Abstract
This study investigated the photocatalytic degradation of chlorothalonil under a range of ultraviolet lamp configurations, and studied the improvement in the photocatalytic degradation efficiency of a reflective material (silver-white aluminium foil). Increasing the number of UV lamps significantly enhanced degradation efficiency, reducing the half-life from 29.95 min with one lamp to 8.15 min with four in a 20 cm enamel bucket. The use of silvery-white aluminium foil further decreased the half-life to 3.86 min, improving degradation rates by up to 262.9%. In larger containers, degradation efficiency increased by up to 414.7% with aluminium foil. Comparisons with black aluminium foil confirmed that silver-white aluminium foil enhanced degradation by reflecting and redistributing UV light, increasing intensity by 252% and reducing the CTL half-life from 150.36 min to 22.9 min in a controlled light box. Further tests confirmed that silver-white aluminium foil amplified UV irradiation, increasing degradation efficiency by up to 555.1%. These improvements might suggest that aluminium foil enhances UV utilisation through direct reflection, refraction, and diffuse reflection, effectively redirecting photons that would otherwise escape the system. Experiments with natural water sources showed similar trends, with half-lives of 55.23 min in ultrapure water, 12.63 min in pond water, and 16.36 min in paddy field water. The addition of silver-white aluminium foil further reduced these times to 23.92 min, 7.13 min, and 12.34 min, respectively. These findings demonstrate that silvery-white aluminium foil significantly enhances CTL photodegradation without increasing energy consumption. While effective, the method faces challenges in acidic or alkaline wastewater due to potential corrosion of system components. Future research should focus on identifying stable, high-reflectivity materials for long-term applications. This study offers practical insights into the optimisation of photodegradation processes, which contributes to improved water treatment strategies and environmental pollution mitigation.
1. Introduction
Chlorothalonil (CTL) is a fungicide with a broad spectrum of activity, it is frequently employed in the prevention and control of fungal diseases in horticultural crops and fruits [1]. Furthermore, CTL has been shown to play a pivotal role in the sterilisation process in a variety of industrial contexts, including wood and paint preservation [2]. In the field of agriculture, CTL has become an indispensable product due to its efficacy in controlling a wide range of crop diseases [3]. The cost-effective production and efficient performance of this process have driven a significant increase in both production and usage, with annual output now surpassing one million kilograms [4]. Consequently, the environmental accumulation of CTL cannot be disregarded [4]. According to the CCME report, the range of CTL concentrations in Canada’s Black Brook Watershed was from 0.1 to 1851.0 μg/L, which exceeded the maximum allowable average concentration for aquatic organisms by 12.9% [5]. In a similar vein, elevated concentrations of CTL were detected in the water and sediment of the Blackwater Estuary in Essex, UK, ranging from 0.36 μg/L to 45.6 μg/L [6]. The environmental impact of CTL has raised concerns due to its persistence and accumulation in various habitats [7]. It has a long half-life in soil and its degradation is significantly influenced by soil properties [8]. CTL can also enter water bodies through runoff, spray drift, or atmospheric deposition [9], leading to potential toxicity in aquatic ecosystems, particularly to marine organisms like crustaceans, invertebrates, and fish [10]. Studies show that CTL induces oxidative stress in the gill tissues of molluscs and affects enzyme activity, such as sodium/potassium ATPase and acetylcholinesterase [11]. In the study of amphibians, CTL exposure alters cortisol levels, immune function, and mortality, with both low and high doses having nonlinear toxic effects [12]. Its impact on the early life stages of Atlantic salmon is also notable, where higher doses reduce hatching rates and increase malformations [13]. It is important to note that CTL poses a significant risk to human health as a potent contact allergen. Workers in the flower cultivation industry have been observed to exhibit allergic dermatitis and conjunctivitis [14]. Similar effects are observed in textiles treated with CTL [15]. Additionally, CTL has been detected in maternal and umbilical cord serum, suggesting potential transfer to the fetus and possible impacts on fetal health and development [16].
In the environment, agrochemical degradation can be broken down into two main categories: microbial and photochemical [17]. The final products of the microbial degradation of agrochemicals are mostly low toxicity, but their degradation efficiency is affected by various environmental factors, making it difficult to achieve efficient application in production [18]. Photochemical degradation is defined as the process of converting agrochemicals into harmless substances through the application of light energy [19]. This process typically occurs on soil surfaces or in water and is influenced by environmental factors such as light intensity, wavelength, temperature, and pH [20,21]. The absorption of light energy by agrochemical molecules can result in a number of chemical reactions, including bond breakage, cyclisation, or structural rearrangement. These reactions ultimately lead to the decomposition of agrochemicals into smaller, non-harmful compounds. Furthermore, the interaction of agrochemicals with photosensitisers, such as nitrate and humic acid, or active substances, such as hydroxyl radicals (∙OH) and singlet oxygen (1 O2) [22,23,24], have been shown to promote the decomposition of agrochemicals. Photochemical degradation has been demonstrated to be an effective method for the breakdown of various types of agrochemicals, including organophosphorus, organochlorine, and sulfonylurea herbicides [25]. Studies have shown that photocatalytically treated agricultural wastewater can meet irrigation standards, with nutrients such as nitrogen and phosphorus being effectively absorbed and utilised by plants [26,27]. Increasing the utilisation rate of light is an effective and cost-effective way to improve the efficiency of photocatalysis. Research has demonstrated that different materials exhibit significant variations in their ability to reflect light across different wavelengths [28]. Theoretical studies indicate that aluminium exhibits a reflectivity of over 88% in the ultraviolet (UV) region, thus rendering it a promising material for the optimisation of light utilisation in photocatalysis [29]. Moreover, replacing galvanized steel with aluminium as a reflective material has been shown to significantly increase UV radiation intensity [30]. Despite these advancements, however, there is currently no research on the use of aluminium to enhance the photocatalytic degradation of organic agrochemicals pollutants.
This study examines the effects of light intensity, wavelength range, and reflective materials on the photocatalytic degradation of CTL under controlled water quality and temperature conditions. Light intensity and wavelength are key factors in photocatalytic efficiency, while reflective materials also play a significant role [31]. The high UV reflectivity of aluminium is expected to increase ultraviolet light intensity in the reaction zone, enhancing CTL interaction with light energy and accelerating the degradation process [32]. This study evaluates aluminium as a reflective material to improve light utilization and degradation efficiency. Additionally, experiments with real water sources are conducted to examine the effect of water quality variations on CTL degradation, unlike traditional photolysis methods, which have limitations such as short lifetimes of photolysis components or secondary light pollution [33]. The aim of this study is to evaluate the effect of light on CTL degradation and to establish a model of an independent closed photodegradation system. This model may have wider applicability and practicality. These findings will inform the optimization of photocatalytic processes, contributing to advances in water treatment technologies and supporting sustainable water resource management and agricultural wastewater treatment.
2. Materials and Methods
2.1. Purpose of Construction
The objective of this study is to develop a simulation platform for mobile water sources to systematically examine the factors influencing the photocatalytic degradation of CTL. By controlling light conditions, reflective materials, and water flow dynamics, the platform enables precise adjustments to variables such as water quality, temperature, light intensity, and coverage. This controlled environment facilitates a more nuanced analysis of how these factors affect degradation efficiency. Additionally, the study places particular emphasis on the role of reflective materials, with a view to identifying optimisation strategies for enhancing CTL photocatalytic degradation.
