Hybrid Nylon-6/Pumice Nonwoven Composites as Nature-Based Adsorbents for Methylene Blue Dye-Contaminated Wastewater: Insights into Monolayer and Multilayer Adsorption Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nylon-6/Pumice Nonwoven Fabrics
2.2.1. Pre-Treatment of Materials
2.2.2. Melt-Compounding Extrusion
2.2.3. Nonwoven Fabrics (NWF)
2.3. Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. X-Ray Diffraction (XRD)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Adsorption Assays
2.3.5. Adsorption Analysis
3. Results
3.1. Morphology and PPw Content
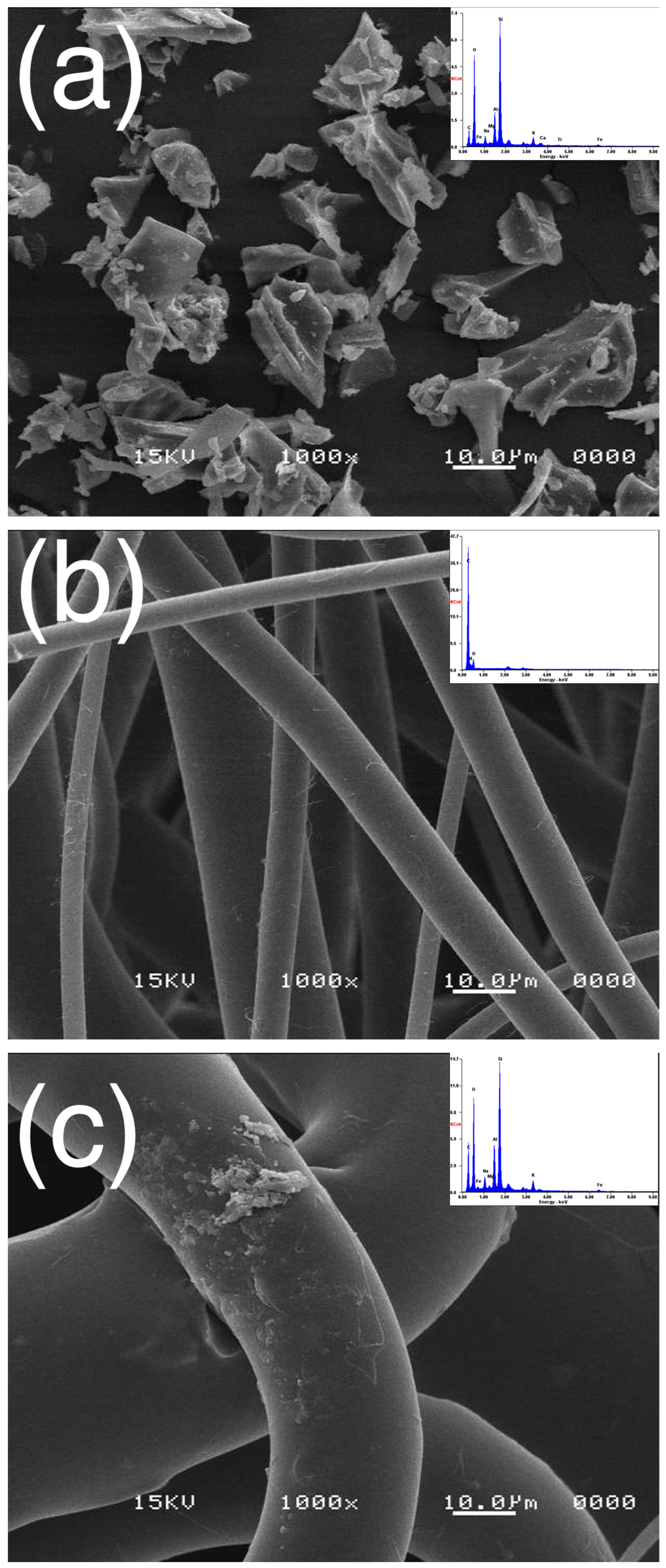
3.1.1. Morphology
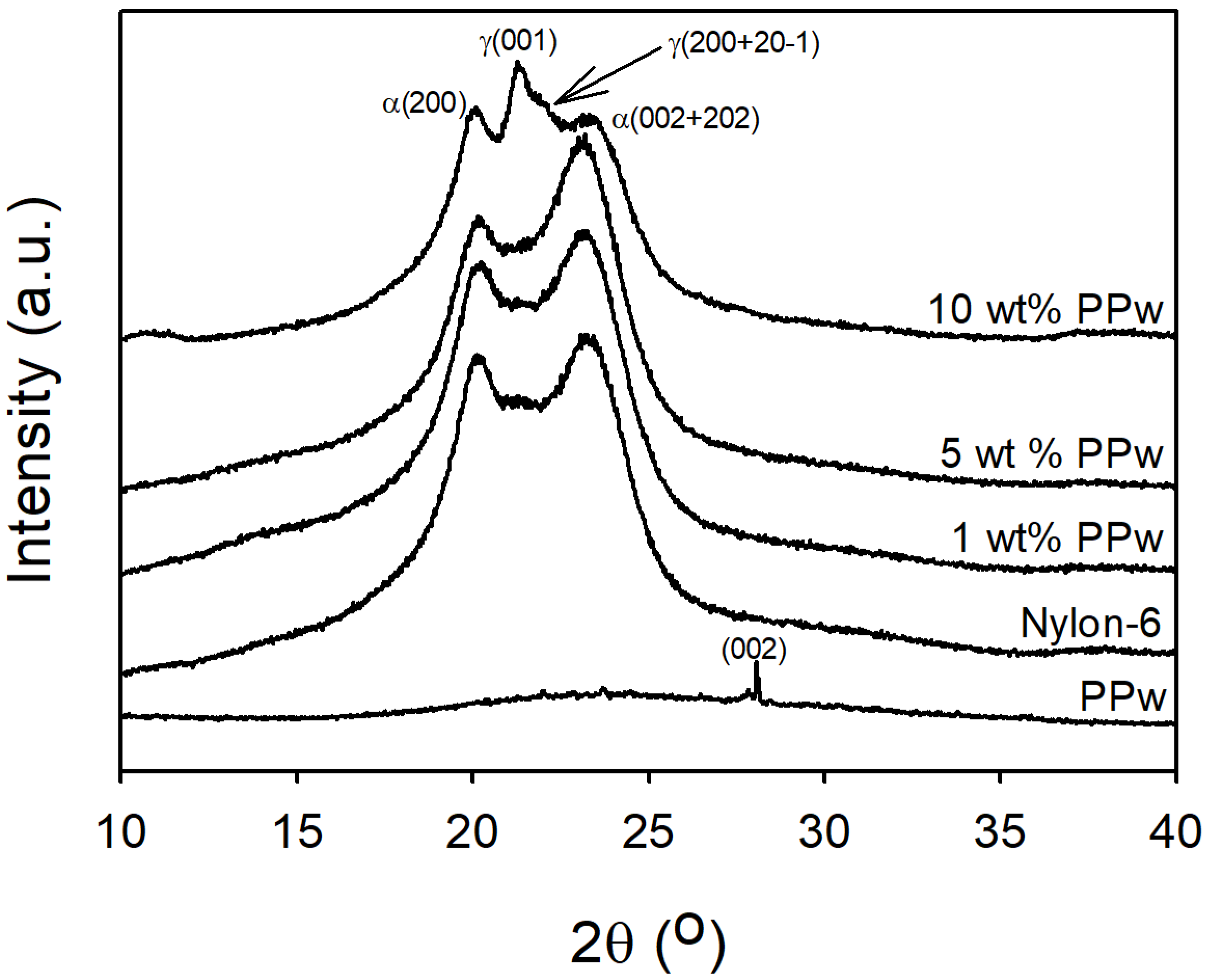
3.1.2. X-Ray Diffraction Analysis (XRD)
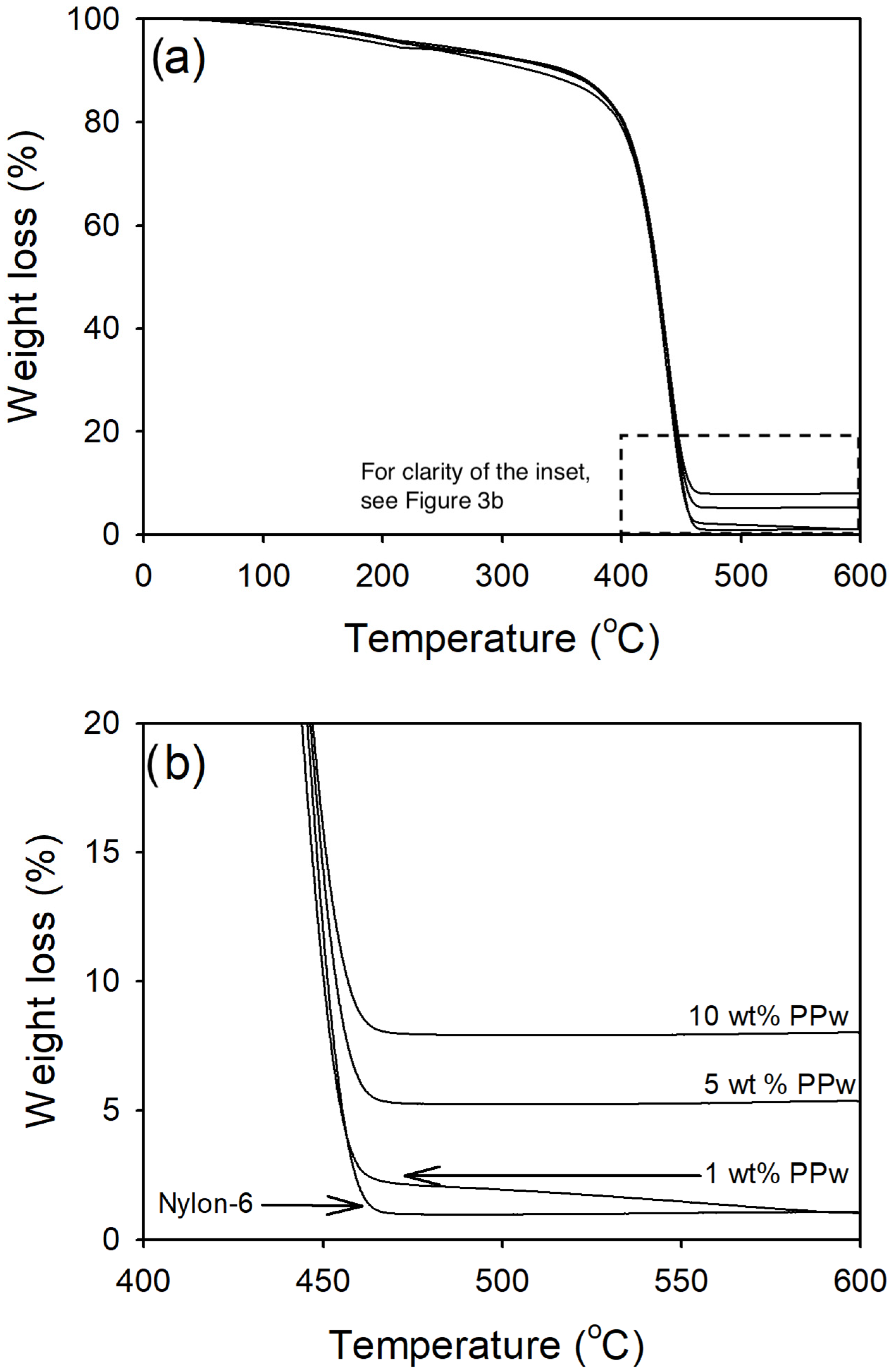
3.1.3. Pumice Powder Content
3.2. Methylene Blue Adsorption Analysis
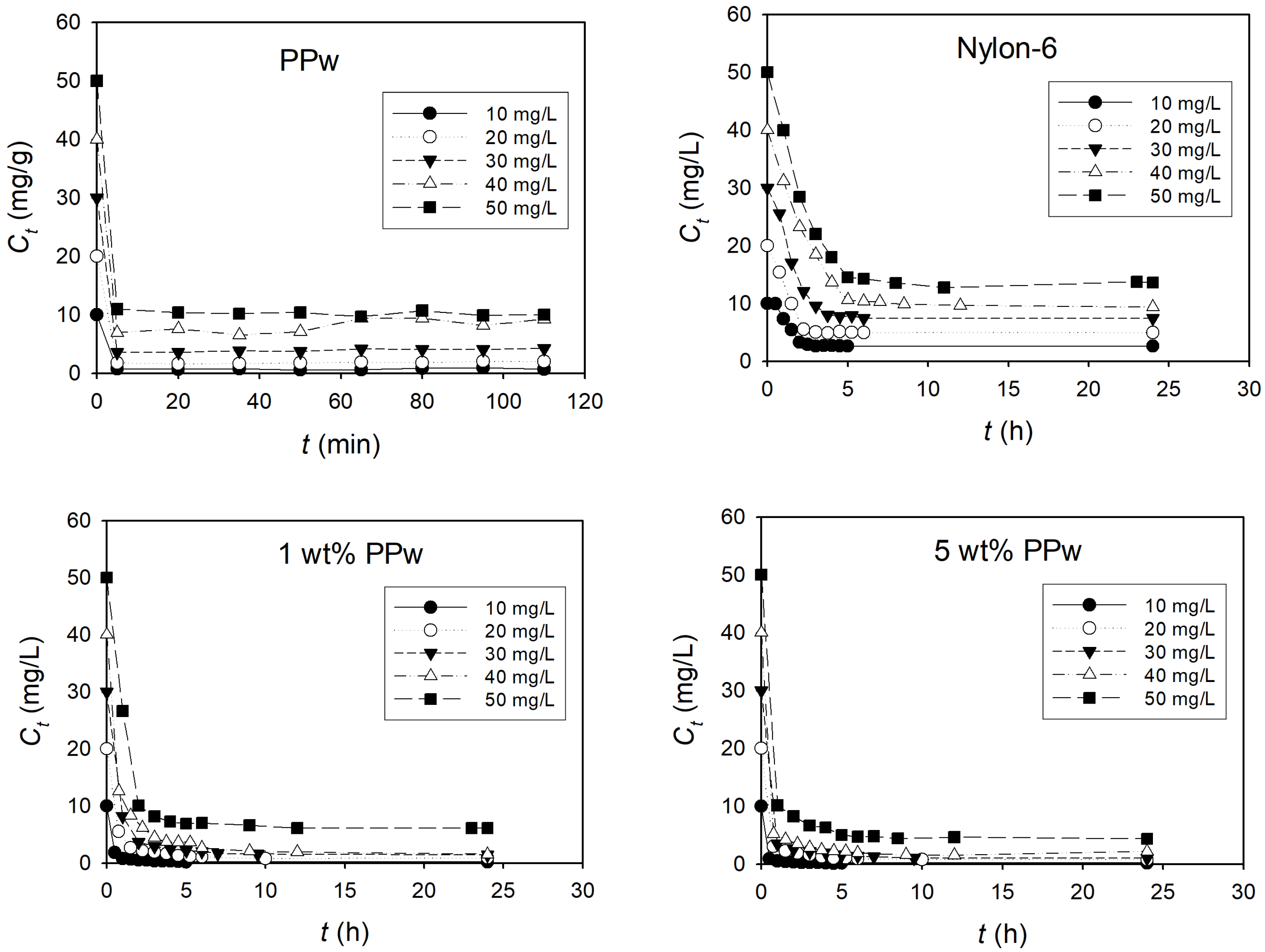
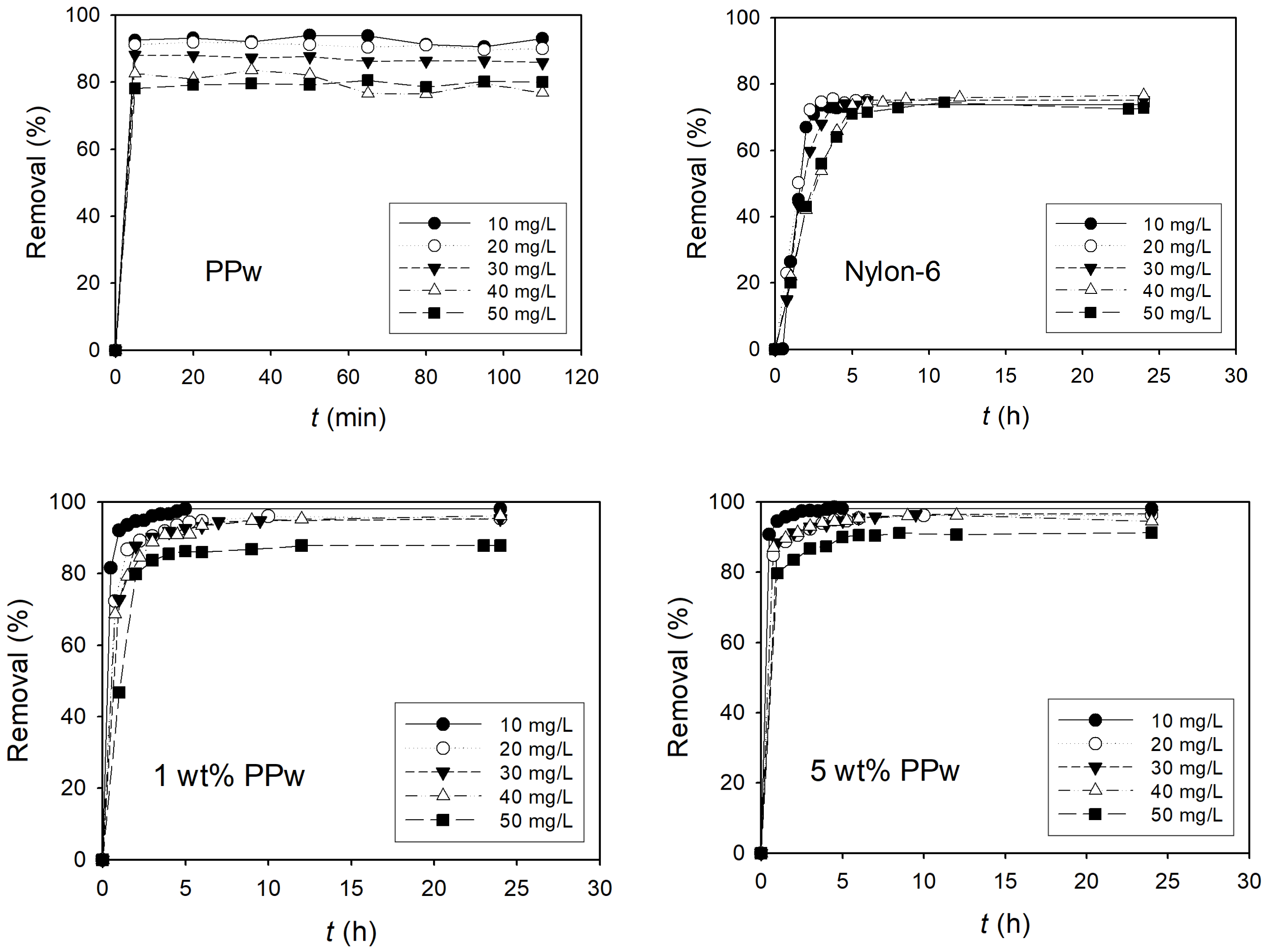
3.2.1. Removal of Dye
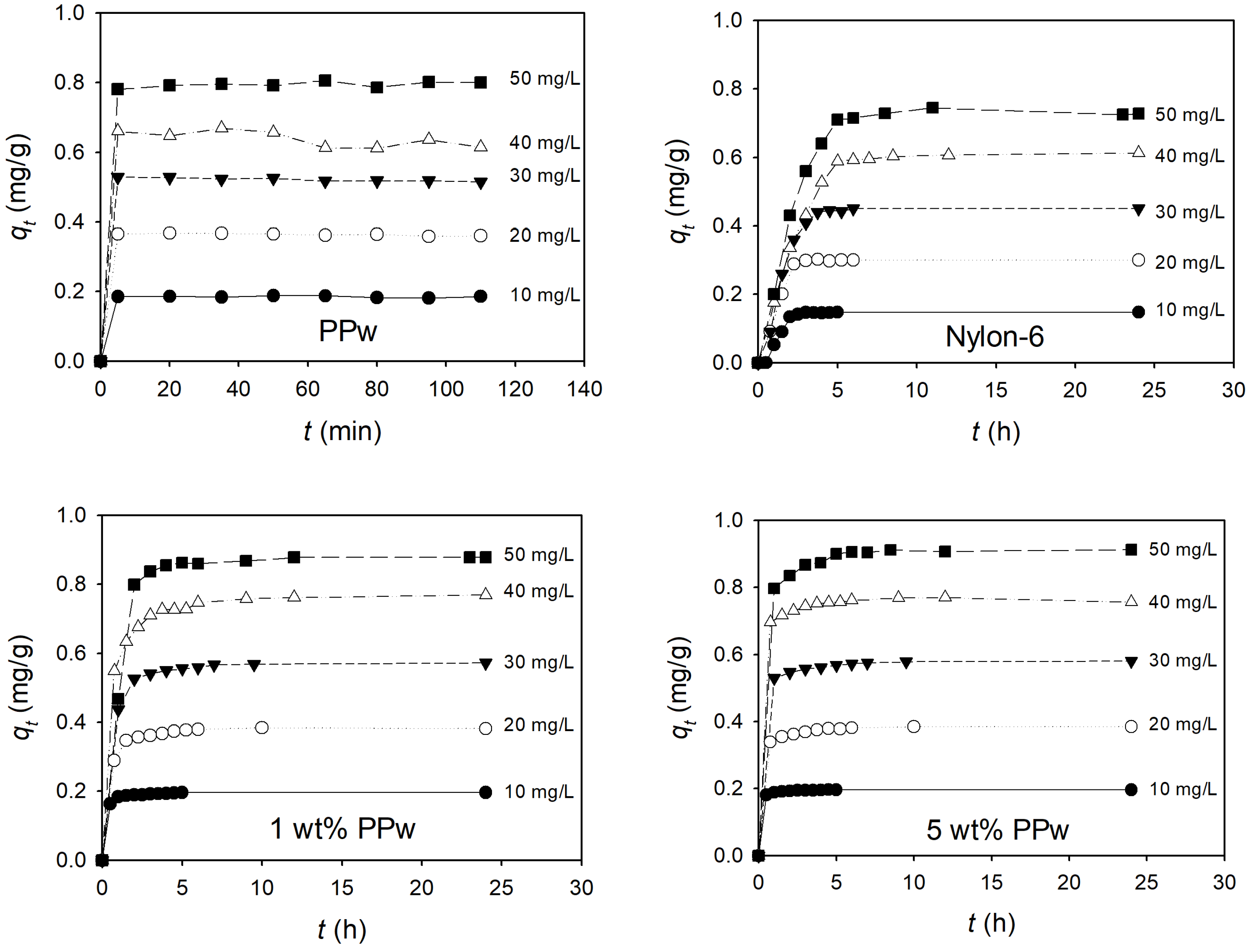
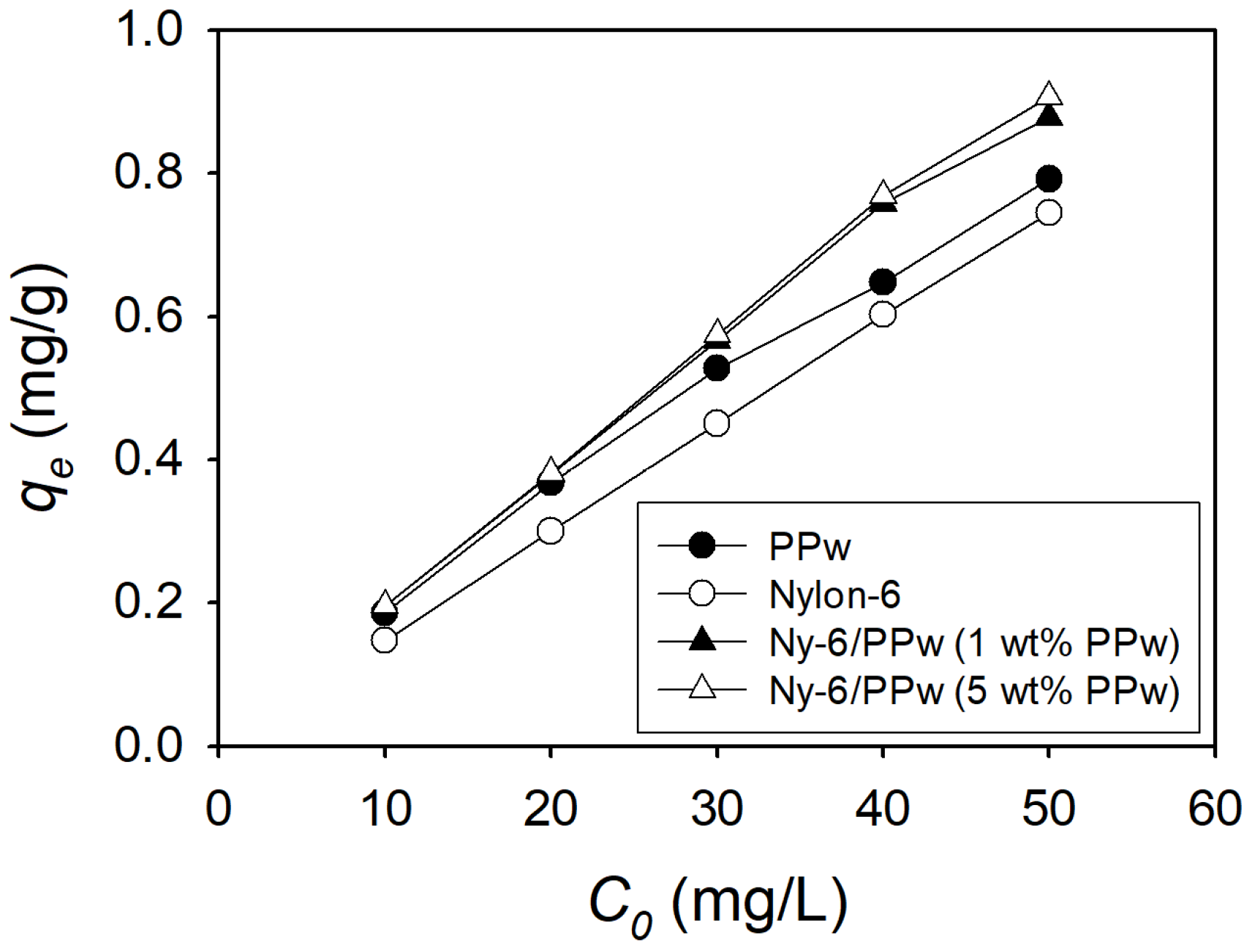
3.2.2. Adsorption Capacity
4. Discussion
4.1. Interpretation of Adsorption Behavior
4.2. Adsorption Mechanisms
4.3. Adsorption Comparison
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MB | Methylene Blue |
| PPw | Pumice Powder |
| Ny-6 | Nylon-6 (Polyamide-6) |
| NWF | Nonwoven Fabric |
| NbS | Nature-Based Solution |
| SDG | Sustainable Development Goal |
| SEM | Scanning Electron Microscopy |
| XRD | X-ray Diffraction |
| TGA | Thermogravimetric Analysis |
| qe | Amount of adsorbate adsorbed at equilibrium (mg/g) |
| qmax | Maximum adsorption capacity (mg/g) |
| Ce | Equilibrium concentration of adsorbate (mg/L) |
| Ct | Concentration at time t (mg/L) |
| KL | Langmuir constant related to adsorption affinity (L/mg) |
| KF | Freundlich constant indicating adsorption capacity ((mg/g)(L/mg)1⁄n) |
| n | Freundlich heterogeneity factor |
| R2 | Correlation coefficient |
| k1 | Pseudo-first-order constant rate (1/min) |
| K2 | Pseudo-second-order constant rate (g/(mg min)) |
| SDG 6 | Clean Water and Sanitation |
| SDG 12 | Responsible Consumption and Production |
References
- UNGA. The Human Right to Water and Sanitation: Resolution/Adopted by the General Assembly, A/RES/64/292. 2010. Available online: https://digitallibrary.un.org/record/687002?ln=es&v=pdf (accessed on 26 October 2025).
