Assessment of Mercury Contamination in the Chalk Aquifer of the Pays de Caux and Its Implications for Public Health (France)
Abstract
1. Introduction
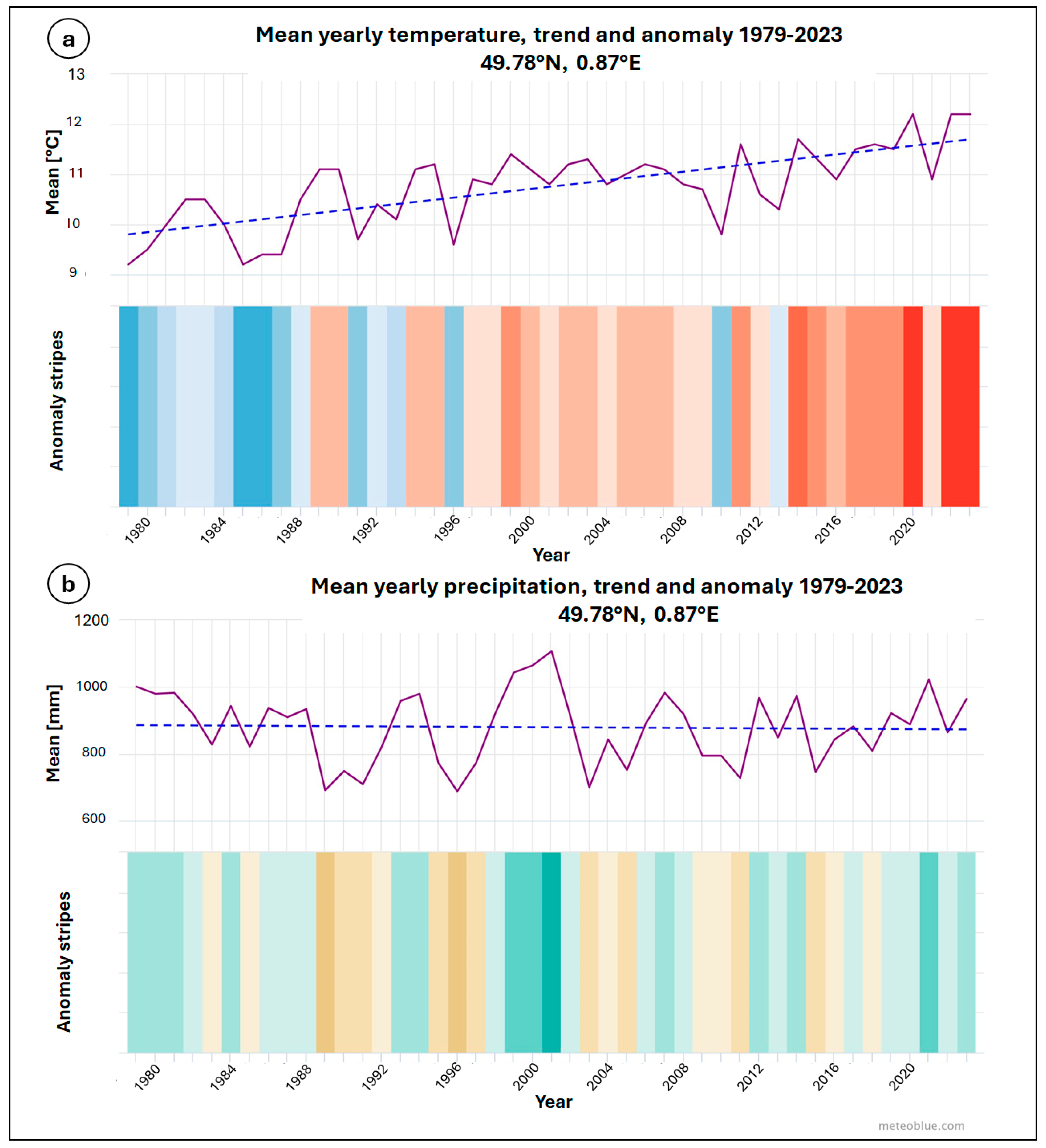
2. Description of the Study Site
3. Description and Characteristics of the Brametot Site (NHWF)
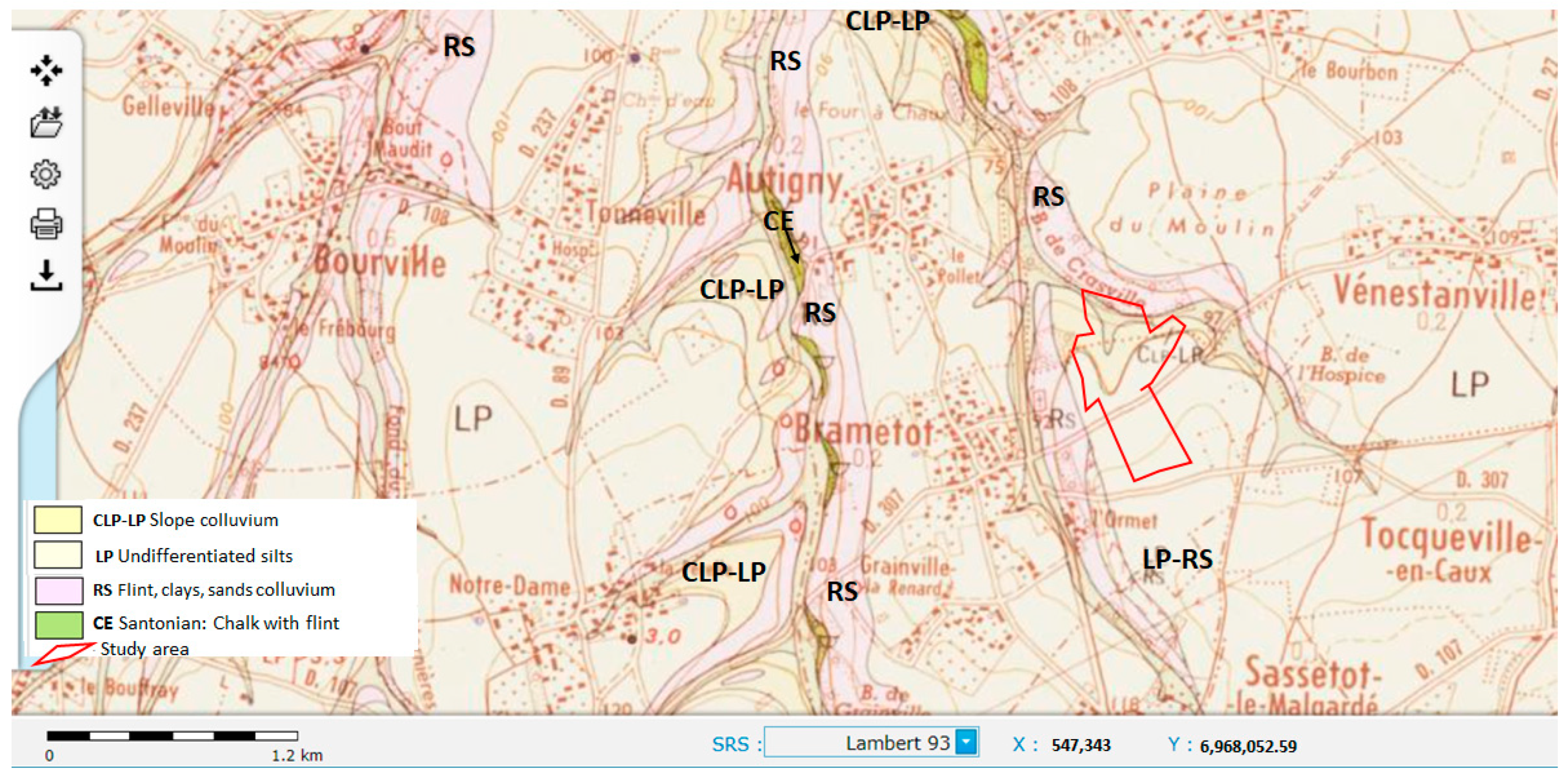
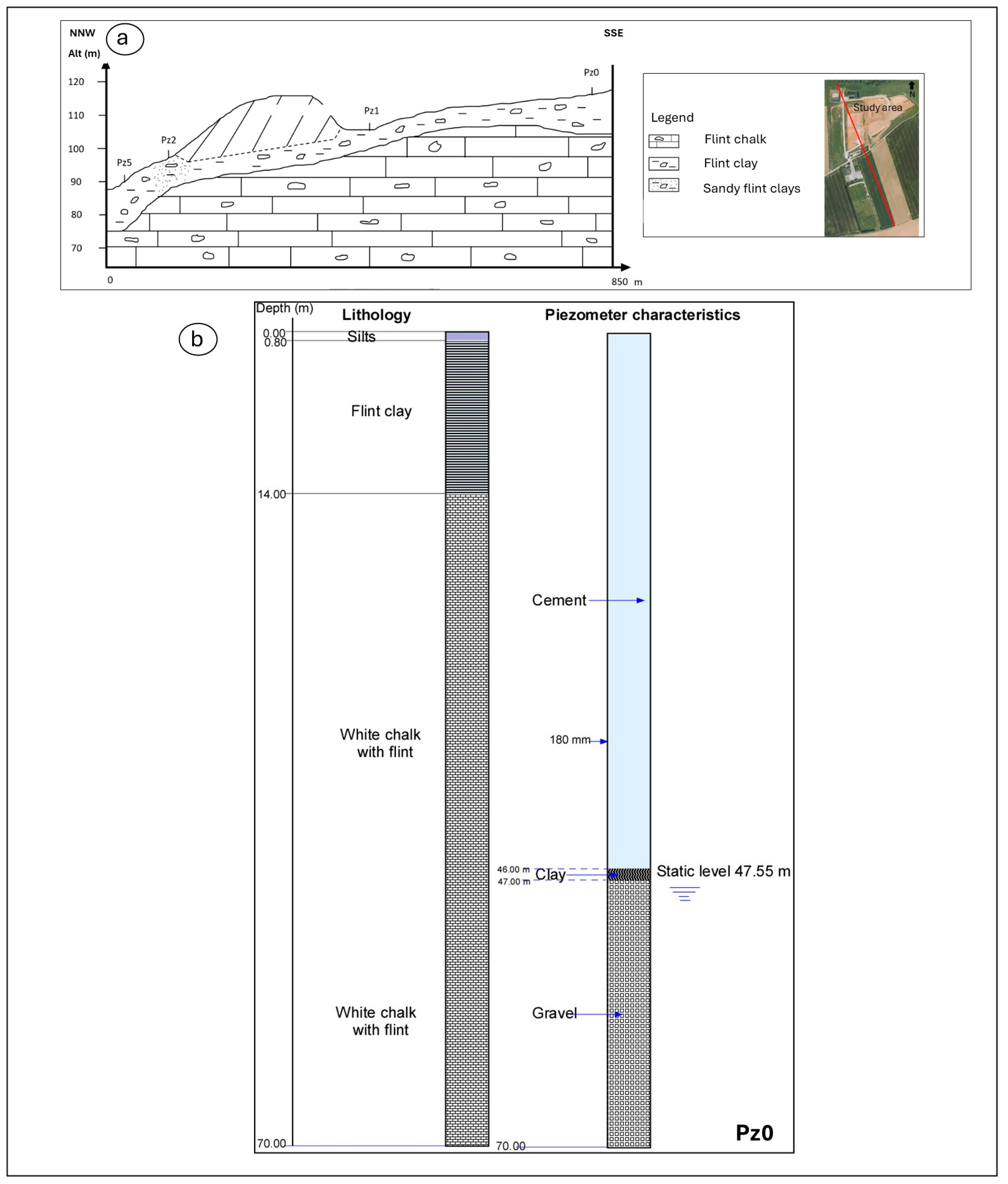
4. Geological and Hydrogeological Settings
5. Materials
6. Campaign Field and Results
6.1. Piezometric Investigations
- Transmissivity values: 10−5 to 10−2 m2/s;
- Storage coefficient: 5% to 10%;
- Cenematic porosity: 0.3 to 1;
- Hydraulic Conductivity: 10−7 to 10−3 m/s;
- Flow rate: 1 m/y to 500 m/h.
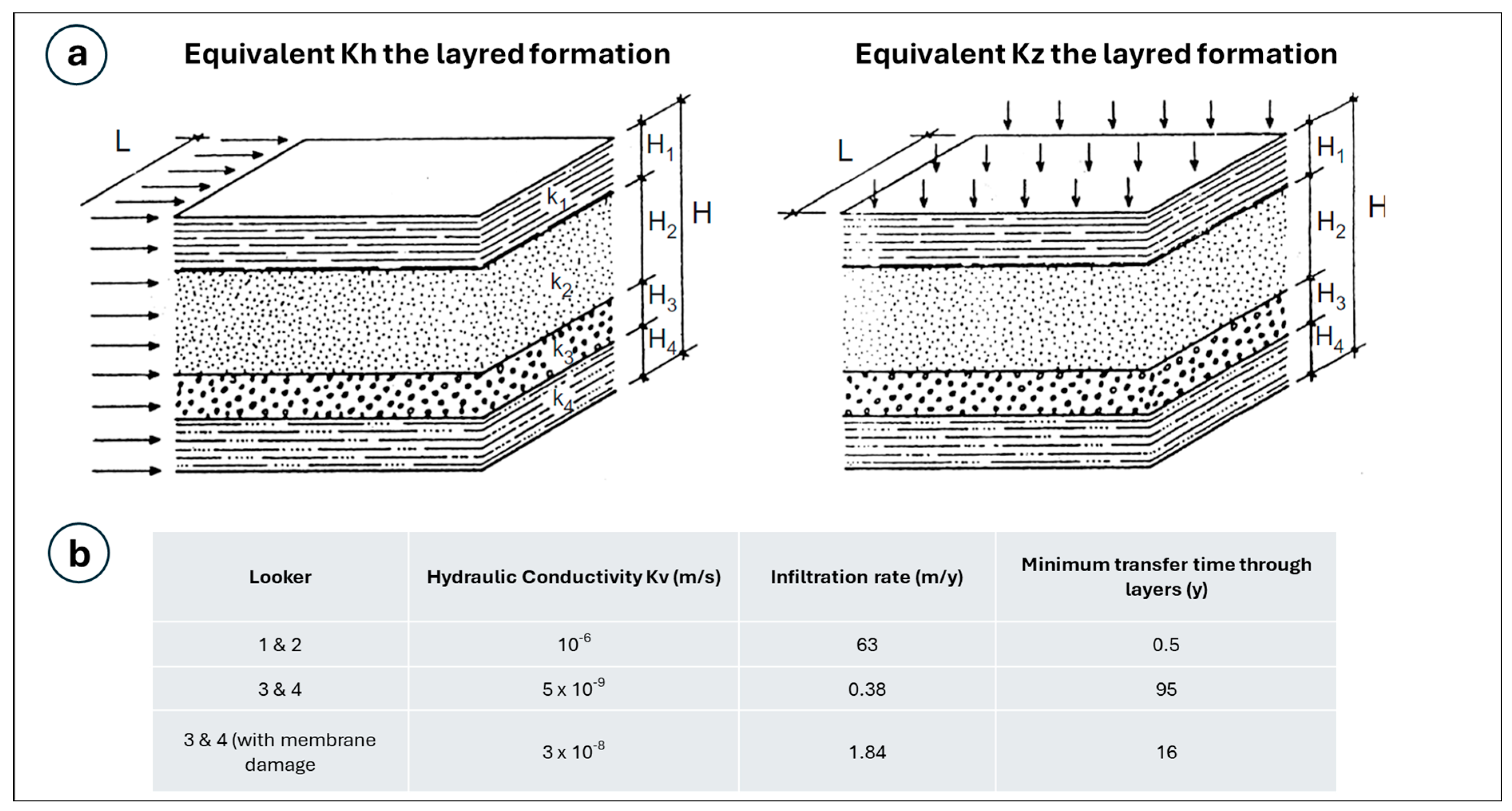
6.2. Velocity of Infiltration
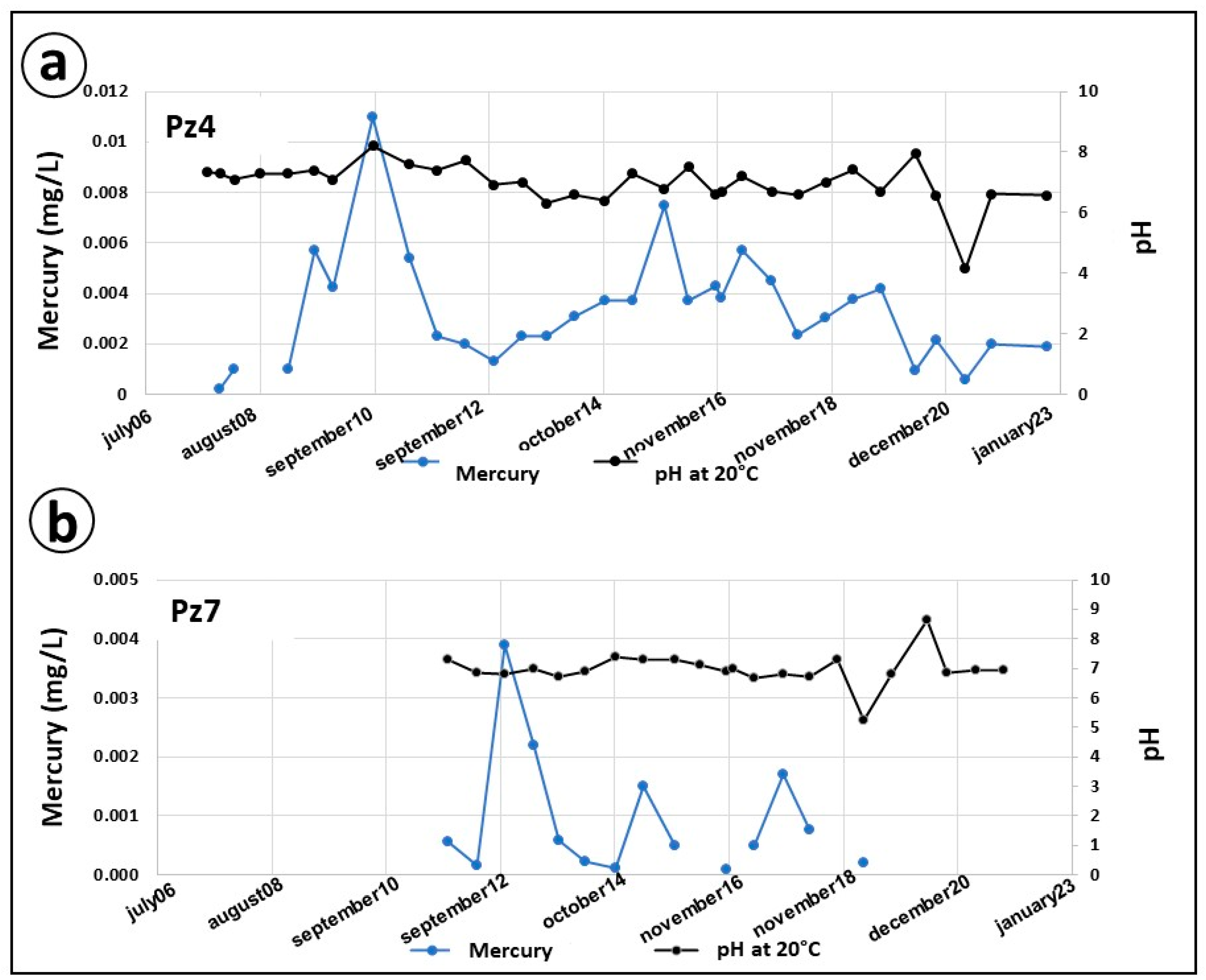
6.3. Correlations of Hg-Ph and Hg-Eh
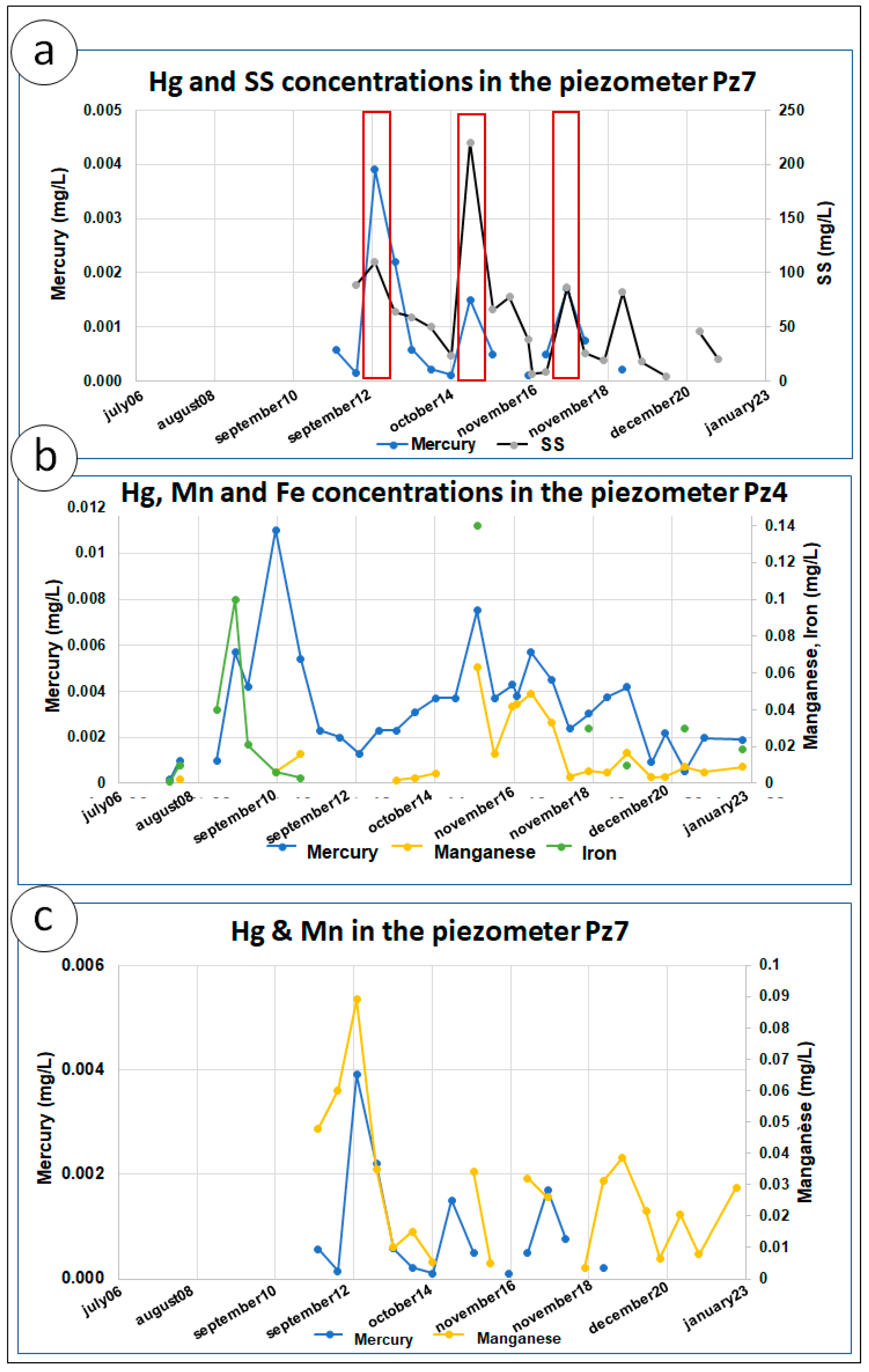
6.4. Evolution of Hg-SS-Metallic Trace Elements (Mn, Fe)
7. Analysis and Discussion
- -
- Discharge of leachates before sealing the basins: The superficial formation, composed of flint clay, is heterogeneous and overlays the chalk formation, which is characterized by a network of fractures and matrix porosity. Regarding hydrodynamic parameters, the Brametot site (NHWF) exhibits various types of porosity. The Brametot non-hazardous waste storage facility (NHWS) is located on the Senonian chalk aquifer, which is distinguished by triple porosity:
