A Complete Mobile Treatment Chain to Produce Drinking Water from Sources Heavily Contaminated by Inorganic and Organic Compounds
Abstract
1. Introduction
2. Materials and Methods
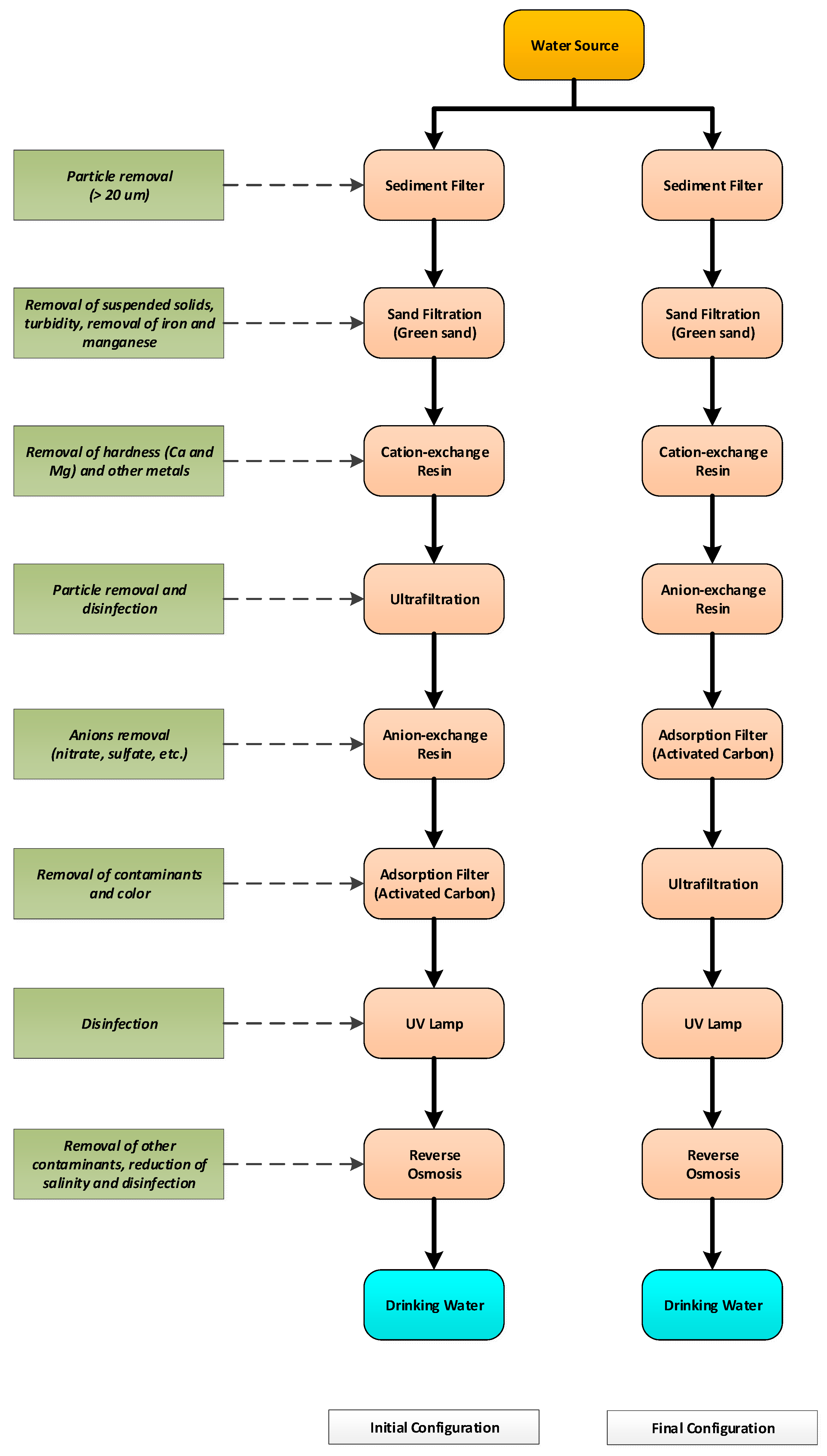
2.1. Description of the Pilot-Scale Drinking Water Production Unit
2.1.1. Sediment Filter
2.1.2. Sand Filter
2.1.3. Cation Exchange Resin
2.1.4. Ultrafiltration
2.1.5. Anion Exchange Resin
2.1.6. Adsorption Filter
2.1.7. UV Disinfection
2.1.8. Reverse Osmosis
2.2. Test Water Preparation and Feed for the Initial System Configuration
2.3. Test Water Preparation and Feed for the Final System Configuration
2.4. Analytical Methods
3. Results and Discussion
3.1. Evaluation of the Performance of the Initial System Configuration
3.1.1. Sediment Filter
3.1.2. Sand Filter
3.1.3. Cation Exchange Resin
3.1.4. Ultrafiltration
3.1.5. Anion Exchange Resin
3.1.6. Adsorption Filter
3.1.7. UV Disinfection
3.1.8. Reverse Osmosis
3.2. Evaluation of the Performance of the Final System Configuration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALARA | As low as reasonably achievable |
| AAHB | Aerobic and anaerobic heterotrophic bacteria |
| AO | Aesthetic objective |
| BTEX | Benzene, toluene, ethylbenzene and xylenes |
| BW30 | Brackish water 30 (a type of reverse osmosis membrane) |
| CFU | Colony-forming unit |
| COD | Chemical oxygen demand |
| DBP | Disinfection by-product |
| DOC | Dissolved organic carbon |
| DWC | Drinking water criteria |
| HAAs | Haloacetic acids |
| HMX | 1,3,5,7-tetranitro-1,3,5,7-tetrazocane |
| HP | Horsepower |
| ICP | Inductively couple plasma |
| INRS | Institut national de la recherche scientifique |
| MTBE | Methyl tert-butyl ether |
| NATO | North Atlantic treaty organization |
| NTU | Nephelometric turbidity unit |
| OG | Operational guidance value |
| PAH | Polycyclic aromatic hydrocarbons |
| PCP | Pentachlorophenol |
| PERC | Tetrachloroethylene |
| PES | Polyethersulfone |
| PFAS | Perfluoroalkyl substances |
| PPCP | Pharmaceuticals and personal care products |
| RDX | 1,3,5-trinitro-1,3,5-triazinane |
| RO | Reverse osmosis |
| ROWPU | Reverse osmosis water purification unit |
| RPM | Revolutions per minute |
| TDS | Total dissolved solids |
| THM | Trihalomethanes |
| TIC | Total inorganic carbon |
| TNT | Trinitrotoluene |
| TOC | Total organic carbon |
| TSS | Total suspended solids |
| UF | Ultrafiltration |
| UV | Ultraviolet |
| VOC | Volatile organic carbon |
| WHO | World health organization |
References
- Katiyar, V.; Tripathi, N.; Patwa, R.; Kotecha, P. Environment friendly packaging plastics. In Polymers for Packaging Applications; Apple Academic Press: Oakville, ON, Canada, 2014; Volume 115. [Google Scholar]
- Boone, C.; Chini, C.M. Comparative life cycle assessment of remote potable water supply for the Department of Defense. Sci. Total Environ. 2024, 949, 174732. [Google Scholar] [CrossRef] [PubMed]
- Gautam, B. Microbiological quality assessment (including antibiogram and threat assessment) of bottled water. Food Sci. Nutr. 2021, 9, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Rhodan, M. Bottled Water Company Issues Recall over Possible E. coli Contamination, TIME. 2015. Available online: https://time.com/3931649/niagara-bottled-water-e-coli/ (accessed on 1 July 2024).
