Analysis of Selected Elements in Rivers and Streams of Papuk Nature Park, Croatia
Abstract
1. Introduction
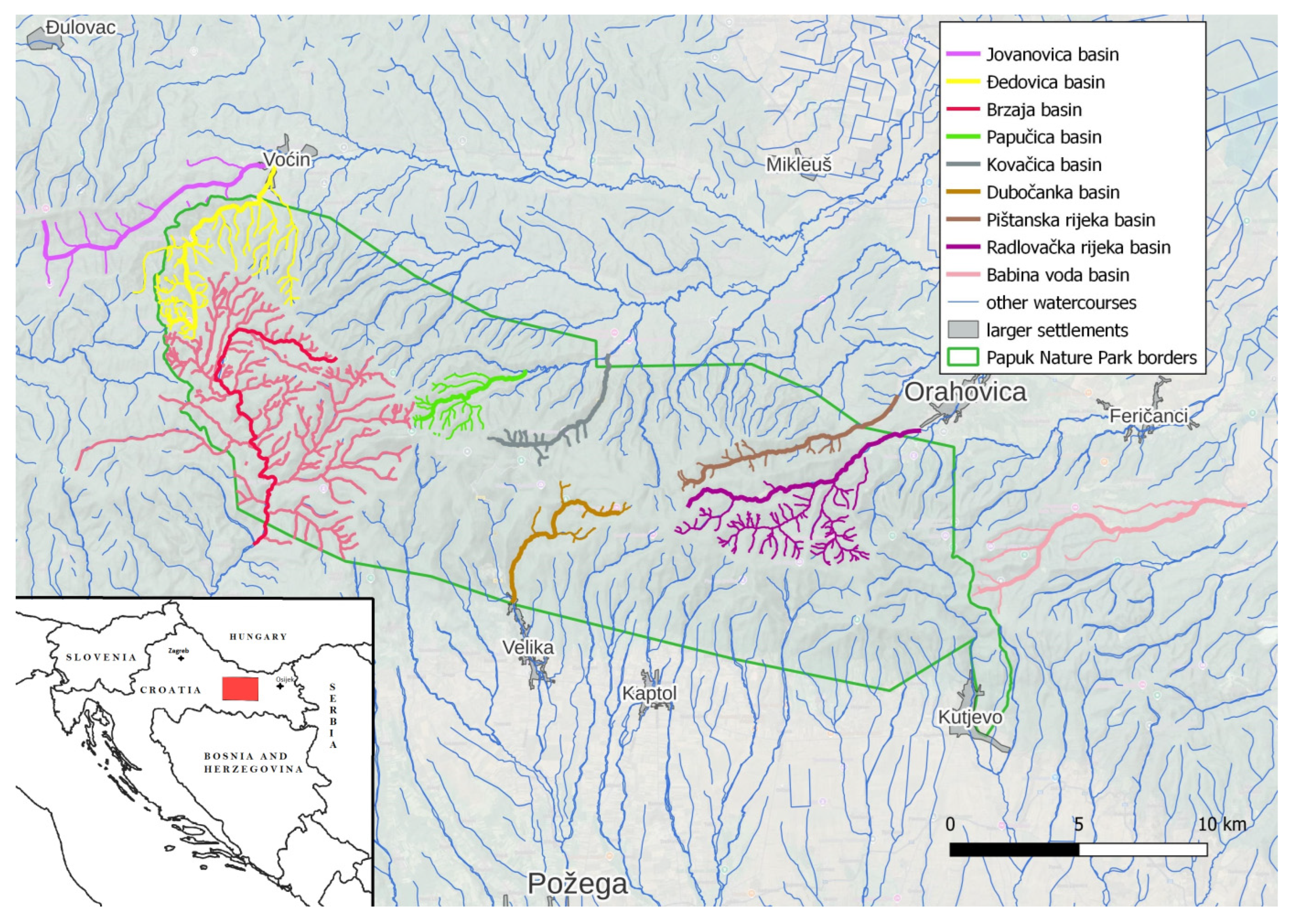
2. Materials and Methods
2.1. Sampling and Analysis
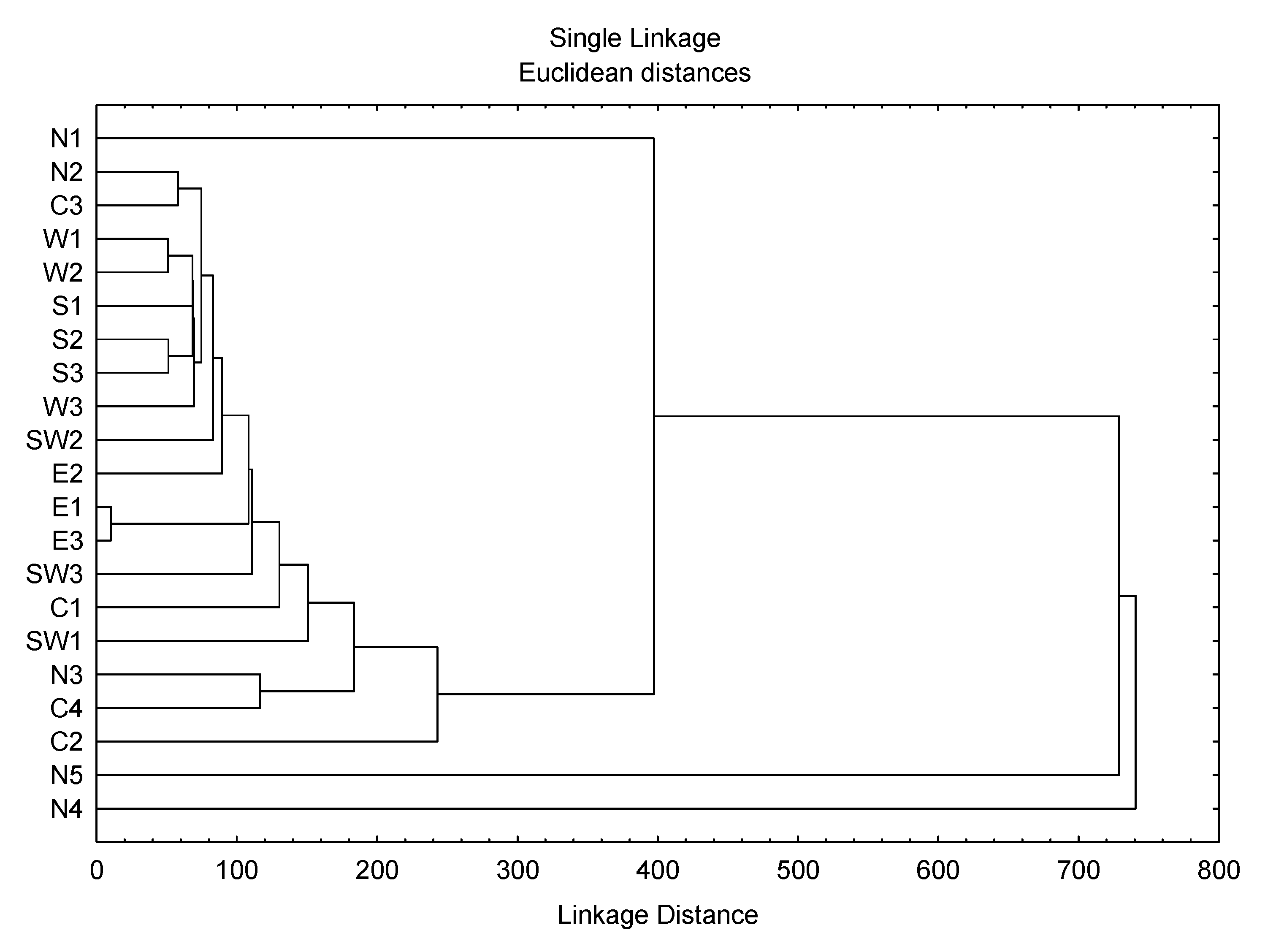
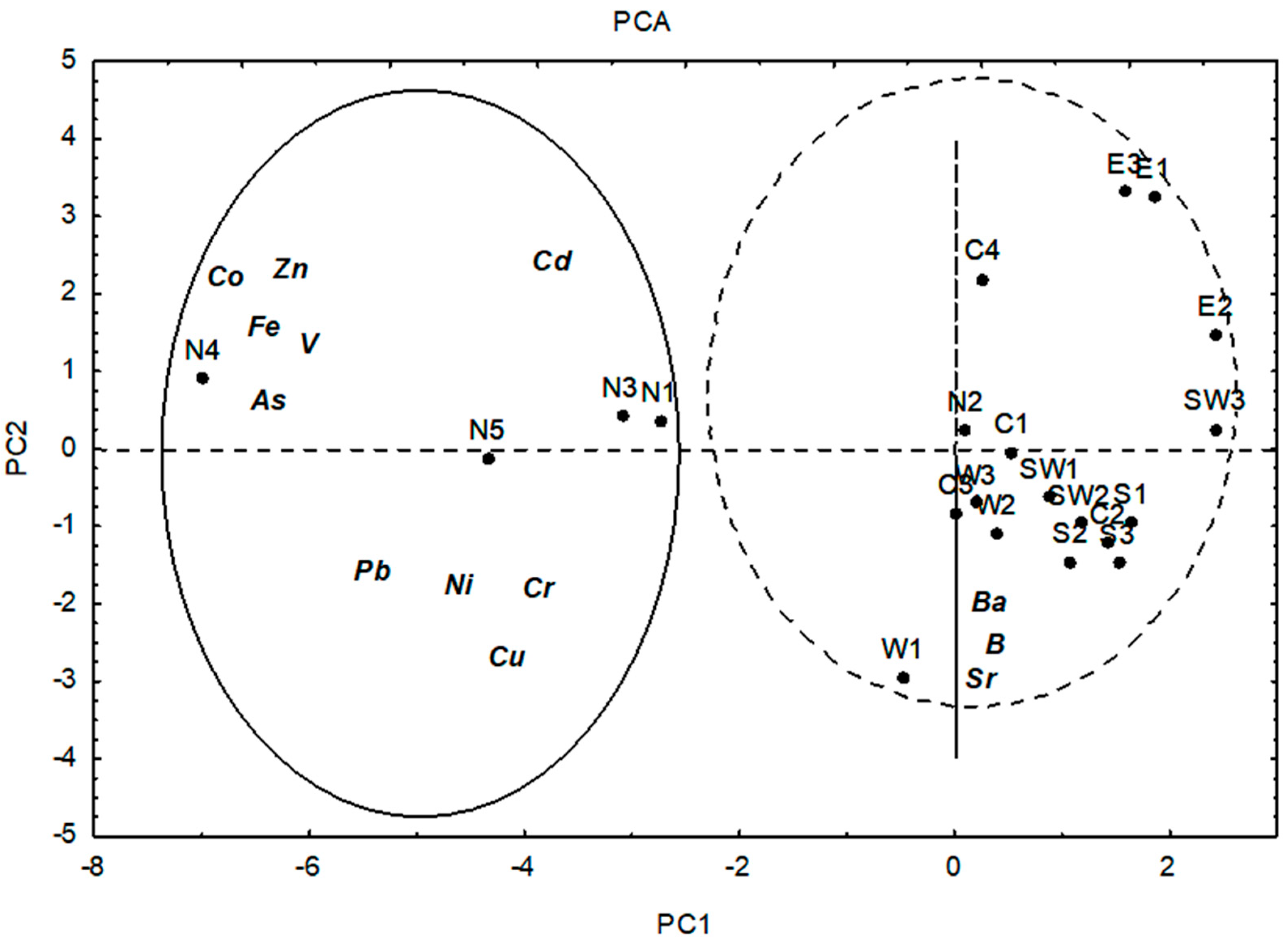
2.2. Data Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrinec, B.; Rašeta, D.; Babić, D. Radiological impact of an active quarry in the Papuk Nature Park, Croatia. Arch. Ind. Hyg. Toxicol. 2022, 73, 15–22. [Google Scholar] [CrossRef]
- Cesar, V.; Lepeduš, H.; Ljubešić, N. Histochemical observations on the needles of norway spruce (Picea abies L. Karst.) trees affected by cement dust pollution. Phyton Horn 2004, 44, 205. [Google Scholar]
- Vidosavljević, D.; Puntarić, D.; Gvozdić, V.; Jergović, M.; Miškulin, M.; Puntarić, I.; Puntarić, E.; Šijanović, S. Soil contamination as a possible long-term consequence of war in Croatia. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2013, 63, 322–329. [Google Scholar] [CrossRef]
