Nature-Based Solution for Wastewater Treatment and Reuse Using Phytoremediation with Floating Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Collection of Aquatic Plants and Establishment of Nursery
2.3. Bench Scale Experiment
2.4. Experimental Treatments
2.5. Sample Collection and Analysis
- pH, total dissolved solids (TDS), electrical conductivity (EC), salinity, temperature, chloride, nitrate, and ammonia using SEBA Hydrometre MPS-16.
- Total hardness determined by titration with EDTA using Eriochrome Black T as an indicator [23].
- Sodium concentration measured using a flame photometer (JENWAY PFP 7) (Figure 6).
2.5.1. Total Dissolved Solids (TDS), Electrical Conductivity, and Salinity
2.5.2. Temperature
2.5.3. pH
2.5.4. Total Hardness
2.5.5. Chloride
2.5.6. Ammonium
2.5.7. Nitrate
2.5.8. Calcium
2.5.9. Sodium Adsorption Ratio (SAR)
2.6. Evapotranspiration and Value Adjustment
2.7. Parameters Used in the Evaluation of Agricultural Water Quality
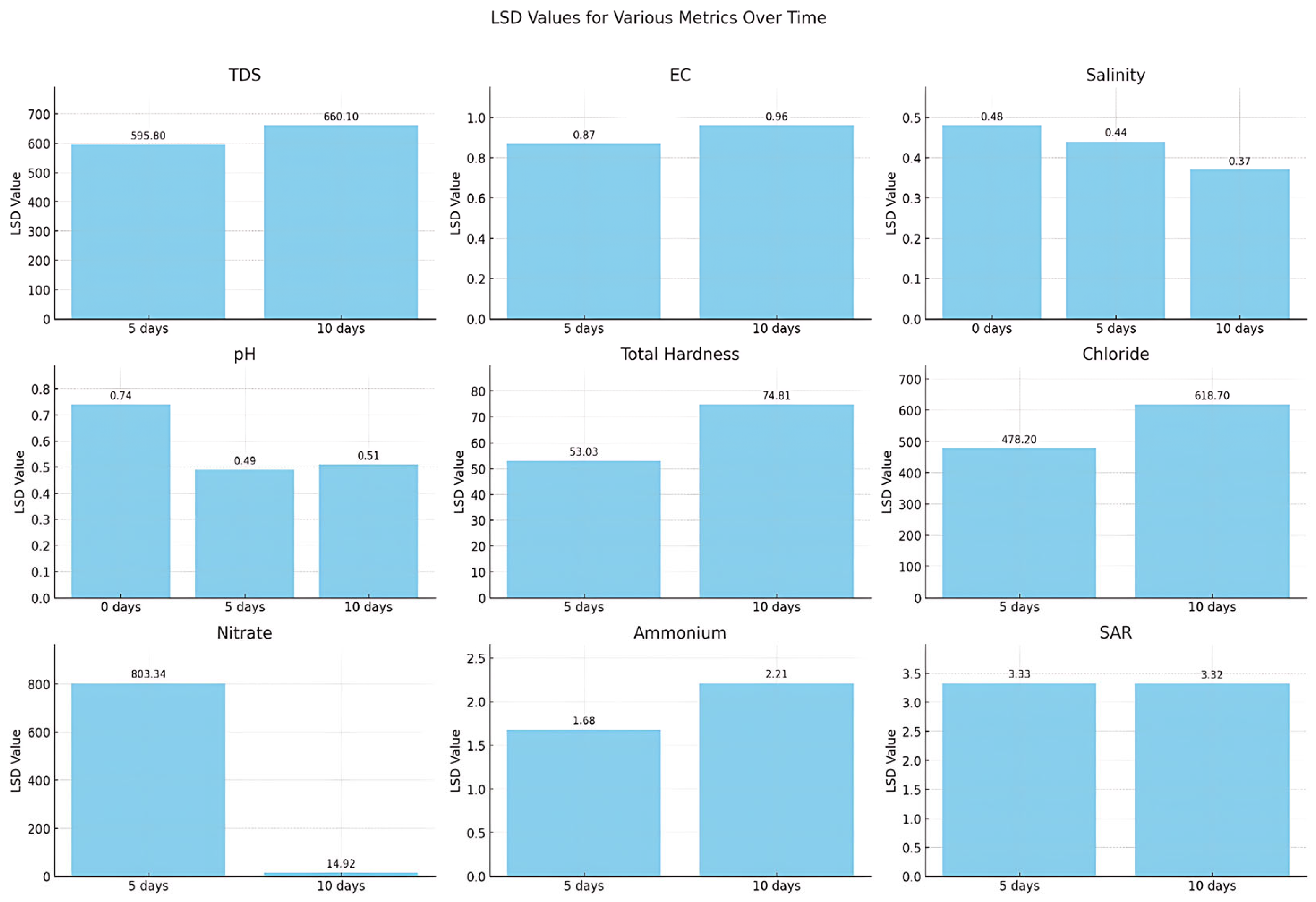
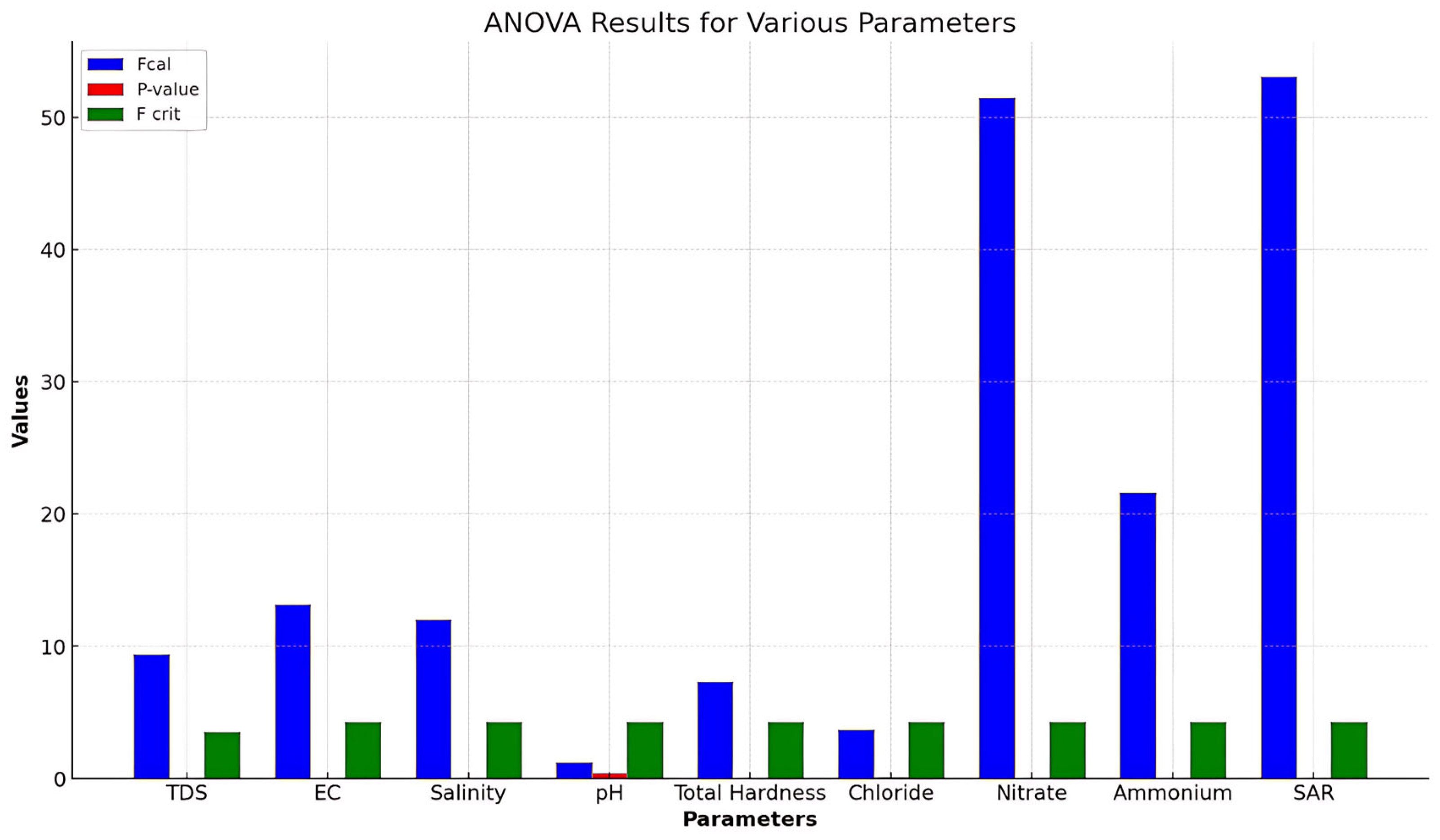
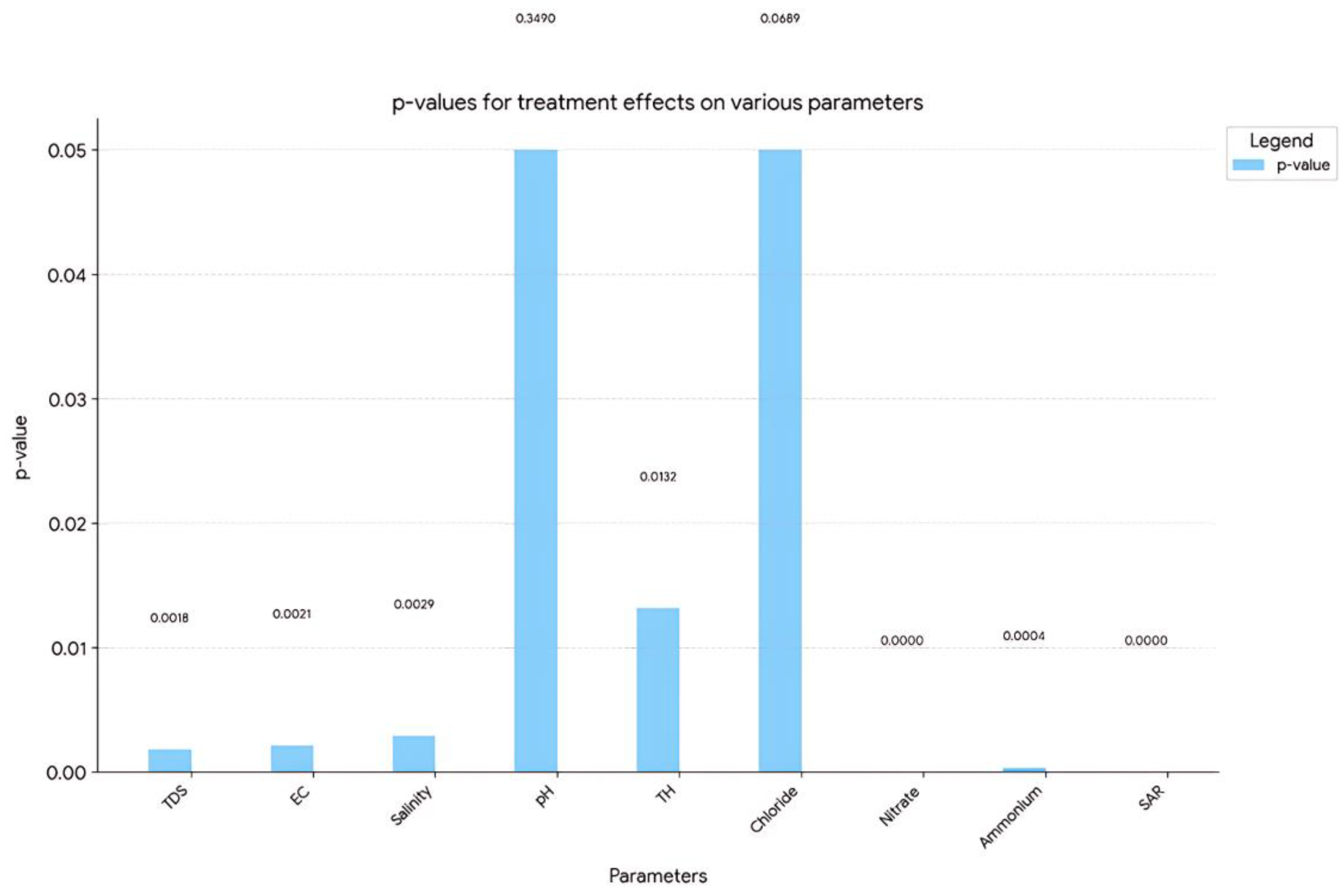
2.8. Statistical Analysis
3. Results
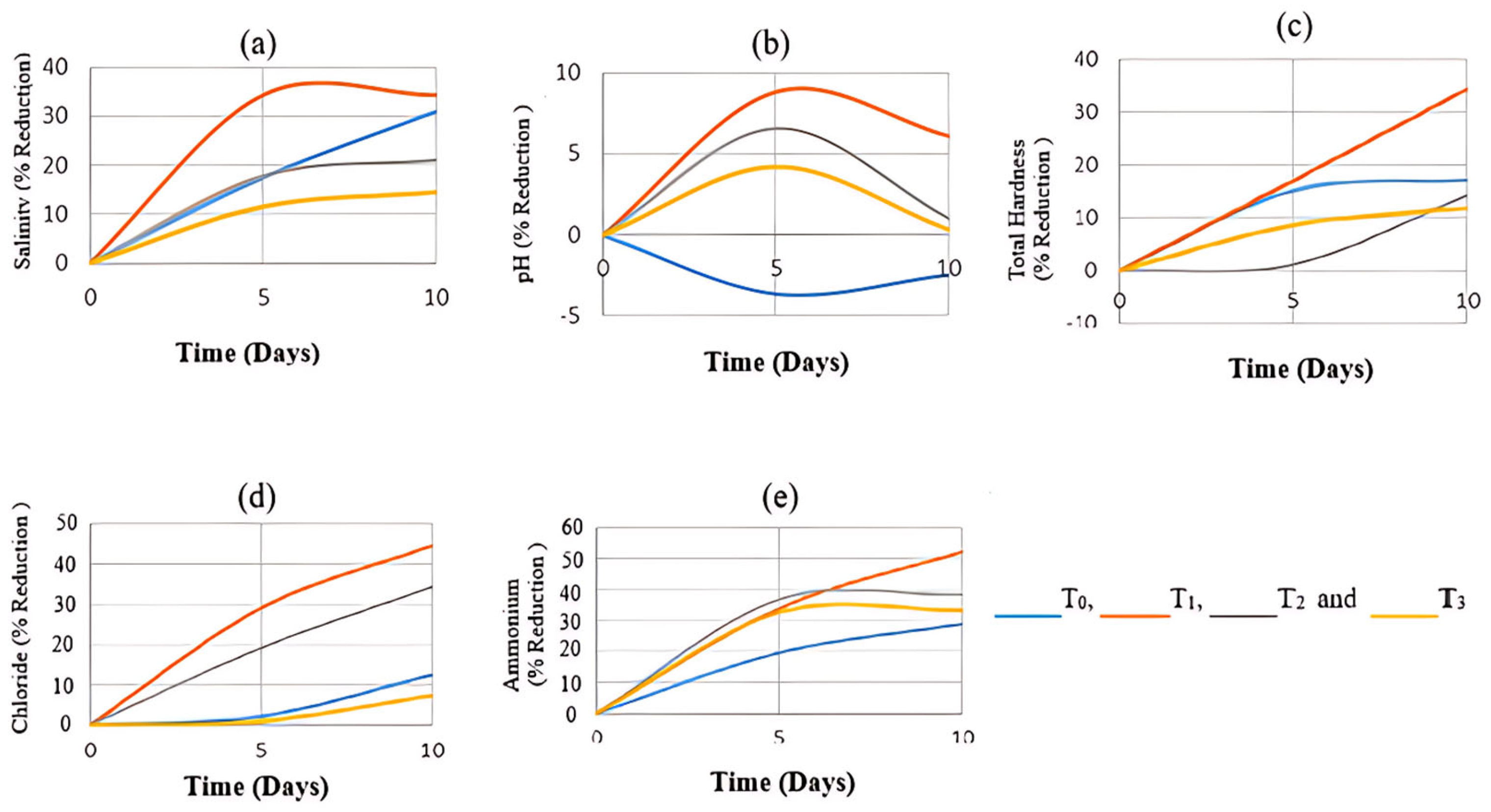
3.1. Effects on Chemical Parameters of Mixed Industrial Wastewater
3.2. Effects on Physical Parameters of Mixed Industrial Wastewater
3.3. Effects on Agricultural Parameters of Mixed Industrial Wastewater
3.4. Statistical Evaluation of Results
4. Discussion
4.1. Superior Pollutant Removal by Eichhornia crassipes
4.2. Nitrate Removal Efficiency
4.3. Comparison with Previous Literature
4.4. Challenges and Real-World Implementation Considerations
- Seasonal Variations: Plant growth rates and pollutant uptake efficiency may vary with seasonal changes in temperature, light availability, and nutrient levels. Cold seasons may reduce metabolic activity, impacting remediation efficiency [42].
