Quantifying Temporal Dynamics of E. coli Concentration and Quantitative Microbial Risk Assessment of Pathogen in a Karst Basin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Analysis
2.3. Discharge and Precipitation Data
2.4. Data Analysis
2.5. Statistical Analysis
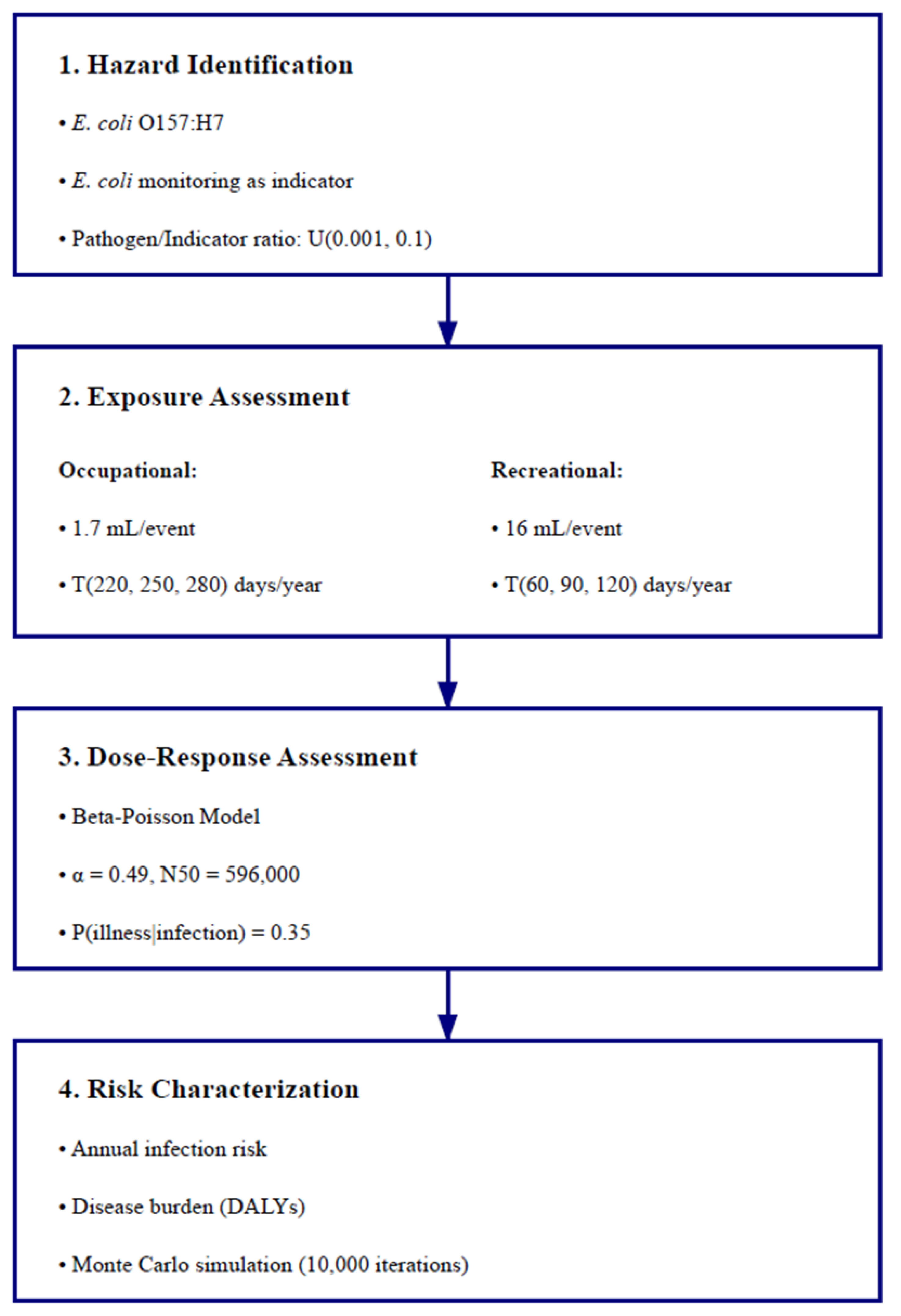
2.6. Quantitative Microbial Risk Assessment
2.6.1. Hazard Identification
2.6.2. Exposure Assessment
2.6.3. Dose–Response Assessment
2.6.4. Risk Characterization
2.6.5. Uncertainty and Sensitivity Analysis
3. Results
3.1. Temporal Dynamics of E. coli and Environmental Parameters
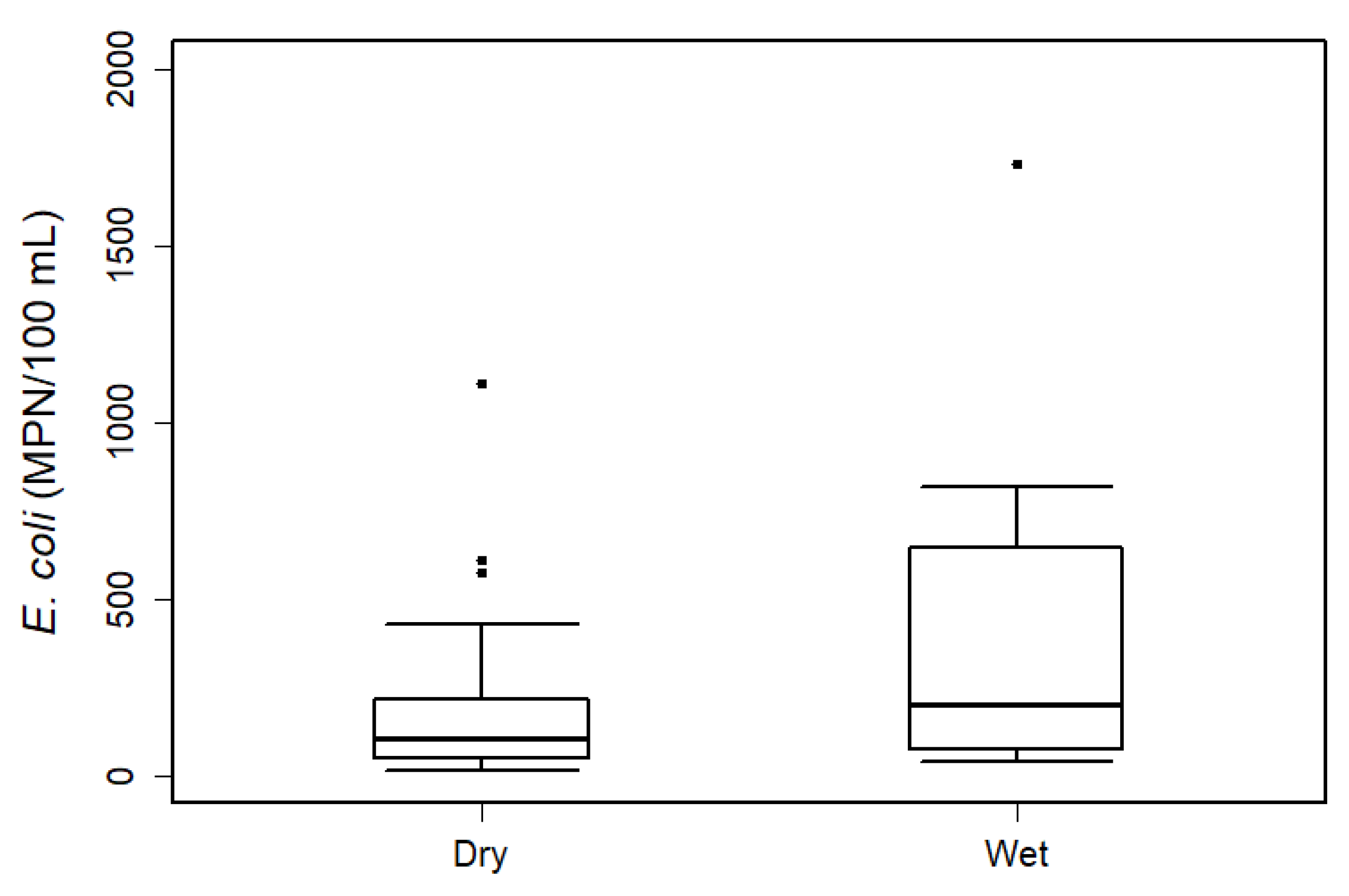
3.2. Influence of Wet Weather Conditions
3.3. Environmental Controls on E. coli Variability
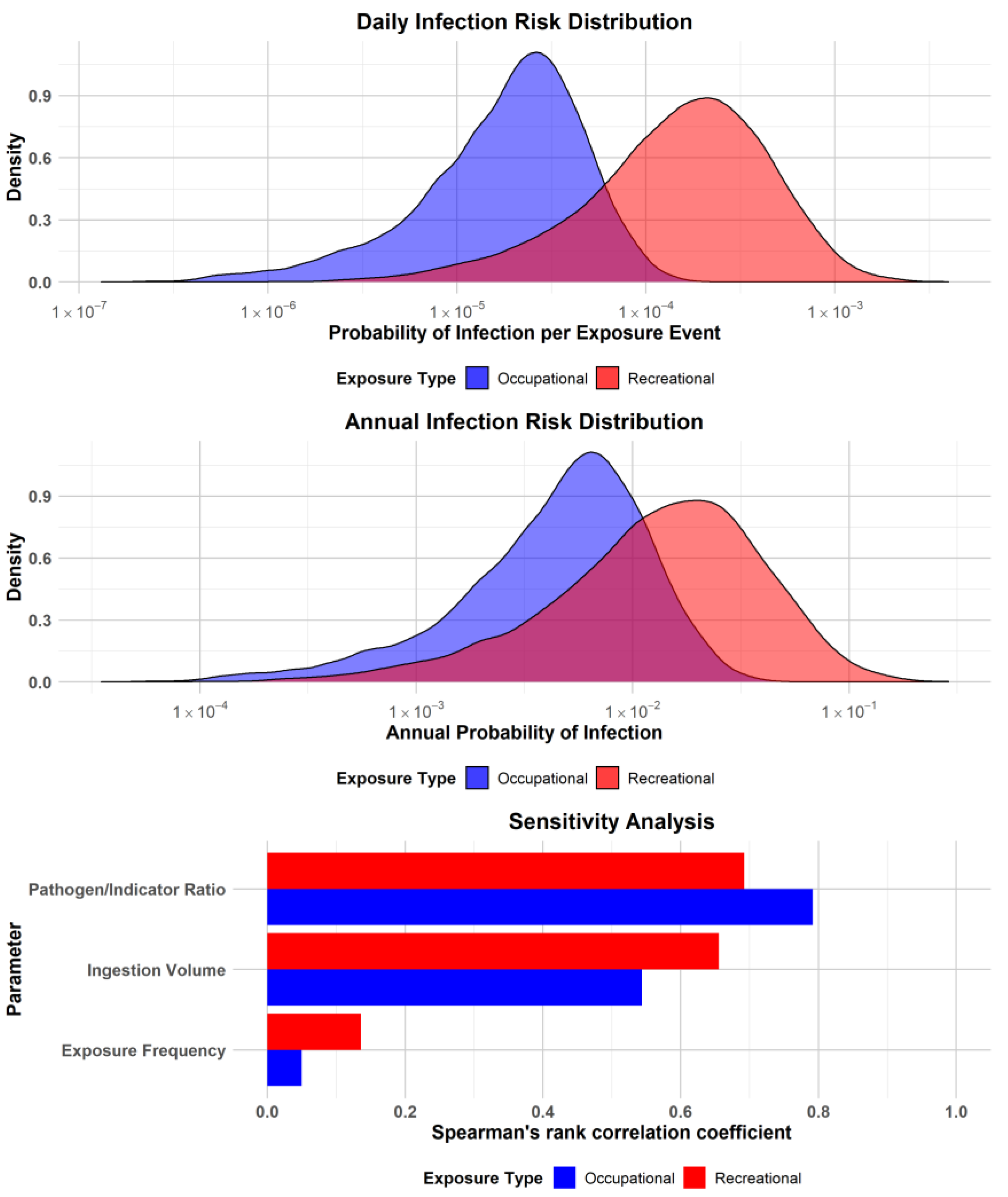
3.4. QMRA Results
4. Discussion
5. Study Limitations and Implications
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- WHO; UNICEF; World Bank. State of the World’s Drinking Water: An Urgent Call to Action to Accelerate Progress on Ensuring Safe Drinking Water for All; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Brouwer, A.F.; Masters, N.B.; Eisenberg, J.N.S. Quantitative microbial risk assessment and infectious disease transmission modeling of waterborne enteric pathogens. Curr. Environ. Health Rep. 2018, 5, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Devane, M.L.; Moriarty, E.; Weaver, L.; Cookson, A.; Gilpin, B. Fecal indicator bacteria from environmental sources; strategies for identification to improve water quality monitoring. Water Res. 2020, 185, 116204. [Google Scholar] [CrossRef]
- Pachepsky, Y.A.; Shelton, D.R. Escherichia coli and fecal coliforms in freshwater and estuarine sediments. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1067–1110. [Google Scholar] [CrossRef]
- Ercumen, A.; Arnold, B.F.; Naser, A.M.; Unicomb, L.; Colford, J.M.; Luby, S.P. Potential sources of bias in the use of Escherichia coli to measure waterborne diarrhoea risk in low-income settings. Trop. Med. Int. Health 2017, 22, 2–11. [Google Scholar] [CrossRef]
