Abstract
This study evaluates the feasibility of using pretreated domestic wastewater (PDW) for food production in a hydroponic system. In the face of increasing water shortage problems and rising fertilizer costs, PDW combined with a limited amount of fertilizer is evaluated for its effects on plant growth, biomass yield, and product safety. The results showed that lettuce grown with PDW and mineral fertilizers reached a fresh weight of 116, while the use of organic fertilizers increased the yield to 127 g, compared to only 54 g with raw water. Nitrate concentration (NO3) was higher in lettuce grown with organic fertilizers (1044.33 ± 144.04 mg/kg) than with mineral fertilizers (623.33 ± 85.62 mg/kg), but the values remained well below the acceptable limit of 5000 mg/kg for safe consumption. Analysis of heavy metals confirmed that levels of arsenic, cadmium, mercury, and lead were significantly lower than the maximum permissible values set by FAO and EU regulations. In addition, no phthalates were detected in the lettuce biomass, confirming the safety of the materials used in the hydroponic system. The use of PDW in hydroponic crops significantly reduces dependence on potable water and synthetic fertilizers, contributing to sustainable resource management. This approach not only reduces production costs, but also reduces the water footprint of crops, which is crucial in the context of global water availability problems. The findings support the validity of using PDW in decentralized food production as a sustainable solution for regions facing water and fertilizer shortages. Further research will focus on optimizing nutrient management and environmental conditions to increase system efficiency and food safety.
1. Introduction
Worldwide, there is a growing contamination of soil and water, caused, among other reasons, by intensive agriculture, which is due to the need to generate ever more significant quantities of food to meet the demands of the growing population [1].
Hydroponics, a rapidly advancing method of cultivation, allows for the growth of vegetables in a soilless culture utilizing artificial lighting, which has been found to have a limited impact on the environment. In hydroponic systems, plants are grown in a medium that supports the root structure, as opposed to soil. This medium is commonly placed in nets on a waterbed and the plants are supplied with nutrients through a nutrient solution [2]. Hydroponic technology presents several benefits, particularly in terms of sustainability. In the context of this study, hydroponic cultivation utilizes low-potential waste heat energy, such as that derived from industrial processes or renewable sources [3,4], providing a system for growing vegetables year-round. The controlled environment eliminates the need to contend with extreme weather conditions, reduces the energy required for tillage, and eliminates the need for pesticides to combat animal pests and weeds. Additionally, the use of domestic wastewater in hydroponic production can be an effective solution in an era of increasing water stress [5]. The reuse of nonrecycled, nutrient-rich hydroponic waste solution and PDW for growing plants in greenhouses is a possible way to control environmental pollution [6]. The benefit of reducing pollutants in wastewater is also important, as well as the utilization of nutrients by plants and therefore reducing burden on the environment. Approximately 25% of water impairments are attributed to nutrient-related issues such as oxygen depletion, algal growth, biological integrity, and turbidity [7]. To enhance the quality of wastewater and reduce the detrimental impact on freshwater resources, it is essential to remove organic pollutants from wastewater [8].
In recent years, antibiotics, becoming the most frequently detected pharmaceutical compounds in the wastewater. Advanced oxidation processes such as non-thermal plasma technologies can be used, which not only generate hydroxyl radicals capable of degrading CEC and inactivating pathogens but also increase the nitrate concentration in purified water [9,10].
In this study, lettuce was chosen as the plant for evaluating the effects of PDW and half-rate fertilizers on growth. Lettuce and other leafy vegetables have a relatively short growth cycle [11], making it possible to conduct multiple experiments in a shorter period. Additionally, due to its quick production cycle and high mass production, lettuce serves as an ideal model plant for studies in vertical farming. It has also been identified as one of the leading leafy plants for commercial production in vertical agriculture systems [12].
On the other hand, lettuce has been known to accumulate nitrate at levels that can be harmful to human consumption if consumed in excessive amounts [13]. The accumulation of nitrate in plants is influenced by both environmental conditions and genetic factors. Among environmental factors, the intensity of light appears to have the strongest impact on the accumulation of nitrates in plant tissues [14]. Previous studies have shown that increasing light intensity within a certain range improves lettuce growth and quality, and it has been suggested that the most efficient light intensity for lettuce in a plant factory is between 200 and 400 µmol m−2 s−1 [15,16,17]. These high levels of nitrate are associated with the rapid growth facilitated by 12 to 14 h light cycles in vertical farming. Additionally, scientific studies have shown that lettuce can be successfully produced with treated greywater without posing a significant health risk from pathogens or metal(loid)s, while also achieving a 5.1 log10 reduction in E. coli in the final effluent [18].
Another area of interest is the fertilizers applied during the growing process. In hydroponic systems, two types of fertilizers are commonly used for vegetable production: mineral and organic fertilizers [19]. Mineral fertilizers, based on chemical fertilizers created from mined, scarce, and nonrenewable sources [20] such as fossil fuels (N fertilizers in the Haber–Bosch process) or fossil ore deposits (phosphate rock) [21], are the most widely used. Previous studies have reported that nitrate accumulation is closely related to the amount of fertilizer applied, with the highest levels of nitrate found in the use of inorganic fertilizers [22]. As concerns about food sources grow, organic fertilizers are gaining popularity. Several studies have begun to investigate the use of organic fertilizers for hydroponic production [23,24,25]. Additionally, research has been conducted on the utilization of byproducts from bioenergy systems as organic fertilizers to improve crop production and maintain soil health [26].