- Retention Time Constraints: Industrial wastewater treatment requires large-scale implementation, where prolonged retention times may not be feasible. Optimization of hydraulic retention time (HRT) to balance treatment efficiency and operational feasibility is necessary [47]. Implementing a hybrid flow regime, integrating both horizontal and vertical flow constructed wetlands, can enhance contaminant removal by optimizing water distribution and retention dynamics [49,50]. Additionally, the strategic incorporation of specialized microbial consortia within these wetlands can significantly improve biodegradation processes, further augmenting treatment efficiency through biological mechanisms [51,52,53].
- Plant Disposal and Biomass Management: Accumulated pollutants in plant tissues pose disposal challenges. Safe disposal or valorization strategies, such as bioenergy production or composting, need to be considered [35]. Incineration of contaminated biomass presents an effective approach to prevent the release of toxic compounds into open water systems, thereby mitigating environmental risks [54]. This controlled thermal degradation not only ensures the safe removal of hazardous substances but can also facilitate energy recovery, aligning with sustainable waste management principles [55].
4.5. Economic Feasibility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wijayaweera, N.; Gunawardhana, L.; Bamunawala, J.; Sirisena, J.; Rajapakse, L.; Patabendige, C.S.; Karunaweera, H. Use of Machine Learning and Indexing Techniques for Identifying Industrial Pollutant Sources: A Case Study of the Lower Kelani River Basin, Sri Lanka. Water 2024, 16, 2766. [Google Scholar] [CrossRef]
- Fatima, B.; Muhammad, A.; Faizan, U.H. Policy Brief Sustainable Development Goal 6: Setting the Course Right; Pakistan Council of Research in Water Resources (PCRWR): Islamabad, Pakistan, 2023; p. 46.
