1. Introduction
Contaminated sediment is a widespread problem with the potential to threaten the health and integrity of aquatic environments [
1]. In the hydrological cycle, around 1% of pollutants are dissolved in water, whereas more than 99% are stored in sediments, which are the major sinks and carriers of contaminants in aquatic environments [
2]. Therefore, knowledge of the sources and types of pollutants in sediments is essential [
1].
The EPA has indicated that the decontamination of sediments will receive the highest priority. Characterization of the metal-contaminated sediments can assist environmental decision-makers in the management of the aquatic ecosystem [
3]. Metals are among the most challenging pollution issues in the ecosystem because of their resistance to decomposition and subsequent bioaccumulation [
4,
5,
6]. Metals are adsorbed and accumulate in sediments due to various mechanisms. As sediments are mobile, the contaminants can travel substantial distances from the pollutant sources [
4,
6].
Heavy metals usually possess significant toxicity to aquatic organisms and human health through bioaccumulation in the food chain. Hence, investigating the transformation and distribution mechanisms of heavy metals in sediment becomes necessary [
7]. Large-scale studies on trace element contaminants in marine [
8,
9], coastal [
10,
11], and freshwater [
12,
13] sediments have been reported from various countries, indicating the large extent of the issue.
Sources of metals in sediments vary and include natural (weathering of soil and rock, erosion, forest fires, and volcanic eruptions) and anthropogenic sources (e.g., industrial effluents, urban wastes, mining and refining, agricultural drainage, domestic discharges, and atmospheric deposition), point and nonpoint sources, and spills [
14,
15]. The main source of heavy metals in the sediments may largely be the result of human activities, with the highest concentrations often measured in rivers, lakes, and reservoirs located in the cities and near industrial parks and towns. Sediments are the ultimate receptor of heavy metals, and the heavy metals sorbed from the water bodies of rivers, lakes, and bays eventually accumulate in the sediments [
16].
The overall objective of heavy metal remediation is to minimize the risk of these toxic compounds on human and ecological health [
17]. Although there is more information on technologies for the remediation of metal-contaminated soil, much less is known about sediment treatment. The properties of sediments including higher clay and organic matter contents can differ significantly from soils, and therefore, technologies that work for soils might not be as efficient for sediments [
18]. The selection of an appropriate remediation technique depends on the characteristics of the site, the level of the metal contamination, and regulatory limits for the heavy metal(s) of concern in that regulatory domain. The remediation methods can be broadly divided into two major strategies: (1) in situ and (2) ex situ.
In-situ strategies focus on improving metal stabilization, which mainly occurs by enhancing metal sorption, precipitation, and complexation capacity of the sediment. Therefore, the potential mobility or bioavailability of the toxic metals to the environment will decrease [
7]. In-situ techniques (such as capping, phytoremediation, resuspension) are logistically favorable, as they are of relatively low cost, less disruptive to the environment, and reduce the need for dredging, handling, or transportation of hazardous substances that generate the need for additional waste disposal [
7,
19,
20].
In ex-situ remediation technologies, the contaminated material is removed from the site for subsequent treatment. This can either take place in an above-ground treatment facility (on-site) or by treatment or disposal elsewhere (off-site) [
21]. In this strategy, polluted sediment is dredged from the bottom, and contaminants are extracted from the sediment through a series of chemical, physical, and biological methods in a specially designed reactor. Ex-situ sediment remediation is often the first choice for the heavily polluted sediments [
7]. After dredging, various remediation approaches (such as electrochemical remediation, washing, and flotation) are available or under development [
7].
Ex-situ remediation is the main viable option for some pollutants, and in-situ techniques are mainly used to reduce the mobility of the contaminants. On the other hand, dredging the contaminated sediment can increase the risk of mobility and availability of heavy metals in the harbors and impact the disposal sites that receive the dredged sediment [
22].
The most popular in-situ approach, capping with or without reactive modifications, is not appropriate for the studied site because the primary issue is shallowness. By capping, the water depth is reduced, and the pollution remains at the location. Reducing water-sediment interactions and immobilizing the contaminants is the only benefit. Furthermore, in certain harbors with fine-grained silt, sand capping is ineffective because the sand layer may be loosened, and the contamination may seep through [
23,
24]. Thus, it would be ideal to create new methods for handling polluted sediment that are more adaptable and cause the least amount of environmental damage. It is important to note that both organic and inorganic contaminants are typically present in harbor areas. As a result, the remediation method should be suitable for both organic and inorganic pollutants at the same time [
22].
