Removal of Methylene Blue from Simulated Wastewater Based upon Hydrothermal Carbon Activated by Phosphoric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Preparation of Hydrothermal Carbon
2.3. Characterization Methods
2.4. Adsorption Experiments
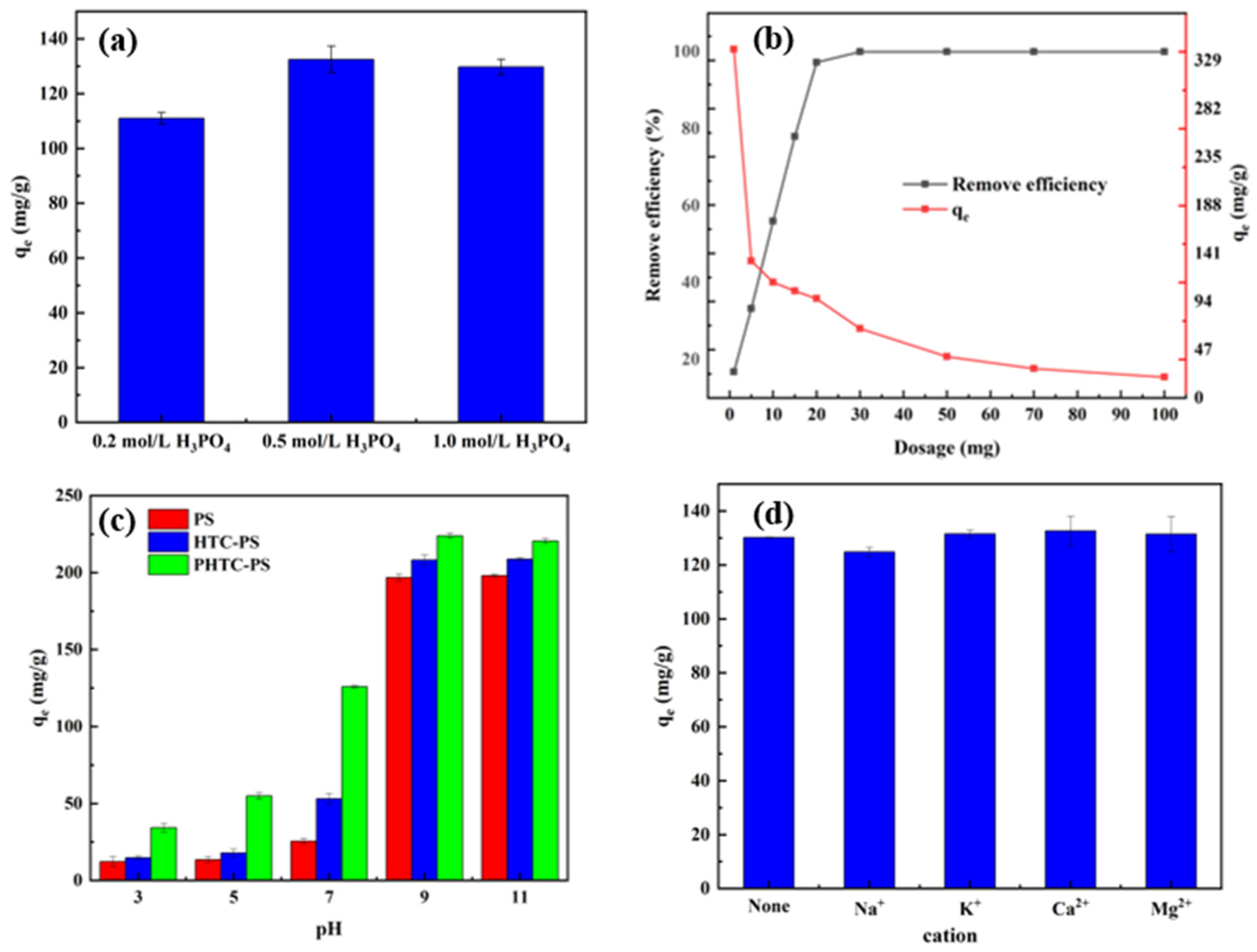
2.4.1. Influencing Factors
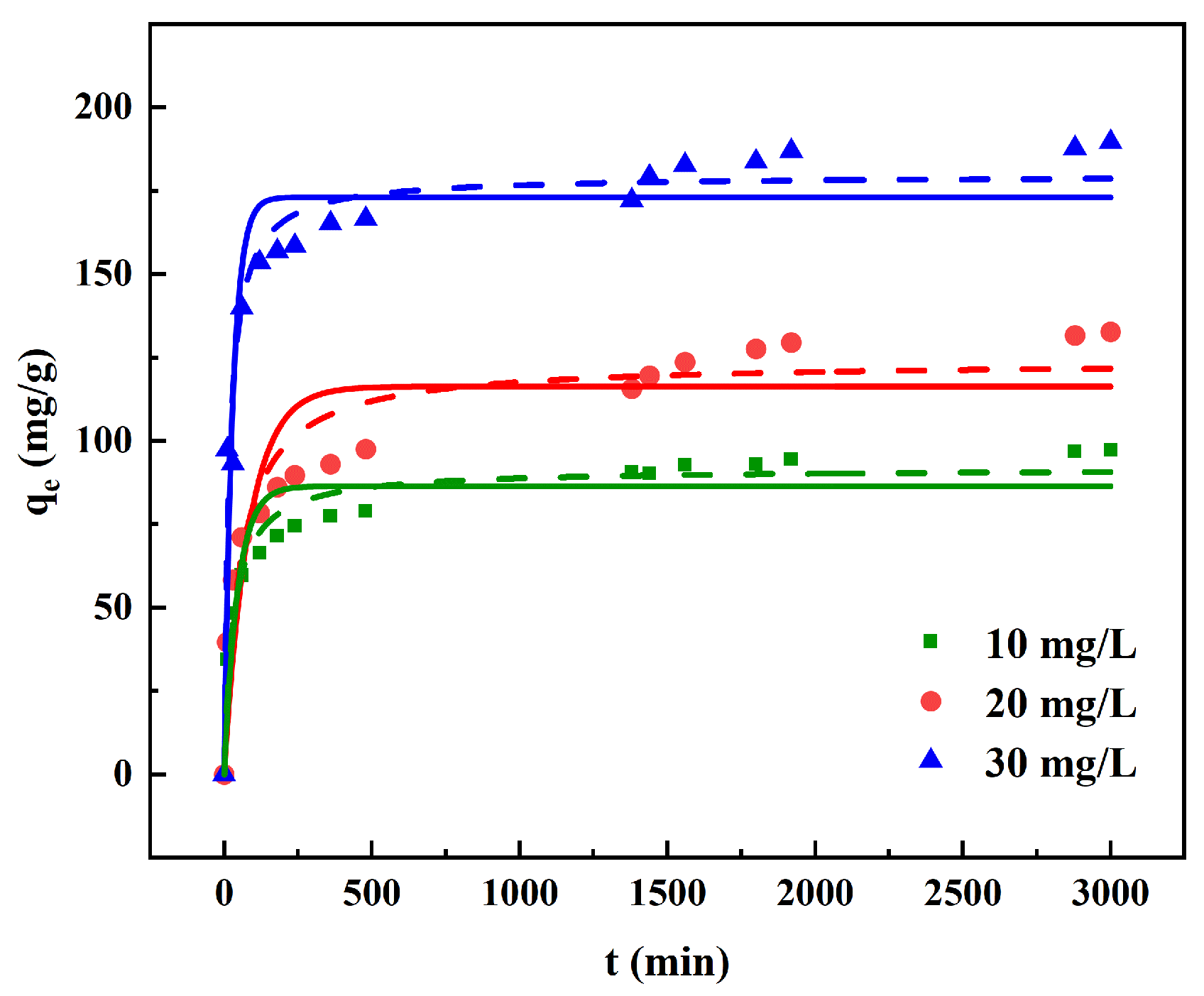
2.4.2. Adsorption Kinetics
2.4.3. Adsorption Isotherms
2.4.4. Adsorption Thermodynamics
3. Results and Discussion
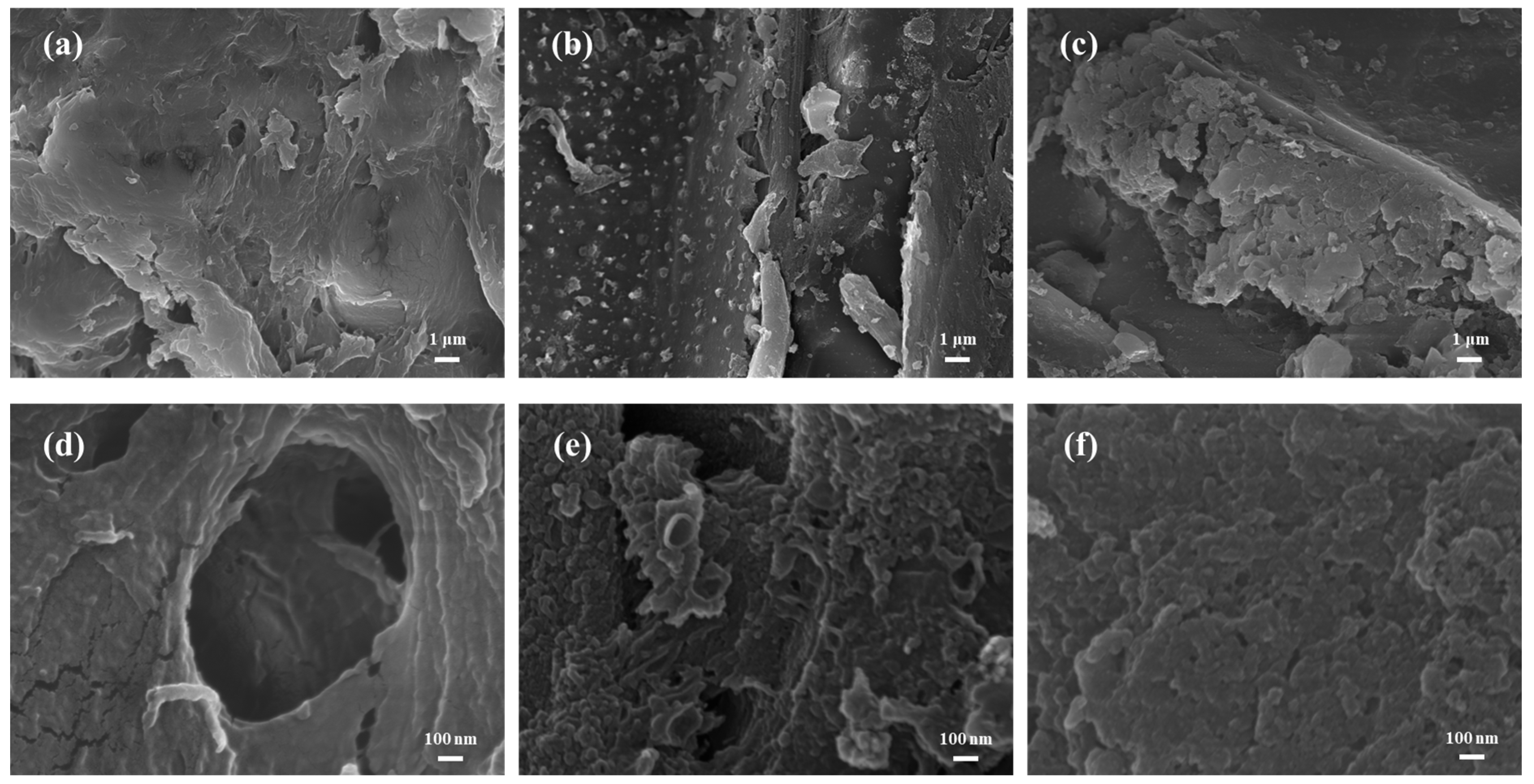
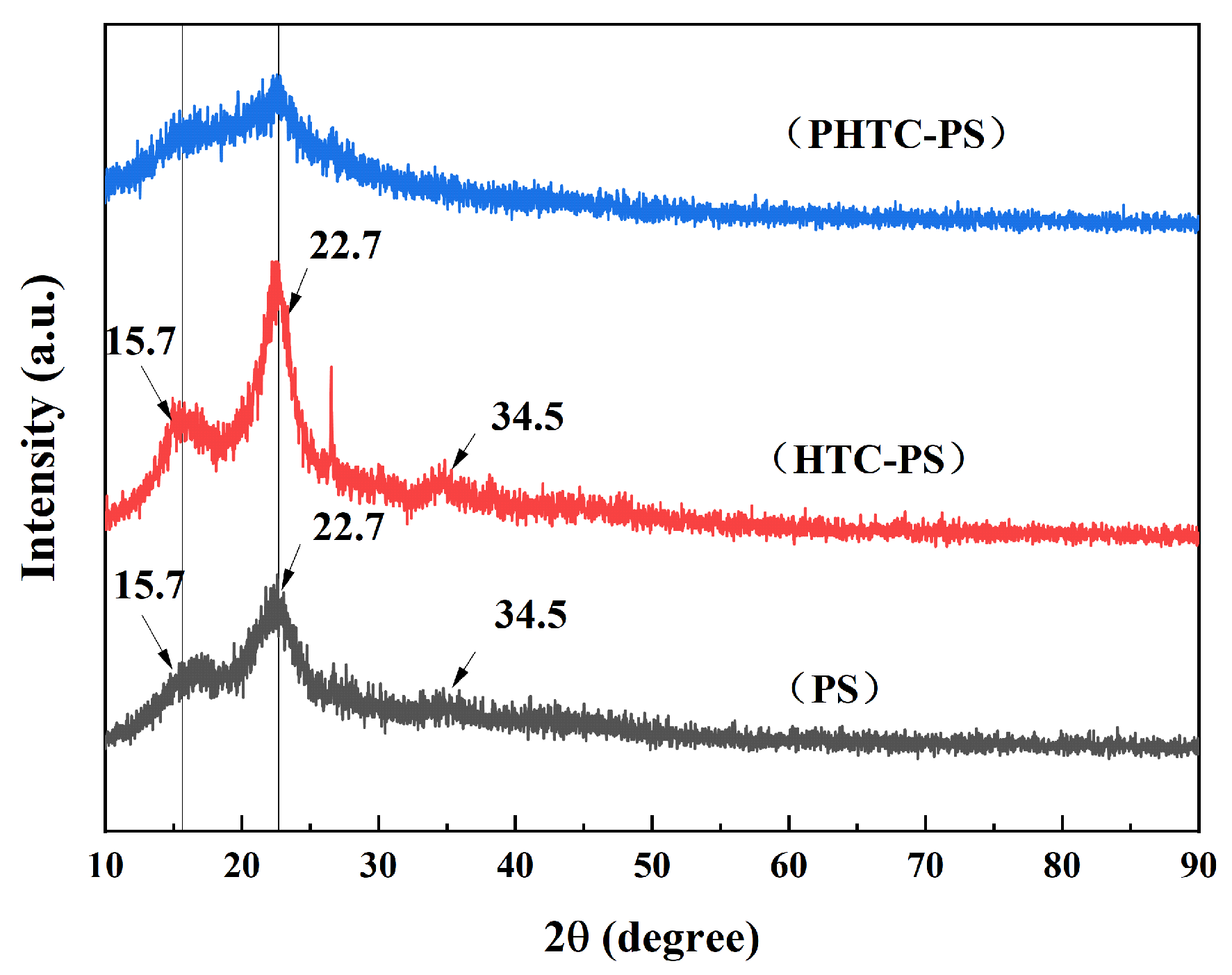
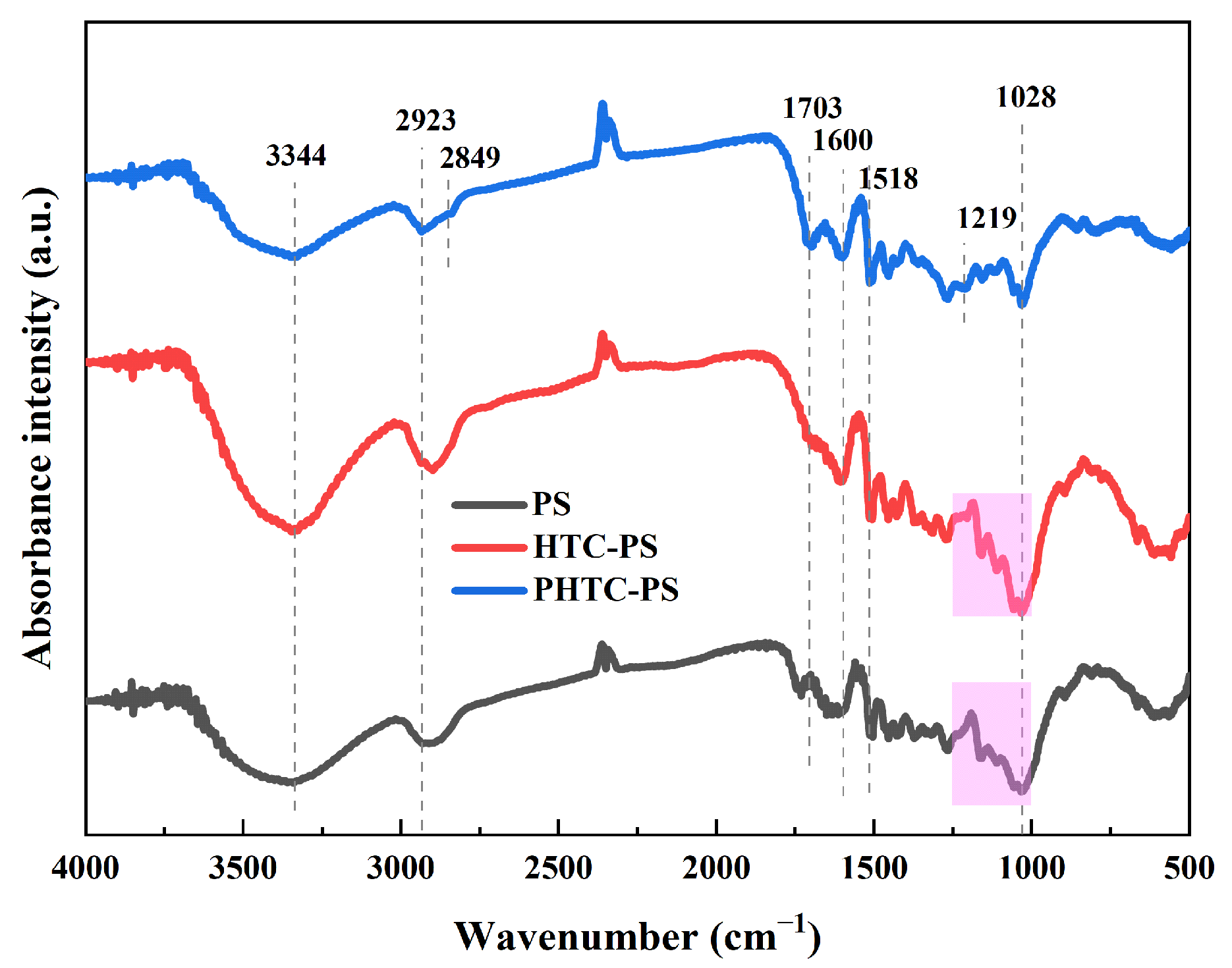
3.1. Characterizations of Materials
3.2. Effects of Different Influencing Factors
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
3.5. Adsorption Thermodynamics
3.6. Adsorption Efficiency of PHTC-PS for Other Dyes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Yu, E.; Sun, S.; Liu, W.; Hu, R.; Xu, L. Fast and Highly Efficient Adsorption of Cationic Dyes by Phytic Acid Crosslinked β-Cyclodextrin. Carbohydr. Polym. 2022, 284, 119231. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Brillas, E. Advances in the Decontamination of Wastewaters with Synthetic Organic Dyes by Electrochemical Fenton-Based Processes. Sep. Purif. Technol. 2023, 316, 123764. [Google Scholar] [CrossRef]
- Liu, Y.; Song, J.-W.; Bao, J.; Shen, X.-J.; Li, C.-L.; Wang, X.; Shao, L.-X. Optimized Removal of Azo Dyes from Simulated Wastewater Through Advanced Plasma Technique with Novel Reactor. Water 2022, 14, 3152. [Google Scholar] [CrossRef]
- Abbas, N.; Hussain, S.; Azeem, F.; Shahzad, T.; Bhatti, S.H.; Imran, M.; Ahmad, Z.; Maqbool, Z.; Abid, M. Characterization of a Salt Resistant Bacterial Strain Proteus Sp. NA6 Capable of Decolorizing Reactive Dyes in Presence of Multi-Metal Stress. World J. Microbiol. Biotechnol. 2016, 32, 181. [Google Scholar] [CrossRef]
- Kausar, A.; Zohra, S.T.; Ijaz, S.; Iqbal, M.; Iqbal, J.; Bibi, I.; Nouren, S.; Messaoudi, N.E.; Nazir, A. Cellulose-Based Materials and Their Adsorptive Removal Efficiency for Dyes: A Review. Int. J. Biol. Macromol. 2023, 224, 1337–1355. [Google Scholar] [CrossRef]
