Toxicity of the Antiretrovirals Tenofovir Disoproxil Fumarate, Lamivudine, and Dolutegravir on Cyanobacterium Microcystis novacekii
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Active Pharmaceutical Ingredients
2.3. Test Organism Culture
2.4. Preparation of Test Substances
2.5. Solvent Control
2.6. Acute and Chronic Toxicity Test
2.7. Metabolic Activity Assay—MTT
2.8. Toxicity Classification on the Test Organism
2.9. Environmental Risk Assessment (ERA)
2.10. Statistical Analysis
3. Results and Discussion
3.1. DTG Toxicity
3.2. TDF Toxicity
3.3. 3TC Toxicity
3.4. ARVs’ Association Toxicity
3.5. Environmental Risk Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Fact Sheet 2024—Global HIV Statistics. UNAIDS. 2024. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 2 February 2025).
- Adeola, A.O.; Forbes, P.B.C. Antiretroviral Drugs in African Surface Waters: Prevalence, Analysis, and Potential Remediation. Environ. Toxicol. Chem. 2022, 41, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Zitha, A.B.; Ncube, S.; Mketo, N.; Nyoni, H.; Madikizela, L.M. Antiretroviral Drugs in Water: An African Challenge with Kenya and South Africa as Hotspots and Plausible Remediation Strategies. Chem. Afr. 2022, 5, 1237–1253. [Google Scholar] [CrossRef]
- Mlunguza, N.Y.; Ncube, S.; Mahlambi, P.N.; Chimuka, L.; Madikizela, L.M. Determination of selected antiretroviral drugs in wastewater, surface water and aquatic plants using hollow fibre liquid phase microextraction and liquid chromatography—Tandem mass spectrometry. J. Hazard. Mater. 2020, 382, 121067. [Google Scholar] [CrossRef]
- Ngumba, E.; Gachanja, A.; Nyirenda, J.; Maldonado, J.; Tuhkanen, T. Occurrence of antibiotics and antiretroviral drugs in source-separated urine, groundwater, surface water and wastewater in the peri-urban area of Chunga in Lusaka, Zambia. Water SA 2020, 46, 278–284. [Google Scholar]
- Kairigo, P.; Ngumba, E.; Sundberg, L.-R.; Gachanja, A.; Tuhkanen, T. Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies. Water 2020, 12, 1376. [Google Scholar] [CrossRef]
- Wooding, M.; Rohwer, E.R.; Naudé, Y. Determination of endocrine disrupting chemicals and antiretroviral compounds in surface water: A disposable sorptive sampler with comprehensive gas chromatography—Time-of-flight mass spectrometry and large volume injection with ultra-high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2017, 1496, 122–132. [Google Scholar] [CrossRef]
- K’oreje, K.O.; Vergeynst, L.; Ombaka, D.; De Wispelaere, P.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere 2016, 149, 238–244. [Google Scholar] [CrossRef]
- Wood, T.P.; Duvenage, C.S.J.; Rohwer, E. The occurrence of anti-retroviral compounds used for HIV treatment in South African surface water. Environ. Pollut. 2015, 199, 235–243. [Google Scholar] [CrossRef]
- Mylan Laboratories Limited. DOLUTEGRAVIR, LAMIVUDINE and TENOFOVIR DISOPROXIL FUMARATE Tablets, for Oral Use; (No. 210787PI—U.S. Food and Drug Administration); Mylan Laboratories Limited: Hyderabad, India, 2017. [Google Scholar]
- Alygizakis, N.A.; Besselink, H.; Paulus, G.K.; Oswald, P.; Hornstra, L.M.; Oswaldova, M.; Medema, G.; Thomaidis, N.S.; Behnisch, P.A.; Slobodnik, J. Characterization of wastewater effluents in the Danube River Basin with chemical screening, in vitro bioassays and antibiotic resistant genes analysis. Environ. Int. 2019, 127, 420–429. [Google Scholar] [CrossRef]
- Almeida, A.; De Mello-Sampayo, C.; Lopes, A.; da Silva, R.C.; Viana, P.; Meisel, L. Predicted Environmental Risk Assessment of Antimicrobials with Increased Consumption in Portugal during the COVID-19 Pandemic; The Groundwork for the Forthcoming Water Quality Survey. Antibiotics 2023, 12, 652. [Google Scholar] [CrossRef]
