Removal of 4-Chloro-3-Methylphenol (p-Chlorocresol) from Tannery Effluent, from Lab to Pilot Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Lab-Scale Trials
2.1.1. Sample Preparation and Tannery Wastewater Collection
2.1.2. Establish Basic Ozone Treatment Conditions
2.1.3. Combined Ozone Treatment with Fenton’s Oxidation
2.2. Pilot-Scale Field Trials
2.2.1. Dissolved Ozone Delivery by Venturi
2.2.2. Direct Ozone Delivery by Air Blower
2.3. Method for p-Chlorocresol Determination
2.4. Kinetic Models
3. Results
3.1. Lab-Scale Optimization
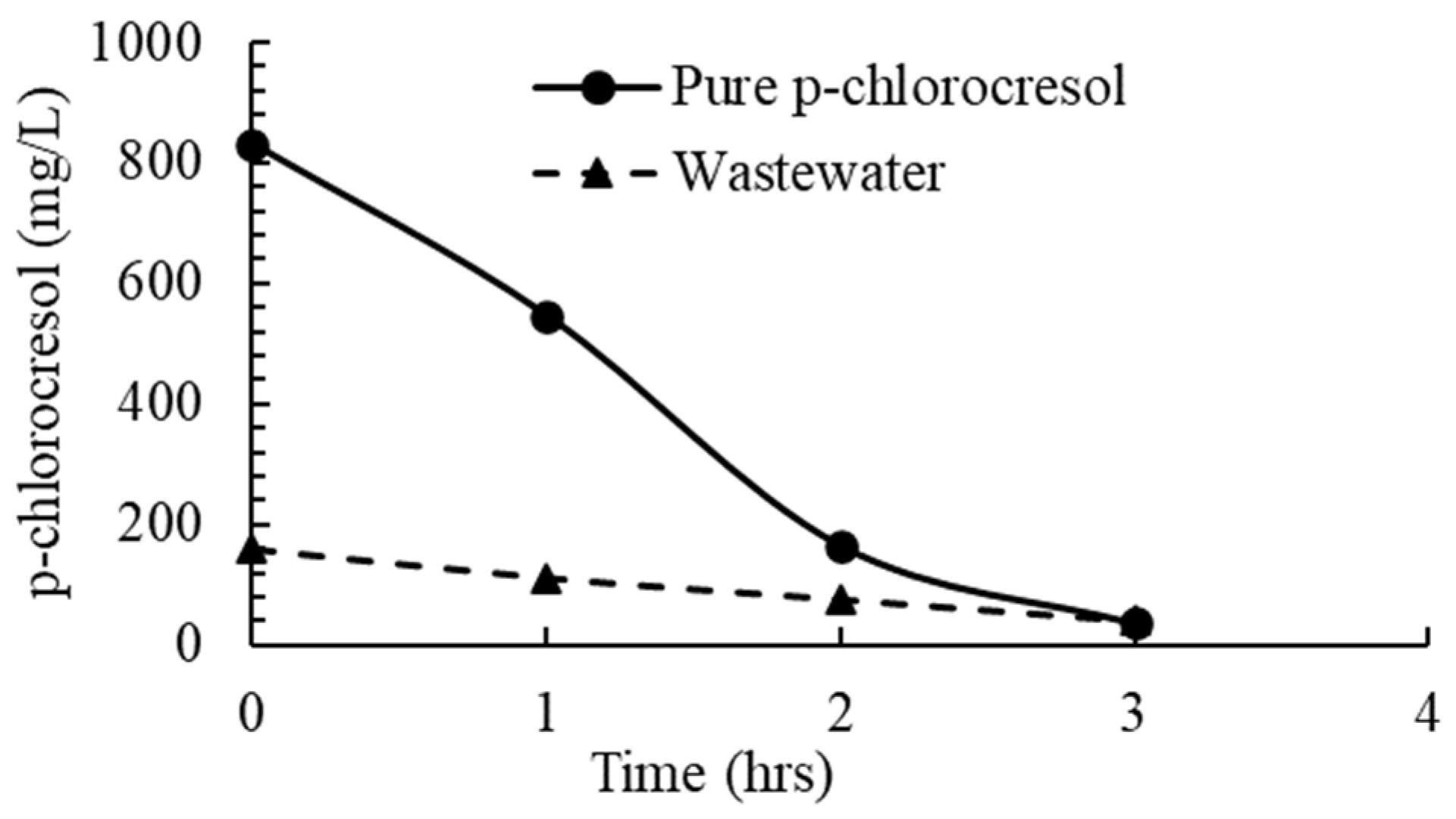
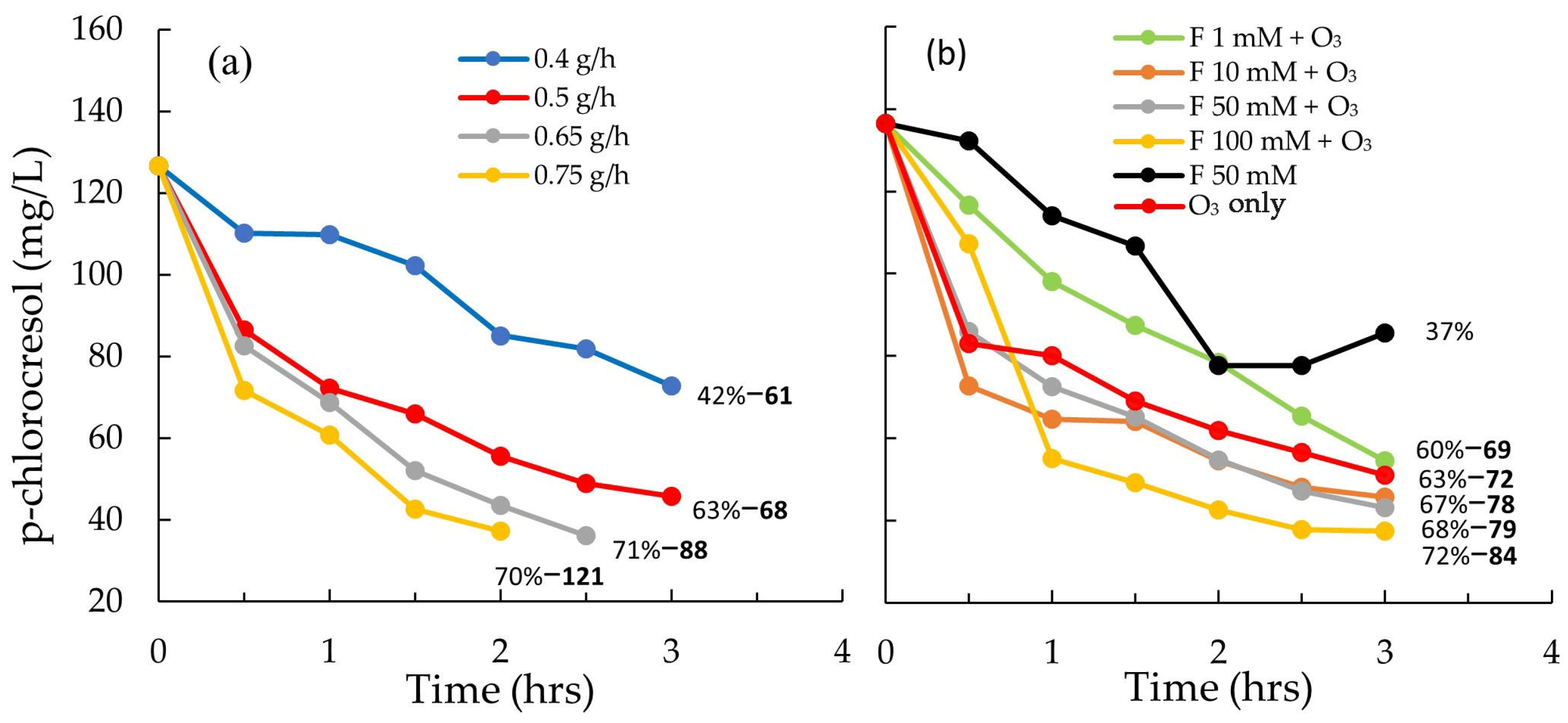
3.1.1. Basic Ozone Treatment
3.1.2. Combined Ozone and Fenton’s Reagent Treatment
3.2. Pilot Field Trials
3.2.1. Dissolved Ozone Delivery
3.2.2. Direct Ozone Delivery
3.3. Kinetics of p-Chlorocresol Removals
4. Discussion
4.1. Influence of Treatment Type on p-Chlorocresol Removal
4.2. Influence of Treatment Type on COD, TOC, and DOC Removal
4.3. Influence of Wastewater pH on p-Chlorocresol Removal
4.4. Influence of Delivery System and Reaction Volume on p-Chlorocresol Removal
4.5. Kinetics of p-Chlorocresol Removal
4.6. Intermediates During p-Chlorocresol Degradation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rajalo, G.; Petrovskaya, T. Selective electrochemical oxidation of sulphides in tannery wastewater. Environ. Technol. 1996, 17, 605–612. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Naumczyk, J.; Zilio-Grandi, F. Electrochemical treatment of tannery wastewater using TiPt and Ti/Pt/Ir electrodes. Water Res. 1995, 29, 517–524. [Google Scholar] [CrossRef]
- Xiao, M.; Qi, Y.; Feng, Q.; Li, K.; Fan, K.; Huang, T.; Qu, P.; Gai, H.; Song, H. P-cresol degradation through Fe (III)-EDDS/H2O2 Fenton-like reaction enhanced by manganese ion: Effect of pH and reaction mechanism. Chemosphere 2021, 269, 129436. [Google Scholar] [CrossRef]
