Evaluation of the Effect of Using the UV + O3 Process with Low- and Medium-Pressure Lamps on the Amount and Properties of Organic Substances in Treated Water
Abstract
1. Introduction
2. Methods and Subjects of Research
3. Results and Discussion
4. Summary and Conclusions
- The diverse properties of organic substances present in these waters influenced the oxidation process and their mineralization, which depended not only on the ozone dose but primarily on the type of UV lamp used.
- The application of a low-pressure UV lamp combined with ozone results in both the mineralization and transformation of organic substances, with the degree being directly proportional to the level of contamination in raw water with high-molecular-weight humic acids. The dominant mechanism for both lamps is the transformation of organic substances, with the type of by-product depending on the wavelength of the emitted light.
- The use of a medium-pressure UV lamp for advanced oxidation provided only limited mineralization of organic substances, which did not exceed 37.8% and was observed for water sample B, characterized by the highest content of organic carbon.
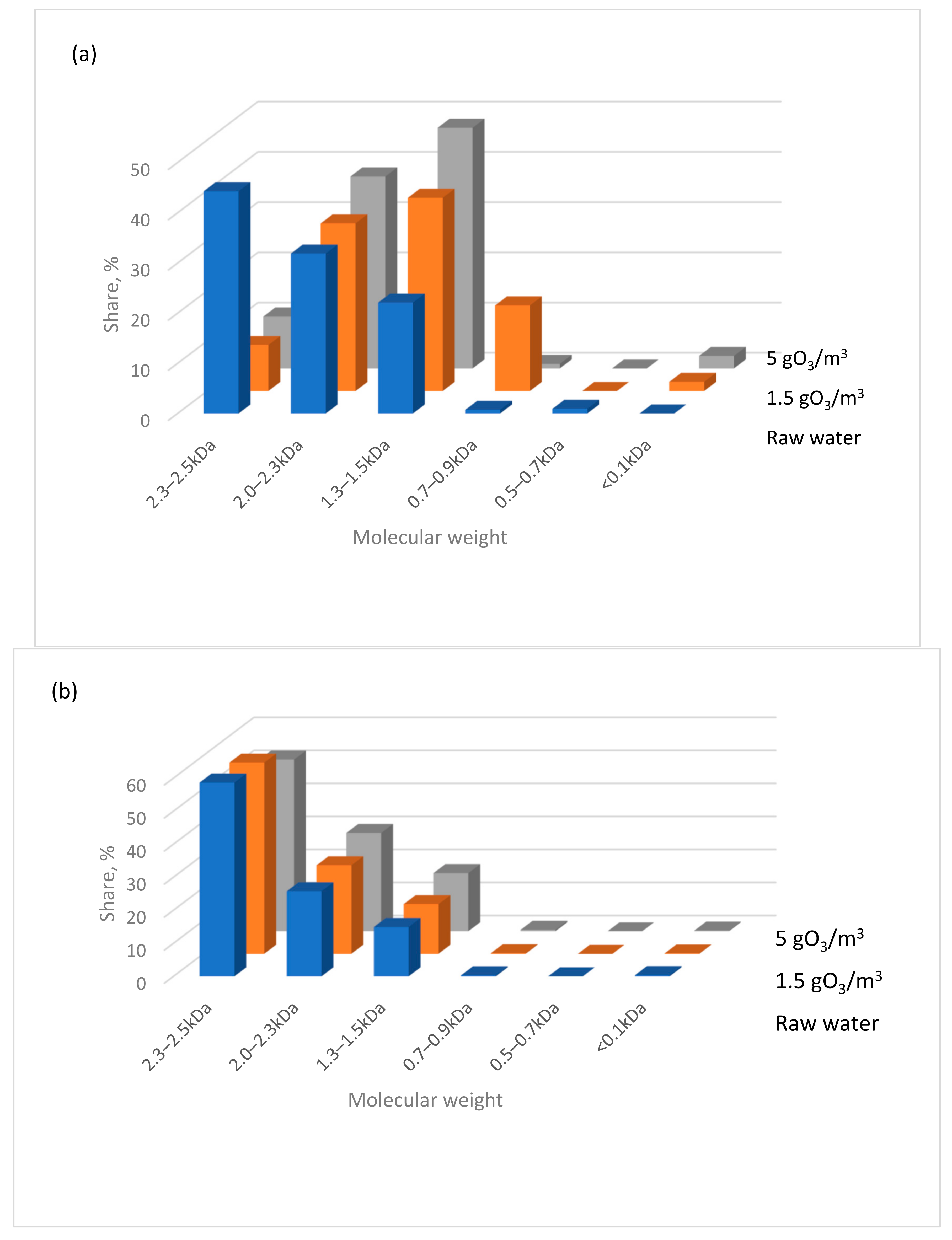
- In the low-pressure UV lamp + ozone system, high-molecular-weight organic substances are transformed into smaller molecules with molecular weights below 0.7 kDa. In contrast, using the same ozone dose but with a medium-pressure UV lamp predominantly produces particles with molecular weights >1.3 kDa.