- Boulard, L.; Dierkes, G.; Ternes, T. Utilization of large volume zwitterionic hydrophilic interaction liquid chromatography for the analysis of polar pharmaceuticals in aqueous environmental samples: Benefits and limitations. J. Chromatogr. A 2018, 1535, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Ngumba, E.; Gachanja, A.; Tuhkanen, T. Occurrence of selected antibiotics and antiretroviral drugs in Nairobi River Basin, Kenya. Sci. Total Environ. 2016, 539, 206–213. [Google Scholar] [CrossRef]
- Gabriel, B.; Hitchcock, M.J.M.; Tomas, C. Assessment of Mitochondrial Toxicity in Human Cells Treated with Tenofovir: Comparison with Other Nucleoside Reverse Transcriptase Inhibitors. Antimicrob. Agents Chemother. 2002, 46, 716–723. [Google Scholar]
- Zhang, X.; Wang, R.; Piotrowski, M.; Zhang, H.; Leach, K.L. Intracellular concentrations determine the cytotoxicity of adefovir, cidofovir and tenofovir. Toxicol. Vitr. 2015, 29, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.J.; Sakowski, E.G.; Preheim, S.P.; Prasse, C. Bacteria exposed to antiviral drugs develop antibiotic cross-resistance and unique resistance profiles. Commun. Biol. 2023, 6, 837. [Google Scholar] [CrossRef]
- Souza-Silva, G.; Souza, C.R.; Pereira, C.A.d.J.; Lima, W.d.S.; Mol, M.P.G.; Silveira, M.R. Toxicological evaluation of antiretroviral Tenofovir Disoproxil Fumarate on the mollusk Biomphalaria glabrata and its hemocytes. Sci. Total. Environ. 2023, 891, 164484. [Google Scholar] [CrossRef] [PubMed]
- Cid, R.S.; Roveri, V.; Vidal, D.G.; Dinis, M.A.P.; Cortez, F.S.; Salgueiro, F.R.; Toma, W.; Cesar, A.; Guimarães, L.L. Toxicity of Antiretrovirals on the Sea Urchin Echinometra lucunter and Its Predicted Environmental Concentration in Seawater from Santos Bay (Brazilian Coastal Zone). Resources 2021, 10, 114. [Google Scholar] [CrossRef]
- Gomes, M.P.; Kubis, G.C.; Kitamura, R.S.A.; Figueredo, C.C.; Nogueira, K.d.S.; Vieira, F.; Navarro-Silva, M.A.; Juneau, P. Do anti-HIV drugs pose a threat to photosynthetic microorganisms? Chemosphere 2022, 307, 135796. [Google Scholar] [CrossRef]
- Almeida, L.C.; Mattos, A.C.; Dinamarco, C.P.G.; Figueiredo, N.G.; Bila, D.M. Chronic toxicity and environmental risk assessment of antivirals in Ceriodaphnia dubia and Raphidocelis subcapitata. Water Sci. Technol. 2021, 84, 1623–1634. [Google Scholar] [CrossRef]
- Omotola, E.O.; Genthe, B.; Ndlela, L.; Olatunji, O.S. Environmental Risk Characterization of an Antiretroviral (ARV) Lamivudine in Ecosystems. Int. J. Environ. Res. Public Health 2021, 18, 8358. [Google Scholar] [CrossRef]
- Xin, X.; Huang, G.; Zhang, B. Review of aquatic toxicity of pharmaceuticals and personal care products to algae. J. Hazard. Mater. 2021, 410, 124619. [Google Scholar] [CrossRef]
- Turek, M.; Różycka-Sokołowska, E.; Koprowski, M.; Marciniak, B.; Bałczewski, P. Can Pharmaceutical Excipients Threaten the Aquatic Environment? A Risk Assessment Based on the Microtox® Biotest. Molecules 2023, 28, 6590. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Santos, L.H.M.L.M.; Delerue-Matos, C.; Figueiredo, S.A. Impact of excipients in the chronic toxicity of fluoxetine on the alga Chlorella vulgaris. Environ. Technol. 2014, 35, 3124–3129. [Google Scholar] [CrossRef]
- Jacob, R.S.; Santos, L.V.d.S.; de Souza, A.F.R.; Lange, L.C. A toxicity assessment of 30 pharmaceuticals using Aliivibrio fischeri: A comparison of the acute effects of different formulations. Environ. Technol. 2016, 37, 2760–2767. [Google Scholar] [CrossRef]
- de Souza, C.R.; Souza-Silva, G.; de Souza Moreira, C.P.; Vasconcelos, O.M.S.R.; Nunes, K.P.; Pereira, C.A.J.; Mol, M.P.G.; Silveira, M.R. Removal of the Active Pharmaceutical Substance Entecavir from Water via the Fenton Reaction or Action by the Cyanobacterium Microcystis novacekii. Toxics 2024, 12, 885. [Google Scholar] [CrossRef]
- Guiry, M.D. AlgaeBase; World-Wide Eletronic Publication; National University of Ireland: Galway, Ireland, 2022; Available online: https://www.algaebase.org/search/species/detail/?species_id=30053 (accessed on 20 July 2023).