- Stauber, C.E.; Wedgworth, J.C.; Johnson, P.; Olson, J.B.; Ayers, T.; Elliott, M.; Brown, J. Associations between self-reported gastrointestinal illness and water system characteristics in community water supplies in rural Alabama: A cross-sectional study. PLoS ONE 2016, 11, e0148102. [Google Scholar] [CrossRef] [PubMed]
- Harwood, V.J.; Staley, C.; Badgley, B.D.; Borges, K.; Korajkic, A. Microbial source tracking markers for detection of fecal contamination in environmental waters: Relationships between pathogens and human health outcomes. FEMS Microbiol. Rev. 2014, 38, 1–40. [Google Scholar] [CrossRef]
- Murphy, H.M.; Prioleau, M.D.; Borchardt, M.A.; Hynds, P.D. Review: Epidemiological evidence of groundwater contribution to global enteric disease, 1948–2015. Hydrogeol. J. 2017, 25, 981–1001. [Google Scholar] [CrossRef]
- Macler, B.A.; Merkle, J.C. Current knowledge on groundwater microbial pathogens and their control. Hydrogeol. J. 2000, 8, 29–40. [Google Scholar] [CrossRef]
- Dura, G.; Pándics, T.; Kádár, M.; Krisztalovics, K.; Kiss, Z.; Bodnár, J.; Asztalos, A.; Papp, E. Environmental health aspects of drinking water-borne outbreak due to karst flooding: Case study. J. Water Health 2010, 8, 513–520. [Google Scholar] [CrossRef]
- Ravbar, N.; Sebela, S. The effectiveness of protection policies and legislative framework with special regard to karst landscapes: Insights from Slovenia. Environ. Sci. Policy 2015, 51, 106–116. [Google Scholar] [CrossRef]
- Pronk, M.; Goldscheider, N.; Zopfi, J. Dynamics and interaction of organic carbon, turbidity and bacteria in a karst aquifer system. Hydrogeol. J. 2006, 14, 473–484. [Google Scholar] [CrossRef]
- Ford, D.; Williams, P. Karst Hydrogeology and Geomorphology; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Mahler, B.J.; Personne, J.C.; Lods, G.F.; Drogue, C. Transport of free and particulate-associated bacteria in karst. J. Hydrol. 2000, 238, 179–193. [Google Scholar] [CrossRef]
- Dussart-Baptista, L.; Massei, N.; Dupont, J.-P.; Jouenne, T. Transfer of bacteria-contaminated particles in a karst aquifer: Evolution of contaminated materials from a sinkhole to a spring. J. Hydrol. 2003, 284, 285–295. [Google Scholar] [CrossRef]
