Influence of Alkalinity Enhancement with Olivine or Steel Slag on a Bacterial Community in Activated Sludge Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. DNA Extraction and High-Throughput Sequencing
2.3. Statistical Analysis
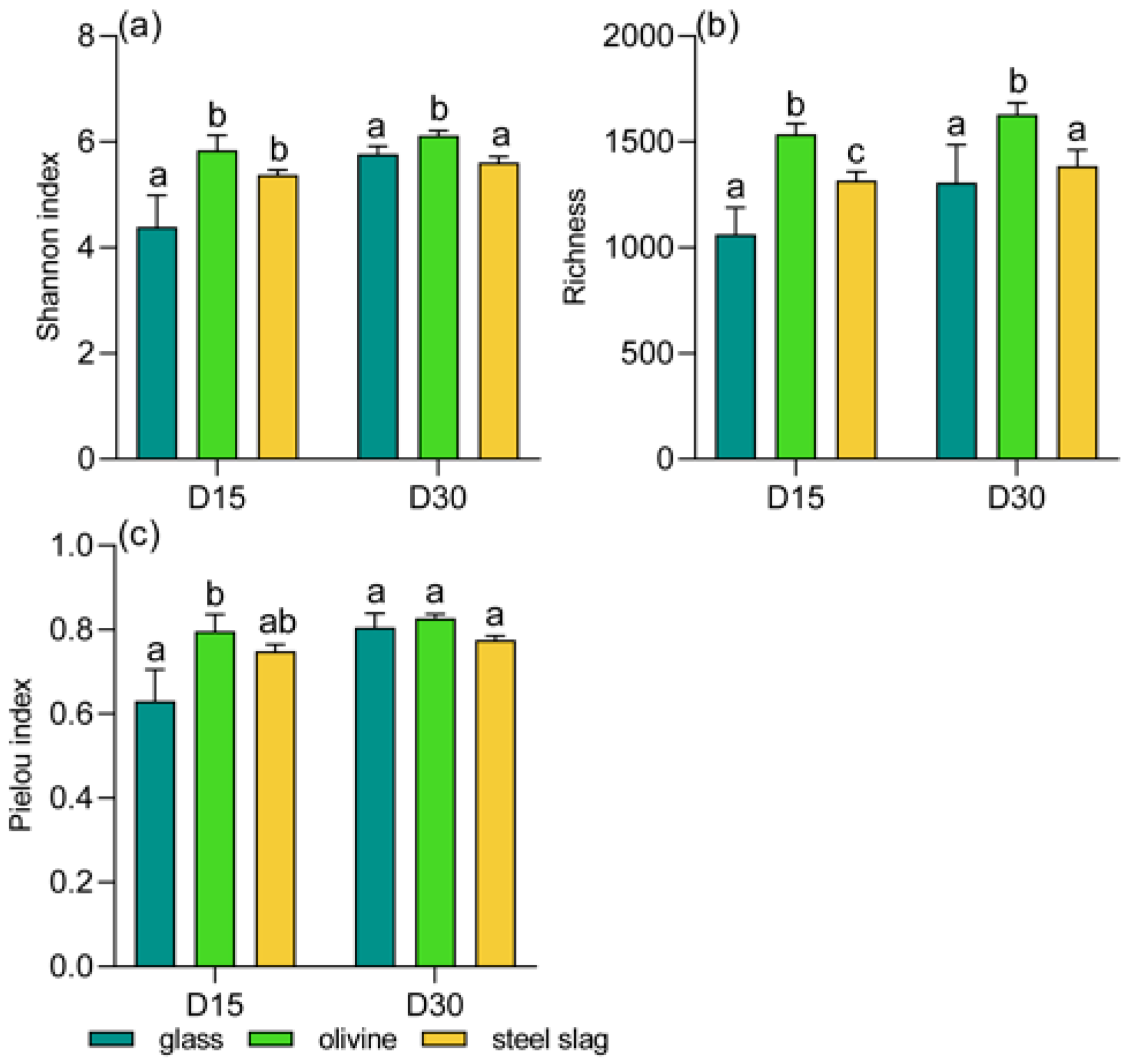
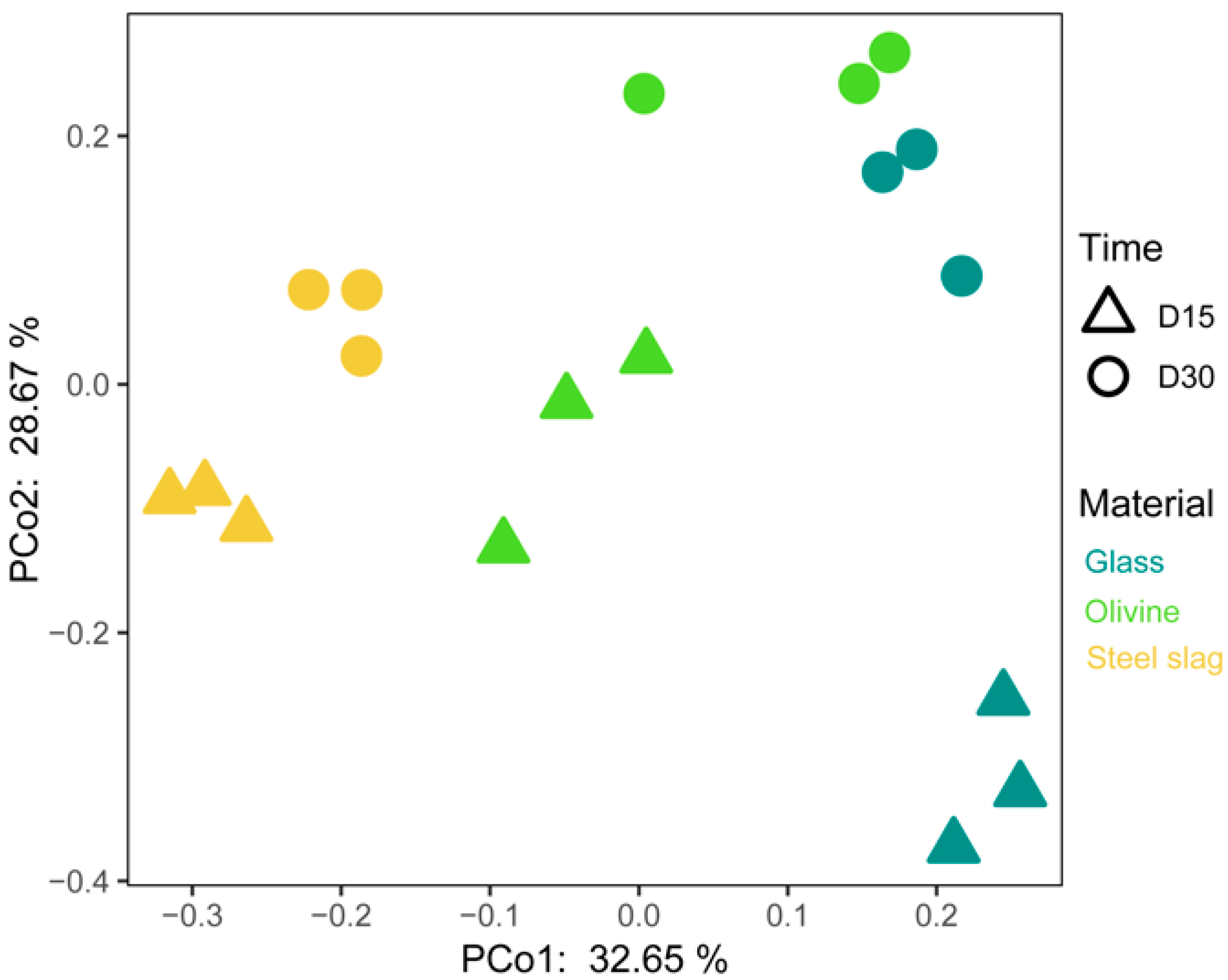
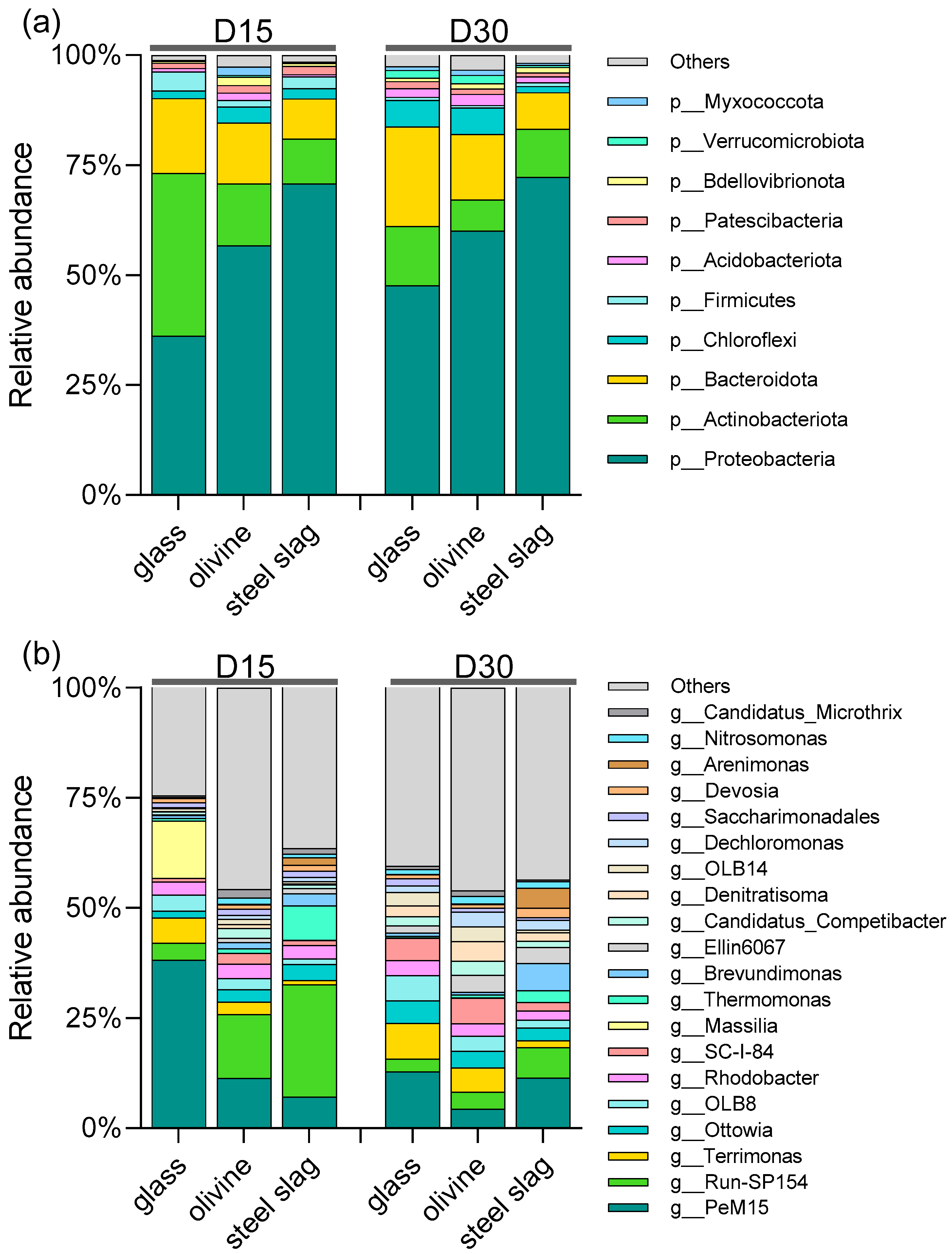
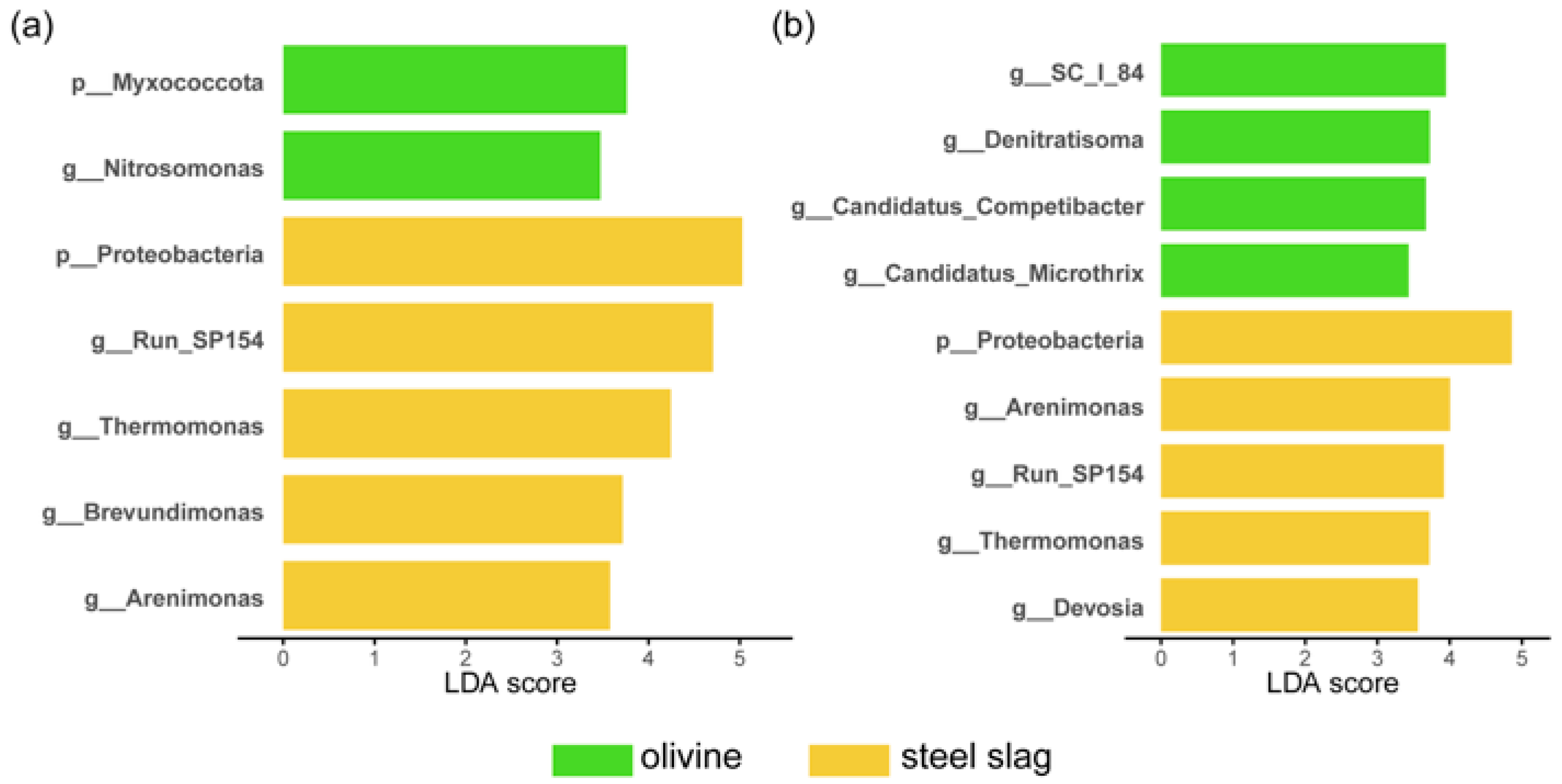
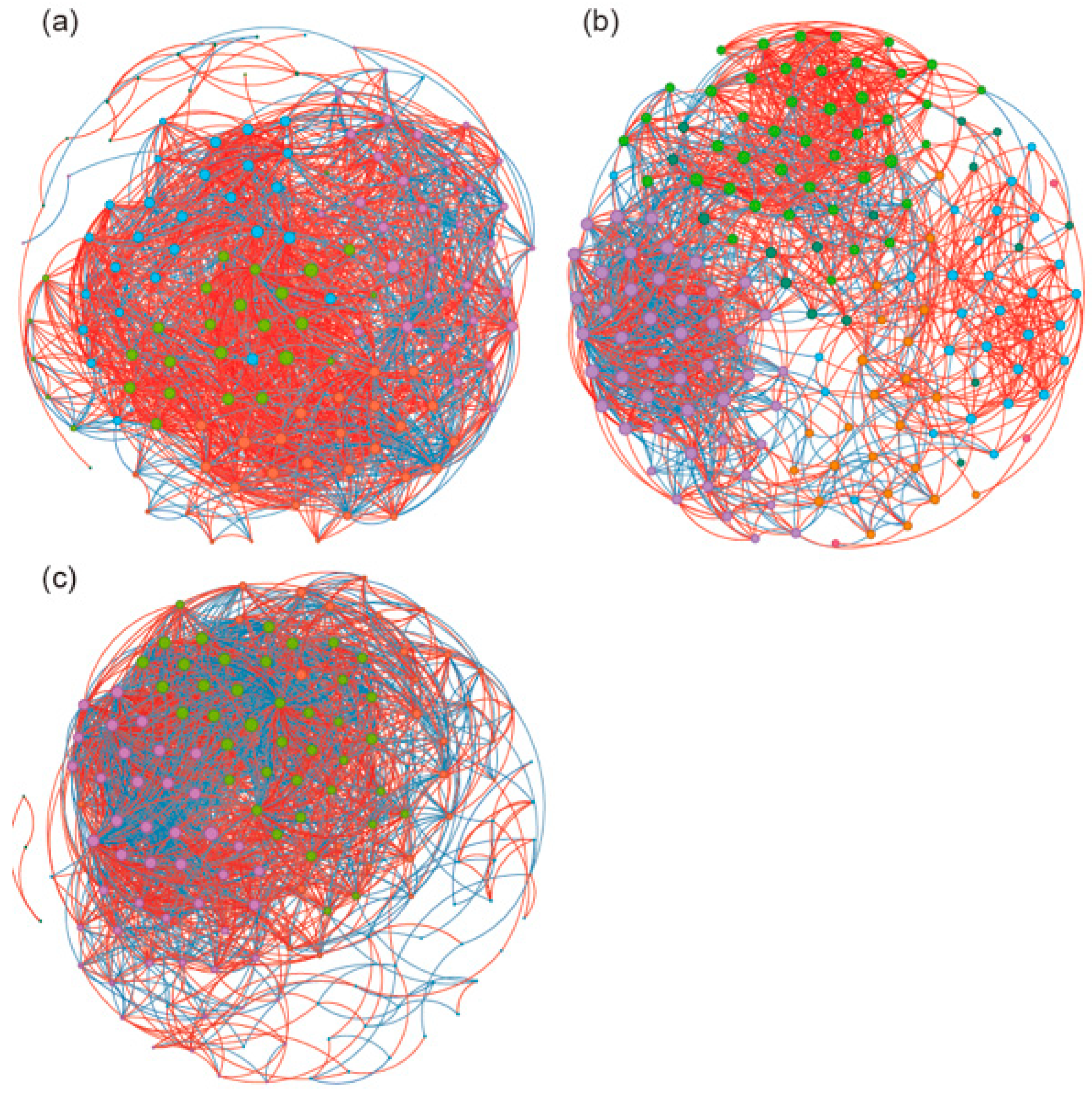
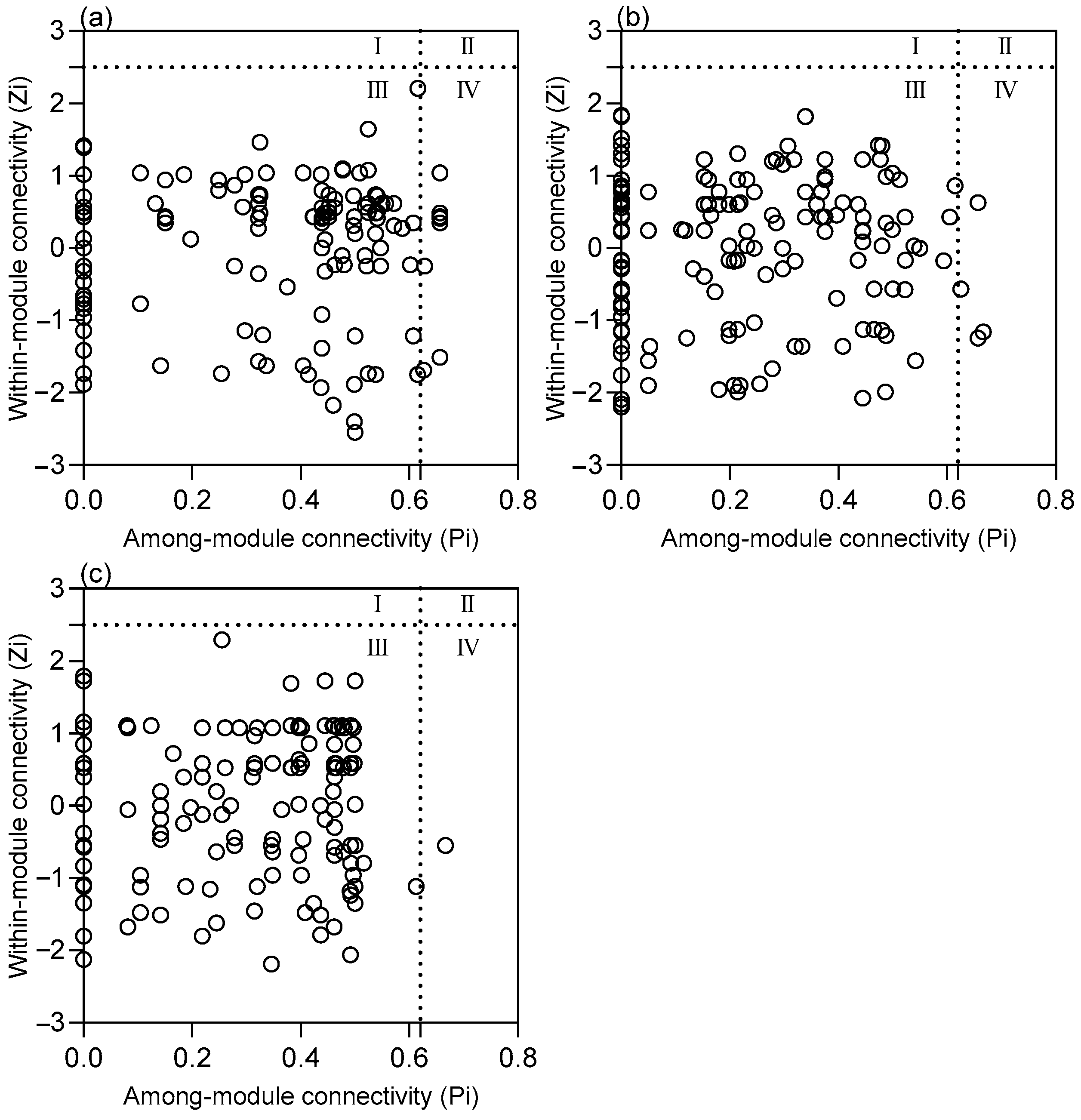
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CO2 | Carbon dioxide |
| TOC | Total organic carbon |
| PCoA | Principal coordinate analysis |
| ANOSIM | Analysis of similarities |
| LEfSe | Linear discriminant analysis effect size |
| Zi | Within-module connectivity |
| Pi | Among-module connectivity |
References
- Tseng, L.Y.; Robinson, A.K.; Zhang, X.; Xu, X.; Southon, J.; Hamilton, A.J.; Sobhani, R.; Stenstrom, M.K.; Rosso, D. Identification of Preferential Paths of Fossil Carbon within Water Resource Recovery Facilities via Radiocarbon Analysis. Environ. Sci. Technol. 2016, 50, 12166–12178. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.; Jacobsen, G.E.; Smith, A.M.; Yuan, Z.; Lant, P. Fossil organic carbon in wastewater and its fate in treatment plants. Water Res. 2013, 47, 5270–5281. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.G.; Townsend-Small, A.; Rosso, D. Impact of direct greenhouse gas emissions on the carbon footprint of water reclamation processes employing nitrification–denitrification. Sci. Total Environ. 2015, 505, 1166–1173. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, Y.; Su, B.; Chen, Q.; Liu, J. The potential of wastewater treatment on carbon storage through ocean alkalinity enhancement. Sci. Adv. 2025, 11, eads0313. [Google Scholar] [CrossRef]