- Slobodiuk, S.; Niven, C.; Arthur, G.; Thakur, S.; Ercumen, A. Does irrigation with treated and untreated wastewater increase antimicrobial resistance in soil and water: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 11046. [Google Scholar] [CrossRef]
- Akhtar, S.; Ahmad, S.; Huifang, W.; Shahbaz, A.; Ghafoor, A.; Imran, S.; Zafar, A. An analysis of wastewater irrigation practices and its impacts on the livelihood generation and food chain contamination in Faisalabad District, Pakistan. ISABB J. Health Environ. Sci. 2018, 5, 33–42. [Google Scholar]
- Nidheesh, P.V.; Ravindran, V.; Gopinath, A.; Kumar, M.S. Emerging technologies for mixed industrial wastewater treatment in developing countries: An overview. Environ. Qual. Manag. 2022, 31, 121–141. [Google Scholar] [CrossRef]
- Oladimeji, T.E.; Oyedemi, M.; Emetere, M.E.; Agboola, O.; Adeoye, J.B.; Odunlami, O.A. Review on the impact of heavy metals from industrial wastewater effluent and removal technologies. Heliyon 2024, 10, e40370. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Wang, Y.; Yu, C.; Hu, X. Biochar-bacteria coupling system enhanced the bioremediation of phenol wastewater-based on life cycle assessment and environmental safety analysis. J. Hazard. Mater. 2024, 480, 136414. [Google Scholar] [CrossRef] [PubMed]
- Zinicovscaia, I. Conventional Methods of Wastewater Treatment. In Cyanobacteria for Bioremediation of Wastewaters; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–25. [Google Scholar]
- Gupta, S.; Mittal, Y.; Panja, R.; Prajapati, K.B.; Yadav, A.K. Conventional wastewater treatment technologies. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 47–75. [Google Scholar]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, Q.; Kumar, A.; Zhang, W.; Yu, Z.; Hui, D.; Zhang, C.; Shan, S. Effect of degradable microplastics, biochar and their coexistence on soil organic matter decomposition: A critical review. TrAC Trends Anal. Chem. 2025, 183, 118082. [Google Scholar] [CrossRef]
- Zhuo, T.; He, L.; Chai, B.; Zhou, S.; Wan, Q.; Lei, X.; Zhou, Z.; Chen, B. Micro-pressure promotes endogenous phosphorus release in a deep reservoir by favouring microbial phosphate mineralisation and solubilisation coupled with sulphate reduction. Water Res. 2023, 245, 120647. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, A.N.; Waris, A.; Ilyas, M.; Zamel, D. Phytoremediation of pollutants from wastewater: A concise review. Open Life Sci. 2022, 17, 488–496. [Google Scholar] [CrossRef]
- Yuliasni, R.; Kurniawan, S.B.; Marlena, B.; Hidayat, M.R.; Kadier, A.; Ma, P.C.; Imron, M.F. Recent Progress of Phytoremediation-Based Technologies for Industrial Wastewater Treatment. J. Ecol. Eng. 2023, 24, 208–220. [Google Scholar]
- Saha, P.; Shinde, O.; Sarkar, S. Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytoremediation 2017, 19, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zeb, B.S.; Mahmood, Q.; Irshad, M.; Zafar, H.; Wang, R. Sustainable treatment of combined industrial wastewater: Synergistic phytoremediation with Eichhornia crassipes, Pistia stratiotes, and Arundo donax in biofilm wetlands. Int. J. Phytoremediation 2025, 27, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, D.; Thiruvengadam, M.; Shruthi, R.M.; Thaiyalnayagi, K.; Modhini, T.M. Influence of Eichhornia crassipes for removing Zn from electroplating industry wastewater. J. Chem. Pharm. Sci. 2015, 8, 775–779. Available online: https://www.jchps.com/ (accessed on 2 April 2015).
