Advanced Oxidation Processes and Adsorption Technologies for the Removal of Organic Azo Compounds: UV, H2O2, and GAC
Abstract
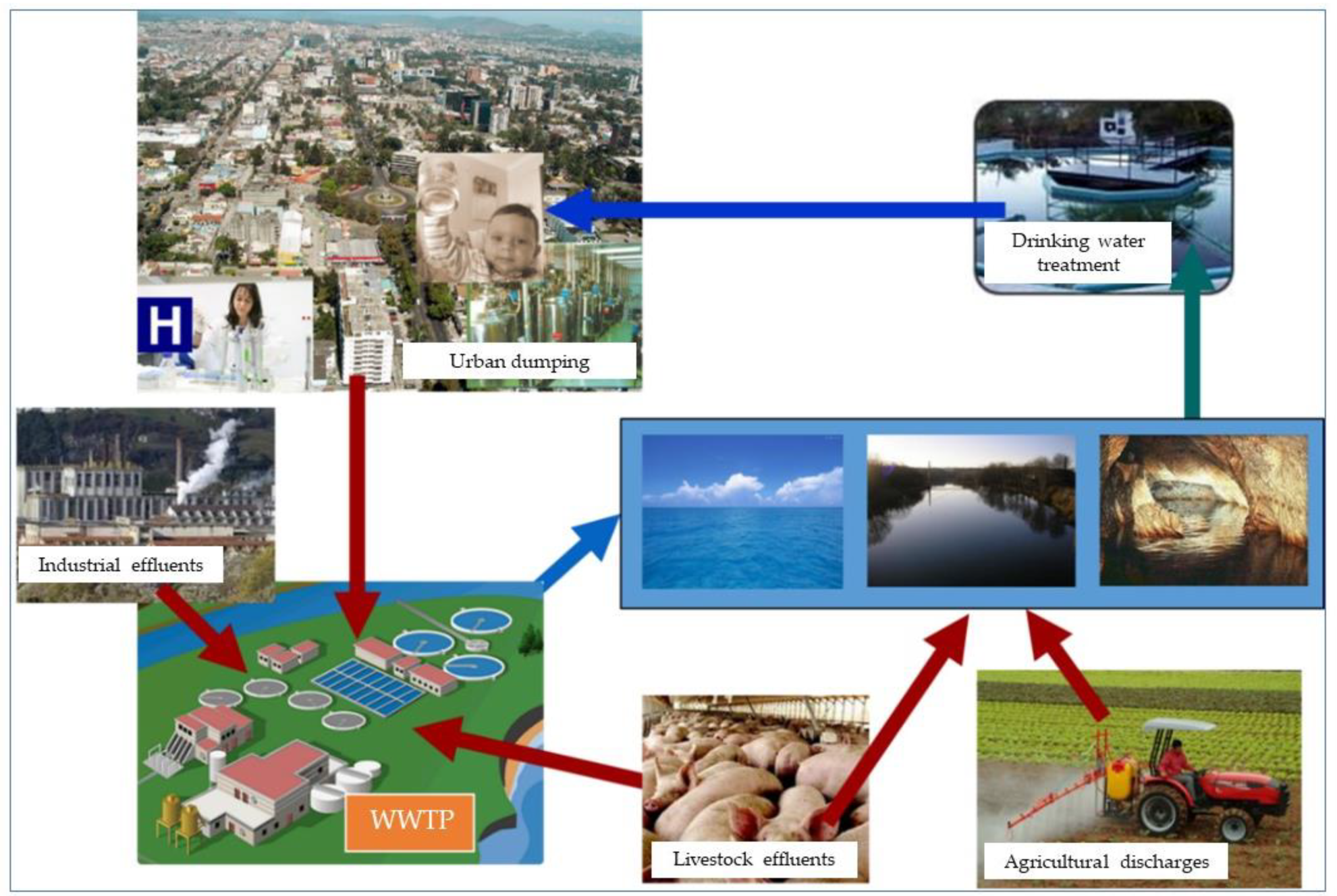
1. Introduction
1.1. Advance Oxidation Processes
1.1.1. Ultraviolet Photolysis
1.1.2. Hydrogen Peroxide
1.2. Adsorption by Activated Carbon
2. Materials and Methods
2.1. Selected Micropollutants
2.2. Analytical Methodology
- Volume injected (µL): 1
- Injection mode: Injection with needle flushing
- Mobile phase: Water (A)/acetonitrile (B)
- Mobile phase flow rate (mL∙min⁻1): 0.4
- Initial oven temperature (°C): 25
- Ramp: 10 min 15% A 85% B, 10.50 min 2% A 98% B, 11.00 min 2% A 98% B, 12.00 min 85% A 15% B
- Time (min): 12
2.3. Aqueous Matrix
2.4. Calculation of the Removal Rate
- E: treatment efficiency (%)
- Ci: concentration of the compound in the influent
- Ce: concentration of the compound in the effluent
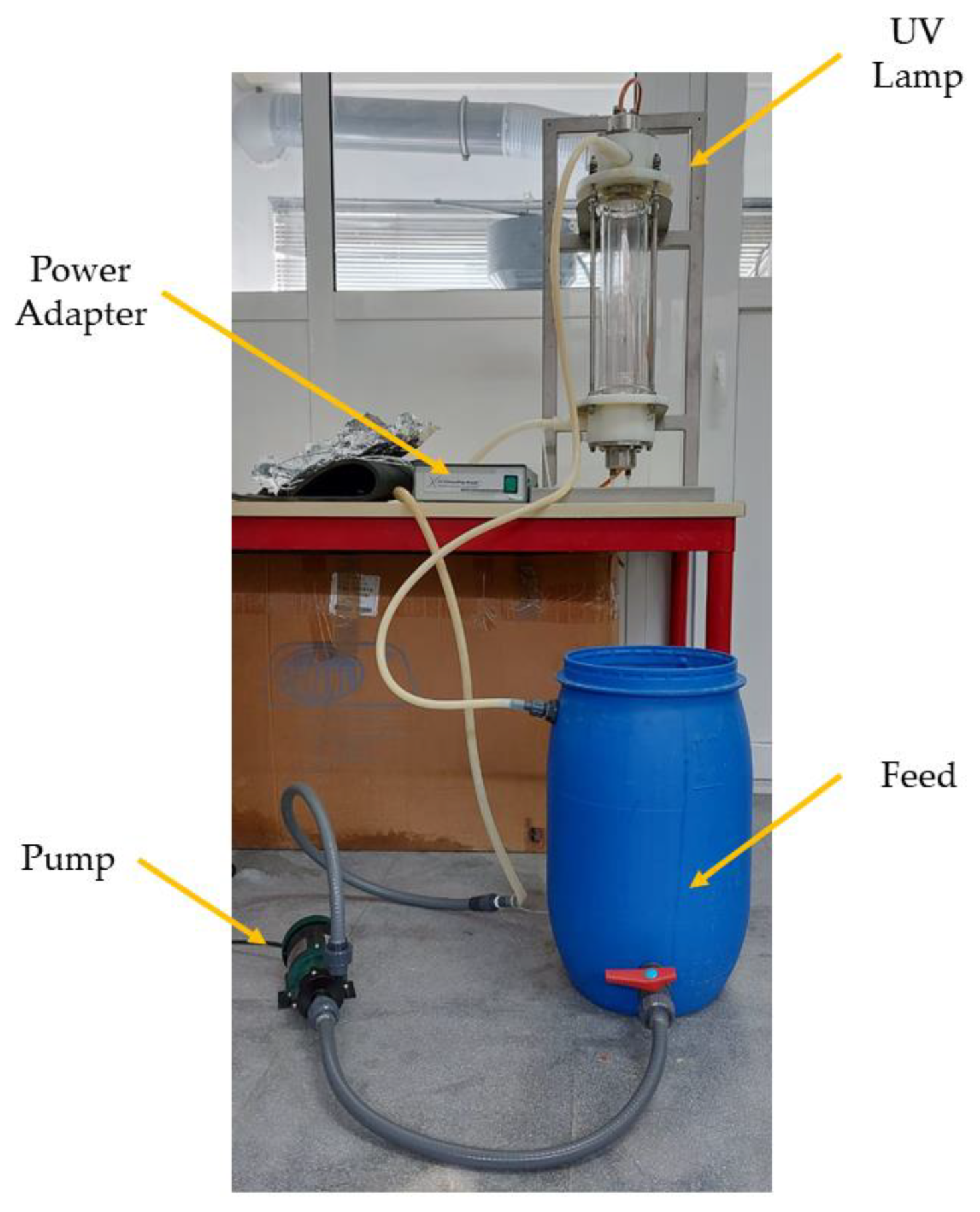
2.5. Ultraviolet Photolysis Tests
2.6. Hydrogen Peroxide Oxidation
2.7. Activated Carbon Adsorption
3. Results and Discussion
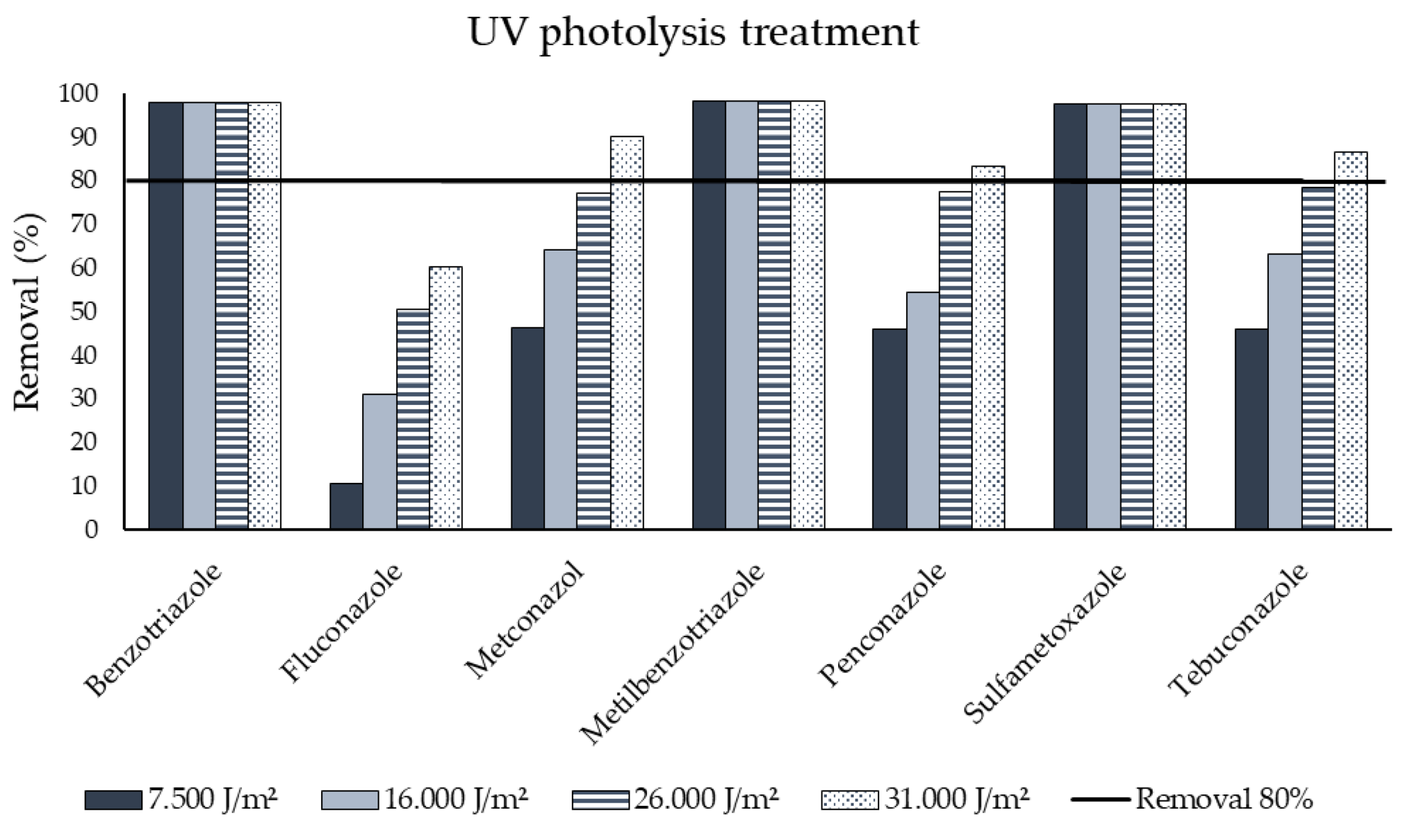
3.1. Ultraviolet Photolysis
3.2. Hydrogen Peroxide
