An Investigation of the Impact of Flocculants on Process Optimization and Floc Properties in Chlorella vulgaris FACHB-15 Harvesting
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Microalgae and Preparation of Flocculants
2.2. Flocculation Trials
2.2.1. Influence of Flocculant Dosage and Standing Duration on Flocculation Effectiveness
2.2.2. Influence of pH on Flocculation Efficacy
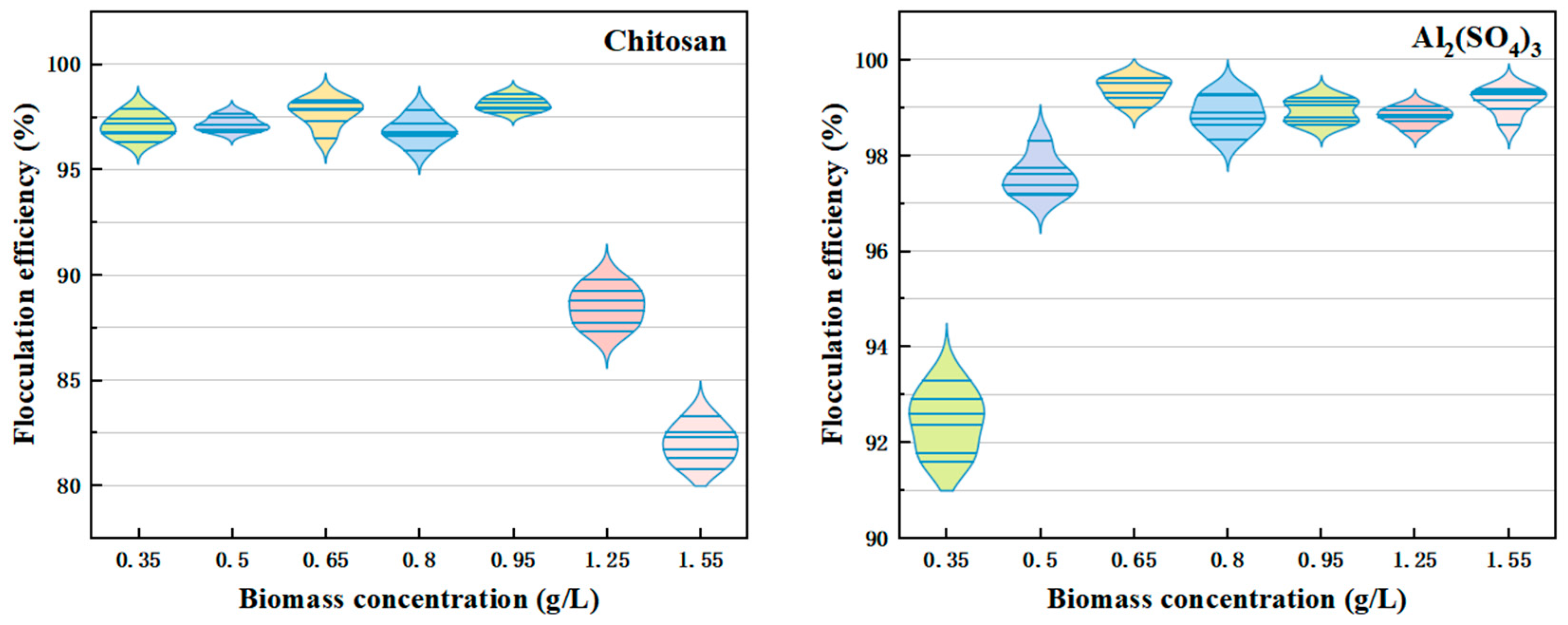
2.2.3. Impact of Biomass on Flocculation Efficacy
2.3. Experimental Design for Response Surfaces
2.4. Assessment of Flocculation Effectiveness
2.5. Extracellular Polymers (EPS)
2.6. Identification of Functional Groupings in Microalgal Cells
3. Results and Discussion
3.1. One-Factor Experiment on C. vulgaris Flocculation
3.1.1. Effect of Dosage
3.1.2. Influence of pH
3.1.3. Impact of Biomass Concentration
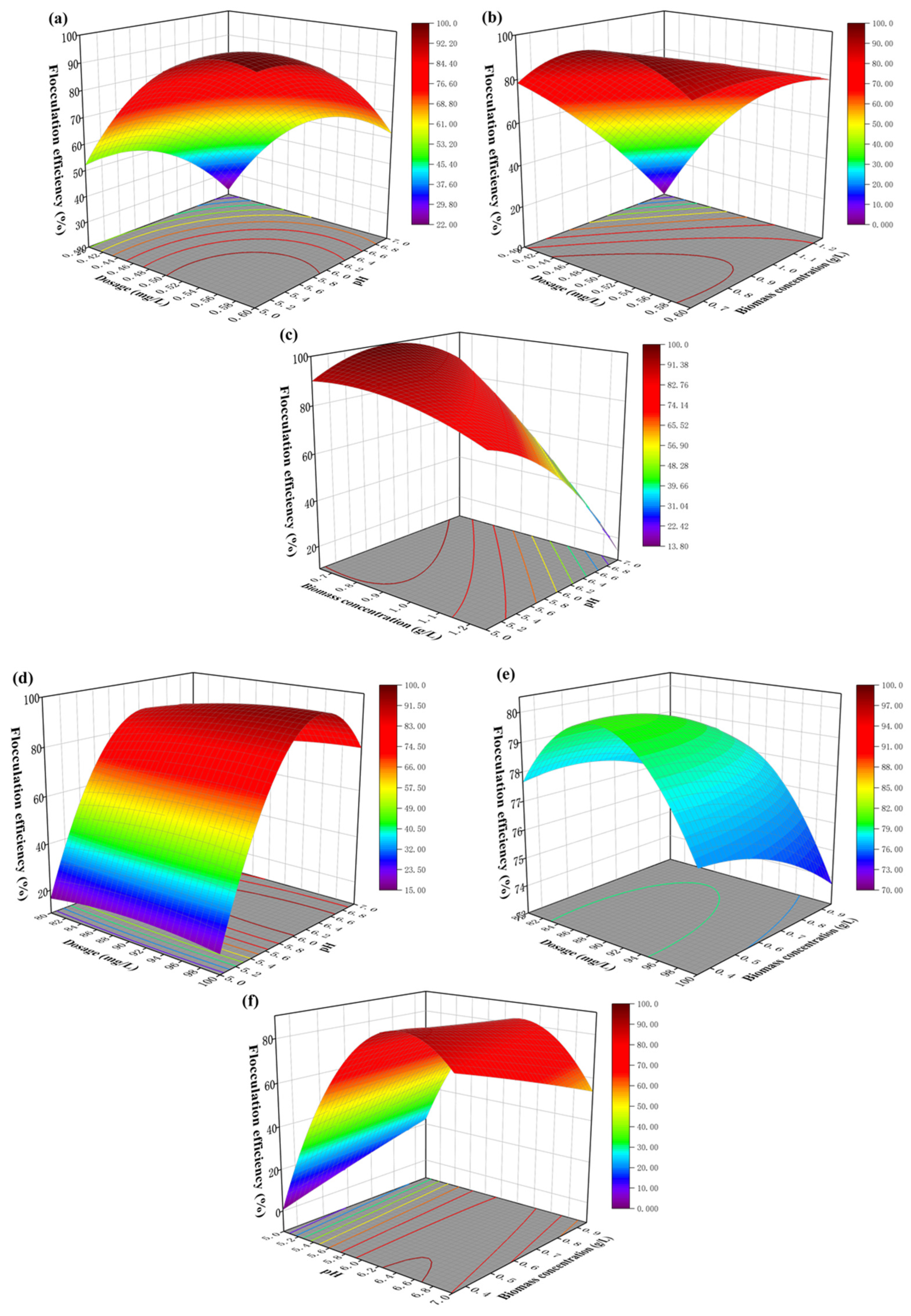
3.2. Response Surface Experimental Design and Analysis
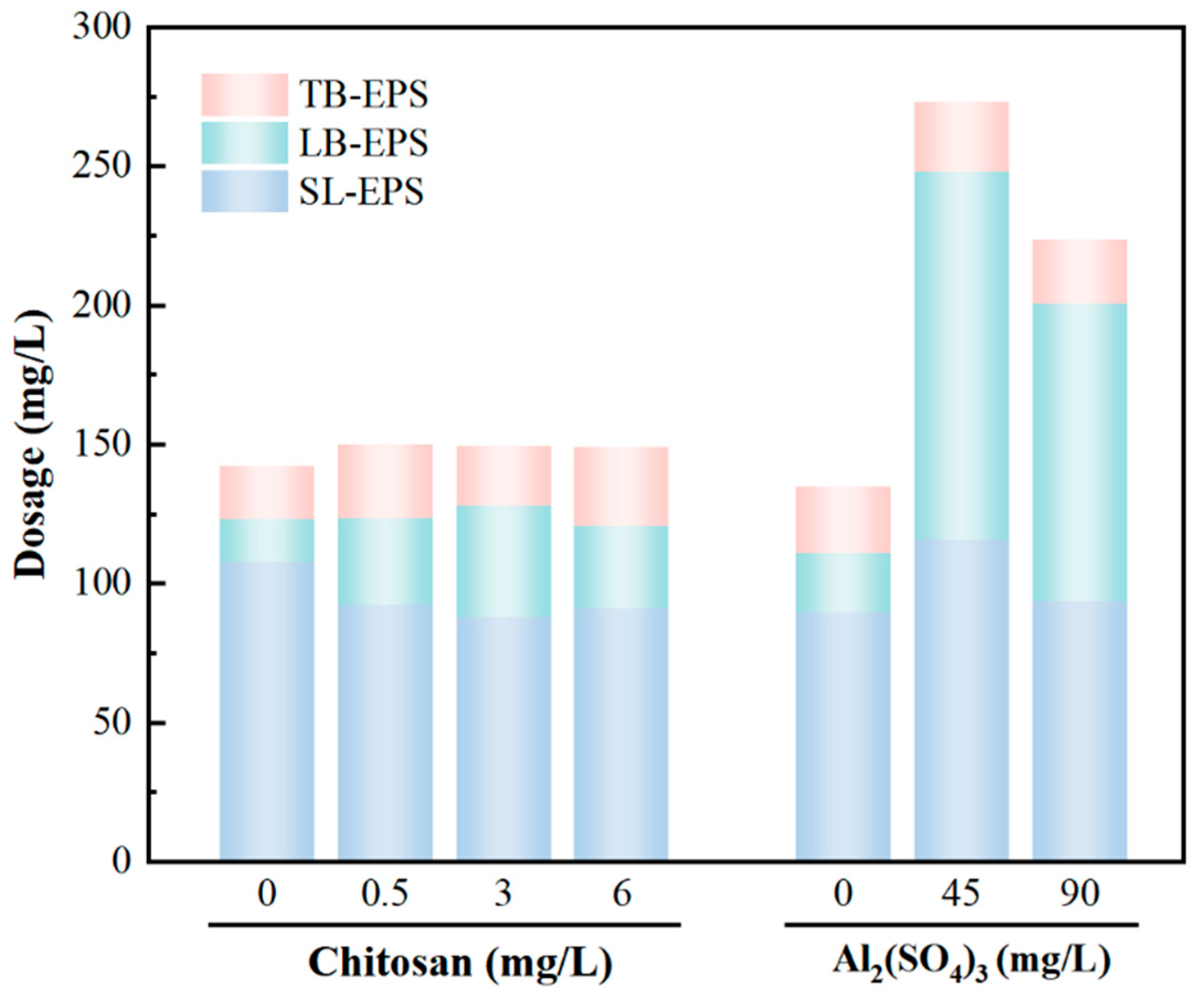
3.3. Composition of the Flocculent
3.3.1. Floc Compaction
3.3.2. Extracellular Polymers of Chlorella
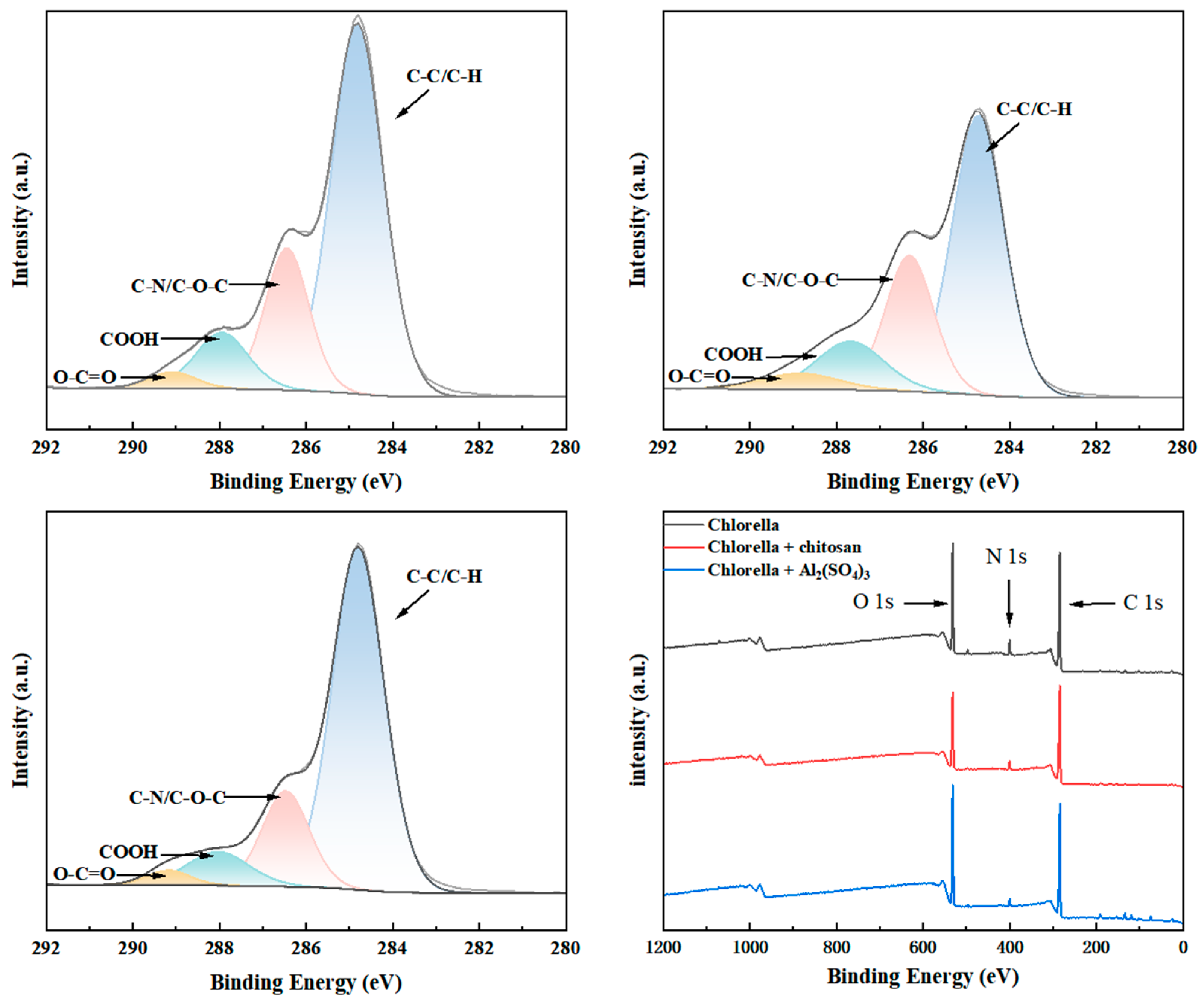
3.3.3. Ratio of Functional Groups on the Surface of Algal Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent developments and key barriers to advanced biofuels: A short review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Choi, H.I.; Sim, S.J. Microalgal fuels: Promising energy reserves for the future. Fuel 2022, 312, 122841. [Google Scholar]
- Jin, Y.; Li, Y.; Qi, Y.; Wei, Q.; Yang, G.; Ma, X. A modified cultivation strategy to enhance biomass production and lipid accumulation of Tetradesmus obliquus FACHB-14 with copper stress and light quality induction. Bioresour. Technol. 2024, 400, 130677. [Google Scholar]
- Cai, Y.; Lim, H.R.; Khoo, K.S.; Ng, H.S.; Cai, Y.; Wang, J.; Tak-Yee Chan, A.; Show, P.L. An integration study of microalgae bioactive retention: From microalgae biomass to microalgae bioactives nanoparticle. Food Chem. Toxicol. 2021, 158, 112607. [Google Scholar] [CrossRef] [PubMed]
