Environmental DNA Reveals Ecologically Relevant Temporal and Spatial Variation of Fish Community in Silver Carp- and Bighead Carp-Dominant Drinking Water Reservoirs
Abstract
:1. Introduction
2. Materials and Methods
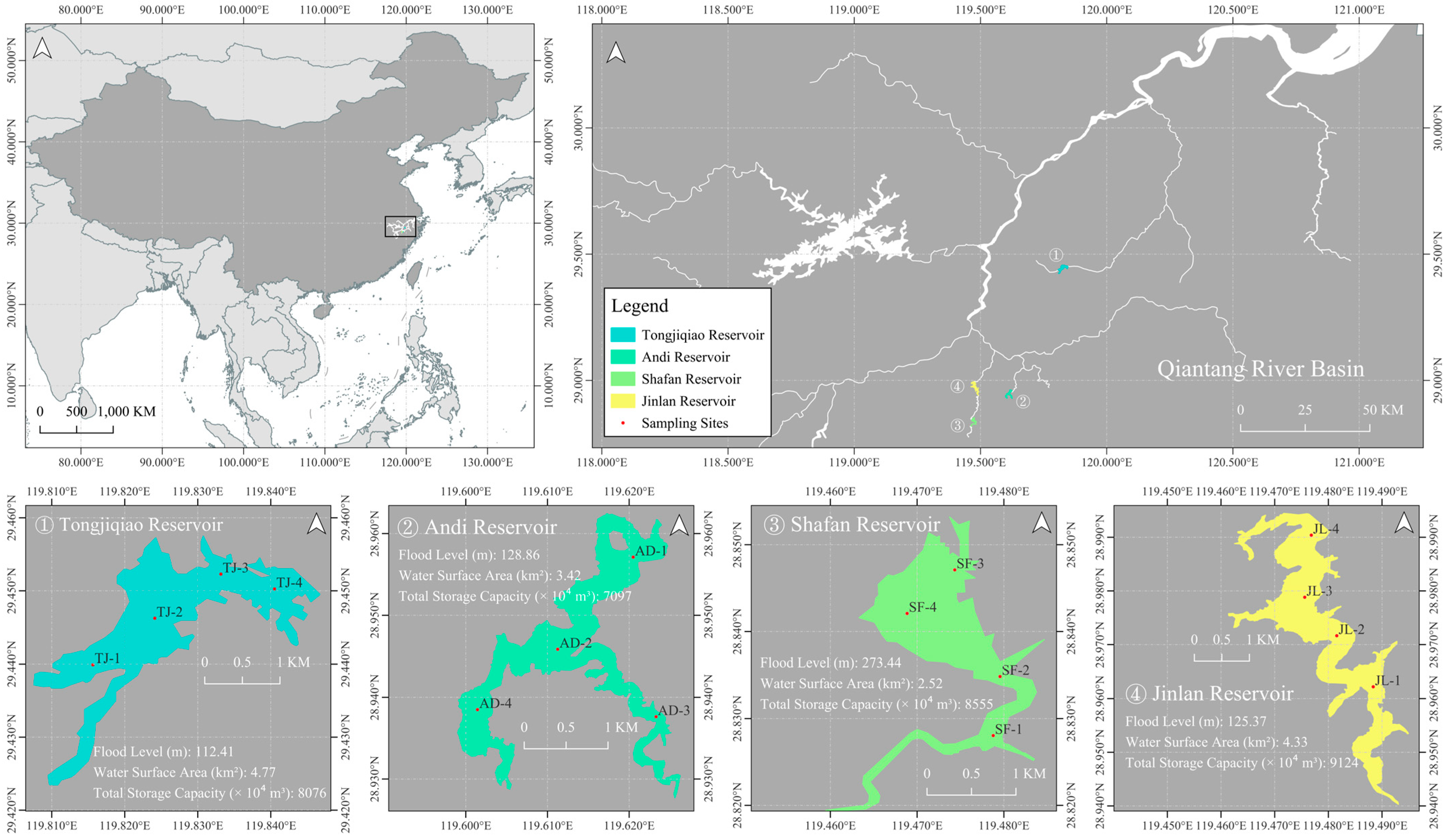
2.1. Study Area
2.2. Sample Collection and Water Quality/eDNA Analysis
2.3. Data Processing and Statistical Analysis
3. Results
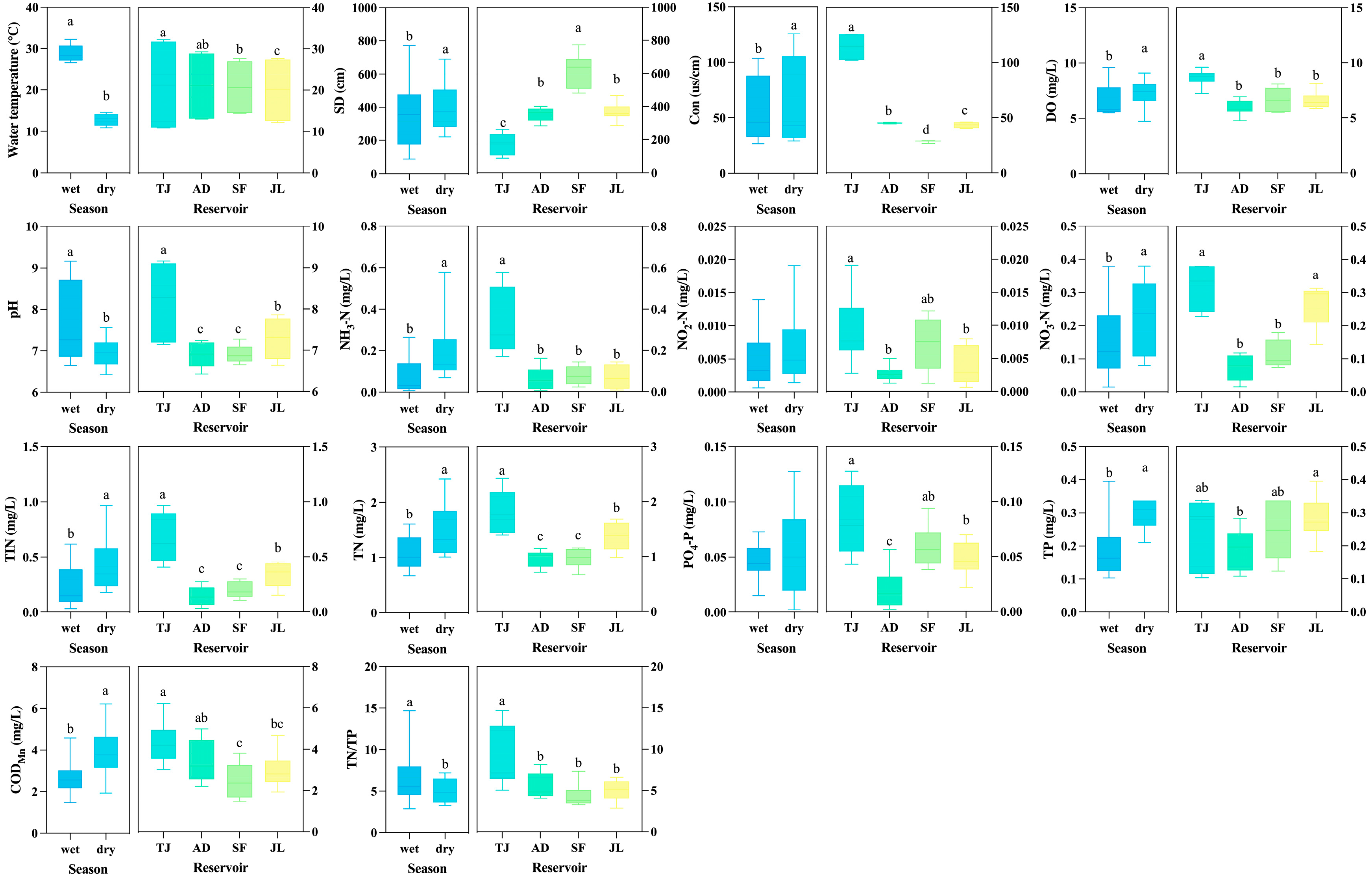
3.1. Water Physicochemical Parameters
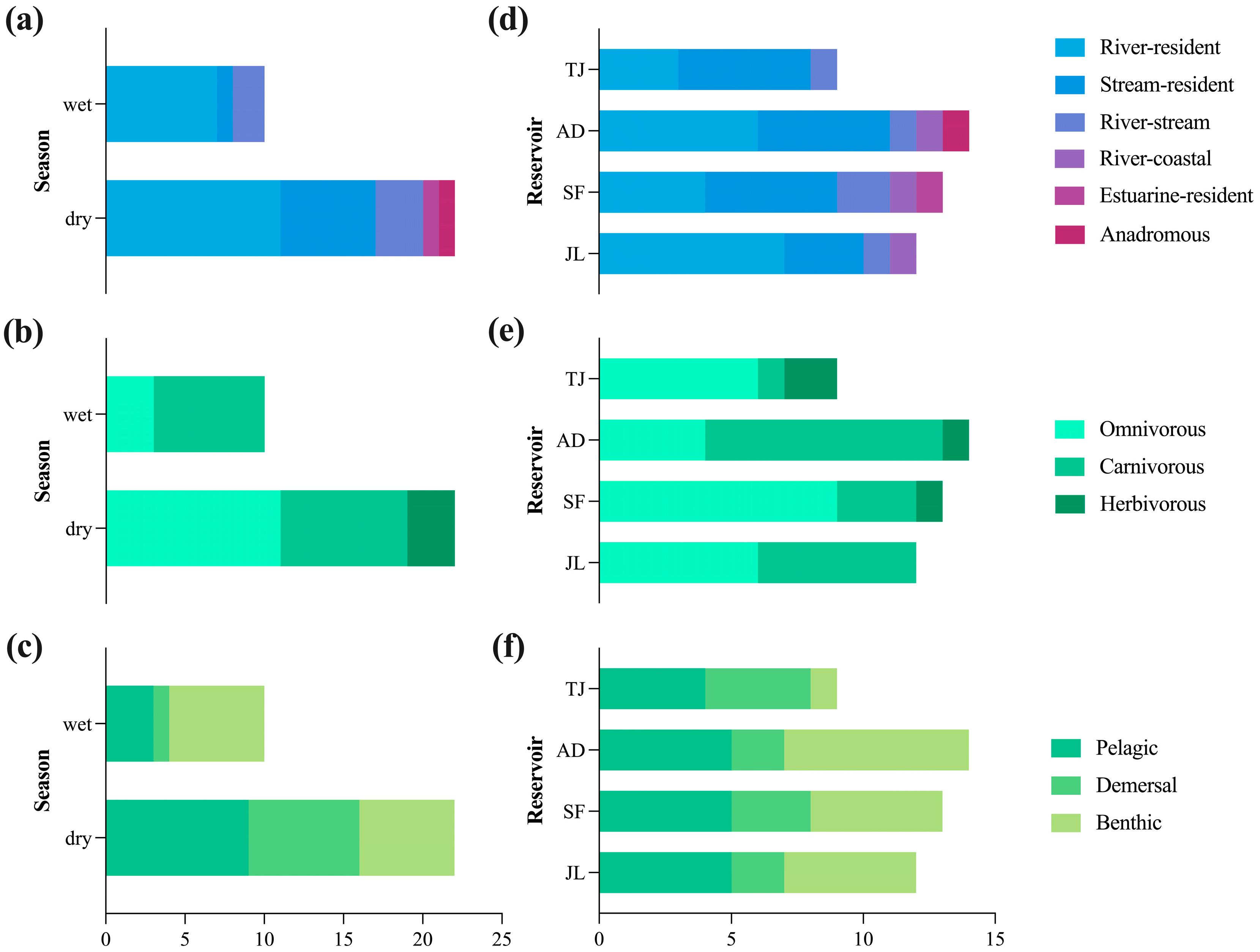
3.2. Fish Species Composition and Ecological Characteristics
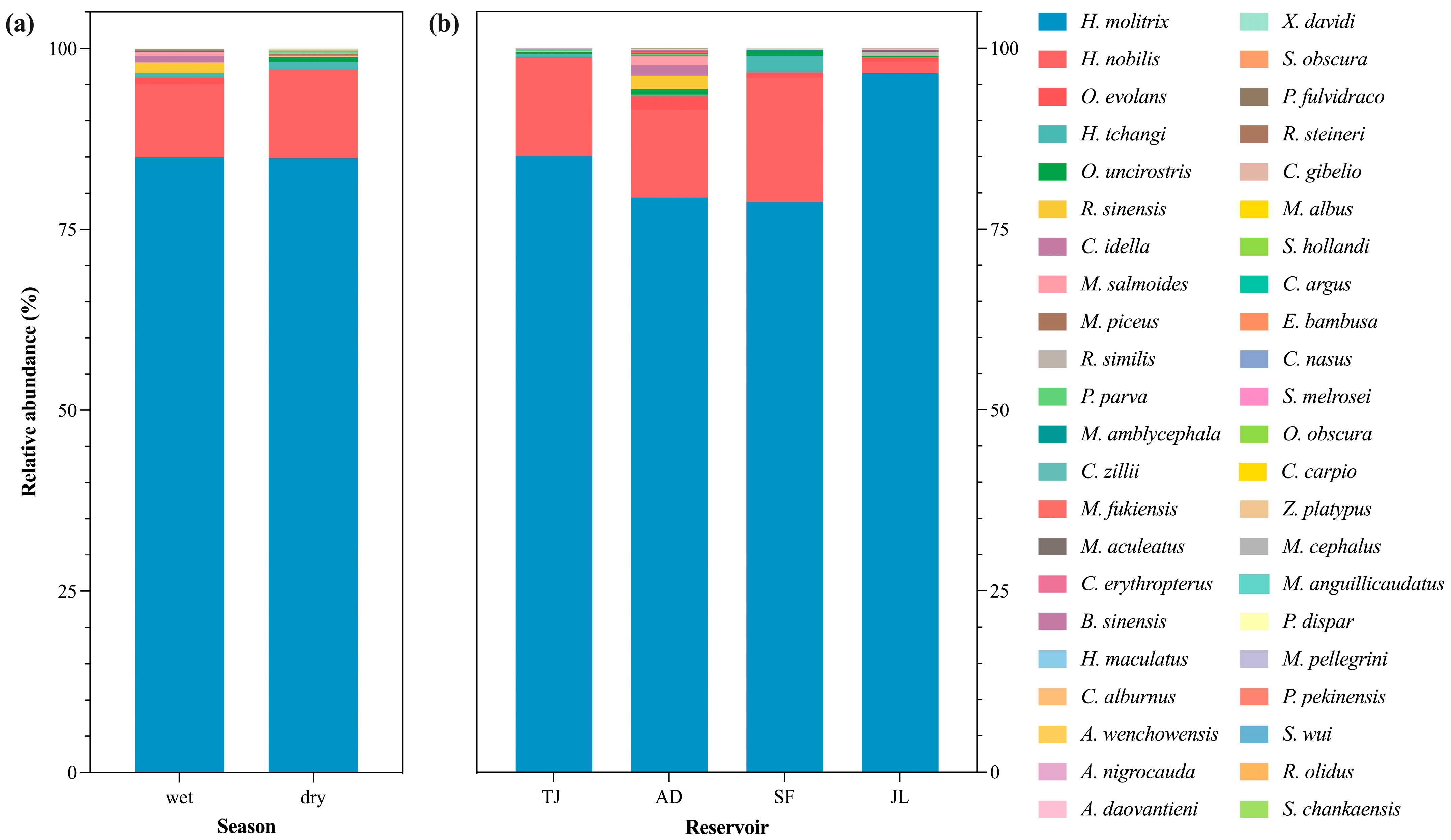
3.3. Relative Abundance of Dominant Fish Species
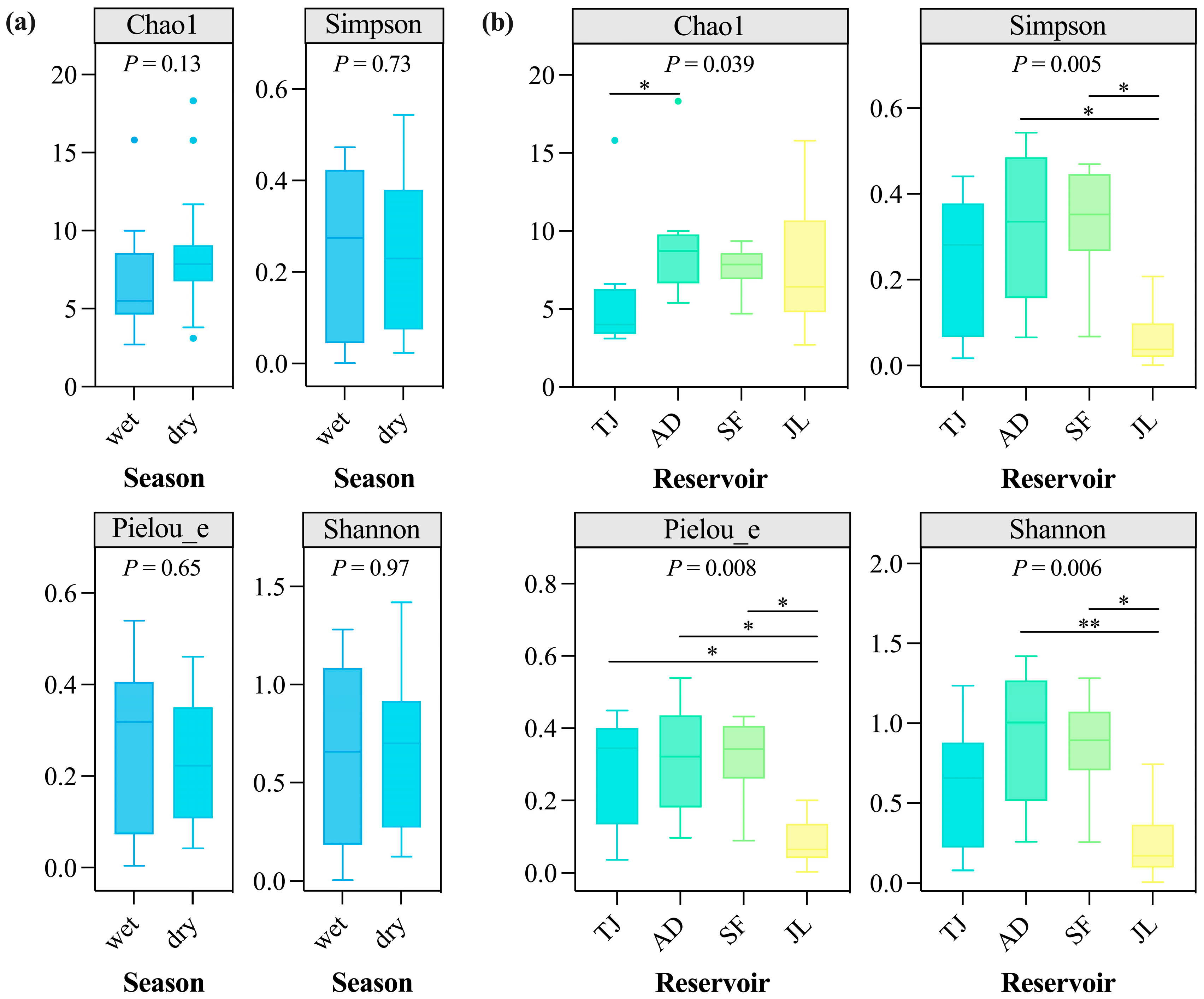
3.4. Seasonal and Spatial Variations in Fish Diversity Indices
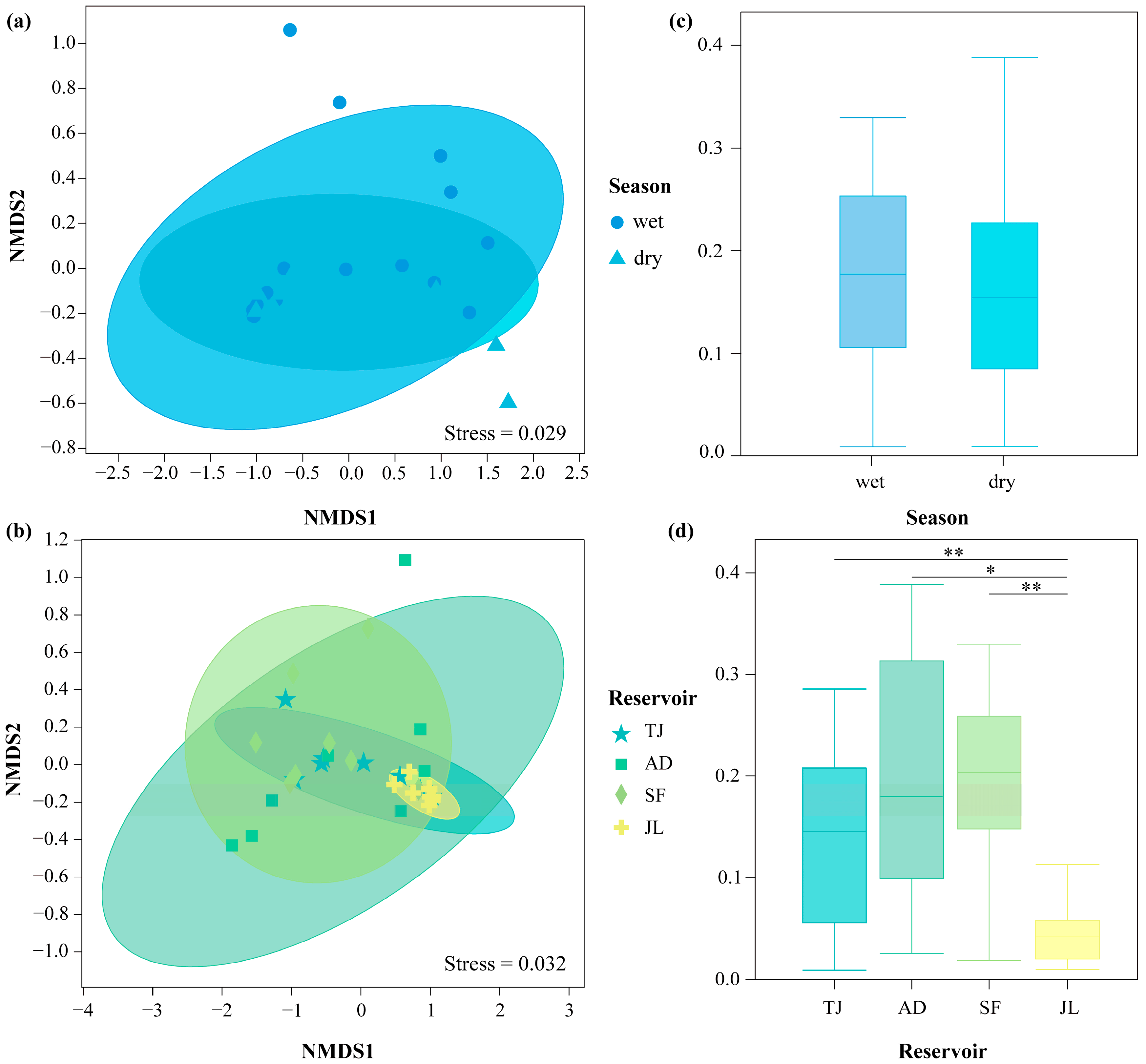
3.5. NMDS and ANOSIM Analysis of Fish Community Composition
3.6. Environmental Drivers of Fish Community Diversity and Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Mézo, P.; Guiet, J.; Scherrer, K.; Bianchi, D.; Galbraith, E. Global Nutrient Cycling by Commercially Targeted Marine Fish. Biogeosciences 2022, 19, 2537–2555. [Google Scholar] [CrossRef]
- Zhou, S.; Fan, C.; Xia, H.; Zhang, J.; Yang, W.; Ji, D.; Wang, L.; Chen, L.; Liu, N. Combined Use of eDNA Metabarcoding and Bottom Trawling for the Assessment of Fish Biodiversity in the Zhoushan Sea. Front. Mar. Sci. 2022, 8, 809703. [Google Scholar] [CrossRef]