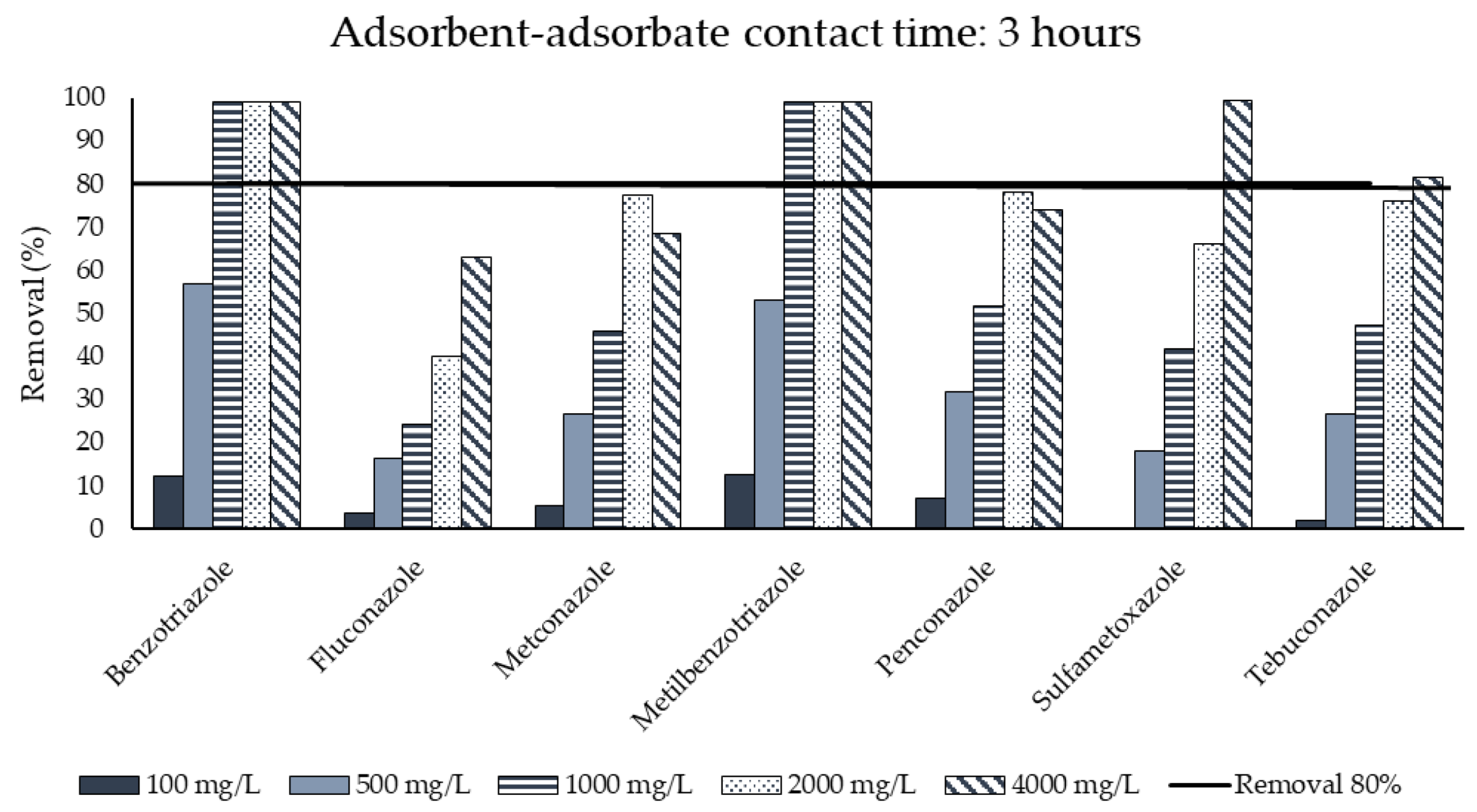
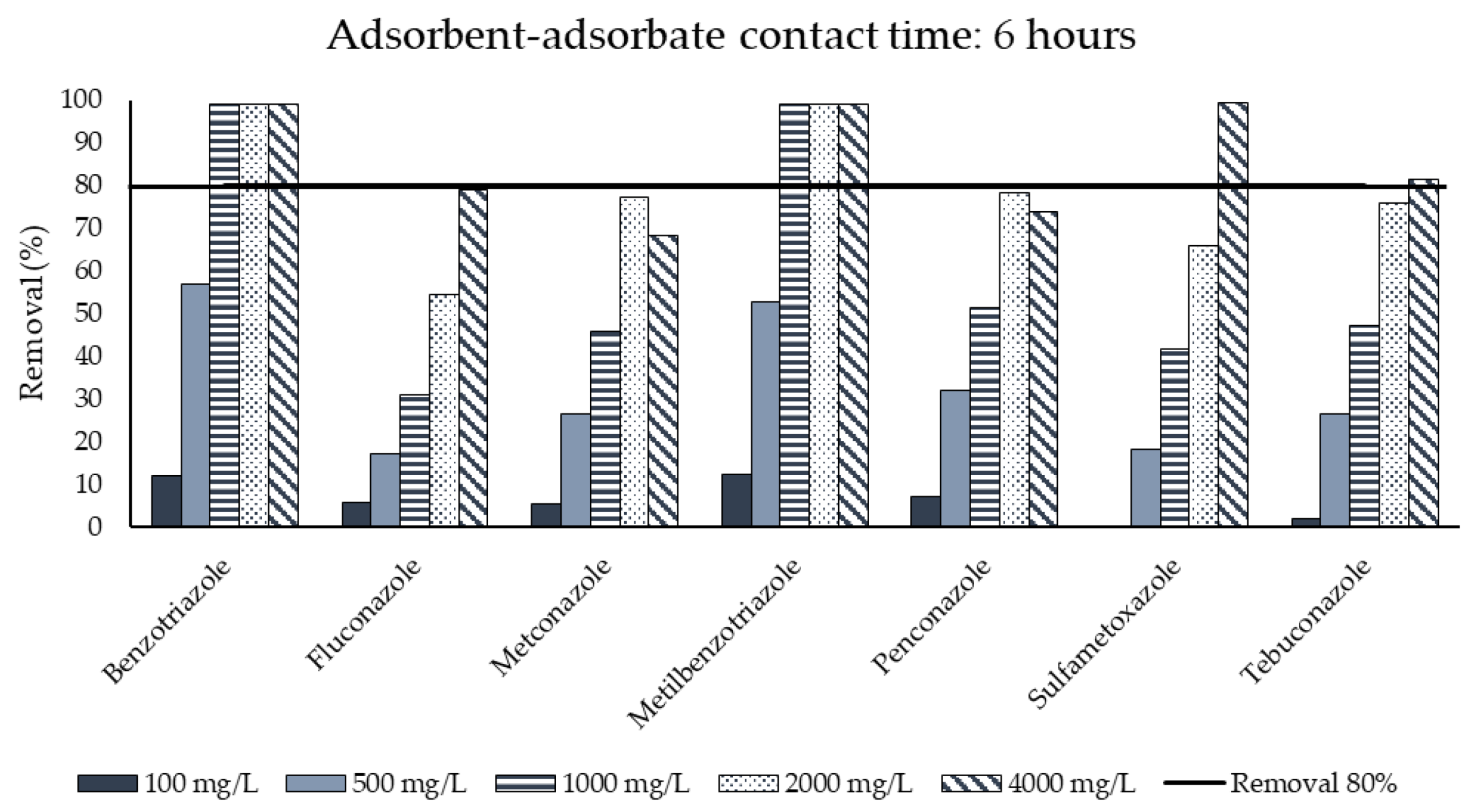
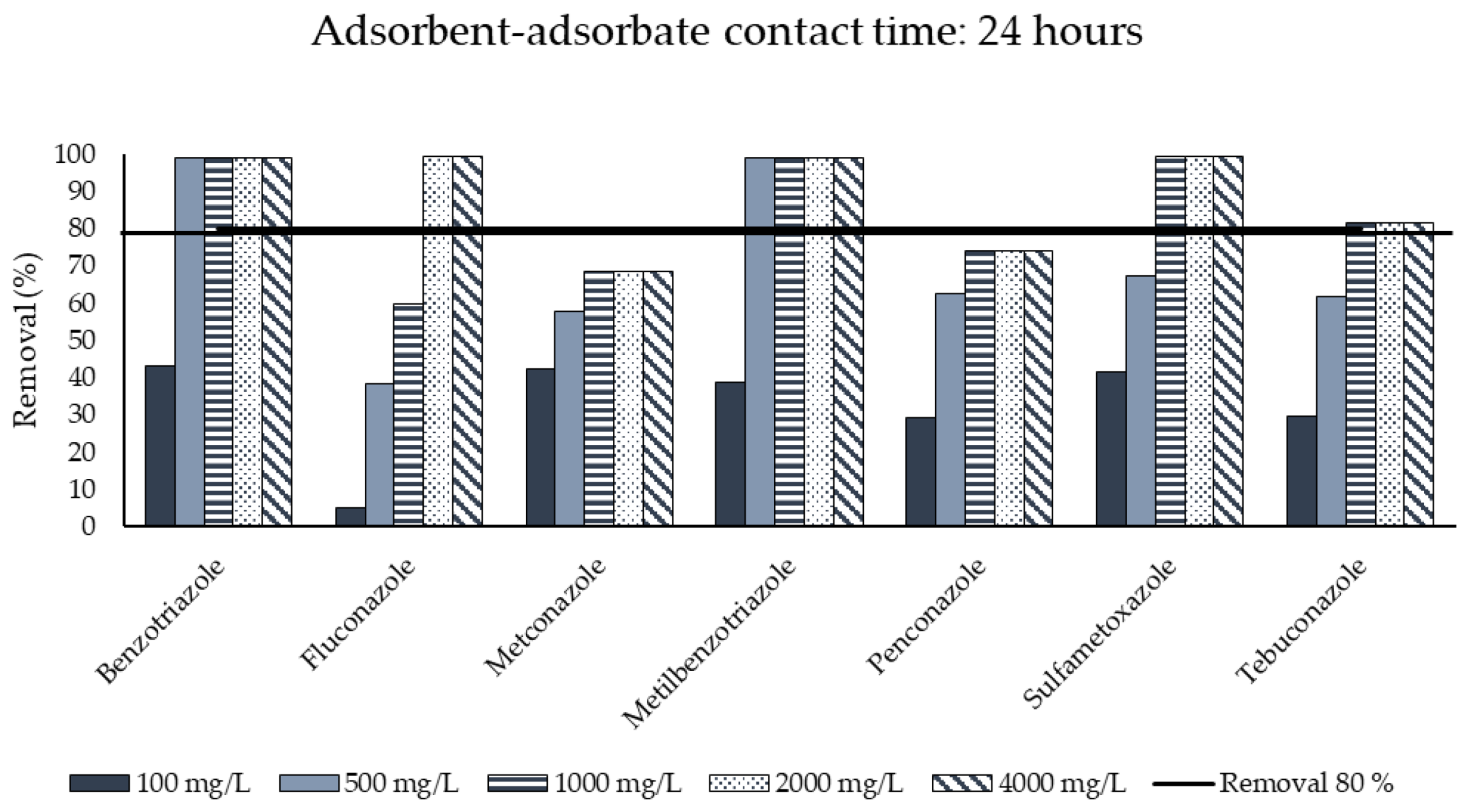
3.3. Activated Carbon Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Walker, G.W.; Muir, D.C.G.; Nagatani-Yoshida, K. Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories. Environ. Sci. Technol. 2020, 54, 2575–2584. [Google Scholar] [CrossRef]
- Dulio, V.; van Bavel, B.; Brorström-Lundén, E.; Harmsen, J.; Hollender, J.; Schlabach, M.; Slobodnik, J.; Thomas, K.; Koschorreck, J. Emerging pollutants in the EU: 10 years of NORMAN in support of environmental policies and regulations. Environ. Sci. Eur. 2018, 30, 5. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment-A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- von der Ohe, P.C.; Dulio, V.; Slobodnik, J.; De Deckere, E.; Kühne, R.; Ebert, R.U.; Ginebreda, A.; De Cooman, W.; Schüürmann, G.; Brack, W. A new risk assessment approach for the prioritization of 500 classical and emerging organic microcontaminants as potential river basin specific pollutants under the European Water Framework Directive. Sci. Total Environ. 