- Bazarnova, J.; Nilova, L.; Trukhina, E.; Bernavskaya, M.; Smyatskaya, Y.; Aktar, T. Use of Microalgae Biomass for Fortification of Food Products from Grain. Foods 2021, 10, 3018. [Google Scholar] [CrossRef]
- García-Pérez, J.S.; Beuckels, A.; Vandamme, D.; Depraetere, O.; Foubert, I.; Parra, R.; Muylaert, K. Influence of magnesium concentration, biomass concentration and pH on flocculation of Chlorella vulgaris. Algal Res. 2014, 3, 24–29. [Google Scholar]
- Deepa, P.; Sowndhararajan, K.; Kim, S. A Review of the Harvesting Techniques of Microalgae. Water 2023, 15, 3074. [Google Scholar] [CrossRef]
- Udom, I.; Zaribaf, B.H.; Halfhide, T.; Gillie, B.; Dalrymple, O.; Zhang, Q.; Ergas, S.J. Harvesting microalgae grown on wastewater. Bioresour. Technol. 2013, 139, 101–106. [Google Scholar]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar]
- Leite, L.d.S.; Hoffmann, M.T.; Daniel, L.A. Coagulation and dissolved air flotation as a harvesting method for microalgae cultivated in wastewater. J. Water Process Eng. 2019, 32, 100947. [Google Scholar]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Ho, Q.N.; Fettweis, M.; Hur, J.; Desmit, X.; Kim, J.I.; Jung, D.W.; Lee, S.D.; Lee, S.; Choi, Y.Y.; Lee, B.J. Flocculation kinetics and mechanisms of microalgae- and clay-containing suspensions in different microalgal growth phases. Water Res. 2022, 226, 119300. [Google Scholar] [PubMed]
- Okoro, V.; Azimov, U.; Munoz, J.; Hernandez, H.H.; Phan, A.N. Microalgae cultivation and harvesting: Growth performance and use of flocculants—A review. Renew. Sustain. Energy Rev. 2019, 115, 109364. [Google Scholar]
- Kuzhiumparambil, U.; Labeeuw, L.; Commault, A.; Vu, H.P.; Nguyen, L.N.; Ralph, P.J.; Nghiem, L.D. Effects of harvesting on morphological and biochemical characteristics of microalgal biomass harvested by polyacrylamide addition, pH-induced flocculation, and centrifugation. Bioresour. Technol. 2022, 359, 127433. [Google Scholar]
- Sanyano, N.; Chetpattananondh, P.; Chongkhong, S. Coagulation–flocculation of marine Chlorella sp. for biodiesel production. Bioresour. Technol. 2013, 147, 471–476. [Google Scholar]
