Abstract
Endocrine disruptors such as 17α-ethinylestradiol pose significant ecological risks in aquatic environments. This study assessed the catalytic performance of Fe- and Cu-impregnated delaminated clays (DCs) and layered double hydroxides (LDHs) in a Fenton-like process for EE2 removal. The effects of key parameters—including hydrogen peroxide concentration, initial contaminant load, and catalyst dosage—were analyzed using HPLC-QqTOF. Delaminated clays (DCs) demonstrated higher removal efficiencies compared to layered double hydroxides (LDHs), reaching 55% with Fe and 47% with Cu, while LDHs achieved 40% and 33% for Fe and Cu, respectively. Ecotoxicity was evaluated using bioassays (L. sativa, S. capricornutum, D. magna) and the Ames test. Notably, S. capricornutum exhibited 100% inhibition at the highest tested concentration, with IC50 values of 11.2–12.4 for Cu and 31.5–32.7 for Fe. L. sativa was inhibited by Cu- and Fe-impregnated LDH/DC, with IC50 values of 71.0 (DC-Cu), 56.6 (DC-Fe), and 58.6 (LDH-Fe). D. magna exhibited 17–75% mortality when exposed to untreated EE2, while LC50 values confirmed Cu’s greater toxicity. The Ames test indicated no mutagenic effects. Integrating the Fenton-like process with complementary techniques is recommended to enhance efficiency. These findings highlight the need to optimize operational parameters for effective removal of 17α-ethinylestradiol.
1. Introduction
Wastewater from hospitals, households, and industries is a significant source of emerging organic contaminants (EOCs), including pharmaceuticals, personal care products, and industrial residues. Since conventional treatment plants do not fully eliminate these pollutants, they persist in surface and groundwater, leading to risks such as bioaccumulation and biomagnification [1]. Several pharmaceutical compounds have been identified as endocrine-disrupting chemicals (EDCs) due to their ability to interfere with the endocrine system. Moreover, they have been linked to cancer, reproductive impairments, immune and nervous system disorders, and developmental abnormalities, among other health concerns [2]. Among the most harmful EDCs for humans and the environment are natural and synthetic steroid hormones, which pose significant environmental risks even at trace concentrations in aquatic ecosystems [3].
The occurrence of emerging contaminants, including pharmaceuticals and hormones, has been widely documented in various environmental matrices. Hormones have been detected at concentrations of 90 ng/L in domestic wastewater, 195 ng/L in surface water, 120 ng/L in groundwater, and 275 ng/L in wastewater treatment plant effluents [4,5]. The presence of pharmaceuticals in aquatic systems was first reported in the 1970s–1990s. For example, caffeine was identified in U.S. wastewater and surface water, estrogens were detected at nanogram-per-liter concentrations in Germany, and theophylline and tetracycline were found in UK rivers at ~1 μg/L. Additionally, phenacetin was detected in Spanish waters, while NSAIDs such as ibuprofen and naproxen appeared in Canadian wastewater. Reports from the U.S. documented salicylic acid (1.83–95.62 μg/L) and clofibric acid (2.54–9.74 μg/L), with the latter also found at 165 ng/L in Germany. Other studies have identified β-blockers, antibiotics (up to 6 μg/L), and barbiturates (205 μg/L) in German and Danish water sources [6]. These findings raise concerns regarding their environmental and human health impacts.
Among synthetic hormones, mestranol (MeEE2) and 17α-ethinylestradiol (EE2) stand out due to their primary sources: oral contraceptives and hormone replacement therapies [7]. Recent studies have detected these hormones in surface water at concerning concentrations. For instance, average levels have been reported at 0.64 µg/L for estrogens [8], 1.2 µg/L for androgens [9], up to 30 µg/L for progestins [10], 11 µg/L for glucocorticoids [11], and 1.0 µg/L for mineralocorticoids [12]. EE2 was not initially considered a contaminant until the 1990s, when it was detected in wastewater and linked to toxicological effects on the environment [13]. Recent data indicate that approximately 35 µg of EE2 is excreted daily, with 80% of these drugs eliminated as unmetabolized conjugates, 30% excreted in feces, and 50% in urine [14]. In addition to being classified as an EDC, EE2 has been associated with negative impacts on aquatic ecosystems, particularly affecting fish reproduction, egg fertilization, and survival rates, ultimately threatening biodiversity [14].
Several treatment strategies have been explored to enhance the removal of this contaminant [15]. Among the most effective are electrochemical methods, including advanced anodic oxidation (EAOP) and electrocoagulation, often integrated with advanced oxidation processes (AOPs). These techniques generate highly reactive oxidizing species, enabling the degradation of diverse contaminants in aqueous solutions [3]. Reported removal efficiencies vary, with electrochemical oxidation achieving 100% [16,17], photoelectrocatalytic oxidation reaching 64% [17], and bioelectrochemical processes attaining 81.9% [18]. However, these methods present challenges such as high energy consumption, strict pH requirements, and the potential formation of toxic byproducts. In particular, electro-Fenton (EF) and photoelectro-Fenton (PEF) processes are only optimal at strongly acidic pH levels (2.8–3.5), which poses environmental concerns, as a final neutralization step may be required to produce tolerable effluents. Additionally, thermal and photo-Fenton processes can lead to the formation of harmful byproducts such as hydroxychlorobiphenyls, dihydroxylated products, 2,4-dichlorophenol, 2,4-dichlorophenylformate, 2,4-dichloro-1-(chloromethoxy)-benzene, 6,8-dichloro-2H-1,4-benzodioxan-3-one, oxalic acid, formic acid, and 1,4-benzoquinone, further complicating wastewater treatment [19]. Fenton-like processes have shown significant promise for removing various organic compounds [20]. However, their implementation often depends on sophisticated or expensive catalysts, limiting scalability. To overcome these challenges, layered materials such as delaminated clays and functionalized layered double hydroxides (LDHs) impregnated with Fe or Cu have emerged as cost-effective and efficient catalyst alternatives for these processes [21,22].
The objective of this study was to evaluate delaminated clays and layered double hydroxides impregnated with Fe or Cu as catalysts in a Fenton-like process under various operational conditions for the removal of 17α-ethinylestradiol. Additionally, this study aimed to assess the environmental safety of the treated effluents using bioassays and evaluate their mutagenic potential.
2. Materials and Methods
2.1. Catalyst Synthesis
Four catalysts were tested in a Fenton-like process to remove the synthetic hormone 17α-ethinylestradiol (Merck, Darmstadt, Germany). Two catalysts were developed using delaminated clays, while the other two employed layered double hydroxides (LDHs) as supports for active Fe and Cu phases.
For clay delamination, bentonite (Bentonitas de Colombia S.A., Bugalagrande, Colombia) was used. The process began with homogenization in a NaCl solution (3 g in 100 mL) under constant stirring for 24 h. The conductivity was then adjusted to 100 µS, followed by the incorporation of polyvinyl alcohol (PVA) at a concentration of 40 g/L. The mixture was refluxed for 24 h, subjected to microwave treatment, dried at 70 °C, and finally calcined at 400 °C [23].
The synthesis of LDHs followed the coprecipitation method. Aqueous solutions of 0.1 M Mg(NO3)2 and 0.1 M Al(NO3)3 were added to a Na2CO3 solution (2.5-fold molar excess relative to Mg2+) under constant agitation (5 g, 1 h). The resulting mixture was then exposed to microwave treatment (20 min, 180 °C, 30 bar) and allowed to age for 24 h. The precipitate was subsequently washed with distilled water until reaching a conductivity of 100 µS, dried at 90 °C for 24 h, and calcined at 500 °C for 6 h [24].
Following synthesis, the supports (DC and LDH) underwent wet impregnation with Fe or Cu, maintaining an active phase content of 5% metal (Supporting Information). This method yielded four distinct catalysts: (i) DC-Fe, (ii) DC-Cu, (iii) LDH-Fe, and (iv) LDH-Cu. The metal content percentages were consistent with previous studies [25,26].
2.2. Catalytic Activity: Degradation of 17α-Ethinylestradiol in a Fenton-like Process
The degradation of 17α-ethinylestradiol (EE2) was assessed under 42 experimental conditions using delaminated clay (Table 1) in a semi-batch reactor, maintaining a controlled temperature of 20 °C, an airflow rate of 2 mL/min, and continuous stirring at 6 g. Aliquots were collected in triplicate at seven time points (0, 20, 40, 60, 80, 100, and 120 min). This experimental setup allowed for a systematic evaluation of the individual effects of hydrogen peroxide concentration, initial contaminant concentration, and catalyst dosage, as well as their interactions with pH. Each parameter was tested at three different pH levels, facilitating a comprehensive analysis of their influence on EE2 degradation and potential synergistic effects.

Table 1.
Treatments used in the catalytic activity for the degradation of 17α-ethinylestradiol in a Fenton-like process, catalyzed by delaminated clays impregnated with Fe or Cu.
At each sampling time, a defined aliquot of the reaction mixture was withdrawn and immediately treated with sodium persulfate to quench the reaction and prevent further degradation. The samples were then centrifuged to remove particulate matter, and the resulting supernatant was filtered through a 0.22 µm polyvinylidene fluoride (PVDF) syringe filter to eliminate any remaining suspended solids before chromatographic analysis.
At each sampling time, potential degradation by-products were analyzed using high-performance liquid chromatography (HPLC, Nexera Series X2, Shimadzu Scientific Instruments, Columbia, IN, USA) coupled with electrospray ionization mass spectrometry (ESI-MS) in positive mode, utilizing a quadrupole-time-of-flight analyzer QqTOF, LCMS-9030, (Shimadzu, Kyoto, Japan). Chromatographic conditions included an isocratic flow for 36 min, with mobile phase A consisting of 80% aqueous formic acid (0.1%) and mobile phase B comprising 20% acetonitrile, maintained at a constant flow rate of 0.15 mL/min at 40 °C. The analysis was conducted using a Shim-pack XR-ODS II column (2.2 µm, dp × 2.0 mm ID × 150 mm L) with a 3 µL injection volume. Detector acquisition parameters were set to positive mode, with an acquisition range of 50–1000 m/z, an interface voltage of 5 kV, an interface temperature of 300 °C, a desolvation line temperature of 250 °C, a nebulizing gas flow (N2) of 3 L/min, and a drying/heating gas flow of 10 L/min.
To evaluate catalyst reusability, the catalyst was recovered after the first cycle, thoroughly washed with water and ethanol, and then dried at 105 °C for 12 h. This process was repeated for four consecutive cycles.
In the case of reactions with layered double hydroxides, a single basic pH (10) was evaluated, considering that the support functions as a buffer. Seven treatments were used for this experiment, each conducted in triplicate (Table 2) and assessed across six groups. Sample collection times and analysis followed the same procedures described for delaminated clays, as well as the catalyst stability assessment mentioned above.