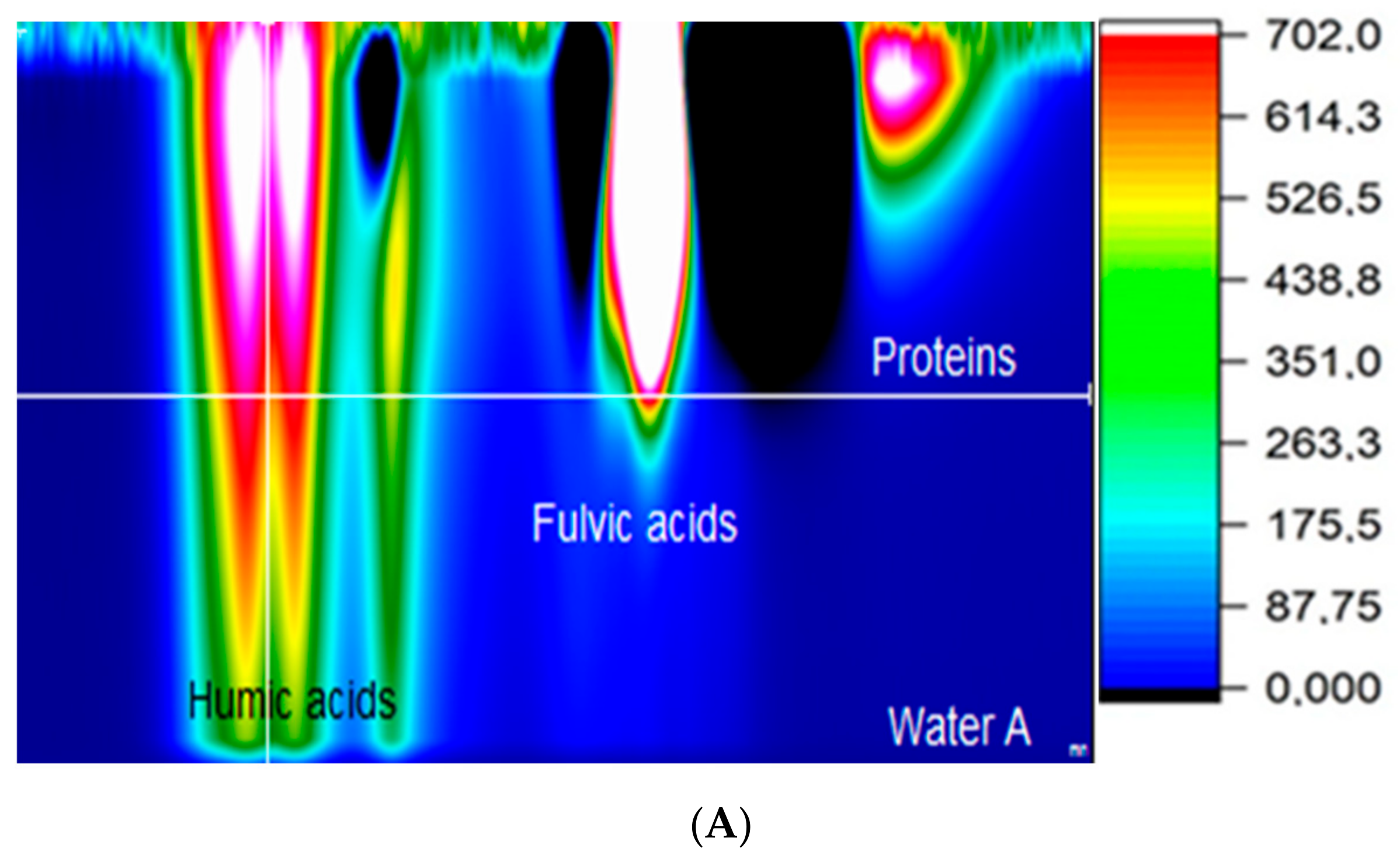
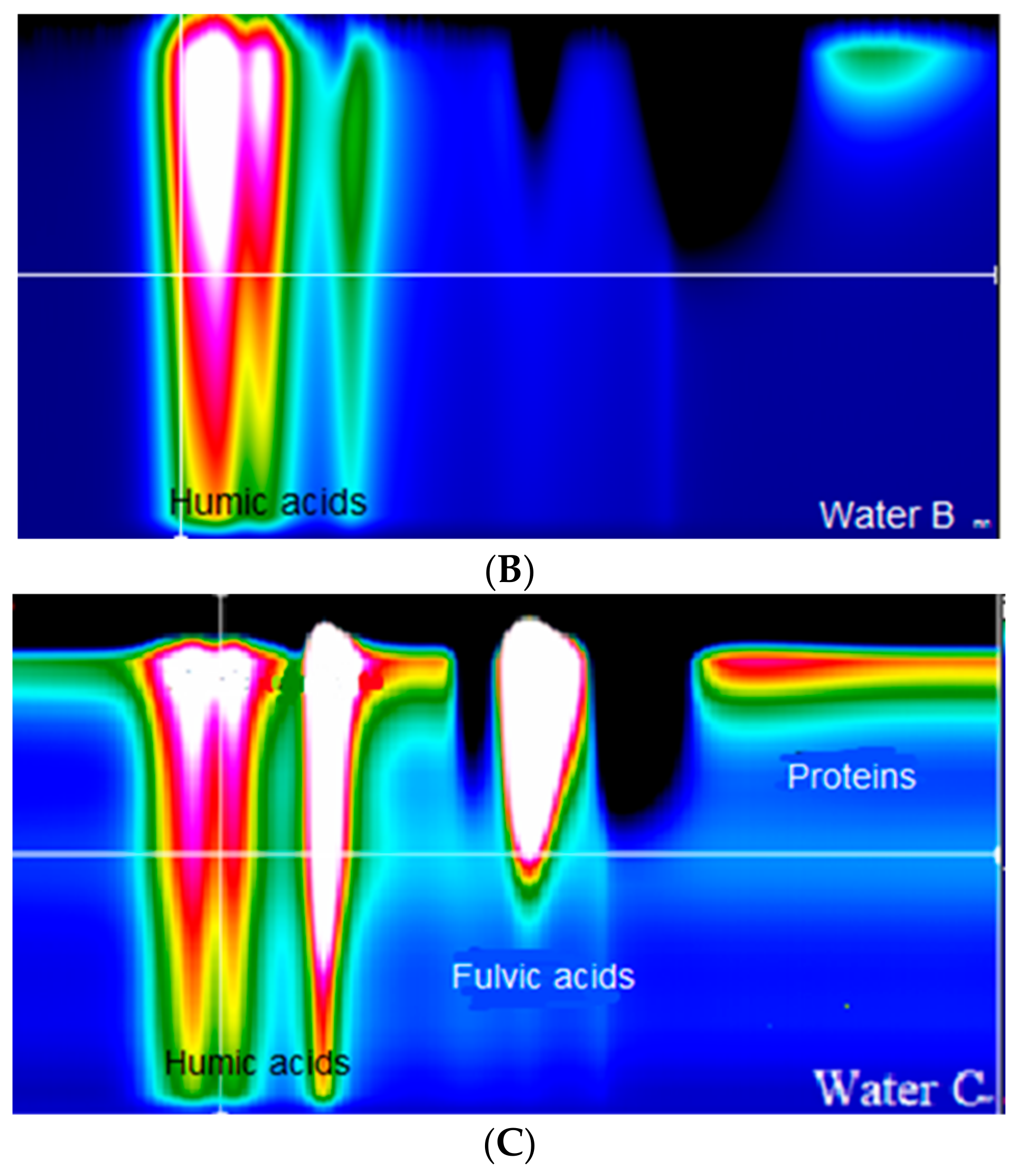
- The process of advanced oxidation (UV + O3) is influenced not only by the content of organic substances in the raw water but also by the type of these substances. Humic acids are the most susceptible to transformations, while fulvic acids are less affected.
- The low-pressure UV lamp ensures greater process efficiency, regardless of the ozone dose applied.