- Dickin, S.K.; Schuster-Wallace, C.J. Assessing changing vulnerability to dengue in northeastern Brazil using a water-associated disease index approach. Glob. Environ. Change-Hum. Policy Dimens. 2014, 29, 155–164. [Google Scholar] [CrossRef]
- Mendez, F.; Piedrahita-Gómez, L.E.; Toro, A.F.; Salazar-Benitez, J.; Zapata, H.; Pena, M. The invisibility of health effects associated with water pollution within disease burden estimates: Analysis from a Colombian Andean watershed. PLoS Water 2024, 3, e0000125. [Google Scholar] [CrossRef]
- Spring, U.O. Aquatic systems and water security in the Metropolitan Valley of Mexico City. Curr. Opin. Environ. Sustain. 2011, 3, 497–505. [Google Scholar] [CrossRef]
- Rubio-Arellano, A.B.; Ramos-Leal, J.A.; Almanza-Tovar, O.G.; Vázquez-Báez, V.M.; Morán-Ramírez, J. Assessment of aquifer vulnerability and water quality: Validated using groundwater flow origin-based regression analysis. Groundw. Sustain. Dev. 2025, 30, 101478. [Google Scholar] [CrossRef]
- Álvarez-Buylla Roces, M.E. 1er Informe Estratégico. Cuenca del Alto Atoyac (Tlaxcala y Puebla): Región de Emergencia Sanitaria y Ambiental; Problemática Socioambiental y Recomendaciones para su Atención Integral; CONAHCYT: Mexico City, Mexico, 2023. [Google Scholar]
- Hernández-Rodríguez, M.d.L. La contaminación del río Atoyac y sus efectos socioambientales en San Mateo Ayecac, Tlaxcala. In Río Atoyac: Hacía una Gestión Integral de una Problemática Multifactorial, 1st ed.; Ávila-Orta, C.A., Hernández-Rodríguez, M.d.L., Lozano-Morales, S.A., Eds.; El Colegio de Tlaxcala, A.C.: Tlaxcala, Mexico, 2021; p. 328. [Google Scholar]
- Metcalf & Eddy, Inc. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- Cohen-Shacham, E.; Andrade, A.; Dalton, J.; Dudley, N.; Jones, M.; Kumar, C.; Maginnis, S.; Maynard, S.; Nelson, C.R.; Renaud, F.G.; et al. Core principles for successfully implementing and upscaling Nature-based Solutions. Environ. Sci. Policy 2019, 98, 20–29. [Google Scholar] [CrossRef]
- Cohen-Shacham, E.; Janzen, C.; Maginnis, S.; Walters, G. Nature-Based Solutions to Address Global Societal Challenges; IUCN, Ed.; IUCN: Gland, Switzerland, 2016. [Google Scholar]