- Pownkumar, V.; Ananthan, P.S.; Ekka, A.; Qureshi, N.W.; T, V. Fisheries as Ecosystem Services: A Case Study of the Cauvery River Basin, India. Front. Environ. Sci. 2022, 10, 892012. [Google Scholar] [CrossRef]
- Port, J.A.; O’Donnell, J.L.; Romero-Maraccini, O.C.; Leary, P.R.; Litvin, S.Y.; Nickols, K.J.; Yamahara, K.M.; Kelly, R.P. Assessing Vertebrate Biodiversity in a Kelp Forest Ecosystem Using Environmental DNA. Mol. Ecol. 2016, 25, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhang, S.; Xu, S.; Xiong, P.; Cao, Y.; Chen, Z.; Li, M. Comparison of Environmental DNA Metabarcoding and Bottom Trawling for Detecting Seasonal Fish Communities and Habitat Preference in a Highly Disturbed Estuary. Ecol. Indic. 2023, 146, 109754. [Google Scholar] [CrossRef]
- Holubová, M.; Čech, M.; Vašek, M.; Peterka, J. On the Use of a Visual Census in Surveying Fish Communities in Lentic Water Bodies. Ecol. Indic. 2019, 105, 1–5. [Google Scholar] [CrossRef]
- Egerton, J.P.; Johnson, A.F.; Turner, J.; LeVay, L.; Mascareñas-Osorio, I.; Aburto-Oropeza, O. Hydroacoustics as a Tool to Examine the Effects of Marine Protected Areas and Habitat Type on Marine Fish Communities. Sci. Rep. 2018, 8, 47. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Miaud, C.; Pompanon, F.; Taberlet, P. Species Detection Using Environmental DNA from Water Samples. Biol. Lett. 2008, 4, 423–425. [Google Scholar] [CrossRef]