- Guo, J.A.; Strzepek, R.F.; Swadling, K.M.; Townsend, A.T.; Bach, L.T. Influence of ocean alkalinity enhancement with olivine or steel slag on a coastal plankton community in Tasmania. Biogeosciences 2024, 21, 2335–2354. [Google Scholar] [CrossRef]
- Flipkens, G.; Fuhr, M.; Fiers, G.; Meysman, F.J.R.; Town, R.M.; Blust, R. Enhanced olivine dissolution in seawater through continuous grain collisions. Geochim. Cosmochim. Acta 2023, 359, 84–99. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Witkamp, G.-J.; Comans, R.N.J. Mineral CO2 Sequestration by Steel Slag Carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, F.; Renforth, P.; Hartmann, J.; Leermakers, M.; Knops, P.; Meysman, F.J. Olivine dissolution in seawater: Implications for CO2 sequestration through enhanced weathering in coastal environments. Environ. Sci. Technol. 2017, 51, 3960–3972. [Google Scholar] [CrossRef]
- Xu, D.; Liu, S.; Chen, Q.; Ni, J. Microbial community compositions in different functional zones of Carrousel oxidation ditch system for domestic wastewater treatment. AMB Express 2017, 7, 40. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Z.; Fang, W.; Gao, G. Composition of bacterial communities in municipal wastewater treatment plant. Sci. Total Environ. 2019, 689, 1181–1191. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.S.; Lalung, J.; Noman, E.A.; Bala, J.D.; Norli, I. Removal of heavy metals and antibiotics from treated sewage effluent by bacteria. Clean Technol. Environ. Policy 2015, 17, 2101–2123. [Google Scholar] [CrossRef]