However, the use of organic fertilizers and pretreated domestic wastewater may lead to heavy metal contamination of the final product. There is growing evidence that organic fertilizers and wastewater may have higher concentrations of trace metals such as As, Cd, Hg, and Pb. Studies have shown that Chinese organic fertilizers can have trace heavy metal content that exceeds typical values [27,28]. With the use of these fertilizers, trace metal accumulation can occur in the grown biomass [29]. While some metals, such as Cu, Co, Fe, Mo, Mn, and Zn, are essential nutrients for proper plant growth, others, such as Hg, Cd, Ni, and Pb, are toxic to plants [30]. One concern is the potential for the higher accumulation of metals in leafy plants compared to root vegetables [31].
Lastly, some concerns arise from the materials used to create the growing platform, such as PVC or plastic PP, as the root structure is in constant contact with the material [32]. The transfer of phthalates to lettuce can occur through contact with packaging materials. These materials are frequently associated with microplastics, phthalates, and heavy metals, which can be deposited on plant roots or in their biomass [33,34]. Phthalates are a type of synthetic organic compound that are widely used as plastic additives to increase the flexibility of plastic polymers [35]. The most detected phthalate additives are di-n-butyl phthalate (DnBP) and di (2-ethyl hexyl) phthalate (DEHP), both of which are included on the priority list produced by the US Environmental Protection Agency (USEPA) as potential endocrine-disrupting compounds [36]. Human interaction with these compounds can lead to oxidative stress, genotoxicity, inflammatory and reproductive diseases, and various forms of cancer [37].
Phthalates are commonly transferred to vegetables through contact with plastic wrapping or packaging [33]. Given that the roots and lower leaves of lettuce in the nutrient film technique (NFT) system are in constant contact with plastic material, our hypothesis was that the lettuce biomass would accumulate phthalates due to prolonged contact with plastics [38].
The need for the evolution of alternative methods of cultivation is always a hot topic, especially to confront the multiple problems that conventional agriculture and conventional wastewater treatment are. It seems that an innovative method, such as hydroponic cultivation, comes to solve many of problems of both area plastics [39,40]. Integrated wastewater treatment and hydroponic plant production systems that solely depend on wastewater to supply nutrients for plants have been reported to be deficient in nutrients such as nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg). This is attributed to the low content of nutrients such as N, P, K, Ca, and Mg in wastewater effluents [41]. Therefore, growing plants effectively in hydroponic systems will require the addition of fertilizer to the wastewater that is used as a supplementary nutrient source. At present, however, there is limited information on the effects of combining PDW with commercial fertilizers for plants grown hydroponically.
2. Materials and Methods
2.1. The Hydroponic Farm with NFT System
The experiment was carried out in a vertical hydroponic laboratory with an NFT closed recirculating system where water containing dissolved nutrients flows over the roots of plants in a thin film, allowing them to absorb nutrients and water directly. This allows for a high degree of control over environmental conditions, including nutrient levels, air temperature, water temperature, light intensity, water circulation, and humidity conditions. We produced eight batches of lettuce, 116 plants with 11.5 kg of biomass. The individual series (batches of lettuce) only varied in the type of feed water, in accordance with the research objective.
The climate of the laboratory was maintained at 21 °C to 22 °C using an air conditioning system that compensated for the change in the external environment. Humidity was kept at 50% to 60% using the same means. The growing system was made up of four floors with twenty plants on each floor. The plants were seeded 20 cm apart in three rows spaced 15 cm apart, each row with ten seedlings. The construction, including the growing space, was made of polypropylene of food certified quality.
Salads were seeded in rock wool blocks, suitable for water temperatures up to 25 °C and a pH of 5.8 to 6. For the source of growth light, LED strips (cold white light) with a correlated color temperature of 6000 K, a luminous flux of 2100 lm/m, 120 LED per meter, a power rating of 20 W/m, and a light degree of 120° were used. The growth area on one floor was 195 × 56 cm, and each floor had four strips of lights, 10 cm apart, with a distance from the growing base 20.5 cm. This resulted in an even spread of the luminous flux across the growing space. The diagram of the research system is shown in Figure 1 and Figure 2.
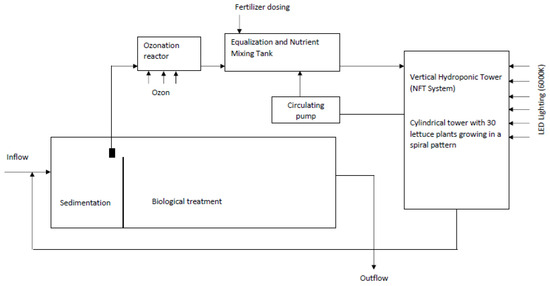
Figure 1.
The diagram of the research unit [own source].

Figure 2.
Hydroponic farm with NFT system in the lab [own source].