- Salahshoori, I.; Namayandeh Jorabchi, M.; Ghasemi, S.; Mirnezami, S.M.S.; Nobre, M.A.L.; Khonakdar, H.A. Assessing Cationic Dye Adsorption Mechanisms on MIL-53 (Al) Nanostructured MOF Materials Using Quantum Chemical and Molecular Simulations: Toward Environmentally Sustainable Wastewater Treatment. J. Water Process Eng. 2023, 55, 104081. [Google Scholar] [CrossRef]
- Paul Nayagam, J.O.; Prasanna, K. Utilization of Shell-Based Agricultural Waste Adsorbents for Removing Dyes: A Review. Chemosphere 2022, 291, 132737. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.; Peng, X.; Sun, J.; Li, C.; Zhang, X.; Zhang, H.; Chen, J.; Zhou, X.; Zeng, H.; et al. Hydrogels for the Removal of the Methylene Blue Dye from Wastewater: A Review. Environ. Chem. Lett. 2022, 20, 2665–2685. [Google Scholar] [CrossRef]
- Liu, X.-J.; Li, M.-F.; Singh, S.K. Manganese-Modified Lignin Biochar as Adsorbent for Removal of Methylene Blue. J. Mater. Res. Technol. 2021, 12, 1434–1445. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Bao, J.; Wang, X.; Yu, W.; Shao, L. Degradation of Azo Dyes with Different Functional Groups in Simulated Wastewater by Electrocoagulation. Water 2022, 14, 123. [Google Scholar] [CrossRef]
- Hou, Y.; Yan, S.; Huang, G.; Yang, Q.; Huang, S.; Cai, J. Fabrication of N-Doped Carbons from Waste Bamboo Shoot Shell with High Removal Efficiency of Organic Dyes from Water. Bioresour. Technol. 2020, 303, 122939. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zhang, P.; Zhang, B.; Zhao, L. Synthesis of Fe3O4/C Composites Derived from Cornstalk by One-Step Hydrothermal Method as a Reusable Adsorbent for Dyes. Inorg. Chem. Commun. 2022, 143, 109762. [Google Scholar] [CrossRef]
- Hu, H.; Wageh, S.; Al-Ghamdi, A.A.; Yang, S.; Tian, Z.; Cheng, B.; Ho, W. NiFe-LDH Nanosheet/Carbon Fiber Nanocomposite with Enhanced Anionic Dye Adsorption Performance. Appl. Surf. Sci. 2020, 511, 145570. [Google Scholar] [CrossRef]
