Effects of Cyanobacteria on Competitive Interactions Between Different-Sized Cladoceran Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Computer Modelling
2.4. Statistical Analysis
3. Results
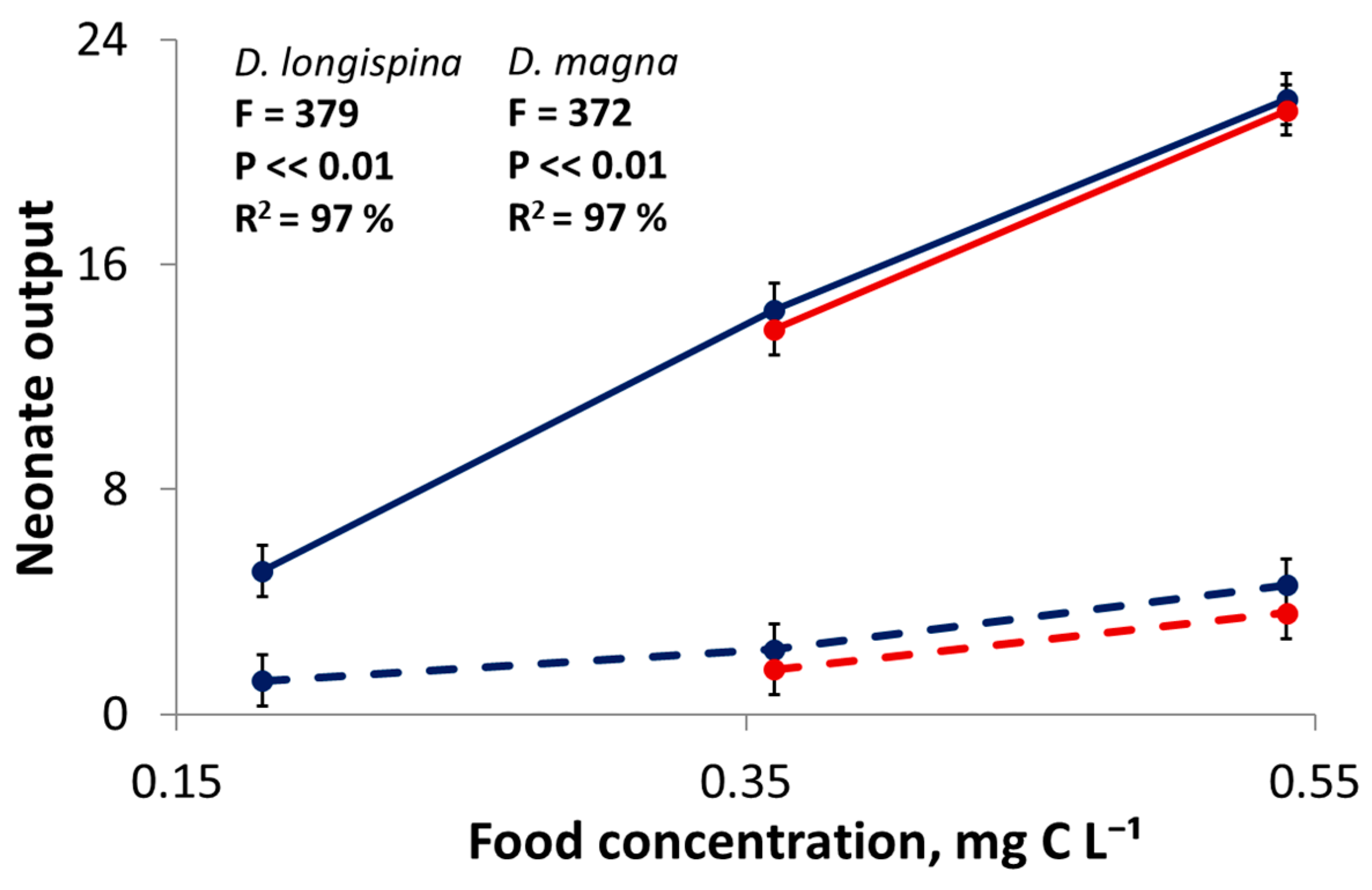
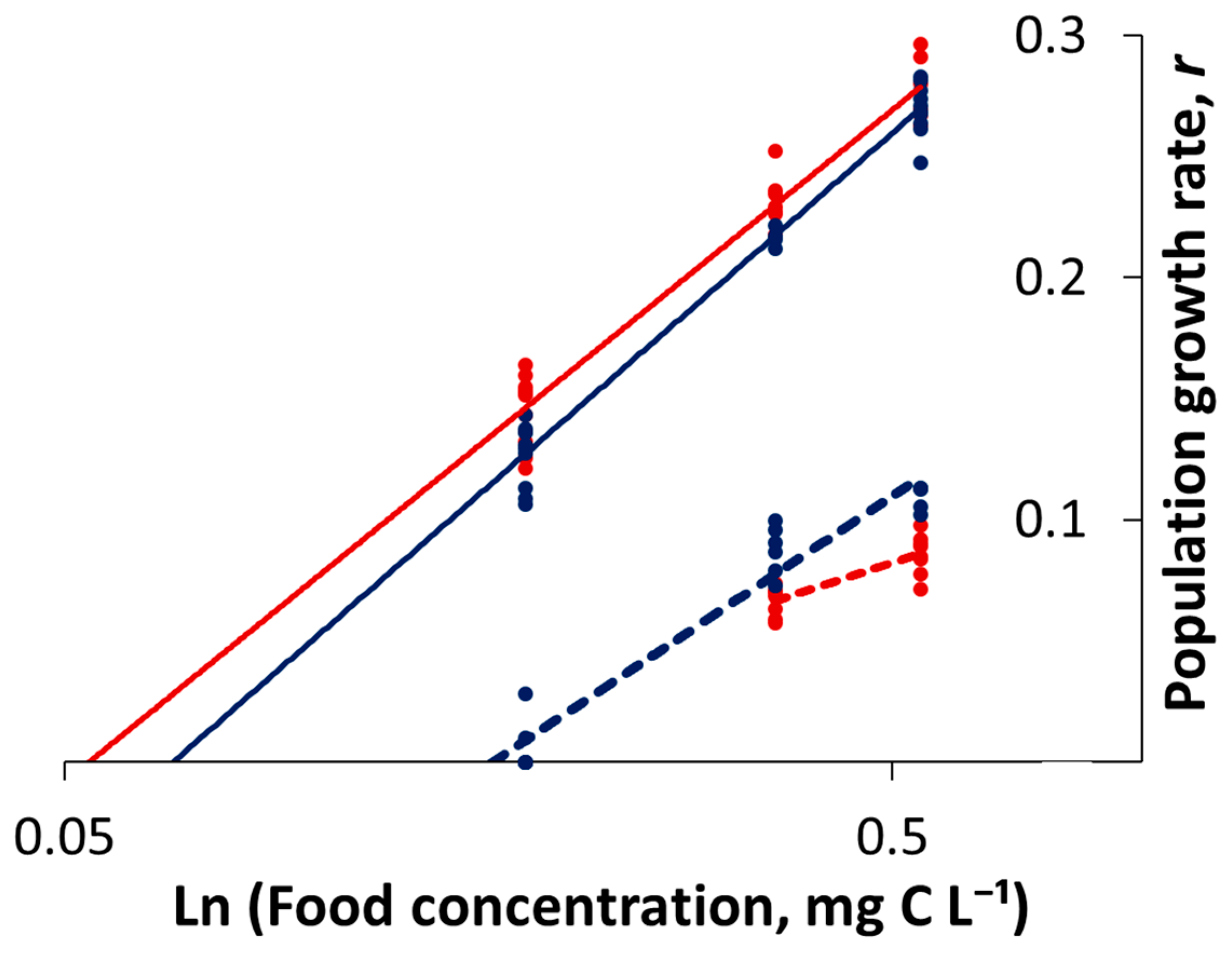
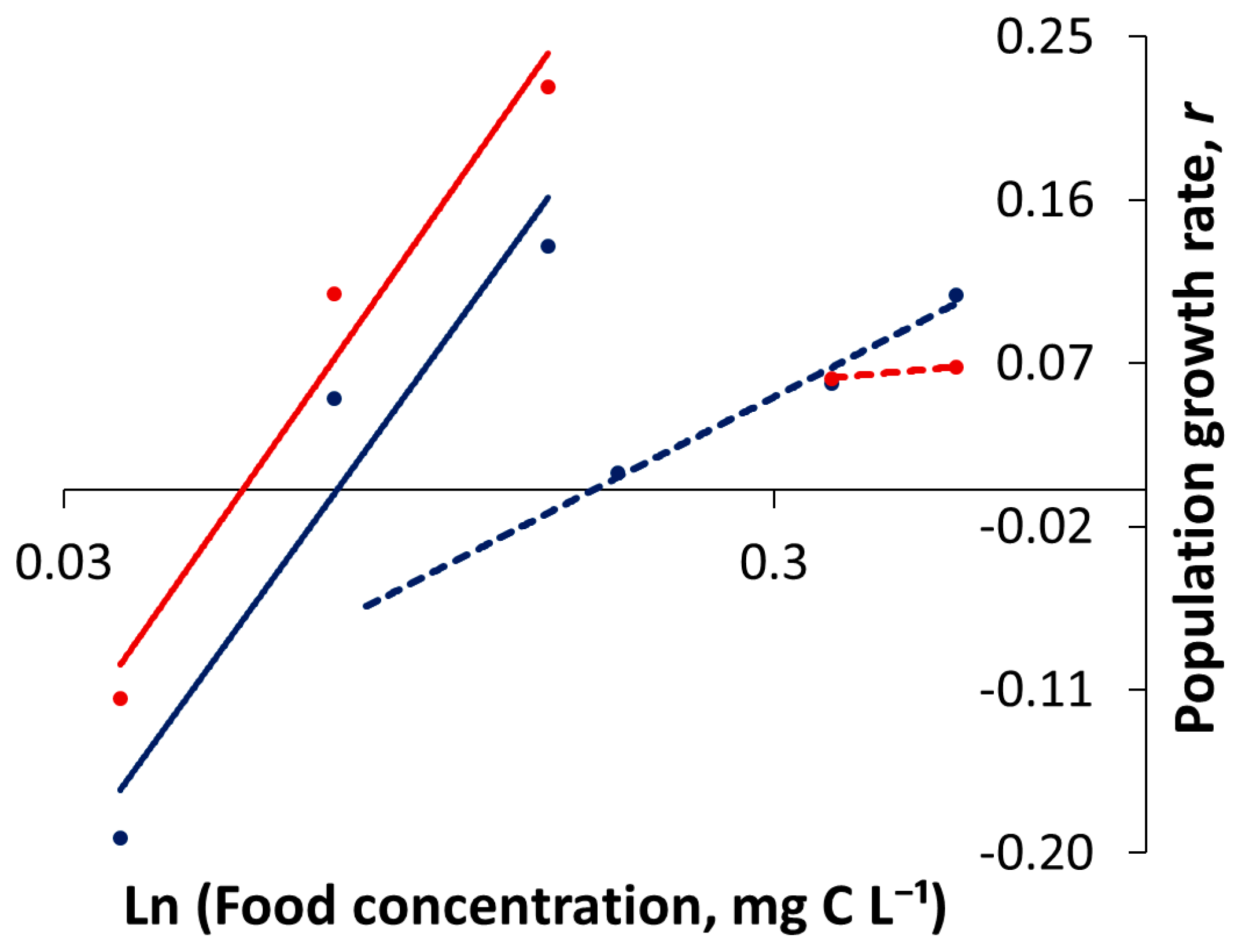
3.1. Life-Table Experiments
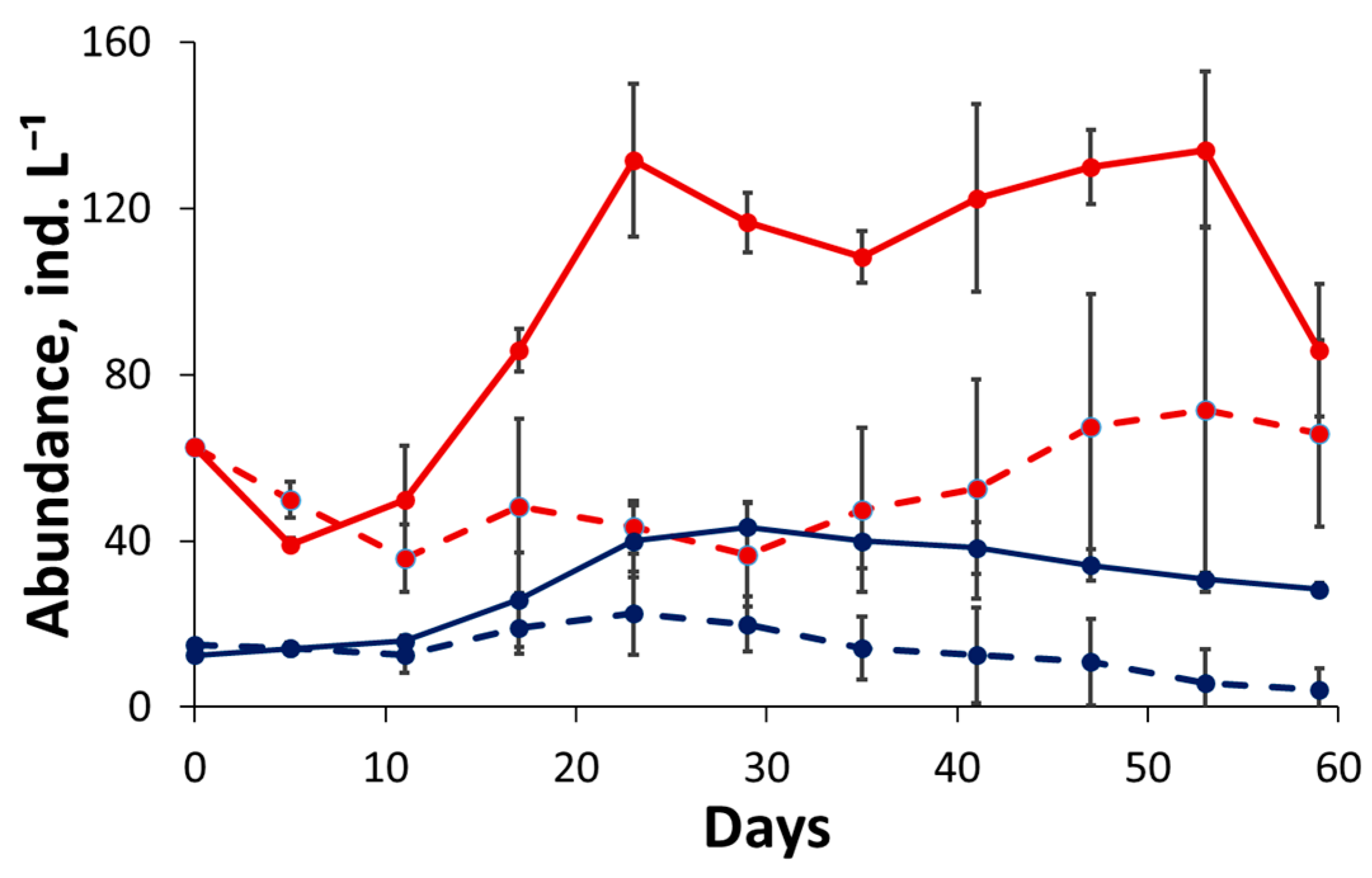
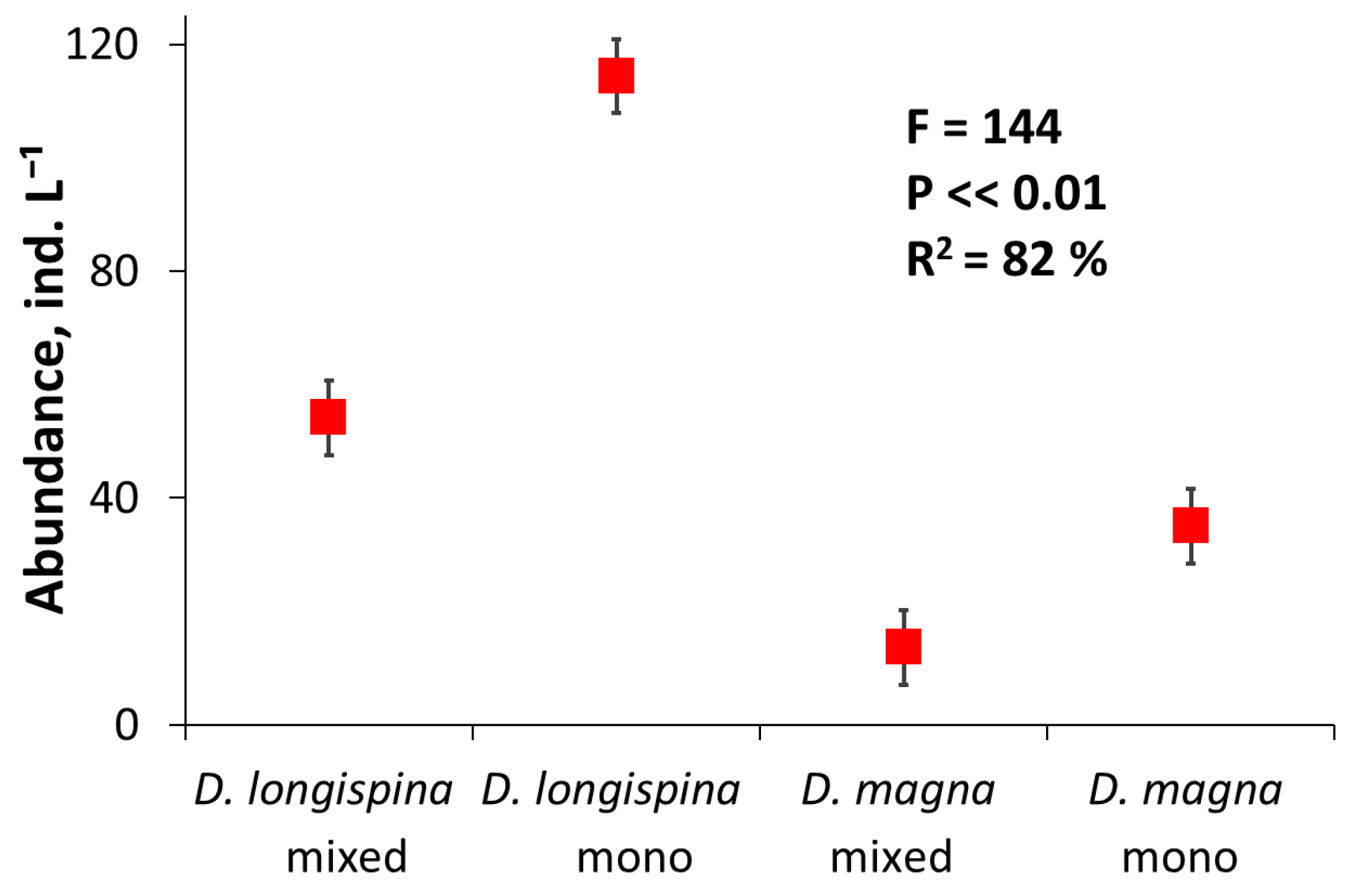
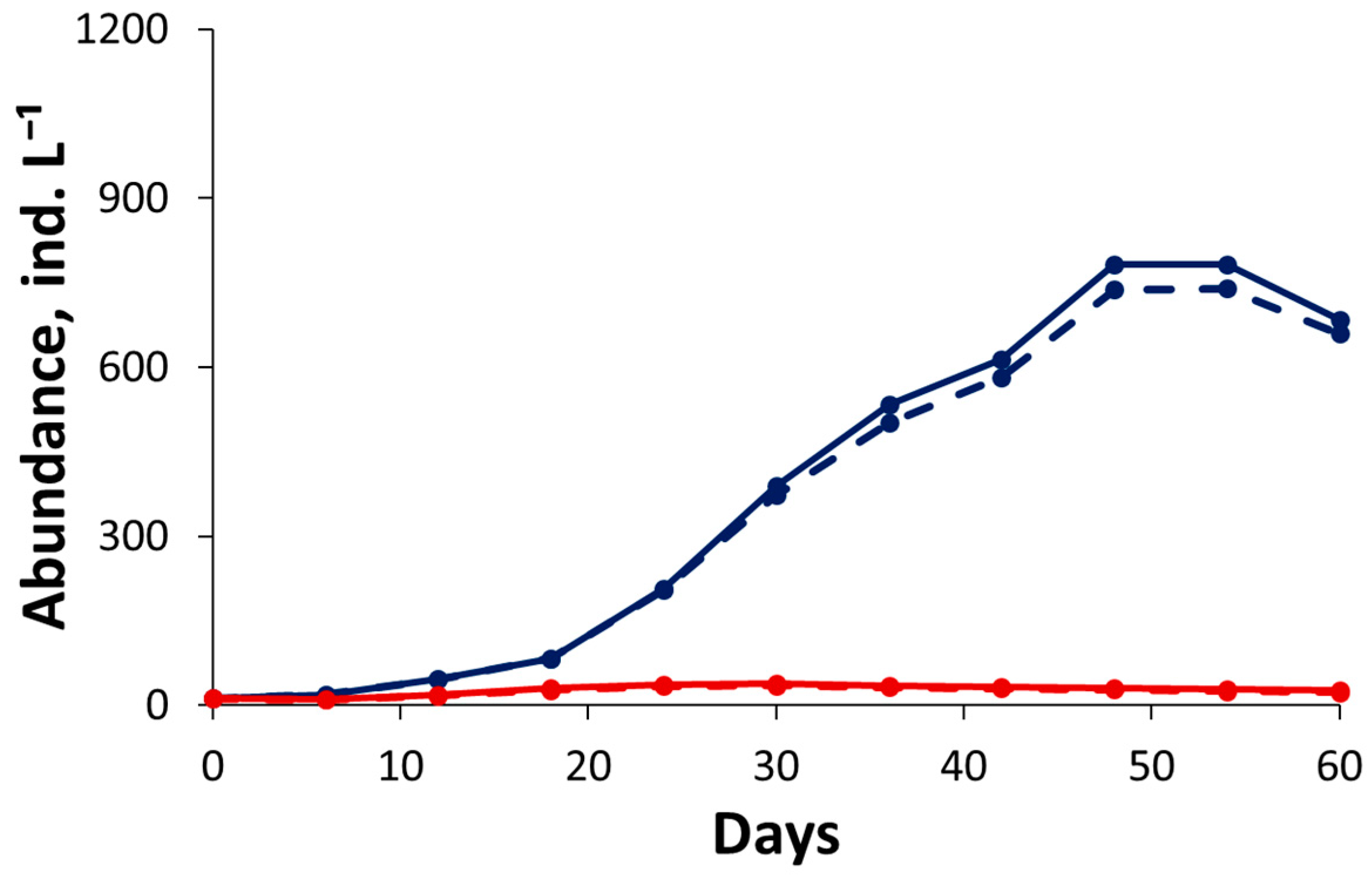
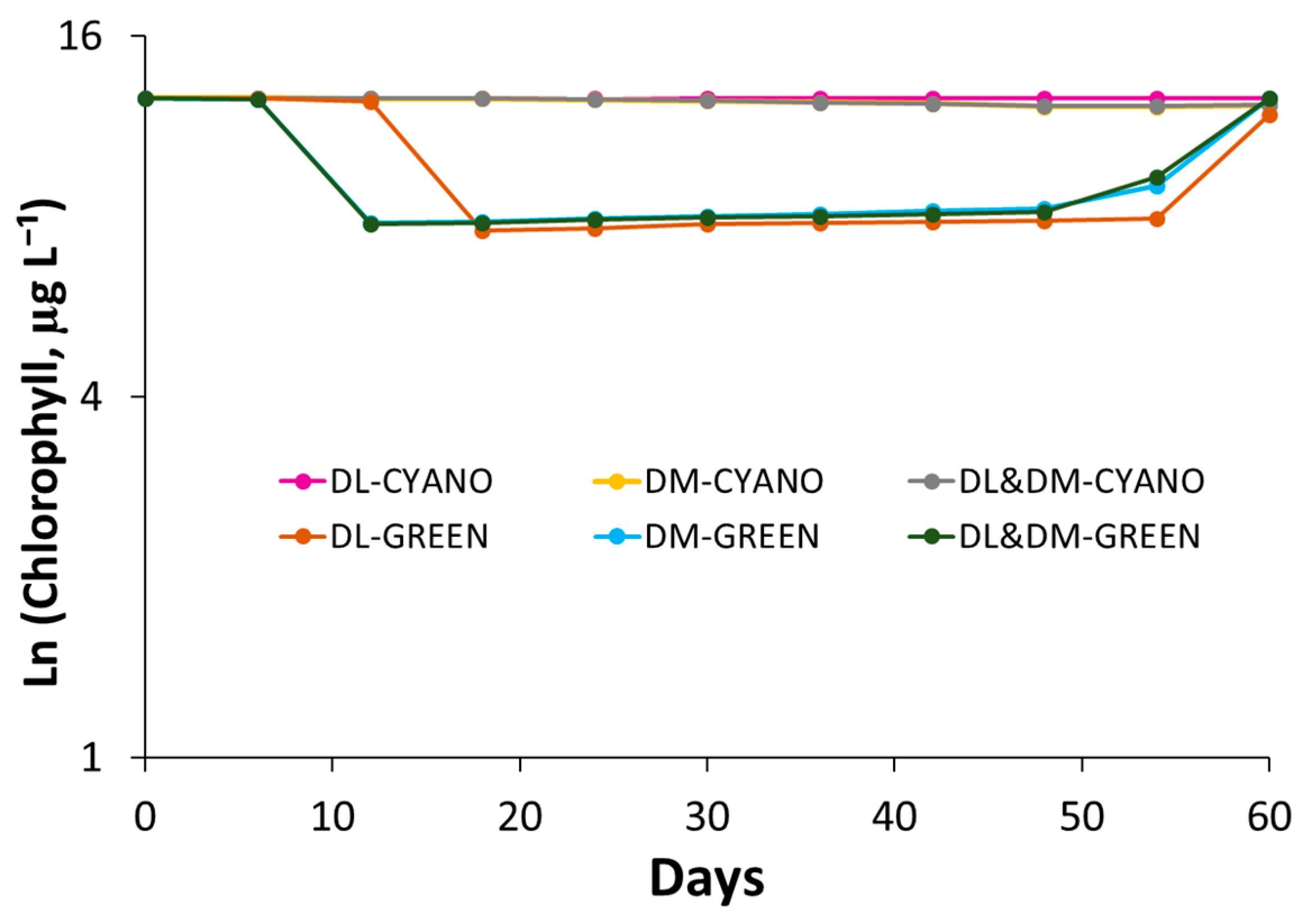
3.2. Competition Experiment
3.3. Computer Competition Experiment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GREEN | Green algae treatment |
| CYANO | Cyanobacteria treatment |
| PUFA | Polyunsaturated fatty acids |
| EPA | Eicosapentaenoic acid |
| R* | Population threshold food concentration |
References
- Francis, G. Poisonous Australian Lake. Nature 1878, 18, 11–12. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Kerbrat, A.S.; Zouher, A.; Pawlowiez, R.; Golubic, S.; Sibat, M.; Darius, H.T.; Chinain, M.; Laurent, D. First evidence of palytoxin and 42-hydroxy-palytoxin in the marine cyanobacterium Trichodesmium. Mar. Drugs 2011, 9, 543–560. [Google Scholar] [CrossRef] [PubMed]