PDW from a small domestic treatment plant has been used, with ozonation treatment afterwards. The wastewater was pretreated mechanically using an Imhoff tank and subsequently subjected to ozonation with a ZY-H135 ozone generator (ECS Piotr Paruszewski Kobyla Góra, Bierzów, Poland) operating at a maximum capacity of 1000 mg/h. The ozonation process was carried out in a reaction chamber with a volume of 0.2 m3. From the reaction chamber, the wastewater flowed into an equalization tank, from where it was supplied to the system feeding the cultivation setup.
As for the quality of pretreated sewage, it was characterized by a pH of 6.5 to 7.4, and the concentration of organic compounds measured as BOD5 at the level of 30–50 mg O2/L. The analysis of the individual elements was carried out in an accredited laboratory. No nutrients were measured in the pretreated sewage because they were treated as substances that affect plant growth and were therefore dosed in addition to fertilizers.
PDW was used in lettuce production where the values determined are as follows: total nitrogen (N) = 55 mg/L and total phosphorus (P) = 8 mg/L. The wastewater was supplemented with two types of fertilizer, the nutrient composition of which is given below according to the producer’s data (General Hydroponics Flora Nova). However, fertilizers were applied at only half the rate recommended by the producer. Their concentration was maintained at the desired ratio per liter of water during the 7-week growth period. The rationale for applying a reduced 50% fertilizer dose was based on the fact that the wastewater itself already contains nitrogen and phosphorus. The research concept aimed not only to utilize the reclaimed water from the wastewater but also, and perhaps more importantly, to recover and reuse the biogenic elements that contribute to plant growth. The implementation of such a system is designed to reduce not only the extraction of water from primary sources but also the demand for synthetic fertilizer production. This approach aligns with sustainable agricultural practices by promoting resource efficiency and minimizing environmental impact.
An additional critical factor in determining the reduced fertilizer dose was the consideration of food safety, particularly for children who may consume the produced lettuce. Excessive nitrate levels in vegetables can pose health risks, especially to vulnerable populations. By lowering the fertilizer input, the study sought to ensure that nitrate concentrations in the lettuce remained within safe limits, adhering to established health and safety standards. This dual focus on sustainability and consumer health highlights the broader implications of integrating wastewater reuse into agricultural production systems. In the first example, mineral fertilizer was used to supply the following components (1.1 mL/L): total nitrogen (N) = 70 mg/L, phosphoric anhydride (P2O5) = 44 mg/L, potassium oxide (K2O) = 110 mg/L, calcium oxide (CaO) = 44 mg/L, magnesium oxide (MgO) = 16 mg/L, sulfur anhydride (SO3) = 11 mg/L, and iron (Fe).
In the second example, the PDW was supplemented with organic fertilizers to supply the following components: total nitrogen (N) = 80 mg/L, anthydride phosphorique (P2O5) = 20 mg/L, potassium oxide (K2O) = 118 mg/L, sulfur anhydride (SO3) = 100 mg/L, magnesium oxide (MgO) = 20 mg/L, copper (Cu) = 0.2 mg/L, and iron (Fe), Zinc (Zn).
It should be emphasized that to maintain microbiological safety, the sewage was subjected to an ozonation process before being introduced into the system, after the pretreatment process.
2.2. Plant Material
The plant material consisted of one type of leafy salad, lettuce, shown in Figure 3 and Figure 4, Gagarin RZ-type lettuce dubacek (Lactuca sativa L. var. crispa) (resistance HR, B1:16–36 EU/Nr:0:). Lettuce is generally grown as an annual autotrophic therophytic herb, though under some conditions it can behave as a biennial. In our case, the harvest was carried out from the first year. The root system consists of the primary spherical root, with which the plant is well fixed in the rock wool cube. Simple or segmented leaves with pronounced ribbed veins alternately grow in the rosette until the plant removes the stem into tiny yellow Asteraceae flowers arranged in a cape. This lettuce forms achenes when fully matured.

Figure 3.
(a) Vertical view and (b) horizontal view of the lettuce produced using mineral fertilizer [own source].
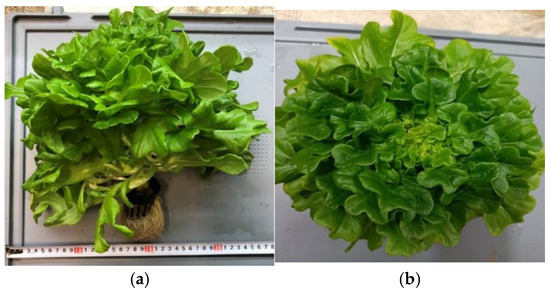
Figure 4.
(a) Vertical view and (b) horizontal view of the lettuce produced using organic fertilizer [own source].
2.3. Methods Used for Elemental and Phthalate Analysis
When lettuce was harvested, the root structure was separated from the stem and leaves. The lettuce stems and leaves were kept in a refrigerator at 4 °C for the next 12 h before being processed into the dry mass required for the tests. The analysis of the individual elements was carried out in ALS Czech Republic, s.r.o. an accredited laboratory using appropriate accredited methods to determine individual concentrations of each element in the lettuce produced.
