Abstract
This study systematically compared the harvesting efficiency and flocculation mechanisms of a bioflocculant (chitosan) and a chemical flocculant (Al2(SO4)3) for Chlorella cells. For the first time, the divergent mechanisms underlying floc structure formation between the two flocculants were elucidated by analyzing the EPS distribution and dynamic changes in microalgal surface functional groups. By optimizing critical operational parameters—including flocculant dosage, flocculation time, pH, and biomass concentration—the optimal dosages of chitosan and Al2(SO4)3 were determined as 0.5 mg/L and 90 mg/L, respectively. Under pH 6, both flocculants achieved over 99% flocculation efficiency within 30 min. Notably, at a dosage of 3 mg/L, chitosan-formed flocs exhibited denser structures, stronger adhesion, and a tendency to aggregate into spherical clusters compared to Al2(SO4)3-induced flocs. Beyond identifying ideal conditions for Chlorella flocculation, this work provides novel insights into the role of EPS and surface functional groups in flocculation mechanisms, offering both theoretical foundations and practical guidance for efficient microalgal harvesting. The findings hold significant implications for optimizing bioflocculant applications and advancing environmentally sustainable harvesting technologies.
1. Introduction
The depletion of petroleum fuels and the impact of greenhouse gas emissions on climate change have made the search for sustainable transportation fuels urgent [1,2]. Microalgal biomass is an attractive third-generation biofuel because it is renewable and environmentally friendly [3]. At the same time, research into microalgal biomass cultivation has gained significant attention from both academic and industrial sectors due to its broad applications in food, feed, and wastewater treatment [4,5].
However, collecting microalgae from the growth medium remains a challenge. Their small size (3–50 μm), low biomass concentration (0.1–5.0 g/L), and negatively charged surfaces (−7.5 to −40.0 mV) make harvesting difficult [6,7]. Harvesting also represents a major technical and economic obstacle in algae production, usually accounting for 20–30% of the total production cost. In some cases, this can rise to 60%, depending on the species and cultivation methods [8]. Therefore, developing efficient and cost-effective harvesting methods is crucial for large-scale algae production.
The main methods used for microalgae harvesting include gravity settling, filtration, centrifugation, air flotation, and flocculation [9,10,11]. Among these, flocculation is one of the most common techniques because it is simple and cost-effective. Flocculation is a physicochemical process where suspended particles aggregate and large flocs break apart [12]. The efficiency of this process depends on the algae species and the type of flocculant used [13], as well as factors like the flocculant dosage, solution pH, and flocculation time [14,15]. Flocculants for microalgae harvesting are generally classified into three categories: (1) inorganic flocculants, (2) synthetic polymers, and (3) natural organic polymers.
Inorganic flocculants, such as ferric chloride and Al2(SO4)3, are cost-effective and highly efficient for flocculation. However, they require large doses, which can contaminate the collected microalgal biomass with inorganic salts. This contamination may interfere with biofuel extraction and subsequent processes [11]. Synthetic polymers, like polyacrylamide (PAM) and polyelectrolytes, tend to be more efficient at lower dosages. However, they are expensive, and their widespread use increases harvesting costs. Additionally, these polymers may contain acrylamide residues, which are harmful to aquatic life and potentially carcinogenic [16,17]. Natural organic polymers, such as chitosan, cationic starch, and bioflocculants, help address biomass quality issues. These polymers are non-toxic and biodegradable, making them a safer option for harvesting [18,19,20]. Al2(SO4)3 is commonly used to flocculate microalgal biomass. It is easily available, cost-effective, and can improve harvesting efficiency [21,22]. The metal cations in Al2(SO4)3 neutralize the negative charge on microalgal cells, reducing repulsion and promoting coagulation through van der Waals forces [23]. The resulting flocs are much denser than individual cells, allowing them to settle easily under gravity. Reyes and Labra [24] tested three flocculants—ferrous sulfate, ferric chloride, and Al2(SO4)3—to harvest Scenedesmus sp. from a pilot-scale photobioreactor. They found that, with a dosage of 1.5 g/L and a pH of 8.5, Al2(SO4)3 achieved the highest harvesting efficiency of 97.9%. Similarly, Chatsungnoen and Chisti [25] determined the optimal Al2(SO4)3 dose for harvesting the marine microalga Nannochloropsis salina to be 229 mg/L. Liandong Zhu also used Al2(SO4)3 to flocculate Chlorella biomass and found the best dosage to be 2.5 g/L, achieving over 90% flocculation efficiency at optimal agitation speeds of 150 rpm and 25 rpm, respectively [23]. Chitosan, a natural organic polymer derived from chitin, has a high charge density and molecular weight [26,27], making it effective for microalgae harvesting [28,29,30]. Toh et al. used chitosan and poly(dimethyl diallyl ammonium chloride) (PDDA) with electrostatic patch flocculation to enhance microalgal cell sedimentation. They achieved cell separation efficiencies of 96.7% and 98.4%, respectively, with chitosan showing superior performance. The chitosan-treated cells settled 4.4 times faster than those undergoing self-flocculation [18]. In other trials, Yunos [25] showed that a 30 mg/L chitosan dose resulted in a 98% harvesting efficiency for Chlorella vulgaris and an 80% biomass recovery [29].
This study aimed to evaluate and compare the efficacy of Al2(SO4)3 (a chemical flocculant) and chitosan (a bioflocculant) in harvesting Chlorella cells, with a focus on optimizing key parameters, including the flocculant dosage, pH, and flocculation time. We systematically quantified the dynamic changes in extracellular polymeric substances (EPS) and surface functional groups during flocculation, providing new insights into the mechanisms underlying floc structure formation and stability. By elucidating the distinct roles of charge neutralization, bridging effects, and EPS redistribution, this work advances the understanding of flocculation mechanisms for both chemical and bioflocculants. Collectively, the findings establish a theoretical foundation for selecting flocculants and designing flocculation processes in microalgal harvesting applications.
2. Materials and Methods
2.1. Cultivation of Microalgae and Preparation of Flocculants
The Chlorella vulgaris FACHB-15 (later referred to as C. vulgaris) strain was acquired from the Institute of Aquatic Biology, Chinese Academy of Sciences (Wuhan). C. vulgaris cultures were cultivated in regular BG-11 media, as detailed in the Supplementary Material. The starting pH was calibrated to 7.0 ± 0.1, and the C. vulgaris were cultivated in 2 L conical flasks at a temperature of 25 ± 1 °C, with a continuous light intensity of 3500.lx and aeration at 1 vvm. C. vulgaris suspensions were obtained from the exponential growth phase (about 21 days) for future flocculation studies.
