Abstract
While temperature, pH, DO, and ammonia nitrogen concentration are known to affect nitrous oxide (N2O) emissions from ammonia-oxidizing bacteria (AOB), the specific responses of individual AOB species to these environmental variables have yet to be fully elucidated. The present study reports the isolation and pure culture of a new AOB strain, designated as N.eA1, from a stable CANON bioreactor. The strain’s denitrification and N2O emission were systematically evaluated through a comprehensive analysis of growth kinetics, morphological characteristics, genetic composition, and nitrogen transformation under various environmental processes. Our results indicated that N.eA1 shares 95.33% sequence homology with Nitrosomonas europaea H1 AOB3, and exhibited higher nitrite (NO2−-N) conversion efficiency. Morphological examination revealed white, semi-transparent spherical colonies. The bacterial growth kinetics included adaptation phase (0–12 h), exponential growth phase (12–36 h), stationary phase (36–72 h) and decline phase (after 72 h). Under optimal cultivation conditions (30 °C, DO concentration of 7.3 mg∙L−1, pH 8.0, and NH4+-N concentration of 260 mg∙L−1), the culture achieved a maximum growth rate of 0.0723 h−1, a maximum ammonia oxidation rate (AOR) of 10.74 mg∙(MLVSS∙h)−1, and a minimum doubling time of 9.59 h. The peak time of nitrogen conversion was earlier than that of N2O emission, with a maximum N2O-N conversion from NH4+-N of 1.039%.
1. Introduction
Global warming has garnered extensive attention in recent years [1], with the reduction of greenhouse gas (GHG) emissions emerging as a primary strategy to address this challenge [2]. Among these gases, N2O, a significant contributor to stratospheric ozone depletion, has attracted increasing scientific scrutiny [3]. Previous studies indicate that N2O exhibits a global warming potential 298 times greater than that of carbon dioxide (CO2) over a 100-year period, with its atmospheric concentration continuing to rise at an annual rate of 0.26% [4]. Wastewater treatment processes have been identified as one of the principal sources contributing to this phenomenon. In these processes, the formation of N2O is driven by multiple microbial processes, including nitrification and denitrification [5,6]. Therefore, understanding the mechanisms of microbial N2O production in biological treatment systems is crucial for regulating N2O emissions in wastewater treatment processes.
Biological treatment has gained prominence in nitrogen removal from wastewater due to its cost-effectiveness and minimal secondary pollution [7,8]. Within these systems, ammonia-oxidizing bacteria (AOB) are crucial. AOB are classified as Gram-negative chemolithoautotrophic microorganisms that utilize ammonia oxidation as their primary energy source [9]. These organisms exhibit distinctive physiological characteristics, including stringent growth requirements with a preference for neutral to slightly alkaline conditions, and grow slowly [10,11]. Therefore, AOB affect the nitrogen content of the entire ecosystem [12,13]. Their significance extends beyond wastewater treatment, as they represent crucial mediators in the global nitrogen cycle and constitute essential members of the microbial consortia present in wastewater treatment processes [10]. AOB participate in both nitrification and denitrification. Nitrification is an important N cycling process in ecosystems [14], which oxidizes ammonia (NH3) to nitrate (NO3−-N) via intermediate products, i.e., hydroxylamine (NH2OH) and nitrite (NO2−-N) in a two-step process, and can consequently emit N2O as a by-product under certain conditions [12,15]. Ammonia oxidation, the oxidation of NH3 to NO2−-N via NH2OH, is the rate-limiting step in nitrification and can be carried out by both AOB and ammonia-oxidizing archaea (AOA). Nitrification-related processes (the oxidation of NH2OH and NO2−-N reduction) mediated by AOB are recognized to be a main pathway of N2O emission [16].
While the role of AOB in nitrogen cycling has been well established, their environmental sensitivity remains a critical factor influencing both their performance and N2O emissions. Environmental parameters, including temperature, DO, and pH, significantly affect AOB activity and N2O production [17]. Jiang [18] demonstrated that low-temperature conditions reduce the activity of AOB by impairing functional gene clusters, thereby suppressing N2O emissions in AOB-dominated communities. Guo [19] utilized online monitoring to link real-time N2O fluxes with microbial community dynamics in an anoxic–oxic (A/O) wastewater treatment system. Their findings underscore the critical role of pH regulation, recommending an influent pH of 6–8 as optimal for minimizing emissions during anaerobic processes. Rathnayake [20] observed a positive correlation between dissolved oxygen (DO) levels (0.5–7.3 mg∙L−1) and N2O production rates in autotrophic partial nitrification (PN) granules, attributing this phenomenon to enhanced ammonia oxidation at higher DO concentrations. Sarkar [21] investigated the effects of historical land use and precipitation regimes on the metabolic niches of ammonia-oxidizing archaea (AOA) and AOB in terrestrial ecosystems, demonstrating that drought stress amplifies N2O fluxes through niche partitioning between these microbial guilds.
These studies provide valuable insights into the environmental adaptability of AOB and their contributions to N2O emissions. However, technical limitations have confined most prior investigations to mixed microbial consortia, inherently masking strain-specific responses to operational parameters [22]. Furthermore, the slow growth rates, fastidious nutritional requirements, and susceptibility of AOB to environmental perturbations have posed substantial challenges in obtaining stable pure cultures [23]. For instance, in partial nitrification systems, elevated N2O fluxes are often ambiguously attributed to AOB-dominated processes, without distinguishing contributions from individual species or their interactions with heterotrophs [24]. Systematic comparisons of AOB ammonia oxidation kinetics and N2O emissions under well-controlled multi-parametric conditions remain scarce. Critical unresolved questions include the following: How do individual AOB strains balance ammonium oxidation efficiency against N2O emission potential across environmental gradients? Can pure culture-derived thresholds for pH, DO, and temperature be directly translated to complex bioreactor ecosystems?
This study aims to address these persistent challenges by unraveling the strain-specific contributions of AOB to N2O emissions. The primary objectives are as follows: establishing axenic cultures of an AOB strain isolated from a CANON bioreactor; systematically quantifying its ammonia oxidation kinetics and emission responses across environmental gradients, including temperature, pH, DO, and NH4+-N concentrations, and exploring the temporal dynamics of nitrogen transformation; developing threshold-based operational guidelines to optimize nitrogen removal efficiency while mitigating N2O emissions. The experimental design progresses sequentially from microbial isolation and genomic characterization to controlled-environment physiological profiling, ultimately exploring the nitrogen transformation pathway. Throughout this workflow, we prioritize decoupling strain-specific behaviors from mixed-culture effects—a critical gap in previous studies on microbial consortia. By isolating AOB strain-specific responses to environmental stressors, this study eliminates confounding interactions with heterotrophic denitrifiers or AOA, thereby resolving conflicting observations in previous mixed-culture research. Furthermore, we offer a predictive framework for optimizing CANON bioreactors. By integrating kinetic modeling with phylogenetic analysis, we demonstrate that phylogenetically similar AOB strains exhibit divergent N2O emission potentials under identical conditions, highlighting the need for strain-level resolution in greenhouse gas inventories. This study provides critical insights for optimizing AOB performance and mitigating N2O emissions, thereby contributing to the development of more sustainable nitrogen removal technologies.