- Ferderer, A.; Chase, Z.; Kennedy, F.; Schulz, K.G.; Bach, L.T. Assessing the influence of ocean alkalinity enhancement on a coastal phytoplankton community. Biogeosciences 2022, 19, 5375–5399. [Google Scholar] [CrossRef]
- Ren, H.; Hu, Y.; Liu, J.; Zhang, Z.; Mou, L.; Pan, Y.; Zheng, Q.; Li, G.; Jiao, N. Response of a coastal microbial community to olivine addition in the Muping Marine Ranch, Yantai. Front. Microbiol. 2022, 12, 805361. [Google Scholar] [CrossRef] [PubMed]
- Shirokova, L.S.; Benezeth, P.; Pokrovsky, O.S.; Gerard, E.; Menez, B.; Alfredsson, H. Effect of the heterotrophic bacterium Pseudomonas reactans on olivine dissolution kinetics and implications for CO2 storage in basalts. Geochim. Cosmochim. Acta 2012, 80, 30–50. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Ren, H.; Liu, J. Potential of CO2 sequestration by olivine addition in offshore waters: A ship-based deck incubation experiment. Mar. Environ. Res. 2024, 201, 106708. [Google Scholar] [CrossRef]
- Chun, S.-J.; Cui, Y.; Baek, S.H.; Ahn, C.-Y.; Oh, H.-M. Seasonal succession of microbes in different size-fractions and their modular structures determined by both macro- and micro-environmental filtering in dynamic coastal waters. Sci. Total Environ. 2021, 784, 147046. [Google Scholar] [CrossRef] [PubMed]
- Seviour, T.W.; Lambert, L.K.; Pijuan, M.; Yuan, Z. Selectively inducing the synthesis of a key structural exopolysaccharide in aerobic granules by enriching for Candidatus “Competibacter phosphatis”. Appl. Microbiol. Biotechnol. 2011, 92, 1297–1305. [Google Scholar] [CrossRef]