- Soldo, S.; Puntarić, D. Injuries in Croatian Army Brigade Soldiers Inflicted in an Offensive Action during the 1991/1992 War in Croatia. Mil. Med. 1998, 163, 420–422. [Google Scholar] [CrossRef]
- Martinić Jerčić, N. Operacija Papuk-91. (oslobađanje šireg papučkog područja u jesen i zimu 1991./1992. godine). Rad. Zavoda Za Znan. Umjetnički Rad U Bjelovar. 2014, 8, 45–69. [Google Scholar]
- Puntarić, D.; Soldo, S.; Prgomet, D.; Vodopija, R. Type, severity, location, and timing of battle casualties in a Croatian Army brigade during an offensive action in 1992. Croat. Med. J. 1999, 40, 88–92. [Google Scholar]
- Vitale, K.; Marijanović Rajcić, M.; Senta, A. Waters in Croatia between practice and needs: Public health challenge. Croat. Med. J. 2002, 43, 485–492. [Google Scholar]
- Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics 2023, 11, 828. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Verma, R.K.; Chopade, R.L.; Pandit, P.P.; Nagar, V.; Aseri, V.; Choudhary, S.K.; Awasthi, G.; Awasthi, K.K.; et al. Heavy Metal Contamination of Water and Their Toxic Effect on Living Organisms. In The Toxicity of Environmental Pollutants; IntechOpen: London, UK, 2022; ISBN 978-1-80355-580-5. [Google Scholar]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef]