2.2. Equipment Set-Up
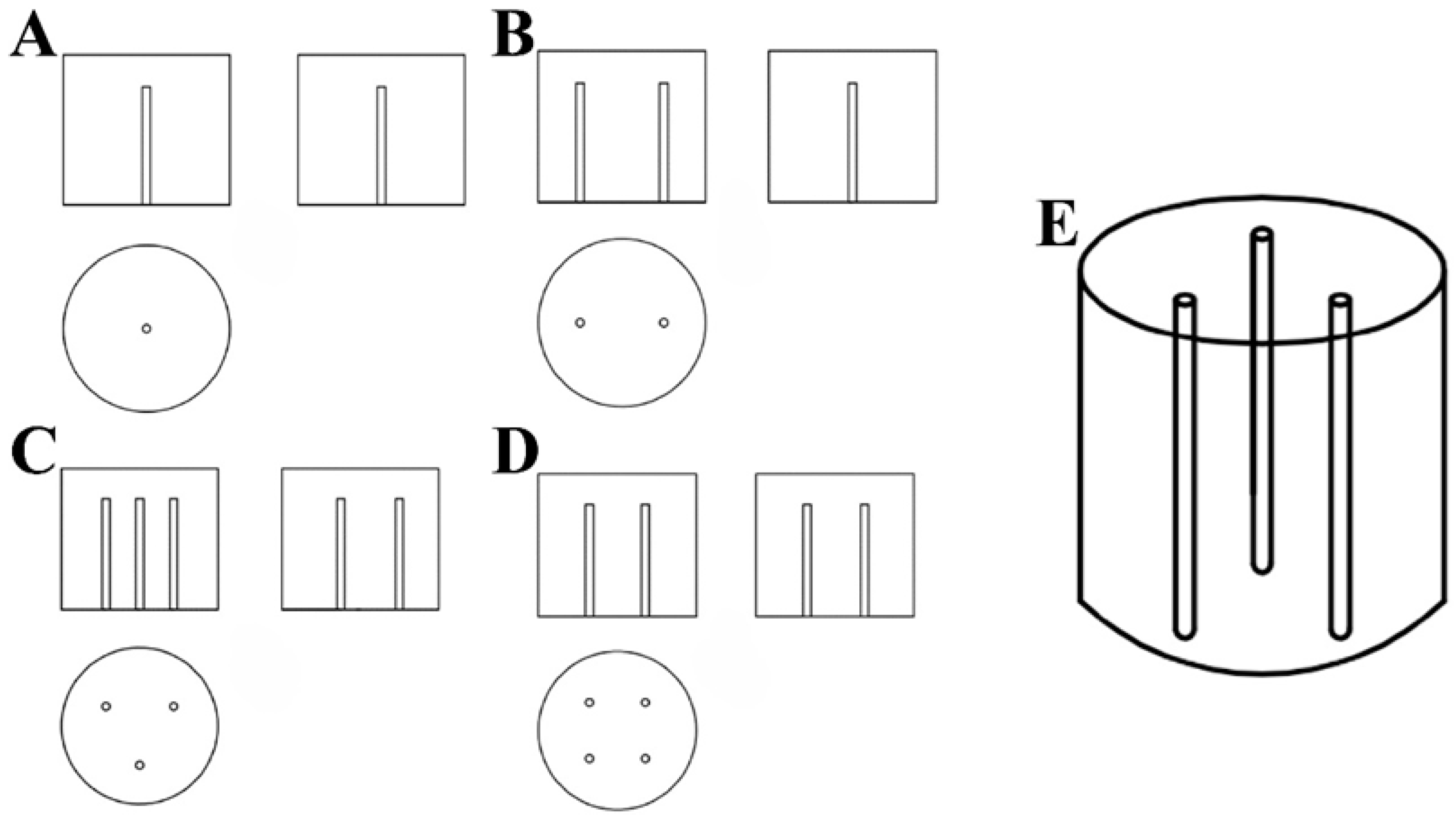
In this experiment, a UV lamp with a 254 nm wavelength (the maximum absorption wavelength of CTL was 232 nm) (Supplementary Figure S1) was utilised as the light source. The lamp tube measured 212 mm in length and had a power output of 10 W. It was fully sealed within a quartz tube, allowing it to be safely immersed in water while maintaining stable light output. This configuration guaranteed the uniformity of UV radiation throughout the experiment, thereby optimising conditions for photochemical reactions. The reaction vessel was a white enamel bucket made of cast iron, coated with an enamel glaze for strong resistance to acids and alkalis and ease of cleaning. Buckets with diameters of 20 cm, 30 cm, and 40 cm were utilised to accommodate varying volumes of reaction solution. For the UV irradiation experiments, a CTL standard solution was diluted to a target concentration of 1.88 μmol/L (the maximum solubility of CTL in water is 0.6 ppm) [34]. The reaction solution volume was adjusted to ensure the UV lamp was fully submerged, thereby facilitating uniform and comprehensive irradiation. The experimental design regulated the installation of the UV lamp, ensuring uniform light distribution and enhancing the precision of the experiment. The configurations are outlined below: Single-Lamp Configuration Figure 1A: A single UV lamp was installed vertically at the centre of the enamel bucket, providing a centralised light source that served as the control for comparative analysis with other configurations. Two-Lamp Configuration Figure 1B: Two UV lamps were installed vertically at the midpoint of the circle’s radius. This configuration ensured uniform illumination over a larger reaction area while maintaining high light intensity at the centre. Three-Lamp Configuration Figure 1C,E: Three UV lamps were installed vertically at the midpoint of the lines connecting the vertices of an equilateral triangle to the centre of the circle. This configuration aimed to achieve more balanced UV distribution and assess the impact of uneven light exposure on the reaction rate. Four-Lamp Configuration Figure 1D: Four UV lamps were installed vertically at the midpoint of the lines connecting the vertices of a square to the centre of the circle. This configuration enhanced irradiation uniformity and ensured comprehensive coverage of a wider reaction area. A mechanical stirrer operating at 240 r/min was utilised to ensure a uniform flow of the reaction solution. This configuration was designed to replicate the dynamic conditions typically observed in natural water bodies, thereby ensuring uniform exposure to UV light while preventing localised shading due to stagnant liquid. To investigate the effect of reflective surfaces on UV irradiation, two types of aluminium foil were applied to the inner walls of the UV light boxes: black and silver-white. The black aluminium foil minimised light reflection, while the silver-white foil, with its high reflectivity, enhanced UV light efficiency. These configurations, designated as black aluminium foil boxes and silver-white aluminium foil boxes, respectively, facilitated a comprehensive study of how different reflective properties influence the photocatalytic degradation of CTL. The light intensity of different diameters of barrels and different inner wall materials irradiated by different amounts of ultraviolet lamps is shown in Table 1. The specifications of the materials used are in accordance with Supplementary Table S1.
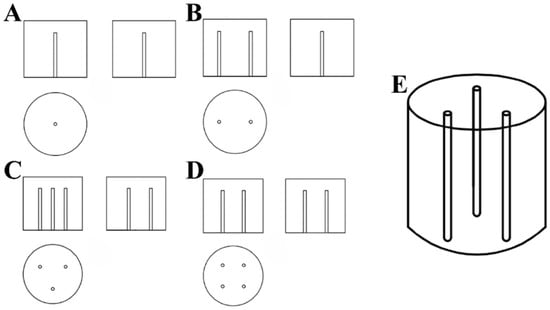
Figure 1.
Three-view diagram of a single UV lamp (A). Three-view diagram of two UV lamps (B). Three-view diagram of three UV lamps (C). Three-view diagram of four UV lamps (D). Installation simulation of three UV lamps (E).