- Dumitru, A.; Wendling, L. Evaluating the Impact of Nature-Based Solutions—A Handbook for Practitioners; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- IUCN. Global Standard for Nature-Based Solutions. A User-Friendly Framework for the Verification, Design and Scaling up of NbS; IUCN: Gland, Switzerland, 2020. [Google Scholar]
- Wu, S.; Wallace, S.; Brix, H.; Kuschk, P.; Kirui, W.K.; Masi, F.; Dong, R. Treatment of industrial effluents in constructed wetlands: Challenges, operational strategies and overall performance. Environ. Pollut. 2015, 201, 107–120. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Rodríguez, S.-R.; Siebe, C.; Komorowski, J.-C.; Abrams, M. The Quetzalapa Pumice: A voluminous late Pleistocene rhyolite deposit in the eastern Trans-Mexican Volcanic Belt. J. Volcanol. Geotherm. Res. 2002, 113, 177–212. [Google Scholar] [CrossRef]
- de Rozari, P.; Krisnayanti, D.S.; Refli; Yordanis, K.V.; Atie, M.R.R. The use of pumice amended with sand media for domestic wastewater treatment in vertical flow constructed wetlands planted with lemongrass (Cymbopogon citratus). Heliyon 2021, 7, e07423. [Google Scholar] [CrossRef]
- Akbal, F. Adsorption of basic dyes from aqueous solution onto pumice powder. J. Colloid Interface Sci. 2005, 286, 455–458. [Google Scholar] [CrossRef]
- Çifçi, D.I.; Meriç, S. A review on pumice for water and wastewater treatment. Desalin. Water Treat. 2016, 57, 18131–18143. [Google Scholar] [CrossRef]
- Goldszal, A.; Bousquet, J. Wet agglomeration of powders: From physics toward process optimization. Powder Technol. 2001, 117, 221–231. [Google Scholar] [CrossRef]
- Wang, J.-P.; Gallo, E.; François, B.; Gabrieli, F.; Lambert, P. Capillary force and rupture of funicular liquid bridges between three spherical bodies. Powder Technol. 2017, 305, 89–98. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; Awwad, N.S.; Abdelgawad, M.A.; Wu, J.; Mo, X.; Gomha, S.M.; Aly, A.A.; Brase, S. Review of the Recent Advances in Electrospun Nanofibers Applications in Water Purification. Polymers 2022, 14, 1594. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Feng, H.; Li, D.; Xia, Z.; Yang, R. Flame Retardancy of Nylon 6 Fibers: A Review. Polymers 2023, 15, 2161. [Google Scholar] [CrossRef]