2011, 409, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Mertová, D. Recast of the Urban Wastewater Treatment Directive brings new challenges not only in the water management sector. Water Manag. Tech. Econ. Inf. J. 2024, 66, 28–31. [Google Scholar]
- Prats, D. Emerging pollutants: Problematic, Legislation, Characterisation and Possible Treatment. Item 2: Water Quality. Master’s Thesis, University of Alicante, Alicante, Spain, 2021. [Google Scholar]
- Parida, V.K.; Saidulu, D.; Majumder, A.; Srivastava, A.; Gupta, B.; Gupta, A.K. Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. J. Environ. Chem. Eng. 2021, 9, 105966. [Google Scholar] [CrossRef]
- Saidulu, D.; Gupta, B.; Gupta, A.K.; Ghosal, P.S. A review on occurrences, eco-toxic effects, and remediation of emerging contaminants from wastewater: Special emphasis on biological treatment based hybrid systems. J. Environ. Chem. Eng. 2021, 9, 105282. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Barros de Souza, A.; Cabooter, D.; De Laet, S.; Van Eyck, K.; Dewil, R. Treatment of antimicrobial azole compounds via photolysis, electrochemical and photoelectrochemical oxidation: Degradation kinetics and transformation products. Environ. Pollut. 2023, 334, 122220. [Google Scholar] [CrossRef] [PubMed]