- Campos, M.M.C.; Faria, V.H.F.; Teodoro, T.S.; Barbosa, F.A.R. Evaluation of the capacity of the cyanobacterium Microcystis novacekii to remove atrazine from a culture medium Evaluation of the capacity of the cyanobacterium Microcystis novacekii to remove atrazine from a culture medium. J. Environ. Sci. Health Part B 2013, 48, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Touliabah, H.E.-S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A Review of Microalgae- and Cyanobacteria-Based Biodegradation of Organic Pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Luo, Y.; Nie, Y.; Sun, J.; Wang, Y.; Qiao, Z. The Distribution Characteristics of Microcystis novacekii Based on 16S rDNA Sequence. In Advances in Applied Biotechnology; Zhang, T.C., Nakajima, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Whitton, B.A.; Potts, M. The Ecology of Cyanobacteria; Klumer Academic Plublishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2002. [Google Scholar]
- Fioravante, I.A.; Barbosa, F.A.R.; Augusti, R.; Magalhães, S.M.S. Removal of methyl parathion by cyanobacteria Microcystis novacekii under culture conditions. J. Environ. Monit. 2010, 12, 1302–1306. [Google Scholar] [CrossRef]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef]
- Fioravante, I.A.; Albergaria, B.; Teodoro, T.S.; Magalhães, S.M.S.; Barbosa, F.; Augusti, R. Cyanobacteria Microcystis novacekii. J. Environ. Monit. 2012, 14, 2362–2366. [Google Scholar] [CrossRef]
- Silva, S.R.; Barbosa, F.A.R.; Mol, M.P.G.; Magalhães, S.M.S. Toxicity for Aquatic Organisms of Antiretroviral Tenofovir Disoproxil. J. Environ. Prot. 2019, 10, 1565–1577. [Google Scholar] [CrossRef]
- Vilar, M.; Ferrão-Filho, A. (Eco)Toxicology of Cyanobacteria and Cyanotoxins: From Environmental Dynamics to Adverse Effects. Toxics 2022, 10, 648. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Gorham, P.R. An improved method for obtaining axenic clones of planktonic blue-green algae. J. Phycol. 1974, 10, 238–240. [Google Scholar] [CrossRef]
- Modrzyński, J.J.; Christensen, J.H.; Brandt, K.K. Evaluation of dimethyl sulfoxide (DMSO) as a co-solvent for toxicity testing of hydrophobic organic compounds. Ecotoxicology 2019, 28, 1136–1141. [Google Scholar] [CrossRef]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Tets. OECD Guidel. Test. Chem. Sect. 2006, 2, 4–7. [Google Scholar]
- Barmshuri, M.; Kholdebarin, B.; Sadeghi, S. Applications of comet and MTT assays in studying Dunaliella algae species. Algal Res. 2023, 70, 103018. [Google Scholar] [CrossRef]
- United-Nations. Globally Harmonized System of Classification and Labelling os Chemicals (GHS), 10th ed.; United Nations Publications: New York, NY, USA; Geneva, Switzerland, 2023. [Google Scholar]
- UBA. Database—Pharmaceuticals in the Environment. Umwelt Bundesamt. 2024. Available online: https://www.umweltbundesamt.de/en/database-pharmaceuticals-in-the-environment-0#background (accessed on 10 December 2024).