- Andrade-Guel, M.; Reyes-Rodríguez, P.Y.; Cabello-Alvarado, C.J.; Cadenas-Pliego, G.; Ávila-Orta, C.A. Influence of Modified Carbon Black on Nylon 6 Nonwoven Fabric and Performance as Adsorbent Material. Nanomaterials 2022, 12, 4247. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Orta, C.A.; Martínez-Colunga, J.G.; Bueno-Baqués, D.; Raudry-López, C.E.; Cruz-Delgado, V.J.; González-Morones, P.; Valdez-Garza, J.A.; Esparza-Juárez, M.E.; Espinoza-González, C.J.; Rodríguez-González, J.A. Proceso Continuo Asistido por Ultrasonido de Frecuencia y Amplitud Variable para la Preparacion de Nanocompuestos a Base de Polimeros y Nanoparticulas. MX Patent No. 323756, 8 April 2014. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Murphy, O.P.; Vashishtha, M.; Palanisamy, P.; Kumar, K.V. A Review on the Adsorption Isotherms and Design Calculations for the Optimization of Adsorbent Mass and Contact Time. ACS Omega 2023, 8, 17407–17430. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Hassani, A.H.; Karri, R.R.; Younesi, B.; Shayeghi, M.; Salari, M.; Zarei, A.; Yousefi, M.; Heidarinejad, Z. Process optimization and enhancement of pesticide adsorption by porous adsorbents by regression analysis and parametric modelling. Sci. Rep. 2021, 11, 11719. [Google Scholar] [CrossRef]
- Murthy, N.S.; Minor, H. General procedure for evaluating amorphous scattering and crystallinity from X-ray diffraction scans of semicrystalline polymers. Polymer 1990, 31, 996–1002. [Google Scholar] [CrossRef]
- Andrade-Guel, M.; Cabello-Alvarado, C.J.; Ávila Orta, C.A.; Cadenas-Pliego, G.; Cruz-Ortiz, B. Functional Technical Textile-Based Polymer Nanocomposites with Adsorbent Properties of Toxins and Dyes also Have Antibacterial Behavior. Materials 2024, 17, 3007. [Google Scholar] [CrossRef]
- Kua, T.L.; Kooh, M.R.R.; Dahri, M.K.; Zaidi, N.A.H.M.; Lu, Y.; Lim, L.B.L. Aquatic plant, Ipomoea aquatica, as a potential low-cost adsorbent for the effective removal of toxic methyl violet 2B dye. Appl. Water Sci. 2020, 10, 243. [Google Scholar] [CrossRef]
- Weber Walter, J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Le, T.P.; Luong, H.V.T.; Nguyen, H.N.; Pham, T.K.T.; Trinh Le, T.L.; Tran, T.B.Q.; Ngo, T.N.M. Insight into adsorption-desorption of methylene blue in water using zeolite NaY: Kinetic, isotherm and thermodynamic approaches. Results Surf. Interfaces 2024, 16, 100281. [Google Scholar] [CrossRef]