- -
- Infiltration of leachate beneath the locker 1 or 2 landfill (Figure 3), resulting from rainwater carrying pollutants from the landfill. Therefore, it is essential to carry out additional geotechnical investigations, including permeability tests, at the site to evaluate the integrity of the locker 2/1 containment.
- -
- Anthropogenic factors through sinkholes and the karst system in the region. The sinkhole phenomenon is observed in the two talwegs bordering the ISDND to the west and east, as well as in the dry valley to the north of the site.
- -
- Transport of mercury concentrations from the soil into the groundwater.
- Immediate Protection Perimeter (IPP): A fenced-off catchment area (unless otherwise exempted), usually owned by a public authority. All activities are prohibited, except those related to the operation and maintenance of the water supply well catchment and the perimeter itself. The primary goal is to protect the integrity of the infrastructure and prevent the discharge of pollutants in the immediate vicinity of the catchment.
- Close Protection Perimeter (CPP): “A larger area (typically a few hectares) where any activity likely to cause pollution is prohibited or subject to special regulations (such as construction, deposits, discharges, etc.). Its purpose is to prevent the migration of pollutants toward the catchment well”.
- Remote Protection Perimeter (RPP): “Optional, this perimeter is established if certain activities are likely to be a significant source of pollution. It generally encompasses the entire catchment basin of the water supply well or catchment area”.
8. Conclusions and Recommendations
8.1. Conclusions
8.2. Recommendations
- (i)
- Accurately identify the heterogeneous layers within the aquifer, particularly at the flint clay and chalk levels.
- (ii)
- Define the geometry of both permeable and impermeable geological formations and characterize structures such as faults and fractures that facilitate groundwater flow and mercury transport. Previous studies have demonstrated the significance of tectonic structures in understanding the hydrogeological behavior (including piezometric variations and transmissivity distribution) of complex aquifer systems, such as coastal, porous, and fractured media. The characterization of clayey layers within the chalk aquifer should be further explored in future work through borehole logging techniques (including measurements of water conductivity, temperature, gamma-ray, as well as central and lateral camera logging).
- (iii)
- Modeling the transport of mercury in the groundwater system, particularly within the chalk aquifer. In this context, the study will focus on defining the hydrogeological parameters that govern groundwater flow within the chalk matrix, including transmissivity, hydraulic conductivity, and storage coefficients. This information will be used to construct hydrodynamic and hydrodispersive models for the site. One promising approach is the application of hydrogeological optimization through the algorithm-based LBM/CMA-ES combination, which offers a solution for improved water management and provides further insights into solute transport dynamics in the chalk aquifer. However, the spatial characterization of these hydrodynamic parameters presents significant challenges due to several factors: (a) the high cost of installing additional piezometers and experimental test wells, especially in deeper boreholes, which requires considerable financial resources and time; and (b) the quality of geophysical survey data (such as Magnetic Resonance Soundings (MRS)) at the Brametot site, which is compromised by interference from field measurement noise originating from surrounding urban infrastructure, including the sewer system, and buried electrical and telecommunication cables.