- Long, Q.; Chen, X.; Feng, Y.; He, X.; Gu, H.; Huang, T.; Zhao, P. Effective harvesting of the microalga Monoraphidium sp. QLY-1: Comparison of different flocculants. J. Appl. Phycol. 2024, 36, 1143–1151. [Google Scholar]
- Nguyen, L.N.; Labeeuw, L.; Commault, A.S.; Emmerton, B.; Ralph, P.J.; Johir, M.A.H.; GUO, W.; Ngo, H.H.; Nghiem, L.D. Validation of a cationic polyacrylamide flocculant for the harvesting fresh and seawater microalgal biomass. Environ. Technol. Innov. 2019, 16, 100466. [Google Scholar]
- Toh, P.Y.; Azenan, N.F.; Wong, L.; Ng, Y.S.; Chng, L.M.; Lim, J.; Chan, D.J.C. The Role of Cationic Coagulant-to-Cell Interaction in Dictating the Flocculation-Aided Sedimentation of Freshwater Microalgae. Arab. J. Sci. Eng. 2018, 43, 2217–2225. [Google Scholar]
- You, Y.; Yang, L.; Sun, X.; Chen, H.; Wang, H.; Wang, N.; Li, S. Synthesized cationic starch grafted tannin as a novel flocculant for efficient microalgae harvesting. J. Clean. Prod. 2022, 344, 131042. [Google Scholar]
- Nguyen, T.D.P.; Le, T.V.A.; Show, P.L.; Tran, M.H.; Tran, T.N.T.; Lee, S.Y. Bioflocculation formation of microalgae-bacteria in enhancing microalgae harvesting and nutrient removal from wastewater effluent. Bioresour. Technol. 2019, 272, 34–39. [Google Scholar]
- Cui, Y.; Yuan, W.; Cheng, J. Understanding pH and Ionic Strength Effects on Aluminum Sulfate-Induced Microalgae Flocculation. Appl. Biochem. Biotechnol. 2014, 173, 1692–1702. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Lin, L.; Zhang, C.; Li, A.; Zhu, Y.; Zhang, Y. Evaluation of several flocculants for flocculating microalgae. Bioresour. Technol. 2015, 197, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hu, T.; Li, S.; Nugroho, Y.K.; Li, B.; Cao, J.; Show, P.-L.; Hiltunen, E. Effects of operating parameters on algae Chlorella vulgaris biomass harvesting and lipid extraction using metal sulfates as flocculants. Biomass Bioenergy 2020, 132, 105433. [Google Scholar] [CrossRef]
- Reyes, J.F.; Labra, C. Biomass harvesting and concentration of microalgae scenedesmus sp. cultivated in a pilot phobioreactor. Biomass Bioenergy 2016, 87, 78–83. [Google Scholar] [CrossRef]