- Gunn, J.; Tranter, J.; Perkins, J.; Hunter, C. Sanitary bacterial dynamics in a mixed karst aquifer. In Karst Hydrology; Leibundgut, C., Gunn, J., Dassargues, A., Eds.; International Association of Hydrological Sciences Publication 247; IAHS Press: Wallingford, UK, 1997; pp. 61–70. [Google Scholar]
- Marshall, D.; Brahana, J.V.; Davis, R.K. Resuspension of viable sediment-bound enteric pathogens in shallow karst aquifers. In Gambling with Groundwater—Physical, Chemical, and Biological Aspects of Aquifer-Stream Relations; Brahana, J.V., Eckstein, Y., Ongley, L.K., Schneider, R., Moore, J.E., Eds.; American Institute of Hydrology: St. Paul, MN, USA, 1998; pp. 179–186. [Google Scholar]
- Sherer, B.M.; Miner, J.R.; Moore, J.A.; Buckhouse, J.C. Resuspending organisms from a rangeland stream bottom. Trans. ASAE 1988, 31, 1217–1222. [Google Scholar] [CrossRef]
- Sherer, B.M.; Miner, J.R.; Moore, J.A.; Buckhouse, J.C. Indicator bacterial survival in stream sediments. J. Environ. Qual. 1992, 21, 591–595. [Google Scholar] [CrossRef]
- O’Reilly, C.E.; Bowen, A.B.; Perez, N.E.; Sarisky, J.P.; Shepherd, C.A.; Miller, M.D.; Hubbard, B.C.; Herring, M.; Buchanan, S.D.; Fitzgerald, C.C.; et al. A waterborne outbreak of gastroenteritis with multiple etiologies among resort island visitors and residents: Ohio, 2004. Clin. Infect. Dis. 2007, 44, 506–512. [Google Scholar] [CrossRef]
- Kresic, N.; Stevanovic, Z. Groundwater Hydrology of Springs: Engineering, Theory, Management, and Sustainability; Butterworth-Heinemann: Oxford, UK, 2010. [Google Scholar]
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Stevanovic, Z.; et al. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar] [CrossRef]
- White, W.B. Geomorphology and Hydrology of Karst Terrains; Oxford University Press: New York, NY, USA, 1988. [Google Scholar]
- Currens, J.C. Kentucky Is Karst Country! What You Should Know About Sinkholes and Springs; Information Circular 4, Series XII; Kentucky Geological Survey, University of Kentucky: Lexington, KY, USA, 2002. [Google Scholar]