Chitosan was acquired from Shanghai McLean Biochemical Technology Co., Ltd., Shanghai, China, exhibiting a deacetylation degree of ≥95% and a viscosity of 100–200 mPa·s. A total of 0.5 g of chitosan powder was dissolved in 500 mL of a 1% glacial acetic acid solution and agitated at 300 rpm for 1 h to yield a chitosan reserve solution of 1 g/L. Al2(SO4)3 was acquired from Tianjin New Technology Industrial Park Chemo Chemical Reagent Co. A total of 5 g of Al2(SO4)3 powder was dissolved in 500 mL of water and agitated at 300 rpm for 5 min to prepare a 10 g/L Al2(SO4)3 stock solution.
2.2. Flocculation Trials
2.2.1. Influence of Flocculant Dosage and Standing Duration on Flocculation Effectiveness
Different dosages of flocculant reserve solution were added to a beaker containing 100 mL of C. vulgaris solution, with chitosan reserve solution dosages varying from 0.2 to 3 mg/L and Al2(SO4)3 reserve solution dosages ranging from 0 to 110 mg/L. The mixture was stirred with a magnetic stirrer at 200 rpm for 2 min to enhance mixing and aggregation, then rotated at 100 rpm for 3 min to encourage flocculation. Subsequent to flocculation, the aggregates were permitted to settle, and the supernatant was analyzed at 30 min, 1 h, and 2 h. The optical density (OD) of the supernatant at 680 nm was assessed using a UV-visible spectrophotometer (UV8000, Shanghai Yuanshui Instrument Co., Ltd.) [31]. The formula for flocculation efficiency is described in Equation (1) [32]:
where ODo and ODi represent the average optical density values of the original algal solution prior to flocculation and the supernatant after flocculation, respectively.
2.2.2. Influence of pH on Flocculation Efficacy
The optimal flocculant dosage and settling duration were determined to design the experimental investigation examining the impact of pH on flocculation efficiency. The test set utilized seven gradient pH values ranging from 4 to 10. The remaining operating conditions were identical to those outlined in the preceding section. The flocculation efficiency was determined using Equation (1).
2.2.3. Impact of Biomass on Flocculation Efficacy
This part applied the optimal flocculant dosage and the ideal flocculation pH. Microalgal dry weight was used to represent the biomass concentration. The samples were centrifuged at 1500× g for 15 min, followed by drying at 60 °C for 24 h until a constant weight was achieved. The dry cell weight of the biomass was determined gravimetrically to quantify the microalgal dry weight. The remaining operating conditions were identical to those outlined in the preceding section. The flocculation efficiency was determined using Equation (1).
2.3. Experimental Design for Response Surfaces
This study began with an initial examination of specific factors, including flocculant dosage, flocculation time, pH, and algal biomass concentration. In order to improve the C. vulgaris flocculation process, 17 experimental sets were conducted using the Box–Behnken design and Response Surface Methodology (RSM), which includes variables and response values. This experiment determined three parameters from the results of a one-way test: flocculant dosage, pH, and algal biomass concentration. The dependent variable was the efficacy of C. vulgaris flocculation. Three optimization levels (−1, 0, and 1) were constructed for each factor, as indicated by the previously mentioned one-way testing. Data were analyzed using Design-Expert® 11 software (Stat-Ease Inc., Minneapolis, MN, USA).
2.4. Assessment of Flocculation Effectiveness
The settable solids volume fraction (SSVF) and concentration factor (CF) of the floc are frequently employed to evaluate the density of the floc and were determined using microalgae column-body sedimentation experiments [28,33]. C. vulgaris was flocculated using optimum conditions, and the flocs were permitted to settle for 30 min after the slow decantation of the flocs with the supernatant into a 100 mL measuring cylinder, where the height of the concentrated flocs was documented [34]. SSVF is the ratio of the C. vulgaris slurry volume to the original C. vulgaris culture volume, with elevated SSVF values signifying a greater C. vulgaris slurry volume and diminished flocculation efficacy; SSVF is computed using the subsequent formula:
where ho represents the beginning height of the C. vulgaris solution, and hf denotes the ultimate height of the concentrated C. vulgaris floc following the settling process.
CF denotes the ratio of the final product to the original concentration, with a low CF value indicating low HE and high SSVF.
The calculation of CF was conducted as follows:
2.5. Extracellular Polymers (EPS)
In a restricted context, EPS typically denotes extracellular polysaccharides, which constitute the predominant element of EPS [35,36]. This study exclusively addressed the EPS content in a narrow context, employing the phenol-sulfuric acid method to quantify the extracellular polysaccharides extracted from algal solutions and microalgal flocs. An appropriate volume of algal suspension was centrifuged at 2500× g for 15 min, and the supernatant was collected to extract soluble EPS (S-EPS). The remaining pellet was resuspended in a 0.01 mol/L Na2EDTA solution, allowed to stand, and then centrifuged at 5000× g for 15 min to collect the supernatant for loosely bound EPS (LB-EPS) extraction. Subsequently, the remaining pellet was treated again with 0.01 mol/L Na2EDTA solution, allowed to stand, and incubated in a water bath at 60 °C for 30 min, followed by centrifugation at 15,000× g for 20 min to collect the supernatant for tightly bound EPS (TB-EPS) extraction. For EPS quantification, 1 mL of the filtered extract was mixed with 1 mL of 5% (w/v) phenol solution, followed by the rapid addition of 5 mL of concentrated sulfuric acid. The mixture was incubated in a water bath at 95 °C for 30 min. The absorbance was measured at a wavelength of 490 nm using a UV spectrophotometer, and the EPS content was calculated based on a standard calibration curve.
2.6. Identification of Functional Groupings in Microalgal Cells
Following flocculation and precipitation, the floc was centrifuged at 4000 rpm for 10 min using centrifuge (model: TDZ4-WS; manufacturer: Hunan Xiangyi Laboratory Instrument Development Co., Ltd., Changsha, China). The supernatant was discarded, ultrapure water was added, and centrifugation was repeated three times. The harvested algal sludge was frozen at −30 °C for 24 h and subsequently placed in a vacuum freeze-dryer to produce algal powder. The ratio of C. vulgaris cellular functional groups was assessed using X-ray photoelectron spectroscopy (XPS, model: K-Alpha+; manufacturer: Thermo Fisher Scientific), calibrated against the C1s peak with a binding energy set at 284.8 eV, to evaluate the impact of C. vulgaris surface functional groups on the flocculation effect.