2. Materials and Methods
2.1. Separation, Purification and Enrichment Culture
Original sludge was sourced from a stable CANON bioreactor operating in a laboratory (Guizhou, China). The inlet water of the bioreactor is simulated industrial sewage, and its water quality is characterized by high NH4+-N and low C/N ratio. The bacterial strain was introduced into 100 mL of liquid culture medium (Table 1) at an inoculation volume ratio of 2% (v/v). Cultivation was performed in a shaking incubator (VRERA, Shanghai, China) maintained at 30 °C with an agitation rate of 120 r∙min−1, without light throughout the incubation period. Medium pH was adjusted to 8.0 using 1 mol∙L−1 hydrochloric acid (HCl) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and sodium hydroxide (NaOH) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) solutions. NO2−-N accumulation was monitored at 24 h intervals. Positive cultures exhibiting significant NO2−-N accumulation were subcultured into fresh medium at a 1% (v/v) inoculation ratio for five consecutive rounds. Subsequently, positive samples were subjected to three rounds of streak/spread plate isolation on solid medium, followed by selection of single colonies for incubation in a shaking incubator (Figure 1). Throughout the experimental period, pH was regularly monitored and maintained at 8.0. For solid medium preparation, 1.5% (w/v) agar was supplemented. The medium was subsequently autoclaved at 121 °C for 30 min and stored until use.

Table 1.
Ammonia-oxidizing bacteria purification medium.
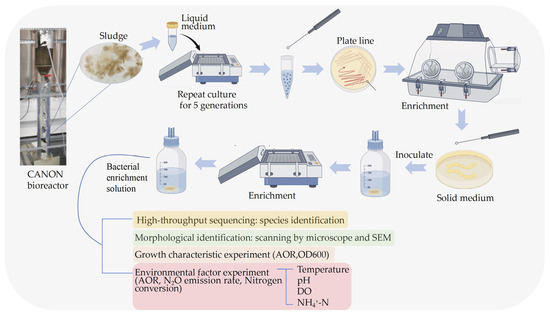
Figure 1.
Experimental methods and procedures.
2.2. Growth Characteristic Experiment
The bacterial enrichment culture obtained from Section 2.1 was inoculated into fresh liquid medium at a 2% (v/v) inoculation ratio. Initial cultivation parameters were established as follows: NH4+-N concentration of 260 mg∙L−1 and pH 8.0. The cultures were maintained in an orbital shaking incubator under light-excluded conditions at 30 °C with continuous agitation at 120 r·min−1. Quantitative measurements of NH4+-N concentration, NO2−-N concentration, and bacterial growth (monitored as optical density at 600 nm) were conducted at 12 h intervals throughout the 96 h cultivation period. All experimental conditions were established with duplicate control groups.
2.3. Environmental Factor Experiment
The bacterial enrichment culture obtained from Section 2.1 was inoculated into fresh liquid medium at a 2% (v/v) inoculation ratio. The experimental design incorporated the investigation of four environmental parameters: temperature, pH, DO concentration and NH4+-N concentration (Table 2). Throughout the environmental factor studies, all parameters were maintained at constant levels except for the specific variable under investigation. All experimental conditions were established with duplicate control groups. In pH experiments, medium pH was adjusted through titration with 1 mol∙L−1 HCl and NaOH solutions. A phosphate buffer system was incorporated to ensure pH stability throughout the experimental period. pH was monitored at 6 h intervals.

Table 2.
Single factor experiment.
For DO experiments, aeration was facilitated through a glass tube apparatus extending to the vessel bottom, with the external air inlet equipped with two serially-connected 0.22 μm filters to prevent microbial contamination from ambient air. DO concentrations were regulated via a controlled aeration system (Aquasystems International, Halle, Belgium), implementing distinct aeration durations (5, 20, 40, and 60 min) at 2-h intervals. A non-aerated control group was maintained throughout the experiment, with DO levels continuously monitored using a calibrated DO meter (YSI, Yellow Springs, OH, USA). Gas samples were collected over 1 min intervals from each experimental group at 6 h intervals during aeration periods. Prior to subsequent aeration cycles, cultures were subjected to agitation at 200 r∙min−1 for 20 min, followed by collection of headspace gas samples. In the NH4+-N concentration experiment, while the theoretical NH4+-N concentrations were set at 50, 100, 200, and 400 mg∙L−1, the actual measured concentrations were 36.5, 71.0, 133.0, and 281.0 mg∙L−1, respectively.
2.4. Bioinfomatics Analysis
The enriched flocculent biomass was placed in 1.5 mL centrifuge tubes and centrifuged at 4500 rpm for 5 min. After discarding the supernatant, sterile water was added to restore the original volume. This centrifugation process was repeated three times. The resulting biomass was stored at −20 °C and sent to Guiyang Weilai Testing Co., Ltd. (Guiyang, China) for bio-electron microscopy analysis.
The enrichment culture from a single colony was centrifuged, and genomic DNA was extracted using the Ezup Column Bacterial Genomic DNA Extraction Kit (Shanghai Sangon Biotech, Shanghai, China). PCR amplification was done using the TC1000-G PCR machine (SCILOGEX, Rocky Hill, CT, USA). PCR amplification was performed using primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1541R (5′-AAGGAGGTGATCCA GCC-3′) as forward and reverse primers, respectively. The PCR products were verified by 1% (w/v) agarose gel electrophoresis and then sent to Sangon Biotech (Shanghai, China) for sequencing. The sequencing results were analyzed using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 14 November 2023) against the NCBI database, and a phylogenetic tree was constructed using the Neighbor-Joining method implemented in MEGA11.
2.5. Analytical Methods
NH4+-N, NO2−-N concentrations and OD600 were measured using a UV-visible spectrophotometer (Shimadzu, Kyoto, Japan). NH4+-N was measured using the Nessler’s reagent spectrophotometric method [25], with a wavelength of 420 nm. NO2−-N was measured using the N-(1-naphthyl)-ethylenediamine spectrophotometric method [26], with a wavelength of 520 nm. N2O was collected at each phase using a gas sampler and analyzed using a GC9790+ gas chromatograph (Foley Instrument Co., LTD, Zhejiang, China). High-purity N2 (99.999%) was used as the carrier gas at a flow rate of 21 mL·min−1. The temperatures of the ECD detector and column oven were set at 250 °C and 70 °C, respectively. Data analyses and visualizations were performed using Python (Version 3.8.5, Python Software Foundation). The maximum growth rate (μmax) was determined through linear regression analysis of the natural logarithm of OD600 values during the exponential growth phase (12–36 h) using scipy.stats module.