- Iliuta, I.; Iliuta, M.C. Intensified phenol and p-cresol biodegradation for wastewater treatment in countercurrent packed-bed column bioreactors. Chemosphere 2022, 286, 131716. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, S.; Kumar, S.; Kumar, A. Biodegradation kinetic studies for the removal of p-cresol from wastewater using Gliomastix indicus MTCC 3869. Biochem. Eng. J. 2008, 40, 293–303. [Google Scholar] [CrossRef]
- Dantas, T.L.P.; José, H.J.; Moreira, R.F.P.M. Fenton and Photo-Fenton oxidation of tannery wastewater. Acta Sci. Technol. 2003, 25, 91–95. [Google Scholar]
- Schrank, S.G.; José, H.J.; Moreira, R.F.P.M.; Schröder, H.F. Applicability of Fenton and H2O2/UV reactions in the treatment of tannery wastewaters. Chemosphere 2005, 60, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Sivagami, K.; Sakthivel, K.P.; Nambi, I.M. Advanced oxidation processes for the treatment of tannery wastewater. J. Environ. Chem. Eng. 2018, 6, 3656–3663. [Google Scholar] [CrossRef]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461–462, 265–281. [Google Scholar] [CrossRef]
- R Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Schrank, S.G.; Jose, H.J.; Moreira, R.F.P.M.; Schröder, H.F. Elucidation of the behavior of tannery wastewater under advanced oxidation conditions. Chemosphere 2004, 56, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Rameshraja, D.; Suresh, S. Treatment of tannery wastewater by various oxidation and combined processes. Int. J. Environ. Res. 2011, 5, 349–360. [Google Scholar]
- Asaithambi, P.; Sajjadi, B.; Aziz, A.R.A. Integrated ozone–photo–Fenton process for the removal of pollutant from industrial wastewater. Chin. J. Chem. Eng. 2017, 25, 516–522. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Hybrid ozonation process for industrial wastewater treatment: Principles and applications: A review. J. Water Process Eng. 2020, 35, 101193. [Google Scholar] [CrossRef]