3. Results and Discussion
3.1. One-Factor Experiment on C. vulgaris Flocculation
3.1.1. Effect of Dosage
To determine the appropriate dosage of the two flocculants, various dosage levels were tested using C. vulgaris cultures with a uniform optical density (OD680 nm = 1). The results of flocculation efficiency are shown in Figure 1. The biomass flocculation efficiencies of chitosan on C. vulgaris ranged from 67.13% to 95.97%, 76.88% to 96.29%, and 78.47% to 96.71% after 30 min, 1 h, and 1.5 h of standing time, respectively. The effectiveness of chitosan increased with higher dosages at low concentrations. Under neutral or slightly acidic conditions, the amino group (−NH2) of chitosan can be protonated to form a positive charge (−NH3⁺). This neutralizes the negative charges on the surface of C. vulgaris (such as carboxyl groups, −COOH, and phosphate groups, −PO42−), reducing electrostatic repulsion and promoting particle aggregation and flocculation. Additionally, chitosan molecules can form “bridges” between particles through physical adsorption or chemical bonding, resulting in larger flocs [37]. At low concentrations, the positive charge of chitosan effectively neutralizes the negative charge on the surface of C. vulgaris, causing a rapid increase in flocculation efficiency, which peaks at around 96% at a concentration of 0.5 mg/L. After this point, flocculation efficiency began to decrease gradually. At a concentration of 3 mg/L, the efficiency dropped to 92.2%. This decline may be due to an excessive amount of chitosan. When chitosan is present in excess, the surplus positive charge on the particle surfaces can increase repulsion between particles, thereby hindering the flocculation process [38].

Figure 1.
Harvesting efficiency relative to flocculant dosage and harvesting duration (pH = 7; biomass concentration: OD680 nm = 1; data presented as means ± standard deviation).
The flocculation efficiency of Al2(SO4)3 on Chlorella biomass ranged from 38.18% to 99.39%, 41.92% to 99.60%, and 43.74% to 99.60% at 30 min, 1 h, and 1.5 h, respectively. The efficiency increased with higher dosages of Al2(SO4)3. As the dosage increased, the ionic charge also grew proportionally. The Al3⁺ ions produced by the hydrolysis of Al2(SO4)3 are positively charged, which neutralizes the negative charge on the surface of C. vulgaris. This reduces electrostatic repulsion between particles and increases intercellular collisions in suspensions with higher concentrations, leading to the formation of more flocs and improved flocculation efficiency [24]. At 90 mg/L of Al2(SO4)3, the flocculation efficiency reached 99.39%. At this concentration, most of the negative charges were neutralized, minimizing electrostatic repulsion between particles and achieving optimal flocculation. Further increases in the Al2(SO4)3 concentration had little effect on flocculation efficiency [37].
This study also showed that longer flocculation times improved flocculation efficiencies, especially at lower flocculant concentrations. The best flocculation results were obtained with optimal dosages of chitosan and Al2(SO4)3. At a chitosan concentration of 0.5 mg/L, the flocculation efficiency of C. vulgaris was 95.97% after 30 min and 96.56% after 1.5 h. With Al2(SO4)3 at 90 mg/L, the flocculation efficiency of C. vulgaris was 98.69% after 30 min and 98.70% after 1.5 h. These findings showed that the flocculation time did not significantly affect the efficiency, as the efficiencies remained stable throughout the measured durations. Therefore, the flocculation time is not a critical factor for optimizing the flocculation process. From a practical standpoint, longer flocculation times are not ideal for commercial applications. As a result, a flocculation duration of 30 min was selected for subsequent trials, offering a balance between efficiency and practicality.
3.1.2. Influence of pH
The pH level is a key factor influencing the effectiveness of chitosan flocculation in C. vulgaris. The surface charge of Chlorella affects flocculation efficiency, the shape of the flocculant, and the chemical processes during the flocculation process. At a lower pH, functional groups like carboxyl and phosphate on the Chlorella surface are partially protonated, reducing the number of negative charges. This decreases electrostatic repulsion between particles, making flocculation easier. As the pH increases, these functional groups progressively deprotonate, increasing the negative charge and causing greater electrostatic repulsion among particles, which hinders flocculation [39].
Figure 2 shows how pH affects flocculation efficiency. Chitosan is a natural polysaccharide, and its solubility and shape are greatly influenced by pH. The protonation of amino groups (−NH2) within chitosan molecules at different pH levels determines its solubility and charge distribution. In acidic conditions, chitosan becomes more soluble, and its amino groups protonate to form a positive charge (−NH3⁺). This positive charge effectively neutralizes the negative charges on the C. vulgaris surface (such as carboxyl, −COOH, and phosphate, −PO42⁻), reducing electrostatic repulsion and facilitating flocculation. The highest flocculation efficiency, 97.9%, was achieved at pH 6. However, when the pH exceeds 7, the amino group in chitosan deprotonates, reducing the solubility of chitosan and potentially forming insoluble precipitates. As the positive charge decreases, it is less able to neutralize the negative charges on the Chlorella surface, causing a sharp decline in flocculation efficiency [32,40].
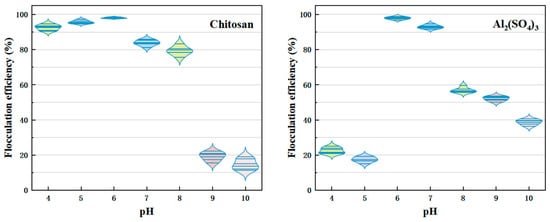
Figure 2.
Flocculation effectiveness of C. vulgaris collected using chitosan and Al2(SO4)3 across varying pH levels. Chitosan concentration: 0.5 mg/L; Al2(SO4)3 concentration: 90 mg/L; biomass concentration: OD680 nm = 1.
Al2(SO4)3 was effective in flocculating C. vulgaris at pH levels 6 and 7, achieving a flocculation efficiency of 98.2% at pH 6. The Al3⁺ ions neutralized the negative charge on the Chlorella surface, promoting flocculation. However, the flocculation efficiency decreased in both acidic (pH < 6) and alkaline (pH > 7) conditions. In acidic environments, Al3⁺ ions may form additional monomers, leading to over-neutralization and increased interparticle repulsion. In alkaline conditions, Al3⁺ ions can produce multinuclear hydroxyl–aluminum complexes (e.g., Al(OH)3), which have a low charge density and reduced capacity to neutralize charges, resulting in lower flocculation efficiency [41].
3.1.3. Impact of Biomass Concentration
Figure 3 shows that at a value of 0.95 g/L, chitosan dispersed uniformly in the solution, achieving complete contact with C. vulgaris particles. This resulted in a peak flocculation efficiency of 98.12%. The success of chitosan at this concentration was due to its ability to effectively coat the Chlorella surfaces, providing sufficient positive charges to neutralize the negative charges, reducing electrostatic repulsion between particles and promoting flocculation. However, at higher biomass concentrations, the number of Chlorella particles increased significantly. As a result, the available chitosan was insufficient to neutralize all the negative charges on the microalgal surfaces. Some particles retained negative charges, reducing the decrease in electrostatic repulsion and hindering the flocculation process. Additionally, the increased biomass concentration raised the solution’s viscosity, which limited the dispersion and uniform diffusion of chitosan molecules, further reducing flocculation efficiency [42,43].
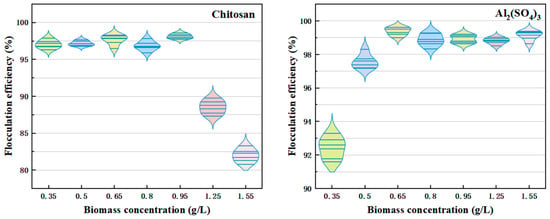
Figure 3.