The determination of nitrates in the lettuce leaves was made using the colorimetric method involved extracting nitrate ions with distilled water, followed by a reaction with salicylic acid in a sulfuric acid medium, resulting in the formation of a yellow complex. The absorbance of this complex was measured at a wavelength of 410 nm using a spectrophotometer, and the nitrate concentration was calculated based on the calibration curve.
For the determination of arsenic (As), cadmium (Cd), lead (Pb), and mercury (Hg) in vegetables, samples were first digested using a mixture of concentrated nitric acid (HNO3) and perchloric acid (HClO4) to break down the organic matter and release metal ions. The digested solution was then introduced into the Atomic Absorption Spectrometer (AAS), where each metal was quantified by measuring its specific light absorption.
Phthalates in vegetable samples were determined using Gas Chromatography–Mass Spectrometry (GC-MS). The samples were homogenized and extracted with an organic solvent, typically hexane or dichloromethane, followed by purification through solid-phase extraction (SPE) to eliminate matrix interferences. The purified extracts were then injected into the GC-MS system, where phthalates were identified and quantified based on their characteristic mass spectra and retention times.
3. Results
3.1. Lettuce Growth, Harvestation, and Biomass Processing
This study employed a procedure in which seeds were planted in rockwool cubes soaked in water or PDW and left to germinate in a dark space for five days until the first leaves developed. Once the first set of seed leaves emerged, the plants were transferred to a location with artificial light to prevent excessive growth. Following this, the plants developed their roots and first true leaves, at which point they were transplanted into net pots and placed in a closed loop NFT system with artificial light and fertilizer.
Harvesting occurred when the lettuce heads were fully formed and prior to the bolting phase, approximately 7 weeks after transplantation to the NFT system (with a 14 h/day light period and a 10 h/day dark period). Fresh biomass (g) was determined by selecting 20 plants from the vertical farm, separating the root structure from the stem, and converting the stem and leaves to a dry mass. The average number of leaves and fresh biomass of plants is shown in Table 1.

Table 1.
Effects of different fertilizers on morphological traits of plant [own source].
The table presented shows the effects of a combination of PDW and two different fertilizers on the morphological traits of a lettuce. The data compare the use of PDW with half-dose mineral fertilizers, organic fertilizers, and then PDW (control group) on the fresh biomass and number of leaves of the plant.
The results indicate that the use of PDW and both fertilizers significantly increased the fresh biomass of the lettuce plant compared to the control group using raw water. Specifically, the use of mineral fertilizer resulted in a fresh biomass of 116 ± 3 g and the use of organic fertilizer resulted in a fresh biomass of 127 ± 3 g. This suggests that both types of fertilizers are effective in promoting growth in lettuce plants, although it was only used at half the dose recommended by the producer.
The number of leaves on the plants did not see any increase when using the combination of PDW and fertilizers, with 39 ± 2 leaves and 44 ± 2 leaves, respectively, compared to 40 ± 3 leaves in the control group using raw water. This suggests that both types of fertilizers may not have any effect on the promotion of leaf growth in the lettuce plants. It is worth noting that the standard errors (SEs) of the means are relatively low, indicating that the results are likely to be reliable and generalizable. We are not comparing the effects of PDW without fertilizers but raw water vs. PDW with fertilizers. Also, we are making the conclusion that PDW with fertilizers do not have an effect on leaf growth in lettuce compared to raw water.
3.2. Effects of Mineral and Organic Fertilizers on Nitrate Uptake
The tables presented the concentration of nitrates (NO3) in hydroponically grown lettuce using PDW supplemented with half the rate of mineral and organic fertilizers and using raw water as a control. The data presented in Table 2 support the initial hypothesis that organic fertilizers promote higher amounts of nitrates absorbed into the grown vegetable. The results of the analysis indicate a difference of 421 mg/kg of nitrates (NO3) in the biomass of the whole lettuce produced in the first and second experiments and 100.33 mg/kg of nitrates in the third and fourth experiments when using mineral and organic fertilizers. The results suggest that the PDW with organic fertilizer has a role in the increase in nitrates in hydroponically grown lettuce. However, it is worth noting that the nitrate increase is not as significant as some previous studies have indicated [22].

Table 2.
Concentration of nitrates NO3 in two hydroponically produced lettuce samples using PDW and mineral and organic fertilizers and using raw water [own source].
The results from Table 2 indicate that the use of raw water results in a significantly lower NO3 concentration in lettuce plants compared to the use of domestic pretreated wastewater with mineral and organic fertilizers. Specifically, the use of raw water results in an average NO3 concentration of 148.67 ± 14.83 mg/kg. This suggests that the use of raw water as a source of water results in lower levels of nitrates in lettuce plants.
A more substantial difference in the concentration of NO3 is present when comparing the pretreated domestic wastewater with raw water (no fertilizers) as shown in Table 2. Raw water resulted in 895.66 to 441 mg/kg less NO3 in the plant compared to PDW mixtures with fertilizers. This further supports the conclusion that the use of domestic pretreated wastewater with both mineral and organic fertilizers results in significantly higher NO3 concentrations in lettuce plants compared to the control group using raw water.
However, using only raw water resulted in a significant decrease in biomass over the same growing period, as shown in Figure 5.