- Dai, L.; Zhu, W.; He, L.; Tan, F.; Zhu, N.; Zhou, Q.; He, M.; Hu, G. Calcium-Rich Biochar from Crab Shell: An Unexpected Super Adsorbent for Dye Removal. Bioresour. Technol. 2018, 267, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L.; Cheng, Z. Removal of Organic Pollutants from Aqueous Solution Using Agricultural Wastes: A Review. J. Mol. Liq. 2015, 212, 739–762. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.; Zhang, H.; Xiao, R. Fast Pyrolysis of Holocellulose for the Preparation of Long-Chain Ether Fuel Precursors: Effect of Holocellulose Types. Bioresour. Technol. 2021, 338, 125519. [Google Scholar] [CrossRef]
- Chávez-Rosales, J.S.; Pintor-Ibarra, L.F.; González-Ortega, N.; Orihuela-Equihua, R.; Ruiz-Aquino, F.; Lujan-Álvarez, C.; Rutiaga-Quiñones, J.G. Basic Chemical Composition of Pinus Spp. Sawdust from Five Regions of Mexico, for Bioenergetic Purposes. BioRes 2020, 16, 816–824. [Google Scholar] [CrossRef]
- Hou, M.; He, Y.; Yang, X.; Yang, Y.; Lin, X.; Feng, Y.; Kan, H.; Hu, H.; He, X.; Liu, C. Preparation of Biomass Biochar with Components of Similar Proportions and Its Methylene Blue Adsorption. Molecules 2023, 28, 6261. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Ahmed, M.J.; Hummadi, E.H. Eucalyptus-Based Materials as Adsorbents for Heavy Metals and Dyes Removal from (Waste) Waters. J. Mol. Liq. 2022, 356, 118864. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Danish, M.; Anastopoulos, I.; Iwuozor, K.O. Recent Progress on Corn (Zea mays L.)-Based Materials as Raw, Chemically Modified, Carbonaceous, and Composite Adsorbents for Aquatic Pollutants: A Review. J. Anal. Appl. Pyrolysis 2023, 172, 106004. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Wang, X.; Khan, M.B.; Lu, M.; Khan, K.Y.; Song, Y.; He, Z.; Yang, X.; Yan, B.; et al. Biochar from Constructed Wetland Biomass Waste: A Review of Its Potential and Challenges. Chemosphere 2022, 287, 132259. [Google Scholar] [CrossRef] [PubMed]