The resuspension technique is based on the concept of the sediment particle–specific surface area and adsorption theory. Finer sediments have a larger specific surface area (i.e., clay and silt), so they have a greater tendency to adsorb the contaminants [
22,
23,
24]. One of the benefits of this method is that the aeration in the water column not only suspends the sediments but also creates an aerobic condition in the lower layers of sediment. Additionally, its primary function is to stop the creation of hydrogen sulfide and eutrophication [
23]. The resuspension’s ability to be used simultaneously for the cleanup of both organic and inorganic pollution is another benefit. Furthermore, this method does not require any chemical reagents. Compared to the large volumes of contaminated sediment recovered by dredging, much lower amounts are removed by this method, reducing transportation and disposal issues [
22].
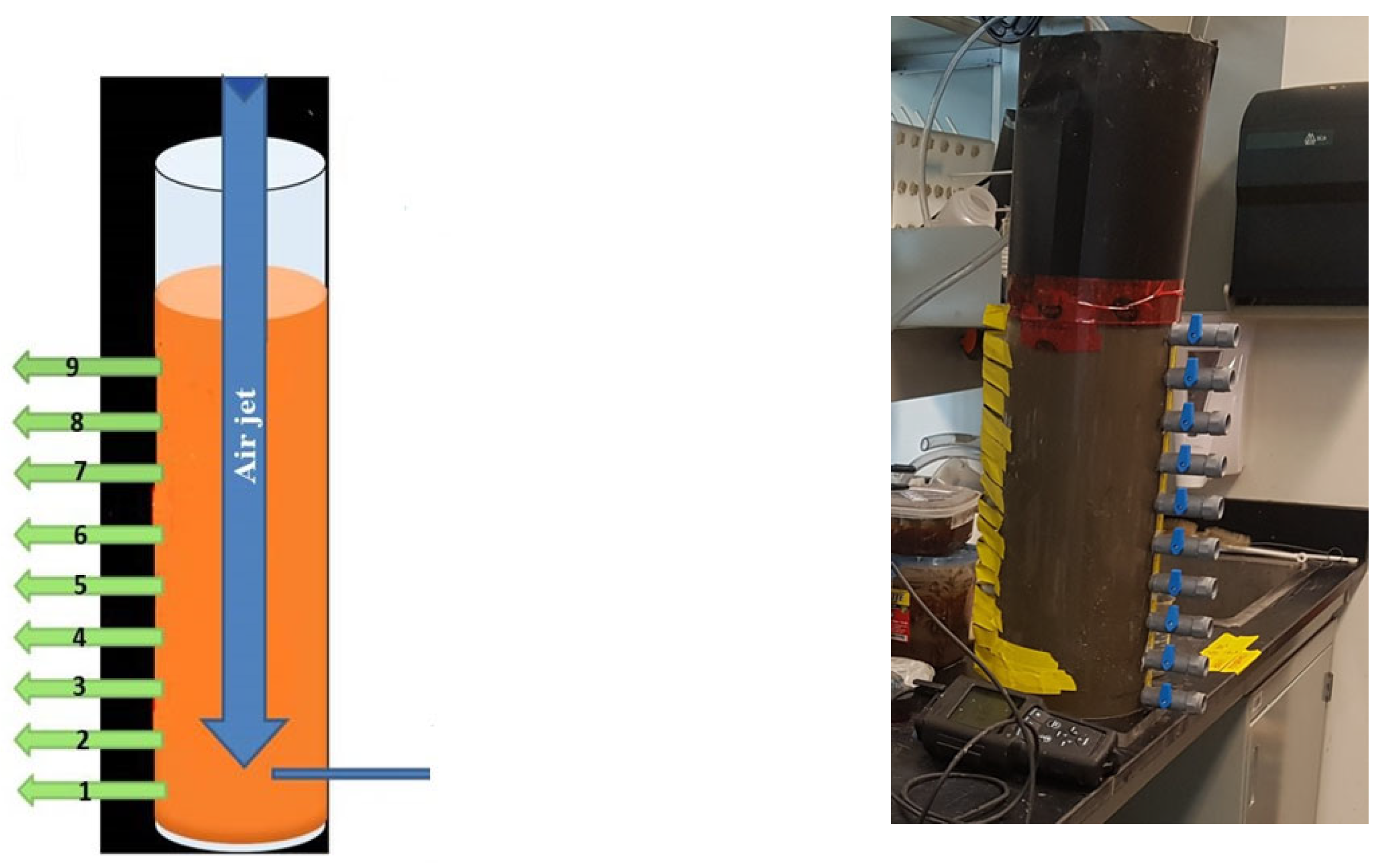
Resuspension was originally used as a remediation method for contaminated sediments used in Fukuyama City Port by Fukue et al. [
23] to enhance the water-sediment quality and slow down the rate of eutrophication. They created a pilot project to selectively remove the sediments in the Fukuyama Canal Port. Instead of using the dredging procedure to remove all of the sediment at the canal’s bottom, they only removed 3% of it. According to their findings, the resuspension successfully decreased the chemical oxygen demand (COD), total phosphorus (T-P), and total nitrogen (T-N) by 31%, 14%, and 27.6%, respectively. However, they suggested that T-N, T-P, and COD may be reduced by roughly 10% by eliminating resuspended sediment. Pourabadehei and Mulligan demonstrated the feasibility of reducing the level of contamination of sediment through removal of finer sediment (clay and silt) that has more tendency to adsorb the contaminants [
22].
The objectives of this study are to (1) determine the level of metal contamination in the sediments of the harbor, (2) assess the extent of contamination of the heavy metals using sediment quality guidelines and enrichment factors, (3) determine the influence of the resuspension technique on metal remediation and the relationship between metals and particle size of the sediment, (4) evaluate the effectiveness of this method for reduction of metal mobility from the contaminated sediment, and (5) assess risk reduction of sediment samples by the resuspension technique.
4. Conclusions