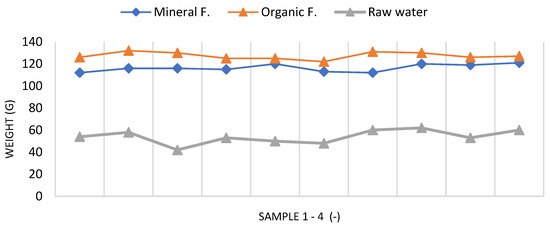
Figure 5.
Fresh biomass of samples 1–4 from randomly selected lettuce produced in the hydroponic system using PDW supplemented with half the dose of organic and mineral fertilizers [own source].
3.3. Heavy Metals
The data presented in Table 3 show the concentrations of heavy metal traces (As, Cd, Hg, Pb) contained in lettuce leaves produced using PDW with fertilizers. The table also includes the maximum permissible concentrations of these heavy metals in lettuce for human consumption established by the European Union and the Food and Agriculture Organization of the United Nations (FAO) [42]. The text highlights that all investigated heavy metals (As, Cd, Hg, and Pb) present in the lettuce produced are below the maximum concentration recommended by the FAO and the EU.

Table 3.
Heavy metal concentration that occurs in hydroponically produced lettuce, using mineral and organic fertilizers [own source].
All investigated heavy metals (As, Cd, Hg, and Pb) present in the lettuce produced are below the maximum concentration recommended by the FAO and the EU. Although cadmium is more solvable compared to other metals, which means that it can accumulate more in vegetable tissue [38], its presence in our produce was the lowest between tested heavy metals. Cadmium was alleviated when using organic fertilizers, while Hg was higher in mineral fertilizers. The variation in these metal concentrations is likely attributed to the standard error in the analysis and errors in the subsequent statistical analysis, especially since all the measured values were below the detection limit of the laboratory equipment.
3.4. Phthalate
The concentration of several types of phthalates occurring in hydroponically grown lettuce using PDW + fertilizer was determined in the laboratory. Its values were often below the limit of detection, in the following range: 0.1 mg/kg (Tributyl Phosphate), 0.3 mg/kg (Butylbynzylphtalate, Di-Butylphthalate, Di-iso-Butyladipate, Di-iso-butylphthalate), 0.5 mg/kg (Di-Butyladipate, Diethyladipate, Diethylhexyladipate, Diethylphthalate, Dimethylphthalate, DINCH, Di-n-octylphthalate, Dipenthylphthalate (sum of I and N), Di(2-propylheptyl) phthalate, Tributyl O-acetylcitrate, Diethylhexyl sebacate), 1 mg/kg (Diethylhexylphthalate), and 5 mg/kg (Di-iso-Decylphthalate, Di-iso-Heptylphthalate, Dinonylphthalate (sum I and N)).
The working hypothesis was the possibility that fertilizers could interact with materials used for the construction of the growing platform and thus alter its stability, which could lead to contamination of the water and the grown biomass by phthalates released from PVC or PP [32]. The second hypothesis was the effects of constant contact of the root structure with the said material and the possibility of uptake of phthalates by the roots [38]. The results of the analysis are given below.
All tested phthalate esters were determined to have a quantification level in the final product. This leads to the conclusion of refutation of both original hypotheses concerning phthalates in this analysis. The results indicate the safety of the material used for hydroponic farming in the vertical farm in an experiment. Similarly, these results could be used to validate the safety of other vertical farms and growing complexes operating under similar conditions.
4. Discussion
The study presented in this article aimed to investigate the effects of using PDW supplemented with half doses of mineral and organic fertilizers on the growth and morphological traits of hydroponically produced lettuce. The results, presented in Table 1, indicate that both mixtures significantly increased the fresh biomass and number of leaves of the lettuce plant compared to the control group using raw water, suggesting that PDW and both types of fertilizers are effective in promoting growth in lettuce plants. Additionally, it is worth noting that the standard errors (SEs) of the means are relatively low, indicating that the results are likely to be reliable and generalizable.
The study also conducted an elemental analysis, which found that NO3 concentrations were substantially lower than the recommendations of the Scientific Committee of Food. The concentrations of arsenic, cadmium, mercury, and lead were particularly important due to their potential harmful effects on human health, which are well documented in previous studies [43,44,45,46]. The study also found no presence of phthalates in the lettuce, which is important due to the potential danger of phthalate contamination of food to human health [47,48] and the increasing use of PVC materials for packaging, storage, and transporting food. The Scientific Committee on Food (SCF) stated in the regulation (EC) 2005 that the total intake of nitrate is normally well below the acceptable daily intake (ADI) of 3.65 mg/kg of body weight. This is also regulated by the FAO [49] and Commission regulation [50], stating that the maximum permissible concentration of nitrates such as NO3 must be 5000 mg/kg for lettuce grown under cover. In our analysis, NO3 was highest at 1044.33 ± 144.04 and 623.33 ± 85.62 mg/kg, respectively, for PDW mixtures, which is 20.89% and 12.47% of the maximum allowed NO3. This would suggest that using raw water would be safer for production of the lettuce on a smaller localized scale compared to the pretreated domestic wastewater. On the other hand, the morphological traits of the plants suffered substantially when no fertilizer was used as shown in Figure 4. This is due to the lack of necessary nutrients in raw water which are separated from the raw water.