- Rowland Helen, A.L.; Omoregie, E.O.; Millot, R.; Jiminez, C.; Mertens, J.; Baciu, C.; Hug, S.J.; Berg, M. Geochemistry and arsenic behaviour in groundwater resources of the Pannonian Basin (Hungary and Romania). Appl. Geochem. 2011, 26, 1–17. [Google Scholar] [CrossRef]
- Ćurković, M.; Sipos, L.; Puntarić, D.; Dodig-Ćurković, K.; Pivac, N.; Kralik, K. Arsenic, Copper, Molybdenum, and Selenium Exposure through Drinking Water in Rural Eastern Croatia. Pol. J. Environ. Stud. 2016, 25, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Gvozdić, V.; Brana, J.; Orešković, K.; Puntarić, D.; Vidosavljević, D.; Jergović, M.; Puntarić, I.; Puntarić, E.; Vidosavljević, M.; Müller, A. Analysis and Assessment of Available Water Sources in Eastern Croatia. Rev. Roum. Chim. 2015, 60, 935–941. [Google Scholar]
- Habuda-Stanic, M.; Kules, M.; Kalajdzic, B.; Romic, Z. Quality of groundwater in eastern Croatia. The problem of arsenic pollution. Desalination 2007, 210, 157–162. [Google Scholar] [CrossRef]