- Pacholak, A.; Burlaga, N.; Frankowski, R.; Zgoła-Grześkowiak, A.; Kaczorek, E. Azole fungicides: (Bio)degradation, transformation products and toxicity elucidation. Sci. Total Environment. 2022, 802, 149917. [Google Scholar] [CrossRef]
- Hendrickson, J.A.; Hu, C.; Aitken, S.L.; Beyda, N. Antifungal Resistance: A Concerning Trend for the Present and Future. Curr. Infect. Dis. Rep. 2019, 21, 47. [Google Scholar] [CrossRef]
- Richter, E.; Roller, E.; Kunkel, U.; Ternes, T.A.; Coors, A. Phytotoxicity of wastewater-born micropollutants—Characterisation of three antimycotics and a cationic surfactant. Environ. Pollut. 2016, 208, 512–522. [Google Scholar] [CrossRef]
- Zarn, J.A.; Brüschweiler, B.J.; Schlatter, J.R. Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14α-demethylase and aromatase. Environ. Health Perspect. 2003, 111, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, Y.; Takada, H.; Sato, H.; Kubota, A.; Terasaki, M.; Takeuchi, S.; Ikeda-Araki, A.; Watanabe, Y.; Kitamura, S.; Kojima, H. An analytical survey of benzotriazole UV stabilizers in plastic products and their endocrine-disrupting potential via human estrogen and androgen receptors. Sci. Total Environ. 2021, 800, 149374. [Google Scholar] [CrossRef]
- Decision 2020/1161/EU. Commission Implementing Decision (Eu) 2020/1161. Off. J. Eur. Union 2020, 257, 32–35. [Google Scholar]
- Council of the European Union. Proposal for a Directive of the European Parliament and of the Council Concerning Urban Wastewater Treatment (Recast); Letter to the Chair of the European Parliament Committee on the Environment, Public Health and Food Safety (ENVI); European Commission: Brussels, Belgium, 2024; pp. 1–148. [Google Scholar]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water—A critical review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A review on emerging water contaminants and the application of sustainable removal technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- David Vásquez Rodríguez, E. Instituto Universitario Del Agua Y De Las Ciencias Ambientales. 2018, pp. 18–27. Available online: http://hdl.handle.net/10045/80430 (accessed on 26 December 2024).