- Benitez, F.J.; Beltrán-Heredia, J.; Acero, J.L.; Rubio, F.J. Rate constants for the reactions of ozone with chlorophenols in aqueous solutions. J. Hazard. Mater. 2000, 79, 271–285. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Esplugas, S.; Gimenez, J.; Contreras, S.; Pascual, E.; Rodríguez, M. Comparison of different advanced oxidation processes for phenol degradation. Water Res. 2002, 36, 1034–1042. [Google Scholar] [CrossRef]
- Sanchis, S.; Meschede-Anglada, L.; Serra, A.; Simon, F.X.; Sixto, G.; Casas, N.; Garcia-Montaño, J. Solar photo-Fenton with simultaneous addition of ozone for the treatment of real industrial wastewaters. Water Sci. Technol. 2018, 77, 2497–2508. [Google Scholar] [CrossRef]
- Schrank, S.G.; Jose, H.J.; Moreira, R.F.P.M.; Schröder, H.F. Comparison of different advanced oxidation process to reduce toxicity and mineralisation of tannery wastewater. Water Sci. Technol. 2004, 50, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Kow, S.H.; Fahmi, M.R.; Abidin, C.Z.A.; Ong, S.A.; Ibrahim, A.H.; Sabri, S.N.; Razali, N.A. Oxidation of p-cresol by ozonation. Sains Malays. 2018, 47, 1085–1091. [Google Scholar] [CrossRef]
- Kavitha, V.; Palanivelu, K. Destruction of cresols by Fenton oxidation process. Water Res. 2005, 39, 3062–3072. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of ozonation for pharmaceuticals and personal care products removal from water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef]
- Zhou, H.; Smith, D.W. Advanced technologies in water and wastewater treatment. Can. J. Civ. Eng. 2001, 28, 49–66. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B Environ. 2011, 99, 27–42. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Walling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Kirmaci, A.; Duyar, A.; Akgul, V.; Akman, D.; Cirik, K. Optimization of combined ozone/Fenton process on olive mill wastewater treatment. Aksaray Univ. J. Sci. Eng. 2018, 2, 52–62. [Google Scholar] [CrossRef]