- Šćavničar, S.; Bermanec, V.; Kniewald, G.; Barišić, D.; Oreščanin, V. Uranium Minerals in the Radlovac Series Metasediments at Mt. Papuk, Croatia. Geol. Croat. 2007, 60, 165–171. [Google Scholar] [CrossRef]
- Bolan, S.; Wijesekara, H.; Amarasiri, D.; Zhang, T.; Ragályi, P.; Brdar-Jokanović, M.; Rékási, M.; Lin, J.-Y.; Padhye, L.P.; Zhao, H.; et al. Boron contamination and its risk management in terrestrial and aquatic environmental settings. Sci. Total Environ. 2023, 894, 164744. [Google Scholar] [CrossRef]
- WHO. “Boron in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality”, Art. No. WHO/HSE/WSH/09.01/2. 2009. Available online: https://iris.who.int/handle/10665/70170 (accessed on 3 December 2024).
- Okay, O.; Güçlü, H.; Soner, E.; Balkaş, T. Boron pollution in the Simav River, Turkey and various methods of boron removal. Water Res. 1985, 19, 857–862. [Google Scholar] [CrossRef]
- NN 64/2023 (14.6.2023.), Pravilnik o Parametrima Sukladnosti, Metodama Analiza i Monitorinzima Vode Namijenjene za Ljudsku Potrošnju—Zakon.hr. Available online: https://www.zakon.hr/cms.htm?id=57136 (accessed on 3 December 2024).