- De la Cruz, N.; Giménez, J.; Esplugas, S.; Grandjean, D.; De Alencastro, L.F.; Pulgarín, C. Degradation of 32 emergent contaminants by UV and neutral photo-fenton in domestic wastewater effluent previously treated by activated sludge. Water Res. 2012, 46, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; Urtiaga, A.M.; Ibáñez, R.; Ortiz, I. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012, 46, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Miralles-Cuevas, S.; Audino, F.; Oller, I.; Sánchez-Moreno, R.; Sánchez Pérez, J.A.; Malato, S. Pharmaceuticals removal from natural water by nanofiltration combined with advanced tertiary treatments (solar photo-Fenton, photo-Fenton-like Fe(III)-EDDS complex and ozonation). Sep. Purif. Technol. 2014, 122, 515–522. [Google Scholar] [CrossRef]
- Liu, Z.; Demeestere, K.; Van Hulle, S. Comparison and performance assessment of ozone-based AOPs in view of trace organic contaminants abatement in water and wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105599. [Google Scholar] [CrossRef]
- Rodriguez, T.; Botelho, D.; Cleto, E. Tratamiento de Efluentes Industriales de Naturaleza Recalcitrante Usando Ozono, Peróxido de Hidrógeno y Radiación Ultravioleta; Revista Facultad de Ingenieria: 2008. pp. 24–38. Available online: http://www.scielo.org.co/scielo.php?pid=S0120-62302008000400003&script=sci_arttext (accessed on 26 December 2024).
- Stasinakis, A.S. Use of selected advanced oxidation processes (AOPs) for wastewater treatment—A mini review. Glob. Nest J. 2008, 10, 376–385. [Google Scholar]
- Moya-Llamas, M.J.; Ferre, M.; Dominguez, E.; Trapote, A.; Prats, D. Tecnologías de Oxidación Avanzada Para la Degradación del Fármaco Carbamazepina: La Ozonización; Seguridad Hídrica: 2023. Available online: http://hdl.handle.net/10045/137481 (accessed on 26 December 2024).
- Kovalova, L.; Siegrist, H.; Von Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; McArdell, C.S. Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.B.; Mielcke, J.; Ali, I.; Dewil, R.; van de Goor, T.; Cabooter, D. Removal of miconazole from water by O3, UV/H2O2 and electrochemical advanced oxidation: Real-time process monitoring and degradation pathway elucidation. J. Environ. Chem. Eng. 2023, 11, 109993. [Google Scholar] [CrossRef]
- Antonio da Silva, D.; Pereira Cavalcante, R.; Batista Barbosa, E.; Machulek Junior, A.; César de Oliveira, S.; Falcao Dantas, R. Combined AOP/GAC/AOP systems for secondary effluent polishing: Optimization, toxicity and disinfection. Sep. Purif. Technol. 2021, 263, 118415. [Google Scholar] [CrossRef]
- Della-Flora, A.; Wilde, M.L.; Lima, D.; Lima, E.C.; Sirtori, C. Combination of tertiary solar photo-Fenton and adsorption processes in the treatment of hospital wastewater: The removal of pharmaceuticals and their transformation products. J. Environ. Chem. Eng. 2021, 9, 105666. [Google Scholar] [CrossRef]
- Cruz, G.; Julcour, C.; Jáuregui, U. El Estado actual y perspectivas de la degradación de pesticidas por procesos avanzados de oxidación State of the art and perspectives of pesticides degradation by advanced oxidation processes Resumen. Rev. Cuba. De Química 2017, 29, 492–516. [Google Scholar]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef] [PubMed]
- Esquerdo, A.A.; Galvañ, P.J.V.; Gadea, I.S.; Rico, D.P. Carbamazepine and diclofenac removal double treatment: Oxidation and adsorption. Int. J. Environ. Res. Public. Health 2021, 18, 7163. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Li, W.K.; Shi, Y.P. Recent advances of carbon materials on pesticides removal and extraction based determination from polluted water. TrAC—Trends Anal. Chem. 2024, 171, 117534. [Google Scholar] [CrossRef]
- Bogeat, Á.E. Utilización de Carbón Activado y Titania en la Ozonación Fotocatalítica de Contaminantes Emergentes del agua (Doctoral Dissertation, Universidad de Extremadura). 2012. Available online: https://dialnet.unirioja.es/servlet/dctes?codigo=180793 (accessed on 26 December 2024).
- Delgado, N.; Capparelli, A.; Navarro, A.; Marino, D. Pharmaceutical emerging pollutants removal from water using powdered activated carbon: Study of kinetics and adsorption equilibrium. J. Environ. Manag. 2019, 236, 301–308. [Google Scholar] [CrossRef]
- Ormad, M.P.; Miguel, N.; Claver, A.; Matesanz, J.M.; Ovelleiro, J.L. Pesticides removal in the process of drinking water production. Chemosphere 2008, 71, 97–106. [Google Scholar] [CrossRef]
- Díaz Viúdez, A. Estudio de la Eliminación de Fármacos Mediante la Combinación de Carbón Activo y SBR. 75. 2016. Available online: http://hdl.handle.net/10251/67612 (accessed on 26 December 2024).