- Germán, S.M.; Gabriela, R.M.; Dora, S.C.; Rubí, R.; Reyna, N. Advanced Oxidation Processes: Ozonation and Fenton Processes Applied to the Removal of Pharmaceuticals. In Ecopharmacovigilance: Multidisciplinary Approaches to Environmental Safety of Medicines; Springer: Berlin/Heidelberg, Germany, 2017; pp. 119–142. [Google Scholar]
- Chang, C.Y.; Hsieh, Y.H.; Cheng, K.Y.; Hsieh, L.L.; Cheng, T.C.; Yao, K.S. Effect of pH on Fenton process using estimation of hydroxyl radical with salicylic acid as trapping reagent. Water Sci. 2008, 58, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Fischbacher, A.; von Sonntag, C.; Schmidt, T.C. Hydroxyl radical yields in the Fenton process under various pH, ligand concentrations and hydrogen peroxide/Fe (II) ratios. Chemosphere 2017, 182, 738–744. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Dai, M.; Niu, Q.; Wu, S.; Lin, Y.; Biswas, J.K.; Yang, C. Hydroxyl radicals in ozone-based advanced oxidation of organic contaminants: A review. Environ. Chem. Lett. 2024, 22, 3059–3106. [Google Scholar] [CrossRef]
- Edwards, J.O.; Curci, R. Fenton type activation and chemistry of hydroxyl radical. In Catalytic Oxidations with Hydrogen Peroxide as Oxidant; Springer: Berlin/Heidelberg, Germany, 1992; pp. 97–151. [Google Scholar]
- Lopez, A.; Mascolo, G.; Detomaso, A.; Lovecchio, G.; Villani, G. Temperature activated degradation (mineralization) of 4-chloro-3-methyl phenol by Fenton’s reagent. Chemosphere 2005, 59, 397–403. [Google Scholar] [CrossRef]
- Rakness, K.L. Ozone in Drinking Water Treatment: Process Design, Operation, and Optimization; American Water Works Association: Denver, CO, USA, 2011. [Google Scholar]
- Fedorov, K.; Dinesh, K.; Sun, X.; Soltani, R.D.C.; Wang, Z.; Sonawane, S.; Boczkaj, G. Synergistic effects of hybrid advanced oxidation processes (AOPs) based on hydrodynamic cavitation phenomenon–A review. Chem. Eng. J. 2022, 432, 134191. [Google Scholar] [CrossRef]