- Gibson, T.I.; Baillie, C.; Collins, R.A.; Wangensteen, O.S.; Corrigan, L.; Ellison, A.; Heddell-Cowie, M.; Westoby, H.; Byatt, B.; Lawson-Handley, L.; et al. Environmental DNA Reveals Ecologically Relevant Spatial and Temporal Variation in Fish Assemblages between Estuaries and Seasons. Ecol. Indic. 2024, 165, 112215. [Google Scholar] [CrossRef]
- Bista, I.; Carvalho, G.R.; Walsh, K.; Seymour, M.; Hajibabaei, M.; Lallias, D.; Christmas, M.; Creer, S. Annual Time-Series Analysis of Aqueous eDNA Reveals Ecologically Relevant Dynamics of Lake Ecosystem Biodiversity. Nat. Commun. 2017, 8, 14087. [Google Scholar] [CrossRef]
- Bessey, C.; Gao, Y.; Truong, Y.B.; Miller, H.; Jarman, S.N.; Berry, O. Comparison of Materials for Rapid Passive Collection of Environmental DNA. Mol. Ecol. Resour. 2022, 22, 2559–2572. [Google Scholar] [CrossRef] [PubMed]
- Roblet, S.; Priouzeau, F.; Gambini, G.; Cottalorda, J.-M.; Gastaldi, J.M.; Pey, A.; Raybaud, V.; Suarez, G.R.; Serre, C.; Sabourault, C.; et al. From Sight to Sequence: Underwater Visual Census vs. Environmental DNA Metabarcoding for the Monitoring of Taxonomic and Functional Fish Diversity. Sci. Total Environ. 2024, 956, 177250. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, K. Metabarcoding Is (Usually) More Cost Effective than Seining or qPCR for Detecting Tidewater Gobies and Other Estuarine Fishes. PeerJ 2024, 12, e16847. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, X.; Shi, C.; Peng, K.; Wang, Z.; Jiang, Z. Fish Community Monitoring in Floodplain Lakes: eDNA Metabarcoding and Traditional Sampling Revealed Inconsistent Fish Community Composition. Ecol. Indic. 2024, 166, 112467. [Google Scholar] [CrossRef]
- Czeglédi, I.; Sály, P.; Specziár, A.; Preiszner, B.; Szalóky, Z.; Maroda, Á.; Pont, D.; Meulenbroek, P.; Valentini, A.; Erős, T. Congruency between Two Traditional and eDNA-Based Sampling Methods in Characterising Taxonomic and Trait-Based Structure of Fish Communities and Community-Environment Relationships in Lentic Environment. Ecol. Indic. 2021, 129, 107952. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Liu, X.; Huang, T.; Ma, B.; Li, N.; Yang, W.; Li, H.; Zhao, K. Novel Insights in Seasonal Dynamics and Co-Existence Patterns of Phytoplankton and Micro-Eukaryotes in Drinking Water Reservoir, Northwest China: DNA Data and Ecological Model. Sci. Total Environ. 2023, 857, 159160. [Google Scholar] [CrossRef]