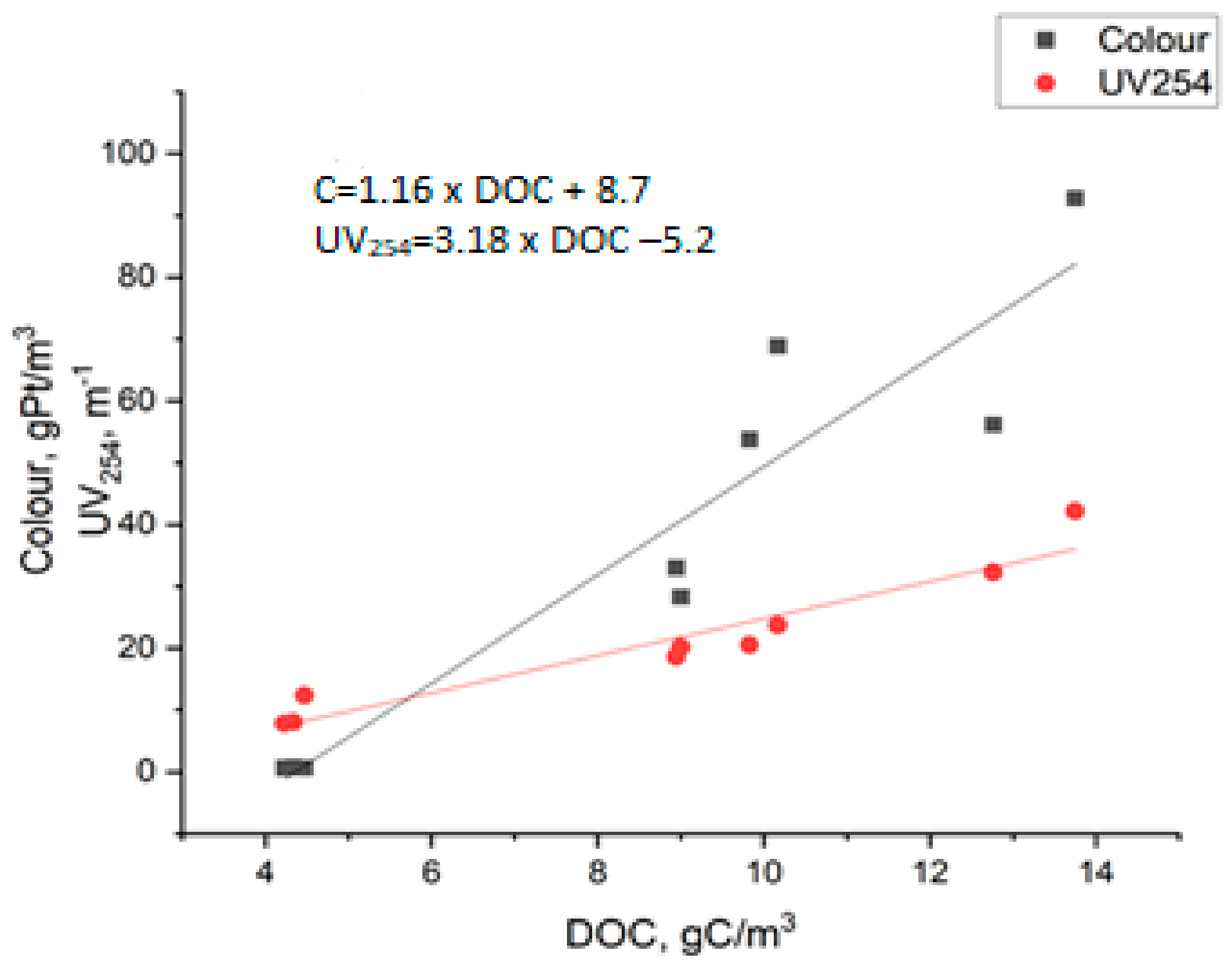
- Regardless of the UV lamp type, the amount of organic substances removed was directly proportional to the reduction in UV 254 nm absorbance, while no such correlation was found for UV absorbance at 272 nm.
- The use of the O3 + UV system for pre-oxidation helps reduce the potential for the formation of chlorinated organic compounds and does not significantly affect their removability in other treatment processes.
- The use of low-pressure lamps is economically justified due to their lower energy consumption.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.; Noh, J.H.; Park, J.W.; Yoon, S.W.; Kim, S.Y.; Son, H.J.; Maeng, S.K. Integrating biological ion exchange with biological activated carbon treatment for drinking water: A novel approach for NOM removal, trihalomethane formation potential, and biological stability. Water Res. 2023, 245, 120598. [Google Scholar] [CrossRef]
- Gonzalez-Perez, A.; Hägg, K.; Duteil, F. Optimizing NOM removal: Impact of calcium chloride. Sustainability 2021, 13, 6338. [Google Scholar] [CrossRef]
- Mustereț, C.P.; Morosanu, I.; Ciobanu, R.; Plavan, O.; Gherghel, A.; Al-Refai, M.; Roman, I.; Teodosiu, C. Assessment of coagulation–flocculation process efficiency for the natural organic matter removal in drinking water treatment. Water 2021, 13, 3073. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Jiang, J.; Yang, T.; Cao, Y. Oxidative treatment of NOM by selective oxidants in drinking water treatment and its impact on DBP formation in postchlorination. Sci. Total Environ. 2023, 858, 159908. [Google Scholar] [CrossRef] [PubMed]
- Jazić, J.M.; Đurkić, T.; Bašić, B.; Watson, M.; Apostolović, T.; Tubić, A.; Agbaba, J. Degradation of a chloroacetanilide herbicide in natural waters using UV activated hydrogen peroxide, persulfate and peroxymonosulfate processes. Environ. Sci. Water Res. Technol. 2020, 6, 2800–2815. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Zhang, J.; Wu, S.; Yang, L.; Chen, L.; Shao, Y. The challenge of micropollutants in surface water of the Yangtze River. Sci. Total Environ. 2021, 780, 146537. [Google Scholar] [CrossRef]
- Kim, M.K.; Zoh, K.D. Occurrence and removals of micropollutants in water environment. Environ. Eng. Res. 2016, 21, 319–332. [Google Scholar] [CrossRef]
- Delpla, I.; Jung, A.V.; Baures, E.; Clement, M.; Thomas, O. Impacts of climate change on surface water quality in relation to drinking water production. Environ. Int. 2009, 35, 1225–1233. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.; Roccaro, P. Removal of poly-and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Deng, Y.; Gao, P.; Wang, L.; Zhang, Y.; Fu, J.; Huang, R.; Zhou, S. Activation of peroxymonosulfate by MnO2 with oxygen vacancies: Degradation of organic compounds by electron transfer nonradical mechanism. J. Environ. Chem. Eng. 2022, 10, 107481. [Google Scholar] [CrossRef]