The maximum specific growth rate (μmax) (h−1) and minimum doubling time (td) (h) were calculated as follows:
td = ln (2)/μmax
The maximum AOR [mg∙(L∙h)−1] was calculated by linear regression of NH4+-N consumption data during the period of highest activity; the unit conversion was performed using the following formula (the specific formula is in Appendix A):
where:
Vmax = v/Cmlvss
Vmax: Maximum AOR [mg∙(MLVSS∙h)−1]
v: Maximum AOR [mg∙(L∙h)−1]
Cmlvss: 0.38—biomass concentration (g∙L−1)
The N2O conversion percentage was determined by the ratio of N2O-N (sum of N2O in liquid and gas phases) to the total nitrogen change (ΔN). The conversion percentage of N2O was calculated as follows (the specific formula is in Appendix A):
where:
P = (N2Oliquid + N2Oair)/ΔN × 100%
N2Oliquid: N2O in liquid (mg∙L−1)
N2Oair: N2O in air (mg∙L−1)
ΔN: Change in total nitrogen (mg∙L−1)
In the measurement of NH4+-N, nitrogen exists in both ammonium ion (NH4+) and free ammonia (FA) forms, which undergo dynamic interconversion. An equilibrium relationship exists between NH4+ and FA in wastewater. The calculation formula for FA concentration was derived by incorporating the ionization constants of ammonia and water into the equilibrium equation [27,28]:
FA = 17 (NH4+-N) × 10pH/14e6334/(273+t) + 10pH
Results are presented as mean values of triplicate experiments with standard deviation (mean ± SD, n = 3). Time series analyses were conducted to evaluate the temporal dynamics of NH4+-N degradation, NO2−-N accumulation, and N2O emissions in different environmental conditions.
Sensitivity Analysis Using Standardized Regression Coefficients (SRC) [29] quantifies the effect of each independent variable on the dependent variable (N2O emissions) in a regression model. This method is widely used to understand how variations in input variables impact model outputs [30]. The regression model can be expressed as follows:
where:
: N2O emissions (dependent variable)
: The environmental factors (temperature, pH, DO, and NH4+-N)
: The regression coefficients
The data for each independent variable (X) were standardized by subtracting the mean and dividing by the standard deviation. This ensures that each variable contributes equally to the analysis, allowing for comparison of their relative effects on N2O emissions.
The Standardized Regression Coefficients (SRC) are given by the following equation:
where:
: The regression coefficient for each independent variable
: The standard deviation of the independent variable
: The standard deviation of the dependent variable
The sensitivity analysis was carried out using Python 3.8 and the statsmodels package for regression analysis. Data were first standardized, and then an Ordinary Least Squares (OLS) regression was performed. The SRC values were calculated to quantify the influence of each environmental factor on N2O emissions.
The R2 value of the regression model was also calculated to ensure the validity of the results. The R2 value indicates the proportion of variance in N2O emissions explained by the model. A value above 0.7 was considered acceptable for determining the effectiveness of the SRC coefficients.
3. Results and Discussion
3.1. Growth Characteristics, Genetic Identification and Morphological Analysis
A comparative analysis on the NCBI website revealed a 95.33% homology with Nitrosomonas europaea H1 AOB3 (Figure 2), and the strain was designated as N.eA1. Colonies appeared as white, semi-translucent, spherical structures, morphologically similar to the Nitrosomonas colonies isolated from activated sludge [7]. SEM images revealed rod-shaped bacterial cells, measuring 8–10 µm × 5–6 µm, enveloped in extracellular polymeric substances (EPS) (Figure 3b,d). Specifically, EPS serves to protect AOB against environmental stressors [31] and provides nutrient reserves during starvation periods, thereby enhancing microbial stability and function [32,33]. However, excessively thick EPS layers can impede oxygen and ammonia diffusion, potentially reducing nitrogen removal efficiency [34]. Therefore, maintaining optimal EPS thickness is crucial for maximizing CANON system performance.
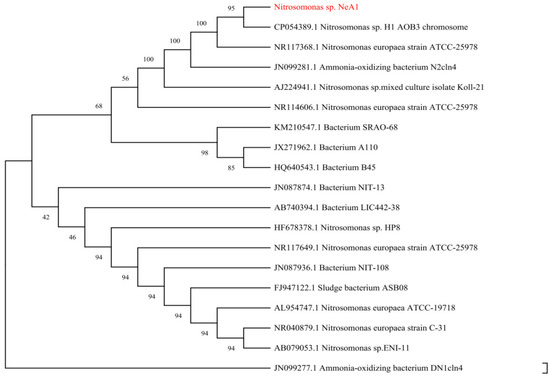
Figure 2.
Evolutionary relationships of taxa. The red text is the target test strain; The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches.
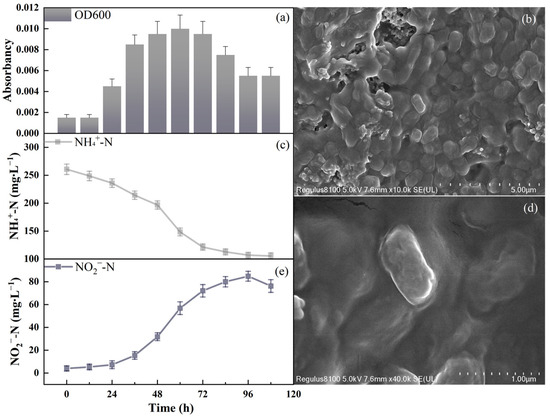
Figure 3.
Strain N.eA1 growth status: (a): OD600 value during the growth of strains; (b,d): SEM images; (c): NH4+-N change during the growth of strains; (e): NO2−-N change during the growth of strains.
The adaptation, exponential growth, stationary, and decline phases occurred at 12 h, 12–36 h, 36–72 h, and above 72 h post-inoculation, respectively. During the logarithmic phase, the strain exhibited enhanced growth rates (Figure 3a), with a maximum growth rate of 0.0723 h−1 and a minimum doubling time of 9.59 h, similar to Nitrosomonas europaea [35,36]. Furthermore, under similar NO2−-N concentrations, the N2O emission rate of the N.eA1 strain (3.494 × 10−5 mg N2O/mg NO2−-N) was higher than that of Nitrosomonas europaea (1.048 × 10−5 mg N2O/mg NO2−-N) [37], suggesting that the N.eA1 strain may maintain a higher metabolic activity state under similar NO2−-N concentrations.
Throughout the growth process, the maximum AOR was 10.74 mg∙(MLVSS∙h)−1, with an average AOR of 5.08 mg∙(MLVSS∙h)−1. When the initial NH4+-N concentration was 260 mg·L−1, more than 50% of NH4+-N degradation occurred within 72 h (Figure 3c). This rapid degradation phase was likely driven by the high metabolic activity of the strain during the initial stages of cultivation, where NH4+-N served as the primary substrate for energy production and growth. This finding is consistent with previous studies indicating that AOB can efficiently oxidize ammonia under optimal conditions [38,39]. Correspondingly, NO2−-N accumulation increased over time (Figure 3e). In the later stages of cultivation, the NH4+-N concentration gradually leveled off until it remained constant, potentially due to NO2−-N accumulation leading to reduced bacterial activity and inhibition of the ammonia oxidation reaction. It was observed that when NO2−-N exceeded 90 mg·L−1, it significantly inhibited AOB activity, as high nitrite levels can lead to toxic conditions for AOB, interfering with their metabolic processes [40]. Interestingly, the decline in NO2−-N during the later stages suggested a possible shift in the metabolic activity of the strain towards denitrification. Increased nitrite reductase (NIR) enzyme expression in response to high NO2−-N concentrations can enhance the conversion of nitrite to gaseous products such as N2O and N2 [41].