- Mainali, K.; Mood, S.H.; Pelaez-Samaniego, M.R.; Sierra-Jimenez, V.; Garcia-Perez, M. Production and Applications of N-Doped Carbons from Bioresources: A Review. Catal. Today 2023, 423, 114248. [Google Scholar] [CrossRef]
- Sivaranjanee, R.; Kumar, P.S.; Rangasamy, G. A Critical Review on Biochar for Environmental Applications. Carbon. Lett. 2023, 33, 1407–1432. [Google Scholar] [CrossRef]
- Huff, M.D.; Kumar, S.; Lee, J.W. Comparative Analysis of Pinewood, Peanut Shell, and Bamboo Biomass Derived Biochars Produced via Hydrothermal Conversion and Pyrolysis. J. Environ. Manag. 2014, 146, 303–308. [Google Scholar] [CrossRef]
- Liu, H.; Basar, I.A.; Nzihou, A.; Eskicioglu, C. Hydrochar Derived from Municipal Sludge through Hydrothermal Processing: A Critical Review on Its Formation, Characterization, and Valorization. Water Res. 2021, 199, 117186. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.-H.; Duc Nguyen, D.; Pugazhendhi, A.; Chang, S.W.; Kumar, G. Review on Sustainable Production of Biochar Through Hydrothermal Liquefaction: Physico-Chemical Properties and Applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef]
- Alharbi, H.A.; Hameed, B.H.; Alotaibi, K.D.; Al-Oud, S.S.; Al-Modaihsh, A.S. Recent Methods in the Production of Activated Carbon from Date Palm Residues for the Adsorption of Textile Dyes: A Review. Front. Environ. Sci. 2022, 10, 996953. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Q.; Abou-Elwafa, S.F.; Alshehri, M.A.; Zhang, T. Hydrothermal Carbonization Technology for Wastewater Treatment under the “Dual Carbon” Goals: Current Status, Trends, and Challenges. Water 2024, 16, 1749. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Li, M.; Chen, Y. Biochar Facilitated Bioprocessing and Biorefinery for Productions of Biofuel and Chemicals: A Review. Bioresour. Technol. 2020, 295, 122252. [Google Scholar] [CrossRef]