Table 2.
Treatments used in the catalytic degradation of 17α-ethinylestradiol using a Fenton-like process catalyzed by double-layer hydroxides impregnated with Fe or Cu.
In all cases, a Shapiro–Wilk test was performed to assess data normality. However, due to the data distribution, non-parametric analyses were conducted, including the Kruskal–Wallis variance test and the modified Wilcoxon median comparison test, with Bonferroni correction for multiple comparisons. All statistical analyses were performed using R Studio (version 2023.12.0+369).
2.3. Bioassays
2.3.1. Acute Toxicity Bioassay with Lactuca sativa
Lactuca sativa, variety Great Lake Batavia, was used following the methodology described by Dutka (1989) [27]. The reaction products from delaminated clays impregnated with Fe or Cu, double-layer hydroxides impregnated with Fe or Cu, and EE2—spiked with the contaminant (5 ppm), hydrogen peroxide (0.1 M), and a catalyst load of 0.12 g—were collected at 120 min and filtered through Whatman No. 1 paper.
Subsequently, serial dilutions of 1, 10, 25, 50, 75, and 100% were prepared, and 4 mL of each dilution was added to Petri dishes lined with Whatman No. 3 filter paper, each containing 25 seeds of uniform size, shape, and color. The samples were incubated in darkness at 22 (±2) °C for five days, in duplicate. After the incubation period, root length was measured in millimeters (mm) to assess the inhibition effect. Finally, the concentration that produced 50% inhibition of root elongation (Inhibitory Concentration 50% (IC50)) was estimated using Probit analysis.
2.3.2. Acute Toxicity Bioassay with Selenastrum capricornutum
The assessment was conducted following the EPA method (1994), with modifications based on Cifuentes [28]. The same reaction products previously tested on Lactuca sativa were used. Serial dilutions of 1, 10, 25, 50, 75, and 100% were prepared in triplicate. Each dilution was inoculated at a 1:1 ratio into a final volume of 10 mL of medium containing Selenastrum capricornutum (UTEX 1648) at a concentration of 104 cells/mL. The cultures were incubated at 23 (±2) °C under a light intensity of 4300 lux (±10), with constant agitation at 100 rpm.
After a 96-h incubation period, the percentage of inhibition was determined for each concentration by comparing turbidity at 750 nm to the control. The concentration required to achieve a 50% inhibition of algal cell growth (IC50) was then calculated using the Probit method, with a significance level of p < 0.05 [29].
2.3.3. Acute Toxicity Bioassay with Daphnia magna
Toxicity evaluation in Daphnia magna was performed following Dutka’s procedure (1989) [27]. Dilutions of 1, 10, 25, 50, 75, and 100% were prepared from the filtrate described in the Lactuca sativa bioassay and mixed with hard reconstituted water (180 mg/L CaCO3). A volume of 25 mL was dispensed into plastic containers, each containing 10 neonates (24 h post-hatch), in triplicate. The organisms were incubated for 48 h at 21 (±1) °C under a 16 h light/8 h dark cycle, with a light intensity of 800 (±100) lux.
At the end of the incubation period, mortality was recorded and expressed as a percentage. Data were analyzed for normality and variance. Based on the recorded mortality counts, the 48-h lethal concentration 50% (LC50) was determined using the Probit method, with a significance level of p < 0.05.
2.3.4. Calculation of LC/IC50
To determine the lethal concentration 50% (LC50) and inhibitory concentration 50% (IC50) values along with their 95% confidence intervals, the Probit method (EPA, Version 1.5) was applied. This parametric approach estimates the effective concentration (LC50 or IC50) by modeling dose–response relationships using probabilistic techniques. LC50 represents the concentration of a substance that causes 50% mortality in an exposed population within a specific time frame, while IC50 refers to the concentration required to inhibit 50% of an organism’s growth or biological activity. Accurate LC50 determination requires intermediate effect values between 0% and 100%. The evaluated effects include inhibition, sub-lethality, lethality, and v/v relationships.
In ecotoxicology, LC50 is widely used in bioassays with aquatic organisms such as D. magna, whereas IC50 is relevant for studies involving microorganisms and plants, including S. capricornutum and L. sativa. These parameters, obtained through Probit regression, are critical for assessing species sensitivity to contaminants and evaluating environmental risks.
2.4. Mutagenicity Index (MI) Using the Ames Test
The mutagenicity assay followed the methodology of Williams (1983) [30], using genetically modified Salmonella Typhimurium strains TA98 (hisD3052, uvrB, rfa, pKM101) and TA100 (hisG46, uvrB, rfa, pKM101). These strains were cultured in Nutrient Broth No. 2 (Oxoid, Basingstokes, UK) at 37 ± 2 °C and 140 ± 10 rpm for 24 h until reaching a concentration of 109 CFU/mL. Filtered supernatants from previous bioassays (Section 2.3) were serially diluted (1–100%) in sterile distilled water. Each dilution (0.1 mL) was mixed with 0.1 mL of bacterial culture and 3.5 mL of histidine–biotin-supplemented agar No. 2 (0.5 mM). The mixture was homogenized and plated onto minimal agar Petri dishes. All assays were performed in triplicate. After 120 h of incubation at 37 ± 2 °C, revertant colonies were counted using an automatic colony counter (Industrial Scientific, Pittsburgh, PA, USA). Mutagenic activity was expressed as revertants per mL of CFU, and the mutagenicity index (MI) was calculated. MI values ≥ 2.0 were considered mutagenic [31]. Sodium azide (0.0375 ppm) and 2-nitrofluorene (7.5 ppm) served as positive controls for TA100 and TA98, respectively, while Nutrient Broth No. 2 (Oxoid, Basingstokes, UK) was used as the negative control.
MI = a/b
MI: Mutagenicity Index. a: Average number of revertant colonies in Petri dishes exposed to each dilution of the samples. b: Average number of revertant colonies in Petri dishes with the negative control (corresponding S. Typhimurium strain without sample).
Formula (1): Revertant colony count for TA98 and TA100.
All bioassays were analyzed using R Studio software, version 2023.12.0+369 for normality tests and analysis of variance.
3. Results and Discussion
3.1. Catalytic Activity
3.1.1. Reaction Controls
To assess the relevance of different components in a system analogous to the Fenton-like process, a series of experiments were conducted under specific initial conditions: an initial contaminant concentration of 5 ppm, a hydrogen peroxide concentration of 0.1 M, and a catalyst mass of 0.12 g.
The following assays were performed: 1. Evaluation without H2O2: This phase examined the reaction in the absence of hydrogen peroxide, a key component responsible for generating radicals that degrade contaminants; 2. Evaluation without exposure to air: The potential influence of oxygen on the reactions was investigated, considering its role in secondary reactions and the formation of reactive species; 3. Evaluation with light: Given the photosensitive nature of certain compounds, the effect of light on the contaminant removal rate was analyzed; 4. Use of a high-purity EE2 standard: Experiments were initially performed with commercially available EE2 containing excipients that may be susceptible to radical action and influence degradation efficiency. To ensure accuracy, a high-purity EE2 standard (98%) was used.
These evaluations aimed to determine the individual contributions of the components in the Fenton-like process and provide a more precise understanding of their impact on EE2 degradation (Figure 1).
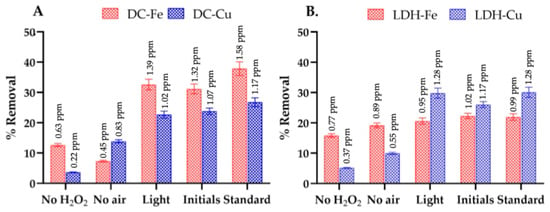
Figure 1.
Evaluation of each component of the Fenton-like process system used for the removal of EE2, utilizing delaminated clays and double-layer hydroxides as supports for Fe or Cu, under reaction conditions of an initial contaminant concentration of 5 ppm, a hydrogen peroxide concentration of 0.1 M, and a catalyst amount of 0.12 g, including (A) Fenton-like process systems catalyzed with DC-Fe and DC-Cu, and (B) Fenton-like process systems catalyzed with LDH-Fe and LDH-Cu.
In Figure 1A,B, reactions performed in the absence of hydrogen peroxide achieved approximately 15% removal with the Fe-based catalyst (DC-Fe) but only about 5% with the Cu-based catalyst (DC-Cu). Although these differences are modest, they highlight the inherent adsorption capacity of both clays and layered double hydroxides, as previously reported [32,33]. Specifically, Fe-based catalysts often exhibit greater affinity for organic molecules due to their surface properties, which facilitate electrostatic interactions or complex formation with EE2’s phenolic group [34]. In contrast, Cu-based catalysts appear to have a lower adsorption capacity under similar conditions, potentially due to differences in surface charge, pore structure, or the specific electronic configuration of the metal center.
Nevertheless, upon the introduction of hydrogen peroxide (H2O2 concentration: 0.1 M), the overall removal of EE2 increases significantly to 31%, 18%, 22%, and 26% for DC-Fe, DC-Cu, LDH-Fe, and LDH-Cu, respectively. This increase in removal efficiency suggests that oxidative pathways overwhelmingly surpass mere adsorption in governing EE2 degradation. While some degree of adsorption persists, it plays a secondary role once hydroxyl radicals or other reactive species form in the presence of H2O2. These findings confirm that oxidative processes are the primary mechanism responsible for EE2 removal, whereas adsorption alone contributes only modestly to total removal more so for Fe catalysts than for Cu catalysts.
Furthermore, Figure 1 illustrates that the absence of air (Figure 1A,B) affects the removal percentages compared to initial treatments conducted with a constant air flow rate (2 mL/min). This suggests that superoxide radical anions (O2−) were generated during the Fenton-like process through interactions between the active metal phases and O2. These reactive species are also associated with the mineralization of organic compounds [35,36].
Additionally, photocatalytic processes have demonstrated efficiency in EE2 removal. Therefore, the Fenton-like process was evaluated under natural light to assess its influence on 17α-ethinylestradiol degradation. Previous studies have reported up to 60% removal under UV light [37]. In this study, natural light achieved removal percentages of 32%, 22%, 20%, and 30% for DC-Fe, DC-Cu, LDH-Fe, and LDH-Cu, respectively. No significant enhancement in removal efficiency was observed, consistent with prior research concluding that hydrogen peroxide in the presence of visible light is insufficient for EE2 oxidation [38].
The comparison between reactions using a commercial medication (Dienille: Dienogest + Ethinylestradiol 2/0.03 mg) and a high-purity (>98%) 17α-ethinylestradiol standard showed higher removal rates with the standard. This difference is attributed to excipients in the commercial formulation, which interact with reactive species, reducing their availability for the mineralization of target compounds [39].