- Çifçi, D.I.; Meriç, S. Optimization of methylene blue adsorption by pumice powder. Adv. Environ. Res. 2016, 5, 37–50. [Google Scholar] [CrossRef]
- Du, J.; Luo, X.; Fu, Z.; Xu, C.; Ren, X.; Gao, W.; Li, Y. Improving the hydrophobicity of nylon fabric by consecutive treatment with poly(acrylic acid), tetraethylorthosilicate, and octadecylamine. J. Appl. Polym. Sci. 2015, 132, 42456. [Google Scholar] [CrossRef]
- Roy, S.; Subrata, G.; Bhowmick, N. Study the effect of denier and fiber cut length on zeta potential of nylon and polyester fibers for sustainable dyeing process. J. Environ. Res. Dev. 2017, 11, 392–397. [Google Scholar]
- Afolabi, O.A.; Ndou, N. Synergy of Hybrid Fillers for Emerging Composite and Nanocomposite Materials—A Review. Polymers 2024, 16, 1907. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Ali, S.; Zaman, W. Innovative Adsorbents for Pollutant Removal: Exploring the Latest Research and Applications. Molecules 2024, 29, 4317. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, M.; Taghizadeh, A.; Ghaedi, M. Chapter 2—Adsorbent. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 71–210. [Google Scholar]
- Ahmed, T.; Al Biruni, M.T.; Azad, S.; Hasan, M. Medical waste incineration fly ash as an effective adsorbent for removing dyes from textile effluent and methylene blue from synthetic aqueous solutions. Case Stud. Chem. Environ. Eng. 2024, 9, 100681. [Google Scholar] [CrossRef]
- Al-Asadi, S.T.; Al-Qaim, F.F.; Al-Saedi, H.F.S.; Deyab, I.F.; Kamyab, H.; Chelliapan, S. Adsorption of methylene blue dye from aqueous solution using low-cost adsorbent: Kinetic, isotherm adsorption, and thermodynamic studies. Environ. Monit Assess 2023, 195, 676. [Google Scholar] [CrossRef]
- An, J.; Nhung, N.T.H.; Ding, Y.; Chen, H.; He, C.; Wang, X.; Fujita, T. Chestnut Shell-Activated Carbon Mixed with Pyrolytic Snail Shells for Methylene Blue Adsorption. Materials 2022, 15, 8227. [Google Scholar] [CrossRef] [PubMed]
- Bo, C.; Jia, Z.; Liu, B.; Dai, X.; Ma, G.; Li, Y. Copolymer-type magnetic graphene oxide with dual-function for adsorption of variety of dyes. J. Taiwan Inst. Chem. Eng. 2022, 138, 104499. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Quesada, H.B.; Baptista, A.T.A.; Gomes, R.G.; Bergamasco, R. Soybean hulls as a low-cost biosorbent for removal of methylene blue contaminant. Environ. Prog. Sustain. Energy 2019, 39, e13328. [Google Scholar] [CrossRef]