- Riva, F.; Zuccato, E.; Davoli, E.; Fattore, E.; Castiglioni, S. Risk assessment of a mixture of emerging contaminants in surface water in a highly urbanized area in Italy. J. Hazard. Mater. 2018, 361, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Ngwenya, P.; Musee, N. Modelling ecological risks of antiretroviral drugs in the environment. Environ. Chem. Ecotoxicol. 2023, 5, 145–154. [Google Scholar] [CrossRef]
- Gajewicz-Skretna, A.; Gromelski, M.; Wyrzykowska, E.; Furuhama, A.; Yamamoto, H.; Suzuki, N. Aquatic toxicity (Pre)screening strategy for structurally diverse chemicals: Global or local classification tree models? Ecotoxicol. Environ. Saf. 2021, 208, 111738. [Google Scholar] [CrossRef]
- Mathieu-Denoncourt, J.; Wallace, S.J.; de Solla, S.R.; Langlois, V.S. Influence of Lipophilicity on the Toxicity of Bisphenol A and Phthalates to Aquatic Organisms. Bull. Environ. Contam. Toxicol. 2016, 97, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Scheunert, I.; Bruggemann, R.; Matthies, M.; Steinberg, C.; Zitko, V.; Kettrup, A.; Garrison, W. The relevance of aquatic organisms’ lipid content to the toxicity of lipophilic chemicals: Toxicity of lindane to different fish species. Ecotoxicol. Environ. Saf. 1994, 28, 53–70. [Google Scholar] [CrossRef]
- Guo, J.; Ren, J.; Chang, C.; Duan, Q.; Li, J.; Kanerva, M.; Yang, F.; Mo, J. Freshwater crustacean exposed to active pharmaceutical ingredients: Ecotoxicological effects and mechanisms. Environ. Sci. Pollut. Res. 2023, 30, 48868–48902. [Google Scholar] [CrossRef]
- Bouzas-Monroy, A.; Wilkinson, J.L.; Melling, M.; Boxall, A.B.A. Assessment of the Potential Ecotoxicological Effects of Pharmaceuticals in the World’s Rivers. Environ. Toxicol. Chem. 2022, 41, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Siciliano, A.; Guida, M.; Andreozzi, R.; Reis, N.M.; Puma, G.L.; Marotta, R. Removal of antiretroviral drugs stavudine and zidovudine in water under UV254 and UV254/H2O2 processes: Quantum yields, kinetics and ecotoxicology assessment. J. Hazard. Mater. 2018, 349, 195–204. [Google Scholar] [CrossRef]
- Stanley, J.K.; Perkins, E.J.; Habib, T.; Sims, J.G.; Chappell, P.; Escalon, B.L.; Wilbanks, M.; Garcia-Reyero, N. The Good, the Bad, and the Toxic: Approaching Hormesis in Daphnia magna Exposed to an Energetic Compound. Environ. Sci. Technol. 2013, 47, 9424–9433. [Google Scholar] [CrossRef]
- Li, S.; Tan, Y. Hormetic response of cholinesterase from Daphnia magna in chronic exposure to triazophos and chlorpyrifos. J. Environ. Sci. 2011, 23, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N.; Streibig, J.C.; Kudsk, P.; Mathiassen, S.K.; Duke, S.O. The Occurrence of Hormesis in Plants and Algae. Dose-Response 2007, 5, 150–162. [Google Scholar] [CrossRef]