- von Elert, E.; Wolffrom, T. Supplementation of cyanobacterial food with polyunsaturated fatty acids does not improve growth of Daphnia. Limnol. Oceanogr. 2001, 46, 1552–1558. [Google Scholar] [CrossRef]
- von Elert, E.; Martin-Creuzburg, D.; Le Coz, J.R. Absence of sterols constrains carbon transfer between cyanobacteria and a freshwater herbivore (Daphnia galeata). Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 1209–1214. [Google Scholar] [CrossRef]
- Müller-Navarra, D.C.; Brett, M.T.; Liston, A.M.; Goldman, C.R. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 2000, 403, 74–77. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; von Elert, E.; Hoffmann, K.H. Nutritional constraints at the cyanobacteria–Daphnia magna interface: The role of sterols. Limnol. Oceanogr. 2008, 5, 456–468. [Google Scholar] [CrossRef]
- Laine, M.B.; Martin-Creuzburg, D.; Litmanen, J.J.; Taipale, S.J. Sterol limitation of Daphnia on eukaryotic phytoplankton: A combined supplementation and compound-specific stable isotope labeling approach. Oikos 2024, 6, e10359. [Google Scholar] [CrossRef]
- Agrelius, T.C.; Dudycha, J.L. Maternal effects in the model system Daphnia: The ecological past meets the epigenetic future. Heredity 2025, 134, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Windisch, H.S.; Fink, P. The molecular basis of essential fatty acid limitation in Daphnia magna: A transcriptomic approach. Mol. Ecol. 2018, 27, 871–885. [Google Scholar] [CrossRef] [PubMed]