- Hamri, N.; Imessaoudene, A.; Hadadi, A.; Cheikh, S.; Boukerroui, A.; Bollinger, J.-C.; Amrane, A.; Tahraoui, H.; Tran, H.N.; Ezzat, A.O.; et al. Enhanced Adsorption Capacity of Methylene Blue Dye onto Kaolin through Acid Treatment: Batch Adsorption and Machine Learning Studies. Water 2024, 16, 243. [Google Scholar] [CrossRef]
- Misran, E.; Pratama, W.; Napitupulu, K.I.K.; Supardan, M.D.; Iryani, D.A.; Pramananda, V. Ultrasonic-assisted adsorption of methylene blue using shrimp shells as a low-cost adsorbent: Evaluation on the adsorption isotherm, kinetics, and thermodynamics. S. Afr. J. Chem. Eng. 2025, 52, 111–126. [Google Scholar] [CrossRef]
- Ma, L.; Liu, W.; Liu, B.; Tang, Y. Removal of methylene blue by acrylic polymer adsorbents loaded with magnetic iron manganese oxides: Synthesis, characterization, and adsorption mechanisms. Chemosphere 2024, 346, 140588. [Google Scholar] [CrossRef]
- Noori, M.; Tahmasebpoor, M.; Foroutan, R. Enhanced adsorption capacity of low-cost magnetic clinoptilolite powders/beads for the effective removal of methylene blue: Adsorption and desorption studies. Mater. Chem. Phys. 2022, 278, 125655. [Google Scholar] [CrossRef]
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax | KL | R2 | n | KF | R2 | |
| PPw | 0.9718 ±0.0007 | 2.9707 ±0.0093 | 0.9830 | 1.9923 ±0.0021 | 0.5496 ±0.0003 | 0.9637 |
| NWF Ny-6 | −14.977 ±0.4151 | −261.36 ±6.9033 | 0.1816 | 0.9657 ±0.0007 | 0.2844 ±0.0002 | 0.9981 |
| NWF Ny-6/PPw, 1% PPw | 1.0631 ±0.0026 | 1.2885 ±0.0145 | 0.9644 | 2.1214 ±0.0159 | 0.6877 ±0.0012 | 0.9389 |
| NWF Ny-6/PPw, 5% PPw | 1.1469 ±0.0014 | 1.2213 ±0.0076 | 0.9414 | 1.9606 ±0.0037 | 0.7202 ±0.0005 | 0.9195 |
| Sample | Langmuir | Freundlich |
|---|---|---|
| PPw | Langmuir-Freundlich | Monolayer adsorption on silanol/aluminosilicate sites; electrostatic interactions |
| NWF Ny-6 | Freundlich | Weak physisorption on a heterogeneous surface; limited electrostatic affinity |
| NWF Ny-6/PPw, 1% PPw | Langmuir-Freundlich | Heterogeneous multilayer adsorption |
| NWF Ny-6/PPw, 5% PPw | Langmuir-Freundlich | Near-monolayer adsorption; surface homogenization via higher pumice content |
| Sample | qe | Pseudo-First-Order | Pseudo-Second-Order | ||
|---|---|---|---|---|---|
| k1 | R2 | k2 | R2 | ||