Table 1.
The light intensities of different diameter barrels and different inner wall materials were irradiated by different numbers of UV lamps.
2.3. Sampling Method
The Bucket Sampling Method was employed, whereby the enamel bucket containing the prepared reaction solution was placed in a dark environment as a control. Following UV illumination, 5 mL of the reaction solution was sampled and transferred to a glass colorimetric tube. An equal volume of acetonitrile (5 mL) was then added, and the mixture was thoroughly blended using a vortex analyser. The solution was then filtered through a 0.22 μm PTEF aqueous filter membrane and transferred to an injection bottle. It should be noted that each sample was prepared in three parallel treatments for accuracy. The Box Sampling Method involved the use of a 100 mL volumetric flask to prepare the CTL reaction solution at a concentration of 1.88 μmol/L. The solution was then diluted with pure water and sonicated for three min to ensure uniform mixing. A 5 mL sample of the prepared solution was transferred into a stoppered quartz tube with an inner wall thickness of 3 mm and placed under UV illumination, with a dark control group set up separately. Following this, 5 mL of acetonitrile was added to each quartz tube. The mixture was then blended using a vortex analyser, filtered through a 0.22 μm PTEF aqueous filter membrane, and transferred to an injection bottle, with three parallel treatments for consistency.
2.4. Different Water Quality
In order to comprehensively evaluate the impact of different water source conditions on the photocatalytic degradation effect of CTL, a pond and a rice field in the Hefei area were selected. A total of 75 L of each water sample was collected and stored in a 4 °C refrigerated room for future use. Prior to the commencement of the experiment, the pure water, pond water, and paddy field water samples were retrieved and placed at a room temperature of 25 °C before being utilised in reaction experiments. The physicochemical properties of the rice paddy and pond water are shown in Table 2.

Table 2.
Properties of paddy, farm ditch, and pond waters.
2.5. Sample Analysis
CTL and 2,4,5-trichlorobenzonitrile were analysed with a Waters e2695 HPLC system (Waters Corporation, Milford, MA, USA), which was equipped with an Agilent XDB-C18 column (4.6 mm × 250 mm, 5 μm) at 30 °C and a UV absorbance detector operated at 232 nm and 242 nm, respectively. CTL and 2,4,5-trichloroisophtalonitrile were eluted with a mixture of 60:40 (v/v) acetonitrile/water,. The flow rate was kept at 0.7 mL/min and the column temperature was at 30 °C. The limit of detection of CTL and 2,4,5-trichloroisophtalonitrile was 0.01 mg/L.
2.6. Calculation
The photodegradation half-life () can be calculated according to the first-order model:
where and are the initial concentrations at = 0 and the CTL residual concentration at time after irradiation, respectively; is the degradation constant.
Photodegradation efficiency was expressed as:
where and represent reaction rate constants of CTL with and without SL, respectively.
2.7. Statistical Analysis of Data
In this study, all recorded data were from three parallel experimental groups. The data were analysed using SPSS (Version 20.0) statistical software, using one-way ANOVA (Duncan’s multiple range tests) for significance testing (p < 0.05). All data are presented in the form of mean ± standard deviation.
3. Results and Discussion
3.1. Photodegradation of CTL Irradiated with Different Numbers of UV Lamps in Different Diameter Barrels and Different Inner Wall Materials
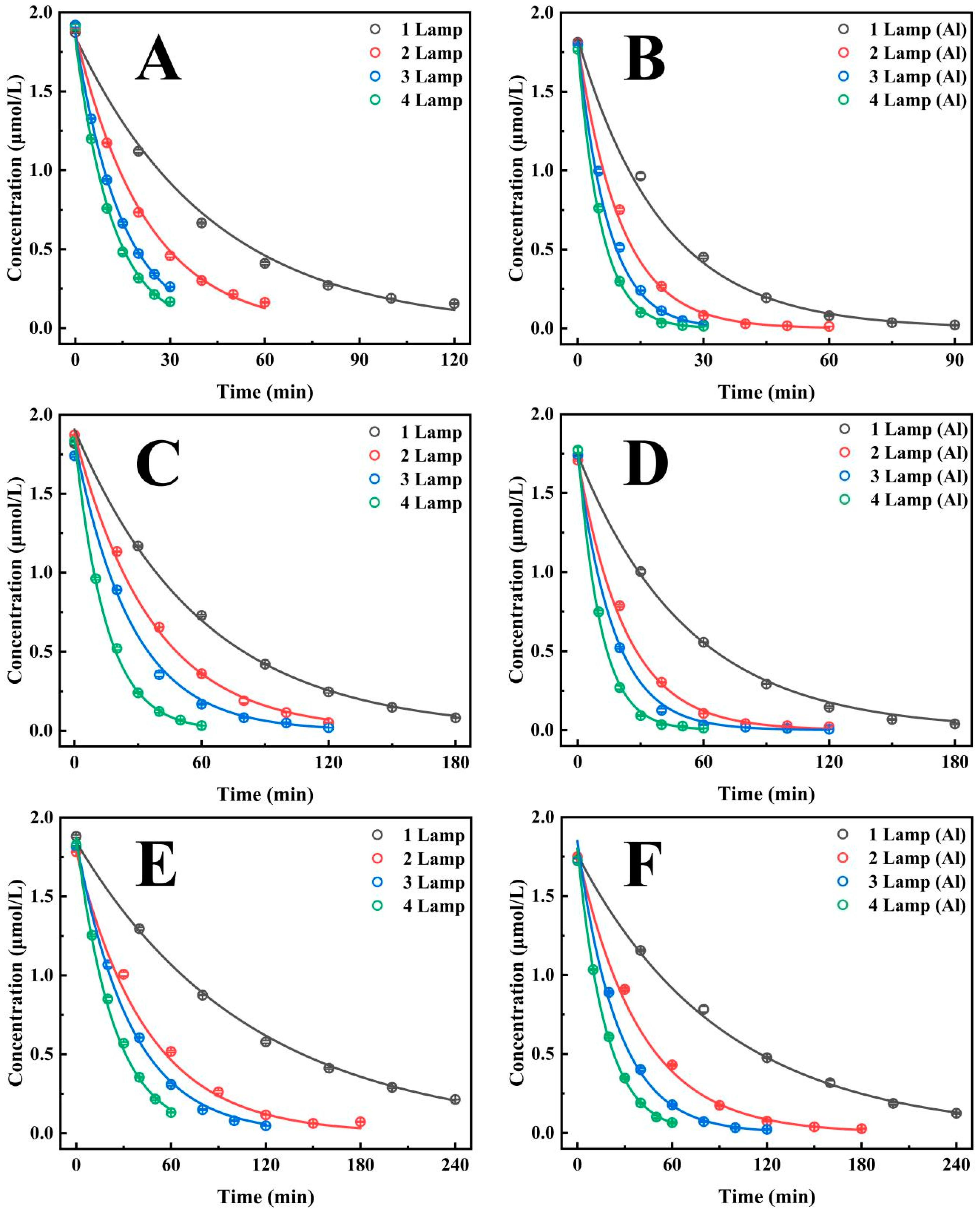
As illustrated in Figure 2A, the half-life of CTL degradation in an enamel bucket with a diameter of 20 cm is shown as a function of the number of UV lamps (Supplementary Table S2). The results show that the degradation rate decreases with increasing numbers of lamps, from 29.95 min for a single lamp to 8.15 min for four lamps. It is evident that as the number of lamps increases, the degradation rate of CTL experiences a significant enhancement, with acceleration rates of 93.6%, 193.8%, and 267.6% observed for lamp ratios of 2:1, 3:1, and 4:1, respectively. These findings indicate that augmenting the quantity of UV lamps has a substantial impact on the acceleration of the CTL degradation process. Moreover, Figure 2B (Supplementary Table S2) demonstrates that following the incorporation of silver-white aluminium foil, the half-life of CTL degradation was diminished to 14.01 min with a single UV lamp, 7.13 min with two, 4.98 min with three, and 3.86 min with four. The degradation rate increased by 96.5%, 180.9%, and 262.9% for two, three, and four lamps, respectively, in comparison with single-lamp irradiation. These findings further confirm that increasing the number of UV lamps significantly accelerates the CTL degradation process. A comparison between the silver-white aluminium foil and enamel inner walls revealed that the former significantly outperformed the latter in terms of degradation efficiency under the same number of UV lamps. Specifically, the degradation rates for the silver-white aluminium foil group increased by 114%, 117.2%, 104.6%, and 111.3% with one, two, three, and four UV lamps, respectively. These findings indicate that the reflective properties of the silver-white aluminium foil inner wall substantially enhance the CTL degradation process.