- von Elert, E.; Fink, P. Global warming: Testing for direct and indirect effects of temperature at the interface of primary producers and herbivores is required. Front. Ecol. Evol. 2018, 6, 87. [Google Scholar] [CrossRef]
- Sikora, A.B.; Petzoldt, T.; Dawidowicz, P.; von Elert, E. Demands of eicosapentaenoic acid (EPA) in Daphnia: Are they dependent on body size? Oecologia 2016, 182, 405–417. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Between Hazards of Starvation and Risk of Predation: The Ecology of Off-Shore Animals; Excellence in Ecology, Book 12; International Ecology Institute: Oldendorf/Luhe, Germany, 2003; 379p. [Google Scholar]
- Tilman, D. Tests of resource competition theory using four species of Lake Michigan algae. Ecology 1981, 62, 802–815. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure; Monogr. Pop. Biol. 17; Princeton University Press: Princeton, NJ, USA, 1982; 296p. [Google Scholar] [CrossRef]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Urabe, J. Competitive outcomes between herbivorous consumers can be predicted from their stoichiometric demands. Ecosphere 2012, 3, 1–16. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Urabe, J. Food quality and food threshold: Implications of food stoichiometry to competitive ability of herbivore plankton. Ecosphere 2012, 3, 1–17. [Google Scholar] [CrossRef]
- Feniova, I.Yu.; Dzialowski, A.R.; Bednarska, A.; Brzeziński, T.; Zilitinkevicz, N.; Piotr Dawidowicz, P. Shifts in competition outcomes between two Daphnia species in response to algal P-content. Oecologia 2025, 207, 32. [Google Scholar] [CrossRef]
- Achenbach, L.; Lampert, W. Effects of elevated temperatures on threshold food concentrations and possible competitive abilities of differently sized cladoceran species. Oikos 1997, 79, 469–476. [Google Scholar] [CrossRef]
- Chen, F.; Ye, J.; Shu, T.; Sun, Y.; Li, J. Zooplankton response to the lake restoration in the drinking-water source in Meiliang Bay of subtropical eutrophic Lake Taihu, China. Limnologica 2012, 42, 189–196. [Google Scholar] [CrossRef]
- Abrantes, N.; Nogueria, A.; Gonçalves, F. Short-term dynamics of cladocerans in a eutrophic shallow lake during a shift in the phytoplankton dominance. Ann. Limnol. Int. J. Limnol. 2009, 45, 237–245. [Google Scholar] [CrossRef][Green Version]
- Jiang, X.; Yang, W.; Xiang, X.; Niu, Y.; Chen, L.; Zhang, J. Cyanobacteria alter competitive outcomes between Daphnia and Bosmina in dependence on environmental conditions. Fundam. Appl. Limnol. 2014, 184, 11–22. [Google Scholar] [CrossRef]