| PPw | |||||
| C0, 10 mg/L | 0.1862 | 0.0585 | 0.0393 | 0.0065 | 0.9993 |
| C0, 20 mg/L | 0.3674 | 0.0536 | 0.0606 | 0.0493 | 0.9999 |
| C0, 30 mg/L | 0.5274 | 0.0513 | 0.2429 | 0.1458 | 0.9999 |
| C0, 40 mg/L | 0.6476 | 0.0412 | 0.0068 | 0.2650 | 0.9981 |
| C0, 50 mg/L | 0.7918 | 0.1152 | 0.1288 | 0.5083 | 0.9998 |
| NWF Ny-6 | |||||
| C0, 10 mg/L | 0.1473 | 0.0081 | 0.4149 | 0.0032 | 0.9928 |
| C0, 20 mg/L | 0.3001 | 0.0007 | 0.5277 | 0.0269 | 0.9937 |
| C0, 30 mg/L | 0.4506 | 0.0097 | 0.6268 | 0.0906 | 0.9822 |
| C0, 40 mg/L | 0.6027 | 0.0078 | 0.5277 | 0.2522 | 0.9890 |
| C0, 50 mg/L | 0.7447 | 0.7447 | 0.5426 | 0.4008 | 0.9914 |
| NWF Ny-6/PPw, 1% PPw | |||||
| C0, 10 mg/L | 0.1959 | 0.0209 | 0.8513 | 0.0075 | 1.0000 |
| C0, 20 mg/L | 0.3787 | 0.0173 | 0.9445 | 0.0546 | 0.9999 |
| C0, 30 mg/L | 0.5665 | 0.0181 | 0.7401 | 0.1827 | 0.9999 |
| C0, 40 mg/L | 0.7575 | 0.0125 | 0.9149 | 0.4373 | 1.0000 |
| C0, 50 mg/L | 0.8777 | 0.0079 | 0.7871 | 0.6747 | 0.9993 |
| NWF Ny-6/PPw, 5% PPw | |||||
| C0, 10 mg/L | 0.1962 | 0.0276 | 0.8195 | 0.0076 | 1.0000 |
| C0, 20 mg/L | 0.3811 | 0.0185 | 0.8950 | 0.0557 | 1.0000 |
| C0, 30 mg/L | 0.5745 | 0.0166 | 0.8859 | 0.1909 | 1.0000 |
| C0, 40 mg/L | 0.7683 | 0.0049 | 0.2181 | 0.4485 | 0.9998 |
| C0, 50 mg/L | 0.9069 | 0.0148 | 0.9574 | 0.7483 | 0.9999 |
| Material | Experimental Conditions * | Maximum MB Adsorption Capacity (mg/g) | Ref |
|---|---|---|---|
| Medical Waste Incineration Fly Ash (MWIFA) | 40 min, pH 10–12,132 Pt-Co, 7.5 g/L, 300 rpm, room temperature | 48.78 | [42] |
| Fig leaf- activated carbon (FLAC-3) | 60 min, pH 7, 80 ppm, 0.032 g/L, --, room temperature | 41.7 | [43] |
| Chestnut Shell-AC | 600 min, pH 12, 1476 ppm, --, --, 25 °C | 1191 | [44] |
| Methacryloyloxyethyl -Fe3O4-GO | 50 min, pH 11, --, 1.5 g/L, --, -- | 205 | [45] |
| Soybean hulls | 180 min, pH 7, 50 ppm, 1 g/L, 120 rpm, 25 °C | 169.90 | [46] |
| Algerian kaolinite (acid treatment) | 24 h, unadjusted, 250 ppm, 100 g/L, 200 rpm, ~25 °C | 64.58 | [47] |
| Shrimp shells | 60 min, pH 8, 50 ppm, 10 g/L, --, 30 °C. | 17.6 | [48] |
| Acrylic polymers loaded with magnetic iron manganese oxides (AP/MIMO) | 120 min, pH 4–10, 1000 ppm, 0.5 g/L, 600 rpm, 50 °C. | 2611.23 | [49] |
| Magnetic clinoptilolite powder (Alg/Clin/Fe3O4) | 60 min, pH 10, 10 ppm, 1 g/L, --, 25 °C | 12.484 | [50] |