- Kasper, M.R.; Lescano, A.G.; Lucas, C.; Gilles, D.; Biese, B.J.; Stolovitz, G.; Reaves, E.J. Diarrhea outbreak during U.S. military training in El Salvador. PLoS ONE 2012, 7, e40404. [Google Scholar] [CrossRef] [PubMed]
- Putnam, S.D.; Sanders, J.W.; Frenck, R.W.; Monteville, M.; Riddle, M.S.; Rockabrand, D.M.; Sharp, T.W.; Frankart, C.; Tribble, D.R. Self-reported description of diarrhea among military populations in operations Iraqi Freedom and Enduring Freedom. J. Travel Med. 2006, 13, 92–99. [Google Scholar] [CrossRef]
- Riddle, M.S.; Rockabrand, D.M.; Schlett, C.; Monteville, M.R.; Frenck, R.W.; Romine, M.; Ahmed, S.F.; Sanders, J.W. A prospective study of acute diarrhea in a cohort of United States military personnel on deployment to the Multinational Force and Observers, Sinai, Egypt. Am. J. Trop. Med. Hyg. 2011, 84, 59–64. [Google Scholar] [CrossRef]
- Cao, F.; Jaunat, J.; Sturchio, N.; Cancès, B.; Morvana, X.; Devos, A.; Barbin, V.; Ollivier, P. Worldwide occurrence and origin of perchlorate ion in waters: A review. Sci. Total Environ. 2019, 661, 737–749. [Google Scholar] [CrossRef]
- Clausen, J.; Robb, J.; Curry, D.; Korte, N. A case study of contaminants on military ranges: Camp Edwards, Massachusetts, USA. Environ. Pollut. 2004, 129, 13–21. [Google Scholar] [CrossRef]
- Bausinger, T.; Bonnaire, E.; Preuß, J. Exposure assessment of a burning ground for chemical ammunition on the Great War battlefields of Verdun. Sci. Total Environ. 2007, 382, 259–271. [Google Scholar] [CrossRef]
- Lafond, S.; Blais, J.F.; Mercier, G.; Martel, R. Counter-current acid leaching process for removal of Cu, Pb, Sb and Zn from shooting range soil. Environ. Technol. 2013, 34, 2377–2387. [Google Scholar] [CrossRef]
- Bhat, S.; Jacobs, J.M.; Hatfield, K.; Prenger, J. Relationships between stream water chemistry and military land use in forested watersheds in Fort Benning, Georgia. Ecol. Indic. 2006, 6, 458–466. [Google Scholar] [CrossRef]
- Iverson, R.M.; Hinckley, B.S.; Webb, R.M.; Hallet, B. Physical effects of vehicular disturbance on arid landscapes. Science 1981, 212, 915–917. [Google Scholar] [CrossRef]
- Whitecotton, R.C.A.; David, M.B.; Darmody, R.G.; Price, D.L. Impact of foot traffic from military training on soil and vegetation properties. Environ. Manag. 2000, 26, 697–706. [Google Scholar] [CrossRef]
- Dong, S.; Page, M.A.; Hur, A.; Hur, K.; Bokenkamp, K.V.; Wagner, E.D.; Plewa, M.J.; Massalha, N. Comparison of estrogenic, spectroscopic, and toxicological analyses of pilot-scale water, wastewaters, and processed wastewaters at select military installations. Environ. Sci. Technol. 2021, 55, 13103–13112. [Google Scholar] [CrossRef] [PubMed]
- Quist, M.C.; Fay, P.A.; Guy, C.S.; Knapp, A.K.; Rubenstein, B.N. Military training effects on terrestrial and aquatic communities on a grassland military installation. Ecol. Appl. 2003, 13, 432–442. [Google Scholar] [CrossRef]
- Stellmann, J.M.; Stellmann, S.D.; Christian, R.; Weber, T.; Tomasallo, C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature 2003, 422, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T.; Feldmann, D.; Reddersen, K.; Altmann, H.J.; Zimmermann, T. Production of drinking water from highly contaminated surface waters: Removal of organic. inorganic. and microbial contaminants applying mobile membrane filtration units. Acta Hydrochim. Hydrobiol. 2002, 30, 24–33. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ghosh, P. Nanofiltration and reverse osmosis membranes: Theory and application in separation of electrolytes. Rev. Chem. Eng. 2004, 20, 111–173. [Google Scholar] [CrossRef]