- Gliwicz, Z.M.; Lampert, W. Food thresholds in Daphnia species in the absence and presence of blue-green filaments. Ecology 1990, 71, 691–702. [Google Scholar] [CrossRef]
- Kurmayer, R. Competitive ability of Daphnia under dominance of non-toxic filamentous cyanobacteria. Hydrobiologia 2001, 442, 279–289. [Google Scholar] [CrossRef]
- Sikora, A.; Dawidowicz, P. Do the presence of filamentous cyanobacteria and an elevated temperature favor small-bodied Daphnia in interspecific competitive interactions? Fundam. Appl. Limnol. 2014, 185, 307–314. [Google Scholar] [CrossRef]
- Brooks, J.L.; Dodson, S.I. Predation, body size, and composition of plankton. Science 1965, 150, 28–35. [Google Scholar] [PubMed]
- Hart, R.; Bychek, E. Body size in freshwater planktonic crustaceans: An overview of extrinsic determinants and modifying influences of biotic interactions. Hydrobiologia 2011, 668, 61–108. [Google Scholar] [CrossRef]
- Maszczyk, P.; Krajewski, K.; Leniowski, K.; Pukos, S.; Wawrzenczak, J.; Wilczynski, W.; Zebrowski, M.L.; Lee, J.S.; Babkiewicz, E. Temperature and hypoxia driven shifts in Daphnia interspecific competition. Limnol. Oceanogr. 2024, 69, 576–588. [Google Scholar] [CrossRef]