3.2. Effect of Temperature on Strain N.eA1
At 30 °C, the AOR and the NO2−-N accumulation rate were significantly higher compared to the other two temperatures. The average AORs at the three temperatures were 3.13 mg∙(MLVSS∙h)−1, 5.00 mg∙(MLVSS∙h)−1 and 4.92 mg∙(MLVSS∙h)−1, respectively. The maximum N2O emission occurred between 24 and 36 h at 35 °C, reaching 1030.21 μg∙L−1. The highest N2O conversion percentage occurred at 24 h, after which the conversion percentage decreased. The total N2O release concentrations after 48 h reached 1223.07 μg∙L−1, 2406.48 μg∙L−1 and 2699.36 μg∙L−1, respectively (Figure 4).
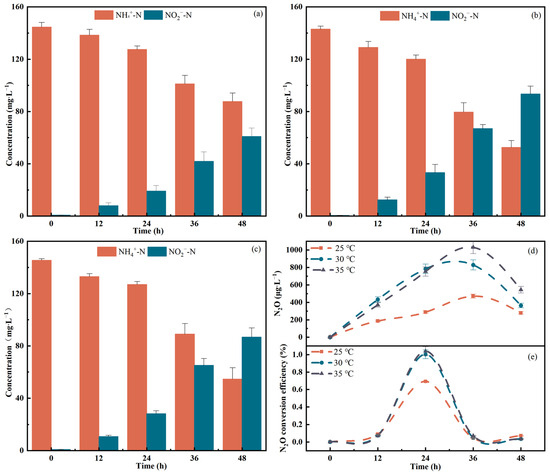
Figure 4.
Effect of temperature on N.eA1: (a): NH4+-N and NO2−-N change at 25 °C; (b): NH4+-N and NO2−-N change at 30 °C; (c): NH4+-N and NO2−-N change at 35 °C; (d): N2O emission concentration at different temperatures; (e): the degree of N2O conversion at different temperatures.
Temperature could be one of the key factors contributing to N2O emissions during nitrification and denitrification processes [42]. The maximum AOR occurs at 30 °C, indicating that increased temperature promoted NH4+-N degradation by the AOB strain. However, the excessively high temperatures inhibited bacterial activity [43]. During the logarithmic growth phase (12–36 h), the bacteria exhibited a high demand for oxygen, which may have resulted in the incomplete oxidation of NH2OH and the release of N2O. Additionally, the high bacterial density during this phase likely contributed to a significant portion of N2O production through the denitrification process. Furthermore, the apparent relationship between N2O emissions and temperature has been investigated in several studies. The N2O solubility decreases as temperature increases (being about two times lower at 25 °C than at 5 °C), therefore the liquid phase N2O is more easily stripped into the gas phase, leading to the enhancement of N2O emissions [44]. The peak N2O conversion percentage was recorded at 24 h. The N2O emission concentration peaked at 36 h, indicating that during this period (24–36 h), although N2O emissions continued to rise, more NH4+-N was being converted into NO, NO2−-N, and NO3−-N. It can also be seen from the change of nitrite concentration that the accumulation of nitrite increased significantly during this period. The maximum AOR also appeared in this period.
3.3. Effect of DO Concentration on Strain N.eA1
As shown in Figure 5, the consumption of NH4+-N and the accumulation of NO2−-N both increased with the rise in DO concentration. In the non-aerated control group, the NH4+-N conversion percentage at 30 h was 9.8%, with a conversion amount of 14.8 mg·L−1, and the accumulated NO2−-N was 8.0 mg·L−1. At DO concentration of 7.3 mg·L−1, the 30 h conversion rate of NH4+-N reached 45.7%, with a conversion amount of 68.8 mg·L−1, and the accumulation of NO2−-N was 35.7 mg·L−1. The N2O emissions during the aeration stage were significantly lower than those during the unaerated stage. During the unaerated stage, N2O emissions increased over time. However, during the aeration stage, when the DO concentration exceeded 4.5 mg·L−1, N2O emissions peaked between 12 and 18 h and subsequently declined, suggesting that high DO concentrations (>4.5 mg·L−1) could significantly reduce N2O emissions while maintaining high ammonia oxidation efficiency. In the non-aerated control group, the N2O release was relatively lower than in other experimental groups.
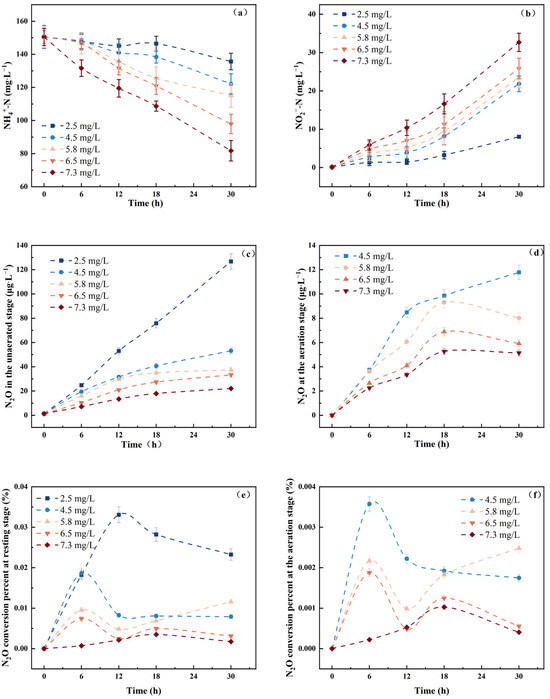
Figure 5.
Effect of DO on N.eA1: (a): changes of NH4+-N at different DO levels; (b): changes of NO2−-N at different DO levels; (c): N2O emission concentration at different DO levels in the resting stage; (d): N2O emission concentration at different DO levels in the aeration stage; (e): N2O conversion percentage at different DO levels in the resting stage; (f): N2O conversion percentage at different DO levels in the aeration stage.