- Shrivas, K.; Maji, P.; Dewangan, K. Onsite-detection of barium and nickel from river, pond and tap water samples using gold nanoparticles as a chemical sensor. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2017, 173, 630–636. [Google Scholar] [CrossRef]
- Chaalal, O.; Zekri, A.Y.; Soliman, A.M. A novel technique for the removal of strontium from water using thermophilic bacteria in a membrane reactor. J. Ind. Eng. Chem. 2015, 21, 822–827. [Google Scholar] [CrossRef]
- Musgrove, M. The occurrence and distribution of strontium in U.S. groundwater. Appl. Geochem. 2021, 126, 104867. [Google Scholar] [CrossRef]
- Oste, L.; Bervoets, A.R.; Behets, G.J.; Dams, G.; Marijnissen, R.L.; Geryl, H.; Lamberts, L.V.; Verberckmoes, S.C.; Van Hoof, V.O.; De Broe, M.E.; et al. Time-evolution and reversibility of strontium-induced osteomalacia in chronic renal failure rats. Kidney Int. 2005, 67, 920–930. [Google Scholar] [CrossRef]
- Toccalino, P.L.; Norman, J.E. Health-based screening levels to evaluate U.S. Geological Survey ground water quality data. Risk Anal. Off. Publ. Soc. Risk Anal. 2006, 26, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Dalai, T.K.; Sarin, M. Trace Determination of Strontium and Barium in River Waters by Inductively Coupled Plasma-Atomic Emission Spectrometry Using an Ultrasonic Nebulizer. 2002. Available online: http://repository.ias.ac.in/86505/ (accessed on 6 December 2024).
- Babaluk, R.J.; John, A. “Technica”, ResearchGate. Available online: https://www.researchgate.net/publication/303607533_The_use_of_strontium_distribution_in_Dolly_Varden_char_otoliths_from_Accomplishment_Creek_Alaska_for_determining_migratory_behaviour (accessed on 10 December 2024).
- Pyrzyńska, K.; Wierzbicki, T. Determination of vanadium species in environmental samples. Talanta 2004, 64, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Huang, F.; Evans, L.; Glasauer, S. Vanadium: Global (bio)geochemistry. Chem. Geol. 2015, 417, 68–89. [Google Scholar] [CrossRef]
- Marczewski, K.; Marczewska, B.; Kuzioła, R. The Importance of Vanadium Concentration in Ground and Deep Ground Water for Spring Water Quality. J. Jpn. Assoc. Phys. Med. Balnelogy Climatol. 2015, 78, 201–208. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Sadeghi Rad, S.; Vilas-Boas, J.A.; Khataee, A. A global systematic review, meta-analysis, and risk assessment of the concentration of vanadium in drinking water resources. Chemosphere 2021, 267, 128904. [Google Scholar] [CrossRef]
- Uppal, J.S.; Zheng, Q.; Le, X.C. Arsenic in drinking water—Recent examples and updates from Southeast Asia. Curr. Opin. Environ. Sci. Health 2019, 7, 126–135. [Google Scholar] [CrossRef]
- Vidosavljević, M.; Puntarić, D.; Gvozdić, V.; Vidosavljević, D.; Šijanović, S.; Šekerija, M.; Venus, M.; Jovičić, M.; Begović, L. Arsenic in Drinking Water and Urine and Its Relationship with Malignant Tumors of Urinary Tract in Osijek-Baranja County, Croatia. Acta Clin. Croat. 2023, 62, 95–103. [Google Scholar] [CrossRef]
- Ćavar, S.; Klapec, T.; Jurišić Grubešić, R.; Valek, M. High exposure to arsenic from drinking water at several localities in eastern Croatia. Sci. Total Environ. 2005, 339, 277–282. [Google Scholar] [CrossRef]
- Council Directive. 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. Communities 1998, 330, 32–54. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th Edition, Incorporating the 1st Addendum; World Health Organization: Geneva, Switzerland. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 7 December 2024).