- Özdemir, K. Investigation of trihalomethane formation after chlorine dioxide preoxidation followed by chlorination of natural organic matter. Environ. Prot. Eng. 2021, 2, 125–137. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Patel, N.; Chaudhary, V.K. Disinfection by-products in drinking water: Occurrence, toxicity and abatement. Environ. Pollut. 2020, 267, 115474. [Google Scholar] [CrossRef] [PubMed]
- Khettaf, S.; Boumaraf, R.; Benmahdi, F.; Bouhidel, K.E.; Bouhelassa, M. Removal of the neutral dissolved organic matter (NDOM) from surface water by coagulation/flocculation and nanofiltration. Anal. Lett. 2021, 54, 2713–2726. [Google Scholar] [CrossRef]
- Bodzek, M. Membrane separation techniques—Removal of inorganic and organic admixtures and impurities from water environment—Review. Arch. Environ. Prot. 2019, 4, 4–19. [Google Scholar] [CrossRef]
- Peters, C.D.; Rantissi, T.; Gitis, V.; Hankins, N.P. Retention of natural organic matter by ultrafiltration and the mitigation of membrane fouling through pre-treatment, membrane enhancement, and cleaning—A review. J. Water Process Eng. 2021, 44, 102374. [Google Scholar] [CrossRef]
- Remucal, C.K.; Salhi, E.; Walpen, N.; von Gunten, U. Molecular-level transformation of dissolved organic matter during oxidation by ozone and hydroxyl radical. Environ. Sci. Technol. 2020, 54, 10351–10360. [Google Scholar] [CrossRef]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef]
- Derco, J.; Gotvajn, A.Ž.; Čižmárová, O.; Dudáš, J.; Sumegová, L.; Šimovičová, K. Removal of micropollutants by ozone-based processes. Processes 2021, 9, 1013. [Google Scholar] [CrossRef]
- Mirnasab, M.A.; Hashemi, H.; Samaei, M.R.; Azhdarpoor, A. Advanced removal of water NOM by Pre-ozonation, Enhanced coagulation and Bio-augmented Granular Activated Carbon. Int. J. Environ. Sci. Technol. 2021, 18, 3143–3152. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoon, H.W.; Lee, M.A.; Kim, Y.H.; Lee, C.J. Impact of UV-C irradiation on bacterial disinfection in a drinking water purification system. J. Microbiol. Biotechnol. 2022, 33, 106. [Google Scholar] [CrossRef]
- Tran, H.D.M.; Boivin, S.; Kodamatani, H.; Ikehata, K.; Fujioka, T. Potential of UV-B and UV-C irradiation in disinfecting microorganisms and removing N-nitrosodimethylamine and 1, 4-dioxane for potable water reuse: A review. Chemosphere 2022, 286, 131682. [Google Scholar] [CrossRef]