- Hadi, P.; Xu, M.; Ning, C.; Sze Ki Lin, C.; McKay, G. A Critical Review on Preparation, Characterization and Utilization of Sludge-Derived Activated Carbons for Wastewater Treatment. Chem. Eng. J. 2015, 260, 895–906. [Google Scholar] [CrossRef]
- Cui, H.-J.; Cai, J.-K.; Zhao, H.; Yuan, B.; Ai, C.; Fu, M.-L. One Step Solvothermal Synthesis of Functional Hybrid γ-Fe2O3/Carbon Hollow Spheres with Superior Capacities for Heavy Metal Removal. J. Colloid. Interface Sci. 2014, 425, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Q.; Qiu, L.; Zhu, M.-Q. Eucommia Ulmoides Oliver Derived Magnetic Activated Carbon for Eliminating Methylene Blue from Dyeing Wastewater and Its Economic Efficiency Assessment. Ind. Crops Prod. 2022, 187, 115537. [Google Scholar] [CrossRef]
- Alhawtali, S.; El-Harbawi, M.; Al-Awadi, A.S.; El Blidi, L.; Alrashed, M.M.; Yin, C.-Y. Enhanced Adsorption of Methylene Blue Using Phosphoric Acid-Activated Hydrothermal Carbon Microspheres Synthesized from a Variety of Palm-Based Biowastes. Coatings 2023, 13, 1287. [Google Scholar] [CrossRef]
- Zhou, F.; Li, K.; Hang, F.; Zhang, Z.; Chen, P.; Wei, L.; Xie, C. Efficient Removal of Methylene Blue by Activated Hydrochar Prepared by Hydrothermal Carbonization and NaOH Activation of Sugarcane Bagasse and Phosphoric Acid. RSC Adv. 2022, 12, 1885–1896. [Google Scholar] [CrossRef]
- Pan, J.; Bai, X.; Li, Y.; Yang, B.; Yang, P.; Yu, F.; Ma, J. HKUST-1 Derived Carbon Adsorbents for Tetracycline Removal with Excellent Adsorption Performance. Environ. Res. 2022, 205, 112425. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Zhang, H.; Liu, Y.; Xing, B. Adsorption of Pb(II) and Cd(II) by Magnetic Activated Carbon and Its Mechanism. Sci. Total Environ. 2021, 757, 143910. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon Composite Lignin-Based Adsorbents for the Adsorption of Dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Tran, H.N.; Chao, H.-P.; Lin, C.-C. Effect of Nitric Acid Oxidation on the Surface of Hydrochars to Sorb Methylene Blue: An Adsorption Mechanism Comparison. Adsorpt. Sci. Technol. 2019, 37, 607–622. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Yao, J.; Zhan, S.; Li, H.; Zhang, J.; Qiu, Z. Synthesis of Polyethyleneimine Modified CoFe2O4-Loaded Porous Biochar for Selective Adsorption Properties towards Dyes and Exploration of Interaction Mechanisms. Sep. Purif. Technol. 2021, 277, 119474. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The Production of Carbon Materials by Hydrothermal Carbonization of Cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Li, F.; Zimmerman, A.R.; Zheng, Y.; Yang, Y.; Huang, J.; Zhang, Y.; Hu, X.; Yu, Z.; Huang, J.; Gao, B. P-Enriched Hydrochar for Soil Remediation: Synthesis, Characterization, and Lead Stabilization. Sci. Total Environ. 2021, 783, 146983. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Cui, K.; Cui, M.; Ding, Y.; Guo, Z.; Chen, Y.; Li, C.; Li, X. Enhanced Norfloxacin Degradation by Iron and Nitrogen Co-Doped Biochar: Revealing the Radical and Nonradical Co-Dominant Mechanism of Persulfate Activation. Chem. Eng. J. 2021, 420, 129902. [Google Scholar] [CrossRef]
- Shen, D.; Wang, K.; Yin, J.; Chen, T.; Yu, X. Effect of Phosphoric Acid as a Catalyst on the Hydrothermal Pretreatment and Acidogenic Fermentation of Food Waste. Waste Manag. 2016, 51, 65–71. [Google Scholar] [CrossRef]
- Lan, Y.; Luo, Y.; Yu, S.; Ye, H.; Zhang, Y.; Xue, M.; Sun, Q.; Yin, Z.; Li, X.; Xie, C.; et al. Cornstalk Hydrochar Produced by Phosphoric Acid-Assisted Hydrothermal Carbonization for Effective Adsorption and Photodegradation of Norfloxacin. Sep. Purif. Technol. 2024, 330, 125543. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced Adsorption of Cu(II) and Cd(II) by Phosphoric Acid-Modified Biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q.; Wang, Y. Comparisons of Porous, Surface Chemistry and Adsorption Properties of Carbon Derived from Enteromorpha Prolifera Activated by H4P2O7 and KOH. Chem. Eng. J. 2013, 232, 582–590. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Z.; Xin, Z.; Hu, Z.; Ji, H. Interfacial Charge Dominating Major Active Species and Degradation Pathways: An Example of Carbon Based Photocatalyst. J. Colloid. Interface Sci. 2019, 554, 743–751. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Socha, R.P.; Gurgul, J.; Wisniewski, M. XPS and NMR Studies of Phosphoric Acid Activated Carbons. Carbon 2008, 46, 2113–2123. [Google Scholar] [CrossRef]
- Chen, M.; He, F.; Hu, D.; Bao, C.; Huang, Q. Broadened Operating pH Range for Adsorption/Reduction of Aqueous Cr(VI) Using Biochar from Directly Treated Jute (Corchorus capsularis L.) Fibers by H3PO4. Chem. Eng. J. 2020, 381, 122739. [Google Scholar] [CrossRef]
- Estrade-Szwarckopf, H. XPS Photoemission in Carbonaceous Materials: A ‘“Defect”’ Peak beside the Graphitic Asymmetric Peak. Carbon 2004, 42, 1713–1721. [Google Scholar] [CrossRef]
- Ullah, H.; Barzgar Vishlaghi, M.; Balkan, T.; Ur Rehman, Z.; Kaya, S. Scaling-up Photocatalytic Activity of CdS from Nanorods to Nanowires for the MB Degradation. Inorg. Chem. Commun. 2021, 130, 108744. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, Z.; Nasir, J.A.; Balkan, T.; Butler, I.S.; Kaya, S.; Rehman, Z.U. Green Synthesis of Mesoporous MoS2 Nanoflowers for Efficient Photocatalytic Degradation of Congo Red Dye. J. Coord. Chem. 2021, 74, 2302–2314. [Google Scholar] [CrossRef]
- Li, H.; Budarin, V.L.; Clark, J.H.; North, M.; Wu, X. Rapid and Efficient Adsorption of Methylene Blue Dye from Aqueous Solution by Hierarchically Porous, Activated Starbons®: Mechanism and Porosity Dependence. J. Hazard. Mater. 2022, 436, 129174. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Viglašová, E.; Galamboš, M. Visible Light-Driven Photocatalytic Rhodamine B Degradation Using CdS Nanorods. Processes 2021, 9, 263. [Google Scholar] [CrossRef]
- Asl, S.H.; Ahmadi, M.; Ghiasvand, M.; Tardast, A.; Katal, R. Artificial Neural Network (ANN) Approach for Modeling of Cr(VI) Adsorption from Aqueous Solution by Zeolite Prepared from Raw Fly Ash (ZFA). J. Ind. Eng. Chem. 2013, 19, 1044–1055. [Google Scholar] [CrossRef]