- Wang, W.; Park, S.; Choi B gyu Oh, J.E. Occurrence and removal of benzotriazole and benzothiazole in drinking water treatment plants. Environ. Pollut. 2023, 316, 120563. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J.; Rehfeld, D.; Träder, K.; Sperlich, A.; Jekel, M. Combination of granular activated carbon adsorption and deep-bed filtration as a single advanced wastewater treatment step for organic micropollutant and phosphorus removal. Water Res. 2016, 92, 131–139. [Google Scholar] [CrossRef]
- Östman, M.; Björlenius, B.; Fick, J.; Tysklind, M. Effect of full-scale ozonation and pilot-scale granular activated carbon on the removal of biocides, antimycotics and antibiotics in a sewage treatment plant. Sci. Total Environ. 2019, 649, 1117–1123. [Google Scholar] [CrossRef]
- ISO 15705:2002; Water Quality—Determination of the Chemical Oxygen Demand Index (ST-COD)—Small-Scale Sealed-Tube Method. International Organization for Standardization: Geneva, Switzerland, 2002.
- Shu, Z.; Bolton, J.R.; Belosevic, M.; Gamal El Din, M. Photodegradation of emerging micropollutants using the medium-pressure UV/H2O2 Advanced Oxidation Process. Water Res. 2013, 47, 2881–2889. [Google Scholar] [CrossRef]
- Tufail, A.; Alharbi, S.; Alrifai, J.; Ansari, A.; Price, W.E.; Hai, F.I. Combining enzymatic membrane bioreactor and ultraviolet photolysis for enhanced removal of trace organic contaminants: Degradation efficiency and by-products formation. Process Saf. Environ. Prot. 2021, 145, 110–119. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Xiao, Y.; Chang, V.W.C.; Lim, T.T. Direct and indirect photodegradation pathways of cytostatic drugs under UV germicidal irradiation: Process kinetics and influences of water matrix species and oxidant dosing. J. Hazard. Mater. 2017, 324, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Véliz Flores, R.R.; Aronés Medina, E.G.; Palomino Malpartida, Y.G.; Huincho Rodríguez, R. Desinfección Del Efluente Secundario De La Planta De Agua Residual De Ayacucho Con Radiación Ultravioleta Para Su Reutilización En Riego Agrícola. Rev. De La Soc. Química Del Perú 2018, 84, 41–56. [Google Scholar] [CrossRef]
- Wang, H.; Zhan, J.; Yao, W.; Wang, B.; Deng, S.; Huang, J.; Yu, G.; Wang, Y. Comparison of pharmaceutical abatement in various water matrices by conventional ozonation, peroxone (O3/H2O2), and an electro-peroxone process. Water Res. 2018, 130, 127–138. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, G.; Deng, S.; Huang, J.; Wang, B. The electro-peroxone process for the abatement of emerging contaminants: Mechanisms, recent advances, and prospects. Chemosphere 2018, 208, 640–654. [Google Scholar] [CrossRef] [PubMed]
- ASTM D3860-98(2020); Standard Practice for Determination of Adsorptive Capacity of Activated Carbon by Aqueous Phase Isotherm Technique. ASTM: West Conshohocken, PA, USA, 2008.
- Requena Molina, M. Diseño de un Sistema Combinado de Carbón Activo y Reactor Biológico Secuencial para Eliminación de Compuestos Farmacéuticos Presentes en Aguas Residuales Urbanas. 2018. Available online: http://hdl.handle.net/10251/108813 (accessed on 26 December 2024).
- Alharbi, S.K.; Kang, J.; Nghiem, L.D.; van de Merwe, J.P.; Leusch, F.D.L.; Price, W.E. Photolysis and UV/H2O2 of diclofenac, sulfamethoxazole, carbamazepine, and trimethoprim: Identification of their major degradation products by ESI–LC–MS and assessment of the toxicity of reaction mixtures. Process Saf. Environ. Prot. 2017, 112, 222–234. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical Processes for Water Treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Chaves, F.P.; Gomes, G.; Della-Flora, A.; Dallegrave, A.; Sirtori, C.; Saggioro, E.M.; Bila, D.M. Comparative endocrine disrupting compound removal from real wastewater by UV/Cl and UV/H2O2: Effect of pH, estrogenic activity, transformation products and toxicity. Sci. Total Environ. 2020, 746, 141041. [Google Scholar] [CrossRef]
- Zhang, A.; Li, Y. Removal of phenolic endocrine disrupting compounds from waste activated sludge using UV, H2O2, and UV/H2O2 oxidation processes: Effects of reaction conditions and sludge matrix. Sci. Total Environ. 2014, 493, 307–323. [Google Scholar] [CrossRef]
- Okolie, J.A.; Savage, S.; Ogbaga, C.C.; Gunes, B. Assessing the potential of machine learning methods to study the removal of pharmaceuticals from wastewater using biochar or activated carbon. Total Environ. Res. Themes 2022, 1–2, 100001. [Google Scholar] [CrossRef]
- Bernal, M.Á. Reducción de Microcontaminantes Mediante Procesos Biológicos, Tratamiento con Membranas y Carbón Activado. 2020. Available online: http://hdl.handle.net/10045/109781 (accessed on 26 December 2024).