- Kebbi, Y.; Muhammad, A.I.; Sant’Ana, A.S.; do Prado-Silva, L.; Liu, D.; Ding, T. Recent advances on the application of UV-LED technology for microbial inactivation: Progress and mechanism. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3501–3527. [Google Scholar] [CrossRef] [PubMed]
- Rougé, V.; von Gunten, U.; Allard, S. Efficiency of pre-oxidation of natural organic matter for the mitigation of disinfection byproducts: Electron donating capacity and UV absorbance as surrogate parameters. Water Res. 2020, 187, 116418. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Xu, B.J.; Wu, H.; Su, H.Z.; Lee, M.Y.; Zhang, Y.X.; Chen, Y.; Liu, M.; Wang, W.L.; Du, Y. A novel ultraviolet light source of microwave discharge electrodeless ultraviolet lamp: Luminescence mechanisms, reactor structures, and environmental applications. J. Clean. Prod. 2020, 436, 140706. [Google Scholar] [CrossRef]
- Granzoto, M.R.; Seabra, I.; Malvestiti, J.A.; Cristale, J.; Dantas, R.F. Integration of ozone, UV/H2O2 and GAC in a multi-barrier treatment for secondary effluent polishing: Reuse parameters and micropollutants removal. Sci. Total Environ. 2021, 759, 143498. [Google Scholar] [CrossRef]
- Sharpless, C.M.; Linden, K.G. Experimental and model comparisons of low-and medium-pressure Hg lamps for the direct and H2O2 assisted UV photodegradation of N-nitrosodimethylamine in simulated drinking water. Environ. Sci. Technol. 2003, 37, 1933–1940. [Google Scholar] [CrossRef]
- Gorito, A.M.; Pesqueira, J.F.; Moreira, N.F.; Ribeiro, A.R.; Pereira, M.F.; Nunes, O.C.; Almeida, C.M.; Silva, A.M. Ozone-based water treatment (O3, O3/UV, O3/H2O2) for removal of organic micropollutants, bacteria inactivation and regrowth prevention. J. Environ. Chem. Eng. 2021, 9, 105315. [Google Scholar] [CrossRef]
- Lu, H.; Li, Q.; Feng, W. Application progress of O3/UV advanced oxidation technology in the treatment of organic pollutants in water. Sustainability 2022, 14, 1556. [Google Scholar] [CrossRef]
- Paul, A.; Dziallas, C.; Zwirnmann, E.; Gjessing, E.T.; Grossart, H.-P. UV irradiation of natural organic matter (NOM): Impact on organic carbon and bacteria. Aquat Sci. 2012, 74, 443–454. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Yang, S.; Song, Y.; Chang, F.; Wang, K. Evaluation of chemistry and key reactor parameters for industrial water treatment applications of the UV/O3 process. Environ. Res. 2020, 188, 109660. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Assessment of sulfate radical-based advanced oxidation processes for water and wastewater treatment: A Review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Zhu, G.; Bian, Y.; Hursthouse, A.S.; Wan, P.; Szymanska, K.; Ma, J.; Wang, X.; Zhao, Z. Application of 3-D Fluorescence: Characterization of Natural Organic Matter in Natural Water and Water Purification Systems. J. Fluoresc. 