3.1.2. Catalytic Activity—Effects of Operating Parameters
Evaluations of operating parameters, including hydrogen peroxide concentration, initial contaminant concentration, and catalyst presence, were conducted under various acidic, neutral, and basic pH conditions [40]. The influence of pH on contaminant removal, regardless of treatment, showed no significant differences for delaminated clays impregnated with Cu (p > 0.05), indicating that the catalytic activity of Cu-based systems remained stable across different pH conditions. However, for delaminated clays impregnated with Fe, significantly higher removal efficiencies were observed (p < 0.001), particularly between pH 3 and pH 7 or 11 (p < 0.05).
pH played a crucial role in modifying catalyst properties, particularly surface charge and the electronic state of active Fe sites. These modifications influenced catalyst–reactant interactions, thereby altering reaction kinetics and overall efficiency [35]. For Fe-impregnated delaminated clays, acidic conditions enhanced Fe3+/Fe2+ redox cycling, increasing hydroxyl radical generation and contaminant degradation. In contrast, at higher pH, surface charge changes likely interfered with hydrogen peroxide activation, reducing radical formation efficiency. In contrast, Cu-impregnated delaminated clays did not exhibit statistically significant differences in removal across the tested pH conditions. This suggests that the catalytic performance of Cu species in the system was less dependent on pH, possibly due to variations in Cu–H2O2 interactions or the stability of active Cu species under different conditions.
pH variations also impacted the physicochemical properties of the catalyst, modifying functional group density and reactivity. Under acidic conditions, protonated hydroxyl and silanol groups enhanced electrostatic interactions with EE2, increasing its availability for oxidation [41]. At neutral and alkaline pH, the deprotonation of surface functional groups might alter the adsorption–desorption equilibrium, affecting the interaction between EE2, the catalyst, and oxidant species [42]. Furthermore, variations in surface charge and electron transfer properties could influence the stability and reactivity of hydroxyl radicals and other reactive oxygen species, thereby impacting the overall efficiency and selectivity of the degradation process [43].
Hydrogen Peroxide Concentration—Delaminated Clay Impregnated with Fe or Cu
The effect of hydrogen peroxide concentration on EE2 degradation was assessed using a Fenton-like process catalyzed by Fe- or Cu-impregnated delaminated clays. As shown in Figure 2A–C, EE2 removal efficiency with DC-Fe was evaluated across a peroxide concentration range of 0.05 M–0.2 M. Increasing the concentration from 0.05 M to 0.1 M under acidic conditions enhanced EE2 degradation from 35% to 49%, likely due to an increased generation of hydroxyl radicals (⋅OH). These radicals are produced via Fenton-like reactions, where Fe(III) is reduced to Fe(II), promoting H2O2 decomposition into highly reactive species that drive contaminant oxidation [44].
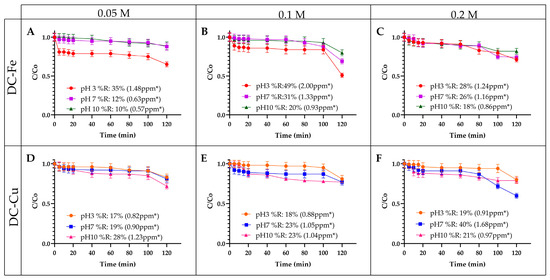
Figure 2.
Influence of hydrogen peroxide concentration on the removal of EE2. (A) AD-Fe—0.05 M H2O2 (B) AD-Fe—0.1 M H2O2 (C) AD-Fe—0.2 M H2O2 (D) AD-Cu—0.05 M H2O2 (E) AD-Cu—0.1 M H2O2 (F) AD-Cu—0.2 M H2O2. Experimental conditions: contaminant concentration 5 ppm and catalyst amount: 0.12 g. * number of ppm removed.
However, at 0.2 M, degradation efficiency declined to 28%, a trend commonly observed in advanced oxidation processes. This decrease is attributed to the scavenging effect, where excess H2O2 reacts with hydroxyl radicals (⋅OH), generating hydroperoxyl radicals (⋅OOH), which have a lower oxidation potential (+1.7 V vs. +2.8 V for hydroxyl radicals). This competitive consumption of reactive oxygen species (ROS) reduces EE2 removal efficiency [44,45]. These findings align with those reported by Zaixing Li (2023) [46], who investigated the critical factors influencing the removal of pharmaceutical compounds. Their study highlighted the significant impact of peroxide concentration and pH on the degradation process [46]. A similar trend was observed, where an initial increase in peroxide concentration enhanced degradation efficiency. However, when the oxidant concentration doubled or exceeded a threshold, especially under low pH conditions, the removal percentages decreased significantly [47].
For reactions catalyzed by DC-Cu (Figure 2D–F), lower peroxide concentrations (0.05 M and 0.1 M) under acidic and neutral pH conditions resulted in the lowest removal percentages. This outcome is attributed to radical deactivation and the conversion of hydrogen peroxide into oxonium ions, which reduces the effective concentration of the oxidant. As a result, the generation of reactive species primarily responsible for contaminant degradation decreased [48]. This behavior aligns with previous studies on Cu-based catalysts, where increasing hydrogen peroxide concentration led to improved degradation percentages [49].
Additionally, reactions with DC-Fe achieved the highest removal percentages under acidic pH conditions, whereas those with DC-Cu performed better at neutral or basic pH levels. This difference stems from the dependence of H2O2 oxidation on the metal’s valence state, with low- or high-valence metals favoring the process under acidic, neutral, or basic conditions, respectively [50].
Initial Contaminant Concentration—Delaminated Clay Impregnated with Fe or Cu
The influence of initial EE2 concentration on catalytic removal was analyzed, as illustrated in Figure 3 Reactions with DC-Fe were conducted at EE2 concentrations of 1 ppm, 5 ppm, and 10 ppm (Figure 3A–C). A significant increase in removal efficiency was observed when the EE2 concentration rose from 1 ppm to 5 ppm across all pH conditions. Specifically, under acidic pH, efficiency sharply increased from 17% to 49%, marking the highest recorded value. However, at 10 ppm, removal efficiency declined, dropping by 34% and 11% under acidic and neutral pH conditions, respectively. This reduction is attributed to catalyst surface saturation, which limits EE2 adsorption and oxidation efficiency. Since adsorption plays a fundamental role in heterogeneous catalytic reactions, higher contaminant loads reduce the number of available active sites, restricting degradation [51]. Additionally, increased contaminant concentrations intensify competition for hydroxyl radicals, diminishing oxidation efficiency [52]. At elevated hydrogen peroxide levels, secondary scavenging reactions generate hydroperoxyl radicals with lower oxidation potential, further reducing EE2 degradation. Mass transfer limitations also contribute to efficiency loss, as high contaminant loads hinder EE2 and hydrogen peroxide diffusion toward the catalyst surface, limiting interactions with reactive species and decreasing overall removal efficiency [53].
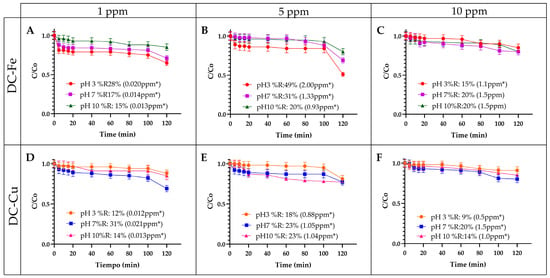
Figure 3.
Influence of the initial contaminant concentration on the removal of EE2. (A) DC-Fe—1 ppm of EE2 (B) DC-Fe—5 ppm of EE2 (C) DC-Fe—10 ppm of EE2 (D) DC-Cu—1 ppm of EE2 (E) DC-Cu—5 ppm of EE2 (F) DC-Cu—10 ppm of EE2. Experimental conditions: hydrogen peroxide concentration 0.1 M and catalyst amount: 0.12 g. * number of ppm removed.
Additionally, the highest removal percentages were achieved under acidic pH conditions. In neutral or alkaline environments, the generated radicals may react with Fe2+ and Fe3+ to form less reactive species. This, combined with the potential inhibition of the Fe2+ and H2O2 reaction, leads to reduced hydroxyl radical generation [45].
In contrast, the system catalyzed by DC-Cu (Figure 3E) exhibited a completely different behavior. The highest removal percentage was observed at an initial contaminant concentration of 1 ppm under neutral pH, reaching approximately 31%. However, removal efficiency decreased as the contaminant concentration increased to 10 ppm, likely due to contaminant deposition on the catalyst surface, which blocks active sites. This trend aligns with findings by Li (2023) [46], who reported higher removal percentages at an initial contaminant concentration of 6 ppm compared to 15 ppm in a heterogeneous Fenton-like process catalyzed by Co-Cu [46].
As observed in Figure 1A,B, a portion of the contaminant removal can be attributed to the catalysts’ inherent adsorption capacity, though adsorption alone does not account for high overall removal. When the initial EE2 concentration increases from 1 ppm to 5 ppm, the ratio of available catalyst surface sites to contaminant molecules appears optimal, enhancing removal efficiency as adsorbed EE2 is more effectively oxidized. However, at 10 ppm, active sites may become saturated or blocked, limiting the overall degradation process. Similarly, catalysts with lower baseline adsorption capacity (e.g., Cu-based systems) may exhibit a more pronounced decline in removal efficiency at higher contaminant concentrations. Therefore, while adsorption plays a crucial role in localizing EE2 on the catalyst surface before oxidation, it is not the sole determinant of removal efficiency.
Catalyst Amount—Delaminated Clay Impregnated with Fe or Cu
The effect of catalyst dosage on 17α-ethinylestradiol (EE2) removal in a Fenton-like process was examined using delaminated clays impregnated with Fe or Cu (Figure 4). Experimental conditions included 0.1 M hydrogen peroxide and an initial EE2 concentration of 5 ppm. As illustrated in Figure 4A–C, Fe-based catalysts exhibited the highest removal efficiency, reaching 49% at a catalyst dosage of 0.12 g under acidic pH conditions. Generally, increasing the catalyst dosage enhances catalytic activity by providing more active sites. However, at 0.24 g, the efficiency dropped to 25%, likely due to hydroxyl radical recombination caused by excess Fe ions, reducing the availability of reactive species for contaminant degradation [35].
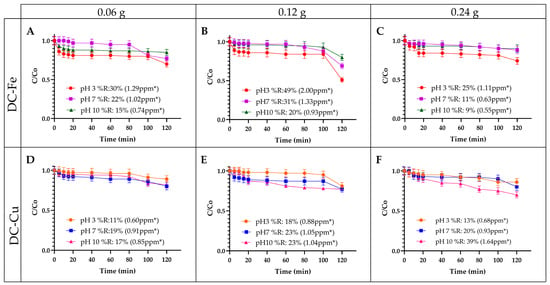
Figure 4.