- Pawlak, Z.; Zak, S.; Zablocki, L. Removal of hazardous metals from groundwater by reverse osmosis. Pol. J. Environ. Stud. 2005, 15, 579–583. [Google Scholar]
- Bhattacharya, A. Remediation of pesticides polluted water through membranes. Sep. Purif. Rev. 2006, 35, 1–38. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.H.; Yu, M.; Heo, M.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Banks, D.; Jun, M.B.; Heo, J.; Her, N.; Park, C.M.; Yoon, Y. Selected advanced water treatment technologies for perfluoroalkyl and polyfluoroalkyl substances: A review. Sep. Purif. Technol. 2020, 231, 115929. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Hosamani, K.M.; Aminabhavi, T.M. Nanofiltration and reverse osmosis thin film composite membrane module for the removal of dye and salts from the simulated mixtures. Desalination 2009, 249, 12–17. [Google Scholar] [CrossRef]
- Ye, L.; You, H.; Su, H. Water treatment technologies for perchlorate: A review. Desalination 2012, 298, 1–12. [Google Scholar] [CrossRef]
- Chen, C.; Guo, L.; Yang, Y.; Oguma, K.; Hou, L. Comparative effectiveness of membrane technologies and disinfection methods for virus elimination in water: A review. Sci. Total Environ. 2021, 801, 149678. [Google Scholar] [CrossRef]
- Park, S.K.; Hu, J.Y. Assessment of the extent of bacterial growth in reverse osmosis system for improving water quality. J. Environ. Sci. Health Part A 2010, 45, 968–977. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, W.; Zhang, W.; Wang, C.; Wang, P. Life cycle assessment of water supply alternatives in water-receiving areas of the south-to-north water diversion project in China. Water Res. 2016, 89, 9–19. [Google Scholar] [CrossRef]
- Tarpani, R.R.Z.; Lapolli, F.R.; Recio, M.A.L.; Gallego-Schmid, A. Comparative life cycle assessment of three alternative techniques for increasing potable water supply in cities in the global south. J. Clean. Prod. 2021, 290, 125871. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Huang, Y.; Nie, Z.; Murray, A.; Li, Y.; Woods-Chabane, G.; Hofmann, R. Experimental validation of a test to estimate the remaining adsorption capacity of granular activated carbon for taste and odour compounds. Environ. Sci. Water Res. Technol. 2019, 5, 609–617. [Google Scholar] [CrossRef]
- Altowayti, W.A.H.; Othman, N.; Shahir, S.; Alshalif, A.F.; Al-Gheethi, A.A.; Al-Towayti, F.A.H.; Saleh, Z.M.; Haris, S.A. Removal of arsenic from wastewater by using different technologies and adsorbents: A review. Int. J. Environ. Sci. Technol. 2022, 19, 9243–9266. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Sakhiya, A.K.; Anand, A.; Pant, K.K.; Kaushal, P. A state-of-the-art review of various adsorption media employed for the removal of toxic polycyclic aromatic hydrocarbons (PAHs): An approach towards a cleaner environment. J. Water Process Eng. 2022, 47, 102674. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Al-Ghouti, M.A.; Kasak, P.; Krupa, I. An updated review on boron removal from water through adsorption processes. Emergent Mater. 2021, 4, 1167–1186. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef]