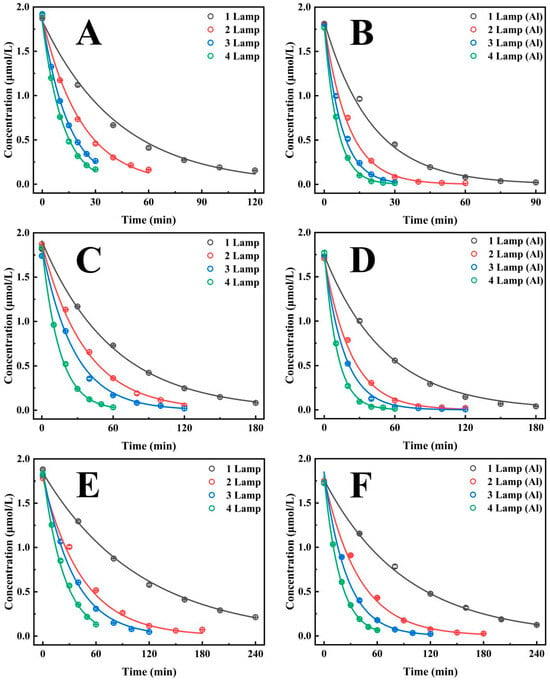
Figure 2.
The effect of installing 1, 2, 3, and 4 UV lamps in containers of different sizes on the photocatalytic degradation efficiency of CTL: (A) The diameter of the container was 20 cm. (B) The container has a diameter of 20 cm and was attached with silver-white aluminium foil. (C) The diameter of the container was 30 cm. (D) The container has a diameter of 30 cm and was attached with silver-white aluminium foil. (E) The diameter of the container was 40 cm. (F) The container has a diameter of 40 cm and was attached with silver-white aluminium foil.
As demonstrated in Figure 2C (Supplementary Table S2), the half-life of CTL degradation was 41.41 min in a 30 cm diameter enamel bucket when exposed to one UV lamp, 25.26 min when exposed to two, 18.53 min when exposed to three, and 10.32 min when exposed to four. The degradation rate exhibited a progressive increase as the number of lamps increased, with enhancements of 63.9%, 123.5%, and 301.4% observed for lamp ratios of 2:1, 3:1, and 4:1, respectively. Under identical conditions, Figure 2D (Supplementary Table S2) demonstrates that the incorporation of silver-white aluminium foil contributed to an additional improvement in the degradation efficiency. The half-life of CTL degradation decreased to 34.09 min for one lamp, 15.7 min for two, 11.92 min for three, and 7.61 min for four. The degradation rates increased by 117.2%, 186.1%, and 348.3% for two, three, and four lamps, respectively, in comparison with single-lamp irradiation. These results indicate a clear trend of accelerating degradation with both an increased number of UV lamps and the use of silver-white aluminium foil to enhance light reflection. Moreover, subsequent analysis showed that the silver-white aluminium foil inner wall significantly enhanced the CTL degradation rate in comparison to the enamel inner wall under an equal number of UV lamps. The degradation rates for the silver-white aluminium foil group increased by 21.5%, 60.9%, 55.5%, and 47.5% with one, two, three, and four UV lamps, respectively. These results indicate that the reflective properties of the silver-white aluminium foil inner wall effectively promote the CTL degradation reaction.
In a 40 cm diameter enamel bucket, Figure 2E (Supplementary Table S2) demonstrates that the half-life of CTL degradation is 76.09 min in the presence of one UV lamp, 30.9 min with two, 23.9 min with three, and 16.41 min with four. It is evident that as the number of UV lamps increased, the degradation rate underwent a progressive acceleration, with increases of 146.2%, 218.3%, and 363.8% observed for lamp ratios of 2:1, 3:1, and 4:1, respectively. In the same conditions, Figure 2F (Supplementary Table S2) demonstrates that the addition of silver-white aluminium foil further accelerated the degradation process, reducing the half-life to 64.00 min for one lamp, 27.31 min for two, 17.61 min for three, and 12.44 min for four. A comparison of the degradation rates resulting from single-lamp irradiation with those resulting from two, three and four lamps reveals increases of 134.3%, 263.4%, and 414.7%, respectively. This finding suggests a trend of accelerating degradation as the number of UV lamps increases. Additionally, analysis revealed that the silver-white aluminium foil inner wall significantly outperformed the enamel inner wall in promoting CTL degradation under the same number of UV lamps. The degradation rate for the silver-white aluminium foil group increased by 18.9%, 13.2%, 35.7%, and 31.9% with one, two, three, and four UV lamps, respectively, compared to the enamel group. This finding underscores the efficacy of the silver-white aluminium foil inner wall in substantially boosting the degradation reaction of CTL. It can be concluded that both an increase in the number of UV lamps and the use of silver-white aluminium foil inner walls have a significant effect on the acceleration of the degradation rate of CTL. The combination of these two factors has been shown to synergistically improve degradation efficiency, underscoring the importance of optimizing UV irradiation conditions and reactor inner wall design to enhance photochemical degradation processes.