- Kentucky Division of Water. Source Water Protection. Available online: https://eec.ky.gov/Environmental-Protection/Water/Protection/Pages/SWP.aspx (accessed on 13 December 2024).
- Kentucky State Data Center. Population and Housing Estimates. Available online: http://ksdc.louisville.edu/data-downloads/estimates/ (accessed on 7 December 2020).
- Department of Biosystems and Agricultural Engineering, University of Kentucky. Cane Run and Royal Spring Watershed-Based Plan; Report Prepared for U.S. Environmental Protection Agency Under Project Number C9994861-06; University of Kentucky: Lexington, KY, USA, 2011. [Google Scholar]
- Lee, S.C. Identifying Hot-Spots of Fecal Contamination in the Royal Spring Karstshed. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2012. Available online: https://uknowledge.uky.edu/ce_etds/2 (accessed on 1 March 2025).
- Currens, B.J.; Hall, A.; Brion, G.M.; Fryar, A.E. Use of acetaminophen and sucralose as co-analytes to differentiate sources of human excreta in surface waters. Water Res. 2019, 157, 1–7. [Google Scholar] [CrossRef]
- Coakley, T.L.; Brion, G.M.; Fryar, A.E. Prevalence of and relationship between two human-associated DNA biomarkers for Bacteroidales in an urban watershed. J. Environ. Qual. 2015, 44, 1694–1698. [Google Scholar] [CrossRef]
- Fryar, A.E.; Currens, B.J.; Alvarez Villa, C.S. Hydrochemical delineation of spring recharge in an urbanized karst basin, central Kentucky. Environ. Eng. Geosci. 2023, 29, 203–216. [Google Scholar] [CrossRef]
- Amraotkar, A.R.; Hargis, C.W.; Cambon, A.C.; Rai, S.N.; Keith, M.C.L.; Ghafghazi, S.; Bolli, R.; DeFilippis, A.P. Comparison of coliform contamination in non-municipal waters consumed by the Mennonite versus the non-Mennonite rural populations. Environ. Health Prev. Med. 2015, 20, 338–346. [Google Scholar] [CrossRef]
- Baughn, C.; Bledsoe, L.A.; Groves, C. Evaluating potential health threats from untreated karst springs as community drinking water sources, Monroe County, Kentucky. In Kentucky Water Resources Annual Symposium Proceedings; Kentucky Water Resources Research Institute, University of Kentucky: Lexington, KY, USA, 2019; pp. 33–34. [Google Scholar]