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef]
- Georgaki, M.-N.; Charalambous, M.; Kazakis, N.; Talias, M.A.; Georgakis, C.; Papamitsou, T.; Mytiglaki, C. Chromium in Water and Carcinogenic Human Health Risk. Environments 2023, 10, 33. [Google Scholar] [CrossRef]
- Sirinawin, W.; Turner, D.R.; Westerlund, S. Chromium(VI) distributions in the Arctic and the Atlantic Oceans and a reassessment of the oceanic Cr cycle. Mar. Chem. 2000, 71, 265–282. [Google Scholar] [CrossRef]
- Idrees, N.; Tabassum, B.; Abd Allah, E.F.; Hashem, A.; Sarah, R.; Hashim, M. Groundwater contamination with cadmium concentrations in some West U.P. Regions, India. Saudi J. Biol. Sci. 2018, 25, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Ferguson, A.; Eyre, B. Behaviour of aluminium and iron in acid runoff from acid sulphate soils in the lower Richmond River catchment. AGSO J. Aust. Geol. Geophys. 1999, 17, 193–202. [Google Scholar]
- Vukosav, P.; Mlakar, M.; Cukrov, N.; Kwokal, Ž.; Pižeta, I.; Pavlus, N.; Špoljarić, I.; Vurnek, M.; Brozinčević, A.; Omanović, D. Heavy metal contents in water, sediment and fish in a karst aquatic ecosystem of the Plitvice Lakes National Park (Croatia). Environ. Sci. Pollut. Res. 2014, 21, 3826–3839. [Google Scholar] [CrossRef]
- Maldini, K.; Cukrov, N.; Pikelj, K.; Matić, N.; Mlakar, M. Geochemistry of Metals and Organic Matter in Water and Sediments of the Karst River Cetina, Croatia. Water 2023, 15, 1429. [Google Scholar] [CrossRef]
- Matić, N.; Maldini, K.; Cuculić, V.; Frančišković-Bilinski, S. Investigations of karstic springs of the Biokovo Mt from the Dinaric karst of Croatia. Chem. Erde-Geochem. 2012, 72, 179–190. [Google Scholar] [CrossRef]
| Parameters | Working Conditions |
|---|---|
| Spray gas flow rate, Argon > 99.99% | 0.88 Lmin−1 |
| Auxiliary gas flow rate | 1.20 Lmin−1 |
| Plasma flow rate | 15.00 Lmin−1 |
| Lens voltage | 8.0 V |
| Power voltage | 1050 W |
| Element | LOD (µgL−1) | LQD (µgL−1) | Certified (µgL−1) | Found (µgL−1) | Recovery (%) |
|---|---|---|---|---|---|
| As | 0.1 | 0.30 | 53 | 52.1 | 98 |
| B | 0.1 | 0.30 | 93 | 101 | 101.8 |
| Ba | 0.07 | 0.21 | 51 | 55 | 107.8 |
| Cd | 0.01 | 0.030 | 20 | 19.1 | 95.5 |
| Co | 0.01 | 0.030 | 25 | 24.8 | 99.2 |
| Cr | 0.01 | 0.03 | 21 | 20.5 | 97.6 |
| Cu | 0.1 | 0.30 | 21 | 22.3 | 106.1 |
| Fe | 3 | 9 | 102 | 110 | 107.8 |
| Ni | 0.04 | 0.12 | 50 | 48.8 | 97.6 |
| Pb | 0.005 | 0.015 | 27 | 25.9 | 95.2 |
| Sr | 0.01 | 0.03 | 97 | 101 | 104.1 |
| V | 0.04 | 0.12 | 51 | 50.1 | 98.2 |
| Zn | 0.5 | 1.5 | 52 | 55 | 105.7 |
| Element (µgL−1) | MAC (µgL−1) | North Median (25–75%) | Central Part Median (25–75%) | West Median (25–75%) | Southwest Median (25–75%) | South Median (25–75%) | East Median (25–75%) |
|---|---|---|---|---|---|---|---|
| As | 10 | 4.22 (4.01–4.93) | 0.50 (0.433–1.63) | 1.62 (0.83–2.57) | 0.69 (0.21–1.18) | 1.04 (0.60–1.88) | 0.82 (0.66–1.48) |