Impact of varying biomass concentrations on the flocculation of C. vulgaris using chitosan and Al2(SO4)3. pH = 6, chitosan concentration: 0.5 mg/L, Al2(SO4)3 concentration: 90 mg/L.
On the other hand, the use of Al2(SO4)3 as a flocculant can improve flocculation efficiency with an increasing biomass concentration, achieving an optimal flocculation efficiency of 99.27% at a biomass concentration of 0.65 g/L. The Al3⁺ ions produced during the hydrolysis of Al2(SO4)3 have a positive charge and can effectively neutralize the negative charge on the surface of C. vulgaris. Even with higher biomass concentrations, the large presence of Al2(SO4)3 molecules was sufficient to provide enough positive charge to neutralize the negative charges on Chlorella surfaces, reducing electrostatic repulsion and maintaining flocculation efficiency. As the biomass concentration rose, the density of Chlorella particles increased, leading to more frequent collisions between particles. These collisions promoted particle aggregation and the formation of more flocs. The increased concentration of Al2(SO4)3 molecules helped to quickly bind the particles together, enhancing the flocculation process.
3.2. Response Surface Experimental Design and Analysis
Following the outcomes of the one-way experiment, the flocculant concentration, pH, and biomass concentration were chosen for subsequent optimization. A three-factor, three-level BBD experiment was performed to examine the combined impact of the independent variables on flocculation efficiency. A quadratic model was employed to evaluate the interactive impact of these circumstances on the microalgal flocculation efficiency. The three independent variables and their corresponding experimental ranges are summarized in Table S2. For comprehensive documentation, the complete Box–Behnken design (BBD) matrices and response data for chitosan and Al2(SO4)3 flocculation optimization are detailed in Tables S3 and S4 of the Supplementary Materials, respectively, ensuring reproducibility of the experimental workflow.
Table 1 presents the analysis of variance (ANOVA) for the predictive model for chitosan flocculation of C. vulgaris. F was employed to evaluate the model equations, where higher F values and lower p-values indicate the significance of the correlation coefficients. The results indicate that the p value of the fitted model is less than 0.001, signifying that the regression model is highly statistically significant. The F-value for the misfit component was 1.19, with a p-value < 0.001, suggesting that the model’s misfit was insignificant. The residuals are ascribed to stochastic errors, highlighting the model’s strong fit. The coefficient of variation (C.V. %) was 6.3703, signifying that the data exhibited relative stability with minimal variability. The Adeq Precision value of 25.2824 demonstrated that the data displayed commendable precision [44,45,46,47]. The F-value indicates that the hierarchy of influence on flocculation efficiency is C > A > B > A2 > AC > BC > B2 > C2 > AB. The primary factors A, B, and C, along with the interaction term AC and the quadratic term A2, are significant simulation variables. The R2Adj of 0.9926 signifies a strong correlation between observed and anticipated response values, effectively accounting for the response’s variability.

Table 1.
Regression analysis of quadratic model for optimization of flocculation efficiency of chitosan flocculated Chlorella.
Similarly, an analysis of variance (ANOVA) was employed to evaluate the statistical significance of the Al2(SO4)3 flocculated Chlorella model. The data presented in Table 2 provide a p-value of < 0.0001, confirming the model’s relevance. The multiple correlation coefficient R2 was 0.9967, signifying a robust association among the three variables. The coefficient of variation (C.V. %) was 3.38, signifying that the data exhibited relative stability and minimal variability. Adeq Precision = 41.5841, signifying that the data exhibited commendable precision. The observed quadratic term B2 (pH), linear term B, and interaction term BC (pH, biomass concentration) were very significant, with the following order of their effect on flocculation efficiency: B2 > B > BC > A > A2 > AB > C > C2 > AC.

Table 2.
Regression analysis of quadratic model for optimization of Chlorella flocculation efficiency of Al2(SO4)3.
The regression equation for the effectiveness of Chlorella flocculation is presented below.
The RSM model for chitosan as the flocculant is presented in Equation (4):
The RSM model for Al2(SO4)3 as the flocculant is provided in Equation (5):
Figure 4 illustrates the three-dimensional response surface plots produced by the aforementioned model to examine the components’ interaction and assess their impact on flocculation efficiency.

Figure 4.
Response surface plots illustrating the interaction effects of three factors on microalgal flocculation efficiency: (a) chitosan concentration versus pH, (b) chitosan concentration versus biomass concentration, and (c) biomass concentration versus pH; as well as (d) Al2(SO4)3 concentration versus pH, (e) Al2(SO4)3 concentration versus biomass concentration, and (f) biomass concentration versus pH.
The 3D response surface plots illustrate the flocculation efficiency as a function of the investigated factors, while the regression equation graphs provide further insights into the relationships between these factors and the response. As shown in Figure 4a, the interaction between chitosan dosage and pH was analyzed under a constant microalgal biomass concentration of 0.95 g/L. The results indicate that flocculation efficiency improved significantly at chitosan concentrations of 0.52–0.60 mg/L and pH values of 5.0–5.7. Figure 4b,c demonstrate that the flocculation effectiveness reached its maximum when the biomass concentration was maintained within the range of 0.65–0.9 g/L. Furthermore, Figure 4d,f reveal that the flocculation efficiency of Chlorella treated with Al2(SO4)3 exhibited a distinct trend: it initially increased but subsequently declined with rising pH, achieving optimal performance at a biomass concentration (optical density) of 0.65 and an Al2(SO4)3 concentration of 90 mg/L. In Figure 4d, with a constant pH of 6, the flocculation efficiency varied with changes in biomass and Al2(SO4)3 content; still, the overall flocculation efficiency exceeded 80%, demonstrating a high degree of effectiveness. A pH value over 80% significantly affects the flocculation efficiency in Chlorella’s Al2(SO4)3 flocculation process. Al2(SO4)3 hydrolyzes in water, producing various aluminum ions that flocculate Chlorella via charge neutralization. The types and quantities of these hydrolysis products are significantly affected by pH, as evidenced in this experiment.
Chlorella flocculation experiments were subsequently conducted under the optimal conditions determined by the Box–Behnken design. Chitosan flocculation achieved a maximum efficiency of 99.98%, with a chitosan concentration of 0.58 mg/L, pH 5.1, and a biomass concentration of 1.046 g/L. Al2(SO4)3 flocculation reached an optimal efficiency of 99.97% with an Al2(SO4)3 concentration of 96.99 mg/L, pH 6.2, and a biomass concentration of 0.902 g/L. The flocculation of Chlorella using Al2(SO4)3 achieved an ideal efficiency of 99.97%.