- McIlroy, S.J.; Nittami, T.; Seviour, E.M.; Seviour, R.J. Filamentous members of cluster III Defluviicoccus have the in situ phenotype expected of a glycogen-accumulating organism in activated sludge. FEMS Microbiol. Ecol. 2010, 74, 248–256. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, W.; Xu, Z.; Liu, F.; Feng, P.; Su, L.; Xu, C.; Zhu, S.; Wang, Z. Nitrogen and phosphorus removal by GAOs and PAOs using nitrate and limited oxygen as electron acceptors simultaneously and the impact of external carbon source in the anoxic phase. J. Environ. Chem. Eng. 2021, 9, 106520. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, X.; Wang, S.; Xiong, J.; Xie, C.; Chen, Y. Divalent manganese stimulates the removal of nitrate by anaerobic sludge. RSC Adv. 2024, 14, 2447–2452. [Google Scholar] [CrossRef]
- Li, J.; Zuo, X.; Chen, Q.; Lin, Y.; Meng, F. Genome-resolved metagenomic analysis reveals a novel denitrifier with truncated nitrite reduction pathway from the genus SC-I-84. Water Res. 2025, 282, 123598. [Google Scholar] [CrossRef]
- Wen, X.; Fan, A.; Wang, J.; Xia, Y.; Chen, S.; Yang, Y. Different Emergency Response Strategies to Oil Spills in Rivers Lead to Divergent Contamination Compositions and Microbial Community Response Characteristics. Microorganisms 2025, 13, 1193. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, Q.; She, Z.; Gao, M.; Zhao, Y.; Guo, L.; Jin, C. Nitrogen removal pathway and dynamics of microbial community with the increase of salinity in simultaneous nitrification and denitrification process. Sci. Total Environ. 2019, 697, 134047. [Google Scholar] [CrossRef] [PubMed]