- Humbert, H.; Gallard, H.; Suty, H.; Croué, J.P. Natural organic matter and pesticides removal using a combination of ion exchange resin and powdered activated carbon (PAC). Water Res. 2008, 42, 1635–1643. [Google Scholar] [CrossRef]
- Yu, B.; Yuan, Z.; Yu, Z.; Xue-Song, F. BTEX in the environment: An update on sources, fate, distribution, pretreatment, analysis, and removal techniques. Chem. Eng. J. 2022, 435, 134825. [Google Scholar] [CrossRef]
- Gul, A.; Ma’amor, A.; Khaligh, N.G.; Julkapli, N.M. Recent advancements in the applications of activated carbon for the heavy metals and dyes removal. Chem. Eng. Res. Des. 2022, 186, 276–299. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of currently available PFAS remediation technologies in water: A review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef]
- El Bouaidi, W.; Enaime, G.; Loudiki, M.; Yaacoubi, A.; Douma, M.; Ounas, A.; Lubken, M. Adsorbents used for microcystin removal from water sources: Current knowledge and future prospects. Processes 2022, 10, 1235. [Google Scholar] [CrossRef]
- Hoslett, J.; Massara, T.M.; Malamis, S.; Ahmad, D.; van den Boogaert, I.; Katsou, E.; Ahmad, B.; Ghazal, H.; Simons, S.; Wrobel, L.; et al. Surface water filtration using granular media and membranes: A review. Sci. Total Environ. 2018, 639, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.E.; Farquharson, E.M.; Hedman, P.C.; Chiu, P. Removal of munition constituents in stormwater runoff: Screening of native and cationized cellulosic sorbents for removal of insensitive munition constituents NTO, DNAN, and NQ, and legacy munition constituents HMX, RDX, TNT, and perchlorate. J. Hazard. Mater. 2022, 424, 127335. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Sorial, G.A. Treatment of perchlorate in drinking water: A critical review. Sep. Purif. Technol. 2009, 69, 7–21. [Google Scholar] [CrossRef]
- Paredes, L.; Fernandez-Fontaina, E.; Lema, J.M.; Omil, F.; Carballa, M. Understanding the fate of organic micropollutants in sand and granular activated carbon biofiltration systems. Sci. Total Environ. 2016, 551–552, 640–648. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Patel, N.; Chaudhary, V.K. Disinfection by-products in drinking water: Occurrence. toxicity and abatement. Environ. Pollut. 2020, 267, 115724. [Google Scholar] [CrossRef]
- Sincero, A.P.; Sincero, G.A. Physical-Chemical Treatment of Water and Wastewater; CRC Press: Boca Raton, FL, USA, 2002; 856p. [Google Scholar] [CrossRef]
- Korak, J.A.; Mungan, A.L.; Watts, L.T. Critical review of waste brine management strategies for drinking water treatment using strong base ion exchange. J. Hazard. Mater. 2023, 441, 129473. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses. bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
- Jamaly, S.; Darwish, N.N.; Amed, I.; Hasan, S.W. A short review on reverse osmosis pretreatment technologies. Desalination 2014, 354, 30–38. [Google Scholar] [CrossRef]
- Health Canada, Guidelines for Canadian Drinking Water Quality—Summary Table, Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, ON, Canada. 2022. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewh-semt/alt_formats/pdf/pubs/water-eau/sum_guide-res_recom/summary-tables-sept-2022-eng.pdf (accessed on 1 July 2024).
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; 541p, ISBN 978-92-4-154995-0. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 1 July 2024).