- Beltran, F.J. Ozone Reaction Kinetics for Water and Wastewater Systems; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Kow, S.H.; Fahmi, M.R.; Abidin, C.Z.A.; Ong, S.A. Mechanistic insight into the degradation pathways of p-cresol in ozonation, peroxone, and ozone-persulfate process. Ozone: Sci. Eng. 2020, 43, 507–519. [Google Scholar] [CrossRef]
- Valsania, M.C.; Fasano, F.; Richardson, S.D.; Vincenti, M. Investigation of the degradation of cresols in the treatments with ozone. Water Res. 2012, 46, 2795–2804. [Google Scholar] [CrossRef]
- Celary, P.; Sobik-Szołtysek, J. Vitrification as an alternative to landfilling of tannery sewage sludge. Waste Manag. 2014, 34, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Gachet, C.; Von Gunten, U. Fate of Cr(III) during ozonation of secondary municipal wastewater effluent. Ozone: Sci. Eng. 2018, 40, 441–447. [Google Scholar] [CrossRef]
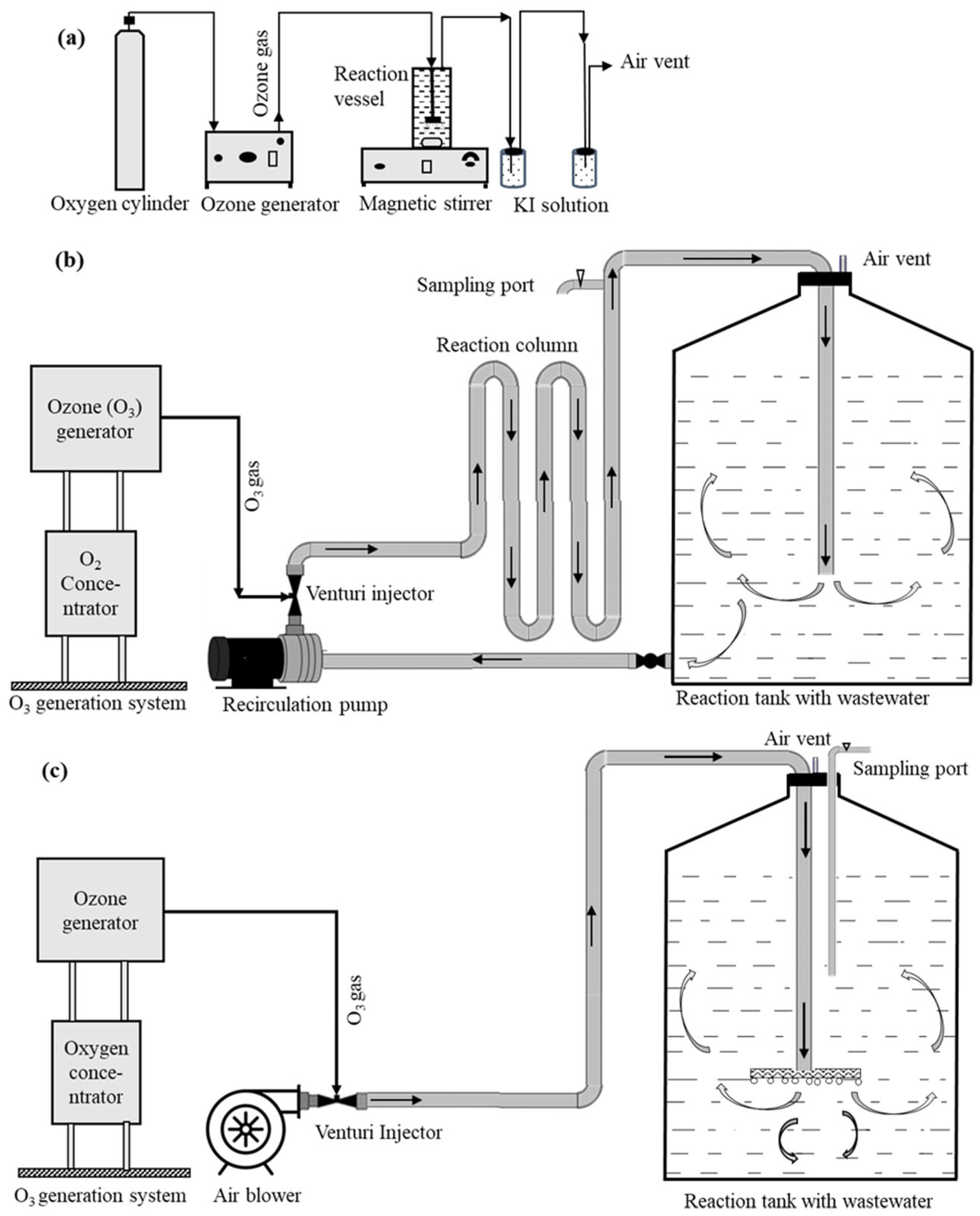


| Delivery System | Treatment | Wastewater (L) | O3 Application (g h−1) | H2O2 (mM) | Fe2+ (mM) |
|---|---|---|---|---|---|
| Venturi delivery with centrifugal pump | O3 | 400 | 5 | - | - |
| O3 + Fenton’s | 400 | 5 | 50 | 0.25 | |
| O3 + Fenton’s | 400 | 5 | 50 | 0.50 | |
| Fenton’s only | 400 | - | 50 | 0.125 | |
| Venturi delivery with screw pump | O3 | 700 | 20 | - | - |
| O3 + Fenton’s | 700 | 20 | 50 | 0.5 | |
| Direct delivery with blower | O3 | 700 | 20 | - | - |
| O3 + Fenton’s | 700 | 20 | 50 | 0.5 |
| Parameter | Concentration |
|---|---|
| pH | 4.09 |
| Conductivity (EC) (mg L−1) | 32.6 |
| Total organic carbon (mg L−1) | 2244 |
| * Total organic carbon (mg L−1) | 1558 |
| p-chlorocresol (mg L−1) | 175.0 ± 12.14 |
| Iron (mg L−1) | 16.0 |
| Aluminum (mg L−1) | 7.77 |
| Chromium (mg L−1) | 457 |
| Manganese (mg L−1) | 0.82 |
| Copper (mg L−1) | <0.001 |
| Zinc (mg L−1) | <0.001 |
| Nickel (mg L−1) | 0.55 |
| Lead (mg L−1) | <0.001 |
| Selenium (mg L−1) | 0.02 |
| Treatment | O3 (g h−1) | H2O2 (mM) | Fe2+ (mM) | Removal * | |
|---|---|---|---|---|---|
| (%) | mg p-Chlorocresol g− O3 h−1 | ||||
| Venturi delivery with centrifugal pump | |||||
| O3 | 5 | - | - | 33.5 | 7.5 |