- Nierychlo, M.; Singleton, C.M.; Petriglieri, F.; Thomsen, L.; Petersen, J.F.; Peces, M.; Kondrotaite, Z.; Dueholm, M.S.; Nielsen, P.H. Low Global Diversity of Candidatus Microthrix, a Troublesome Filamentous Organism in Full-Scale WWTPs. Front. Microbiol. 2021, 12, 690251. [Google Scholar] [CrossRef] [PubMed]
- Hesselsoe, M.; Nielsen, J.L.; Roslev, P.; Nielsen, P.H. Isotope Labeling and Microautoradiography of Active Heterotrophic Bacteria on the Basis of Assimilation of 14CO2. Appl. Environ. Microbiol. 2005, 71, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Maza-Márquez, P.; Castellano-Hinojosa, A.; González-Martínez, A.; Juárez-Jiménez, B.; González-López, J.; Rodelas, B. Abundance of total and metabolically active Candidatus Microthrix and fungal populations in three full-scale wastewater treatment plants. Chemosphere 2019, 232, 26–34. [Google Scholar] [CrossRef]
- Silyn-Roberts, G.; Lewis, G. In situ analysis of Nitrosomonas spp. in wastewater treatment wetland biofilms. Water Res. 2001, 35, 2731–2739. [Google Scholar] [CrossRef]
- Gómez-Acata, S.; Esquivel-Ríos, I.; Pérez-Sandoval, M.V.; Navarro-Noya, Y.; Rojas-Valdez, A.; Thalasso, F.; Luna-Guido, M.; Dendooven, L. Bacterial community structure within an activated sludge reactor added with phenolic compounds. Appl. Microbiol. Biotechnol. 2017, 101, 3405–3414. [Google Scholar] [CrossRef]
- Parente, C.E.T.; Brito, E.M.S.; Caretta, C.A.; Cervantes-Rodríguez, E.A.; Fábila-Canto, A.P.; Vollú, R.E.; Seldin, L.; Malm, O. Bacterial diversity changes in agricultural soils influenced by poultry litter fertilization. Braz. J. Microbiol. 2021, 52, 675–686. [Google Scholar] [CrossRef]