- Souza-Silva, G.; de Souza, C.R.; Pereira, C.A.d.J.; Lima, W.d.S.; Mol, M.P.G.; Silveira, M.R. Using freshwater snail Biomphalaria glabrata (Say, 1818) as a biological model for ecotoxicology studies: A systematic review. Environ. Sci. Pollut. Res. 2023, 30, 28506–28524. [Google Scholar] [CrossRef]
- Agrahari, V.; Putty, S.; Mathes, C.; Murowchick, J.B.; Youan, B.C. Evaluation of degradation kinetics and physicochemical stability of tenofovir. Drug Test. Anal. 2015, 7, 207–213. [Google Scholar] [CrossRef]
- Silva, S.R.; Souza-Silva, G.; Moreira, C.P.d.S.; Vasconcelos, O.M.d.S.R.; Silveira, M.R.; Barbosa, F.A.R.; Magalhães, S.M.S.; Mol, M.P.G. Biodegradation of the Antiretroviral Tenofovir Disoproxil by a Cyanobacteria/Bacterial Culture. Toxics 2024, 12, 729. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.D.S.; Quadra, G.R.; de Oliveira Souza, H.; Amaral, V.S.D.; Navoni, J.A. The link between pharmaceuticals and cyanobacteria: A review regarding ecotoxicological, ecological, and sanitary aspects. Environ. Sci. Pollut. Res. 2021, 28, 41638–41650. [Google Scholar] [CrossRef] [PubMed]
- Spilsbury, F.; Kisielius, V.; Bester, K.; Backhaus, T. Ecotoxicological mixture risk assessment of 35 pharmaceuticals in wastewater effluents following post-treatment with ozone and/or granulated activated carbon. Sci. Total Environ. 2024, 906, 167440. [Google Scholar] [CrossRef]
- Castellino, S.; Moss, L.; Wagner, D.; Borland, J.; Song, I.; Chen, S.; Lou, Y.; Min, S.S.; Goljer, I.; Culp, A.; et al. Metabolism, Excretion, and Mass Balance of the HIV-1 Integrase Inhibitor Dolutegravir in Humans. Antimicrob. Agents Chemother. 2013, 57, 3536–3546. [Google Scholar] [CrossRef]
- Ramalwa, N.R. The Potential Risks of Long Term Exposure to Low Concentrations of Antiretrovirals in Treated and Untreated Water Sources in Gauteng, South Africa. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2021. [Google Scholar]
- Souza, C.R.; Ribeiro, M.d.F.; Soares, M.d.F.; dos Santos, S.F.; Pereira, C.A.d.J.; Mol, M.P.G.; Silveira, M.R. Environmental elimination estimate and literature review of ecotoxicological aspects of the main widely used antiretrovirals in Brazil. Res. Soc. Dev. 2022, 11, e368111032975. [Google Scholar] [CrossRef]
- Vergeynst, L.; Haeck, A.; De Wispelaere, P.; Van Langenhove, H.; Demeestere, K. Multi-residue analysis of pharmaceuticals in wastewater by liquid chromatography–magnetic sector mass spectrometry: Method quality assessment and application in a Belgian case study. Chemosphere 2015, 119, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Strotmann, U.; Thouand, G.; Pagga, U.; Gartiser, S.; Heipieper, H.J. Toward the future of OECD/ISO biodegradability testing-new approaches and developments. Appl. Microbiol. Biotechnol. 2023, 107, 2073–2095. [Google Scholar] [CrossRef]