- Chatsungnoen, T.; Chisti, Y. Continuous flocculation-sedimentation for harvesting Nannochloropsis salina biomass. J. Biotechnol. 2016, 222, 94–103. [Google Scholar] [CrossRef]
- Chae, K.-S.; Shin, C.-S.; Shin, W.-S. Characteristics of cricket (Gryllus bimaculatus) chitosan and chitosan-based nanoparticles. Food Sci. Biotechnol. 2018, 27, 631–639. [Google Scholar] [CrossRef]
- Yin, Z.; Chu, R.; Zhu, L.; Li, S.; Mo, F.; Hu, D.; Liu, C. Application of chitosan-based flocculants to harvest microalgal biomass for biofuel production: A review. Renew. Sustain. Energy Rev. 2021, 145, 111159. [Google Scholar] [CrossRef]
- Lama, S.; Muylaert, K.; Karki, T.B.; Foubert, I.; Henderson, R.K.; Vandamme, D. Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresour. Technol. 2016, 220, 464–470. [Google Scholar] [CrossRef]
- Yunos, F.H.M.; Nasir, N.M.; Jusoh, H.H.W.; Khatoon, H.; Lam, S.S.; Jusoh, A. Harvesting of microalgae (Chlorella sp.) from aquaculture bioflocs using an environmental-friendly chitosan-based bio-coagulant. Int. Biodeterior. Biodegrad. 2017, 124, 243–249. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Christwardana, M.; Widayat, W.; Jati, A.K.; Laes, S.I. Optimization of flocculation efficiency and settling time using chitosan and eggshell as bio-flocculant in Chlorella pyrenoidosa harvesting process. Environ. Technol. Innov. 2021, 24, 101959. [Google Scholar] [CrossRef]
- Nascimento, W.J., Jr.; Silva, M.; Vieira, M. Competitive fixed-bed biosorption of Ag(I) and Cu(II) ions on Sargassum filipendula seaweed waste. J. Water Process Eng. 2020, 36, 101294. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Miao, L. Harvesting freshwater microalgae with natural polymer flocculants. Algal Res. 2021, 57, 102358. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [PubMed]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Meesschaert, B.; Muylaert, K. Flocculation of Chlorella vulgaris induced by high pH: Role of magnesium and calcium and practical implications. Bioresour. Technol. 2012, 105, 114–119. [Google Scholar]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar]