- Lyu, J.; Li, W.; Shi, T.; Zhu, C.; Yu, X.; Zheng, T.; Cao, Y.; Ma, Y.; Shi, Z.; Li, P.; et al. Characterization of biofilm bacterial communities in rural drainage system ancillary facilities. Results Eng. 2023, 20, 101623. [Google Scholar] [CrossRef]
- Sun, W.; Zheng, Z. Research on removal of fluoroquinolones in rural domestic wastewater by vertical flow constructed wetlands under different hydraulic loads. Chemosphere 2022, 303, 135100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, J.; Li, F.; Li, X. Performance of Denitrifying Microbial Fuel Cell with Biocathode over Nitrite. Front. Microbiol. 2016, 7, 344. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zeng, Q.; Liu, J.; Hou, Y.; Xu, J.; Li, H.; Shi, S.; Ma, F. Enhanced treatment performance of phenol wastewater and membrane antifouling by biochar-assisted EMBR. Bioresour. Technol. 2020, 306, 123147. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wei, S.; Chen, S.; Wang, K.; Holmes, D.E.; Yuan, Q. Anaerobic stabilization and landscape utilization of rural sewage sludge from the enhanced membrane coagulation process. Sci. Total Environ. 2025, 958, 177902. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, C.; Zhou, H.; Jiang, M.; Chen, G.; Wang, C.; Zhang, Z.; Zhao, X.; Jiang, L.-M.; Zhou, Z. Comparison on biological nutrient removal and microbial community between full-scale anaerobic/anoxic/aerobic process and its upgrading processes. Bioresour. Technol. 2023, 374, 128757. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Cheng, Y.; Jiang, M.; Li, X.; Xue, G. Iron Robustly Stimulates Simultaneous Nitrification and Denitrification Under Aerobic Conditions. Environ. Sci. Technol. 2018, 52, 1404–1412. [Google Scholar] [CrossRef]