- Slusarczyk, M.; Ochocka, A.; Cichocka, D. The prevalence of diapause response to risk of size-selective predation in small-and large-bodied prey species. Aquat. Ecol. 2012, 46, 1–8. [Google Scholar] [CrossRef]
- An, M.; Mou, S.; Zhang, X.; Zheng, Z.; Ye, N.; Wang, D.; Zhang, W.; Miao, J. Expression of fatty acid desaturase genes and fatty acid accumulation in Chlamydomonas sp. ICE-L under salt stress. Bioresour. Technol. 2013, 149, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M. Purification and properties of unicellular blue-green algae (Order Chroococcales). Bacterial. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Martin-Creuzburg, D.; Sperfeld, E.; Wacker, A. Colimitation of a freshwater herbivore by sterols and polyunsaturated fatty acids. Proc. R. Soc. Biol. Sci. 2009, 276, 1805–1814. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; Wacker, A.; von Elert, E. Life history consequences of sterol availability in the aquatic keystone species Daphnia. Oecologia 2005, 144, 362–372. [Google Scholar] [CrossRef]
- Urich, K. Vergleichende Biochemie der Tiere, 1st ed.; Gustav Fischer: Stuttgart, Germany, 1990; 710p. [Google Scholar]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Book Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar] [CrossRef]