3.2. Mechanism of Action of Silver-White Aluminum Foil to Promote CTL Degradation
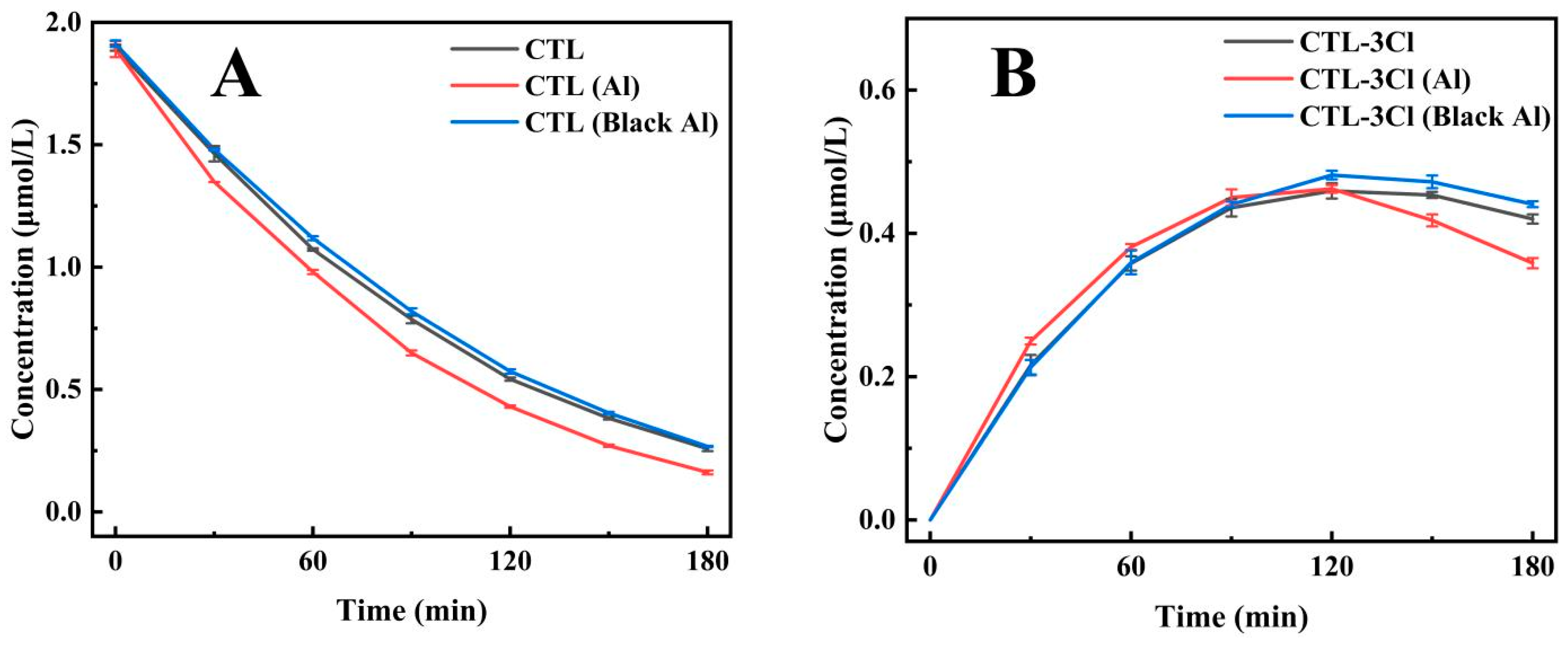
Silver-white aluminium foil has been shown to exhibit extremely high ultraviolet reflectivity, while black aluminium foil has been demonstrated to have strong light absorption properties [35,36]. In this study, a 30 cm diameter bucket was employed, with a silver-white and a black aluminium foil lining, respectively, and a UV lamp positioned vertically for CTL photodegradation experiments. The results obtained from this experiment demonstrated that the photodegradation half-lives of CTL were 57.38 min for the enamel inner wall, 44.4 min for the silver-white aluminium foil inner wall, and 56.77 min for the black aluminium foil inner wall. Of particular note is the observation that the half-lives for the enamel and black aluminium foil inner walls were nearly identical, as demonstrated in Figure 3A (Supplementary Table S3). This finding suggests that black aluminium foil does not significantly influence the catalytic process of CTL photodegradation. Additional analysis of 2, 4, 5-trichlorobenzonitrile (CTL-3Cl), a key photolysis product of CTL, provided additional insights. In both the enamel and black aluminium foil groups, the content of this compound remained relatively stable over time, exhibiting a similar trend (Figure 3B). Conversely, the silver-white aluminium foil group exhibited a rapid increase in the compound’s concentration within the first 90 min, followed by a sharp decline between 90 and 180 min. This finding suggests that silver-white aluminium foil significantly enhances the photolysis rate of CTL by improving UV reflection, leading to substantial and rapid changes in photolysis products. In summary, the photolysis half-life of CTL shows no significant difference between the enamel inner wall and black aluminium foil inner wall, indicating that black aluminium foil lacks catalytic effects on the photolysis process of CTL. Conversely, silver-white aluminium foil, with its high UV reflectivity, significantly accelerates the photolysis reaction, demonstrating higher photolysis efficiency. Consequently, silver-white aluminium foil is evidently advantageous in terms of its promotion of the photocatalytic degradation of CTL.
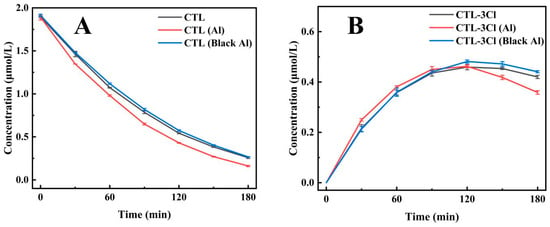
Figure 3.
The impact of installing a single lamp tube within a 30 cm diameter container, with inner walls composed of enamel, silver-white aluminium foil (Al), and black aluminium foil (Black Al), on the degradation of CTL (A) and the subsequent formation of its product CTL-3Cl (B).
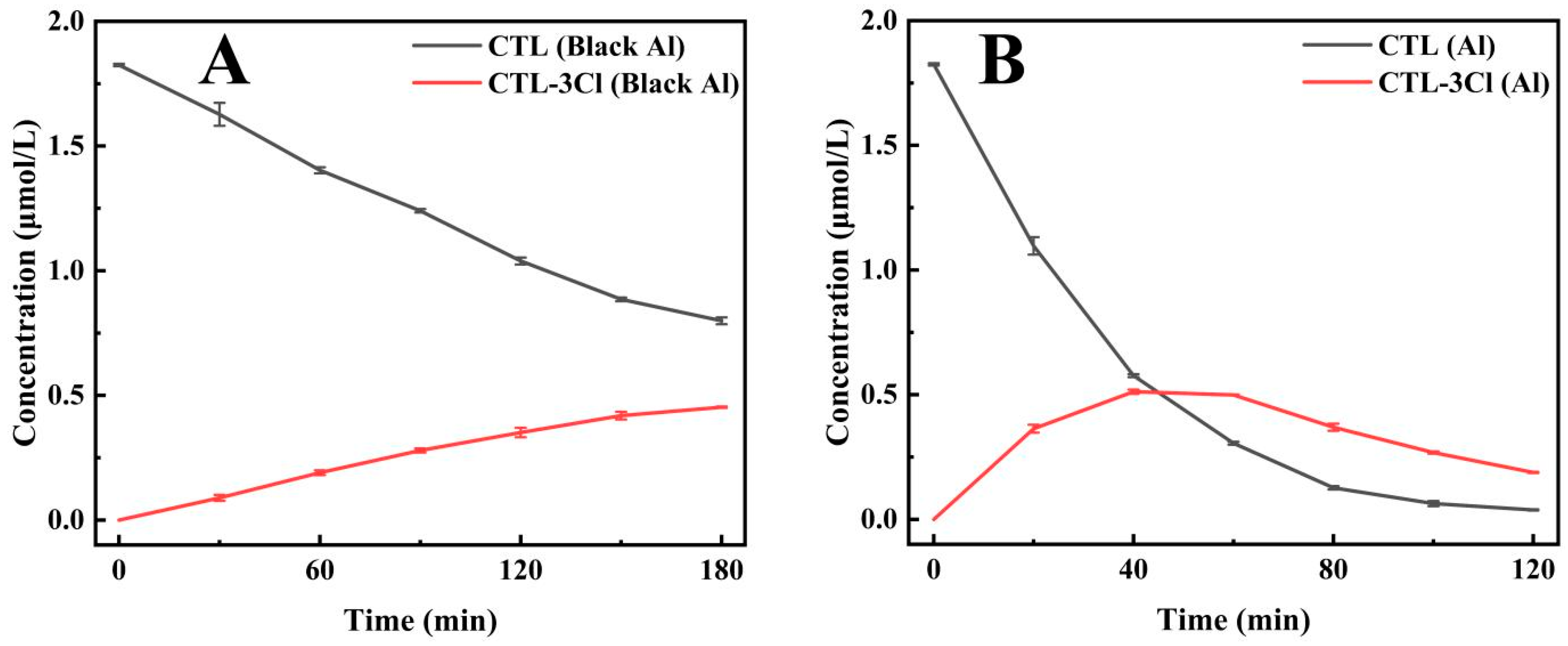
In the modified UV light box, the photodegradation half-lives of CTL were 150.36 min for the black aluminium foil group and 22.9 min for the silver-white aluminium foil group (Figure 4A) (Supplementary Table S3). This represents a 555.1% increase in the degradation rate for the silver-white aluminium foil group, thereby demonstrating its significant enhancement of CTL photodegradation. In addition, the formation and subsequent breakdown of the photodegradation product 2, 4, 5-trichlorobenzonitrile occurred at a faster rate in the silver-white aluminium foil group (Figure 4B) (Supplementary Table S3). This finding experimentally substantiates the hypothesis that silver-white aluminium foil accelerates the generation and transformation of CTL degradation products, thereby underscoring its role in enhancing the photolysis process. Moreover, light intensity measurements taken at the quartz tube’s illuminated position revealed that the silver-white aluminium foil group exhibited approximately 252% higher light intensity than the black aluminium foil group. This finding underscores the silver-white aluminium foil ability to effectively reflect ultraviolet light and enhance the irradiation intensity within the reaction system, thereby accelerating the photodegradation of CTL. Despite the absence of direct contact between the reaction solution and the aluminium foil, the silver-white aluminium foil significantly enhanced the photodegradation rate of CTL. This phenomenon can be attributed to the superior UV reflection capability of the silver-white aluminium foil, which plays a pivotal role in the optimisation of the photodegradation process.