- Bergeisen, G.H.; Hinds, M.W.; Skaggs, J.W. A waterborne outbreak of hepatitis A in Meade County, Kentucky. Am. J. Public Health 1985, 75, 161–164. [Google Scholar] [CrossRef]
- Haas, C.N.; Rose, J.B.; Gerba, C.P. Quantitative Microbial Risk Assessment, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Howard, G.; Pedley, S.; Tibatemwa, S. Quantitative microbial risk assessment to estimate health risks attributable to water supply: Can the technique be applied in developing countries with limited data? J. Water Health 2006, 4, 49–65. [Google Scholar] [CrossRef]
- Soller, J.A.; Schoen, M.E.; Bartrand, T.; Ravenscroft, J.E.; Ashbolt, N.J. Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of faecal contamination. Water Res. 2010, 44, 4674–4691. [Google Scholar] [CrossRef] [PubMed]
- Fuhrimann, S.; Winkler, M.S.; Stalder, M.; Niwagaba, C.B.; Babu, M.; Kabatereine, N.B.; Halage, A.A.; Utzinger, J.; Cissé, G.; Nauta, M. Disease burden due to gastrointestinal pathogens in a wastewater system in Kampala, Uganda. Microb. Risk Anal. 2016, 4, 16–28. [Google Scholar] [CrossRef]
- Stupar, Z.; Levei, E.A.; Neag, E.; Baricz, A.; Szekeres, E.; Moldovan, O.T. Microbial water quality and health risk assessment in karst springs from Apuseni Mountains, Romania. Front. Environ. Sci. 2022, 10, 931893. [Google Scholar] [CrossRef]
- Robb, K.; Null, C.; Teunis, P.; Yakubu, H.; Armah, G.; Moe, C.L. Assessment of fecal exposure pathways in low-income urban neighborhoods in Accra, Ghana: Rationale, design, methods, and key findings of the SaniPath study. Am. J. Trop. Med. Hyg. 2017, 97, 1020–1032. [Google Scholar] [CrossRef]
- World Health Organization. Quantitative Microbial Risk Assessment: Application for Water Safety Management; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Dapkus, R. Tryptophan-Like Fluorescence and Non-Point Source Pollution in Karst Basins, Inner Bluegrass Region, Kentucky. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2022. Available online: https://uknowledge.uky.edu/ees_etds/96/ (accessed on 1 March 2025).