- Fang, Y.-K.; Wang, H.-C.; Han, J.-L.; Li, Z.-L.; Wang, A.-J. Enhanced nitrogen removal of constructed wetlands by coupling with the bioelectrochemical system under low temperature: Performance and mechanism. J. Clean. Prod. 2022, 350, 131365. [Google Scholar] [CrossRef]
- Quan, X.; Zhang, H.; Liu, H.; Chen, L.; Li, N. Remediation of nitrogen polluted water using Fe-C microelectrolysis and biofiltration under mixotrophic conditions. Chemosphere 2020, 257, 127272. [Google Scholar] [CrossRef]
- Xing, W.; Li, J.; Li, D.; Hu, J.; Deng, S.; Cui, Y.; Yao, H. Stable-Isotope Probing Reveals the Activity and Function of Autotrophic and Heterotrophic Denitrifiers in Nitrate Removal from Organic-Limited Wastewater. Environ. Sci. Technol. 2018, 52, 7867–7875. [Google Scholar] [CrossRef]
- Parter, M.; Kashtan, N.; Alon, U. Environmental variability and modularity of bacterial metabolic networks. BMC Evol. Biol. 2007, 7, 169. [Google Scholar] [CrossRef]
- Girvan, M.S.; Campbell, C.D.; Killham, K.; Prosser, J.I.; Glover, L.A. Bacterial diversity promotes community stability and functional resilience after perturbation. Environ. Microbiol. 2005, 7, 301–313. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, Y.; Gong, Y.; Luo, W.; Tang, X. Extreme trophic tales: Deciphering bacterial diversity and potential functions in oligotrophic and hypereutrophic lakes. BMC Microbiol. 2024, 24, 348. [Google Scholar] [CrossRef] [PubMed]