- Zhang, C.; Zhong, R.; Wang, Z.; Montaña, C.G.; Song, Y.; Pan, K.; Wang, S.; Wu, Y. Intra-Annual Variation of Zooplankton Community Structure and Dynamics in Response to the Changing Strength of Bio-Manipulation with Two Planktivorous Fishes. Ecol. Indic. 2019, 101, 670–678. [Google Scholar] [CrossRef]
- Pergl, J.; Sádlo, J.; Petrusek, A.; Laštůvka, Z.; Musil, J.; Perglová, I.; Šanda, R.; Šefrová, H.; Šíma, J.; Vohralík, V.; et al. Black, Grey and Watch Lists of Alien Species in the Czech Republic Based on Environmental Impacts and Management Strategy. NeoBiota 2016, 28, 1–37. [Google Scholar] [CrossRef]
- Eichmiller, J.J.; Hamilton, M.J.; Staley, C.; Sadowsky, M.J.; Sorensen, P.W. Environment Shapes the Fecal Microbiome of Invasive Carp Species. Microbiome 2016, 4, 44. [Google Scholar] [CrossRef]
- Slater, M.; James, P. Low Trophic Species in Aquaculture-Growth and Research Challenges. J. World Aquac. Soc. 2023, 54, 4–6. [Google Scholar] [CrossRef]
- Ma, C.; Xu, X.; Yang, J.; Cheng, L. Safety Monitoring and Management of Reservoir and Dams. Water 2023, 15, 1078. [Google Scholar] [CrossRef]
- Dai, Y.; Xie, N.; Dai, Y.; Guo, W.; Tang, J.; Wang, Y. Length-Weight and Length-Length Relations of 14 Freshwater Fish Species from the Qiantang River, China. J. Appl. Ichthyol. 2024, 2024, 4101501. [Google Scholar] [CrossRef]
- GB3838-2002; Environmental Quality Standard for Surface Water. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2002.