- Durairaj, S. Sorption capacity of Eichhornia crassipes (Mart.) Solms for zinc removal from electroplating industry wastewater. Environ. Sci. Pollut. Res. 2024, 31, 30849–30866. [Google Scholar]
- Ali, M.; Aslam, A.; Qadeer, A.; Javied, S.; Nisar, N.; Hassan, N.; Hussain, A.; Ali, B.; Iqbal, R.; Chaudhary, T.; et al. Domestic wastewater treatment by Pistia stratiotes in constructed wetland. Sci. Rep. 2024, 14, 7553. [Google Scholar]
- Iqbal, J.; Javed, A.; Baig, M.A. Growth and nutrient removal efficiency of duckweed (Lemna minor) from synthetic and dumpsite leachate under artificial and natural conditions. PLoS ONE 2019, 14, e0221755. [Google Scholar]
- Sahi, W.; Megateli, S. Evaluation of Lemna minor phytoremediation performance for the treatment of dairy wastewater. Water Pract. Technol. 2023, 18, 1138–1147. [Google Scholar] [CrossRef]
- Farid, M.; Irshad, M.; Fawad, M.; Ali, Z.; Eneji, A.E.; Aurangzeb, N.; Mohammad, A.; Ali, B. Effect of Cyclic Phytoremediation with Different Wetland Plants on Municipal Wastewater. Int. J. Phytoremediation 2014, 16, 572–581. [Google Scholar]
- Dhoke, S.K. Determination of total hardness of water sample by titration using double burette method: An economical approach. Chem. Teach. Int. 2024. [Google Scholar] [CrossRef]
- Timalsina, R.; Acharya, S.; Đurin, B.; Awasthi, M.P.; Pant, R.R.; Joshi, G.R.; Byanju, R.M.; Panthi, K.P.; Joshi, S.; Kumar, A.; et al. An Assessment of Seasonal Water Quality in Phewa Lake, Nepal, by Integrating Geochemical Indices and Statistical Techniques: A Sustainable Approach. Water 2025, 17, 238. [Google Scholar] [CrossRef]
- Corwin, D.L.; Yemoto, K. Salinity: Electrical conductivity and total dissolved solids. Soil Sci. Soc. Am. J. 2020, 84, 1442–1461. [Google Scholar] [CrossRef]
- Alagha, O.; Abuhajar, O. Numerical Modeling of Beach Well Intake as Pre-Treatment for a Desalination Plant. Water 2020, 12, 2420. [Google Scholar] [CrossRef]
- Liu, M.; Tian, X.; Yan, Z.; Zhao, Y.; Li, S. Diurnal Variation of Carbon Dioxide Concentration and Flux in a River–Lake Continuum of a Mega City. Water 2025, 17, 306. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, J.; Lei, Y.; Qin, D.; Cai, W.; Chen, X.; Ma, C.; Zhu, X.; Zhang, S.; Sun, Q. Impacts of Reclaimed Water Irrigation on Soil Salinity, Nutrient Cycling, and Landscape Plant Growth in a Coastal Monsoon Environment. Water 2025, 17, 337. [Google Scholar] [CrossRef]
- Sheldon, A.R.; Dalal, R.C.; Kirchhof, G.; Kopittke, P.M.; Menzies, N.W. The effect of salinity on plant-available water. Plant Soil 2017, 418, 477–491. [Google Scholar] [CrossRef]
- Han, T.; Zhang, M.; Feng, W.; Li, T.; Liu, X.; Wang, J. Effects of Aeration Intensity on Water Quality, Nutrient Cycling, and Microbial Community Structure in the Biofloc System of Pacific White Shrimp Litopenaeus vannamei Culture. Water 2024, 17, 41. [Google Scholar] [CrossRef]
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater Treatment and Reuse: A Review of its Applications and Health Implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Apha, A.W. Standard Methods For the Examination of Water and Wastewater, 22nd ed.; American Public Heath Association: Washington, DC, USA, 2012; Volume 5. [Google Scholar]
- Gusti Wibowo, Y.; Tyaz Nugraha, A.; Rohman, A. Phytoremediation of several wastewater sources using Pistia stratiotes and Eichhornia crassipes in Indonesia. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100781. [Google Scholar] [CrossRef]
- Akinbile, C.O.; Yusoff, M.S. Assessing Water Hyacinth (Eichhornia crassopes) and Lettuce (Pistia stratiotes) Effectiveness in Aquaculture Wastewater Treatment. Int. J. Phytoremediation 2012, 14, 201–211. [Google Scholar] [CrossRef]
- Mohd Nizam, N.U.; Mohd Hanafiah, M.; Mohd Noor, I.; Abd Karim, H.I. Efficiency of Five Selected Aquatic Plants in Phytoremediation of Aquaculture Wastewater. Appl. Sci. 2020, 10, 2712. [Google Scholar] [CrossRef]