- Tessier, A.J.; Woodruff, P.A. Trading off the ability to exploit rich versus poor food quality. Ecol. Lett. 2002, 5, 685–692. [Google Scholar] [CrossRef]
- Kot, M. Elements of Mathematical Ecology; Cambridge University Press: Cambridge, UK, 2001; 453p. [Google Scholar]
- Fujiwara, M.; Diaz-Lopez, J. Constructing stage-structured matrix population models from life tables: Comparison of methods. PeerJ 2017, 5, e3971. [Google Scholar] [CrossRef]
- Suschchenya, L.M. Quantitative Aspects of Feeding in Crustaceans; Nauka i Tekhnika: Minsk, Belarus, 1975; 208p. (In Russian) [Google Scholar]
- Porter, K.G.; Gerritsen, J.; Orcutt, J.D., Jr. The effect of food concentration on swimming patterns of feeding behavior, ingestion, assimilation, and respiration by Daphnia. Limnol. Oceanogr. 1982, 27, 935–949. [Google Scholar] [CrossRef]
- Romanovsky, Y.E.; Feniova, I.Y. Competition among Cladocera: Effect of different levels of food supply. Oikos 1985, 44, 243–252. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall/Pearson: Upper Saddle River, NJ, USA, 2010; 944p. [Google Scholar]
- R Core Team. R: The R Project for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2024; Available online: http://www.R-project.org/ (accessed on 29 February 2024).