- Strotmann, U.; Durand, M.-J.; Thouand, G.; Eberlein, C.; Heipieper, H.J.; Gartiser, S.; Pagga, U. Microbiological toxicity tests using standardized ISO/OECD methods—Current state and outlook. Appl. Microbiol. Biotechnol. 2024, 108, 454. [Google Scholar] [CrossRef]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total. Environ. 2021, 764, 142319. [Google Scholar] [CrossRef]
| Information | DTG | TDF | 3TC |
|---|---|---|---|
| CAS number | 1051375-19-9 | 147127-20-6 | 134678-17-4 |
| Molecular weight | 441.4 g/moL | 519.5 g/moL | 229.3 g/moL |
| Chemical formula | C20H18F2N3NaO5 | C19H30N5O10P | C8H11N3O3S |
| pKa | −0.5/10.1 | 4.1/18.6 | 4.3/14.3 |
| Log KOW | 1.6 | −1.9 | −0.9 |
| Water solubility | 269 mg/L | 13,400 mg/L | 70,000 mg/L |
| Melting point | 190–193 °C | 113–115 °C | 160–162 °C |
| Chemical structure | 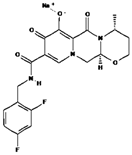 | 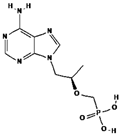 | 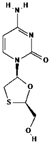 |
| Test substance | Classification | ARV Test Concentrations (mg/L) ** | API Purity |
|---|---|---|---|
| DTG | Medication | 45; 35; 25; 15; 10; 5; 0.5 and 0.1 | INOA |
| TDF + 3TC * | Medication | 256; 128; 64; 32; 16; 8; 4; 2 and 1 | INOA |
| 3TC | Medication | 300; 200; 100; 50; 25; 10; 5 and 1 | INOA |
| TDF | Medication | 256; 128; 64; 32; 16; 8; 4; 2 and 1 | INOA |
| DTG | API | 50; 40; 30; 20; 10; 1; 0.1 and 0.01 | >95.0% |
| 3TC | API | 400; 300; 200; 100; 50; 25; 10; 5 and 1 | >99.5% |
| TDF | API | 512; 256; 128; 64; 32; 16; 8; 4 and 2 | >99.8% |
| EC50 Value | Toxicity Classification |
|---|---|
| ≤1 mg/L | High toxicity |
| >1–≤10 mg/L | Toxic |
| >10–≤100 mg/L | Low toxicity |
| >100 mg/L | Virtually non-toxic |
| ARV | Time | EC50 | Statistical Model | Classification * |
|---|---|---|---|---|
| Active Pharmaceutical Ingredient (API) | ||||
| DTG | 4 days | 1.7 ± 0.3 mg/L | Weibull | Toxic |
| DTG | 14 days | 0.9 ± 0.4 mg/L | Weibull | Toxic |
| TDF | 4 days | 130.7 ± 5.8 mg/L | log-normal | Virtually non-toxic |
| TDF | 14 days | 147.0 ± 7.3 mg/L | Weibull | Virtually non-toxic |
| 3TC | 4 days | 184.8 ± 22.1 mg/L | Weibull | Virtually non-toxic |
| 3TC | 14 days | >400 mg/L | log-logistic | Virtually non-toxic |
| DTG + TDF | 4 days | 1.1 ± 0.4/11.7 ± 4.4 mg/L | log-normal | Toxic |
| DTG + TDF | 14 days | 21.0 ± 3.2/214.6 ± 32.8 mg/L | Weibull | Low toxicity |
| DTG + 3TC | 4 days | 1.4 ± 0.8/11.4 ± 6.2 mg/L | Weibull | Toxic |
| DTG + 3TC | 14 days | 9.6 ± 1.0/77.0 ± 7.8 mg/L | log-normal | Toxic |
| TDF + 3TC | 4 days | >256 mg/L | Weibull | Virtually non-toxic |
| TDF + 3TC | 14 days | >256 mg/L | log-logistic | Virtually non-toxic |
| DTG + TDF + 3TC | 4 days | 0.9 ± 0.1 mg/L | log-logistic | High toxicity |
| DTG + TDF + 3TC | 14 days | 3.5 ± 0.5 mg/L | Weibull | Toxic |
| Commercial medication | ||||
| DTG | 4 days | 27.6 ± 5.4 mg/L | Weibull | Low toxicity |
| DTG | 14 days | 19.5 ± 1.8 mg/L | Weibull | Low toxicity |
| TDF | 4 days | >256 mg/L | Weibull | Virtually non-toxic |