Influence of catalyst amount on the removal of EE2. (A) 0.06 g of DC-Fe (B) 0.12 g of DC-Fe (C) 0.24 g of DC-Fe (D) 0.06 g of DC-Cu (E) 0.12 g of DC-Cu (F) 0.24 g of DC-Cu. Experimental conditions: contaminant concentration 5 ppm and hydrogen peroxide concentration 0.1 M. * number of ppm removed.
This trend is consistent with previous studies examining the effect of catalyst dosage on pharmaceutical removal via Fenton-like processes [54]. Additionally, reactions conducted under acidic pH conditions with Fe catalysts achieved the highest removal efficiencies. In contrast, neutral or alkaline pH conditions reduced the reactivity between Fe3+ and H2O2, leading to lower hydroxyl radical generation [50].
Figure 4D–F display the results for reactions catalyzed by Cu-based active phases (DC-Cu). Most treatments demonstrated an increase in degradation percentages as the catalyst dosage increased from 0.06 g to 0.12 g under acidic and neutral pH conditions. However, under alkaline conditions, the degradation percentage increased by 7% when the catalyst dosage was raised from 0.12 g to 0.24 g. The highest degradation percentage with DC-Cu was 30%, achieved under alkaline pH conditions. This phenomenon is attributed to an increased number of active sites for radical generation with 0.24 g [55]. This behavior is consistent with other studies that examined different catalyst dosages for removing highly recalcitrant contaminants, observing higher removal rates and percentages as the Cu-based catalyst dosage increased [56].
To identify the most efficient treatment regardless of pH, the 48 treatments were categorized into seven groups, and normality tests were conducted. Since none of the comparisons followed a normal distribution, non-parametric analyses were performed. A Kruskal–Wallis variance test was applied, followed by a modified Wilcoxon median comparison test with Bonferroni correction to account for multiple comparisons.
From these results, no statistically significant differences were observed among the delaminated clays impregnated with Cu or Fe (Figure 5). However, for Cu-impregnated clays, Treatment 3 exhibited a trend of higher contaminant removal, albeit with high data variability, whereas Treatment 2 showed less dispersion. In contrast, for Fe-impregnated clays, Treatment 2 had the highest median value, indicating the highest removal percentage. Although no statistical differences were detected, the observed trends in removal percentages align with the findings discussed throughout this document.
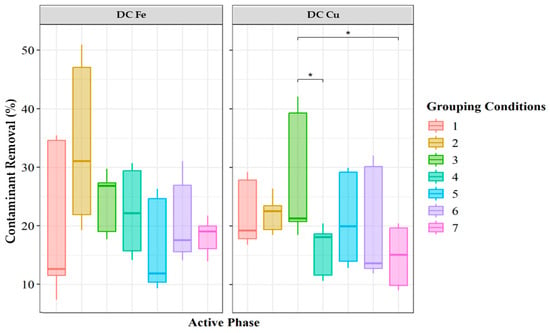
Figure 5.
Percentage removal of 17α-ethinylestradiol (EE2) under seven different catalytic conditions, each differing in contaminant concentration, hydrogen peroxide concentration, and catalyst dosage. The experimental groups were defined as follows: The grouped was: Group 1: Contaminant concentration: 5 ppm, hydrogen peroxide concentration: 0.05 M, catalyst dosage: 0.12 g. Group 2: Contaminant concentration: 5 ppm, hydrogen peroxide concentration: 0.1 M, catalyst dosage: 0.12 g. Group 3: Contaminant concentration: 5 ppm, hydrogen peroxide concentration: 0.2 M, catalyst dosage: 0.12 g. Group 4: Contaminant concentration: 5 ppm, hydrogen peroxide concentration: 0.1 M, catalyst dosage: 0.06 g. Group 5: Contaminant concentration: 5 ppm, hydrogen peroxide concentration: 0.1 M, catalyst dosage: 0.24 g. Group 6: Contaminant concentration: 1 ppm, hydrogen peroxide concentration: 0.1 M, catalyst dosage: 0.12 g. Group 7: Contaminant concentration: 10 ppm, hydrogen peroxide concentration: 0.1 M, catalyst dosage: 0.12 g. Significant differences between groups, as determined by Tukey’s test, are indicated with * (p < 0.05).
3.2. Catalytic Activity—Operational Parameters: Layered Double Hydroxides Impregnated with Fe or Cu
The operational parameters analyzed in this study included hydrogen peroxide concentration, initial contaminant load, and catalyst dosage, using layered double hydroxides (LDHs) impregnated with Fe or Cu as catalysts. All reactions involving LDH were conducted at pH 10 due to their inherent buffering capacity. LDH materials can maintain an alkaline environment by releasing or adsorbing hydroxyl ions, thereby stabilizing the pH throughout the reaction [57]. Figure 6A–C present the results obtained with Fe-impregnated layered double hydroxides (LDH-Fe), while Figure 6D–F display the results for Cu-impregnated layered double hydroxides (LDH-Cu).
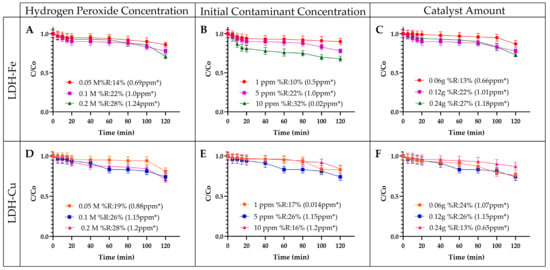
Figure 6.
Influence of operating parameters. This figure illustrates the influence of operational parameters, including hydrogen peroxide concentration (0.05 M, 0.1 M, 0.2 M), initial contaminant concentration (1 ppm, 5 ppm, 10 ppm), and catalyst dosage (0.06 g, 0.12 g, 0.24 g). Figure (A–C) present the results obtained under these conditions with LDH-Fe as the catalyst. Figure (D–F) display the outcomes using LDH-Cu as the catalytic system. * number of ppm removed.
Figure 6A,D illustrate the removal percentages of EE2 as a function of hydrogen peroxide concentration (0.05 M, 0.1 M, and 0.2 M) using LDH-Fe and LDH-Cu, respectively. In Fe-catalyzed reactions, removal efficiency increased from 14% to 28% as the hydrogen peroxide concentration rose from 0.05 M to 0.2 M. A similar trend was observed in Cu-catalyzed reactions, where removal percentages ranged from 19% to 28%. These findings align with previous studies, such as those by Melo Costa-Sergue (2021), which reported improved removal efficiency when hydrogen peroxide concentration increased from 2 mmol/L to 4 mmol/L [58]. This behavior can be attributed to the activation of hydrogen peroxide by Fe or Cu, leading to the generation of reactive oxygen species (ROS), including hydroxyl radicals (•OH), superoxide radicals (O2•−), and singlet oxygen (1O2). These ROS play a crucial role in the oxidation of the organic contaminant EE2 [59].
Figure 6B,E illustrate the impact of varying initial EE2 concentrations on removal efficiency. For LDH-Fe, increasing the EE2 concentration from 1 ppm to 10 ppm resulted in an improvement in removal efficiency, rising from 10% to 32%. In contrast, for Cu-catalyzed reactions, removal efficiency increased from 17% to 26% as the EE2 concentration increased from 1 ppm to 5 ppm. However, a further increase to 10 ppm led to a decline in removal efficiency, dropping to 16%. These findings align with previous studies investigating EE2 removal under varying contaminant concentrations. In those studies, a higher contaminant concentration generally led to increased removal percentages. However, the methodologies differed, as TiO2-based photocatalysts were used in photocatalytic processes, which yielded higher removal efficiencies than the Fenton-like process employed in this study with Fe- or Cu-impregnated laminar structures [10]. This difference is primarily due to the use of electrical currents in photocatalysis, which enhances catalytic performance by generating simultaneous photoreactions across different regions of the material, thereby accelerating reaction speed and improving overall process efficiency [11,12].
Finally, Figure 6C,F illustrate the impact of varying catalyst dosage on EE2 removal. For LDH-Fe, an increase in catalyst dosage from 0.06 g to 0.24 g led to an improvement in removal efficiency, rising from 13% to 27%. In contrast, for LDH-Cu, no significant change was observed when the dosage increased from 0.06 g to 0.12 g. However, a further increase to 0.24 g resulted in a notable decline in removal efficiency, dropping from 26% to 13%. This behavior can be attributed to the balance between reactive radical generation and radical recombination or scavenging. Within an optimal catalyst dosage range, more reactive species are produced, enhancing contaminant degradation. However, excessive catalyst loading can promote radical recombination or adsorption effects that reduce the availability of reactive species, ultimately lowering removal efficiency [60,61]. Similar trends have been reported in the literature, such as in the work of Kaur (2023), who investigated Au-supported carbon nitride-modified layered double hydroxides for pharmaceutical degradation [62].
The LDH-Fe catalysts achieved a maximum EE2 removal of 32%, while LDH-Cu reached 26%. These results align with findings from the scientific literature on the Fenton process, where the use of hydrogen peroxide alone as an oxidant typically does not exceed 50% removal efficiency. This limitation arises from the need for additional reactive species, such as sulfate radicals (SO4•−), hydroxyl radicals (•OH), and superoxide radicals (O2•−), to enhance EE2 degradation [36]. For instance, Liu (2022) reported higher removal efficiencies by incorporating sodium peroxydisulfate into a Fenton-like mechanism [63]. Similarly, He (2020) highlighted that a single treatment technology is often insufficient for effective EE2 removal [64]. Their study evaluated photolysis, electrolysis, and catalysis individually, ultimately concluding that photoelectrocatalysis was the most efficient process for degrading 17α-ethinylestradiol [64,65]. Comparing Cu- and Fe-impregnated LDH across different treatments (Figure 7) revealed significant statistical differences in Treatments 3, 4, and 5 (p < 0.01, p < 0.0001, and p < 0.01, respectively).