DO concentration simultaneously affects the rates of both nitrification and denitrification [45], and DO is considered an important parameter affecting N2O emissions [46]. According to Park [47], the higher the DO concentration, the faster the accumulation rate of NO2−-N and the lower the amount of N2O released. In another study, Law [48] investigated how varying DO concentrations affected AOR and N2O production in an enriched AOB culture. They observed minimal N2O production at low DO levels (0.05–0.2 mg·L−1), which increased as DO levels rose (2.4 mg·L−1). This finding differs from our results, possibly due to different DO concentration settings. In low DO conditions (<2.4 mg·L−1), increased DO promoted AOR, leading to more rapid production of intermediates (NH2OH, NO). The accumulation of these intermediates resulted in increased N2O production. In high DO conditions, sufficient oxygen favored complete oxidation, reducing the risk of intermediate accumulation and thereby suppressing the expression of denitrification-related enzymes, ultimately leading to decreased N2O emissions. The DO control group exhibited higher N2O accumulation and conversion percentage, as the low-oxygen environment induced denitrification by AOB, and the absence of the NoS encoding gene led to the accumulation of N2O [49]. In the aeration stage, when DO concentration exceeded 4.5 mg·L−1, the N2O emissions decreased in the later stages. This may have been due, in part, to the more complete oxidation of N2O at high DO concentrations, reducing the emission of some N2O. Additionally, high DO concentrations limited the denitrification of AOB [50]. This further demonstrated that high DO concentrations were beneficial for reducing N2O emissions in ammonia oxidation processes in AOB pure culture systems [51].
3.4. Effect of pH on Strain N.eA1
At pH 8.0, the degradation rate of NH4+-N and accumulation rate of NO2−-N reached their maximum values. Within 84 h, 98% of NH4+-N was degraded, while NO2−-N accumulated to 158.1 mg∙L−1. N2O emission peaked between 24 and 36 h, reaching 228.86 μg∙L−1, with a conversion rate of 0.021%. At pH 9.5, the AOR decreased significantly. After 84 h, only 42.1% was degraded, with a conversion amount of 82 mg∙L−1 and NO2−-N accumulation of 39.9 mg∙L−1. This indicates that high pH inhibits the strain’s ammonia oxidation capacity, resulting in significantly reduced N2O emissions. The cumulative N2O release was only 103.8 μg∙L−1, with a maximum conversion rate of 0.017% (Figure 6).
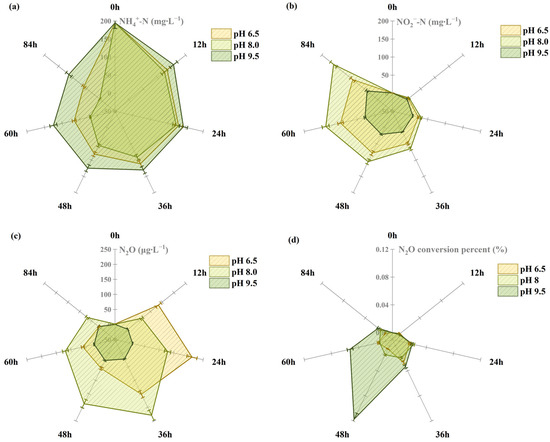
Figure 6.
Effect of pH on N.eA1: (a): changes of NH4+-N at different pH levels; (b): changes of NO2−-N at different pH levels; (c): N2O emission concentration at different pH levels; (d): N2O conversion percentage at different pH levels.
The influence of pH on N2O production during denitrification can be mechanistically explained through the structure of enzymes [52]. The NoS enzyme contains two copper centers (CuA and CuZ), where the electron transfer between these sites significantly impacts N2O reduction efficiency. Within the pH range of 4–8, this electron transfer serves as the rate-limiting step in N2O reduction [53]. Previous studies have established that maintaining pH between 7 and 8 during denitrification is optimal for minimizing N2O emissions [52]. Consistent with previous research, we observed that NO2−-N accumulation inhibited subsequent ammonia oxidation, likely due to its toxic effects on AOB and the consequent reduction in available substrate [54]. Notably, at pH 9.5, the N2O emissions were significantly reduced and the conversion rate of N2O was increased, exhibiting significantly reduced ammonia oxidation capacity. FA formation is favored at higher pH levels, which has been demonstrated to substantially inhibit bacterial activity and consequently reduce ammonia oxidation rates [55]. On the other hand, given that nitrification and denitrification pathways constitute the primary routes for N2O production [56], elevated pH levels can compromise these processes through NOs enzyme inactivation and suppression of microbial activity [57].
3.5. Effect of NH4+-N Concentration on Strain N.eA1
At initial NH4+-N concentrations of 36, 71, 133 and 281 mg∙L−1, the degradation amounts after 48 h were 34.8, 55.9, 81 and 73 mg∙L−1, respectively, while NO2−-N accumulated to 42.6, 50.6, 111.3, and 89.9 mg∙L−1, respectively. Respective N2O emissions at 36 h reached 111.91, 195.11, 142.08 and 249.02 μg∙L−1, with emission peaks observed at 36 h across all concentrations (Figure 7). The experimental group with an initial NH4+-N concentration of 281 mg∙L−1 exhibited the highest ammonia oxidation rate of 5.07 mg∙(MLVSS∙h)−1, corresponding to the maximum NO2−-N accumulation. Calculations using Formulas (2)–(4) yielded theoretical FA values of 3.08, 6.24, 11.55, and 24.48 mg∙L−1 for the four respective NH4+-N concentrations.
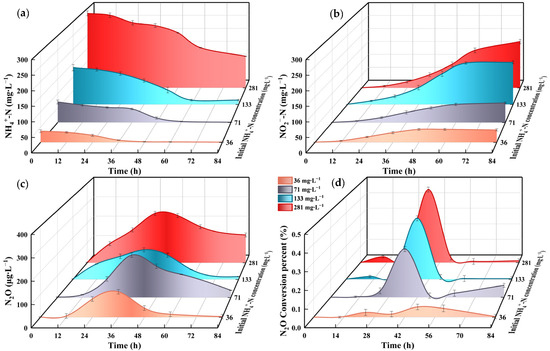
Figure 7.
Effect of NH4+-N on N.eA1: (a): changes of NH4+-N at different NH4+-N levels; (b): changes of NO2−-N at different NH4+-N levels; (c): N2O emission concentration at different NH4+-N levels; (d): N2O conversion percentage at different NH4+-N levels.
At high NH4+-N concentration (281 mg∙L−1), the strain exhibited slightly higher AOR compared to those at 133 mg∙L−1. Nitrosomonas europea can tolerate elevated NH4+-N concentrations [58], where higher NH4+-N levels promote increased ammonia oxidation rates, leading to enhanced AMO gene expression and NH2OH accumulation, subsequently resulting in N2O formation through oxidative pathways [59]. Peak N2O emissions were consistently observed at 36–48 h across all experimental groups. This phenomenon can be attributed to both the logarithmic growth phase of the strain and substrate concentration dynamics [60]. N2O production was relatively high during the initial reaction phase but declined significantly following substantial NH4+-N consumption. AOB demonstrated higher activity during the early stages of the reaction; however, this activity gradually decreased due to limitations in nutrient availability and DO, consequently affecting N2O production. Maximum N2O conversion occurs at 36 h. Maximum AOR also occurs during this period (36–48 h). Notably, at an initial NH4+-N concentration of 133 mg∙L−1, the maximum AOR reached 63.12% of that observed at 281 mg∙L−1. This finding provides valuable economic implications for N2O emission reduction strategies.