- (iv)
- Confirm the influence of geological structures on the presence of the dividing axis (Figure 7). Additionally, the role of microbial activity in reducing mercury concentrations will be further explored. Specifically, under reducing conditions, microbial activity contributes to the reductive dissolution of FeOOH, which, in turn, releases sorbed Hg2+ into the groundwater.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, R.-P.; Xu, Y.-N.; Rui, H.-C.; El-Wardany, R.M.; Dong, Y. Migration and speciation transformation mechanisms of mercury in undercurrent zones of the Tongguan gold mining area, Shaanxi Loess Plateau and impact on the environment. China Geol. 2021, 4, 311–328. [Google Scholar]
- Georgin, J.; Franco, D.S.P.; Dehmani, Y.; Nguyen-Tri, P.; El Messaoudi, N. Current status of advancement in remediation technologies for the toxic metal mercury in the environment: A critical review. Sci. Total Environ. 2024, 947, 174501. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Wang, J.; Guo, S.; Yang, L. Mercury-induced toxicity: Mechanisms, molecular pathways, and gene regulation. Sci. Total Environ. 2024, 943, 173577. [Google Scholar] [CrossRef]
- Aleku, D.L.; Lazareva, O.; Pichler, T. Mercury in groundwater—Source, transport and remediation. Appl. Geochem. 2024, 170, 106060. [Google Scholar] [CrossRef]
- Liu, B.; Tian, K.; He, Y.; Hu, W.; Huang, B.; Zhang, X.; Zhao, L.; Teng, Y. Dominant roles of torrential floods and atmospheric deposition revealed by quantitative source apportionment of potentially toxic elements in agricultural soils around a historical mercury mine. Southwest China Ecotoxicol. Environ. Safety 2022, 242, 113854. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-S.; Kim, Y.-M.; Lee, K.-E. Methylmercury Exposure and Health Effects. J. Prev. Med. Public Health 2012, 45, 353–363. [Google Scholar] [CrossRef]
- Mahaffey, K.R.; Clickner, R.P.; Bodurow, C.C. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ. Health Perspect. 2004, 112, 562–570. [Google Scholar]
- Marcello, M.V.; Omotayo, F. A review of the failed attempts to curb mercury use at artisanal gold mines and a proposed solution. Extr. Ind. Soc. 2020, 7, 1135–1146. [Google Scholar] [CrossRef]
- Feng, X.; Dai, Q.Q.; Qiu, G.L.; Li, G.H.; He, L.; Wang, D.Y. Gold mining related mercury contamination in Tongguan, Shaanxi Province, PR China. Appl. Geochem. 2006, 21, 1955–1968. [Google Scholar] [CrossRef]
- Feng, X.; Li, G.; Qiu, G. A preliminary study on mercury contamination to the environment from artisanal zinc smelting using indigenous methods in Hezhang County, Guizhou, China: Part 2. Mercury contaminations to soil and crop. Sci. Total Environ. 2006, 368, 47–55. [Google Scholar] [CrossRef]
- Ferrara, R.; Mazzolai, B.; Lanzillota, E.; Nucaro, E.; Pirrone, N. Volcanoes as emission sources of atmospheric mercury in the Mediterranean basin. Sci Total Environ. 2000, 259, 115–121. [Google Scholar] [PubMed]
- Fiorentino, J.C.; Enzweiler, J.; Angélica, R.S. Geochemistry of mercury along a soil profile compared to other elements and to the parental rock: Evidence of external input. Water Air Soil Pollut. 2011, 221, 63–75. [Google Scholar]
- Peña-Rodríguez, S.; Pontevedra-Pombal, X.; Fernández-Calviño, D.; Taboada, T.; Arias-Estévez, M.; Martínez-Cortizas, A.; Nóvoa-Muñoz, J.C. Mercury content in volcanic soils across Europe and its relationship with soil properties. J. Soils Sediments 2012, 12, 542–555. [Google Scholar] [CrossRef]
- Kotalik, C.J.; Willacker, J.J.; Wesner, J.S.; Johnson, B.L.; Flanagan Pritz, C.M.; Nelson, S.J.; Walters, D.M.; Eagles-Smith, C.A. Ecosystem Drivers of Freshwater Mercury Bioaccumulation Are Con-text-Dependent: Insights from Continental-Scale Modeling. Environ. Sci. Technol. 2025, 59, 1780–1789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johannesson, K.H.; Neumann, K. Geochemical cycling of mercury in a deep, confined aquifer: Insights from biogeochemical reactive transport modeling Geochem. Cosmochim. Acta. 2013, 106, 25–43. [Google Scholar]
- Grassi, S.; Netti, R. Sea water intrusion and mercury pollution of some coastal aquifers in the province of Grosseto (Southern Tuscany–Italy). J. Hydrol. 2000, 237, 198–211. [Google Scholar]
- Bagnato, E.; Aiuppa, A.; Parello, F.; D’alessandro, W.; Allard, P. Calabrese Mercury concentration, speciation and budget in volcanic aquifers: Italy and Guadeloupe (Lesser Antilles). J. Volcanol. Geoth. Res. 2009, 179, 96–106. [Google Scholar]
- Smith, K.D.; Lacerda, L.D.; Mendes, M. Mercury in the environment: Biological, geological, and environmental perspectives. Sci. Total Environ. 2014, 476, 102–116. [Google Scholar]
- Bashir, M.J.K.; Zhang, Z.; Lu, X. Mercury in the environment: A review of its sources, toxicology, and remediation techniques. Environ. Sci. Pollut. Res. 2019, 26, 7479–7494. [Google Scholar]
- Moussa, B.; Yahia, H. Contamination by mercury of groundwater of the North Numidian zone of Azzaba, North East Algeria. Effect of inorganic mercury contamination of population. Int. J. Environ. Waste Manag. 2012, 9, 347–357. [Google Scholar]
- Charvát, P.; Klimeš, L.; Pospíšil, J.; Klemeš, J.J.; Varbanov, P.S. An overview of mercury emissions in the energy industry—A step to mercury footprint assessment. J. Clean. Prod. 2020, 267, 122087. [Google Scholar]
- Wiederhold, J.G.; Skyllberg, U.; Drott, A.; Jiskra, M.; Jonsson, S.; Bjorn, E.; Kretzschmar, R. Mercury Isotope Signatures in Contaminated Sediments as a Tracer for Local Industrial Pollution Sources. Environ. Sci. Technol. Environ. 2015, 49, 177–185. [Google Scholar]
- Lacerda, L.D.; Salomons, W. Mercury from gold and silver mining: A global as-sessment. In Mercury in the Environment; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–18. [Google Scholar]
- Brgm-Georisques, Les Risques Près de Chez Moi. Brametot (76). In: Géorisques [17/12/2023.], 223. Date de Consultation. 17 December 2023. Available online: https://www.georisques.gouv.fr/mes-risques/connaitre-les-risques-pres-de-chez-moi (accessed on 1 April 2025).
- Sauter, M. Impact sur les Eaux Souterraines d’une Installation de Compostage et de Mise en Décharge D’ordures Ménagères, Le cas de Brametot (76); BRGM: Paris, France, 1989. 20p. Available online: http://infoterre.brgm.fr/rapports/RR-30145-FR.pdf (accessed on 1 April 2025).
- Eberentz, P. Notice d’impact de la Décharge D’ordures Ménagères et de la Zone de Compostage de Brametot (76); Bureau de Recherches Géologiques et Minières, Service Géologique National: Orléans, France, 1978; 18p. [Google Scholar]
- Bureau de Recherches Géologiques et Minières. Carte et notice 0058. In Doudeville; BGRM: Paris, France; ISBN 9782715910584.
- Chevrier, B.; Lallier, S. Rapport D’expertise: Recherche de L’origine de la Qualité des Eaux des Piézomètres ISDND Brametot (76). BRGMRP-58895-FR. 2010. 19p. Available online: http://infoterre.brgm.fr/rapports/RP-58895-FR.pdf (accessed on 1 April 2025).