- Dapkus, R.T.; Fryar, A.E.; Tobin, B.W.; Byrne, D.M.; Sarker, S.K.; Bettel, L.; Fox, J.F. Utilization of tryptophan-like fluorescence as a proxy for E. coli contamination in a mixed-land-use karst basin. Hydrology 2023, 10, 74. [Google Scholar] [CrossRef]
- Husic, A.; Fox, J.; Agouridis, C.; Currens, J.; Ford, W.; Taylor, C. Sediment carbon fate in phreatic karst (Part 1): Conceptual model development. J. Hydrol. 2017, 549, 179–193. [Google Scholar] [CrossRef]
- Thrailkill, J.; Sullivan, S.B.; Gouzie, D.R. Flow parameters in a shallow conduit-flow carbonate aquifer, Inner Bluegrass Karst Region, Kentucky, USA. J. Hydrol. 1991, 129, 87–108. [Google Scholar] [CrossRef]
- Sawyer, A.H.; Zhu, J.; Currens, J.C.; Atcher, C.; Binley, A. Time-lapse electrical resistivity imaging of solute transport in a karst conduit. Hydrol. Process. 2015, 29, 4968–4976. [Google Scholar] [CrossRef]
- Brugger, K. World Map of the Köppen-Geiger Climate Classification Updated Map for the United States of America. Available online: http://koeppen-geiger.vu-wien.ac.at/usa.htm (accessed on 13 December 2024).
- U.S. Climate Data—Monthly Averages. Available online: https://www.usclimatedata.com/ (accessed on 13 December 2024).
- cli-MATE: MRCC Application Tools Environment. Available online: https://mrcc.purdue.edu/CLIMATE/ (accessed on 13 December 2024).
- Sims, R.; Preston, D.; Richarson, A.; Newton, J.; Isgrig, D. Soil Survey of Fayette County, Kentucky; U.S. Government Printing Office: Washington, DC, USA, 1968. [Google Scholar]
- QIAGEN. DNeasy® PowerWater® Kit Handbook; QIAGEN: Germantown, MD, USA, 2022. [Google Scholar]
- Li, B.; Liu, H.; Wang, W. Multiplex real-time PCR assay for detection of Escherichia coli O157:H7 and screening for non-O157 Shiga toxin-producing E. coli. BMC Microbiol. 2017, 17, 215. [Google Scholar] [CrossRef]
- Herlemann, D.P.R.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Royal Springs at Georgetown, KY. Available online: https://waterdata.usgs.gov/monitoring-location/03288110/ (accessed on 13 December 2024).