- Levchuk, I.; Marquez, J.J.R.; Sillanpaa, M. Removal of natural organic matter (NOM) from water by ion exchange—A review. Chemosphere 2018, 192, 90–104. [Google Scholar] [CrossRef]
- Hu, J.; Martin, A.; Shang, R.; Siegers, W.; Cornelissen, E.; Hejman, B.; Rietveld, L. Anionic exchange for NOM removal and the effects on micropollutant adsorption competition on activated carbon. Sep. Purif. Technol. 2014, 129, 25–31. [Google Scholar] [CrossRef]
- Apell, J.N.; Boyer, T.H. Combined ion exchange treatment for removal of dissolved organic matter and hardness. Water Res. 2010, 44, 2419–2430. [Google Scholar] [CrossRef]
- Kumar, K.S.; Kavitha, S.; Parameswari, K.; Sakunthala, A. Environmental occurrence, toxicity and remediation of perchlorate—A review. Chemosphere 2023, 311, 137017. [Google Scholar] [CrossRef]
- Hu, Z.; Jia, Y.; Wu, Y.; Zhang, Y. Occurrence and removal technologies of perchlorate in water: A systematic review and bibliometric analysis. Chemosphere 2024, 364, 143119. [Google Scholar] [CrossRef]
- CEAEQ. Centre D’expertise en Analyse Environnementale du Québec, Méthode D’analyse, Ministère de l’Environnement et Lutte Contre les Changements Climatiques, Gouvernement du Québec, Québec, QC, Canada. 2023. Available online: https://www.ceaeq.gouv.qc.ca/methodes/methode_numer.htm (accessed on 1 July 2024).
- US EPA. United States Environmental Protection, Method EPA 6010B, Inductively Coupled Plasma Atomic Emission Spectrometry. 1996. Available online: https://archive.epa.gov/epawaste/hazard/testmethods/web/html/6_series.html (accessed on 1 July 2024).
- US EPA. United States Environmental Protection, Method EPA 8330B, Nitroaromatics, Nitramines, and Nitrate Esters by High Performance Liquid Chromatography (HPLC). 2006. Available online: https://www.epa.gov/esam/epa-method-8330b-sw-846-nitroaromatics-nitramines-and-nitrate-esters-high-performance-liquid (accessed on 1 July 2024).
| Parameters | Units | Raw Water | Sediment Filter | Sand Filtration | Cation Exchange Resin | Ultra-Filtration | Anion Exchange Resin | Adsorption Filter | UV Lamp | Reverse Osmosis | Global Removal (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 6.66 ± 0.12 | 6.66 ± 0.12 | 6.61 ± 0.23 | 6.91 ± 0.39 | 6.91 ± 0.12 | 8.89 ± 0.03 | 9.58 ± 0.74 | 9.65 ± 0.69 | 7.87 | ND | |
| Conductivity | mS/cm | 1.98 ± 0.02 | 1.95 ± 0.10 | 1.94 ± 0.02 | 2.02 ± 0.02 | 2.04 ± 0.02 | 2.06 ± 0.02 | 1.91 ± 0.17 | 1.95 ± 0.23 | 0.08 | 96.0 |
| Turbidity | NTU | 54.9 ± 2.9 | 46.5 ± 16.0 | 5.42 ± 3.46 | 2.45 ± 0.33 | 0.36 ± 0.21 | 0.35 ± 0.19 | 0.94 ± 1.11 | 0.90 ± 0.96 | 0.82 | 98.5 |
| COD | mgO2/L | 41.9 ± 1.6 | ND | ND | ND | ND | ND | <5 | <5 | <5 | >88.1 |
| DOC | mg/L | 15.0 ± 5.2 | ND | ND | ND | ND | 9.40 ± 1.56 | 4.61 ± 3.80 | 6.10 ± 5.95 | 2.30 | 84.7 |
| TSS | mg/L | 85 ± 6 | 72 ± 17 | 5.0 ± 4.2 | ND | ND | ND | ND | <1 | <1 | >98.8 |
| TDS | mg/L | 1420 ± 112 | 1593 ± 33 | 1519 ± 43 | ND | ND | ND | ND | 973 ± 310 | <5 | >99.9 |