| Chemical Structure | Chemical and Physical Properties | ||
|---|---|---|---|
| Benzotriazole | |||
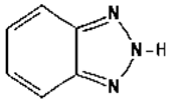 | CAS Number: | 95-14-7 | |
| Molecular formula: | C6H5N3 | ||
| Use: | Antifouling basic product | ||
| Molecular weight (g/mol): | 119,12 | ||
| Solubility (g/L): | 19 (25 °C) | ||
| 2H-benzotriazole | Log Kow: | 1.34 | |
| pKa: | 8.37 (20 °C) | ||
| Fluconazole | |||
 | CAS Number: | 86386-73-4 | |
| Molecular formula: | C13H12F2N6O | ||
| Use: | Animycotic drug | ||
| Molecular weight (g/moL): | 306.27 | ||
| Solubility (g/L): | 4,4 × 10−3 (25 °C) | ||
| 2-(2,4-difluorophenyl)-1,3-bis(1,2,4-triazol-1-yl)propan-2-ol | Log Kow: | 0.5 | |
| pKa: | 1.76 (20 °C) | ||
| Metconazole | |||
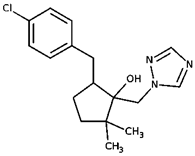 | CAS Number: | 125116-23-6 | |
| Molecular formula: | C17H22ClN3O | ||
| Use: | Pesticide | ||
| Molecular weight (g/moL): | 319.83 | ||
| Solubility (g/L): | 30.4 (20 °C) | ||
| 5-[(4-chlorophenyl)methyl]-2,2-dimethyl-1-(1,2,4-triazol-1-ylmethyl)cyclopentan-1-ol | Log Kow: | 3.85 | |
| pKa: | - | ||
| MetilBenzotriazole | |||
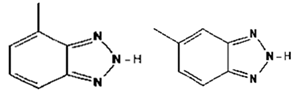 | CAS Number: | 29878-31-7 | |
| Molecular formula: | C7H7N3 | ||
| Use: | Antifouling basic product | ||
| Molecular weight (g/moL): | 133.15 | ||
| Solubility (g/L): | 4.05 (25 °C) | ||
| 4-methyl-2H-benzotriazole 5-methyl-2H-benzotriazole | Log Kow: | 1.08 | |
| pKa: | 8.85 (20 °C) | ||
| Chemical Structure | Chemical and Physical Properties | ||
| Penconazole | |||
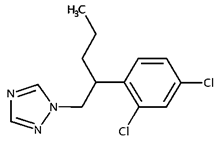 | CAS Number: | 66246-88-6 | |
| Molecular formula: | C13H15Cl2N3 | ||
| Use: | Pesticide | ||
| Molecular weight (g/moL): | 284.18 | ||
| Solubility (g/L): | 0.073 (20 °C) | ||
| 1-[2-(2,4-dichlorophenyl)pentyl]-1,2,4-triazole | Log Kow: | 3.72 | |
| pKa: | - | ||
| Sulfametoxazole | |||
 | CAS Number: | 723-46-6 | |
| Molecular formula: | C10H11N3O3S | ||
| Use: | Antibiotic drug | ||
| Molecular weight (g/moL): | 253.28 | ||
| Solubility (g/L): | 0.61 (37 °C) | ||
| 4-amino-N-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide | Log Kow: | 0.89 | |
| pKa: | 1.6 | ||
| Tebuconazole | |||
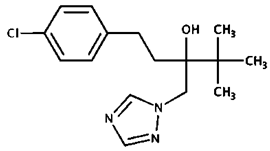 | CAS Number: | 107534-96-3 | |
| Molecular formula: | C16H22ClN3O | ||
| Use: | Pesticide | ||
| Molecular weight (g/moL): | 307.82 | ||
| Solubility (g/L): | 0.036 (20 °C) | ||
| 1-(4-chlorophenyl)-4,4-dimethyl-3-(1,2,4-triazol-1-ylmethyl)pentan-3-ol | Log Kow: | 3.7 | |
| pKa: | 2.3 | ||
| Operational Parameters | ||
|---|---|---|
| Volume of water | L | 15 |
| Initial concentration of each CEC | µg/L | 10 |
| Water flow rate | L/min | 8 |
| Radiation intensity | J/m2 | 1.069 |
| Duration of test | min | 58 |
| Operational Parameters | ||
|---|---|---|
| Volume of water in each reactor | L | 0.5 |
| Concentration of CEC | µg/L | 10 |
| Initial Concentration of H2O2 | mg/L | 10, 50, 500 and 1000 |
| Test time | min | 30 |
| Stirring rate | rpm | 150 |
| H2O2 Entered (mg/L) | H2O2 Residual (mg/L) | H2O2 Consumed (%) |
|---|---|---|
| 10 | 0 | 100 |
| 50 | 0 | 100 |
| 500 | 170 | 66 |
| 1000 | 170 | 83 |
| Specification | AquaSorb CS |
|---|---|
| Iodine number | min. 1000 mg/g |
| Moisture content, as packed | max. 5% |
| Ash content | max. 4% |
| Ball-pan hardness | min. 98% |
| Surface are (BET) | 1050 m2/g |
| Apparent density | 540 kg/m3 |
| Operational Parameters | ||
|---|---|---|
| Volume of water in each reactor | L | 0.5 |
| Concentration of CEC | µg/L | 10 |
| Concentration of GAC | mg/L | 100, 500, 1000, 2000 and 4000 |
| Stirring rate | rpm | 150 |
| Duration of test | h | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferre, M.; Moya-Llamas, M.J.; Dominguez, E.; Ortuño, N.; Prats, D. Advanced Oxidation Processes and Adsorption Technologies for the Removal of Organic Azo Compounds: UV, H2O2, and GAC. Water 2025, 17, 212. https://doi.org/10.3390/w17020212
Ferre M, Moya-Llamas MJ, Dominguez E, Ortuño N, Prats D. Advanced Oxidation Processes and Adsorption Technologies for the Removal of Organic Azo Compounds: UV, H2O2, and GAC. Water. 2025; 17(2):212. https://doi.org/10.3390/w17020212
Chicago/Turabian StyleFerre, M., M. J. Moya-Llamas, E. Dominguez, Nuria Ortuño, and D. Prats. 2025. "Advanced Oxidation Processes and Adsorption Technologies for the Removal of Organic Azo Compounds: UV, H2O2, and GAC" Water 17, no. 2: 212. https://doi.org/10.3390/w17020212
APA StyleFerre, M., Moya-Llamas, M. J., Dominguez, E., Ortuño, N., & Prats, D. (2025). Advanced Oxidation Processes and Adsorption Technologies for the Removal of Organic Azo Compounds: UV, H2O2, and GAC. Water, 17(2), 212. https://doi.org/10.3390/w17020212