- Terry, A. (Georgetown Municipal Water and Sewer Service, Georgetown, KY, USA). Personal communication, 2024. [Google Scholar]
- Helsel, D.R. Statistics for Censored Environmental Data Using Minitab and R, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kentucky Administrative Regulations. Surface Water Standards. Available online: https://apps.legislature.ky.gov/law/kar/titles/401/010/031/ (accessed on 3 March 2025).
- U.S. Environmental Protection Agency. Recreational Water Quality Criteria; Office of Water 820-F-12-058; U.S. EPA: Washington, DC, USA, 2012.
- NOAA Online Weather Data (NOWData)—Frequently Asked Questions. Available online: https://www.weather.gov/climateservices/nowdatafaq (accessed on 24 February 2025).
- Tooth, A.F.; Fairchild, I.J. Soil and karst aquifer hydrological controls on the geochemical evolution of speleothem-forming drip waters, Crag Cave, southwest Ireland. J. Hydrol. 2003, 273, 51–68. [Google Scholar] [CrossRef]
- Kentucky Pollutant Discharge Elimination System Permit No. KYR100000. Available online: https://www.hkywater.org/DocumentCenter/View/183/Kentucky-Pollutant-Discharge-Elimination-System-KPDES-PDF (accessed on 13 December 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.1.2; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Dufour, A.P.; Behymer, T.D.; Cantú, R.; Magnuson, M.; Wymer, L.J. Ingestion of swimming pool water by recreational swimmers. J. Water Health 2017, 15, 429–437. [Google Scholar] [CrossRef]
- Pronk, M.; Goldscheider, N.; Zopfi, J. Microbial communities in karst groundwater and their potential use for biomonitoring. Hydrogeol. J. 2009, 17, 37–48. [Google Scholar] [CrossRef]
- Bandy, A.M.; Cook, K.; Fryar, A.E.; Zhu, J. Differential transport of Escherichia coli isolates compared to abiotic tracers in a karst aquifer. Groundwater 2020, 58, 70–78. [Google Scholar] [CrossRef]
- Teunis, P.; Takumi, K.; Shinagawa, K. Dose response for infection by Escherichia coli O157:H7 from outbreak data. Risk Anal. 2004, 24, 401–407. [Google Scholar] [CrossRef]
- Teunis, P.F.M.; Ogden, I.D.; Strachan, N.J.C. Hierarchical dose response of E. coli O157:H7 from human outbreaks incorporating heterogeneity in exposure. Epidemiol. Infect. 2008, 136, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Machdar, E.; van der Steen, N.P.; Raschid-Sally, L.; Lens, P.N.L. Application of Quantitative Microbial Risk Assessment to analyze the public health risk from poor drinking water quality in a low income area in Accra, Ghana. Sci. Total Environ. 2013, 449, 134–142. [Google Scholar] [CrossRef]
- Frank, S.; Goeppert, N.; Goldscheider, N. Multiple-parameter approach to characterize dynamics of organic carbon, faecal bacteria and particles at alpine karst springs. Sci. Total Environ. 2018, 615, 1446–1459. [Google Scholar] [CrossRef] [PubMed]
- Vucinic, L.; O’Connell, D.; Dubber, D.; Coxon, C.; Gill, L. Multiple fluorescence approaches to identify rapid changes in microbial indicators at karst springs. J. Contam. Hydrol. 2023, 254, 104129. [Google Scholar] [CrossRef]
- Sinreich, M.; Pronk, M.; Kozel, R. Microbiological monitoring and classification of karst springs. Environ. Earth Sci. 2014, 71, 563–572. [Google Scholar] [CrossRef]
- Covington, M.D.; Gibson, K.E.; Rodriguez, J. Comparative microbial community dynamics in a karst aquifer system and proximal surface stream in northwest Arkansas. In Arkansas Bulletin of Water Research; Arkansas Water Resources Center, University of Arkansas: Fayetteville, AR, USA, 2018; pp. 3–8. Available online: https://scholarworks.uark.edu/awrcbwr/2 (accessed on 1 March 2025).