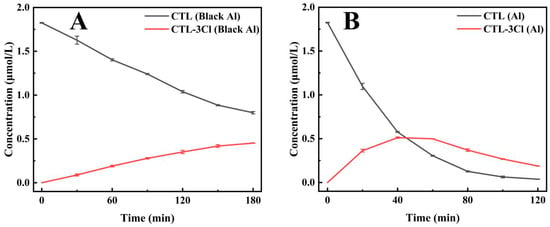
Figure 4.
The impact of black aluminium foil containers (Black Al) on the degradation of CTL and its product CTL-3Cl (A). The impact of silver-white aluminium foil containers (Al) on the degradation of CTL and its product CTL-3Cl (B).
3.3. Photodegradation Effect of Aluminum Foil on CTL in Natural Water
In this study, experiments were conducted using natural water sources to determine whether silver-white aluminium foil can enhance the photodegradation of water pollutants under natural conditions. The experimental setup comprised two containers: a 20 cm diameter enamel container and an silver-white aluminium foil-lined container. Each container was fitted with a UV lamp. The water samples included ultrapure water, pond water, and paddy field water. The photodegradation half-life of the CTL aqueous solution is presented in Table 3. In the enamel container, the half-life for ultrapure water, pond water, and paddy field water was 55.23 min, 12.63 min, and 16.36 min, respectively. Conversely, in the silver-white aluminium foil-lined container, the photodegradation half-life was reduced to 23.92 min, 7.13 min, and 12.34 min for the same water types. This finding underscores the efficacy of silver-white aluminium foil in expediting photodegradation across diverse water sources. Additional analysis revealed that the use of silver-white aluminium foil as the inner lining of the container significantly enhanced the photodegradation rate of the CTL aqueous solution. The degradation rate increased by 1.3 times in ultrapure water, 0.77 times in pond water, and 0.33 times in paddy field water. These findings demonstrate that silver-white aluminium foil effectively promotes CTL photodegradation across various water types. This study demonstrates that silver-white aluminium foil significantly enhances the photodegradation of CTL under ultraviolet light, with consistent effects observed across different water types. These findings suggest that silver-white aluminium foil has potential as a material for water treatment, offering a practical solution for improving pollutant degradation efficiency.

Table 3.
Photodegradation effect of silver-white aluminium foil in buckets on CTL in water from different sources under UV lamp irradiation.
4. Conclusions
This study systematically investigated the effects of ultraviolet (UV) irradiation conditions and reactor wall materials on the photodegradation of chlorothalonil (CTL) in aqueous systems. The results demonstrated that increasing the number of UV lamps and incorporating a reflective silvery-white aluminium foil inner wall significantly enhanced the degradation rate of CTL. This study aimed to develop a preliminary design model for the photocatalytic degradation of CTL in a controlled system, reducing secondary light pollution and allowing degradation simulations in different environments.
In a 20 cm enamel bucket, increasing the number of UV lamps reduced the degradation half-life from 29.95 min with one lamp to 8.15 min with four, improving degradation rates by 93.6%, 193.8%, and 267.6% for two, three, and four lamps, respectively. When lined with silver-white aluminium foil, the half-life further decreased to 14.01 min, 7.13 min, 4.98 min, and 3.86 min, with corresponding rate increases of 96.5%, 180.9%, and 262.9% compared to a single-lamp setup. Silver -white aluminium foil improved degradation efficiency by 104.6% to 117.2% over enamel surfaces. Similar trends were observed in larger buckets. In a 30 cm enamel bucket, the half-life dropped from 41.41 min with one lamp to 10.32 min with four, with degradation rates increasing by 63.9%, 123.5%, and 301.4%. Silver-white aluminium foil further lowered the half-life to 34.09 min, 15.7 min, 11.92 min, and 7.61 min, improving rates by 117.2%, 186.1%, and 348.3%. Compared to enamel, silver-white aluminium foil increased degradation efficiency by 21.5% to 60.9%. In a 40 cm bucket, the half-life decreased from 76.09 min with one lamp to 16.41 min with four, with rate improvements of 146.2%, 218.3%, and 363.8%. With silver-white aluminium foil, the half-life dropped further to 64.00 min, 27.31 min, 17.61 min, and 12.44 min, boosting rates by 134.3%, 263.4%, and 414.7%. The silver-white aluminium foil lining improved efficiency by 13.2% to 35.7% over enamel. These results confirm that increasing UV lamps and using silver-white aluminium foil significantly enhance CTL degradation, with a synergistic effect observed across all conditions. These results confirm that both an increased number of UV lamps and the presence of silver-white aluminium foil significantly accelerate CTL degradation, demonstrating a synergistic effect.
The mechanism underlying the enhancement of CTL degradation by aluminium foil was further examined using silver-white and black aluminium foil. The photodegradation half-life of CTL in a 30 cm bucket was 57.38 min for the enamel inner wall, 44.4 min for the silver-white aluminium foil, and 56.77 min for the black aluminium foil. The similarity in results between the enamel and black foil groups suggests that black aluminium foil lacks a catalytic effect, while silver-white aluminium foil significantly enhances photodegradation. The analysis of 2,4,5-trichlorobenzonitrile (CTL-3Cl), a primary degradation product, further supports this conclusion. In the enamel and black foil groups, its concentration remained relatively stable, whereas in the silver-white aluminium foil group, it increased rapidly before declining sharply, indicating accelerated photolysis. Additional experiments in a modified UV light box revealed that the degradation half-life of CTL was 150.36 min in the black aluminium foil setup and 22.9 min in the silver-white aluminium foil setup, corresponding to a 555.1% increase in the degradation rate. Light intensity measurements confirmed that silver-white aluminium foil increased UV intensity by approximately 252% compared to black foil, further explaining its role in accelerating CTL degradation. These improvements might suggest that silver-white aluminium foil enhances UV utilisation through direct reflection, refraction, and diffuse reflection, effectively redirecting photons that would otherwise escape the system [37]. Notably, the silver-white aluminium foil does not introduce any additional energy consumption, making it a practical improvement. Furthermore, the use of a low power 10 watt UV lamp highlighted the potential for achieving efficient CTL degradation with minimal energy input. Given its low cost and wide applicability, silver-white aluminium foil represented a viable strategy for improving the efficiency of photocatalytic degradation in water treatment systems.