2017, 27, 2069–2094. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Henzie, J.; Jiang, B.; Xia, W.; Chen, T.; Bando, Y.; Kang, Y.M.; Hossain, M.S.; Sugahara, Y.; et al. Mesoporous iron-doped MoS2/CoMo2S4 heterostructures through organic-metal cooperative interactions on spherical micelles for electrochemical water splitting. ACS Nano 2020, 14, 4141–4152. [Google Scholar] [CrossRef]
- Du, J.; Wang, C.; Zhao, Z.; Liu, J.; Deng, X.; Cui, F. Mineralization, characteristics variation, and removal mechanism of algal extracellular organic matter during vacuum ultraviolet/ozone process. Sci. Total Environ. 2022, 820, 153298. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Xiang, Y.; Jiang, J.; Yin, R. Role of UV-based advanced oxidation processes on NOM alteration and DBP formation in drinking water treatment: A state-of-the-art review. Chemosphere 2023, 311, 136870. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A.; Puma, G.L. Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics. Sep. Purif. Technol. 2010, 72, 235–241. [Google Scholar] [CrossRef]
- Sharma, V.K.; Graham, N.J. Oxidation of amino acids, peptides and proteins by ozone: A review. Ozone Sci. Eng. 2020, 32, 81–90. [Google Scholar] [CrossRef]
- Wang, H.W.; Li, X.Y.; Hao, Z.P.; Sun, Y.J.; Wang, Y.N.; Li, W.H.; Tsang, Y.F. Transformation of dissolved organic matter in concentrated leachate from nanofiltration during ozone-based oxidation processes (O3, O3/H2O2 and O3/UV). J. Environ. Manag. 2017, 191, 244–251. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Tobiason, J.E. Enhanced Coagulation: US Requirements and a Broader View. Water Sci. Technol. 1999, 40, 63–70. [Google Scholar] [CrossRef]
- Zhang, P.; Jian, L. Ozone-enhanced photocatalytic degradation of natural organic matter in water. Water Sci. Technol. Water Supply 2006, 6, 53–61. [Google Scholar] [CrossRef]
- Mołczan, M.; Szlachta, M.; Karpińska, A.; Biłyk, A. Zastosowanie absorbancji włąściwej w nadfiolecie (SUVA) w ocenie jakości wody. Ochr. Sr. 2006, 28, 11–16. [Google Scholar]
- Li, G.Q.; Wang, W.L.; Huo, Z.Y.; Lu, Y.; Hu, H.Y. Comparison of UV-LED and low pressure UV for water disinfection: Photoreactivation and dark repair of Escherichia coli. Water Res. 2017, 126, 134–143. [Google Scholar] [CrossRef]
- Beck, S.E.; Wright, H.B.; Hargy, T.M.; Larason, T.C.; Linden, K.G. Action spectra for validation of pathogen disinfection in medium-pressure ultraviolet (UV) systems. Water Res. 2015, 70, 27–37. [Google Scholar] [CrossRef]