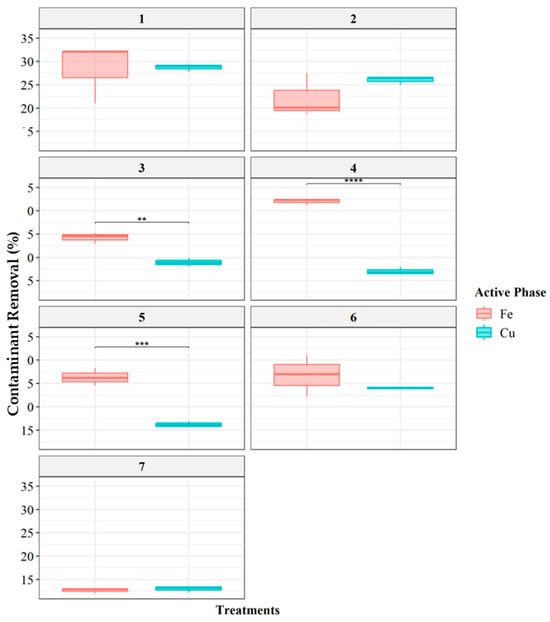
Figure 7.
Percentage removal comparison of catalytic activity for the degradation of EE2 in a Fenton-like process between Cu- and Fe-impregnated layered double hydroxides. Group 1: Contaminant concentration 5 ppm, Hydrogen peroxide concentration 0.05 M, Catalyst load 0.12 g; Group 2: Contaminant concentration 5 ppm, Hydrogen peroxide concentration 0.1 M, Catalyst load 0.12 g; Group 3: Contaminant concentration 5 ppm, Hydrogen peroxide concentration 0.2 M, Catalyst load 0.12 g; Group 4: Contaminant concentration 1 ppm, Hydrogen peroxide concentration 0.1 M, Catalyst load 0.12 g; Group 5: Contaminant concentration 10 ppm, Hydrogen peroxide concentration 0.1 M, Catalyst load 0.12 g; Group 6: Contaminant concentration 5 ppm, Hydrogen peroxide concentration 0.1 M, Catalyst load 0.06 g; Group 7: Contaminant concentration 5 ppm, Hydrogen peroxide concentration 0.1 M, Catalyst load 0.24 g. Significance levels by Tukey’s test: ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001).
The results indicate that Fe-impregnated LDH exhibits the highest EE2 degradation efficiency. The optimal conditions for achieving maximum removal include a contaminant concentration between 1 and 5 ppm, a hydrogen peroxide concentration of 0.1–0.2 M, and a catalyst dosage of 0.12 g. Under these conditions, Fe-based catalysts demonstrate superior degradation performance. For Cu-impregnated LDH, the highest degradation trend was observed at 5 ppm EE2, with hydrogen peroxide concentrations ranging between 0.05 and 1 M, and a catalyst dosage of 0.12 g. When comparing different treatments within the same catalyst type, LDH-Cu showed the highest degradation efficiency in Treatments 1 and 2, followed by Treatments 3, 4, and 5, with significant differences (p < 0.05). Similarly, for LDH-Fe, Treatments 1 and 2 exhibited significantly higher removal efficiencies compared to the other treatments (Figure 8).
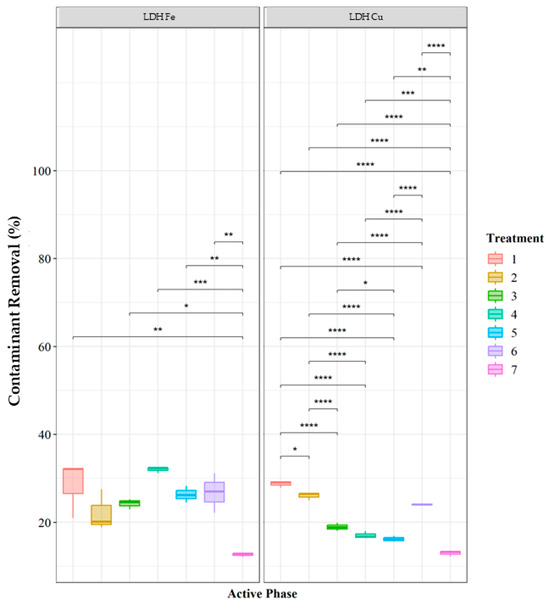
Figure 8.
Percentage removal catalytic activity for the degradation of EE2 using a Fenton-like process with a support of Cu and Fe impregnated double-layer hydroxides. Group 1: Contaminant concentration 5 ppm, hydrogen peroxide concentration 0.05 M, catalyst load 0.12 g; Group 2: Contaminant concentration 5 ppm, hydrogen peroxide concentration 0.1 M, catalyst load 0.12 g; Group 3: Contaminant concentration 5 ppm, hydrogen peroxide concentration 0.2 M, catalyst load 0.12 g; Group 4: Contaminant concentration 1 ppm, hydrogen peroxide concentration 0.1 M, catalyst load 0.12 g; Group 5: Contaminant concentration 10 ppm, hydrogen peroxide concentration 0.1 M, catalyst load 0.12 g; Group 6: Contaminant concentration 5 ppm, hydrogen peroxide concentration 0.1 M, catalyst load 0.06 g; Group 7: Contaminant concentration 5 ppm, hydrogen peroxide concentration 0.1 M, catalyst load 0.24 g. Significance values from Tukey’s test: * (p < 0.05); ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001).
3.3. Degradation Byproducts
Figure 9 illustrates the degradation pathway of ethinylestradiol (EE2) through several chemical transformation steps. The process starts with the dehydrogenation of EE2, forming an intermediate with an m/z of 297. This intermediate is then oxidized to produce a compound with an m/z of 313. Two main pathways emerge from the m/z 313 intermediate. In the first pathway, the m/z 313 intermediate undergoes further oxidation to yield a product with an m/z of 327. This product then experiences dehydroxylation, forming an intermediate with an m/z of 311, which subsequently undergoes oxidative demethylation to yield a compound with an m/z of 301. In the second pathway, the m/z 313 intermediate undergoes dehydroxylation to produce a compound with an m/z of 295. This product then undergoes another dehydroxylation to form a subproduct with an m/z of 275. These degradation mechanisms and the identified byproducts align with findings reported in the literature. For instance, Pan et al. observed similar transformation pathways during the photodegradation of EE2 using TiO2-functionalized zeolites [37]. Additionally, Dong and colleagues identified comparable m/z values during the removal of EE2 via advanced oxidation processes, such as ozonation [66].
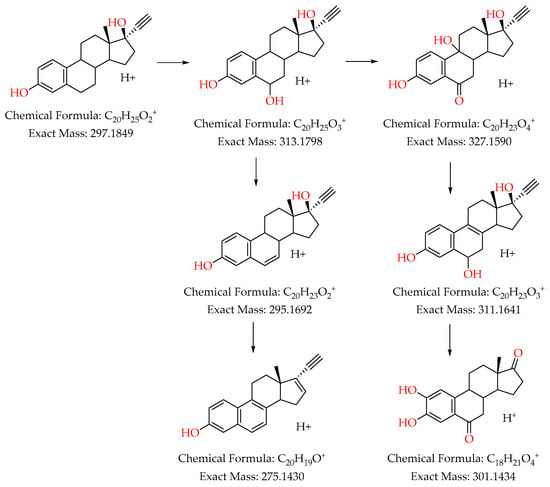
Figure 9.
Possible degradation pathway of 17α-ethinylestradiol (EE2) using Fenton-like processes catalyzed by delaminated clays impregnated with Fe.
3.4. Catalyst Reusability
Once the optimal reaction conditions were determined (Table 3), reusability studies were conducted to evaluate the long-term performance of the catalysts. Figure 10 illustrates the reusability of catalysts supported on delaminated clays (DCs) and layered double hydroxides (LDHs) over four consecutive cycles. A progressive decline in removal efficiency was observed for all materials, though the extent varied between Fe- and Cu-based systems. For instance, AD-Fe initially achieved approximately 55% removal, but this dropped to 23% by the third cycle and below 1% by the fourth. AD-Cu exhibited a similar trend, decreasing from 47% to 20%, ultimately falling below 1%. In contrast, LDH-Fe started at around 40% removal and retained approximately 2% efficiency by the third cycle. Notably, LDH-Cu demonstrated the most sustained performance, declining from 33% to 23% in the second cycle and 14% in the third, while still maintaining nearly 13% efficiency in the fourth. This decline in removal efficiency is primarily attributed to the accumulation of oxidation byproducts on the catalyst surface, which progressively blocks active sites and inhibits hydroxyl radical generation [67,68]. These byproducts may also form chelating species or deposits that reduce surface accessibility for EE2 molecules, further hindering interactions with reactive oxygen species [67].

Table 3.
Optimal operating parameters for the removal of 17α-ethinylestradiol.
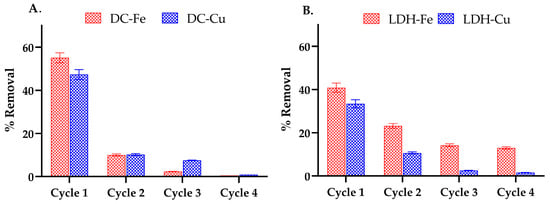
Figure 10.
Reusability of the catalysts evaluated through reuse cycles in a Fenton-like process under the best conditions (Table 3). (A) Cycles catalyzed DC-Fe and DC-Cu (DC-Fe and DC-Cu). (B) Cycles catalyzed with LDH-Fe and LDH-Cu. The amount of 17 α-ethinylestradiol removed in the first cycle was 2.2 ppm with AD-Fe, 1.9 ppm with AD-Cu, 1.6 ppm with LDH-Fe, and 1.4 ppm with LDH-Cu.
3.5. Acute Toxicity Bioassays
The microalga S. capricornutum exhibited complete inhibition (100%) in all treatments at 100% (v/v), whereas EE2 alone showed a significantly lower inhibitory effect of 31%. Inhibition progressively decreased with dilution, reaching its lowest values at 10% (v/v) for LDH-Fe (13%), LDH-Cu (37%), DC-Fe (11%), and DC-Cu (65%). Notably, DC-Cu still exhibited residual inhibition at 1% (v/v) (37%), indicating its persistent toxic effect (Figure 11A).
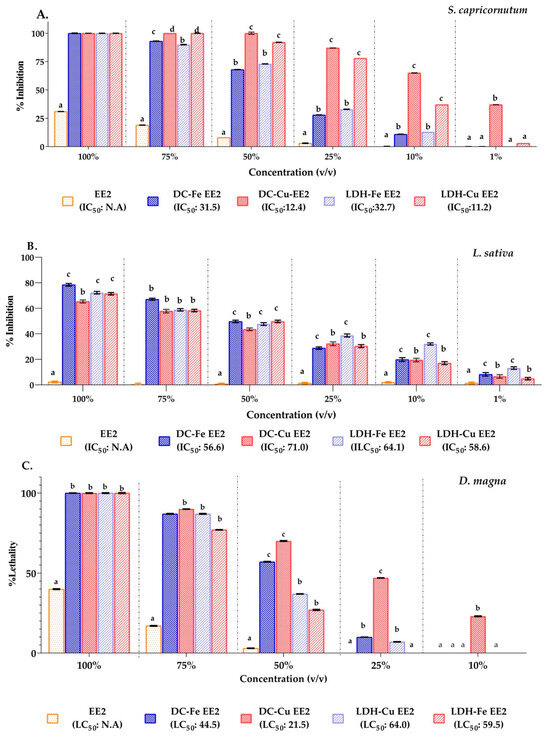
Figure 11.
