Lubberding, H.J.; Hooijmans, C.M.; Gijzen, H.J.; van Loosdrecht, M.C.M. Long term effects of salt on activity, population structure and floc characteristics in enriched bacterial cultures of nitrifiers. Water Res. 2006, 40, 1377–1388. [Google Scholar] [CrossRef]
- Pronk, M.; Bassin, J.P.; de Kreuk, M.K.; Kleerebezem, R.; van Loosdrecht, M.C.M. Evaluating the main and side effects of high salinity on aerobic granular sludge. Appl. Microbiol. Biotechnol. 2014, 98, 1339–1348. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Gao, M.; Wang, Y.; Yang, Z.; Xu, H.; Wang, X.-H. Enhanced aerobic granulation at low temperature by stepwise increasing of salinity. Sci. Total Environ. 2020, 722, 137660. [Google Scholar] [CrossRef]
- Sivasubramanian, R.; Chen, G.-h.; Mackey, H.R. Shock effects of monovalent cationic salts on seawater cultivated granular sludge. J. Hazard. Mater. 2021, 403, 123646. [Google Scholar] [CrossRef]
- Badireddy, A.R.; Chellam, S.; Gassman, P.L.; Engelhard, M.H.; Lea, A.S.; Rosso, K.M. Role of extracellular polymeric substances in bioflocculation of activated sludge microorganisms under glucose-controlled conditions. Water Res. 2010, 44, 4505–4516. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, Y.; Wan, P.; Yu, X.; Chen, H.; Lv, W. New perspectives on enhancing waste activated sludge dewaterability through extracellular polymeric substances (EPS) biodegradation: Investigating the impact of EPS stratification and conversion. J. Water Process Eng. 2024, 65, 105803. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, M.; Wang, Z.; She, Z.; Chang, Q.; Sun, C.; Zhang, J.; Ren, Y.; Yang, N. Effect of salinity on extracellular polymeric substances of activated sludge from an anoxic–aerobic sequencing batch reactor. Chemosphere 2013, 93, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.J.; Baudon, A.; Chow, A.T. Improved Fluorescence Excitation-Emission Matrix Regional Integration to Quantify Spectra for Fluorescent Dissolved Organic Matter. J. Environ. Qual. 2013, 42, 925–930. [Google Scholar] [CrossRef]
- Gao, M.; Yang, J.; Liu, Y.; Zhang, J.; Li, J.; Liu, Y.; Wu, B.; Gu, L. Deep insights into the anaerobic co-digestion of waste activated sludge with concentrated leachate under different salinity stresses. Sci. Total Environ. 2022, 838, 155922. [Google Scholar] [CrossRef]
- Miao, L.; Wang, S.; Li, B.; Cao, T.; Zhang, F.; Wang, Z.; Peng, Y. Effect of carbon source type on intracellular stored polymers during endogenous denitritation (ED) treating landfill leachate. Water Res. 2016, 100, 405–412. [Google Scholar] [CrossRef]
- Wen, X.; Cui, L.; Lin, H.; Zhu, W.; Shao, Z.; Wang, Y. Comparison of nitrification performance in SBR and SBBR with response to NaCl salinity shock: Microbial structure and functional genes. Environ. Res. 2024, 252, 118917. [Google Scholar] [CrossRef]
- Liu, J.; Hu, M.; Hu, M.; Wang, J.; Zhang, T.; Wang, Y.; Wang, X. Responses of suspended sludge and biofilm in a SNAD system under C/N elevation: Microbial activity, nitrogen conversion flux and molecular ecological network. Sci. Total Environ. 2024, 954, 176236. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Fu, H.-M.; Shen, Y.; Wang, J.; Wang, N.; Chen, Y.-P.; Yan, P. Biosynthetic potential of uncultured anammox community bacteria revealed through multi-omics analysis. Bioresour. Technol. 2024, 401, 130740. [Google Scholar] [CrossRef]
- Rubio-Rincón, F.J.; Lopez-Vazquez, C.M.; Welles, L.; van Loosdrecht, M.C.M.; Brdjanovic, D. Cooperation between Candidatus Competibacter and Candidatus Accumulibacter clade I, in denitrification and phosphate removal processes. Water Res. 2017, 120, 156–164. [Google Scholar] [CrossRef]
- Zhang, K.; Miao, Y.; Zou, J.; Wang, S.; Hu, C.; Wang, Z. The start-up of anammox system inoculated with sludge overwhelmingly dominated by Candidatus Competibacter: System performance and microbial community succession. J. Water Process Eng. 2024, 57, 104605. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Ren, S.; Qiu, J.; Zhang, Q.; Li, X.; Peng, Y. Enrichment and retention of key functional bacteria of partial denitrification-Anammox (PD/A) process via cell immobilization: A novel strategy for fast PD/A application. Bioresour. Technol. 2021, 326, 124744. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Sun, L.; Chen, Z.; Guo, X.; Qu, Y. Simultaneous selenite reduction and nitrogen removal using Paracoccus sp.: Reactor performance, microbial community, and mechanism. Environ. Res. 2024, 240, 117564. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Chi, Y.; Wang, Y.; Shi, X.; Jin, X.; Jin, P. Diversified metabolism makes novel Thauera strain highly competitive in low carbon wastewater treatment. Water Res. 2021, 206, 117742. [Google Scholar] [CrossRef]




| Component | Concentration (mg/L) | Component | Concentration (mg/L) |
|---|---|---|---|
| Sodium acetate | 5120 | Iron (III) chloride | 1500 |
| Magnesium sulfate | 90 | Copper (II) sulfate | 30 |
| Calcium chloride | 14 | Potassium iodide | 180 |
| Ammonium chloride | 1340 | Manganese chloride | 153 |
| Dipotassium hydrogen phosphate | 184 | Sodium molybdate | 60 |
| Yeast extract | 10 | Boric acid | 150 |
| Cobalt chloride hexahydrate | 150 | Zinc sulfate | 120 |
| EDTA | 10,000 |
| Parameter Indicator | Analytical Method |
|---|---|
| CODcr | Rapid digestion spectrophotometry (HJ/T 399-2007) |
| NH4+-N | Nessler’s reagent spectrophotometry (HJ 535-2009) |
| NO3−-N | UV spectrophotometry (SL 84-1994) |
| NO2−-N | Spectrophotometry (GB 7493-1987) |
| TN | Alkaline persulfate digestion–UV spectrophotometry (HJ 636-2012) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Cui, P.; Wang, S.; Long, J.; Yang, X. Effects of High Salinity on Nitrogen Removal Efficiency and Microbial Community Structure in a Three-Stage AO System. Water 2025, 17, 1112. https://doi.org/10.3390/w17081112
Shi S, Cui P, Wang S, Long J, Yang X. Effects of High Salinity on Nitrogen Removal Efficiency and Microbial Community Structure in a Three-Stage AO System. Water. 2025; 17(8):1112. https://doi.org/10.3390/w17081112
Chicago/Turabian StyleShi, Shengyu, Pengfei Cui, Shasha Wang, Jun Long, and Xiaojun Yang. 2025. "Effects of High Salinity on Nitrogen Removal Efficiency and Microbial Community Structure in a Three-Stage AO System" Water 17, no. 8: 1112. https://doi.org/10.3390/w17081112
APA StyleShi, S., Cui, P., Wang, S., Long, J., & Yang, X. (2025). Effects of High Salinity on Nitrogen Removal Efficiency and Microbial Community Structure in a Three-Stage AO System. Water, 17(8), 1112. https://doi.org/10.3390/w17081112