The concentration of mercury is not specified by the FAO [49] for the norms for leafy vegetables, only for fish, while the EU regulation (EC) [51] specifies the permissible amount of mercury at 0.01 mg/kg. Neither the FAO [49] nor the EU [50] specifically specify arsenic concentrations in leafy vegetables or lettuce. Cadmium has stricter regulation in the EU [52] at 0.1 mg/kg compared to FAO regulations (2019) at 0.2 mg/kg. Lastly, lead is regulated at 0.3 mg/kg in both the FAO [49] and the EU regulation [53,54].
The results of this study contained minimal amounts of As, Cd, Hg, and Pb in the lettuce produced. Table 3 shows that the concentrations were below the permissible levels (maximum allowed concentrations of heavy metals in leafy vegetables). In the case of Cd, however, the resulting value is too close to the permissible value. Therefore, it cannot be generalized that there is a high safety of growing lettuce in PDW mixtures against the presence of heavy metals.
5. Conclusions
The study clearly confirms the potential for the safe and effective use of partially treated domestic wastewater (PDW) in food production within hydroponic systems. The application of PDW, supplemented with half doses of organic or mineral fertilizers, resulted in satisfactory lettuce growth, comparable to traditional cultivation methods. Despite the reduced fertilizer dosage, both biomass accumulation and leaf count were adequate, demonstrating that PDW can effectively support food production.
Analysis of lettuce biomass revealed no presence of heavy metals or other harmful contaminants, confirming the safety of using PDW mixed with fertilizers in hydroponic cultivation. Additionally, prolonged exposure to the NFT hydroponic system did not lead to phthalate migration into the plants, further emphasizing the safety of this technology.
The use of PDW in food production not only offers a viable alternative to conventional water sources but also reduces synthetic fertilizer consumption, lowers production costs, and significantly decreases the water footprint of crops. This approach enables efficient management of water and nutrient resources, aligning with global sustainable agriculture goals.
Future research should focus on further improving this technology by analyzing the effects of PDW without fertilizer supplements and optimizing environmental conditions, such as lighting. However, the current findings provide a solid foundation for developing innovative, safe, and sustainable food production methods based on wastewater reuse.
Author Contributions
Conceptualization, L.V., M.G. and I.K.-B.; methodology, L.V. and H.S.; validation, L.V. and M.G.; formal analysis, H.S.; investigation, L.V. and M.G.; writing-original draft preparation, L.V. and I.K.-B.; writing-review and editing, M.G. and H.S.; visualization, H.S.; supervision, I.K.-B. and M.G.; data curation L.V. All authors have read and agreed to the published version of the manuscript.
Funding
SGS Grant from VSB-Technical University of Ostrava: SP2025/031.
Data Availability Statement
Data available on request due to restrictions e.g., privacy or ethical. The data presented in this study are available on request from the corresponding author.
Acknowledgments
This research was developed for the sustainability of the project Extension of the graduates competencies in the areas of sustainable electrical power engineering and environment (EPEN), No. CZ.11.3.119/0.0/0.0/17 _027/0001671, from the program Interreg V-A Czech Republic–Poland. The project is supported by the European Union from the European Regional Development Fund.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Turcios, A.; Papenbrock, J. Sustainable Treatment of Aquaculture Effluents—What Can We Learn from the Past for the Future? Sustainability 2014, 6, 836–856. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; van Os, E.; Anseeuw, D.; Van Havermaet, R.; Ranka, J. Hydroponic Technologies. In Aquaponics Food Production Systems; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Lisy, M.; Balas, M.; Spilacek, M.; Skala, Z. Technical and economic optimization of cogeneration technology using com-bustion and gasification. Acta Polytech. Hung. 2014, 54, 42–51. [Google Scholar] [CrossRef]
- Bartela, Ł.; Waniczek, S.; Lutyński, M. Concept of the thermal integration of the compressed air energy storage system with the power plant. In Proceedings of the 32nd International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, ECOS 2019, Wroclaw, Poland, 23–28 June 2019; pp. 4753–4766. [Google Scholar]
- Jaramillo, M.; Restrepo, I. Wastewater Reuse in Agriculture: A Review about Its Limitations and Benefits. Sustainability 2017, 9, 1734. [Google Scholar] [CrossRef]
- Kumar, R.R.; Cho, J.Y. Reuse of hydroponic waste solution. Environ. Sci. Pollut. Res. 2014, 21, 9569–9577. [Google Scholar] [CrossRef]
- U.S. EPA. National Section 303(d) List Fact Sheet. 2007. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/2007_03_08_tmdl_mercury5m_mercury5m.pdf (accessed on 20 June 2024).