Ecotoxicity results of untreated and treated effluents: (A) Inhibition percentages in S. capricornutum. (B) Inhibition percentages in L. sativa (17α-ethinylestradiol, EE2). (C) Mortality percentages in D. magna. Lowercase letters (a,b,c,d) represent grouping according to significance level using the Tukey test. This analysis was performed for each removal percentage. IC50: Inhibitory Concentration 50%. LC50: lethal concentration 50%.
The IC50 values further highlighted significant differences between treatments. Cu-based catalysts had IC50 values ranging from 11.2 to 12.4, whereas Fe-based catalysts exhibited significantly higher values (31.5–32.7). This indicates that Cu-based treatments were considerably more toxic, achieving 50% inhibition at much lower concentrations compared to Fe-based counterparts (Figure 11A).
Studies on the reduction in estrogenic activity in effluents from Wastewater Treatment Plants (WWTPs) have reported varying levels of toxicity depending on the treatment method. Silva (2021) and Rivas Ibáñez (2017) found an absence of toxicity in waters treated using Photo-Fenton processes (LED photo-Fenton and solar photo-Fenton) [69,70]. These findings were supported by toxicity assessments using Raphidocelis subcapitata and Vibrio fischeri [71].
In contrast, studies on Photo-Fenton oxidation processes and solar radiation, conducted by Frontistis (2011) [16] and Cédat (2016) [71], as well as UV-H2O and UV photolysis, classified under Effective Concentration 50% (EC50) by Velegraki (2010) [72], reported low toxicity levels in water samples before and after treatment. These studies evaluated the degradation of various hormones, including estrone (E1), 17β-estradiol (E2), and 17α-ethinylestradiol (EE2).
Lactuca sativa (L. sativa) exhibited inhibition only in response to treatments with LDH and DC impregnated with Cu and Fe. The highest inhibition was observed with LDH-Cu, reaching 17% at 10% (v/v) (IC50 = 58.6). In contrast, DC-Cu, DC-Fe, and LDH-Fe showed inhibition values ranging from 6.58% to 13.15% at 1% (v/v), with IC50 values of 71.0, 56.6, and 64.1, respectively (Figure 11B). Studies on the removal of hormones from pharmaceutical industry effluents using electrochemical processes have demonstrated a reduction in toxicity after treatment. Morais (2019) [73] reported these findings through bioassays conducted with L. sativa, Cucumis sativus, and Artemia salina.
The toxicity of 17α-ethinylestradiol (EE2) was evaluated using Daphnia magna (D. magna), revealing a concentration-dependent lethality. At 100% (v/v), EE2 alone induced a 40% mortality rate, which decreased to 17% at 75% (v/v) and 3% at 50% (v/v), with no lethality at lower concentrations. However, Cu-based catalysts exhibited significantly higher toxicity. DC-Cu caused mortality rates from 100% at 100% (v/v) to 47% at 25% (v/v), whereas LDH-Cu showed a similar decline from 100% at 100% (v/v) to 7% at 25% (v/v). Likewise, Fe-based catalysts demonstrated substantial toxicity. DC-Fe reduced lethality from 100% at 100% (v/v) to 57% at 50% (v/v), while LDH-Fe showed a decline from 100% at 100% (v/v) to 27% at 50% (v/v). At 25% (v/v) or lower concentrations, Fe-based catalysts exhibited no lethality. The 50% Lethal Concentration (LC50) values further highlighted the toxicity differences among treatments, with LDH-Cu and LDH-Fe recording LC50 values of 64.0 and 59.5, respectively, while DC-Fe and DC-Cu showed significantly lower LC50 values of 44.5 and 21.5, respectively (Figure 11C).
Test de Ames
The mutagenicity indices (MI) recorded for Salmonella Typhimurium TA100 and TA98 in response to 17α-ethinylestradiol (EE2), as well as treatments with delaminated clays (DCs) or layered double hydroxides (LDHs) impregnated with Fe and Cu, remained below 2.0. This result indicates that neither the treated water nor the contaminant itself exhibited mutagenic effects (Figure 12) [31].
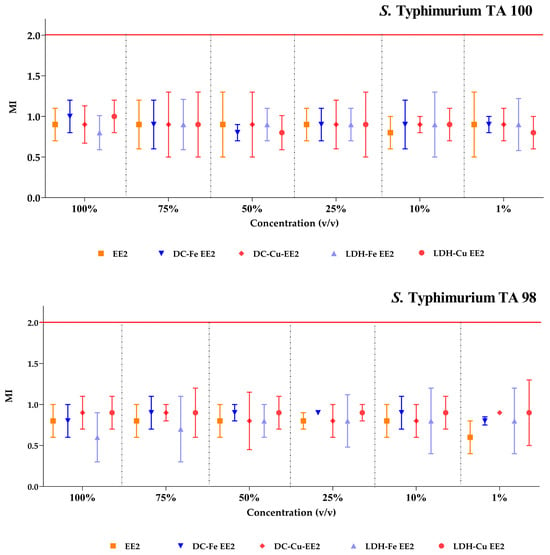
Figure 12.
Mutagenicity index (MI) in S. Typhimurium (TA98 and TA100) for treated and untreated contaminant solutions. MI refers to the mutagenicity index [31].
Consistent with these findings, Lugo (2023) [74] reported that mutagenic effects were only observed in the removal of Amoxicillin (AMX), while no such effects were detected for glyphosate. Similarly, He (2021) [75] found that advanced oxidation treatments effectively reduced secondary effects and eliminated the carcinogenic potential of water contaminated with estrogenic compounds such as benzo[a]pyrene (BaP) and 17β-estradiol (E2).
4. Conclusions
Key operational parameters, namely hydrogen peroxide concentration, initial EE2 concentration, and catalyst dosage significantly influenced the Fenton-like process using Fe- or Cu-impregnated delaminated clays and layered double hydroxides (LDHs). The highest removal efficiencies were achieved with Fe-impregnated (55%) and Cu-impregnated (47%) delaminated clays. The optimal conditions for EE2 degradation using DC-Fe were 0.1 M hydrogen peroxide, pH 3, an initial EE2 concentration of 5 ppm, and a catalyst dosage of 0.12 g. However, the incomplete degradation observed suggests that additional treatment strategies, such as adsorption or complementary advanced oxidation processes, may be required to achieve complete mineralization of EE2.
The ecotoxicological assessment revealed that S. capricornutum exhibited higher susceptibility to inhibition when exposed to LDH-Cu (IC50 = 11.2 mg/L) and DC-Cu (IC50 = 12.4 mg/L), with complete inhibition (100%) observed in Cu-based treatments at 100% (v/v). In contrast, EE2 alone caused only 31% inhibition, suggesting that oxidation byproducts and catalyst residues contribute significantly to toxicity. Although Fe-based catalysts exhibited lower toxicity, their IC50 values (31.5–32.7 mg/L) still indicate moderate inhibition, with LDH-Fe and DC-Fe treatments causing up to 73% and 68% inhibition at 50% (v/v), respectively.
In L. sativa, Cu- and Fe-impregnated LDH/DC caused moderate phytotoxicity, with IC50 values ranging from 56.6 to 71.0 mg/L. However, DC-Cu exhibited the highest IC50 (71.0 mg/L), suggesting lower toxicity compared to other catalysts. D. magna displayed high sensitivity to Cu-based catalysts, with 100% mortality at the highest concentrations, reinforcing concerns regarding their potential environmental impact. Ames test results confirmed the absence of mutagenicity in all treatments (MI < 2.0 for S. Typhimurium TA98/TA100), demonstrating that these processes do not produce genotoxic byproducts. However, the observed ecotoxicity highlights the need for additional post-treatment strategies to ensure the safe discharge of treated effluents, particularly when using Cu-based catalysts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17071043/s1.
Author Contributions
L.L. conceived and designed the study, synthesized the catalysts, conducted Fenton-like process reactions, characterized the final effluents, analyzed results, wrote the article, and edited the manuscript. C.V. processed bioassay samples, analyzed results, wrote the article, processed additional samples, and edited the manuscript. J.D. analyzed and interpreted mass spectra and determined byproducts. S.A.D.-G. conducted statistical analysis, interpreted results, and contributed to writing the article. A.B. performed diagramming, wrote the article, analyzed results, and edited the manuscript. F.-J.V., A.P.-F., S.M. and C.C.-Z. reviewed and revised the article draft. A.P.-F. and C.C.-Z. conceived and designed the study, secured funding, and supervised the research. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to express our gratitude to Pontificia Universidad Javeriana for its support through the projects “Evaluation of Solids with Lamellar Structure in the Synthesis of Fe and Cu Catalysts for the Removal of the Emerging Contaminant Amoxicillin Using the Fenton-Like Process Technique” (Project No. 20078), as well as the Research Support Project related to the Encyclical Laudato Si’ (VRI14-19).