| Chloride | mg/L | 99.0 ± 2.7 | ND | ND | ND | 96.0 ± 3.0 | 506 ± 13 | 467 ± 69 | 458 ± 50 | 1.90 | 98.1 |
| Cyanide | mg/L | 0.21 ± 0.02 | ND | ND | ND | 0.91 ± 0.01 | 0.08 ± 0.10 | <0.005 | <0.005 | <0.005 | >97.6 |
| Fluoride | mg/L | 1.02 ± 0.01 | ND | ND | ND | 0.28 ± 0.10 | <0.002 | <0.002 | 0.20 ± 0.03 | <0.002 | >99.8 |
| Nitrate | mg/L | 473 ± 7 | ND | ND | ND | 466 ± 1 | 15.5 ± 11.8 | 4.20 ± 0.50 | 5.19 ± 1.05 | 0.75 | 99.8 |
| Nitrite | mg/L | 33.2 ± 0.7 | ND | ND | ND | 29.0 ± 2.8 | 0.20 ± 0.05 | 2.85 ± 0.42 | 2.88 ± 0.17 | <0.003 | >99.9 |
| Sulfate | mg/L | 445 ± 4 | ND | ND | ND | 437 ± 6 | 276 ± 45 | 240 ± 49 | 231 ± 67 | 1.15 | 99.7 |
| Ammonium | mg/L | <0.007 | ND | ND | ND | ND | ND | <0.007 | <0.007 | <0.007 | ND |
| Aluminum | mg/L | 0.81 ± 0.04 | 0.68 ± 0.11 | <0.1 | <0.1 | <0.1 | ND | 0.70 ± 0.11 | 0.72 ± 0.05 | <0.1 | >87.7 |
| Antimony | µg/L | 40.0 ± 10.0 | ND | ND | ND | ND | ND | ND | <1 | <1 | >97.1 |
| Arsenic | µg/L | 40 ± 20 | ND | ND | ND | ND | ND | ND | <0.6 | <0.6 | >98.5 |
| Boron | mg/L | 9.89 ± 0.05 | 9.81 ± 0.17 | 9.16 ± 0.04 | 9.12 ± 0.15 | 8.99 ± 0.06 | ND | 1.17 ± 1.61 | 1.03 ± 1.41 | <0.08 | >99.2 |
| Barium | mg/L | 8.94 ± 0.97 | 6.78 ± 0.26 | 0.17 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.00 | ND | 0.06 ± 0.02 | 0.06 ± 0.01 | <0.006 | >99.7 |
| Beryllium | µg/L | 120 ± 10 | 120 ± 0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | ND | 3.0 ± 1.0 | 2.0 ± 1.0 | <0.2 | >99.8 |
| Cadmium | µg/L | 110 ± 10 | 120 ± 0 | 10 ± 0 | 10 ± 0 | 10 ± 0 | ND | <1 | <1 | <1 | >99.1 |
| Calcium | mg/L | 122 ± 1 | 120 ± 3 | 111 ± 6 | <0.03 | <0.03 | ND | 2.62 ± 1.24 | 2.82 ± 1.11 | <0.03 | >99.9 |
| Chromium | µg/L | 500 ± 10 | 500 ± 0 | 350 ± 20 | 350 ± 30 | 350 ± 20 | ND | <0.5 | 0.9 | <0.5 | >99.9 |
| Copper | mg/L | 20.6 ± 0.2 | 19.6 ± 0.2 | 0.11 ± 0.01 | <0.04 | <0.04 | ND | <0.04 | <0.04 | <0.04 | >99.9 |
| Iron | mg/L | 0.76 ± 0.06 | 0.65 ± 0.09 | 0.03 ± 0.01 | <0.03 | <0.03 | ND | <0.03 | <0.03 | <0.03 | >96.1 |
| Lead | µg/L | 60 | ND | ND | ND | ND | ND | <0.8 | <0.8 | <0.8 | >98.7 |
| Magnesium | mg/L | 16.6 ± 0.2 | 16.6 ± 0.3 | 16.0 ± 0.3 | 0.1 ± 0.0 | 0.11 ± 0.01 | ND | 0.35 ± 0.17 | 0.45 ± 0.24 | 0.02 | 99.9 |
| Manganese | mg/L | 1.22 ± 0.01 | 1.22 ± 0.04 | 0.17 ± 0.04 | <0.01 | <0.01 | ND | <0.01 | 0.02 ± 0.02 | <0.01 | >99.2 |
| Mercury | µg/L | 10.0 ± 1.0 | ND | ND | ND | ND | ND | ND | <0.001 | <0.001 | >99.9 |
| Molybdenum | µg/L | 760 ± 60 | 760 ± 100 | <1 | <1 | <1 | ND | <1 | <1 | <1 | >99.9 |
| Nickel | mg/L | 0.69 ± 0.01 | 0.69 ± 0.03 | 0.52 ± 0.06 | <0.001 | <0.001 | ND | <0.001 | <0.001 | <0.001 | >99.9 |
| Selenium | mg/L | 0.49 ± 0.01 | ND | ND | ND | ND | ND | <0.02 | <0.02 | <0.02 | >95.9 |
| Sodium | mg/L | 169 ± 2 | 169 ± 4 | 158 ± 0 | 456 ± 1 | 449 ± 1 | ND | 421 ± 27 | 413 ± 43 | <0.1 | >99.9 |
| Strontium | mg/L | 68.2 ± 1.5 | 65.8 ± 1.1 | 54.7 ± 1.4 | <0.02 | <0.02 | ND | <0.02 | 0.053 | <0.02 | >99.9 |