- Tang, J.-Y.; Dai, Y.-X.; Wang, Y.; Qin, J.G.; Li, Y.-M. Improvement of Fish and Pearl Yields and Nutrient Utilization Efficiency through Fish–Mussel Integration and Feed Supplementation. Aquaculture 2015, 448, 321–326. [Google Scholar] [CrossRef]
- Siegenthaler, A.; Wangensteen, O.S.; Soto, A.Z.; Benvenuto, C.; Corrigan, L.; Mariani, S. Metabarcoding of Shrimp Stomach Content: Harnessing a Natural Sampler for Fish Biodiversity Monitoring. Mol. Ecol. Resour. 2019, 19, 206–220. [Google Scholar] [CrossRef]
- Ni, Y.; Zhu, C. Fishes of the Taihu Lake; Shanghai Scientific & Technical Publishers: Shanghai, China, 2005. [Google Scholar]
- Chen, M.K.; Tong, H.Y.; Yu, T.J.; Diao, Z.S. The Fish Resources of Qiantang Rive]; Shanghai Scientific and Technological Literature Publishing House: Shanghai, China, 1990; ISBN 7-80513-458-8. [Google Scholar]
- Mao, J.R.; Xu, S.S.; Zheng, G.S.; Zheng, M.L. Fauna of Zhejiang: Freshwater Fishes; Zhejiang Science and Technology Press: Hangzhou, China, 1991. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. Version (10/2023). Available online: www.fishbase.org (accessed on 20 January 2025).
- De Santana, C.D.; Parenti, L.R.; Dillman, C.B.; Coddington, J.A.; Bastos, D.A.; Baldwin, C.C.; Zuanon, J.; Torrente-Vilara, G.; Covain, R.; Menezes, N.A.; et al. The Critical Role of Natural History Museums in Advancing eDNA for Biodiversity Studies: A Case Study with Amazonian Fishes. Sci. Rep. 2021, 11, 18159. [Google Scholar] [CrossRef] [PubMed]
- Lamy, T.; Pitz, K.J.; Chavez, F.P.; Yorke, C.E.; Miller, R.J. Environmental DNA Reveals the Fine-Grained and Hierarchical Spatial Structure of Kelp Forest Fish Communities. Sci. Rep. 2021, 11, 14439. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Q.; Wang, Y.; Wang, X.; Zhao, J.; Yao, M. Assessment of Fish Communities Using Environmental DNA: Effect of Spatial Sampling Design in Lentic Systems of Different Sizes. Mol. Ecol. Resour. 2020, 20, 242–255. [Google Scholar] [CrossRef]
- Xiang, T.; Dong, X.; Grenouillet, G. Ecological and Biological Traits of Non-Native Freshwater Fish Species Differentiate Them from Native Species in China. Ecol. Indic. 2021, 131, 108218. [Google Scholar] [CrossRef]
- Pfauserová, N.; Slavík, O.; Horký, P.; Turek, J.; Randák, T. Spatial Distribution of Native Fish Species in Tributaries Is Altered by the Dispersal of Non-Native Species from Reservoirs. Sci. Total Environ. 2021, 755, 143108. [Google Scholar] [CrossRef]
- Spear, M.J.; Embke, H.S.; Krysan, P.J.; Vander Zanden, M.J. Application of eDNA as a Tool for Assessing Fish Population Abundance. Environ. DNA 2021, 3, 83–91. [Google Scholar] [CrossRef]
- Gao, H.; Qu, W.; Ren, Z.; Zhang, B.; Liu, J.; Duan, Z. Fish Communities and Diversity in River Ecosystems on the Qinghai-Tibet Plateau Revealed by Environmental DNA (eDNA) Method. Ecol. Indic. 2023, 156, 111185. [Google Scholar] [CrossRef]
- Suzuki, J.; Nakano, D.; Kobayashi, S. Characteristics of Diurnal and Seasonal Changes in Fish Detection Patterns Using Environmental DNA Metabarcoding in a Mountain Stream. Limnologica 2022, 93, 125955. [Google Scholar] [CrossRef]
- Zou, K.; Chen, J.; Ruan, H.; Li, Z.; Guo, W.; Li, M.; Liu, L. eDNA Metabarcoding as a Promising Conservation Tool for Monitoring Fish Diversity in a Coastal Wetland of the Pearl River Estuary Compared to Bottom Trawling. Sci. Total Environ. 2020, 702, 134704. [Google Scholar] [CrossRef] [PubMed]
- Littlefair, J.E.; Hrenchuk, L.E.; Blanchfield, P.J.; Rennie, M.D.; Cristescu, M.E. Thermal Stratification and Fish Thermal Preference Explain Vertical eDNA Distributions in Lakes. Mol. Ecol. 2021, 30, 3083–3096. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Wang, J.; Ye, S.; Li, W.; Correa, S.B.; Zhang, T.; Liu, J. Multifaceted Fish Diversities Respond Differently to Impounding Age and Longitudinal Location along a Reservoir Cascade. Front. Ecol. Evol. 2022, 10, 955053. [Google Scholar] [CrossRef]
- Lian, Y.; Ye, S.; Godlewska, M.; Huang, G.; Wang, J.; Liu, J.; Li, Z. Comparison of Two Types of Survey Designs for Acoustic Estimates of Fish Resources in the Three Gorges Reservoir, China. Water 2022, 14, 2745. [Google Scholar] [CrossRef]
- Tao, J.P.; Gong, Y.T.; Tan, X.C.; Yang, Z.; Chang, J.B. Spatiotemporal patterns of the fish assemblages downstream of the Gezhouba Dam on the Yangtze River. Sci. China Life Sci. 2012, 55, 626–636. [Google Scholar] [CrossRef]