- Luffman, I.; Tran, L. Risk factors for E. coli O157 and cryptosporidiosis infection in individuals in the karst valleys of East Tennessee, USA. Geosciences 2014, 4, 202–218. [Google Scholar] [CrossRef]
- McBride, G.B.; Stott, R.; Miller, W.; Bambic, D.; Wuertz, S. Discharge-based QMRA for estimation of public health risks from exposure to stormwater-borne pathogens in recreational waters in the United States. Water Res. 2013, 47, 5282–5297. [Google Scholar] [CrossRef]
- Korajkic, A.; McMinn, B.R.; Harwood, V.J. Relationships between microbial indicators and pathogens in recreational water settings. Int. J. Environ. Res. Public Health 2018, 15, 2842. [Google Scholar] [CrossRef]
- Zhang, Q.; Gallard, J.; Wu, B.; Harwood, V.J.; Sadowsky, M.J.; Hamilton, K.A.; Ahmed, W. Synergy between quantitative microbial source tracking (qMST) and quantitative microbial risk assessment (QMRA): A review and prospectus. Environ. Int. 2019, 130, 104703. [Google Scholar] [CrossRef]
- Ferguson, A.S.; Layton, A.C.; Mailloux, B.J.; Culligan, P.J.; Williams, D.E.; Smartt, A.E.; Sayler, G.S.; Feighery, J.; McKay, L.; Knappett, P.S.K.; et al. Comparison of fecal indicators with pathogenic bacteria and rotavirus in groundwater. Sci. Total Environ. 2012, 431, 314–322. [Google Scholar] [CrossRef]


| Variable | Coefficient | Standard Error | t | p |
| Intercept | −438.63 | 270.93 | −1.619 | 0.113 |
| Discharge | 52.05 | 93.50 | 0.557 | 0.581 |
| AMC 48-h | 232.80 | 102.59 | 2.269 | 0.028 |
| Temperature | 37.65 | 14.32 | 2.629 | 0.012 |
| Model Statistics | Value | |||
| R-squared | 0.2356 | |||
| F-statistic | 5.52 | |||
| p-value | 0.002 | |||
| Residual-standard error | 273.6 | |||
| Degrees of freedom | 41 |
| Occupational Exposure | Recreational Exposure | |||||
|---|---|---|---|---|---|---|
| Risk Metric | Median | 95% CI Lower | 95% CI Upper | Median | 95% CI Lower | 95% CI Upper |
| Daily Infection Risk | 2.06 × 10−5 | 1.41 × 10−6 | 8.11 × 10−5 | 1.64 × 10−4 | 1.01 × 10−5 | 9.04 × 10−4 |
| Annual Infection Risk | 5.11 × 10−3 | 3.49 × 10−4 | 2.02 × 10−2 | 1.45 × 10−2 | 8.93 × 10−4 | 8.03 × 10−2 |
| Annual Illness Risk | 1.79 × 10−3 | 1.22 × 10−4 | 7.09 × 10−3 | 5.08 × 10−3 | 3.12 × 10−4 | 2.81× 10−2 |
| DALYs | 2.33 × 10−6 | 1.59 × 10−7 | 9.21 × 10−6 | 6.60 × 10−6 | 4.06 × 10−7 | 3.65 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarker, S.K.; Dapkus, R.T.; Byrne, D.M.; Fryar, A.E.; Hutchison, J.M. Quantifying Temporal Dynamics of E. coli Concentration and Quantitative Microbial Risk Assessment of Pathogen in a Karst Basin. Water 2025, 17, 745. https://doi.org/10.3390/w17050745
Sarker SK, Dapkus RT, Byrne DM, Fryar AE, Hutchison JM. Quantifying Temporal Dynamics of E. coli Concentration and Quantitative Microbial Risk Assessment of Pathogen in a Karst Basin. Water. 2025; 17(5):745. https://doi.org/10.3390/w17050745
Chicago/Turabian StyleSarker, Shishir K., Ryan T. Dapkus, Diana M. Byrne, Alan E. Fryar, and Justin M. Hutchison. 2025. "Quantifying Temporal Dynamics of E. coli Concentration and Quantitative Microbial Risk Assessment of Pathogen in a Karst Basin" Water 17, no. 5: 745. https://doi.org/10.3390/w17050745
APA StyleSarker, S. K., Dapkus, R. T., Byrne, D. M., Fryar, A. E., & Hutchison, J. M. (2025). Quantifying Temporal Dynamics of E. coli Concentration and Quantitative Microbial Risk Assessment of Pathogen in a Karst Basin. Water, 17(5), 745. https://doi.org/10.3390/w17050745