| O3 + Fenton’s | 5 | 50 | 0.25 | 39.8 | 9.0 |
| O3 + Fenton’s | 5 | 50 | 0.50 | 47.9 | 11.5 |
| Fenton’s only | - | 50 | 0.125 | 32.3 | - |
| Venturi delivery with screw pump | |||||
| O3 | 20 | - | - | 46 ± 3.5 | 3.8 ± 1.5 |
| O3 + Fenton’s | 20 | 50 | 0.5 | 66 ± 2.1 | 6.7 ± 1.4 |
| Direct delivery with blower | |||||
| O3 | 20 | - | - | 37 ± 3.1 | 4.1 ± 0.42 |
| O3 + Fenton’s | 20 | 50 | 0.5 | 55 ± 7.1 | 5.3 ± 0.28 |
| Treatment | Pseudo-First Order | Pseudo-Second Order | Elovich | |||||
|---|---|---|---|---|---|---|---|---|
| K1 (h−1) | R2 | qe (mg L−1) | K2 (L mg−1 h−1) | R2 | qe (mg L−1) | R2 | qe (mg L−1) | |
| Venturi delivery * | ||||||||
| O3 | 0.0767 | 0.9468 | 78.96 | 0.0006 | 0.7505 | 98.8 | 0.9369 | 61.3 |
| O3 + Fenton’s | 0.3433 | 0.8388 | 109.95 | 0.0101 | 0.9955 | 115.0 | 0.7288 | 106.5 |
| Direct delivery | ||||||||
| O3 | 0.0618 | 0.8503 | 71.45 | 0.0005 | 0.4607 | 88.0 | 0.9311 | 56.3 |
| O3 + Fenton’s | 0.3102 | 0.8683 | 94.63 | 0.0266 | 0.9988 | 93.0 | 0.4117 | 87.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.S.; Brushett, D.; Rose, A.; McIntosh, S.; Erler, D. Removal of 4-Chloro-3-Methylphenol (p-Chlorocresol) from Tannery Effluent, from Lab to Pilot Scale. Water 2025, 17, 3238. https://doi.org/10.3390/w17223238
Rahman MS, Brushett D, Rose A, McIntosh S, Erler D. Removal of 4-Chloro-3-Methylphenol (p-Chlorocresol) from Tannery Effluent, from Lab to Pilot Scale. Water. 2025; 17(22):3238. https://doi.org/10.3390/w17223238
Chicago/Turabian StyleRahman, Md Sydur, Don Brushett, Andrew Rose, Shane McIntosh, and Dirk Erler. 2025. "Removal of 4-Chloro-3-Methylphenol (p-Chlorocresol) from Tannery Effluent, from Lab to Pilot Scale" Water 17, no. 22: 3238. https://doi.org/10.3390/w17223238
APA StyleRahman, M. S., Brushett, D., Rose, A., McIntosh, S., & Erler, D. (2025). Removal of 4-Chloro-3-Methylphenol (p-Chlorocresol) from Tannery Effluent, from Lab to Pilot Scale. Water, 17(22), 3238. https://doi.org/10.3390/w17223238