- Babiak, W.; Krzemińska, I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Blockx, J.; Verfaillie, A.; Thielemans, W.; Muylaert, K. Unravelling the Mechanism of Chitosan-Driven Flocculation of Microalgae in Seawater as a Function of pH. ACS Sustain. Chem. Eng. 2018, 6, 11273–11279. [Google Scholar]
- Endrawati, H.; Widianingsih, W.; Nuraini, R.; Hartati, R.; Redjeki, S.; Riniatsih, I.; Mahendrajaya, R. The effect of chitosan concentration on flocculation efficiency microalgae Porphyridium cruentum (Rhodhophyta). IOP Conf. Ser. Earth Environ. Sci. 2021, 919, 012052. [Google Scholar]
- Van Haver, L.; Nayar, S. Polyelectrolyte flocculants in harvesting microalgal biomass for food and feed applications. Algal Res. 2017, 24, 167–180. [Google Scholar]
- Wang, Q.; Oshita, K.; Takaoka, M. Harvesting Nannochloropsis oculata by Chitosan and AlCl3-Induced Flocculation: Effects of Microalgal Condition on Flocculation Performance. BioEnergy Res. 2021, 14, 924–939. [Google Scholar] [CrossRef]
- Liu, S.; Hajar, H.A.A.; Riefler, G.; Stuart, B.J. Investigation of electrolytic flocculation for microalga Scenedesmus sp. using aluminum and graphite electrodes. RSC Adv. 2018, 8, 38808–38817. [Google Scholar] [PubMed]
- Kirnev, P.C.S.; de Carvalho, J.C.; Miyaoka, J.T.; Cartas, L.C.; Vandenberghe, L.P.S.; Soccol, C.R. Harvesting Neochloris oleoabundans using commercial organic flocculants. J. Appl. Phycol. 2018, 30, 2317–2324. [Google Scholar] [CrossRef]
- Lupa, D.; Płaziński, W.; Michna, A.; Wasilewska, M.; Pomastowski, P.; Gołębiowski, A.; Buszewski, B.; Adamczyk, Z. Chitosan characteristics in electrolyte solutions: Combined molecular dynamics modeling and slender body hydrodynamics. Carbohydr. Polym. 2022, 292, 119676. [Google Scholar] [CrossRef]
- Wei, Q.; Yuan, T.; Li, Z.; Zhao, D.; Wang, C.; Yang, G.; Tang, W.; Ma, X. Investigating cultivation strategies for enhancing protein content in Auxenochlorella pyrenoidosa FACHB-5. Bioresour. Technol. 2024, 402, 130828. [Google Scholar] [CrossRef]