| Zinc | mg/L | 54.8 ± 0.4 | 54.9 ± 1.6 | 28.9 ± 10.4 | <0.03 | <0.03 | ND | <0.03 | <0.03 | <0.03 | >99.9 |
| Pentachlorophenol | µg/L | 591 ± 22 | ND | ND | ND | ND | 3.63 ± 0.23 | 1.31 ± 0.34 | 1.23 ± 0.21 | 0.98 | 99.8 |
| Atrazine | µg/L | 82.7 ± 5.6 | ND | ND | ND | ND | 45.8 ± 19.5 | <0.5 | <0.5 | <0.5 | >99.4 |
| 1,2-dichlorobenzene | µg/L | 1633 ± 666 | ND | ND | ND | ND | 5.6 ± 2.1 | <0.4 | <0.4 | <0.4 | >99.9 |
| 1,2-dichloroethane | µg/L | 68.3 ± 14.2 | ND | ND | ND | ND | 12.5 ± 14.9 | <0.4 | <0.4 | <0.4 | >99.4 |
| Ethylbenzene | µg/L | 1967 ± 723 | ND | ND | ND | ND | 106 ± 147 | <0.4 | <0.4 | <0.4 | >99.9 |
| Tetrachloroethene | µg/L | 113 ± 33 | ND | ND | ND | ND | <2.0 | <0.4 | <0.4 | <0.4 | >99.6 |
| Toluene | µg/L | 460 ± 89 | ND | ND | ND | ND | 41.0 ± 55.2 | <0.4 | <0.4 | <0.4 | >99.9 |
| Trichloroethene | µg/L | 73.0 ± 11.8 | ND | ND | ND | ND | <2.0 | <0.1 | <0.1 | <0.1 | >99.9 |
| Xylenes | µg/L | 730 ± 108 | ND | ND | ND | ND | 57.5 ± 78.5 | <0.1 | <0.1 | <0.1 | >99.9 |
| Parameters | Units | Raw Water | Sediment Filter | Sand Filtration | Cation Exchange Resin | Anion Exchange Resin | Adsorption Filter | Ultra-Filtration | UV Lamp | Reverse Osmosis | Global Removal (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 7.58 | 7.79 ± 0.10 | 7.74 ± 0.10 | 8.01 ± 0.07 | 8.03 ± 0.16 | 8.06 ± 0.21 | 7.99 ± 0.21 | 8.07 ± 0.23 | 7.05 ± 0.43 | ND | |
| Conductivity | mS/cm | 1.53 | 1.53 ± 0.00 | 1.53 ± 0.01 | 1.54 ± 0.02 | 1.53 ± 0.02 | 1.54 ± 0.02 | 1.52 ± 0.02 | 1.53 ± 0.02 | 0.02 ± 0.01 | 98.7 |
| Turbidity | NTU | 3.76 | 2.66 ± 0.59 | 1.57 ± 0.53 | 0.72 ± 0.19 | 0.55 ± 0.15 | 0.45 ± 0.15 | 0.23 ± 0.09 | ND | 0.16 ± 0.07 | 95.7 |
| TSS | mg/L | 25 | 2.0 ± 4.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ND | 0.0 ± 0.0 | 100 |
| TIC | mg C/L | 19.7 | ND | ND | ND | ND | ND | ND | ND | 0.58 ± 0.00 | 97.1 |
| TOC | mg C/L | 5.40 | ND | ND | ND | ND | ND | ND | ND | 4.15 ± 0.92 | 23.1 |
| Fluoride | mg/L | 44.1 ± 7.4 | ND | 46.2 ± 0.8 | 47.4 ± 0.6 | 47.6 ± 1.2 | 48.0 ± 1.5 | 47.2 ± 1.1 | ND | 0.24 ± 0.06 | 99.5 |
| Chloride | mg/L | 200 ± 4 | ND | 196 ± 2 | 199 ± 2 | 212 ± 27 | 218 ± 37 | 214 ± 32 | ND | 1.37 ± 0.57 | 99.3 |
| Nitrate | mg/L | 182 ± 3 | ND | 180 ± 2 | 180 ± 2 | 164 ± 46 | 157 ± 68 | 160 ± 58 | ND | 2.84 ± 1.29 | 98.4 |
| Nitrite | mg/L | 0.12 ± 0.01 | ND | 0.09 ± 0.01 | 0.11 ± 0.04 | 0.16 ± 0.02 | 0.13 ± 0.06 | 0.15 ± 0.07 | ND | 0.008 ± 0.002 | 93.3 |
| Iodine | mg/L | 64.6 ± 2.6 | ND | 63.2 ± 3.9 | 61.8 ± 2.5 | 43.4 ± 26.4 | 39.8 ± 27.1 | 38.8 ± 26.5 | ND | 0.16 ± 0.11 | 99.8 |
| Perchlorate | µg/L | 215 ± 23 | ND | 198 ± 16 | 206 ± 9 | 93 ± 101 | 32.4 ± 39.7 | 46.2 ± 45.0 | ND | <0.5 | >99.8 |
| Aluminum | mg/L | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | ND |
| Arsenic | µg/L | 931 ± 16 | 964 ± 17 | 846 ± 78 | 848 ± 85 | 714 ± 390 | 687 ± 404 | 680 ± 398 | ND | 3.86 ± 0.98 | 99.6 |
| Calcium | mg/L | 26.9 ± 1.5 | 26.7 ± 1.5 | 26.4 ± 1.3 | 0.31 ± 0.34 | 0.30 ± 0.26 | 0.30 ± 0.24 | 0.30 ± 0.23 | ND | <0.03 | >99.9 |