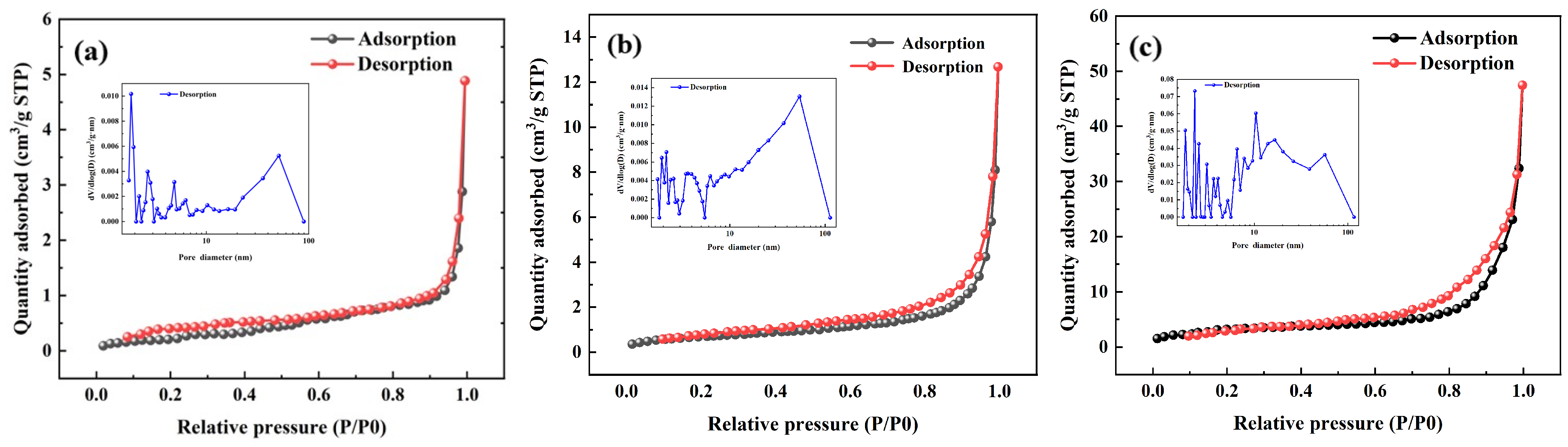



| Samples | BET Surface Area (m2/g) | Total Pore Volume (mL/g) | Average Pore Size (nm) |
|---|---|---|---|
| PS | 1.041 | 0.0057 | 21.90 |
| HTC-PS | 2.440 | 0.0138 | 22.62 |
| PHTC-PS | 11.56 | 0.0549 | 19.00 |
| MB Concentration | Pseudo-First Order | Pseudo-Seond-Order | ||||
|---|---|---|---|---|---|---|
| k1 | qe (mg/g) | R2 | k2 | qe (mg/g) | R2 | |
| 10 mg/L | 0.0221 | 86.38 | 0.860 | 3.48 × 10−4 | 91.52 | 0.950 |
| 20 mg/L | 0.0012 | 116.3 | 0.806 | 1.53 × 10−4 | 123.8 | 0.914 |
| 30 mg/L | 0.0350 | 173.0 | 0.872 | 3.34 × 10−4 | 179.6 | 0.942 |
| Temperature (Kelvin) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (min−1) | R2 | KF (mg/g) | 1/n | R2 | |
| 298 | 268.4 | 0.293 | 0.978 | 92.50 | 0.178 | 0.956 |
| 308 | 339.7 | 0.267 | 0.965 | 106.54 | 0.193 | 0.947 |
| 318 | 376.6 | 0.311 | 0.987 | 123.32 | 0.183 | 0.964 |
| Temperature (Kelvin) | KD (L/g) | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (J·mol−1·K−1) |
|---|---|---|---|---|
| 298 | 3.900 | −3.372 | 14.79 | 61.04 |
| 308 | 4.878 | −4.058 | ||
| 318 | 5.674 | −4.590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Bao, J.; Liu, Y. Removal of Methylene Blue from Simulated Wastewater Based upon Hydrothermal Carbon Activated by Phosphoric Acid. Water 2025, 17, 733. https://doi.org/10.3390/w17050733
Hong J, Bao J, Liu Y. Removal of Methylene Blue from Simulated Wastewater Based upon Hydrothermal Carbon Activated by Phosphoric Acid. Water. 2025; 17(5):733. https://doi.org/10.3390/w17050733
Chicago/Turabian StyleHong, Jing, Jia Bao, and Yang Liu. 2025. "Removal of Methylene Blue from Simulated Wastewater Based upon Hydrothermal Carbon Activated by Phosphoric Acid" Water 17, no. 5: 733. https://doi.org/10.3390/w17050733
APA StyleHong, J., Bao, J., & Liu, Y. (2025). Removal of Methylene Blue from Simulated Wastewater Based upon Hydrothermal Carbon Activated by Phosphoric Acid. Water, 17(5), 733. https://doi.org/10.3390/w17050733