- Wang, Q.; Oshita, K.; Takaoka, M. Evaluation of flocculation performance of amphoteric flocculant when harvesting microalgae Coccomyxa sp. KJ by response surface methodology. J. Environ. Manag. 2021, 277, 111449. [Google Scholar]
- Hadiyanto, H.; Widayat, W.; Pratiwi, M.E.; Christwardana, M.; Muylaert, K. Effect of pH, cationic inducer, and clam shells as bio-flocculant in the optimization of the flocculation process for enhanced microalgae harvesting using response surface methodology. Environ. Pollut. Bioavailab. 2022, 34, 338–351. [Google Scholar]
- Li, Z.; Yuan, T.; Zhao, J.; Wang, C.; Wei, Q.; Ma, X.; Yang, G. Unraveling non-linear dynamics of biomass, photosynthesis efficiency, and lipid accumulation in Chlorella vulgaris under mixotrophic cultivation. J. Clean. Prod. 2024, 448, 141692. [Google Scholar]
- Li, J.; Wang, M.; Li, W.; Zhang, P.; Yu, F.; Li, P. The impact of cell-bound exopolysaccharide on flocculation of the cyanobacterium Synechocystis PCC6803 with ferric chloride and chitosan. J. Appl. Phycol. 2021, 33, 2947–2955. [Google Scholar]
- Shi, Y.; Huang, J.; Zeng, G.; Gu, Y.; Chen, Y.; Hu, Y.; Tang, B.; Zhou, J.; Yang, Y.; Shi, L. Exploiting extracellular polymeric substances (EPS) controlling strategies for performance enhancement of biological wastewater treatments: An overview. Chemosphere 2017, 180, 396–411. [Google Scholar]
- Zhou, Y.; Cui, X.; Wu, B.; Wang, Z.; Liu, Y.; Ren, T.; Xia, S.; Rittmann, B.E. Microalgal extracellular polymeric substances (EPS) and their roles in cultivation, biomass harvesting, and bioproducts extraction. Bioresour. Technol. 2024, 406, 131054. [Google Scholar] [CrossRef]
- Demir, I.; Blockx, J.; Dague, E.; Guiraud, P.; Thielemans, W.; Muylaert, K.; Formosa-Dague, C. Nanoscale Evidence Unravels Microalgae Flocculation Mechanism Induced by Chitosan. ACS Appl. Bio Mater. 2020, 3, 8446–8459. [Google Scholar] [CrossRef]
- Ding, Y.; He, R.; Wang, C.; Wei, Q.; Ma, X.; Yang, G. Efficient separation of Cd2+ and Pb2+ by Tetradesmus obliquus: Insights from cultivation conditions with competitive adsorption modeling. J. Water Process Eng. 2024, 60, 105207. [Google Scholar]