- Ravet, J.L.; Persson, J.; Brett, M.T. Threshold dietary polyunsaturated fatty acid concentrations for Daphnia pulex growth and reproduction. Inland Waters 2012, 2, 199–209. [Google Scholar] [CrossRef]
- Becker, C.; Boersma, M. Differential effects of phosphorus and fatty acids on Daphnia magna growth and reproduction. Limnol. Oceanogr. 2005, 50, 388–397. [Google Scholar] [CrossRef]
- Wacker, A.; Martin-Creuzburg, D. Allocation of essential lipids in Daphnia magna during exposure to poor food quality. Funct. Ecol. 2007, 21, 738–747. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; von Elert, E. Good food versus bad food: The role of sterols and polyunsaturated fatty acids in determining growth and reproduction of Daphnia magna. Aquat. Ecol. 2009, 43, 943–950. [Google Scholar] [CrossRef]
- Goad, L.J. Sterol biosynthesis and metabolism in marine invertebrates. Pure Appl. Chem. 1981, 51, 837–852. [Google Scholar] [CrossRef]
- Seda, J.; Petrusek, A.; Machacek, J.; Smilauer, P. Spatial distribution of the Daphnia longispina species complex and other planktonic crustaceans in the heterogeneous environment of canyon-shaped reservoirs. J. Plankton Res. 2007, 29, 619–628. [Google Scholar] [CrossRef][Green Version]
- Peltomaa, E.T.; Aalto, S.L.; Vuorio, K.M.; Taipale, S.J. The importance of phytoplankton biomolecule availability for secondary production. Front. Ecol. Evol. 2017, 5, 128. [Google Scholar]
- Geller, W.; Müller, H. The filtration apparatus of Cladocera: Filter mesh-sizes and their implications on food selectivity. Oecologia 1981, 49, 316–321. [Google Scholar] [CrossRef]
- Gophen, M.; Geller, W. Filter mesh size and food particle uptake by Daphnia. Oecologia 1984, 64, 408–412. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Food thresholds and body size in cladocerans. Nature 1990, 34, 638–640. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Urabe, J. Phosphorus acquisition and competitive abilities of two herbivorous zooplankton, Daphnia pulex and Ceriodaphnia quadrangula. Ecol. Res. 2010, 25, 619–627. [Google Scholar] [CrossRef]
- Summons, R.E.; Bradley, A.S.; Jahnke, L.L.; Waldbauer, J.R. Steroids, triterpenoids and molecular oxygen. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 951–968. [Google Scholar] [CrossRef]
- Milbrink, G.; Kruse, M.-L.; Bengtsson, J. Competitive ability and life history strategies in four species of Daphnia: D. obtusa, D. magna, D. pulex and D. longispina. Arch. Hydrobiol. 2003, 57, 433–453. [Google Scholar] [CrossRef]
- Feniova, I.Y.; Brzeziński, T.; Dzialowski, A.R.; Petrosyan, V.G.; Bednarska, A.; Zilitinkevicz, N.S.; Dawidowicz, P.D. Combined effects of food quality and temperature on competitive interactions between small- and large-bodied cladoceran species. Limnologica 2024, 109, 126203. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Anishchenko, O.V.; Makhutova, O.N.; Kolmakov, V.I.; Kalachova, G.S.; Kolmakova, A.A.; Dubovskaya, O.P. Efficiency of transfer of essential polyunsaturated fatty acids versus organic carbon from producers to consumers in a eutrophic reservoir. Oecologia 2011, 165, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Taipale, S.J.; Vuorio, K.; Strandberg, U.; Kahilainen, K.K.; Järvinen, M.; Hiltunen, M.; Peltomaa, E.; Kankaala, P. Lake eutrophication and brownification downgrade availability and transfer of essential fatty acids for human consumption. Environ. Int. 2016, 96, 156–166. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feniova, I.Y.; Brzeziński, T.; Bednarska, A.; Dzialowski, A.R.; Petrosyan, V.G.; Zilitinkevich, N.; Dawidowicz, P. Effects of Cyanobacteria on Competitive Interactions Between Different-Sized Cladoceran Species. Water 2025, 17, 1014. https://doi.org/10.3390/w17071014
Feniova IY, Brzeziński T, Bednarska A, Dzialowski AR, Petrosyan VG, Zilitinkevich N, Dawidowicz P. Effects of Cyanobacteria on Competitive Interactions Between Different-Sized Cladoceran Species. Water. 2025; 17(7):1014. https://doi.org/10.3390/w17071014
Chicago/Turabian StyleFeniova, Irina Yu., Tomasz Brzeziński, Anna Bednarska, Andrew R. Dzialowski, Varos G. Petrosyan, Natalia Zilitinkevich, and Piotr Dawidowicz. 2025. "Effects of Cyanobacteria on Competitive Interactions Between Different-Sized Cladoceran Species" Water 17, no. 7: 1014. https://doi.org/10.3390/w17071014
APA StyleFeniova, I. Y., Brzeziński, T., Bednarska, A., Dzialowski, A. R., Petrosyan, V. G., Zilitinkevich, N., & Dawidowicz, P. (2025). Effects of Cyanobacteria on Competitive Interactions Between Different-Sized Cladoceran Species. Water, 17(7), 1014. https://doi.org/10.3390/w17071014