- Jorgensen, L.N.; Andersen, P.M. Integrated study of the Kraka Field. In Proceedings of the Offshore European, Aberdeen, UK, 3–6 September 1991. [Google Scholar] [CrossRef]
- Huerta-Diaz, M.; Tessier, A.; Carrigan, R. Geochemistry of trace metals associated with iron monosulphides and pyrite in organic freshwater sediment. Environ. Sci. Technol. 1996, 72, 2367–2372. [Google Scholar]
- Morse, J.W. Interaction of trace metals with authigenic sulfide minerals: Implications for their bioavailability. Mar. Chem. 1994, 46, 187–199. [Google Scholar]
- Bono, A. The Partitioning of Mercury Among the Solid Components of Sediments of the Saguenay Fjord, Quebec. Master Thesis, McGill University, Montreal, QC, Canada, 1997. [Google Scholar]
- Cossa, D.; Coquery, M.; Gobeil, C.; Martin, J.M. Mercury Fluxes at the Ocean Margins. In Global and Regional Mercury Cycles: Sources, Fluxes and Mass Balances; Baeyens, W., Ebinghaus, R., Vasiliev, O., Eds.; NATO ASI Series; Springer: Berlin/Heidelberg, Germany, 1996; Volumn 21, pp. 229–247. [Google Scholar] [CrossRef]
- Quémerais, B.; Cossa, D.; Rondeau, B.; Pham, T.T.; Fortin, B. Mercury Distribution in relation to Iron and Manganese in the Waters of the St. Lawrence River. Sci. Total Environ. 1998, 213, 193–201. [Google Scholar] [PubMed]
- Roulet, M.; Lucotte, M. Geochemistry of mercury in pristine and flooded ferralitic soils of a tropical rainforest in French Guiana, South America. Water Air Soil Pollut. 1995, 80, 1079–1088. [Google Scholar] [CrossRef]
- Domenico, P.A. Schwartz, F W. Physical and Chemical Hydrogeology; John Wiley & Sons: Hoboken, NJ, USA, 1998; 505p. [Google Scholar]
- Allen, D.J.; Brewerton, L.J.; Coleby, L.M.; Gibbs, B.R.; Lewis, M.A.; MacDonald, A.M.; Wagstaff, S.J.; Williams, A.T. The Physical Properties of Major Aquifers in England and Wales; British Geological Survey Technical Report WD/97/34; Environment Agency R&D Publication: Washington, DC, USA, 1997; p. 312. [Google Scholar]
- Price, M.; Low, R.G.; McCann, C. Mechanisms of water storage and flow in the un-saturated zone of the Chalk aquifer. J. Hydrol. 2000, 233, 54–71. [Google Scholar]
- Thomassin, J.F.; Touze, S.; Baranger, P.; Freyssinet, P. Le Mercure et ses Composés Comportement dans les Sols, les Eaux et les Boues de Sédiments; BRGM: Orléans, France, 2003; BRGM/RP 51980 Fr; 119p. [Google Scholar]
- Valentin-Rouy, N.; Huet, S. Comportement de certains micropolluants métalliques dans le sol et les produits agricoles après épandage de boues urbaines, Suivi parallèle de l’azote et du phosphore 1995–2004. In Aspects Sanitaires et Environnementaux de L’épandage Agricole des Boues D’épuration Urbaines; ADEME Journées Tech: Montrouge, France, 1997; 320p. [Google Scholar]
- Sigg, L.; Behra, P.; Stumm, W. Chimie des Milieux Aquatiques; Dunod: Paris, France, 2014; 514p, ISBN 978-2-10-058801-5. [Google Scholar]
- McLean, J.E. Behavior of Metals in Soils; Technology Innovation Office, Office of Solid Waste and Emergency Response: Washington, DC, USA, 1992. [Google Scholar]
- Rae, J.E.; Aston, S.R. The role of suspended solids in the estuarine geochemistry of mercury. Water Res. 1982, 16, 649–654. [Google Scholar] [CrossRef]

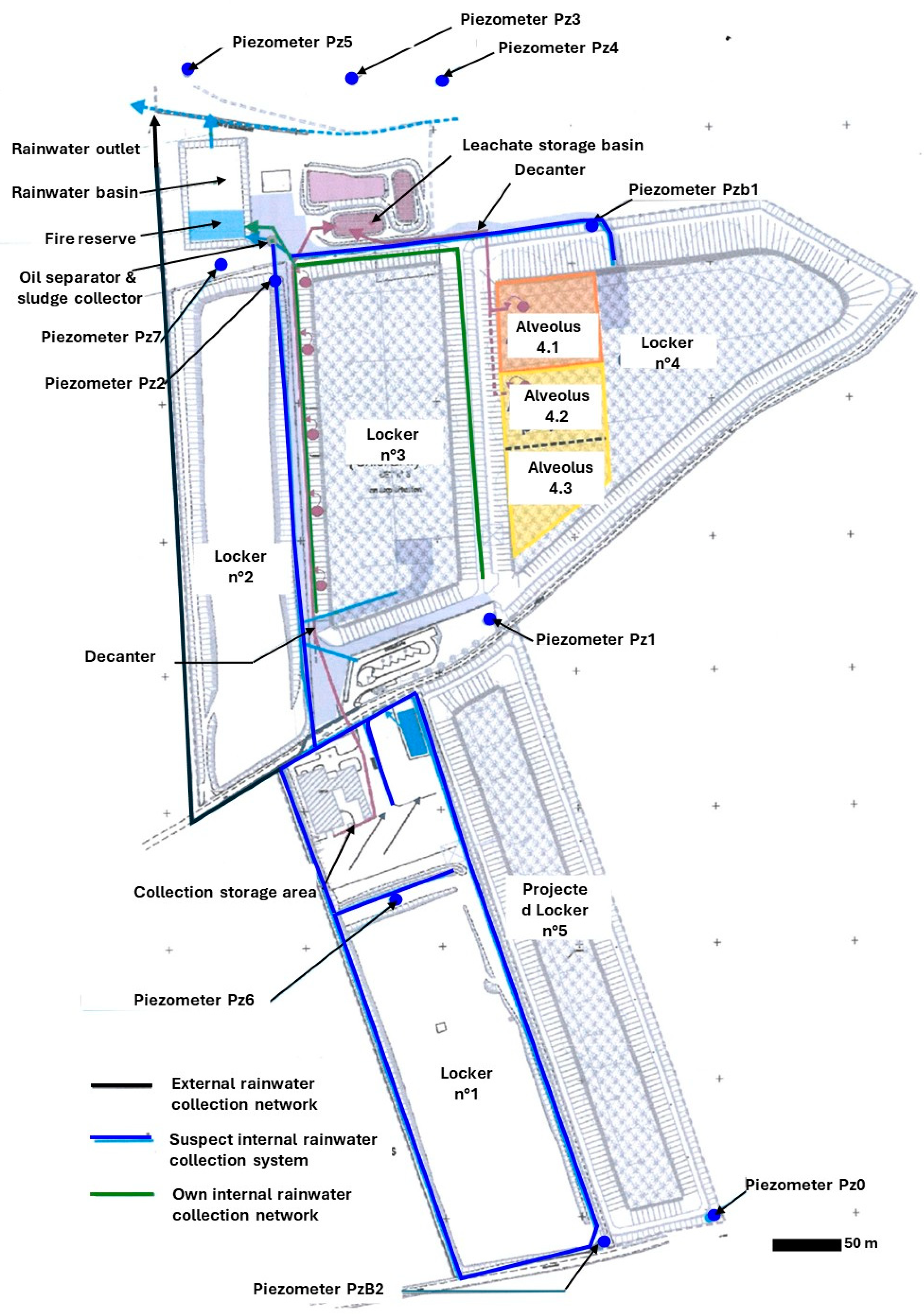
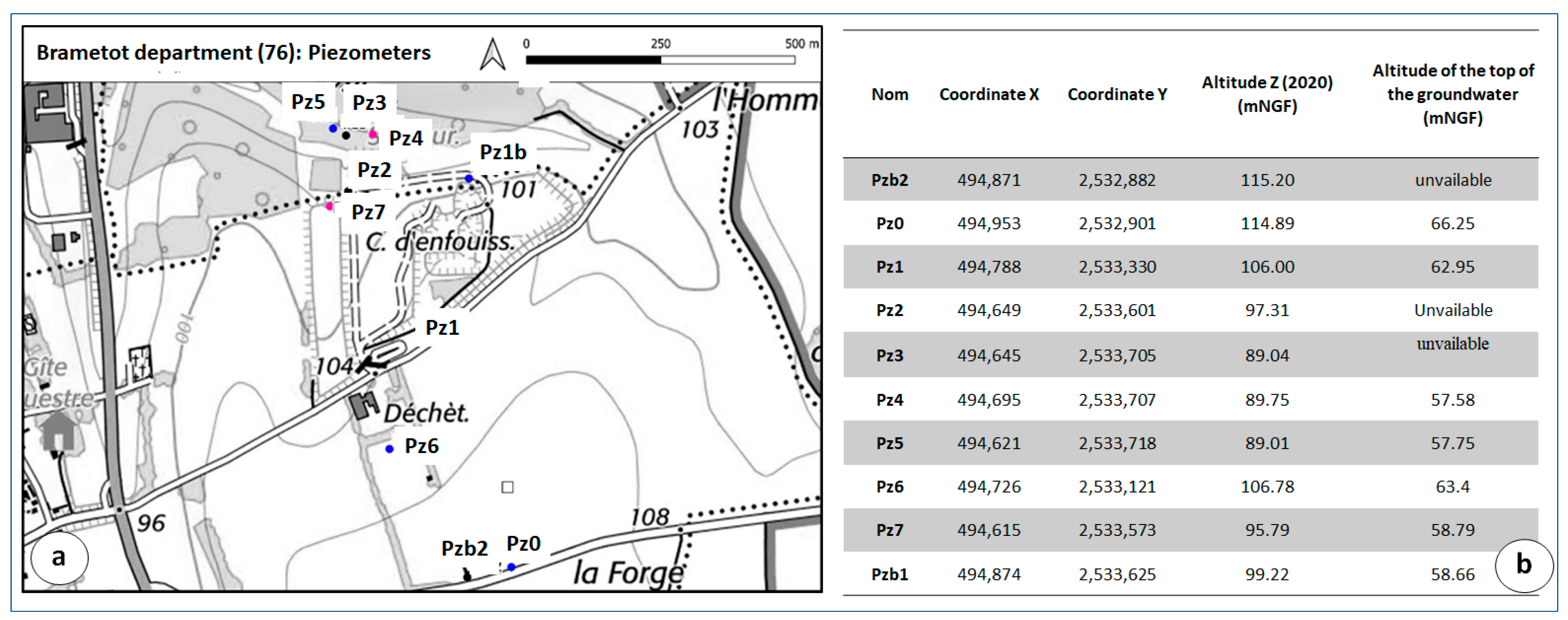

| Piezometer | Diameter (mm) | Pipe Height (m) | Screen Tube Height (m) |
|---|---|---|---|
| Pzb2 | 112/125 | no info | no info |
| Pz0 | 80/90 | 0–50 | 50–70 |
| Pz1 | 80/90 | 0–33.6 | 33.6–47 |
| Pz2 | 112/125 | no info | no info |
| Pz3 | 112/125 | no info | no info |
| Pz4 | 80/90 | 0–40 | 40–65 |
| Pz5 | 80/90 | 0–33.5 | 33.5–58.5 |