| TDF | 14 days | >256 mg/L | log-normal | Virtually non-toxic |
| 3TC | 4 days | 60.3 ± 2.7 mg/L | log-normal | Low toxicity |
| 3TC | 14 days | 18.2 ± 3.5 mg/L | Weibull | Low toxicity |
| DTG + TDF | 4 days | 4.79 ± 0.73/28.71 ± 4.35 mg/L | log-logistic | Toxic |
| DTG + TDF | 14 days | 12.07 ± 1.34/72.42 ± 8.01 mg/L | Weibull | Low toxicity |
| DTG + 3TC | 4 days | 10.9 ± 0.7/65.5 ± 3.9 mg/L | log-logistic | Low toxicity |
| DTG + 3TC | 14 days | >100 mg/L | Weibull | Virtually non-toxic |
| TDF + 3TC | 4 days | 5.6 ± 0.3 mg/L | log-logistic | Toxic |
| TDF + 3TC | 14 days | 4.5 ± 0.2 mg/L | log-logistic | Toxic |
| TDF + 3TC ** | 4 days | 51.6 ± 2.2 mg/L | log-logistic | Low toxicity |
| TDF + 3TC ** | 14 days | 68.7 ± 3.7 mg/L | log-normal | Low toxicity |
| DTG + TDF + 3TC | 4 days | 5.6 ± 0.6/14.9 ± 0.2 mg/L | log-normal | Toxic |
| DTG + TDF + 3TC | 14 days | 14.9 ± 0.2/89.5 ± 1.1 mg/L | Weibull | Low toxicity |
| Classification | Test Concentrations (mg/L) | Absorbance (680 nm) | ||
|---|---|---|---|---|
| 0 d | 4 d | 14 d | ||
| NC (0.0) | 0.119 | 0.261 | 2.027 | |
| Medication | 0.1 | 0.118 | 0.260 | 2.043 |
| 0.5 | 0.120 | 0.259 | 1.771 | |
| 5.0 | 0.117 | 0.233 | 1.168 | |
| 10.0 | 0.119 | 0.219 | 0.806 | |
| 15.0 | 0.120 | 0.198 | 0.466 | |
| 25.0 | 0.119 | 0.170 | 0.207 | |
| 35.0 | 0.118 | 0.058 | 0.076 | |
| 45.0 | 0.120 | 0.052 | 0.057 | |
| NC (0.0) | 0.122 | 0.272 | 2.066 | |
| API | 0.01 | 0.118 | 0.262 | 2.068 |
| 0.1 | 0.120 | 0.269 | 2.061 | |
| 1.0 | 0.117 | 0.182 | 0.502 | |
| 10.0 | 0.116 | 0.142 | 0.155 | |
| 20.0 | 0.118 | 0.072 | 0.077 | |
| 30.0 | 0.112 | 0.077 | 0.057 | |
| 40.0 | 0.122 | 0.058 | 0.076 | |
| 50.0 | 0.126 | 0.052 | 0.057 | |
| Concentration | Group | Absorbance (570 nm) | Metabolic Inhibition | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Mean | |||
| 0.0 mg/L | Control | 0.635 | 0.608 | 0.662 | 0.635 | 0.0% |
| 0.1 mg/L | DTG | 0.284 | 0.302 | 0.255 | 0.280 | 55.85% |
| 10 mg/L | TDF | 0.578 | 0.555 | 0.542 | 0.558 | 12.07% |
| 100 mg/L | 3TC | 0.647 | 0.633 | 0.624 | 0.635 | 0.05% |
| Classification | Test Concentrations (mg/L) | Absorbance (680 nm) | ||
|---|---|---|---|---|
| 0 d | 4 d | 14 d | ||
| NC (0.0) | 0.194 | 0.304 | 2.108 | |
| Medication | 1.0 | 0.195 | 0.316 | 2.131 |
| 2.0 | 0.195 | 0.310 | 2.094 | |
| 4.0 | 0.194 | 0.310 | 2.132 | |
| 8.0 | 0.196 | 0.313 | 2.125 | |
| 16.0 | 0.195 | 0.309 | 2.142 | |
| 32.0 | 0.195 | 0.312 | 2.127 | |
| 64.0 | 0.194 | 0.311 | 2.135 | |
| 128.0 | 0.194 | 0.318 | 2.127 | |
| 256.0 | 0.196 | 0.308 | 2.107 | |
| NC (0.0) | 0.179 | 0.286 | 2.000 | |
| API | 2.0 | 0.180 | 0.288 | 1.993 |
| 4.0 | 0.180 | 0.288 | 1.997 | |
| 8.0 | 0.180 | 0.288 | 1.996 | |
| 16.0 | 0.181 | 0.282 | 1.970 | |
| 32.0 | 0.179 | 0.271 | 1.961 | |
| 64.0 | 0.181 | 0.264 | 1.822 | |
| 128.0 | 0.179 | 0.225 | 1.179 | |
| 256.0 | 0.180 | 0.128 | 0.089 | |