3.6. Sensitivity Analysis of Environmental Factors to N2O Emission
The Standardized Regression Coefficients (SRC) for each environmental factor were calculated to evaluate their relative impacts on N2O emissions. The results are summarized in Table 3, showing the SRC values for temperature, pH, DO, and NH4+-N concentration.

Table 3.
SRC for environmental factors affecting N2O emission.
The sensitivity analysis reveals a distinct hierarchical control of environmental parameters over N2O emissions in the isolated AOB strain, with DO (SRC = 0.85) emerging as the predominant regulatory factor. This pronounced dependence on DO aligns with enzymatically constrained ammonia oxidation pathways, where elevated O2 levels accelerate hydroxylamine accumulation through AMO activity, thereby promoting N2O production via nitrifier denitrification [52]. Temperature exhibits secondary yet substantial influence (SRC = 0.62), displaying Arrhenius-type kinetic behavior between 25 and 35 °C, reflecting the thermal acclimation of ammonia mono-oxygenase [61,62]. The high model determination coefficient (R2 = 0.946) underscores the robustness of these univariate relationships, providing a solid theoretical foundation for practical operations.
Thus, maintaining DO between 4.5 and 7.3 mg∙L−1 is beneficial to reduce N2O emissions without AOR reduction. This could be achieved via high-precision DO sensors and adaptive aeration. Thermal management should consider the non-linear temperature effects on N2O emissions. Although increasing the temperature from 25 °C to 30 °C can enhance nitrogen removal efficiency (ΔAOR = +38% in this study), continuous operation above 35 °C may destabilize emission pathways.
4. Conclusions
In this study, we found that the N.eA1 strain demonstrated high homology with Nitrosomonas europaea H1 AOB3, and exhibited comparable growth kinetics patterns. The N.eA1 strain showed a higher risk of N2O emission than that of Nitrosomonas europaea at similar NO2−-N concentration. Environmental factors significantly affected the N2O emission and ammonia oxidation rate of N.eA1; the conditions helpful to the AOR were pH maintained at 7–8, temperature kept at 25–30 °C, and influent NH4+-N concentration maintained above 133 mg∙L−1.
Additionally, the control of DO concentration is crucial. DO concentrations (4.5–7.3 mg∙L−1) promoted enhanced ammonia oxidation while simultaneously reducing N2O emissions. In the process of nitrogen conversion under different environmental conditions, peak N2O emissions consistently occurred during the logarithmic growth phase (12–36 h), but the peak of maximum conversion of N2O was earlier than this time. The maximum AOR time always occurs in this phase (between the timing of maximum N2O conversion and maximum N2O emissions). This finding helps us understand the timing of N2O emissions and nitrogen conversion. However, several limitations remain. This study was conducted under controlled laboratory conditions, and the effects of field-scale variations in environmental parameters (e.g., fluctuations in DO concentrations over time) were not assessed. Additionally, the specific metabolic pathways contributing to elevated N2O emissions in the N.eA1 strain, particularly in comparison to Nitrosomonas europaea, require further investigation. Future research should focus on the interactions between multiple environmental factors and explore the role of microbial community dynamics in shaping N2O emission patterns and AOR. Given that microbial consortia may exhibit distinct behaviors compared to isolated strains, we aim to extend these findings to mixed AOB communities, ultimately contributing to the development of more efficient and environmentally sustainable nitrogen removal technologies.
Author Contributions
Conceptualization, K.L. and Y.L.; methodology, Y.L.; software, H.Y.; validation, Z.Y., H.Y. and X.L.; formal analysis, Y.L.; investigation, Y.L. and Z.Y.; resources, K.L.; data curation, Z.R.; writing—original draft preparation, Y.L. and Z.R.; writing—review and editing, K.L.; visualization, Z.Y. and X.L.; supervision, K.L.; project administration, K.L.; funding acquisition, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the National Natural Science Foundation of China (42107104); (42207086), the Science and Technology Foundation of Guizhou Province ([2019]1153), and Guizhou Provincial Basic Research Program (Natural Science) No. Qiankehe Foundation-ZK (2021) General 226. Additionally, technical support for this research was provided by the Shanghai Shenggong Company and the Wela Detection Co., Ltd.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ongoing work under a national funding project, which has not yet been completed.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| CANON | Completely autotrophic nitrogen removal over nitrite |
| AOR | Ammonia oxidation rate |
Appendix A
Biomass concentration as follows:
where:
: 0.000361—Weight of dried bacteria (g);
x: 0.0095—OD600 value;
: 0.1—Medium volume (L)
N2O concentration in liquid as follows:
where:
: N2O concentration (ppm);
β: 0.544—The Ostwald solubility coefficient of N2O;
: 28—Molar mass of N2O-N (g∙mol−1);
P: 1—Atmospheric pressure (atm);
: 50—Liquid volume in the bottle (mL);
R: 0.08206—Gas constant [L∙atm(K∙mol)−1];
T: 273.15—Temperature (K);
N2O concentration in air as follows:
where:
: N2O concentration (ppm);
: 50—Air volume in the bottle (mL);
: 28—Molar mass of N2O-N (g∙mol−1);
: 22.4—Molar volume of gas (L∙mol−1);
The conversion percentage of N2O as follows:
where:
: 14—Molar mass of N (g∙mol−1);
: 50—Liquid volume in the bottle (mL);
: Initial NO2−-N concentration (mg∙L−1);
: 18—Molar mass of NH4+-N(g∙mol−1);
: Initial NO2−-N concentration (mg∙L−1);
: 28—Molar mass of N2O-N (g∙mol−1);
: NH4+-N concentration after a period of cultivation (mg∙L−1);