- Boguniewicz-Zablocka, J.; Klosok-Bazan, I.; Capodaglio, A.G.; Ploskonka, J. Planning the optimal solution for wastewater management in rural areas—Case study. In Proceedings of the 3rd Scientific Conference Environmental Challenges in Civil Engineering, ECCE 2018, Opole, Poland, 23–25 April 2018. [Google Scholar] [CrossRef]
- Nikitin, D.; Kaur, B.; Preis, S.; Dulova, N. Degradation of Antibiotic Vancomycin by UV Photolysis and Pulsed Corona Discharge Combined with Extrinsic Oxidants. Catalysts 2023, 13, 466. [Google Scholar] [CrossRef]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal Plasma Inactivation of Food-Borne Pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Botany. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Touliatos, D.; Dodd, I.C.; Mcainsh, M. Vertical farming increases lettuce yield per unit area compared to conventional horizontal hydroponics. Food Energy Secur. 2016, 5, 184–191. [Google Scholar] [CrossRef]
- Cometti, N.N.; Martins, M.Q.; Bremenkamp, C.A.; Nunes, J.A. Nitrate concentration in lettuce leaves depending on photosynthetic photon flux and nitrate concentration in the nutrient solution. Hortic. Bras. 2011, 29, 548–553. [Google Scholar] [CrossRef]
- Chen, Z.; Shah Jahan, M.; Mao, P.; Wang, M.; Liu, X.; Guo, S. Functional growth, photosynthesis, and nutritional property analyses of lettuce grown under different temperature and light intensity. J. Hortic. Sci. Biotechnol. 2020, 96, 53–61. [Google Scholar] [CrossRef]
- Ferrón-Carrillo, F.; Guil-Guerrero, J.L.; González-Fernández, M.J.; Lyashenko, S.; Battafarano, F.; da Cunha-Chiamolera, T.P.L.; Urrestarazu, M. LED Enhances Plant Performance and Both Carotenoids and Nitrates Profiles in Lettuce. Plant Foods Hum. Nutr. 2021, 76, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Nicole, C.C.S.; Charalambous, F.; Martinakos, S.; Van De Voort, S.; Li, Z.; Verhoog, M.; Krijn, M. Lettuce growth and quality optimization in a plant factory. Acta Hortic. 2016, 1134, 231–238. [Google Scholar] [CrossRef]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortiulturae 2016, 198, 277–283. [Google Scholar] [CrossRef]
- Jesse, S.D.; Zhang, Y.; Margenot, A.J.; Davidson, P.C. Hydroponic Lettuce Production Using Treated Post-Hydrothermal Liquefaction Wastewater. Sustainability 2019, 11, 3605. [Google Scholar] [CrossRef]
- Ezziddine, M.; Liltved, H.; Seljåsen, R. Hydroponic Lettuce Cultivation Using Organic Nutrient Solution from Aerobic Digested Aquacultural Sludge. Agronomy 2021, 11, 1484. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhai, H. Evaluation of growth and quality of hydroponic lettuce at harvest as affected by the light intensity, photoperiod, and light quality at seedling stage. Sci. Hortic. 2019, 248, 138–144. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Michels, E.; Crappé, S.; Buysens, S.; Tack, F.M.G.; Meers, E. Utilization of derivatives from nutrient recovery processes as alternatives for fossil-based mineral fertilizers in commercial greenhouse production of Lactuca sativa L. Sci. Hortic. 2016, 198, 267–276. [Google Scholar] [CrossRef]
- Amr, A.; Hadidi, N. Effect of cultivar and harvest date on nitrate (NO3) and nitrite (NO2) content of selected vegetables grown under open field and greenhouse conditions in Jordan. J. Food Compos. Anal. 2001, 14, 59–67. [Google Scholar] [CrossRef]
- Kechasov, D.; Verheul, M.J.; Paponov, M.; Panosyan, A.; Paponov, I.A. Organic Waste-Based Fertilizer in Hydroponics Increases Tomato Fruit Size but Reduces Fruit Quality. Front. Plant Sci. 2021, 12, 680030. [Google Scholar] [CrossRef]
- Atkin, K.; Nichols, M.A. Organic hydroponics. Acta Hortic. 2004, 121–127. [Google Scholar] [CrossRef]
- Kano, K.; Kitazawa, H.; Suzuki, K.; Widiastuti, A.; Odani, H.; Zhou, S.; Chinta, Y.D.; Eguchi, Y.; Shinohara, M.; Sato, T. Effects of Organic Fertilizer on Bok Choy Growth and Quality in Hydroponic Cultures. Agronomy 2021, 11, 491. [Google Scholar] [CrossRef]
- Gell, K.; van Groenigen, J.W.; Cayuela, M.L. Residues of bioenergy production chains as soil amendments: Immediate and temporal phytotoxicity. J. Hazard. Mater. 2011, 186, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Q.; Tang, Z.; Zhang, W.; Yu, G.; Shen, Q.; Zhao, F.J. Heavy metal concentrations and arsenic speciation in animal manure composts in China. Waste Manag. 2017, 64, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, S.; Wang, X.; Wang, M. Contents of heavy metal in commercial organic fertilizers and organic wastes. J. Agro-Environ. Sci. 2005, 24, 392–397. [Google Scholar]
- Lopes, C.; Herva, M.; Franco-Uría, A.; Roca, E. Inventory of heavy metal content in organic waste applied as fertilizer in agriculture: Evaluating the risk of transfer into the food chain. Environ. Sci. Pollut. Res. 2011, 18, 918–939. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Principles of Plant Nutrition; Kluwer Academic: Dordrecht, The Netherlands, 1987. [Google Scholar]