| Pz6 | 80/90 | 0–29 | 29–71 |
| Pz7 | 80/90 | 0–29 | 29–71 |
| Pzb1 | 112/125 | 0–33.6 | 33.6–72.6 |
| Eh Average | |||||||
|---|---|---|---|---|---|---|---|
| Pz0 | Pz1 | Pz4 | Pz5 | Pz6 | Pz7 | Pzb1 | |
| Total averages of the Hg (µg/L) | 0.10 | 0.09 | 3.32 | 0.55 | 0.10 | 0.93 | 0.20 |
| Total averages of the Eh (mV) | 208 | 46 | 234 | 184 | 205 | 161 | 194 |
| 2004 | 15 | 18 | |||||
| 2005 | 18 | 18 | |||||
| 2006 | 19 | 21 | |||||
| 2007 | 85 | 225 | 237 | 225 | |||
| 2008 | 296 | 226 | 202 | 168 | 187 | ||
| 2009 | 13 | 36 | 83 | 80 | 85 | ||
| 2010 | 304 | 294 | 229 | 241 | 380 | 280 | 272 |
| 2011 | 199 | 149 | 216 | 153 | 193 | 62 | 213 |
| 2012 | 138 | −19 | 204 | 176 | 215 | 129 | 172 |
| 2013 | 263 | −62 | 343 | 370 | 349 | 139 | 269 |
| 2014 | 219 | −42 | 204 | 113 | 112 | 156 | 138 |
| 2015 | 144 | −77 | 138 | 102 | 76 | 144 | 146 |
| 2016 | 36 | −47 | 189 | 93 | 80 | 0 | 168 |
| 2017 | 257 | 92 | 358 | 280 | 388 | 257 | 411 |
| 2018 | 281 | 36 | 342 | 187 | 227 | 277 | 307 |
| 2019 | 53 | 43 | 36 | 340 | 50 | 50 | |
| 2020 | 4 | 33 | 26 | 14 | 5 | 7 | |
| 2021 | 2 | 82 | 8 | 11 | 87 | 33 | 100 |
| 2022 | 12 | ||||||
| Average of the SS Content for All Piezometers | |||||||
|---|---|---|---|---|---|---|---|
| Pz0 | Pz1 | Pz4 | Pz5 | Pz6 | Pz7 | Pzb1 | |
| Total averages of the Hg (µg/L) | 0.10 | 0.09 | 3.32 | 0.55 | 0.10 | 0.93 | 0.20 |
| Total averages of the SS (mg/L) | 25 | 1563 | 204 | 73 | 341 | 56 | 1159 |
| 2004 | 370 | ||||||
| 2005 | 150 | 2620 | |||||
| 2006 | 57 | 9055 | |||||
| 2007 | 38 | 9611 | 47 | 7 | 1424 | ||
| 2008 | 63 | 1445 | 3 | 71 | 1845 | ||
| 2009 | 32 | 5730 | 5 | 531 | 1830 | ||
| 2010 | 34 | 370 | 9 | 230 | |||
| 2011 | 3 | 340 | 120 | 19 | |||
| 2012 | 254 | 1351 | 53 | 150 | 100 | 248 | |
| 2013 | 6 | 39 | 2 | 95 | 87 | 62 | 15 |
| 2014 | 28 | 69 | 3 | 30 | 260 | 37 | 28 |
| 2015 | 1423 | 10 | 13 | 655 | 143 | 97 | |
| 2016 | 4 | 68 | 33 | 997 | 41 | 98 | |
| 2017 | 6 | 56 | 38 | 375 | 48 | 730 | |
| 2018 | 3 | 126 | 3 | 36 | 117 | 23 | 490 |
| 2019 | 53 | 43 | 36 | 340 | 50 | 50 | |
| 2020 | 4 | 33 | 26 | 14 | 5 | 7 | |
| 2021 | 2 | 82 | 8 | 11 | 87 | 33 | 100 |
| 2022 | 12 | ||||||
| Compounds and parameters analyzed | Piezometer | PZ0 | PZ6 | PZB1 | PZ7 | PZ5 | PZ4 | PZ1 | Standards ’Guidelines for drinking water quality (Order of 11/1/01/07/Annex 1 (France)) |
| Unit | |||||||||
| In-situ parameters | |||||||||
| Oxygene (%) | % | 82.5 | 62.2 | 78.1 | 76.7 | 69 | 89.2 | - | |
| Temperature (°C) | °C | 13.16 | 14.31 | 12.56 | 13.38 | 12.05 | 13.09 | - | |
| Conductiviy | µS/cm | - | 401 | 407 | - | - | - | - | |
| pH | - | 7.9 | 7.91 | 7.89 | 7.85 | 7.81 | 7.77 | - | |
| Redox Potentiel (Eh) | mV | 4 | −0.1 | 0.5 | 3 | 5.2 | 7.7 | - | |
| Major and Minor Elements | |||||||||
| Phosphorus (P) | mg/L | 0.15 | 1.41 | 0.34 | 1.43 | 0.28 | <0.00015 | 0.39 | |
| Sodium (Na) | mg/L | 12.09 | 10.45 | 10.56 | 22.22 | 64.55 | 12.47 | 13.63 | 200 mg/L |
| Potatium (K) | mg/L | 0.42 | 0.67 | 1.10 | 1.48 | 5.26 | 0.78 | 1.04 | |
| Iron (Fe) | µg/L | 778 | 756 | 626 | 5801 | 1059 | 31 | 8191 | 200 µg/L |
| Magnesium (Mg) | mg/L | 4.20 | 4.73 | 5.00 | 7.33 | 5.34 | 4.79 | 5.45 | |
| Manganese (Mn) | µg/L | 147.7 | 575.5 | 213 | 252.5 | 842.7 | 9.3 | 586.3 | 50 µg/L |
| Aluminium (Al) | µg/L | 953 | 1101 | 806 | 692 | 926 | 343 | 920 | 200 µg/L |
| Calcium (Ca) | mg/L | 172.90 | 659.70 | 273.70 | 499.10 | 138.40 | 136.20 | 175.10 | |
| Rubidium (Rb) | µg/L | 0.97 | 1.36 | 1.30 | 1.76 | 1.44 | 0.44 | 1.44 | |
| Cesium (Cs) | µg/L | 0.11 | 0.10 | 0.09 | 0.09 | 0.04 | 0.01 | 0.05 | |