- Gu, S.; Lan, C.Q. Effects of culture pH on cell surface properties and biosorption of Pb(II), Cd(II), Zn(II) of green alga Neochloris oleoabundans. Chem. Eng. J. 2023, 468, 143579. [Google Scholar]
- Hou, X.; Liu, S.; Zhang, Z. Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge. Water Res. 2015, 75, 51–62. [Google Scholar]
- Yang, Z.; Hou, T.; Ma, J.; Yuan, B.; Tian, Z.; Yang, W.; Graham, N.J. Role of moderately hydrophobic chitosan flocculants in the removal of trace antibiotics from water and membrane fouling control. Water Res. 2020, 177, 115775. [Google Scholar] [PubMed]
- Wang, H.; Roman, M. Effects of Chitosan Molecular Weight and Degree of Deacetylation on Chitosan−Cellulose Nanocrystal Complexes and Their Formation. Molecules 2023, 28, 1361. [Google Scholar] [CrossRef]
- Wu, X.; Ge, X.; Wang, D.; Tang, H. Distinct mechanisms of particle aggregation induced by alum and PACl: Floc structure and DLVO evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 56–63. [Google Scholar]
- Rwehumbiza, V.M.; Harrison, R.; Thomsen, L. Alum-induced flocculation of preconcentrated Nannochloropsis salina: Residual aluminium in the biomass, FAMEs and its effects on microalgae growth upon media recycling. Chem. Eng. J. 2012, 200–202, 168–175. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, Q.; Xu, G.; Wang, D. Flocculation–Dewatering Behavior of Microalgae at Different Growth Stages under Inorganic Polymeric Flocculant Treatment: The Relationships between Algal Organic Matter and Floc Dewaterability. ACS Sustain. Chem. Eng. 2018, 6, 11087–11096. [Google Scholar]


| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 13,735.29 | 9 | 1526.14 | 64.97 | <0.0001 | significant |
| A-Dosage | 3630.23 | 1 | 3630.23 | 154.54 | <0.0001 | |
| B-ph | 2168.11 | 1 | 2168.11 | 92.3 | <0.0001 | |
| C-Biomass concentration | 3743.29 | 1 | 3743.29 | 159.35 | <0.0001 | |
| AB | 8.41 | 1 | 8.41 | 0.358 | 0.5685 | |
| AC | 1230.26 | 1 | 1230.26 | 52.37 | 0.0002 | |
| BC | 939.42 | 1 | 939.42 | 39.99 | 0.0004 | |
| A2 | 1237.39 | 1 | 1237.39 | 52.68 | 0.0002 | |
| B2 | 498.46 | 1 | 498.46 | 21.22 | 0.0025 | |
| C2 | 112.82 | 1 | 112.82 | 4.8 | 0.0645 | |