3.3. Composition of the Flocculent
3.3.1. Floc Compaction
Chitosan and Al2(SO4)3 functioned as flocculants, with optimal flocculation conditions established through column sedimentation studies (see Figure S1). The chitosan-flocculated particles settled uniformly as small particles, but the Al2(SO4)3-flocculated particles aggregated as loose flocs after 10 min of sedimentation. The precipitation concluded primarily after 30 min of settling; the flocculation and sedimentation height for chitosan was roughly 1.5 cm, exhibiting a flocculation efficiency of 87.04%, an SSVF of 0.015, and a CF of 58.03. In contrast, Al2(SO4)3 exhibited a flocculation and sedimentation height of around 6 cm, a flocculation efficiency of 91.15%, SSVF of 0.06, and CF of 15.25. The structural states of Chlorella before flocculation and the flocs after flocculation were observed using electron microscopy (see Figure S2). Before flocculation, the Chlorella cells exhibited a dispersed and free-floating state. After flocculation, the chitosan-flocculated Chlorella demonstrated strong sedimentation and concentration capabilities. The cells formed dense floc structures through charge neutralization and bridging mechanisms, resulting in tightly connected aggregates, which facilitated rapid settling and concentration of the flocs. In contrast, when Al2(SO4)3 was used as the flocculant, the microalgae primarily aggregated through charge neutralization, leading to looser floc structures with more distinct intercellular connections. Compared to the flocs formed by chitosan, those formed by Al2(SO4)3 exhibited a lower density.
Chitosan flocculation of Chlorella exhibits superior settling and concentrating capabilities compared to Al2(SO4)3, likely due to creating a more compact microalgal structure resulting from charge neutralization and the bridging action of chitosan. This may occur because chitosan creates a denser floc via charge neutralization and bridging, facilitating swift sedimentation and concentration. In contrast, Al2(SO4)3 generates a less dense floc through charge neutralization and the production of hydroxy aluminum complexes. In contrast, Al2(SO4)3 generates a less dense floc through charge neutralization and the production of hydroxy aluminum complexes. This finding is consistent with previous research results. As reported by Li [48], due to the influence of flocculation mechanisms and factors such as microalgal EPS, ferric chloride-induced flocs typically range in size from tens of micrometers, while chitosan-induced flocs can reach several hundred micrometers in size and exhibit a more compact structure. The experiment noted that when the flocculant dose was beyond the ideal concentration, the chitosan-flocculated Chlorella exhibited clustering behavior, with the clustering effect being most pronounced at a concentration of 3 mg/L. As the concentration of chitosan increased, the aggregated flocs appeared to disperse. Conversely, the Al2(SO4)3 flocs disseminate more uniformly across the substrate (refer to Figure S3).
The EPS content and its functional groups were assessed following Chlorella flocculation to examine the structural differences between the two flocculants.
3.3.2. Extracellular Polymers of Chlorella
EPS is a polymer consisting of intricate polysaccharides, proteins, nucleic acids, and lipids released by microalgal cells, characterized by a high abundance of functional groups that significantly affect microalgal flocculation [49]. Figure 5 illustrates the variations in EPS content resulting from the flocculation of Chlorella using two flocculants at varying doses.

Figure 5.
EPS content generated by Chlorella at varying doses of both flocculants.
There is minimal variation in the overall EPS change when employing chitosan for Chlorella flocculation. The reduction in S-EPS and the augmentation of LB-EPS and TB-EPS on the microalgal surface suggest that chitosan adheres to C. vulgaris via physical and chemical adsorption, interacting with a portion of the S-EPS in the supernatant to establish a bridge between the particles, thereby immobilizing them and converting them into LB-EPS and TB-EPS. This finding aligns with the results reported by Zhou [50]. They highlighted that microalgal EPS, which coats the cell surface, can alter the surface charge and hydrophobicity of the cells. The negatively charged functional groups (e.g., carboxyl and phosphate groups) within EPS can bind with positively charged chitosan, thereby significantly enhancing flocculation efficiency. Similarly, Demir [51] further corroborated this from a microscopic perspective, demonstrating that the long-chain molecules of chitosan can simultaneously bind to multiple microalgal cells, bridging dispersed cells together to form larger and more compact flocs.
At a chitosan concentration of 3 mg/L, the LB-EPS on the Chlorella surface attains a maximum value of 39.88 mg/L. Chlorella flocs aggregated into spheres. As the chitosan concentration increased, LB-EPS diminished, and flocculation effectiveness declined due to the neutralization of excess chitosan charge, resulting in the loosening of flocculated spheres [48].
Adding Al2(SO4)3 for flocculation resulted in a minor rise in S-EPS and a substantial increase in LB-EPS. The addition of Al2(SO4)3 likely enhanced the release of certain EPSs, mainly loosely bound EPSs, which may subsequently re-adsorb onto the particle surfaces, forming additional loosely bound EPSs. The stability of TB-EPS was more excellent, suggesting that Al2(SO4)3 had less impact on the firmly bound EPSs. The Al2(SO4)3 flocs exhibited a loose structure with no observable clustering tendency, suggesting that floc clustering was mainly attributed to the bridging function of chitosan [52].
3.3.3. Ratio of Functional Groups on the Surface of Algal Cells
To further examine the impact of functional groups on the efficiency and effectiveness of Chlorella flocculation, the pertinent components of the EPS of the cells and Chlorella flocs were studied qualitatively and quantitatively using X-ray photoelectron spectroscopy (XPS). The complete XPS spectra (Figure 6) indicated that carbon was the predominant element in Chlorella. The elemental forms of carbon in EPS were further investigated using high-resolution XPS C1s spectra. Four carbon peaks were identified in the high-resolution C1s spectrum of microalgal extracellular polymeric substances, corresponding to the functional groups O-C=O, −COOH, C-O-C, and C-C/H, respectively [53]. Following flocculation with several flocculants, the ratio of EPS functional groups in Chlorella exhibited considerable alterations (Table 3). The functional group C-(C/H) often signifies the hydrophobic component of EPS, whereas the functional groups −COOH, C-O-C, and O-C=O generally denote the hydrophilic component of EPS [54].
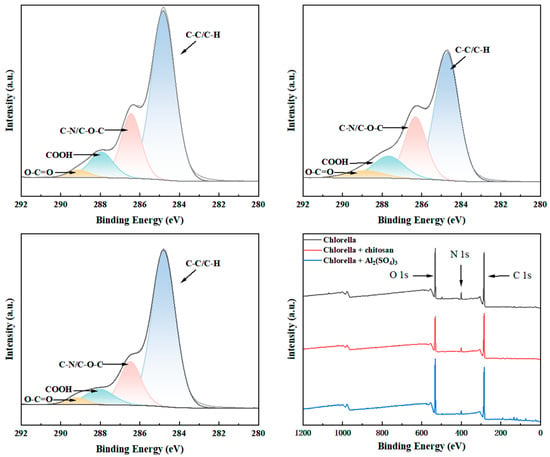
Figure 6.
X-ray photoelectron spectroscopy (XPS) entire spectrum of Chlorella flocculent, including C1s spectra.

Table 3.
High-resolution C1s energy spectra of Chlorella flocculent EPS obtained via XPS.