- Brezinski, K.; Gorczyca, B. An overview of the uses of high performance size exclusion chromatography (HPSEC) in the characterization of natural organic matter (NOM) in potable water, and ion-exchange applications. Chemosphere 2019, 217, 122–139. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, R.; Li, H.; Li, J.; Zhao, D.; Chang, N.; Shi, J. Synergistic effect of ferrous ion-activated hydrogen peroxide and persulfate on the degradation of phthalates in aquatic environments by non-target analysis. Chem. Eng. J. 2023, 451, 139100. [Google Scholar] [CrossRef]
- Abbas, M. Removal of brilliant green (BG) by activated carbon derived from medlar nucleus (ACMN)–Kinetic, isotherms and thermodynamic aspects of adsorption. Adsorpt. Sci. Technol. 2020, 38, 464–482. [Google Scholar] [CrossRef]
- Remy, L. Modifications Chimiques de Polysaccharides en Milieu Aqueux pour Générer de Nouveaux Matériaux Biosourcés Fonctionnels. Ph.D. Thesis, INSA de Lyon, Villeurbanne, France, 2024. [Google Scholar]
- Yan, M.; Shen, X.; Gao, B.; Guo, K.; Yue, Q. Coagulation-ultrafiltration integrated process for membrane fouling control: Influence of Al species and SUVA values of water. Sci. Total Environ. 2021, 793, 148517. [Google Scholar] [CrossRef]

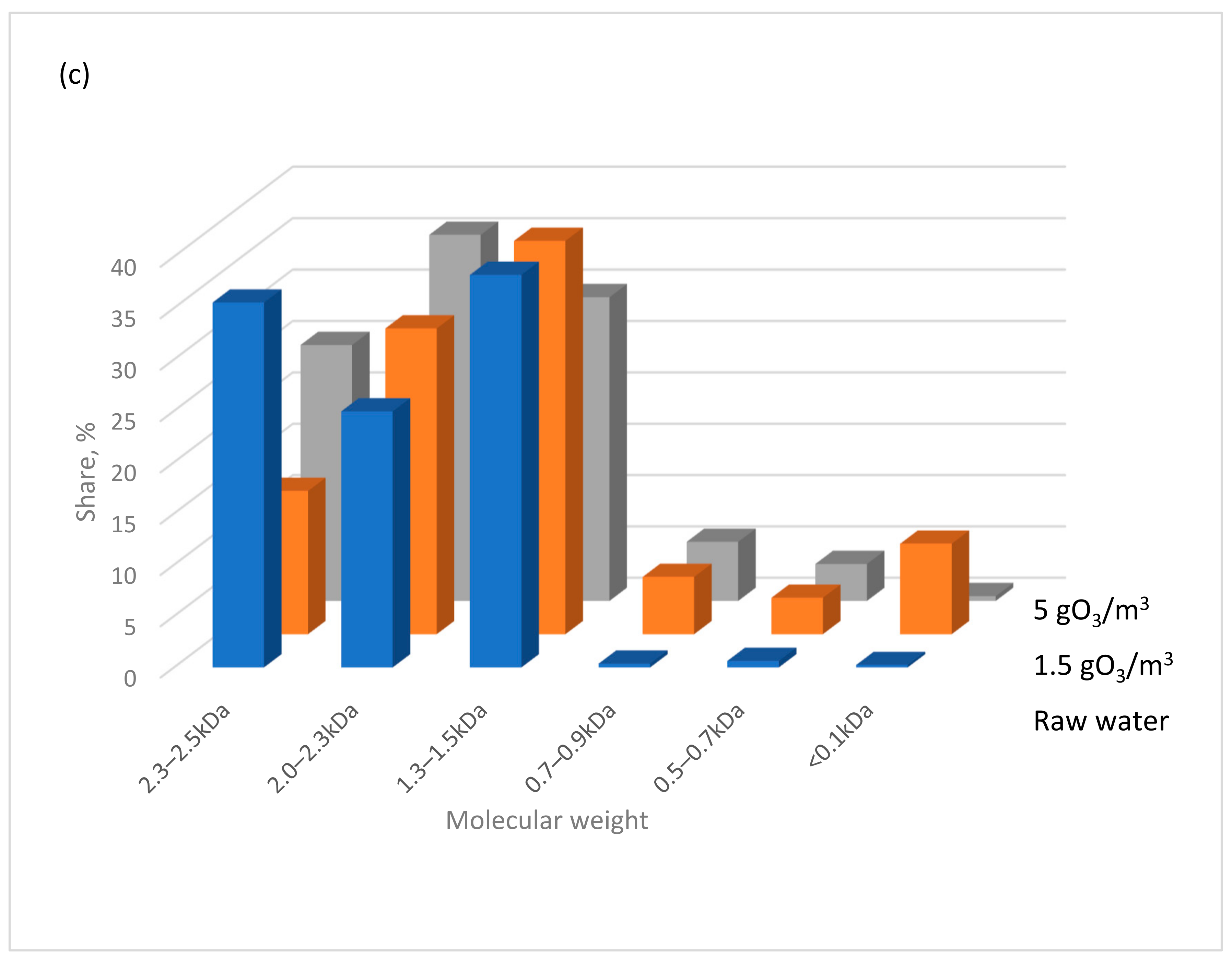

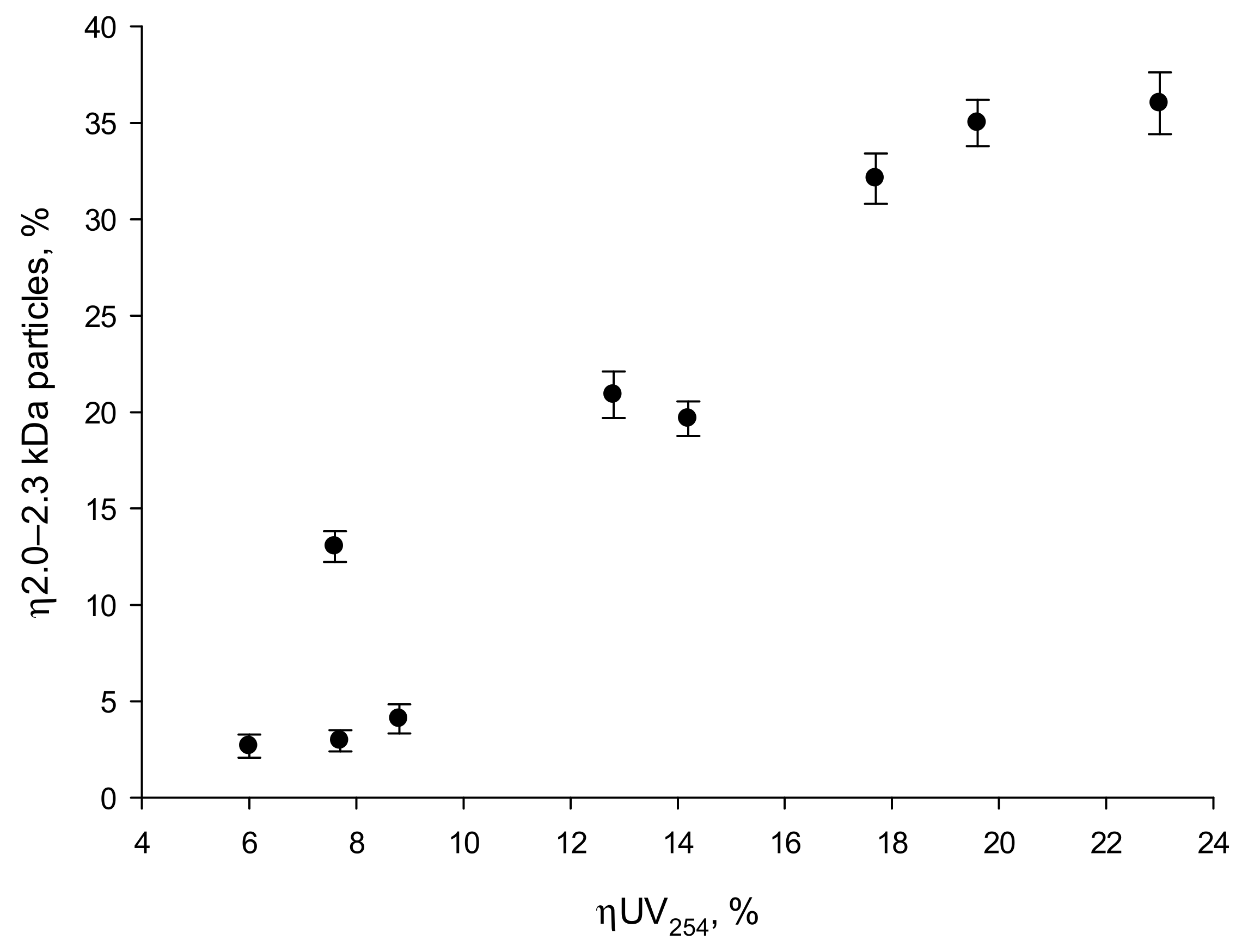
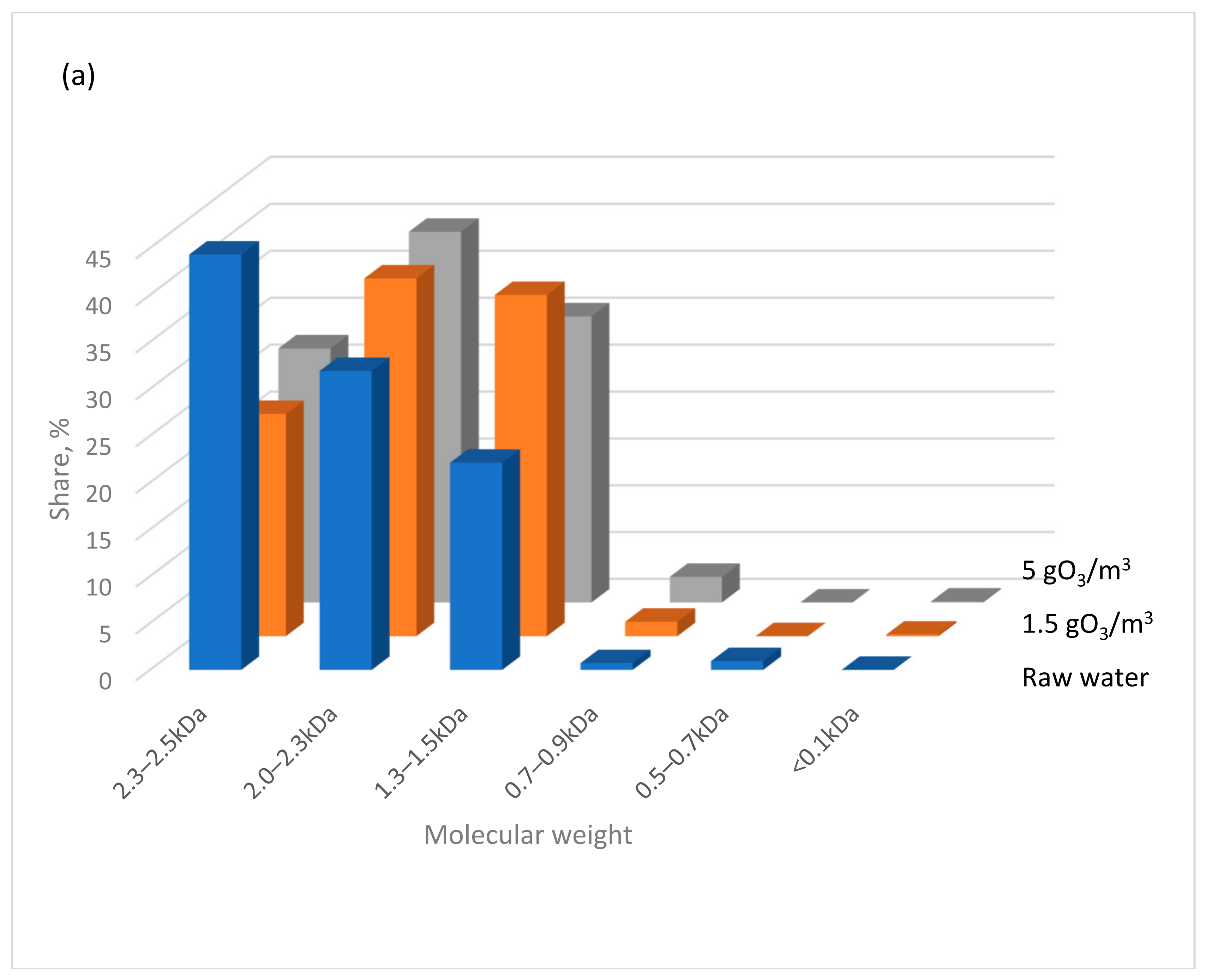

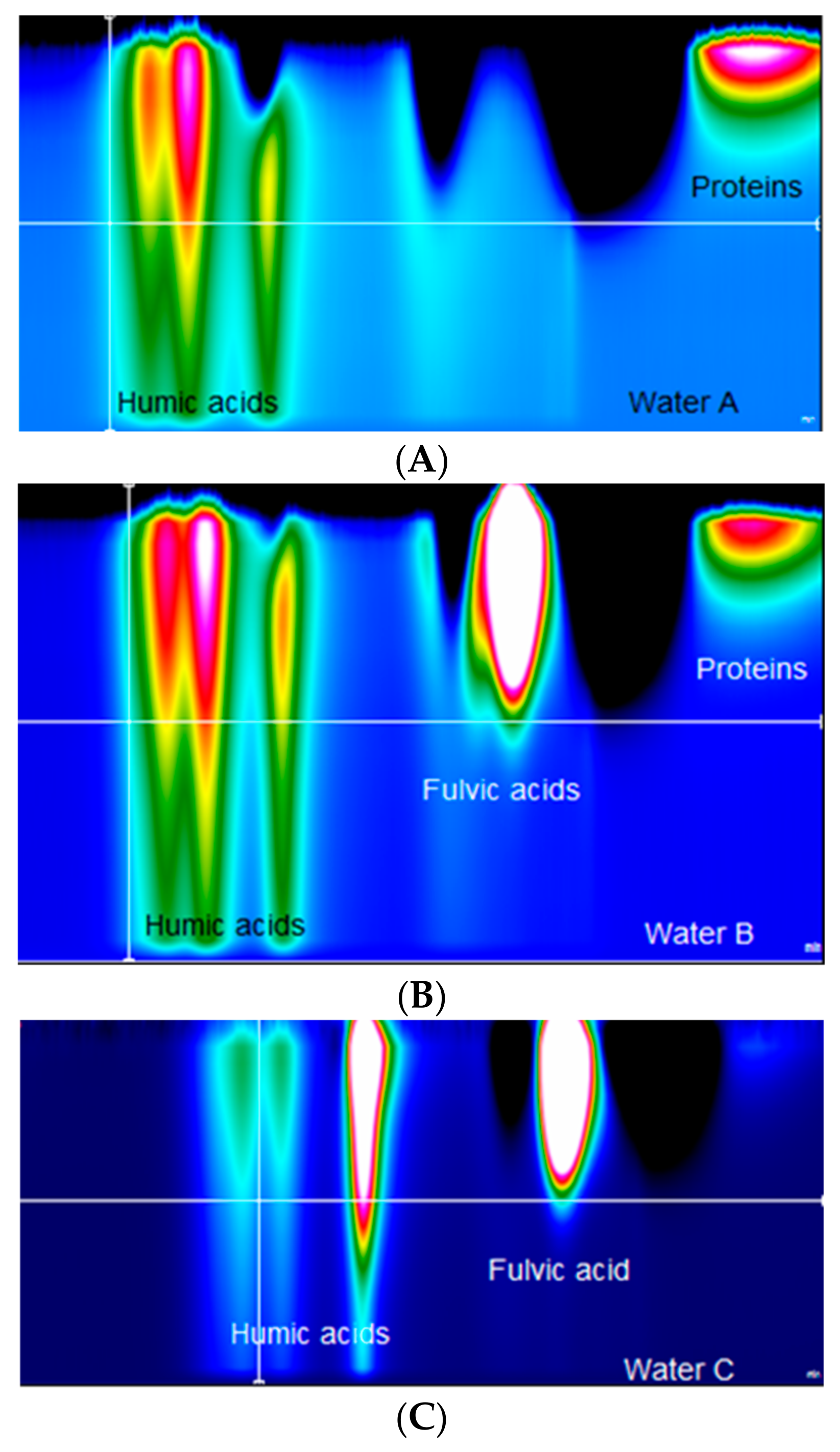
| Parameter | Unit | Water A | Water B | Water C |
|---|---|---|---|---|
| pH | 7.69 | 8.6 | 7.29 | |
| Turbidity | NTU | 3.88 | 6.47 | 5.48 |
| Colour | gPt/m3 | 46.23 | 133.2 | 71.7 |
| TOC | gC/m3 | 5.88 | 19.62 | 11.249 |
| DOC | gC/m3 | 5.257 | 19.12 | 10.807 |
| 2.3–2.5 kDa | g/m3 | 24.145 | 118.541 | 38.935 |
| 2.0–2.3 kDa | g/m3 | 17.390 | 52.033 | 27.346 |
| 1.3–1.5 kDa | g/m3 | 12.057 | 30.174 | 41.885 |
| 0.7–0.9 kDa | g/m3 | 0.397 | 0.590 | 0.409 |
| 0.5–0.7 kDa | g/m3 | 0,.512 | 0.000 | 0.702 |
| <0.1 kDa | g/m3 | 0.000 | 0.710 | 0.266 |
| UV254 | m−1 | 15.1 | 45.1 | 25.3 |
| UV272 | m−1 | 12.6 | 36.5 | 20.1 |
| UV350 | m−1 | 3.9 | 9.4 | 4.8 |
| SUVA | m3/g⋅m | 2.87 | 2.36 | 2.34 |
| NH4+ | gNH4+/m3 | 0.87 | 0.62 | 0.39 |
| Parameter | Water A | Water B | Water C | |||
|---|---|---|---|---|---|---|
| Ozone Dose | Ozone Dose | Ozone Dose | ||||
| 1.5 gO3/m3 | 5.0 gO3/m3 | 1.5 gO3/m3 | 5.0 gO3/m3 | 1.5 gO3/m3 | 5.0 gO3/m3 | |
| Turbidity | 74.7 | 85.6 | 33.8 | 57.8 | 56.6 | 65.9 |
| Colour | 97.6 | 98.5 | 38.4 | 86.5 | −38.2 | 88.2 |
| TOC | 16.8 | 20.6 | 33.1 | 44.9 | 10.7 | 19.8 |
| DOC | 18.0 | 20.5 | 33.6 | 44.0 | 9.9 | 21.3 |
| 2.3–2.5 kDa | 95.3 | 98.5 | 34.3 | 78.6 | 64.2 | 92.6 |
| 2.0–2.3 kDa | 76.4 | 84.7 | 30.8 | 59.8 | 27.2 | 52.4 |