| Traces Elements | |||||||||
| Berylium (Be) | µg/L | 0.20 | 0.19 | 0.37 | 0.15 | 0.04 | 0.0038 | 0.04 | |
| Strontium (Sr) | µg/L | 273.90 | 928.93 | 385.26 | 728.38 | 241.69 | 215.75 | 346.62 | |
| Baryum (Ba) | µg/L | 24.76 | 41.33 | 25.58 | 44.27 | 43.98 | 23.28 | 49.16 | 700 µg/L |
| Titanium (Ti) | µg/L | < 0.10 | < 0.10 | < 0.10 | < 0.10 | < 0.10 | < 0.10 | < 0.10 | |
| Vanadium (V) | µg/L | 1.65 | 6.24 | 2.41 | 4.50 | 2.87 | 0.43 | 2.14 | |
| Chrome (Cr) | µg/L | 19.53 | 7.52 | 2.81 | 5.36 | 2.49 | 1.50 | 3.80 | 50 µg/L |
| Cobalt (Co) | µg/L | 14.94 | 5.63 | 1.36 | 2.80 | 2.22 | 0.08 | 3.71 | |
| Nickel (Ni) | µg/L | 53.28 | 10.46 | 5.75 | 12.80 | 5.06 | 1.86 | 13.69 | 20 µg/L |
| Copper (Cu) | µg/L | 4.42 | 2.19 | 1.81 | 1.65 | 2.10 | 0.50 | 10.44 | 1000 µg/L |
| Zinc (Zn) | µg/L | 13.27 | 20.05 | 18.63 | 26.90 | 9.17 | 3.25 | 27.08 | 5000 µg/L |
| Cadmium (Cd) | µg/L | 0.13 | 0.53 | 0.36 | 0.33 | 0.20 | 0.02 | 0.04 | 5 µg/L |
| Mercury (Hg) | µg/L | <0.5 | 0.71 | <0.5 | <0.5 | <0.5 | 1.07 | <0.5 | 1 µg/L |
| Gallium (Ga) | µg/L | 0.73 | 0.48 | 0.34 | 0.36 | 0.24 | 0.06 | 0.25 | |
| Indium (In) | µg/L | 0.001 | 0.004 | 0.002 | 0.003 | 0.001 | < 0.01 | 0.003 | |
| Plomb (Pb) | µg/L | 1.77 | 3.31 | 1.70 | 2.08 | 1.39 | 0.08 | 3.58 | 10 µg/L |
| Bismuth (Bi) | µg/L | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | |
| Metalloides | |||||||||
| Silicium (Si) | mg/L | 6.06 | 7.60 | 5.65 | 6.48 | 6.38 | 5.88 | 5.81 | |
| Lanthanide Elements | |||||||||
| Lanthane (La) | ng/L | 7381 | 8940 | 4465 | 6024 | 859 | 54 | 752 | |
| Cerium (Ce) | ng/L | 6961 | 9711 | 5182 | 9020 | 2046 | 65 | 2031 | |
| Praseodyme (Pr) | ng/L | 2556 | 1760 | 994 | 1220 | 217 | 11 | 192 | |
| Neodyme (Nd) | ng/L | 10820 | 7065 | 4069 | 4874 | 817 | 45 | 753 | |
| Samarium (Sm) | ng/L | 2565 | 1517 | 956 | 1058 | 191 | 9 | 160 | |
| Europium (Eu) | ng/L | 591 | 360 | 233 | 263 | 43 | 3 | 35 | |
| Gadolinium (Gd) | ng/L | 2400 | 1706 | 1040 | 1248 | 205 | 13 | 169 | |
| Terbium (Tb) | ng/L | 327 | 233 | 143 | 167 | 27 | 2 | 24 | |
| Dysprosium (Dy) | ng/L | 1785 | 1409 | 857 | 1014 | 157 | 9 | 131 | |
| Holmium (Ho) | ng/L | 323 | 282 | 179 | 202 | 29 | 2 | 25 | |
| Erbium (Er) | ng/L | 834 | 788 | 486 | 560 | 80 | 6 | 67 | |
| Thulium (Tm) | ng/L | 96 | 90 | 61 | 64 | 10 | 1 | 8 | |
| Ytterbium (Yb) | ng/L | 636 | 601 | 391 | 426 | 65 | 4 | 53 | |
| Lutécium (Lu) | ng/L | 85 | 85 | 57 | 62 | 10 | 0.3 | 8 | |
| Actinide Elements | |||||||||
| Thorium (Th) | µg/L | 0.13 | 0.10 | 0.12 | 0.18 | 0.02 | 0.003 | 0.10 | |
| Uranium (U) | µg/L | 1 | 2 | 1 | 1 | 1 | 0.5 | 0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouhri, L.; Delépine, J.; Zouhri, L. Assessment of Mercury Contamination in the Chalk Aquifer of the Pays de Caux and Its Implications for Public Health (France). Water 2025, 17, 1087. https://doi.org/10.3390/w17071087
Zouhri L, Delépine J, Zouhri L. Assessment of Mercury Contamination in the Chalk Aquifer of the Pays de Caux and Its Implications for Public Health (France). Water. 2025; 17(7):1087. https://doi.org/10.3390/w17071087
Chicago/Turabian StyleZouhri, Lahcen, Jacques Delépine, and Lockman Zouhri. 2025. "Assessment of Mercury Contamination in the Chalk Aquifer of the Pays de Caux and Its Implications for Public Health (France)" Water 17, no. 7: 1087. https://doi.org/10.3390/w17071087
APA StyleZouhri, L., Delépine, J., & Zouhri, L. (2025). Assessment of Mercury Contamination in the Chalk Aquifer of the Pays de Caux and Its Implications for Public Health (France). Water, 17(7), 1087. https://doi.org/10.3390/w17071087