Chlorella flocculation with 0.5 mg/L of chitosan decreased the hydrophobic component C-C/C-H by 9.03%, as the chitosan surface, abundant in positively charged protonated amino (−NH2) groups, engaged with C-C/C-H to promote the aggregation of bigger cellular flocs [55,56]. The concentrations of the functional groups C-N/C-O-C, COOH, and O-C=O increased by 4.27%, 2.54%, and 2.22%, respectively, possibly due to the incorporation of chitosan, which facilitates the release of extracellular polymeric compounds, especially those that are loosely bound. The flocs were small at an EPS concentration of 0.5 mg/L, and flocculation was not completely realized. Functional groups such as COOH and O-C=O demonstrated superior binding sites, and an increase in chitosan content resulted in bigger flocs, causing incomplete flocculation. An elevated chitosan concentration led to enhanced flocculation, which was consistent with the experimental observations.
The application of 90 mg/L Al2(SO4)3 for the flocculation of Chlorella results in the generation of Al3⁺ ions by the hydrolysis of Al2(SO4)3, which can interact with the hydrophilic components C-N/C-O-C and COOH on the surface of Chlorella, facilitating floc formation [57,58]. In comparison to the control group, following Al2(SO4)3 flocculation of Chlorella, the proportions of C-N/C-O-C and COOH content diminished by 3.27% and 1.78%, respectively, whereas the content of C-C/C-H (hydrophilic component) augmented by 4.99%. Consequently, the overall hydrophobicity of algal cells increased, facilitating intercellular bonding flocculation and sedimentation separation. The study by Zhang [59] further supports this finding. They demonstrated that aluminum ions preferentially bind to hydrophilic functional groups (e.g., −COOH and −OH) on the surface of microalgal cells, forming stable complexes. This binding not only reduces electrostatic repulsion forces between cells, facilitating cellular aggregation, but also increases the relative abundance of hydrophobic functional groups (e.g., C-C/C-H) on the cell surface, thereby further enhancing cellular hydrophobicity.
4. Conclusions
The self-flocculation of Chlorella is typically slow and inefficient, often necessitating the use of flocculants to enhance the harvesting process. This study evaluated the effectiveness of two flocculants—chitosan and Al2(SO4)3—in the flocculation of Chlorella. Chitosan achieved a maximum flocculation efficiency of 99.98% under optimal conditions, including a concentration of 0.598 mg/L, a pH of 5.11, and a biomass concentration of 1.046 g/L. In contrast, Al2(SO4)3 attained an optimal efficiency of 99.97% at a concentration of 96.99 mg/L, a pH of 6.22, and a biomass concentration of 0.902 g/L. The primary mechanisms for chitosan-induced flocculation were charge neutralization and bridging, facilitated by extracellular polymeric substances on the Chlorella surface. However, an increasing chitosan concentration could lead to supersaturation, affecting flocculation efficiency. Al2(SO4)3 flocculation, on the other hand, mainly relied on charge neutralization, with the balance between hydrophobic and hydrophilic components of the flocculant being crucial for optimal performance. Both chitosan and Al2(SO4)3 proved to be effective flocculants for Chlorella harvesting, with each flocculant operating through different mechanisms. These findings provide valuable insights into selecting and optimizing flocculants for large-scale algae harvesting.
However, this study has certain limitations. First, the experiments were conducted under controlled laboratory conditions, and scalability to pilot or industrial-scale systems remains to be validated. Second, while Al2(SO4)3 demonstrated high efficiency, its environmental risks (e.g., aluminum ion residues) necessitate further investigation into eco-friendly alternatives for sustainable applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17070932/s1, Table S1: Components of BG11 Medium and recipe for C. vulgaris cultivation; Table S2: Ranges and levels of the Box-Behnken Design for C. vulgaris flocculation; Table S3: Response Surface Methodology (RSM) Experimental Design for Chitosan-Induced Flocculation of Chlorella; Table S4: Response Surface Methodology (RSM) Experimental Design for Al2(SO4)3-Induced Flocculation of Chlorella; Figure S1: Sedimentation experiment of floc column; Figure S2: Structural states of Chlorella before and after flocculation. (a) Before flocculation; (b) Flocculation using chitosan; (c) Flocculation using Al2(SO4)3; Figure S3: Flocculants in different states due to the type and dose of flocculants. (a):Chitosan 3mg/L; (b):Chitosan 6mg/L; (c):Al2(SO4)3 90mg/L; (d):Al2(SO4)3 120mg/L.
Author Contributions
Y.L.: methodology, experiment design, validation, formal analysis, writing—original draft. Y.Q.: experiment design, validation, formal analysis. Q.W.: conceptualization, experiment design, supervision, writing—original draft, writing—review and editing. X.M.: conceptualization, methodology, experiment design, supervision, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received support from the National Natural Science Foundation of China (No. 52360008) and the Natural Science Foundation of Guangxi Province (No. AD21220064).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 52360008) and the Natural Science Foundation of Guangxi Province (No. AD21220064).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent developments and key barriers to advanced biofuels: A short review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Choi, H.I.; Sim, S.J. Microalgal fuels: Promising energy reserves for the future. Fuel 2022, 312, 122841. [Google Scholar]
- Jin, Y.; Li, Y.; Qi, Y.; Wei, Q.; Yang, G.; Ma, X. A modified cultivation strategy to enhance biomass production and lipid accumulation of Tetradesmus obliquus FACHB-14 with copper stress and light quality induction. Bioresour. Technol. 2024, 400, 130677. [Google Scholar]
- Cai, Y.; Lim, H.R.; Khoo, K.S.; Ng, H.S.; Cai, Y.; Wang, J.; Tak-Yee Chan, A.; Show, P.L. An integration study of microalgae bioactive retention: From microalgae biomass to microalgae bioactives nanoparticle. Food Chem. Toxicol. 2021, 158, 112607. [Google Scholar] [CrossRef] [PubMed]