- Grenouillet, G.; Pont, D.; Hérissé, C. Within-Basin Fish Assemblage Structure: The Relative Influence of Habitat versus Stream Spatial Position on Local Species Richness. Can. J. Fish. Aquat. Sci. 2004, 61, 93–102. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Evans, N.T.; Olds, B.P.; Renshaw, M.A.; Turner, C.R.; Li, Y.; Jerde, C.L.; Mahon, A.R.; Pfrender, M.E.; Lamberti, G.A.; Lodge, D.M. Quantification of Mesocosm Fish and Amphibian Species Diversity via Environmental DNA Metabarcoding. Mol. Ecol. Resour. 2016, 16, 29–41. [Google Scholar] [CrossRef]
- Xie, P.; Liu, J. Practical Success of Biomanipulation Using Filter-Feeding Fish to Control Cyanobacteria Blooms: A Synthesis of Decades of Research and Application in a Subtropical Hypereutrophic Lake. Sci. World J. 2001, 1, 337–356. [Google Scholar] [CrossRef]
- Mao, Z.; Cao, Y.; Gu, X.; Zeng, Q.; Chen, H.; Jeppesen, E. Response of Zooplankton to Nutrient Reduction and Enhanced Fish Predation in a Shallow Eutrophic Lake. Ecol. Appl. 2023, 33, e2750. [Google Scholar] [CrossRef] [PubMed]
- Chick, J.H.; Gibson-Reinemer, D.K.; Soeken-Gittinger, L.; Casper, A.F. Invasive Silver Carp Is Empirically Linked to Declines of Native Sport Fish in the Upper Mississippi River System. Biol. Invasions 2020, 22, 723–734. [Google Scholar] [CrossRef]
- Roa-Fuentes, C.A.; Casatti, L. Influence of Environmental Features at Multiple Scales and Spatial Structure on Stream Fish Communities in a Tropical Agricultural Region. J. Freshw. Ecol. 2017, 32, 281–295. [Google Scholar] [CrossRef]
- Súarez, Y.R.; Petrere Jr, M.; Catella, A.C. Factors Determining the Structure of Fish Communities in Pantanal Lagoons (MS, Brazil). Fish. Manag. Ecol. 2001, 8, 173–186. [Google Scholar] [CrossRef]
- Qian, M.-M.; Wang, Z.-Y.; Zhou, Q.; Wang, J.; Shao, Y.; Qiao, Q.; Fan, J.-T.; Yan, Z.-G. Environmental DNA Unveiling the Fish Community Structure and Diversity Features in the Yangtze River Basin. Environ. Res. 2023, 239, 117198. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Y.; Zhan, A.; Dong, C.; Zhao, J.; Yao, M. Environmental DNA Captures Native and Non-Native Fish Community Variations across the Lentic and Lotic Systems of a Megacity. Sci. Adv. 2022, 8, eabk0097. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Havens, K.E.; Anneville, O.; Carvalho, L.; Coveney, M.F.; Deneke, R.; Dokulil, M.T.; Foy, B.; et al. Lake Responses to Reduced Nutrient Loading—An Analysis of Contemporary Long-term Data from 35 Case Studies. Freshw. Biol. 2005, 50, 1747–1771. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic Shifts in Ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Wu, Y.; Yu, Y.; Sun, J.; Mao, D.; Zhang, G. Intensified Effect of Nitrogen Forms on Dominant Phytoplankton Species Succession by Climate Change. Water Res. 2024, 264, 122214. [Google Scholar] [CrossRef]
- Vadeboncoeur, Y.; Jeppesen, E.; Zanden, M.J.V.; Schierup, H.; Christoffersen, K.; Lodge, D.M. From Greenland to Green Lakes: Cultural Eutrophication and the Loss of Benthic Pathways in Lakes. Limnol. Oceanogr. 2003, 48, 1408–1418. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Cole, J.J.; Hodgson, J.R.; Kitchell, J.F.; Pace, M.L.; Bade, D.; Cottingham, K.L.; Essington, T.E.; Houser, J.N.; Schindler, D.E. Trophic Cascades, Nutrients, and Lake Productivity: Whole-Lake Experiments. Ecol. Monogr. 2001, 71, 163–186. [Google Scholar] [CrossRef]

| Order/Family | Species | Ecological Characteristics | Season | Reservoir | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ecological Types | Feeding Habit | Habitat Habit | Wet | Dry | TJ | AD | SF | JL | ||
| Clupeiformes | ||||||||||
| Engraulidae | Coilia nasus | Anadromous | Carnivorous | Pelagic | − | + | − | + | − | − |
| Cypriniformes | ||||||||||
| Cyprinidea | ||||||||||
| Danioninae | Opsariichthys uncirostris | Stream-resident | Carnivorous | Pelagic | + | − | + | + | + | + |
| Opsariichthys evolans | Stream-resident | Carnivorous | Pelagic | + | + | + | + | + | + | |
| Rasbora steineri * | Stream-resident | Omnivorous | Demersal | − | + | − | − | − | + | |
| Zacco platypus | Stream-resident | Omnivorous | Pelagic | + | + | + | − | + | − | |
| Leuciscinae | Ctenopharyngodon idella | River-resident | Herbivorous | Demersal | − | + | + | + | + | + |
| Elopichthys bambusa | River-resident | Carnivorous | Pelagic | − | + | − | + | − | + | |
| Mylopharyngodon piceus | River-resident | Carnivorous | Benthic | + | − | − | + | − | − | |
| Cultrinae | Ancherythroculter daovantieni * | River-resident | Carnivorous | Pelagic | − | + | − | + | + | − |
| Ancherythroculter nigrocauda | River-resident | Carnivorous | Pelagic | + | + | + | + | + | + | |
| Culter alburnus | River-resident | Carnivorous | Pelagic | + | − | + | + | + | + | |
| Chanodichthys erythropterus | River-resident | Carnivorous | Pelagic | − | + | − | − | − | + | |