- Abou-Elela, S.I.; Elekhnawy, M.A.; Khalil, M.T.; Hellal, M.S. Factors affecting the performance of horizontal flow constructed treatment wetland vegetated with Cyperus papyrus for municipal wastewater treatment. Int. J. Phytoremediation 2017, 19, 1023–1028. [Google Scholar] [PubMed]
- Mohebi, Z.; Nazari, M. Phytoremediation of wastewater using aquatic plants, A review. J. Appl. Res. Water Wastewater 2022, 8, 50–58. [Google Scholar]
- Shi, M.; Li, J.; Zhang, W.; Zhou, Q.; Niu, Y.; Zhang, Z.; Gao, Y.; Yan, S. Contrasting impact of elevated atmospheric CO2 on nitrogen cycle in eutrophic water with or without Eichhornia crassipes (Mart.) Solms. Sci. Total Environ. 2019, 666, 285–297. [Google Scholar] [PubMed]
- Ting, W.H.T.; Tan, I.A.W.; Salleh, S.F.; Wahab, N.A. Application of water hyacinth (Eichhornia crassipes) for phytoremediation of ammoniacal nitrogen: A review. J. Water Process Eng. 2018, 22, 239–249. [Google Scholar]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar]
- Churko, E.E.; Nhamo, L.; Chitakira, M. Phytoremediation Capacity of Water Hyacinth (Eichhornia crassipes) as a Nature-Based Solution for Contaminants and Physicochemical Characterization of Lake Water. Water 2023, 15, 2540. [Google Scholar] [CrossRef]
- Hernández-Vásquez, L.A.; Romo-Gómez, C.; Alvarado-Lassman, A.; Prieto-García, F.; Camacho-López, C.; Acevedo-Sandoval, O.A. Artificial Floating Islands for the Removal of Nutrients and Improvement of the Quality of Urban Wastewater. Water 2024, 16, 1443. [Google Scholar] [CrossRef]
- Vymazal, J. Plants used in constructed wetlands with horizontal subsurface flow: A review. Hydrobiologia 2011, 674, 133–156. [Google Scholar]
- Bhutiani, R.; Rai, N.; Kumar, N.; Rausa, M.; Ahamad, F. Treatment of industrial waste water using Water hyacinth (Eichornia crassipus) and Duckweed (Lemna minor): A Comparative study. Environ. Conserv. J. 2019, 20, 15–25. [Google Scholar]
- Adelodun, A.A.; Olajire, T.; Afolabi, N.O.; Akinwumiju, A.S.; Akinbobola, E.; Hassan, U.O. Phytoremediation potentials of Eichhornia crassipes for nutrients and organic pollutants from textile wastewater. Int. J. Phytoremediation 2021, 23, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cui, H.; Huang, M.; He, Y. Artificial floating islands for water quality improvement. Environ. Rev. 2017, 25, 350–357. [Google Scholar] [CrossRef]
- Shehzadi, M.; Fatima, K.; Imran, A.; Mirza, M.S.; Khan, Q.M.; Afzal, M. Ecology of bacterial endophytes associated with wetland plants growing in textile effluent for pollutant-degradation and plant growth-promotion potentials. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2016, 150, 1261–1270. [Google Scholar] [CrossRef]
- Patel, D.K.; Kanungo, V.K. Phytoremediation Potential of Duckweed (Lemna minor L: A Tiny Aquatic Plant) in the Removal of Pollutants from Domestic Wastewater with Special Reference to Nutrients. Bioscan 2010, 5, 355–358. [Google Scholar]
- Martel-Rodríguez, G.; Millán-Gabet, V.; Mendieta-Pino, C.; García-Romero, E.; Sánchez-Ramírez, J. Long-Term Performance of a Hybrid-Flow Constructed Wetlands System for Urban Wastewater Treatment in Caldera de Tirajana (Santa Lucía, Gran Canaria, Spain). Int. J. Environ. Res. Public Health 2022, 19, 14871. [Google Scholar] [CrossRef]
- Marzec, M.; Listosz, A.; Malik, A.; Kulik, M.; Jóźwiakowski, K. Organic Pollutants Removal in a Hybrid Constructed Wetland Wastewater Treatment Plant with an Aeration System. Water 2024, 16, 947. [Google Scholar] [CrossRef]
- Cao, Z.; Yan, W.; Ding, M.; Yuan, Y. Construction of microbial consortia for microbial degradation of complex compounds. Front. Bioeng. Biotechnol. 2022, 10, 1051233. [Google Scholar] [CrossRef]
- Aziz, T.; Rasheed, S.; Shah, A.H.; Nasir, H.; Fariq, A.; Jamil, A.; Jannat, S. Bioremediation Potential of Plant-Bacterial Consortia for Chlorpyrifos Removal Using Constructed Wetland. Front. Environ. Sci. 2022, 10, 880807. [Google Scholar] [CrossRef]