- Bazarnova, J.; Nilova, L.; Trukhina, E.; Bernavskaya, M.; Smyatskaya, Y.; Aktar, T. Use of Microalgae Biomass for Fortification of Food Products from Grain. Foods 2021, 10, 3018. [Google Scholar] [CrossRef]
- García-Pérez, J.S.; Beuckels, A.; Vandamme, D.; Depraetere, O.; Foubert, I.; Parra, R.; Muylaert, K. Influence of magnesium concentration, biomass concentration and pH on flocculation of Chlorella vulgaris. Algal Res. 2014, 3, 24–29. [Google Scholar]
- Deepa, P.; Sowndhararajan, K.; Kim, S. A Review of the Harvesting Techniques of Microalgae. Water 2023, 15, 3074. [Google Scholar] [CrossRef]
- Udom, I.; Zaribaf, B.H.; Halfhide, T.; Gillie, B.; Dalrymple, O.; Zhang, Q.; Ergas, S.J. Harvesting microalgae grown on wastewater. Bioresour. Technol. 2013, 139, 101–106. [Google Scholar]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar]
- Leite, L.d.S.; Hoffmann, M.T.; Daniel, L.A. Coagulation and dissolved air flotation as a harvesting method for microalgae cultivated in wastewater. J. Water Process Eng. 2019, 32, 100947. [Google Scholar]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Ho, Q.N.; Fettweis, M.; Hur, J.; Desmit, X.; Kim, J.I.; Jung, D.W.; Lee, S.D.; Lee, S.; Choi, Y.Y.; Lee, B.J. Flocculation kinetics and mechanisms of microalgae- and clay-containing suspensions in different microalgal growth phases. Water Res. 2022, 226, 119300. [Google Scholar] [PubMed]
- Okoro, V.; Azimov, U.; Munoz, J.; Hernandez, H.H.; Phan, A.N. Microalgae cultivation and harvesting: Growth performance and use of flocculants—A review. Renew. Sustain. Energy Rev. 2019, 115, 109364. [Google Scholar]
- Kuzhiumparambil, U.; Labeeuw, L.; Commault, A.; Vu, H.P.; Nguyen, L.N.; Ralph, P.J.; Nghiem, L.D. Effects of harvesting on morphological and biochemical characteristics of microalgal biomass harvested by polyacrylamide addition, pH-induced flocculation, and centrifugation. Bioresour. Technol. 2022, 359, 127433. [Google Scholar]
- Sanyano, N.; Chetpattananondh, P.; Chongkhong, S. Coagulation–flocculation of marine Chlorella sp. for biodiesel production. Bioresour. Technol. 2013, 147, 471–476. [Google Scholar]
- Long, Q.; Chen, X.; Feng, Y.; He, X.; Gu, H.; Huang, T.; Zhao, P. Effective harvesting of the microalga Monoraphidium sp. QLY-1: Comparison of different flocculants. J. Appl. Phycol. 2024, 36, 1143–1151. [Google Scholar]
- Nguyen, L.N.; Labeeuw, L.; Commault, A.S.; Emmerton, B.; Ralph, P.J.; Johir, M.A.H.; GUO, W.; Ngo, H.H.; Nghiem, L.D. Validation of a cationic polyacrylamide flocculant for the harvesting fresh and seawater microalgal biomass. Environ. Technol. Innov. 2019, 16, 100466. [Google Scholar]
- Toh, P.Y.; Azenan, N.F.; Wong, L.; Ng, Y.S.; Chng, L.M.; Lim, J.; Chan, D.J.C. The Role of Cationic Coagulant-to-Cell Interaction in Dictating the Flocculation-Aided Sedimentation of Freshwater Microalgae. Arab. J. Sci. Eng. 2018, 43, 2217–2225. [Google Scholar]
- You, Y.; Yang, L.; Sun, X.; Chen, H.; Wang, H.; Wang, N.; Li, S. Synthesized cationic starch grafted tannin as a novel flocculant for efficient microalgae harvesting. J. Clean. Prod. 2022, 344, 131042. [Google Scholar]
- Nguyen, T.D.P.; Le, T.V.A.; Show, P.L.; Tran, M.H.; Tran, T.N.T.; Lee, S.Y. Bioflocculation formation of microalgae-bacteria in enhancing microalgae harvesting and nutrient removal from wastewater effluent. Bioresour. Technol. 2019, 272, 34–39. [Google Scholar]
- Cui, Y.; Yuan, W.; Cheng, J. Understanding pH and Ionic Strength Effects on Aluminum Sulfate-Induced Microalgae Flocculation. Appl. Biochem. Biotechnol. 2014, 173, 1692–1702. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Lin, L.; Zhang, C.; Li, A.; Zhu, Y.; Zhang, Y. Evaluation of several flocculants for flocculating microalgae. Bioresour. Technol. 2015, 197, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hu, T.; Li, S.; Nugroho, Y.K.; Li, B.; Cao, J.; Show, P.-L.; Hiltunen, E. Effects of operating parameters on algae Chlorella vulgaris biomass harvesting and lipid extraction using metal sulfates as flocculants. Biomass Bioenergy 2020, 132, 105433. [Google Scholar] [CrossRef]
- Reyes, J.F.; Labra, C. Biomass harvesting and concentration of microalgae scenedesmus sp. cultivated in a pilot phobioreactor. Biomass Bioenergy 2016, 87, 78–83. [Google Scholar] [CrossRef]
- Chatsungnoen, T.; Chisti, Y. Continuous flocculation-sedimentation for harvesting Nannochloropsis salina biomass. J. Biotechnol. 2016, 222, 94–103. [Google Scholar] [CrossRef]
- Chae, K.-S.; Shin, C.-S.; Shin, W.-S. Characteristics of cricket (Gryllus bimaculatus) chitosan and chitosan-based nanoparticles. Food Sci. Biotechnol. 2018, 27, 631–639. [Google Scholar] [CrossRef]
- Yin, Z.; Chu, R.; Zhu, L.; Li, S.; Mo, F.; Hu, D.; Liu, C. Application of chitosan-based flocculants to harvest microalgal biomass for biofuel production: A review. Renew. Sustain. Energy Rev. 2021, 145, 111159. [Google Scholar] [CrossRef]
- Lama, S.; Muylaert, K.; Karki, T.B.; Foubert, I.; Henderson, R.K.; Vandamme, D. Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresour. Technol. 2016, 220, 464–470. [Google Scholar] [CrossRef]
- Yunos, F.H.M.; Nasir, N.M.; Jusoh, H.H.W.; Khatoon, H.; Lam, S.S.; Jusoh, A. Harvesting of microalgae (Chlorella sp.) from aquaculture bioflocs using an environmental-friendly chitosan-based bio-coagulant. Int. Biodeterior. Biodegrad. 2017, 124, 243–249. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Christwardana, M.; Widayat, W.; Jati, A.K.; Laes, S.I. Optimization of flocculation efficiency and settling time using chitosan and eggshell as bio-flocculant in Chlorella pyrenoidosa harvesting process. Environ. Technol. Innov. 2021, 24, 101959. [Google Scholar] [CrossRef]