- Flipkens, G.; Dujardin, V.; Salden, J.; T’Jollyn, K.; Town, R.M.; Blust, R. Olivine avoidance behaviour by marine gastropods (Littorina littorea L.) and amphipods (Gammarus locusta L.) within the context of ocean alkalinity enhancement. Ecotoxicol. Environ. Saf. 2024, 270, 115840. [Google Scholar] [CrossRef] [PubMed]
- Flipkens, G.; Horoba, K.; Bostyn, K.; Geerts, L.J.J.; Town, R.M.; Blust, R. Acute bioaccumulation and chronic toxicity of olivine in the marine amphipod Gammarus locusta. Aquat. Toxicol. 2023, 262, 106662. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Xie, G.; Liu, Y.; Liu, H.; Wang, S.; Yang, X.; Zhang, C.; Liu, X.; Zhang, L.; Liu, J.; et al. Influence of Alkalinity Enhancement with Olivine or Steel Slag on a Bacterial Community in Activated Sludge Systems. Water 2025, 17, 3355. https://doi.org/10.3390/w17233355
Ren H, Xie G, Liu Y, Liu H, Wang S, Yang X, Zhang C, Liu X, Zhang L, Liu J, et al. Influence of Alkalinity Enhancement with Olivine or Steel Slag on a Bacterial Community in Activated Sludge Systems. Water. 2025; 17(23):3355. https://doi.org/10.3390/w17233355
Chicago/Turabian StyleRen, Hongwei, Gang Xie, Yunjie Liu, Hua Liu, Suhua Wang, Xuena Yang, Chuanxing Zhang, Xingmin Liu, Lianbao Zhang, Jian Liu, and et al. 2025. "Influence of Alkalinity Enhancement with Olivine or Steel Slag on a Bacterial Community in Activated Sludge Systems" Water 17, no. 23: 3355. https://doi.org/10.3390/w17233355
APA StyleRen, H., Xie, G., Liu, Y., Liu, H., Wang, S., Yang, X., Zhang, C., Liu, X., Zhang, L., Liu, J., & Yu, X. (2025). Influence of Alkalinity Enhancement with Olivine or Steel Slag on a Bacterial Community in Activated Sludge Systems. Water, 17(23), 3355. https://doi.org/10.3390/w17233355