| PPw | 20 min, pH 7, 50 ppm, 50 g/L, 250 rpm, 25 °C | 0.9718 | This study |
| NWF Ny-6 | 11 h, pH 7, 50 ppm, 50 g/L, 250 rpm, 25 °C | −14.9977 | |
| NWF Ny-6/PPw, 1% PPw | 12 h, pH 7, 50 ppm, 50 g/L, 250 rpm, 25 °C | 1.0631 | |
| NWF Ny-6/PPw, 5% PPw | 12 h, pH 7, 50 ppm, 50 g/L, 250 rpm, 25 °C | 1.1460 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Orta, C.A.; Alvarado-Tenorio, G.; Ramírez-López, E.R.; Cadenas-Pliego, G.; Cruz-Delgado, V.J.; Hernández-Rodríguez, M.d.L.; Cano-Salazar, L.F.; Pérez-García, Y.; Pérez-Flores, F.; Sevilla-Vargas, K.I.; et al. Hybrid Nylon-6/Pumice Nonwoven Composites as Nature-Based Adsorbents for Methylene Blue Dye-Contaminated Wastewater: Insights into Monolayer and Multilayer Adsorption Mechanisms. Water 2025, 17, 3382. https://doi.org/10.3390/w17233382
Ávila-Orta CA, Alvarado-Tenorio G, Ramírez-López ER, Cadenas-Pliego G, Cruz-Delgado VJ, Hernández-Rodríguez MdL, Cano-Salazar LF, Pérez-García Y, Pérez-Flores F, Sevilla-Vargas KI, et al. Hybrid Nylon-6/Pumice Nonwoven Composites as Nature-Based Adsorbents for Methylene Blue Dye-Contaminated Wastewater: Insights into Monolayer and Multilayer Adsorption Mechanisms. Water. 2025; 17(23):3382. https://doi.org/10.3390/w17233382
Chicago/Turabian StyleÁvila-Orta, Carlos Alberto, Germán Alvarado-Tenorio, Erick Ricardo Ramírez-López, Gregorio Cadenas-Pliego, Víctor Javier Cruz-Delgado, María de Lourdes Hernández-Rodríguez, Lucía Fabiola Cano-Salazar, Yesenia Pérez-García, Fernando Pérez-Flores, Karla Itzel Sevilla-Vargas, and et al. 2025. "Hybrid Nylon-6/Pumice Nonwoven Composites as Nature-Based Adsorbents for Methylene Blue Dye-Contaminated Wastewater: Insights into Monolayer and Multilayer Adsorption Mechanisms" Water 17, no. 23: 3382. https://doi.org/10.3390/w17233382
APA StyleÁvila-Orta, C. A., Alvarado-Tenorio, G., Ramírez-López, E. R., Cadenas-Pliego, G., Cruz-Delgado, V. J., Hernández-Rodríguez, M. d. L., Cano-Salazar, L. F., Pérez-García, Y., Pérez-Flores, F., Sevilla-Vargas, K. I., & Soria-Argüello, G. (2025). Hybrid Nylon-6/Pumice Nonwoven Composites as Nature-Based Adsorbents for Methylene Blue Dye-Contaminated Wastewater: Insights into Monolayer and Multilayer Adsorption Mechanisms. Water, 17(23), 3382. https://doi.org/10.3390/w17233382