- Baker, A.J.M.; Reeves, R.D.; McGrath, S.P. In Situ Decontamination of Heavy Metal Polluted Soils Using Crops of Metal-accumulating Plants: A Feasibility Study. In Proceedings of the Abstracts International Symposium on In Situ and On Site Bioreclamation, San Diego, CA, USA, 19–21 March 1991. [Google Scholar]
- Yong, L.; Huangqian, Y.; Liu, Q.; Li, X.; Ge, J.; Yu, X. Accumulation and transport patterns of six phthalic acid esters (PAEs) in two leafy vegetables under hydroponic conditions. Chemosphere 2020, 249, 126457. [Google Scholar] [CrossRef]
- Gallart-Ayala, H.; Núñez, O.; Lucci, P. Recent advances in LC-MS analysis of food-packaging contaminants. TrAC Trends Anal. Chem. 2013, 42, 99–124. [Google Scholar] [CrossRef]
- Hochman, G.; Hochman, E.; Naveh, N.; Zilberman, D. The Synergy between Aquaculture and Hydroponics Technologies: The Case of Lettuce and Tilapia. Sustainability 2018, 10, 3479. [Google Scholar] [CrossRef]
- Alexander, P.D.; Alloway, B.J.; Dourado, A.M. Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ. Pollut. 2006, 144, 736–745. [Google Scholar] [CrossRef]
- Ma, T.T.; Teng, Y.; Christie, P.; Luo, Y.M. Phytotoxicity in seven higher plant species exposed to di-n-butyl phthalate or bis (2-ethylhexyl) phthalate. Front. Environ. Sci. Eng. 2014, 9, 259–268. [Google Scholar] [CrossRef]
- Poursafa, P.; Ataei, E.; Kelishadi, R. A systematic review on the effects of environmental exposure to some organohalogens and phthalates on early puberty. J. Res. Med. Sci. 2015, 20, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, H.; Yu, X. Uptake and accumulation of di-n-butyl phthalate in six leafy vegetables under hydroponic conditions. Food Prod. Process. Nutr. 2019, 1, 9. [Google Scholar] [CrossRef]
- Manos, D.P.; Xydis, G. Hydroponics: Are we moving towards that direction only because of the environment? A discussion on forecasting and a systems review. Environ. Sci. Pollut. Res. 2019, 26, 12662–12672. [Google Scholar] [CrossRef]
- da Silva, J.S.; Dias, N.d.S.; Jales, G.D.; Rges, L.B.L.; de Freitas, J.M.C.; Umbelino, B.F.; Alves, T.R.C.; da Silva, A.A.; dos Santos Fernandes, C.; de Paiva, E.P.; et al. Physiological responses and production of mini-watermelon irrigated with reject brine in hydroponic cultivation with substrates. Environ. Sci. Pollut. Res. 2021, 29, 11116–11129. [Google Scholar] [CrossRef]
- Magwaza, S.T.; Magwaza, L.S.; Odindo, A.O.; Mditshwav, A.; Buckley, C. Partially treated domestic wastewater as a nutrient source for tomatoes (Lycopersicum solanum) grown in a hydroponic system: Effect on nutrient absorption and yield. Heliyon 2020, 6, e05745. [Google Scholar] [CrossRef]
- Chaudhery, M.H.; Rüstem, K. Modern Environmental Analysis Techniques for Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–36. [Google Scholar]
- Jordan, R.A.; Ribeiro, E.F.; de Oliveira, F.C.; Geisenhoff, L.O.; Martins, E.A.S. Yield of lettuce grown in hydroponic and aquaponic systems using different substrates. Rev. Bras. Eng. Agric. Ambient. 2018, 22, 525–529. [Google Scholar] [CrossRef]
- Panda, S.K.; Upadhyay, R.K.; Nath, S. Arsenic stress in plants. J. Agron. Crop Sci. 2010, 196, 161–174. [Google Scholar] [CrossRef]
- Bernard, A. Cadmium & its adverse effects on human health. Indian J. Med. Res. 2008, 128, 557–564. [Google Scholar]
- Díez, S. Human health effects of methylmercury exposure. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef]
- FAO. General Standard for Contaminants and Toxins in Food and Feed; FAO: Rome, Italy, 2019. [Google Scholar]
- Commission Regulation (EU) 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 20 June 2024).
- Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC (OJ L 70,16.3.2005, p. 1) as Last Amended by Regulation (EC) No 178/2006 (OJ L 29, 2.2.2006, p. 3). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:070:0001:0016:en:PDF (accessed on 20 June 2024).
- Commission Regulation (EU) 2018/73 of 16 January 2018 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Mercury Compounds in or on Certain Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R0073 (accessed on 20 June 2024).
- Commission Regulation (EU) 2021/1317 of 9 August 2021 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Lead in Certain Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2021/1317/oj/eng (accessed on 20 June 2024).
- Commission Regulation (EU) 2021/1323 of 10 August 2021 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Cadmium in Certain Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2021/1323/oj/eng (accessed on 20 June 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).