| Hemiculter tchangi * | River-resident | Omnivorous | Pelagic | + | + | + | + | + | + | |
| Megalobrama amblycephala | River-resident | Herbivorous | Demersal | + | + | + | + | + | + | |
| Megalobrama pellegrini * | River-resident | Omnivorous | Pelagic | − | + | − | − | − | + | |
| Parabramis pekinensis | River-resident | Herbivorous | Demersal | − | + | + | − | − | − | |
| Pseudohemiculter dispar | River-resident | Omnivorous | Pelagic | − | + | − | − | − | + | |
| Sinibrama melrosei * | River–stream | Omnivorous | Pelagic | + | − | + | − | + | − | |
| Sinibrama wui | River–stream | Omnivorous | Pelagic | − | + | − | − | − | + | |
| Xenocyprinae | Xenocypris davidi | River-resident | Omnivorous | Benthic | − | + | − | − | + | + |
| Barbinae | Acrossocheilus wenchowensis | Stream-resident | Herbivorous | Pelagic | − | + | + | + | + | − |
| Spinibarbus hollandi | River–stream | Omnivorous | Demersal | − | + | − | − | + | − | |
| Cyprininae | Carassius gibelio | River-resident | Omnivorous | Benthic | + | + | + | + | + | + |
| Cyprinus carpio | River–stream | Omnivorous | Benthic | − | + | − | + | − | − | |
| Gobioninae | Hemibarbus maculatus | River–stream | Carnivorous | Benthic | + | − | + | + | + | + |
| Microphysogobio fukiensis | Stream-resident | Omnivorous | Demersal | − | + | + | + | + | − | |
| Pseudorasbora parva | Stream-resident | Omnivorous | Pelagic | − | + | + | + | + | − | |
| Squalidus chankaensis * | River-resident | Omnivorous | Demersal | − | + | + | − | − | − | |
| Acheilognathinae | Rhodeus sinensis | River-resident | Omnivorous | Pelagic | + | + | + | + | + | + |
| Hypophthalmichthyinae | Hypophthalmichthys molitrix | River-resident | Planktivorous | Pelagic | + | + | + | + | + | + |
| Hypophthalmichthys nobilis | River-resident | Planktivorous | Pelagic | + | + | + | + | + | + | |
| Cobitidae | Misgurnus anguillicaudatus | River-resident | Omnivorous | Benthic | + | − | − | − | + | − |
| Perciformes | ||||||||||
| Callionymidae | Repomucenus olidus | Estuarine-resident | Omnivorous | Benthic | − | + | − | − | + | − |
| Eleotridae | Bostrychus sinensis | Estuarine-resident | Carnivorous | Benthic | + | + | + | + | + | + |
| Odontobutidae | Odontobutis obscura * | River-resident | Carnivorous | Benthic | − | + | − | + | − | − |
| Gobiidae | Rhinogobius similis * | Stream-resident | Carnivorous | Benthic | − | + | + | + | − | + |
| Mastacembelidae | Macrognathus aculeatus | River-resident | Carnivorous | Benthic | − | + | − | + | − | − |
| Serranidae | Siniperca obscura | Stream-resident | Carnivorous | Demersal | − | + | − | + | + | + |
| Channidae | Channa argus | River-resident | Carnivorous | Benthic | + | − | − | − | − | + |
| Centrarchidae | Micropterus salmoides | River-resident | Carnivorous | Demersal | + | + | + | + | + | + |
| Cichlidae | Coptodon zillii * | River-resident | Omnivorous | Demersal | + | − | + | − | − | − |
| Siluriformes | ||||||||||
| Bagridae | Pelteobagrus fulvidraco | River-resident | Carnivorous | Benthic | + | − | − | + | − | + |
| Mugiliformes | ||||||||||
| Mugilidae | Mugil cephalus | River–coastal | Omnivorous | Benthic | + | + | − | + | + | + |
| Synbranchiformes | ||||||||||
| Synbranchidae | Monopterus albus | River-resident | Carnivorous | Benthic | + | − | − | − | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Dai, Y.; Li, M.; Tian, L.; Lou, B.; Huang, F.; Xie, Z.; Dai, Y.; He, W. Environmental DNA Reveals Ecologically Relevant Temporal and Spatial Variation of Fish Community in Silver Carp- and Bighead Carp-Dominant Drinking Water Reservoirs. Water 2025, 17, 1057. https://doi.org/10.3390/w17071057
Tang J, Dai Y, Li M, Tian L, Lou B, Huang F, Xie Z, Dai Y, He W. Environmental DNA Reveals Ecologically Relevant Temporal and Spatial Variation of Fish Community in Silver Carp- and Bighead Carp-Dominant Drinking Water Reservoirs. Water. 2025; 17(7):1057. https://doi.org/10.3390/w17071057
Chicago/Turabian StyleTang, Jinyu, Yangxin Dai, Ming Li, Lei Tian, Bao Lou, Fuyong Huang, Zhigang Xie, Yulai Dai, and Wenfang He. 2025. "Environmental DNA Reveals Ecologically Relevant Temporal and Spatial Variation of Fish Community in Silver Carp- and Bighead Carp-Dominant Drinking Water Reservoirs" Water 17, no. 7: 1057. https://doi.org/10.3390/w17071057
APA StyleTang, J., Dai, Y., Li, M., Tian, L., Lou, B., Huang, F., Xie, Z., Dai, Y., & He, W. (2025). Environmental DNA Reveals Ecologically Relevant Temporal and Spatial Variation of Fish Community in Silver Carp- and Bighead Carp-Dominant Drinking Water Reservoirs. Water, 17(7), 1057. https://doi.org/10.3390/w17071057