| 512.0 | 0.179 | 0.055 | 0.056 | |
| Classification | Test Concentrations (mg/L) | Absorbance (680 nm) | ||
|---|---|---|---|---|
| 0 d | 4 d | 14 d | ||
| NC (0.0) | 0.139 | 0.216 | 1.888 | |
| Medication | 1.0 | 0.140 | 0.219 | 1.922 |
| 5.0 | 0.140 | 0.217 | 1.885 | |
| 10.0 | 0.138 | 0.208 | 0.493 | |
| 25.0 | 0.139 | 0.197 | 0.371 | |
| 50.0 | 0.140 | 0.173 | 0.298 | |
| 100.0 | 0.140 | 0.159 | 0.125 | |
| 200.0 | 0.138 | 0.141 | 0.115 | |
| 300.0 | 0.140 | 0.101 | 0.088 | |
| NC (0.0) | 0.135 | 0.331 | 2.108 | |
| API | 1.0 | 0.133 | 0.336 | 2.121 |
| 5.0 | 0.134 | 0.319 | 2.111 | |
| 10.0 | 0.132 | 0.318 | 2.081 | |
| 25.0 | 0.131 | 0.296 | 2.097 | |
| 50.0 | 0.134 | 0.293 | 2.112 | |
| 100.0 | 0.131 | 0.233 | 2.107 | |
| 200.0 | 0.136 | 0.214 | 2.103 | |
| 300.0 | 0.132 | 0.127 | 2.123 | |
| 400.0 | 0.132 | 0.113 | 2.107 | |
| ARV | Local | Concentration (µg/L) | PNEC (µg/L) | RQs |
|---|---|---|---|---|
| DTG * | Portugal | Min. = 0.004 Max. = 0.071 | 1.72 | 0.002 [2] 0.004 [2] |
| TDF | South Africa | Min. = 0.110 Max. = 0.250 | 130.72 | <0.001 [1] 0.002 [2] |
| 3TC | Kenya | Min. = 0.700 Max. = 228.30 | 184.82 | 0.004 [2] 1.235 [4] |
| 3TC | South Africa | Min. = 0.040 Max. = 33.99 | 184.82 | <0.001 [1] 0.183 [3] |
| 3TC | U.S.A | Min. = 0.016 Max. = 0.150 | 184.82 | <0.001 [1] <0.001 [1] |
| 3TC | Germany | Min. = 0.020 Max. = 0.060 | 184.82 | <0.001 [1] <0.001 [1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza-Silva, G.; Alcantara, M.D.; Souza, C.R.d.; Moreira, C.P.d.S.; Nunes, K.P.; Pereira, C.A.d.J.; Mol, M.P.G.; Silveira, M.R. Toxicity of the Antiretrovirals Tenofovir Disoproxil Fumarate, Lamivudine, and Dolutegravir on Cyanobacterium Microcystis novacekii. Water 2025, 17, 815. https://doi.org/10.3390/w17060815
Souza-Silva G, Alcantara MD, Souza CRd, Moreira CPdS, Nunes KP, Pereira CAdJ, Mol MPG, Silveira MR. Toxicity of the Antiretrovirals Tenofovir Disoproxil Fumarate, Lamivudine, and Dolutegravir on Cyanobacterium Microcystis novacekii. Water. 2025; 17(6):815. https://doi.org/10.3390/w17060815
Chicago/Turabian StyleSouza-Silva, Gabriel, Mariângela Domingos Alcantara, Cléssius Ribeiro de Souza, Carolina Paula de Souza Moreira, Kenia Pedrosa Nunes, Cíntia Aparecida de Jesus Pereira, Marcos Paulo Gomes Mol, and Micheline Rosa Silveira. 2025. "Toxicity of the Antiretrovirals Tenofovir Disoproxil Fumarate, Lamivudine, and Dolutegravir on Cyanobacterium Microcystis novacekii" Water 17, no. 6: 815. https://doi.org/10.3390/w17060815
APA StyleSouza-Silva, G., Alcantara, M. D., Souza, C. R. d., Moreira, C. P. d. S., Nunes, K. P., Pereira, C. A. d. J., Mol, M. P. G., & Silveira, M. R. (2025). Toxicity of the Antiretrovirals Tenofovir Disoproxil Fumarate, Lamivudine, and Dolutegravir on Cyanobacterium Microcystis novacekii. Water, 17(6), 815. https://doi.org/10.3390/w17060815