| 1.3–1.5 kDa | 60.9 | 72.3 | 33.0 | 58.0 | 39.6 | 71.3 |
| 0.7–0.9 kDa | −427.2 | 83.9 | 16.7 | 61.3 | −801.7 | −664.3 |
| 0.5–0.7 kDa | 100.0 | 100.0 | - | - | −234.2 | −187.4 |
| <0.1 kDa | - | - | 69.0 | 80.3 | −2091.4 | 2.3 |
| UV254 | 41.7 | 60.3 | 49.0 | 71.6 | 22.5 | 70.0 |
| UV272 | 45.2 | 61.1 | 46.6 | 71.0 | 7.0 | 71.6 |
| UV350 | 20.5 | 46.2 | 35.1 | 66.0 | 4.2 | 79.2 |
| NH4+ | −13.8 | 58.6 | −46.8 | −40.3 | −476.9 | −400.0 |
| Parameter | Water A | Water B | Water C | |||
|---|---|---|---|---|---|---|
| Ozone Dose | Ozone Dose | Ozone Dose | ||||
| 1.5 gO3/m3 | 5.0 gO3/m3 | 1.5 gO3/m3 | 5.0 gO3/m3 | 1.5 gO3/m3 | 5.0 gO3/m3 | |
| Turbidity | 82.4 | 88.3 | 38.1 | 47.6 | 44.8 | 52.6 |
| Colour | 99.0 | 99.2 | 30.0 | 77.9 | 4.3 | 51.4 |
| TOC | 13.7 | 18.4 | 27.9 | 38.4 | 6.4 | 19.4 |
| DOC | 14.1 | 20.3 | 28.5 | 41.0 | 6.2 | 16.8 |
| 2.3–2.5 kDa | 77.6 | 98.4 | 70.6 | 94.1 | 6.9 | 39.9 |
| 2.0–2.3 kDa | 58.4 | 94.2 | 47.5 | 79.2 | −14.2 | 14.2 |
| 1.3–1.5 kDa | 48.2 | 91.2 | 36.2 | 68.5 | −2.3 | 26.4 |
| 0.7–0.9 kDa | −44.2 | 85.3 | −57.2 | −23.8 | −1050.0 | −248.0 |
| 0.5–0.7 kDa | 100.0 | 100.0 | - | - | −806.0 | −6.8 |
| <0.1 kDa | - | - | 61.5 | 78.7 | −4.8 | 34.8 |
| UV254 | 18.4 | 47.8 | 6.7 | 53.4 | 5.8 | 28.4 |
| UV272 | 20.9 | 53.2 | 7.7 | 57.3 | 9.3 | 29.9 |
| UV350 | 29.4 | 52.4 | 3.4 | 63.4 | 24.9 | 68.7 |
| NH4+ | 64.8 | 80.4 | −39.4 | −3.7 | −652.0 | −210.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolska, M.; Kabsch-Korbutowicz, M.; Solipiwko-Pieścik, A.; Sperczyńska, E. Evaluation of the Effect of Using the UV + O3 Process with Low- and Medium-Pressure Lamps on the Amount and Properties of Organic Substances in Treated Water. Water 2025, 17, 701. https://doi.org/10.3390/w17050701
Wolska M, Kabsch-Korbutowicz M, Solipiwko-Pieścik A, Sperczyńska E. Evaluation of the Effect of Using the UV + O3 Process with Low- and Medium-Pressure Lamps on the Amount and Properties of Organic Substances in Treated Water. Water. 2025; 17(5):701. https://doi.org/10.3390/w17050701
Chicago/Turabian StyleWolska, Małgorzata, Małgorzata Kabsch-Korbutowicz, Anna Solipiwko-Pieścik, and Elżbieta Sperczyńska. 2025. "Evaluation of the Effect of Using the UV + O3 Process with Low- and Medium-Pressure Lamps on the Amount and Properties of Organic Substances in Treated Water" Water 17, no. 5: 701. https://doi.org/10.3390/w17050701
APA StyleWolska, M., Kabsch-Korbutowicz, M., Solipiwko-Pieścik, A., & Sperczyńska, E. (2025). Evaluation of the Effect of Using the UV + O3 Process with Low- and Medium-Pressure Lamps on the Amount and Properties of Organic Substances in Treated Water. Water, 17(5), 701. https://doi.org/10.3390/w17050701