| B | 1500 | 65.78 (60.23–66.36) | 40.39 (19.68–59.15) | 141.78 (85.11–188.58) | 140.52 (95.05–149.92) | 96.78 (54.90–138.78) | 1.90 (0.64–4.76) |
| Ba | 700 | 32.3 (31.2–44.4) | 60.01 (9.12–191.14) | 18.89 (13.15–23.28) | 25.62 (21.77–25.83) | 35.02 (26.58–37.50) | 0.31 (0.30–59.20) |
| Cd | 5 | 0.37 (0.32–0.66) | 0.29 (0.27–0.46) | 0.21 (0.14–0.26) | 0.19 (0.17–0.20) | 0.16 (0.099–0.20) | 0.57 (0.095–0.60) |
| Co | NR | 0.62 (0.50–0.85) | 0.18 (0.08–0.31) | 0.15 (0.13–0.30) | 0.15 (0.14–0.16) | 0.095 (0.090–0.097) | 0.13 (0.062–0.26) |
| Cr | 25 | 6.66 (5.20–6.94) | 6.27 (3.67–9.23) | 6.26 (5.75–20.44) | 2.07 (0.34–2.16) | 1.52 (0.62–2.32) | 0.18 (0.17–0.50) |
| Cu | 2000 | 15.89 (13.34–16.42) | 8.77 (8.10–9.57) | 12.94 (11.40–14.39) | 11.09 (3.35–12.67) | 11.74 (7.57–16.15) | 1.59 (1.56–1.99) |
| Fe | 200 | 968.20 (557.10–1692.20) | 189.42 (116.20–434.30) | 171.68 (168.10–198.80) | 250.89 (12.10–401.20) | 155.42 (106.30–183.60) | 190.12 (78.80–199.50) |
| Ni | 20 | 8.21 (7.54–8.71) | 6.04 (2.84–11.90) | 4.24 (4.03–13.37) | 1.85 (0.95–2.22) | 1.60 (0.86–2.23) | 0.26 (0.19–0.40) |
| Pb | 5 | 4.78 (4.44–4.86) | 2.93 (1.38–3.44) | 2.60 (2.47–2.80) | 1.73 (0.14–2.52) | 1.38 (1.17–1.87) | 0.0170 (0.015–0.85) |
| Sr | NR HBSL (4000 μgL−1) | 62.4 (61.24–65.22) | 86.35 (38.94–95.64) | 71.69 (66.96–78.88) | 65.22 (59.43–67.56) | 126.09 (105.41–130.69) | 0.039 (0.038–38.06) |
| V | 5 μgL−1 | 2.53 (1.89–3.33) | 0.53 (0.50–0.59) | 1.08 (0.87–1.17) | 0.73 (0.14–0.98) | 0.48 (0.33–0.51) | 0.57 (0.44–1.04) |
| Zn | 3000 μgL−1 | 25.95 (24.99–109.86) | 17.99 (15.06–19.24) | 16.84 (11.30–27.64) | 17.63 (10.11–18.81) | 13.91 (12.85–18.16) | 3.01 (2.49–3.86) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gvozdić, V.; Vidosavljević, M.; Venus, M.; Puntarić, D.; Užarević, Z.; Puntarić, E.; Begović, M.; Danolić, D.; Vidosavljević, D. Analysis of Selected Elements in Rivers and Streams of Papuk Nature Park, Croatia. Water 2025, 17, 902. https://doi.org/10.3390/w17060902
Gvozdić V, Vidosavljević M, Venus M, Puntarić D, Užarević Z, Puntarić E, Begović M, Danolić D, Vidosavljević D. Analysis of Selected Elements in Rivers and Streams of Papuk Nature Park, Croatia. Water. 2025; 17(6):902. https://doi.org/10.3390/w17060902
Chicago/Turabian StyleGvozdić, Vlatka, Marina Vidosavljević, Miroslav Venus, Dinko Puntarić, Zvonimir Užarević, Eda Puntarić, Mario Begović, Damir Danolić, and Domagoj Vidosavljević. 2025. "Analysis of Selected Elements in Rivers and Streams of Papuk Nature Park, Croatia" Water 17, no. 6: 902. https://doi.org/10.3390/w17060902
APA StyleGvozdić, V., Vidosavljević, M., Venus, M., Puntarić, D., Užarević, Z., Puntarić, E., Begović, M., Danolić, D., & Vidosavljević, D. (2025). Analysis of Selected Elements in Rivers and Streams of Papuk Nature Park, Croatia. Water, 17(6), 902. https://doi.org/10.3390/w17060902