To evaluate the practical applicability of these findings, photodegradation experiments were conducted using natural water sources, including pond water and paddy field water. In a 20 cm diameter enamel container, the CTL half-life was 55.23 min in ultrapure water, 12.63 min in pond water, and 16.36 min in paddy field water. When silver-white aluminium foil was introduced, the half-life reduced to 23.92 min, 7.13 min, and 12.34 min, respectively. These results demonstrate that silver-white aluminium foil enhances photodegradation across different water sources, with degradation rates increasing by 1.3 times in ultrapure water, 0.77 times in pond water, and 0.33 times in paddy field water.
This study confirms that both an increased number of UV lamps and the use of silver-white aluminium foil inner walls significantly enhance the photodegradation of CTL. The reflective properties of silver-white aluminium foil contribute to higher UV intensity, accelerating degradation rates and improving pollutant removal efficiency. These findings provide valuable insights for optimizing UV-based water treatment systems and suggest that incorporating reflective materials can be an effective strategy for improving the photodegradation of environmental contaminants. Unfortunately, this model also has its drawbacks. In large-scale industrial applications, this model cannot be directly applied to acidic or alkaline wastewater. Acidic or alkaline effluent can accelerate the corrosion of system components and ultimately lead to system failure. If aluminium oxide material is used instead of silver-white aluminium foil, its degradation data are not as good as silver-white aluminium foil, except for acid and alkali resistance. It is therefore essential to find materials that are more stable and have higher reflectivity. For all that, this study still provides a new catalytic approach for the photolysis of organic wastewater.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17071032/s1. Figure S1: The absorption wavelength of Chlorothalonil; Figure S2: The effect of UV light exposure and temperature rise on the degradation rate of Chlorothalonil; Table S1: Details of Materials and Equipment; Table S2: The degradation coefficient of Chlorothalonil in containers of different sizes.; Table S3: The degradation coefficient of Chlorothalonil in each reactor (Black aluminum foil and silver-white aluminum foil for enamel barrels; Black aluminum foil and silver-white aluminum foil for conventional photocatalytic reactors).
Author Contributions
Conceptualization, J.X., T.S., R.H. and Y.H.; Methodology, J.X., S.C., T.S., H.W., Z.L., R.H. and Y.H.; Software, J.X., X.M. and R.H.; Validation, J.X. and R.H.; Formal analysis, J.X. and Y.H.; Investigation, J.X. and S.C.; Resources, Z.L.; Data curation, J.X., S.C., X.M., T.S. and H.W.; Writing—original draft, J.X. and Y.H.; Writing—review & editing, J.X. and Y.H.; Visualization, J.X., X.M. and Y.H.; Supervision, R.H. and Y.H.; Project administration, Z.L., R.H. and Y.H.; Funding acquisition, Z.L. and R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was under the financial aid of the National Natural Science Foundation of China (31972314), the Natural Science Foundation of the Higher Education Institutions of Anhui Province (2024AH051352), School-level key projects of Chizhou College (CZ2023ZRZ01), and the Natural Students’ Innovation and Entrepreneurship Training Program (X202411306019).
Data Availability Statement
The data presented in this study are available on request from the corresponding author (Y.H.) due to Data Standardization and Privacy.
Conflicts of Interest
The authors declare that they have no conflicts of interest in the contents of this manuscript.
References
- Yu, G.-B.; Chen, R.-N.; Chen, Q.-S.; Chen, F.-Q.; Liu, H.-L.; Ren, C.-Y.; Zhang, Y.-X.; Yang, F.-J.; Wei, J.-P. Jasmonic acid promotes glutathione assisted degradation of chlorothalonil during tomato growth. Ecotoxicol. Environ. Saf. 2022, 233, 113296. [Google Scholar] [PubMed]
- Boman, A.; Montelius, J.; Rissanen, R.L.; Lidén, C. Sensitizing potential of chlorothalonil in the guinea pig and the mouse. Contact Dermat. 2000, 43, 273–279. [Google Scholar]
- Authority, E.F.S.; Arena, M.; Auteri, D.; Barmaz, S.; Bellisai, G.; Brancato, A.; Brocca, D.; Bura, L.; Byers, H.; Chiusolo, A. Peer review of the pesticide risk assessment of the active substance chlorothalonil. EFSA J. 2018, 16, e05126. [Google Scholar]
- Zhang, C.; Zhao, X.; Pan, X.; Zaya, G.; Lyu, B.; Li, S.; Li, J.; Zhao, Y.; Wu, Y.; Chen, D. The mother-offspring transfer of chlorothalonil through human breast milk: A multi-city cross-sectional study. Sci. Total Environ. 2024, 941, 173511. [Google Scholar]
- Xing, Z.; Chow, L.; Cook, A.; Benoy, G.; Rees, H.; Ernst, B.; Meng, F.; Li, S.; Zha, T.; Murphy, C. Pesticide application and detection in variable agricultural intensity watersheds and their river systems in the maritime region of Canada. Arch. Environ. Contam. Toxicol. 2012, 63, 471–483. [Google Scholar] [PubMed]
- Voulvoulis, N.; Scrimshaw, M.D.; Lester, J.N. Occurrence of four biocides utilized in antifouling paints, as alternatives to organotin compounds, in waters and sediments of a commercial estuary in the UK. Mar. Pollut. Bull. 2000, 40, 938–946. [Google Scholar]
- Gallo, A.; Tosti, E. Reprotoxicity of the antifoulant chlorothalonil in ascidians: An ecological risk assessment. PLoS ONE 2015, 10, e0123074. [Google Scholar]
- Báez, M.E.; Sarkar, B.; Peña, A.; Vidal, J.; Espinoza, J.; Fuentes, E. Effect of surfactants on the sorption-desorption, degradation, and transport of chlorothalonil and hydroxy-chlorothalonil in agricultural soils. Environ. Pollut. 2023, 327, 121545. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Meseguer, J.; Esteban, M.A. Risks of using antifouling biocides in aquaculture. Int. J. Mol. Sci. 2012, 13, 1541–1560. [Google Scholar] [CrossRef]
- Verween, A.; Vincx, M.; Degraer, S. Comparative toxicity of chlorine and peracetic acid in the biofouling control of Mytilopsis leucophaeata and Dreissena polymorpha embryos (Mollusca, Bivalvia). Int. Biodeterior. Biodegrad. 2009, 63, 523–528. [Google Scholar]
- Haque, M.N.; Eom, H.-J.; Nam, S.-E.; Shin, Y.K.; Rhee, J.-S. Chlorothalonil induces oxidative stress and reduces enzymatic activities of Na+/K+-ATPase and acetylcholinesterase in gill tissues of marine bivalves. PLoS ONE 2019, 14, e0214236. [Google Scholar]