Data Availability Statement
Data will be available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mukhopadhyay, A.; Duttagupta, S.; Mukherjee, A. Emerging Organic Contaminants in Global Community Drinking Water Sources and Supply: A Review of Occurrence, Processes and Remediation. J. Environ. Chem. Eng. 2022, 10, 107560. [Google Scholar]
- Ng, B.; Quinete, N.; Maldonado, S.; Lugo, K.; Purrinos, J.; Briceño, H.; Gardinali, P. Understanding the Occurrence and Distribution of Emerging Pollutants and Endocrine Disruptors in Sensitive Coastal South Florida Ecosystems. Sci. Total Environ. 2021, 757, 143720. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.H.; Santos, G.d.O.S.; Romanholo Ferreira, L.F.; Américo-Pinheiro, J.H.P.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Environmental Aspects of Hormones Estriol, 17β-Estradiol and 17α-Ethinylestradiol: Electrochemical Processes as next-Generation Technologies for Their Removal in Water Matrices. Chemosphere 2021, 267, 128888. [Google Scholar] [PubMed]
- Akanyeti, İ.; Kraft, A.; Ferrari, M.C. Hybrid Polystyrene Nanoparticle-Ultrafiltration System for Hormone Removal from Water. J. Water Process Eng. 2017, 17, 102–109. [Google Scholar] [CrossRef]
- Koyuncu, I.; Arikan, O.A.; Wiesner, M.R.; Rice, C. Removal of Hormones and Antibiotics by Nanofiltration Membranes. J. Memb. Sci. 2008, 309, 94–101. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Fijalkowski, K. Emerging Contaminants in Sludge (Endocrine Disruptors, Pesticides, and Pharmaceutical Residues, Including Illicit Drugs/Controlled Substances, etc.). In Industrial and Municipal Sludge: Emerging Concerns and Scope for Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 455–473. ISBN 9780128159071. [Google Scholar]
- Hu, Y.; Yan, X.; Shen, Y.; Di, M.; Wang, J. Occurrence, Behavior and Risk Assessment of Estrogens in Surface Water and Sediments from Hanjiang River, Central China. Ecotoxicology 2019, 28, 143–153. [Google Scholar] [CrossRef]
- Liu, S.; Ying, G.G.; Zhao, J.L.; Chen, F.; Yang, B.; Zhou, L.J.; Lai, H.J. Trace Analysis of 28 Steroids in Surface Water, Wastewater and Sludge Samples by Rapid Resolution Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 1367–1378. [Google Scholar] [CrossRef]
- Liu, S.; Ying, G.G.; Zhang, R.Q.; Zhou, L.J.; Lai, H.J.; Chen, Z.F. Fate and Occurrence of Steroids in Swine and Dairy Cattle Farms with Different Farming Scales and Wastes Disposal Systems. Environ. Pollut. 2012, 170, 190–201. [Google Scholar] [CrossRef]
- Shen, X.; Chang, H.; Sun, Y.; Wan, Y. Determination and Occurrence of Natural and Synthetic Glucocorticoids in Surface Waters. Environ. Int. 2020, 134, 105278. [Google Scholar] [CrossRef]
- Ammann, A.A.; Macikova, P.; Groh, K.J.; Schirmer, K.; Suter, M.J.F. LC-MS/MS Determination of Potential Endocrine Disruptors of Cortico Signalling in Rivers and Wastewaters. Anal. Bioanal. Chem. 2014, 406, 7653–7665. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, Z.H.; Wang, H.; Dang, Z.; Liu, Y. A Review of 17α-Ethynylestradiol (EE2) in Surface Water across 32 Countries: Sources, Concentrations, and Potential Estrogenic Effects. J. Environ. Manag. 2021, 292, 112804. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, E.; Dryden, I.L.; Wattis, J.A.D.; Twycross, J.; Scrimshaw, M.D.; Gomes, R.L. Modelling Emerging Pollutants in Wastewater Treatment: A Case Study Using the Pharmaceutical 17A−ethinylestradiol. Comput. Chem. Eng. 2019, 128, 477–487. [Google Scholar] [CrossRef]
- Dong, X.; He, L.; Hu, H.; Liu, N.; Gao, S.; Piao, Y. Removal of 17 β -Estradiol by Using Highly Adsorptive Magnetic Biochar Nanoparticles from Aqueous Solution. Chem. Eng. J. 2018, 352, 371–379. [Google Scholar] [CrossRef]
- Frontistis, Z.; Brebou, C.; Venieri, D.; Mantzavinos, D.; Katsaounis, A. BDD Anodic Oxidation as Tertiary Wastewater Treatment for the Removal of Emerging Micro-Pollutants, Pathogens and Organic Matter. J. Chem. Technol. Biotechnol. 2011, 86, 1233–1236. [Google Scholar] [CrossRef]
- Oliveira, H.G.; Ferreira, L.H.; Bertazzoli, R.; Longo, C. Remediation of 17-α-Ethinylestradiol Aqueous Solution by Photocatalysis and Electrochemically-Assisted Photocatalysis Using TiO2 and TiO2/WO3 Electrodes Irradiated by a Solar Simulator. Water Res. 2015, 72, 305–314. [Google Scholar] [CrossRef]
- He, H.; Huang, B.; Fu, G.; Du, Y.; Xiong, D.; Lai, C.; Pan, X. Coupling Electrochemical and Biological Methods for 17A-Ethinylestradiol Removal from Water by Different Microorganisms. J. Hazard. Mater. 2017, 340, 120–129. [Google Scholar] [CrossRef]
- Seibert, D.; Zorzo, C.F.; Borba, F.H.; de Souza, R.M.; Quesada, H.B.; Bergamasco, R.; Baptista, A.T.; Inticher, J.J. Occurrence, Statutory Guideline Values and Removal of Contaminants of Emerging Concern by Electrochemical Advanced Oxidation Processes: A Review. Sci. Total Environ. 2020, 748, 141527. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A Review on Fenton-like Processes for Organic Wastewater Treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Chaillot, D.; Bennici, S.; Brendlé, J. Layered Double Hydroxides and LDH-Derived Materials in Chosen Environmental Applications: A Review. Environ. Sci. Pollut. Res. 2021, 28, 24375–24405. [Google Scholar] [CrossRef]
- Occelli, M.L. Surface Properties and Cracking Activity of Delaminated Clay Catalysts. Catal. Today 1988, 2, 339–355. [Google Scholar] [CrossRef]
- Pérez, A.; Montes, M.; Molina, R.; Moreno, S. Modified Clays as Catalysts for the Catalytic Oxidation of Ethanol. Appl. Clay Sci. 2014, 95, 18–24. [Google Scholar] [CrossRef]
- Hincapié, G.; López, D.; Moreno, A. Infrared Analysis of Methanol Adsorption on Mixed Oxides Derived from Mg/Al Hydrotalcite Catalysts for Transesterification Reactions. Catal. Today 2018, 302, 277–285. [Google Scholar] [CrossRef]
- Rahayu, I.; Darmawan, W.; Nawawi, D.S.; Prihatini, E.; Ismail, R.; Laksono, G.D. Physical Properties of Fast-Growing Wood-Polymer Nano Composite Synthesized through TiO2 Nanoparticle Impregnation. Polymers 2022, 14, 4463. [Google Scholar] [CrossRef] [PubMed]
- Weldeslase, M.G.; Benti, N.E.; Desta, M.A.; Mekonnen, Y.S. Maximizing Biodiesel Production from Waste Cooking Oil with Lime-Based Zinc-Doped CaO Using Response Surface Methodology. Sci. Rep. 2023, 13, 4430. [Google Scholar] [CrossRef]
- Dutka, B.J. Short-Term Root Elongation Toxicity Bioassay. In Methods for Toxicological Analysis of Waters, Wastewaters and Sediments; National Water Research Institute (NWRI), Environment Canada: Burlington, ON, Canada, 1989; pp. 127–140. [Google Scholar]
- Cifuentes, A.; Silva, J.; Bay-Schmith, E.; Larrain, A. Selección de Cepas de Microalgas Para Ser Utilizadas En Bioensayos de Toxicidad. Gayana Ocean. 1998, 61, 1–9. [Google Scholar]
- Bohórquez-Echeverry, P.; Duarte-Castañeda, M.; León-López, N.; Caicedo-Carrascal, F.; Vásquez-Vásquez, M.; Campos-Pinilla, C. Selection of a Bioassay Battery to Assess Toxicity in the Affluents and Effluents of Three Water-Treatment Plants. Univ. Sci. 2012, 17, 152–166. [Google Scholar]
- Williams, L.; Preston, J. Interim Procedures for Conducting the ’Salmonella’/Microsomal Mutagenicity Assay (Ames Test); U.S. Environmental Protection Agency: Washington, DC, USA, 1983; EPA/600/4-82/068 (NTIS PB88205380). Available online: https://archive.epa.gov/region1/info/testmethods/web/pdf/ames-test.pdf (accessed on 18 August 2024).
- Ames, B.N.; Kammen, H.O.; Yamasaki, E. Hair Dyes Are Mutagenic: Identification of a Variety of Mutagenic Ingredients. Proc. Natl. Acad. Sci. USA 1975, 72, 2423–2427. [Google Scholar]
- Feng, X.; Long, R.; Wang, L.; Liu, C.; Bai, Z.; Liu, X. A Review on Heavy Metal Ions Adsorption from Water by Layered Double Hydroxide and Its Composites. Sep. Purif. Technol. 2022, 284, 120099. [Google Scholar]
- Zhao, T.; Xu, S.; Hao, F. Differential Adsorption of Clay Minerals: Implications for Organic Matter Enrichment. Earth Sci. Rev. 2023, 246, 104598. [Google Scholar]
- Feng, Y.; Zhang, Z.; Gao, P.; Su, H.; Yu, Y.; Ren, N. Adsorption Behavior of EE2 (17α-Ethinylestradiol) onto the Inactivated Sewage Sludge: Kinetics, Thermodynamics and Influence Factors. J. Hazard. Mater. 2010, 175, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, Y.; Wang, C.; Guo, R.; You, J.; Zhang, H. Optimizing the Performance of Fe-Based Metal-Organic Frameworks in Photo-Fenton Processes: Mechanisms, Strategies and Prospects. Chemosphere 2023, 339, 139673. [Google Scholar]
- Zhao, Z. Hydroxyl Radical Generations Form the Physiologically Relevant Fenton-like Reactions. Free Radic. Biol. Med. 2023, 208, 510–515. [Google Scholar] [PubMed]
- Pan, Z.; Stemmler, E.A.; Cho, H.J.; Fan, W.; LeBlanc, L.A.; Patterson, H.H.; Amirbahman, A. Photocatalytic Degradation of 17α-Ethinylestradiol (EE2) in the Presence of TiO2-Doped Zeolite. J. Hazard. Mater. 2014, 279, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Xiunan, C.; Ling, T.; Meifei, C.; Yijun, L.; Wei, W.; Junhao, L.; Yanjuan, Z.; Gan, T.; Huayu, H.; Zuqiang, H. Construction of a C-Decorated and Cu-Doped (Fe,Cu)S/CuFe2O4 Solid Solution for Photo-Fenton Degradation of Hydrophobic Organic Contaminant: Enhanced Electron Transfer and Adsorption Capacity. Chemosphere 2022, 296, 134005. [Google Scholar] [CrossRef]