- Nascimento, W.J., Jr.; Silva, M.; Vieira, M. Competitive fixed-bed biosorption of Ag(I) and Cu(II) ions on Sargassum filipendula seaweed waste. J. Water Process Eng. 2020, 36, 101294. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Miao, L. Harvesting freshwater microalgae with natural polymer flocculants. Algal Res. 2021, 57, 102358. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [PubMed]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Meesschaert, B.; Muylaert, K. Flocculation of Chlorella vulgaris induced by high pH: Role of magnesium and calcium and practical implications. Bioresour. Technol. 2012, 105, 114–119. [Google Scholar]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar]
- Babiak, W.; Krzemińska, I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Blockx, J.; Verfaillie, A.; Thielemans, W.; Muylaert, K. Unravelling the Mechanism of Chitosan-Driven Flocculation of Microalgae in Seawater as a Function of pH. ACS Sustain. Chem. Eng. 2018, 6, 11273–11279. [Google Scholar]
- Endrawati, H.; Widianingsih, W.; Nuraini, R.; Hartati, R.; Redjeki, S.; Riniatsih, I.; Mahendrajaya, R. The effect of chitosan concentration on flocculation efficiency microalgae Porphyridium cruentum (Rhodhophyta). IOP Conf. Ser. Earth Environ. Sci. 2021, 919, 012052. [Google Scholar]
- Van Haver, L.; Nayar, S. Polyelectrolyte flocculants in harvesting microalgal biomass for food and feed applications. Algal Res. 2017, 24, 167–180. [Google Scholar]
- Wang, Q.; Oshita, K.; Takaoka, M. Harvesting Nannochloropsis oculata by Chitosan and AlCl3-Induced Flocculation: Effects of Microalgal Condition on Flocculation Performance. BioEnergy Res. 2021, 14, 924–939. [Google Scholar] [CrossRef]
- Liu, S.; Hajar, H.A.A.; Riefler, G.; Stuart, B.J. Investigation of electrolytic flocculation for microalga Scenedesmus sp. using aluminum and graphite electrodes. RSC Adv. 2018, 8, 38808–38817. [Google Scholar] [PubMed]
- Kirnev, P.C.S.; de Carvalho, J.C.; Miyaoka, J.T.; Cartas, L.C.; Vandenberghe, L.P.S.; Soccol, C.R. Harvesting Neochloris oleoabundans using commercial organic flocculants. J. Appl. Phycol. 2018, 30, 2317–2324. [Google Scholar] [CrossRef]
- Lupa, D.; Płaziński, W.; Michna, A.; Wasilewska, M.; Pomastowski, P.; Gołębiowski, A.; Buszewski, B.; Adamczyk, Z. Chitosan characteristics in electrolyte solutions: Combined molecular dynamics modeling and slender body hydrodynamics. Carbohydr. Polym. 2022, 292, 119676. [Google Scholar] [CrossRef]
- Wei, Q.; Yuan, T.; Li, Z.; Zhao, D.; Wang, C.; Yang, G.; Tang, W.; Ma, X. Investigating cultivation strategies for enhancing protein content in Auxenochlorella pyrenoidosa FACHB-5. Bioresour. Technol. 2024, 402, 130828. [Google Scholar] [CrossRef]
- Wang, Q.; Oshita, K.; Takaoka, M. Evaluation of flocculation performance of amphoteric flocculant when harvesting microalgae Coccomyxa sp. KJ by response surface methodology. J. Environ. Manag. 2021, 277, 111449. [Google Scholar]
- Hadiyanto, H.; Widayat, W.; Pratiwi, M.E.; Christwardana, M.; Muylaert, K. Effect of pH, cationic inducer, and clam shells as bio-flocculant in the optimization of the flocculation process for enhanced microalgae harvesting using response surface methodology. Environ. Pollut. Bioavailab. 2022, 34, 338–351. [Google Scholar]
- Li, Z.; Yuan, T.; Zhao, J.; Wang, C.; Wei, Q.; Ma, X.; Yang, G. Unraveling non-linear dynamics of biomass, photosynthesis efficiency, and lipid accumulation in Chlorella vulgaris under mixotrophic cultivation. J. Clean. Prod. 2024, 448, 141692. [Google Scholar]
- Li, J.; Wang, M.; Li, W.; Zhang, P.; Yu, F.; Li, P. The impact of cell-bound exopolysaccharide on flocculation of the cyanobacterium Synechocystis PCC6803 with ferric chloride and chitosan. J. Appl. Phycol. 2021, 33, 2947–2955. [Google Scholar]
- Shi, Y.; Huang, J.; Zeng, G.; Gu, Y.; Chen, Y.; Hu, Y.; Tang, B.; Zhou, J.; Yang, Y.; Shi, L. Exploiting extracellular polymeric substances (EPS) controlling strategies for performance enhancement of biological wastewater treatments: An overview. Chemosphere 2017, 180, 396–411. [Google Scholar]
- Zhou, Y.; Cui, X.; Wu, B.; Wang, Z.; Liu, Y.; Ren, T.; Xia, S.; Rittmann, B.E. Microalgal extracellular polymeric substances (EPS) and their roles in cultivation, biomass harvesting, and bioproducts extraction. Bioresour. Technol. 2024, 406, 131054. [Google Scholar] [CrossRef]
- Demir, I.; Blockx, J.; Dague, E.; Guiraud, P.; Thielemans, W.; Muylaert, K.; Formosa-Dague, C. Nanoscale Evidence Unravels Microalgae Flocculation Mechanism Induced by Chitosan. ACS Appl. Bio Mater. 2020, 3, 8446–8459. [Google Scholar] [CrossRef]
- Ding, Y.; He, R.; Wang, C.; Wei, Q.; Ma, X.; Yang, G. Efficient separation of Cd2+ and Pb2+ by Tetradesmus obliquus: Insights from cultivation conditions with competitive adsorption modeling. J. Water Process Eng. 2024, 60, 105207. [Google Scholar]
- Gu, S.; Lan, C.Q. Effects of culture pH on cell surface properties and biosorption of Pb(II), Cd(II), Zn(II) of green alga Neochloris oleoabundans. Chem. Eng. J. 2023, 468, 143579. [Google Scholar]
- Hou, X.; Liu, S.; Zhang, Z. Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge. Water Res. 2015, 75, 51–62. [Google Scholar]
- Yang, Z.; Hou, T.; Ma, J.; Yuan, B.; Tian, Z.; Yang, W.; Graham, N.J. Role of moderately hydrophobic chitosan flocculants in the removal of trace antibiotics from water and membrane fouling control. Water Res. 2020, 177, 115775. [Google Scholar] [PubMed]
- Wang, H.; Roman, M. Effects of Chitosan Molecular Weight and Degree of Deacetylation on Chitosan−Cellulose Nanocrystal Complexes and Their Formation. Molecules 2023, 28, 1361. [Google Scholar] [CrossRef]
- Wu, X.; Ge, X.; Wang, D.; Tang, H. Distinct mechanisms of particle aggregation induced by alum and PACl: Floc structure and DLVO evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 56–63. [Google Scholar]
- Rwehumbiza, V.M.; Harrison, R.; Thomsen, L. Alum-induced flocculation of preconcentrated Nannochloropsis salina: Residual aluminium in the biomass, FAMEs and its effects on microalgae growth upon media recycling. Chem. Eng. J. 2012, 200–202, 168–175. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, Q.; Xu, G.; Wang, D. Flocculation–Dewatering Behavior of Microalgae at Different Growth Stages under Inorganic Polymeric Flocculant Treatment: The Relationships between Algal Organic Matter and Floc Dewaterability. ACS Sustain. Chem. Eng. 2018, 6, 11087–11096. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).