As sediment pollution by metals is a global issue, efficient remediation methods are needed. To tackle this issue, environmental managers need effective, scientific methods to assess the possible effects of sediment-bound chemicals on different resource applications (e.g., aquatic organisms or wildlife that consume aquatic organisms). This study has specifically concentrated on the problem of harbors characterized by a combination of shallow depths and sediment contamination due to metals. As dredging sediment leads to a rise in oxygen and turbidity levels, which heightens the risk of metal mobilization into the water, a different management approach to dredging for polluted sediment is necessary to decrease contaminant levels and the potential risk to the aquatic ecosystem.
The sediment quality was evaluated using the Canadian sediment quality guidelines. The overall levels of Cr, Cu, and Zn exceeded the recommended limits, indicating that the sediment is contaminated with metals and could potentially have negative impacts on this region. The exceeding level (EL) indicated that sediments examined in this research were significantly contaminated by Cu, Cd, Pb, and Zn while being moderately contaminated by other metals. The resuspension technique effectively lowered the levels of 7 specific metals (As, Cd, Cr, Cu, Pb, Ni, and Zn) by eliminating only 2.63% of the polluted sediment. Average removal efficiency values were beneficial and ranged from 3.48% for Cd to 32.4% for Cu. The impact of the resuspension method on the fractionation of metals in sediment and SPM fractions was assessed. The results of the sequential extraction indicated that SPM with elevated EF values had higher contaminant concentrations in less stable fractions. Hence, the risk of metal mobilization might be decreased after the resuspension technique and removal from the environment of this SPM. The water quality following treatment was also evaluated. The metal contents (As, Cd, Cr, Ni, Pb, and Zn) were lower than the criterion continuous concentration (CCC or chronic effect level) in both standards, while Cu was the only metal that exceeded the chronic effect level for certain sensitive species. This indicates that the resuspension is a low risk to the water environment.
This study was performed as a laboratory-scale experiment, although the sediment samples used were from the site (St. Lawrence River). Scaling up the equipment for pilot tests is recommended. Moreover, performing the resuspension technique on different types of sediments with different size distributions and various contamination levels is recommended.