: NO2−-N concentration at measuring time (mg∙L−1);
References
- Cui, Z.; Yue, S.; Wang, G.; Meng, Q.; Wu, L.; Yang, Z.; Zhang, Q.; Li, S.; Zhang, F.; Chen, X. Closing the yield gap could reduce projected greenhouse gas emissions: A case study of maize production in China. Glob. Change Biol. 2013, 19, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Chao, Q.; Huang, L. The Core Conclusions and Interpretation of Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Chin. J. Urban Environ. Stud. 2015, 3, 1550003. [Google Scholar] [CrossRef]
- Kuang, W.; Gao, X.; Tenuta, M.; Gui, D.; Zeng, F.J.B.; Soils, F.O. Relationship between soil profile accumulation and surface emission of N2O: Effects of soil moisture and fertilizer nitrogen. Biol. Fertil. Soils 2019, 55, 97–107. [Google Scholar] [CrossRef]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- Guo, J.; Ling, N.; Chen, H.; Zhu, C.; Kong, Y.; Wang, M.; Shen, Q.; Guo, S. Distinct drivers of activity, abundance, diversity and composition of ammonia-oxidizers: Evidence from a long-term field experiment. Soil Biol. Biochem. 2017, 115, 403–414. [Google Scholar] [CrossRef]
- Xu, S.; Feng, S.; Sun, H.; Wu, S.; Zhuang, G.; Deng, Y.; Bai, Z.; Jing, C.; Zhuang, X. Linking N2O emissions from biofertilizer-amended soil of tea plantations to the abundance and structure of N2O-reducing microbial communities. Environ. Sci. Technol. 2018, 52, 11338–11345. [Google Scholar] [CrossRef]
- Abe, T.; Ushiki, N.; Fujitani, H.; Tsuneda, S. A rapid collection of yet unknown ammonia oxidizers in pure culture from activated sludge. Water Res. 2017, 108, 169–178. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Liu, B.; Li, Y.-Y.; Yasui, H. Competitive dynamics of anaerobes during long-term biological sulfate reduction process in a UASB reactor. Bioresour. Technol. 2019, 280, 173–182. [Google Scholar] [CrossRef]
- Pei, Q.; Chen, M.; Li, J.; Liu, J.; Wu, N.; Chen, K.; Chen, X.; Liu, Y.; Feng, Y.; Ren, G.; et al. The protection of ammonia-oxidizing bacteria (AOB) using PDDA/GO composite materials in high salinity wastewater. J. Water Process Eng. 2022, 49, 102998. [Google Scholar] [CrossRef]
- Deepanshi, R.; Anshu, B.; Akshay, K.; Vinod, K.; Gunda, M.; Kashyap, K.D. Applications of autotrophic ammonia oxidizers in bio-geochemical cycles. Chem. Eng. J. 2023, 471, 144318. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, C.; Lin, H.; Liu, W.; Qin, W.; Chen, T.; Yang, T.; Wen, X. Differences in distributions, assembly mechanisms, and putative interactions of AOB and NOB at a large spatial scale. Front. Environ. Sci. Eng. 2023, 17, 122. [Google Scholar] [CrossRef]
- Li, Z.; He, H.; Ding, J.; Zhang, Z.; Leng, Y.; Liao, M.; Xiong, W. Effects of Three Antibiotics on Nitrogen-Cycling Bacteria in Sediment of Aquaculture Water. Water 2024, 16, 1256. [Google Scholar] [CrossRef]
- Zajac, O.; Sudol, M.Z.; Godzieba, M.; Ciesielski, S. Changes in Nitrification Kinetics and Diversity of Canonical Nitrifiers and Comammox Bacteria in a Moving Bed Sequencing Batch Biofilm Reactor—A Long-Term Study. Water 2024, 16, 534. [Google Scholar] [CrossRef]
- Wan, X.; Baeten, J.E.; Volcke, E.I.P. Effect of operating conditions on N2O emissions from one-stage partial nitritation-anammox reactors. Biochem. Eng. J. 2018, 143, 24–33. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; Van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Hakata, M.; Takahashi, M.; Zumft, W.; Sakamoto, A.; Morikawa, H. Conversion of the Nitrate Nitrogen and Nitrogen Dioxide to Nitrous Oxides in Plants. Acta Biotechnol. 2003, 23, 249–257. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, S.; Liao, Y.; Li, S.; Wang, S.; Zhu, X.; Ji, G. Effect of low temperature on contributions of ammonia oxidizing archaea and bacteria to nitrous oxide in constructed wetlands. Chemosphere 2023, 313, 137585. [Google Scholar] [CrossRef]
- Guo, J.; Cong, Q.; Zhang, J.; Zhang, L.; Meng, L.; Liu, M.; Ma, F. Nitrous oxide emission in a laboratory anoxic-oxic process at different influent pHs: Generation pathways and the composition and function of bacterial community. Bioresour. Technol. 2021, 328, 124844. [Google Scholar] [CrossRef]
- Rathnayake, R.M.L.D.; Oshiki, M.; Ishii, S.; Segawa, T.; Satoh, H.; Okabe, S. Effects of dissolved oxygen and pH on nitrous oxide production rates in autotrophic partial nitrification granules. Bioresour. Technol. 2015, 197, 15–22. [Google Scholar] [CrossRef]
- Sarkar, S.; Kazarina, A.; Hansen, P.M.; Ward, K.; Hargreaves, C.; Reese, N.; Ran, Q.; Kessler, W.; de Souza, L.F.; Loecke, T.D.; et al. Metabolism diversification of ammonia-oxidizing archaea and bacteria under different precipitation gradients and land legacies. Appl. Soil Ecol. 2025, 206, 105831. [Google Scholar] [CrossRef]
- Lin, X.; Al-Dhabi, N.A.; Li, F.; Wang, N.; Peng, H.; Chen, A.; Wu, G.; Zhang, J.; Zhang, L.; Huang, H. Relative contribution of ammonia-oxidizing bacteria and denitrifying fungi to N2O production during rice straw composting with biochar and biogas residue amendments. Bioresour. Technol. 2023, 390, 129891. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Cai, C.; Wang, J.; Xu, X.; Zheng, P.; Jetten, M.S.; Hu, B. A novel denitrifying methanotroph of the NC10 phylum and its microcolony. Sci. Rep. 2016, 6, 32241. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, W.; Pei, Z.; Jiao, L. Start-Up and Bacterial Enrichment of an Anammox Reactor with Polyurethane Porous Material: Performance and Microbial Community. Water 2024, 16, 2116. [Google Scholar] [CrossRef]
- HJ 535-2009; Water Quality. Determination of Ammonia Nitrogen. Nessler’s Reagent Spectrophotometry. Ministry of Ecology and Environment: Beijing, China, 2009.
- GB/T 11889-1989; Water Quality—Determination of Aniline Compounds—Spectrophotometric Method with N-(1-naphthyl) Ethylenediamine. State Bureau of Technical Supervision of China: Beijing, China, 1989.