| Residual | 164.43 | 7 | 23.49 | |||
| Lack of Fit | 77.62 | 3 | 25.87 | 1.19 | 0.4188 | not significant |
| Pure Error | 86.81 | 4 | 21.7 | |||
| Cor Total | 13,899.72 | 16 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 14,622.06 | 9 | 1624.67 | 238.5 | <0.0001 | significant |
| A-Dosage | 85.81 | 1 | 85.81 | 12.6 | 0.0094 | |
| B-ph | 6006.08 | 1 | 6006.08 | 881.69 | <0.0001 | |
| C-Biomass concentration | 8.13 | 1 | 8.13 | 1.19 | 0.3107 | |
| AB | 25 | 1 | 25 | 3.67 | 0.0969 | |
| AC | 0.64 | 1 | 0.64 | 0.094 | 0.7681 | |
| BC | 571.21 | 1 | 571.21 | 83.85 | <0.0001 | |
| A2 | 32.11 | 1 | 32.11 | 4.71 | 0.0665 | |
| B2 | 7777.42 | 1 | 7777.42 | 1141.72 | <0.0001 | |
| C2 | 1.41 | 1 | 1.41 | 0.2067 | 0.6631 | |
| Residual | 47.68 | 7 | 6.81 | |||
| Lack of Fit | 47.45 | 3 | 15.82 | 272.71 | <0.0001 | significant |
| Pure Error | 0.232 | 4 | 0.058 | |||
| Cor Total | 14,669.74 | 16 |
| C-C/C-H (%) | C-N/C-O-C (%) | COOH (%) | O-C=O (%) | |
|---|---|---|---|---|
| Chlorella | 65.85 | 21.39 | 10.2 | 2.56 |
| Chlorella + Chitosan | 56.82 | 25.66 | 12.74 | 4.78 |
| Chlorella + Al2(SO4)3 | 70.84 | 18.12 | 8.46 | 2.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qi, Y.; Wei, Q.; Ma, X. An Investigation of the Impact of Flocculants on Process Optimization and Floc Properties in Chlorella vulgaris FACHB-15 Harvesting. Water 2025, 17, 932. https://doi.org/10.3390/w17070932
Li Y, Qi Y, Wei Q, Ma X. An Investigation of the Impact of Flocculants on Process Optimization and Floc Properties in Chlorella vulgaris FACHB-15 Harvesting. Water. 2025; 17(7):932. https://doi.org/10.3390/w17070932
Chicago/Turabian StyleLi, Yinting, Yingying Qi, Qun Wei, and Xiangmeng Ma. 2025. "An Investigation of the Impact of Flocculants on Process Optimization and Floc Properties in Chlorella vulgaris FACHB-15 Harvesting" Water 17, no. 7: 932. https://doi.org/10.3390/w17070932
APA StyleLi, Y., Qi, Y., Wei, Q., & Ma, X. (2025). An Investigation of the Impact of Flocculants on Process Optimization and Floc Properties in Chlorella vulgaris FACHB-15 Harvesting. Water, 17(7), 932. https://doi.org/10.3390/w17070932