- Kannan, P.; Verma, I.; Banerjee, B.; Saleena, L.M. Unveiling bacterial consortium for xenobiotic biodegradation from Pichavaram mangrove forest soil: A metagenomic approach. Arch. Microbiol. 2024, 206, 27. [Google Scholar] [CrossRef]
- Tangri, N. Waste incinerators undermine clean energy goals. PLoS Clim. 2023, 2, e0000100. [Google Scholar] [CrossRef]
- Luo, Y.; Ye, M.; Zhou, Y.; Su, R.; Huang, S.; Wang, H.; Dai, X. Assessing the Environmental Impact of Municipal Waste on Energy Incineration Technology for Power Generation Using Life Cycle Assessment Methodology. Toxics 2024, 12, 786. [Google Scholar] [CrossRef] [PubMed]
- Flafel, H.M.; Rafatullah, M.; Lalung, J.; Kapoor, R.T.; Siddiqui, M.R.; Qutob, M. Enhancing the efficiency of phytoremediation using water hyacinth (Eichhornia crassipes) for 2,4-dichlorophenoxyacetic acid removal with modified biochar as an assisted agent. Chemosphere 2024, 367, 143591. [Google Scholar] [PubMed]








| S/No | Parameter | Unit | PEQS * | FAO ** |
|---|---|---|---|---|
| 1 | pH | - | 6–9 | 6.5–8.4 |
| 2 | TDS | mg/L | <3500 | 0–2000 |
| 3 | EC | - | 0–3 mS/cm | |
| 4 | Salinity | - | - | - |
| 5 | Temperature | °C | <30 | - |
| 6 | Chloride | mg/L | <1000 | <1065 |
| 7 | Nitrate | mg/L | 5–30 | 10 |
| 8 | Ammonium | mg/L | 40 | 0–5 |
| 9 | Calcium | mg/L | - | 0–400 |
| 10 | Sodium | mg/L | - | 0–920 |
| 11 | Magnesium | mg/L | - | 0–60.75 |
| 12 | Total Hardness | mg/L | - | - |
| 13 | SAR | Ratio | - | 0–15 |
| Treatment | Retention Time (Days) | TDS (mg/L) | EC mS/cm | Salinity | pH | Total Hardness (mg/L) | Chloride (mg/L) | Nitrate (mg/L) | Ammonium (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| T0 | 0 | 4071 | 6.07 | 3.04 | 8.5 | 460 | 2188 | 2091 | 13.8 |
| 5 | 3313 | 4.94 | 2.51 | 8.81 | 390 | 2141 | 1408 | 11.09 | |
| 10 | 2732 | 4.07 | 2.1 | 8.71 | 381 | 1913 | 26.05 | 9.8 | |
| T1 | 0 | 4071 | 6.07 | 3.04 | 8.5 | 460 | 2188 | 2091 | 13.8 |
| 5 | 2675 | 3.99 | 2 | 7.75 | 382 | 1552 | 1374 | 9.1 | |
| 10 | 2600 | 3.88 | 2 | 7.98 | 302 | 1214 | 5 | 6.6 | |
| T2 | 0 | 4071 | 6.07 | 3.04 | 8.5 | 460 | 2188 | 2091 | 13.8 |
| 5 | 3385 | 5 | 2.5 | 7.94 | 455 | 1769 | 327 | 8.7 | |
| 10 | 3280 | 4.8 | 2.4 | 8.41 | 395 | 1437 | 12 | 8.5 | |
| T3 | 0 | 4071 | 6.07 | 3.04 | 8.5 | 460 | 2188 | 2091 | 13.8 |
| 5 | 3508 | 5.22 | 2.69 | 8.14 | 420 | 1907 | 1081 | 9.3 | |
| 10 | 3449 | 5.14 | 2.6 | 8.47 | 405 | 1648 | 20.9 | 9.2 |
| Pollutant | Eichhornia crassipes (This Study) | Eichhornia crassipes (Previous Studies) | Pistia stratiotes | Lemna minor |
|---|---|---|---|---|
| TDS (%) | 83.4 | 80–85 [44] | 75.6 | |
| EC (%) | Significant reduction | Reduction with increased HRT [47] | Moderate reduction | Low reduction |
| Chloride (%) | 933.1 | 53.3 [45] | Low absorption [48] | Minimal impact |
| Nitrate (%) | 99 | 90–95 [14,41] | Moderate removal | Moderate removal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.N.; Anjum, L.; Arshad, A.; Ali, S.; Aleem, M.; Nasir, A. Nature-Based Solution for Wastewater Treatment and Reuse Using Phytoremediation with Floating Plants. Water 2025, 17, 1080. https://doi.org/10.3390/w17071080
Khan SN, Anjum L, Arshad A, Ali S, Aleem M, Nasir A. Nature-Based Solution for Wastewater Treatment and Reuse Using Phytoremediation with Floating Plants. Water. 2025; 17(7):1080. https://doi.org/10.3390/w17071080
Chicago/Turabian StyleKhan, Shahbaz Nasir, Lubna Anjum, Arfan Arshad, Saqib Ali, Mannan Aleem, and Abdul Nasir. 2025. "Nature-Based Solution for Wastewater Treatment and Reuse Using Phytoremediation with Floating Plants" Water 17, no. 7: 1080. https://doi.org/10.3390/w17071080
APA StyleKhan, S. N., Anjum, L., Arshad, A., Ali, S., Aleem, M., & Nasir, A. (2025). Nature-Based Solution for Wastewater Treatment and Reuse Using Phytoremediation with Floating Plants. Water, 17(7), 1080. https://doi.org/10.3390/w17071080