- McMahon, T.A.; Halstead, N.T.; Johnson, S.; Raffel, T.R.; Romansic, J.M.; Crumrine, P.W.; Boughton, R.K.; Martin, L.B.; Rohr, J.R. The fungicide chlorothalonil is nonlinearly associated with corticosterone levels, immunity, and mortality in amphibians. Environ. Health Perspect. 2011, 119, 1098–1103. [Google Scholar] [PubMed]
- Du Gas, L.M.; Ross, P.S.; Walker, J.; Marlatt, V.L.; Kennedy, C.J. Effects of atrazine and chlorothalonil on the reproductive success, development, and growth of early life stage sockeye salmon (Oncorhynchus nerka). Environ. Toxicol. Chem. 2017, 36, 1354–1364. [Google Scholar]
- Bruynzeel, D.P.; van Ketel, W.G. Contact dermatitis due to chlorothalonil in floriculture. Contact Dermat. (01051873) 1986, 14, 67. [Google Scholar]
- Lensen, G.; Jungbauer, F.; Gonçalo, M.; Coenraads, P.J. Airborne irritant contact dermatitis and conjunctivitis after occupational exposure to chlorothalonil in textiles. Contact Dermat. 2007, 57, 181–186. [Google Scholar]
- Barr, D.B.; Ananth, C.V.; Yan, X.; Lashley, S.; Smulian, J.C.; Ledoux, T.A.; Hore, P.; Robson, M.G. Pesticide concentrations in maternal and umbilical cord sera and their relation to birth outcomes in a population of pregnant women and newborns in New Jersey. Sci. Total Environ. 2010, 408, 790–795. [Google Scholar] [PubMed]
- Wang, T.; Zhou, Y.; Wang, L.; Sui, J.; Chen, F.; Jia, Y.; Chen, S.; Cui, X.; Yang, Y.; Zhang, W. Assessing the biotic and abiotic degradation of malathion in the environment: Current strategies and advances. J. Environ. Chem. Eng. 2025, 13, 115429. [Google Scholar]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent strategies for bioremediation of emerging pollutants: A review for a green and sustainable environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef]
- Xiong, J.; Li, G.; Gelman, F.; Ronen, Z.; An, T. Mechanism investigation and stable isotope change during photochemical degradation of tetrabromobisphenol A (TBBPA) in water under LED white light irradiation. Chemosphere 2020, 258, 127378. [Google Scholar]
- Barnes, P.W.; Robson, T.M.; Zepp, R.G.; Bornman, J.F.; Jansen, M.; Ossola, R.; Wang, Q.-W.; Robinson, S.; Foereid, B.; Klekociuk, A. Interactive effects of changes in UV radiation and climate on terrestrial ecosystems, biogeochemical cycles, and feedbacks to the climate system. Photochem. Photobiol. Sci. 2023, 22, 1049–1091. [Google Scholar]
- El-Saeid, M.H.; Alotaibi, M.O.; Alshabanat, M.; Al-Anazy, M.M.; Alharbi, K.R.; Altowyan, A.S. Impact of photolysis and TiO2 on pesticides degradation in wastewater. Water 2021, 13, 655. [Google Scholar] [CrossRef]
- Wang, Y.; Roddick, F.A.; Fan, L. Direct and indirect photolysis of seven micropollutants in secondary effluent from a wastewater lagoon. Chemosphere 2017, 185, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Katagi, T. Direct photolysis mechanism of pesticides in water. J. Pestic. Sci. 2018, 43, 57–72. [Google Scholar] [CrossRef]
- Wu, C.; Linden, K.G. Phototransformation of selected organophosphorus pesticides: Roles of hydroxyl and carbonate radicals. Water Res. 2010, 44, 3585–3594. [Google Scholar] [CrossRef] [PubMed]
- Goon, A.; Bhattacharyya, A.; Ghosh, B.; Rakshit, R.; Das, A.; Choudury, S.R.; Kundu, C.; Ganguly, P.; Hossain, A. Photodegradation of flucetosulfuron, a sulfonylurea-based herbicide in the aqueous media is influenced by ultraviolet irradiation. J. Xenobiotics 2021, 11, 142–154. [Google Scholar] [CrossRef]
- Aydin, M.I.; Ozaktac, D.; Yuzer, B.; Doğu, M.; Inan, H.; Okten, H.E.; Coskun, S.; Selcuk, H. Desalination and detoxification of textile wastewater by novel photocatalytic electrolysis membrane reactor for ecosafe hydroponic farming. Membranes 2021, 12, 10. [Google Scholar] [CrossRef]
- Crovella, T.; Paiano, A. Assessing the Sustainability of Photodegradation and Photocatalysis for Wastewater Reuse in an Agricultural Resilience Context. Water 2023, 15, 2758. [Google Scholar] [CrossRef]
- Papernov, S.; Schmid, A. Laser-induced surface damage of optical materials: Absorption sources, initiation, growth, and mitigation. Laser-Induc. Damage Opt. Mater. 2008 2008, 7132, 469–496. [Google Scholar]
- Wu, H.Y.; Huang, S.R.; Shih, C.H.; Hsiao, L.J.; Chen, H.W.; Cheng, M.C.; Hsu, J.C. Highly Reflective Silver-Enhanced Coating with High Adhesion and Sulfurization Resistance for Telescopes. Nanomaterials 2022, 12, 1054. [Google Scholar] [CrossRef]
- Cisterna-Osorio, P.; Galvez-Gonzalez, M.; Moraga-Chaura, M.; Quijada-Vera, S. Increase by Substitution of Galvanized Steel for Aluminum Mirrors in the UV Solar Radiation in Canal with Fins and Side Panels That Disinfect Wastewater. Processes 2022, 11, 84. [Google Scholar] [CrossRef]
- Xu, X.; Ji, F.; Fan, Z.; He, L. Degradation of glyphosate in soil photocatalyzed by Fe3O4/SiO2/TiO2 under solar light. Int. J. Environ. Res. Public Health 2011, 8, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Wang, C.; Huang, H.; Li, W.; Du, D.; Han, D.; Qiu, T.; Chu, P.K. Aluminum plasmonic photocatalysis. Sci. Rep. 2015, 5, 15288. [Google Scholar]
- Denny, F.; Scott, J.; Pareek, V.; Peng, G.D.; Amal, R. CFD modelling for a TiO2-coated glass-bead photoreactor irradiated by optical fibres: Photocatalytic degradation of oxalic acid. Chem. Eng. Sci. 2009, 64, 1695–1706. [Google Scholar] [CrossRef]
- Lv, P.; Min, S.; Wang, Y.; Zheng, X.; Wu, X.; Li, Q.X.; Hua, R. Flavonoid-sensitized photolysis of chlorothalonil in water. Pest Manag. Sci. 2020, 76, 2972–2977. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Wu, Y.H.; Ting, Y.Y.; Wu, C.C.; Wu, J.S.; Lin, S.D. Nano- to atomic-scale epitaxial aluminum films on Si substrate grown by molecular beam epitaxy. AIP Adv. 2019, 9, 105001. [Google Scholar] [CrossRef]
- Pashchanka, M.; Cherkashinin, G. A Strategy towards Light-Absorbing Coatings Based on Optically Black Nanoporous Alumina with Tailored Disorder. Materials 2021, 14, 5827. [Google Scholar] [CrossRef]
- Lindsley, W.G.; McClelland, T.L.; Neu, D.T.; Martin, S.B.; Mead, K.R.; Thewlis, R.E.; Noti, J.D. Ambulance disinfection using Ultraviolet Germicidal Irradiation (UVGI): Effects of fixture location and surface reflectivity. J. Occup. Environ. Hyg. 2018, 15, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).