- Ghosh, S.; Pourebrahimi, S.; Malloum, A.; Ajala, O.J.; AlKafaas, S.S.; Onyeaka, H.; Nnaji, N.D.; Oroke, A.; Bornman, C.; Christian, O.; et al. A Review on Ciprofloxacin Removal from Wastewater as a Pharmaceutical Contaminant: Covering Adsorption to Advanced Oxidation Processes to Computational Studies. Mater. Today Commun. 2023, 37, 107500. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent Advances in Nano-Fenton Catalytic Degradation of Emerging Pharmaceutical Contaminants. J. Mol. Liq. 2019, 290, 111177. [Google Scholar]
- Zhuravlev, L.T. The Surface Chemistry of Amorphous Silica. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar]
- Bernal, V.; Erto, A.; Giraldo, L.; Moreno-Piraján, J.C. Effect of Solution PH on the Adsorption of Paracetamol on Chemically Modified Activated Carbons. Molecules 2017, 22, 1032. [Google Scholar] [CrossRef]
- Shanmugavel, S.P.; Kumar, G. Recent Progress in Mineralization of Emerging Contaminants by Advanced Oxidation Process: A Review. Environ. Pollut. 2024, 341, 122842. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L. Kinetic Study of Hydroxyl Radical Formation in a Continuous Hydroxyl Generation System. RSC Adv. 2018, 8, 40632–40638. [Google Scholar] [CrossRef]
- Yang, C.; Xu, G.; Liu, Q. Adsorption and Heterogenous Fenton-like Reactivity of NH2-MIL-88B towards Toxic Dyes Removal. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132405. [Google Scholar] [CrossRef]
- Li, Z.; Liu, R.; Gao, W.; Zhang, W.; Li, C.; Liu, X.; Wang, N. CuFe2O4/Penicillin Residue Biochar Heterogeneous Fenton-like Catalyst Used in the Treatment of Antibiotic Wastewater: Synthesis, Performance and Working Mechanism. J. Water Process Eng. 2023, 55, 104124. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Zhang, Y.; Xu, X.; Liu, Y.; Zhang, Y.; He, Z.; Wang, J.; Liang, Y. Effect of Interaction between Dissolved Organic Matter and Iron/Manganese (Hydrogen) Oxides on the Degradation of Organic Pollutants by in-Situ Advanced Oxidation Techniques. Sci. Total Environ. 2024, 918, 170351. [Google Scholar] [CrossRef] [PubMed]
- Amedlous, A.; Amadine, O.; Essamlali, Y.; Maati, H.; Semlal, N.; Zahouily, M. Copper Loaded Hydroxyapatite Nanoparticles as Eco-Friendly Fenton-like Catalyst to Effectively Remove Organic Dyes. J. Environ. Chem. Eng. 2021, 9, 105501. [Google Scholar] [CrossRef]
- Silva, M.; Baltrus, J.P.; Williams, C.; Knopf, A.; Zhang, L.; Baltrusaitis, J. Heterogeneous Photo-Fenton-like Degradation of Emerging Pharmaceutical Contaminants in Wastewater Using Cu-Doped MgO Nanoparticles. Appl. Catal. A Gen. 2022, 630, 118468. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Sun, Q.; Yuan, M.; Sun, Z.; Chen, L.; Zhang, Y.; Xia, S.; Zhao, J. Enhanced Activation of Peroxymonosulfate by a Floating FeMo3Ox/C3N4 Photocatalyst under Visible-Light Assistance for Oxytetracycline Degradation: Performance, Mechanisms and Comparison with H2O2 Activation. Environ. Pollut. 2023, 316, 120668. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemannt, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Park, C.M.; Shim, J.J. Ultrasound-Assisted Heterogeneous Fenton-like Process for Efficient Degradation of Tetracycline over SmFeO3/Ti3C2Tx Catalyst. J. Water Process Eng. 2022, 50, 103235. [Google Scholar] [CrossRef]
- Qiao, X.; Xu, Y.; Liu, X.; Chen, S.; Zhong, Z.; Li, Y.; Lü, J. Nitrogen–Doped Titanium Dioxide/Schwertmannite Nanocomposites as Heterogeneous Photo–Fenton Catalysts with Enhanced Efficiency for the Degradation of Bisphenol A. J. Environ. Sci. 2023, 143, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, F.; Wu, J.; Huang, Z.; Song, L.; Yuan, S. Full Utilization of Cu Single Atoms on Carbon Nitride Nanofibers for Enhanced Fenton-like Degradation of Methylene Blue. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132708. [Google Scholar] [CrossRef]
- Goh, K.H.; Lim, T.T.; Dong, Z. Application of Layered Double Hydroxides for Removal of Oxyanions: A Review. Water Res. 2008, 42, 1343–1368. [Google Scholar]
- de Melo Costa-Serge, N.; Gonçalves, R.G.L.; Rojas-Mantilla, H.D.; Santilli, C.V.; Hammer, P.; Nogueira, R.F.P. Fenton-like Degradation of Sulfathiazole Using Copper-Modified MgFe-CO3 Layered Double Hydroxide. J. Hazard. Mater. 2021, 413, 125388. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, L.; Ye, J.; Li, N.; Yan, B.; Chen, G. Influence and Mechanism of Water Matrices on H2O2-Based Fenton-like Oxidation Processes: A Review. Sci. Total Environ. 2023, 888, 164086. [Google Scholar]
- Ali, J.; Li, D.; Shahzad, A.; Wajid Ullah, M.; Ifthikar, J.; Asif, M.; Yanan, C.; Lei, X.; Chen, Z.; Wang, S. MoS4-LDH: A Dual Centre Fe-Based Layered Double Hydroxide Catalyst for Efficient Atrazine Removal and Peroxymonsulfate Activation. Chem. Eng. J. 2023, 456, 141161. [Google Scholar] [CrossRef]
- Gonçalves, R.G.L.; Mendes, H.M.; Bastos, S.L.; D’Agostino, L.C.; Tronto, J.; Pulcinelli, S.H.; Santilli, C.V.; Neto, J.L. Fenton-like Degradation of Methylene Blue Using Mg/Fe and MnMg/Fe Layered Double Hydroxides as Reusable Catalysts. Appl. Clay Sci. 2020, 187, 105477. [Google Scholar] [CrossRef]
- Kaur, J.; Pal, B.; Singh, S.; Kaur, H. Fabrication of Highly Efficient Au and Layered Double Hydroxide Modified G-C3N4 Ternary Composites for Degradation of Pharmaceutical Drug: Pathways and Mechanism. Surf. Interfaces 2023, 36, 102583. [Google Scholar] [CrossRef]
- Liu, M.; Lin, M.; Owens, G.; Chen, Z. Fenton-like Oxidation Mechanism for Simultaneous Removal of Estriol and Ethinyl Estradiol by Green Synthesized Mn3O4 NPs. Sep. Purif. Technol. 2022, 301, 121978. [Google Scholar] [CrossRef]
- He, H.; Sun, S.; Gao, J.; Huang, B.; Zhao, T.; Deng, H.; Wang, X.; Pan, X. Photoelectrocatalytic Simultaneous Removal of 17α-Ethinylestradiol and E. Coli Using the Anode of Ag and SnO2-Sb 3D-Loaded TiO2 Nanotube Arrays. J. Hazard. Mater. 2020, 398, 122805. [Google Scholar] [CrossRef]
- Du, X.; Liu, L.; Dong, Z.; Cui, Z.; Sun, X.; Wu, D.; Ma, Z.; Fang, Z.; Liu, Y.; An, Y. Accelerated Redox Cycles of Fe(III)/Fe(II) and Cu(III)/Cu(II) by Photo-Induced Electron from n-Cqds for Enhanced Photo-Fenton Capability of CuFe-LDH. Catalysts 2020, 10, 960. [Google Scholar] [CrossRef]
- Dong, X.; Yu, S.; Yang, W.; Cheng, L.; Tang, Y.; Chen, D. Homogeneous Oxidation of EE2 by Ozone: Influencing Factors, Degradation Pathway, and Toxicity Assessment. J. Environ. Chem. Eng. 2024, 12, 112360. [Google Scholar] [CrossRef]
- Amiri, S.; Anbia, M. Insights into the Effect of Parameters and Pathway of Visible-Light Photodegradation of Glyphosate and Diazinon by C-TiO2/Clinoptilolite Nanocomposite. J. Photochem. Photobiol. A Chem. 2024, 446, 115146. [Google Scholar] [CrossRef]
- Barzoki, H.R.; Dargahi, A.; Shabanloo, A.; Ansari, A.; Bairami, S. Electrochemical Advanced Oxidation of 2,4-D Herbicide and Real Pesticide Wastewater with an Integrated Anodic Oxidation/Heterogeneous Electro-Fenton Process. J. Water Process Eng. 2023, 56, 104429. [Google Scholar] [CrossRef]
- Silva, M.; Baltrusaitis, J. Destruction of Emerging Organophosphate Contaminants in Wastewater Using the Heterogeneous Iron-Based Photo-Fenton-like Process. J. Hazard. Mater. Lett. 2021, 2, 100012. [Google Scholar]
- Rivas Ibáñez, G.; Bittner, M.; Toušová, Z.; Campos-Mañas, M.C.; Agüera, A.; Casas López, J.L.; Sánchez Pérez, J.A.; Hilscherová, K. Does Micropollutant Removal by Solar Photo-Fenton Reduce Ecotoxicity in Municipal Wastewater? A Comprehensive Study at Pilot Scale Open Reactors. J. Chem. Technol. Biotechnol. 2017, 92, 2114–2122. [Google Scholar] [CrossRef]
- Cédat, B.; de Brauer, C.; Métivier, H.; Dumont, N.; Tutundjan, R. Are UV Photolysis and UV/H2O2 Process Efficient to Treat Estrogens in Waters? Chemical and Biological Assessment at Pilot Scale. Water Res. 2016, 100, 357–366. [Google Scholar] [CrossRef]
- Velegraki, T.; Balayiannis, G.; Diamadopoulos, E.; Katsaounis, A.; Mantzavinos, D. Electrochemical Oxidation of Benzoic Acid in Water over Boron-Doped Diamond Electrodes: Statistical Analysis of Key Operating Parameters, Kinetic Modeling, Reaction by-Products and Ecotoxicity. Chem. Eng. J. 2010, 160, 538–548. [Google Scholar] [CrossRef]
- Morais, R.L.; Santiago, M.F.; Zang, J.W.; Fonseca-Zang, W.A.; Schimidt, F. Removal of Synthetic Sex Hormones by Hydrothermal Carbonization. Acad. Bras. Cienc. 2018, 90, 1327–1336. [Google Scholar] [CrossRef]
- Lugo, L.; Venegas, C.; Guarin Trujillo, E.; Diaz Granados-Ramírez, M.A.; Martin, A.; Vesga, F.J.; Pérez-Flórez, A.; Celis, C. Ecotoxicology Evaluation of a Fenton—Type Process Catalyzed with Lamellar Structures Impregnated with Fe or Cu for the Removal of Amoxicillin and Glyphosate. Int. J. Environ. Res. Public. Health 2023, 20, 7172. [Google Scholar] [CrossRef]
- He, Y.; Patterson-Fortin, L.; Boutros, J.; Smith, R.; Goss, G.G. Removal of Biological Effects of Organic Pollutants in Municipal Wastewater by a Novel Advanced Oxidation System. J. Environ. Manag. 2021, 280, 111855. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).