| Cesium | µg/L | 1304 ± 21 | 1343 ± 28 | 1330 ± 27 | 1007 ± 915 | 979 ± 935 | 943 ± 924 | 934 ± 913 | ND | 9.12 ± 6.81 | >99.3 |
| Copper | mg/L | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | <0.04 | ND | <0.04 | ND |
| Iron | mg/L | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | ND | <0.03 | ND |
| Lead | µg/L | 168 ± 37 | 189 ± 58 | 10.4 ± 6.2 | 13.7 ± 5.2 | 4.70 ± 1.21 | 4.04 ± 1.43 | <0.8 | ND | <0.8 | >99.5 |
| Magnesium | mg/L | 4.13 ± 0.18 | 4.11 ± 0.17 | 4.08 ± 0.17 | 0.07 ± 0.07 | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.05 ± 0.02 | ND | <0.01 | >99.8 |
| Manganese | mg/L | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ND | <0.01 | ND |
| Mercury | µg/L | 2.91 ± 4.86 | ND | ND | 3.53 ± 4.06 | 0.028 ± 0.010 | 0.005 ± 0.001 | 0.007 ± 0.005 | ND | <0.001 | >99.9 |
| Potassium | mg/L | 3.81 ± 0.22 | 3.79 ± 0.07 | 3.84 ± 0.15 | 3.80 ± 1.85 | 4.08 ± 1.93 | 3.98 ± 1.75 | 3.91 ± 1.82 | ND | <0.5 | >86.9 |
| Sodium | mg/L | 145 ± 3 | 144 ± 5 | 144 ± 5 | 163 ± 6 | 163 ± 6 | 165 ± 5 | 164 ± 4 | ND | 1.73 ± 0.18 | 98.8 |
| Zinc | mg/L | <0.07 | <0.07 | <0.07 | <0.07 | <0.07 | <0.07 | <0.07 | ND | <0.07 | ND |
| Benzene | µg/L | 87.0 ± 35.6 | ND | ND | 108 ± 18 | 150 ± 71 | <5 | <5 | ND | <5 | >94.3 |
| CHCl3 | µg/L | 1080 ± 45 | ND | ND | 1116 ± 91 | 1200 ± 245 | <2 | <2 | ND | <2 | >99.8 |
| CCl4 | µg/L | <100 | ND | ND | <20 | <20 | <20 | <20 | ND | <20 | >80.0 |
| MTBE | µg/L | 85.0 ± 24.1 | ND | ND | 94.0 ± 27.3 | 97.0 ± 17.8 | <10 | <10 | ND | <10 | >96.0 |
| Total coliforms | CFU/ 100 mL | ND | ND | ND | ND | ND | ND | ND | 0 | 0 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blais, J.-F.; Taillard, V.; Rioux, G.; Dionne, J.; Lévesque, R.; Abolhosseini, P.; Tran, L.H.; Martel, R. A Complete Mobile Treatment Chain to Produce Drinking Water from Sources Heavily Contaminated by Inorganic and Organic Compounds. Water 2025, 17, 3246. https://doi.org/10.3390/w17223246
Blais J-F, Taillard V, Rioux G, Dionne J, Lévesque R, Abolhosseini P, Tran LH, Martel R. A Complete Mobile Treatment Chain to Produce Drinking Water from Sources Heavily Contaminated by Inorganic and Organic Compounds. Water. 2025; 17(22):3246. https://doi.org/10.3390/w17223246
Chicago/Turabian StyleBlais, Jean-François, Vincent Taillard, Geneviève Rioux, Justine Dionne, Richard Lévesque, Pejman Abolhosseini, Lan Huong Tran, and Richard Martel. 2025. "A Complete Mobile Treatment Chain to Produce Drinking Water from Sources Heavily Contaminated by Inorganic and Organic Compounds" Water 17, no. 22: 3246. https://doi.org/10.3390/w17223246
APA StyleBlais, J.-F., Taillard, V., Rioux, G., Dionne, J., Lévesque, R., Abolhosseini, P., Tran, L. H., & Martel, R. (2025). A Complete Mobile Treatment Chain to Produce Drinking Water from Sources Heavily Contaminated by Inorganic and Organic Compounds. Water, 17(22), 3246. https://doi.org/10.3390/w17223246