- Lan, D. Solid-contact Potentiometric Sensor for the Determination of Total Ammonia Nitrogen in Seawater. Int. J. Electrochem. Sci. 2017, 12, 3296–3308. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, T.; Wang, S.; Wang, L.; Li, Z.; Li, W.; Wang, B. Low alkalinity, free ammonia, and free nitrous acid cooperatively stabilize partial nitrification under excessive aeration condition. Chemosphere 2024, 352, 141447. [Google Scholar] [CrossRef]
- Pianosi, F.; Beven, K.; Freer, J.; Hall, J.W.; Rougier, J.; Stephenson, D.B.; Wagener, T. Sensitivity analysis of environmental models: A systematic review with practical workflow. Environ. Model. Softw. 2016, 79, 214–232. [Google Scholar] [CrossRef]
- Shengkui, D.; Li, Z.; Ling, H.W. Sensitivity Analysis of Coastal City Tourism and Environmental Systems Based on Coupling Model. Sens. Mater. 2020, 32, 1913–1923. [Google Scholar] [CrossRef]
- Flemming, H.-C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2022, 21, 70–86. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. Int. Rev. J. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Pellicer-Nàcher Carles Smets, F.B. Structure, composition, and strength of nitrifying membrane-aerated biofilms. Water Res. 2014, 57, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Pérez, J.; Kreft, J.-U. Why is metabolic labour divided in nitrification? Trends Microbiol. 2006, 14, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, C.J.; Giguere, A.T.; Dobie, M.D.; Mellbye, B.L.; Ferrell, R.V.; Woebken, D.; Sayavedra-Soto, L.A.; Bottomley, P.J.; Daims, H.; Wagner, M. Transcriptomic Response of Nitrosomonas europaea Transitioned from Ammonia- to Oxygen-Limited Steady-State Growth. mSystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Inamori, Y.; Wu, X.-L.; Mizuochi, M. N2O producing capability of Nitrosomonas europaea, Nitrobacter winogradskyi and Alcaligenes faecalis. Water Sci. Technol. 1997, 36, 65–72. [Google Scholar] [CrossRef]
- Zhongjun, J.; Ralf, C. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef]
- Arp, D.J.; Chain, P.S.G.; Klotz, M.G. The Impact of Genome Analyses on Our Understanding of Ammonia-Oxidizing Bacteria. Annu. Rev. Microbiol. 2007, 61, 503–528. [Google Scholar] [CrossRef]
- Blackbune, R.; Vadivelu, V.M.; Yuan, Z.; Keller, J. Determination of growth rate and yield of nitrifying bacteria by measuring carbon dioxide uptake rate. Water Environ. Res. A Res. Publ. Water Environ. Fed. 2007, 79, 2437–2445. [Google Scholar] [CrossRef]
- Stein, L.Y.; Klotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, R94–R98. [Google Scholar] [CrossRef]
- Gruber, W.; Villez, K.; Kipf, M.; Wunderlin, P.; Siegrist, H.; Vogt, L.; Joss, A. N2O emission in full-scale wastewater treatment: Proposing a refined monitoring strategy. Sci. Total Environ. 2020, 699, 134157. [Google Scholar] [CrossRef]
- Lu, H.; Ulanov, A.V.; Nobu, M.; Liu, W.-T. Global metabolomic responses of Nitrosomonas europaea 19718 to cold stress and altered ammonia feeding patterns. Appl. Microbiol. Biotechnol. 2016, 100, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Hulle, S.W.H.V.; Vandeweyer, H.J.P.; Meesschaert, B.D.; Vanrolleghem, P.A.; Dejans, P.; Dumoulin, A. Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chem. Eng. J. 2010, 162, 1–20. [Google Scholar] [CrossRef]
- Pijuan, M.; Zhao, Y. Full-Scale Source, Mechanisms and Factors Affecting Nitrous Oxide Emissions; IWA Publishing: London, UK, 2022. [Google Scholar]
- Kampschreur, M.J.; Kleerebezem, R.; de Vet, W.W.; van Loosdrecht, M.C. Reduced iron induced nitric oxide and nitrous oxide emission. Water Res. 2011, 45, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-D.; Noguera, D.R. Evaluating the effect of dissolved oxygen on ammonia-oxidizing bacterial communities in activated sludge. Water Res. 2004, 38, 3275–3286. [Google Scholar] [CrossRef]
- Law, Y.; Ni, B.-J.; Lant, P.; Yuan, Z. N2O production rate of an enriched ammonia-oxidising bacteria culture exponentially correlates to its ammonia oxidation rate. Water Res. 2012, 46, 3409–3419. [Google Scholar] [CrossRef]
- Fux, C.; Siegrist, H. Nitrogen removal from sludge digester liquids by nitrification/denitrification or partial nitritation/anammox: Environmental and economical considerations. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2004, 50, 19–26. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; vand der Star, W.R.L.; Wieldiers, H.A.; Mulder, J.W.; Jetten, M.S.M.; van Loosdrecht, M.C.M. Dynamics of nitric oxide and nitrous oxide emission during full-scale reject water treatment. Water Res. 2008, 42, 812–826. [Google Scholar] [CrossRef]
- Kunming, F.; Yihao, B.; Fan, Y.; Jian, X.; Fuguo, Q. Achieving partial nitrification: A strategy for washing NOB out under high DO condition. J. Environ. Manag. 2023, 347, 119186. [Google Scholar] [CrossRef]
- Blum, J.M.; Su, Q.; Ma, Y.; Valverde-Pérez, B.; Domingo-Félez, C.; Jensen, M.M.; Smets, B.F. The pH dependency of N-converting enzymatic processes, pathways and microbes: Effect on net N2O production. Environ. Microbiol. 2018, 20, 1623–1640. [Google Scholar] [CrossRef]
- Gorelsky, S.I.; Ghosh, S.; Solomon, E.I. Mechanism of N2O reduction by the μ4-S tetranuclear CuZ cluster of nitrous oxide reductase. J. Am. Chem. Soc. 2006, 128, 278–290. [Google Scholar] [CrossRef]
- Qian, W.; Peng, Y.; Li, X.; Zhang, Q.; Ma, B. The inhibitory effects of free ammonia on ammonia oxidizing bacteria and nitrite oxidizing bacteria under anaerobic condition. J. Bioresour. Technol. 2017, 243, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta-Morley, L.E. Ammonia oxidation: Ecology, physiology, biochemistry and why they must all come together. FEMS Microbiol. Lett. 2018, 365, fny058. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Porro, J.; Nopens, I. Quantification and Modelling of Fugitive Greenhouse Gas Emissions from Urban Water Systems; IWA publishing: London, UK, 2022. [Google Scholar]
- Poghosyan, L.; Lehtovirta-Morley, L.E. Investigating microbial and environmental drivers of nitrification in alkaline forest soil. ISME Commun. 2024, 4, ycae093. [Google Scholar] [CrossRef] [PubMed]
- Mastroleo, F.; Arnau, C.; Verbeelen, T.; Mysara, M.; Gòdia, F.; Leys, N.; Van Houdt, R. Metaproteomics, Heterotrophic Growth, and Distribution of Nitrosomonas europaea and Nitrobacter winogradskyi after Long-Term Operation of an Autotrophic Nitrifying Biofilm Reactor. Appl. Microbiol. 2022, 2, 272–287. [Google Scholar] [CrossRef]
- Chandran, K.; Stein, L.Y.; Klotz, M.G.; Van Loosdrecht, M.C.M. Nitrous oxide production by lithotrophic ammonia-oxidizing bacteria and implications for engineered nitrogen-removal systems. Biochem. Soc. Trans. 2011, 39, 1832–1837. [Google Scholar] [CrossRef]
- Kool, D.M.; Wrage, N.; Zechmeister-Boltenstern, S.; Pfeffer, M.; Brus, D.; Oenema, O.; Van Groenigen, J. Nitrifier denitrification can be a source of N2O from soil: A revised approach to the dual-isotope labelling method. Eur. J. Soil Sci. 2010, 61, 759–772. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius equation revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef]
- Carvalho-Silva, V.H.; Coutinho, N.D.; Aquilanti, V. Temperature dependence of rate processes beyond Arrhenius and Eyring: Activation and Transitivity. Front. Chem. 2019, 7, 380. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).