Investigation of Nutrient Removal Capacity and Growth Rate of Duckweed (Lemna minor) Under Different Harvesting Protocols in Aquaponics
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Environment
2.2. Experimental Stock
2.3. Treatments
2.4. Initial Water Composition and Replacement
2.5. Water Quality Measurement
2.6. Carp and Duckweed Production Parameters
2.7. Feeding
2.8. Sampling
2.9. Water Quality Sampling Protocol
2.10. Statistical Analysis
3. Results
3.1. Fish Production Parameters
3.2. Water Quality Parameters
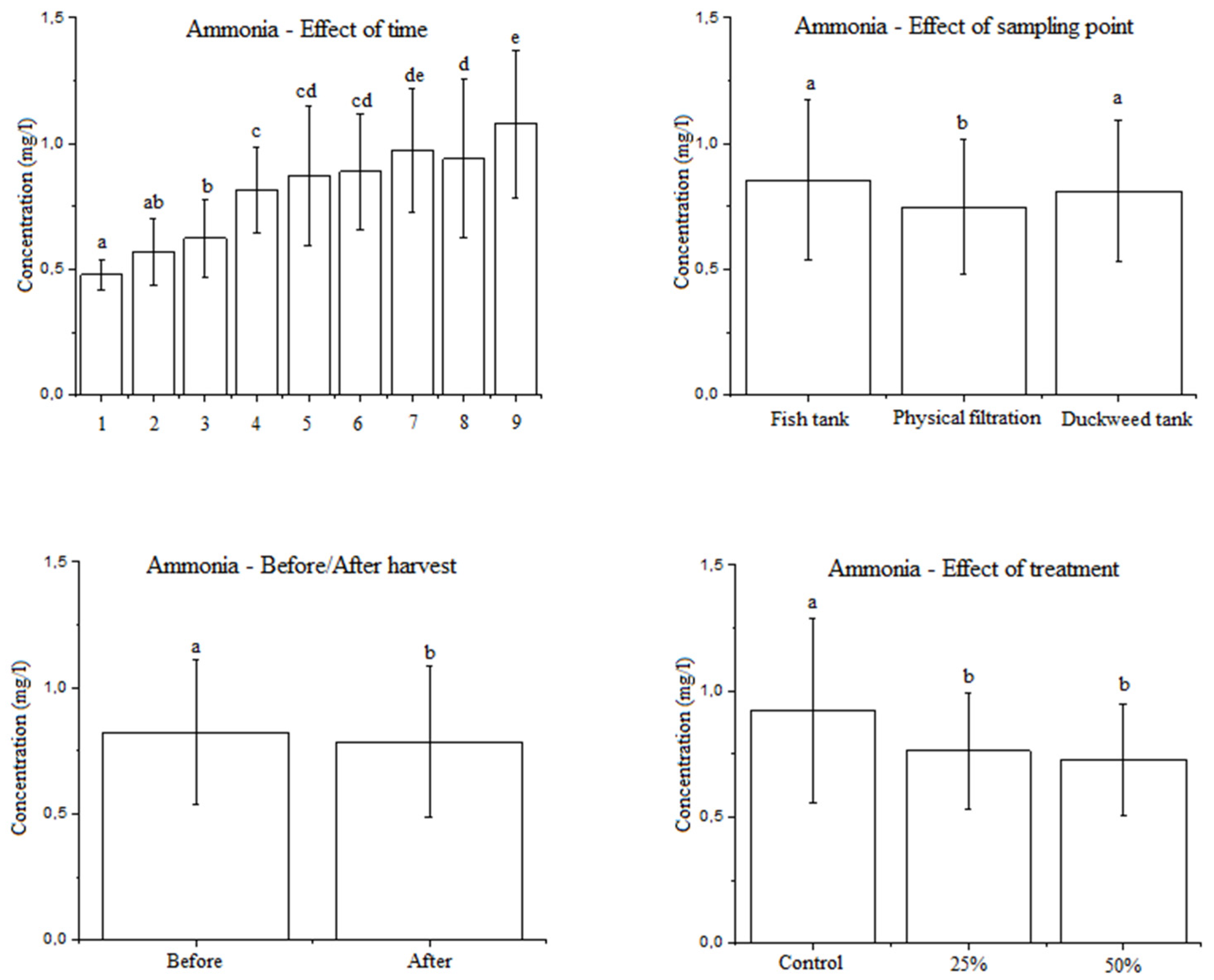
3.2.1. Effect of the Factors Examined on the Ammonia Concentration in Water
3.2.2. Effect of the Factors Studied on the Nitrite Concentration in Water
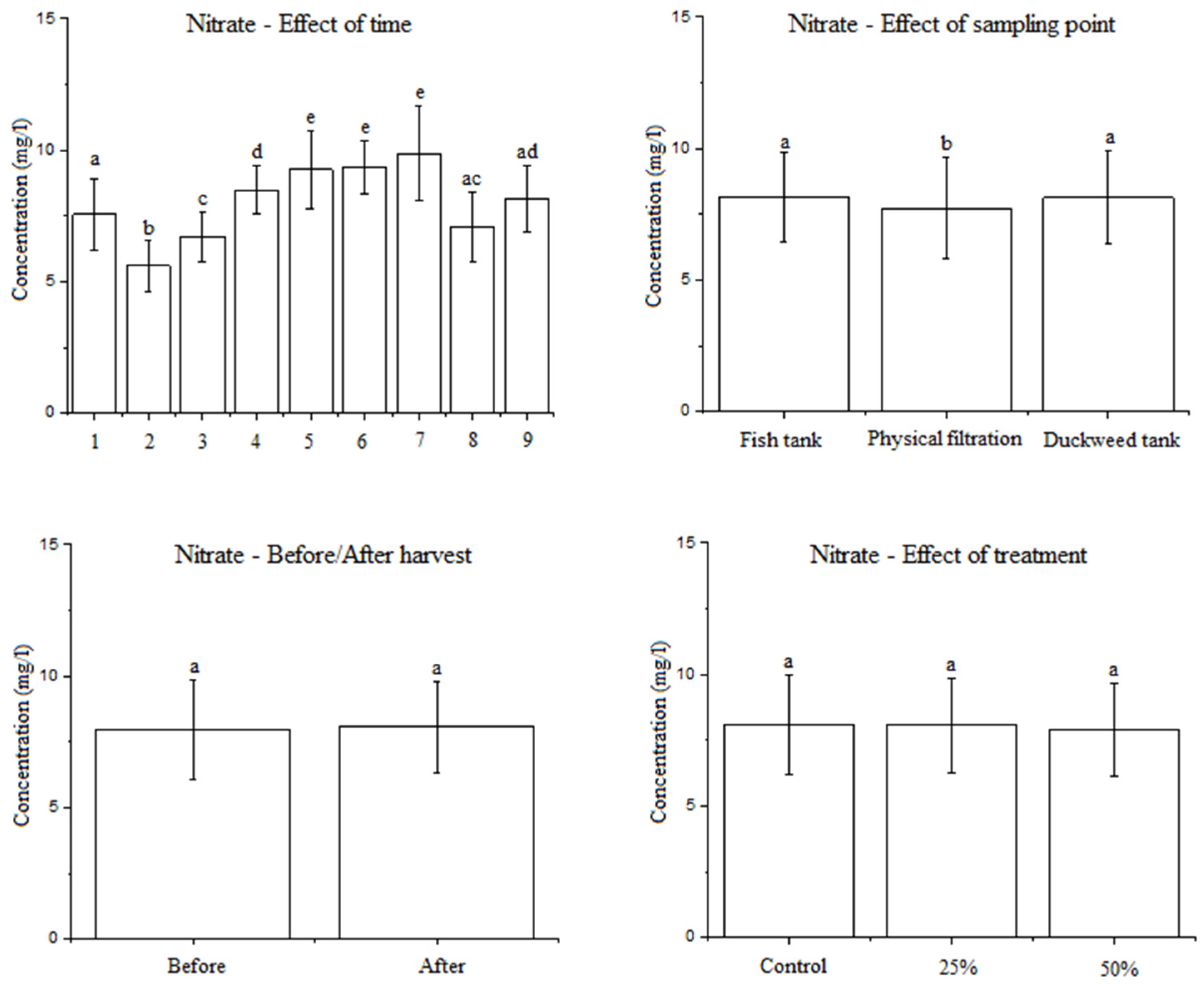
3.2.3. Effect of the Factors Examined on the Nitrate Concentration in Water
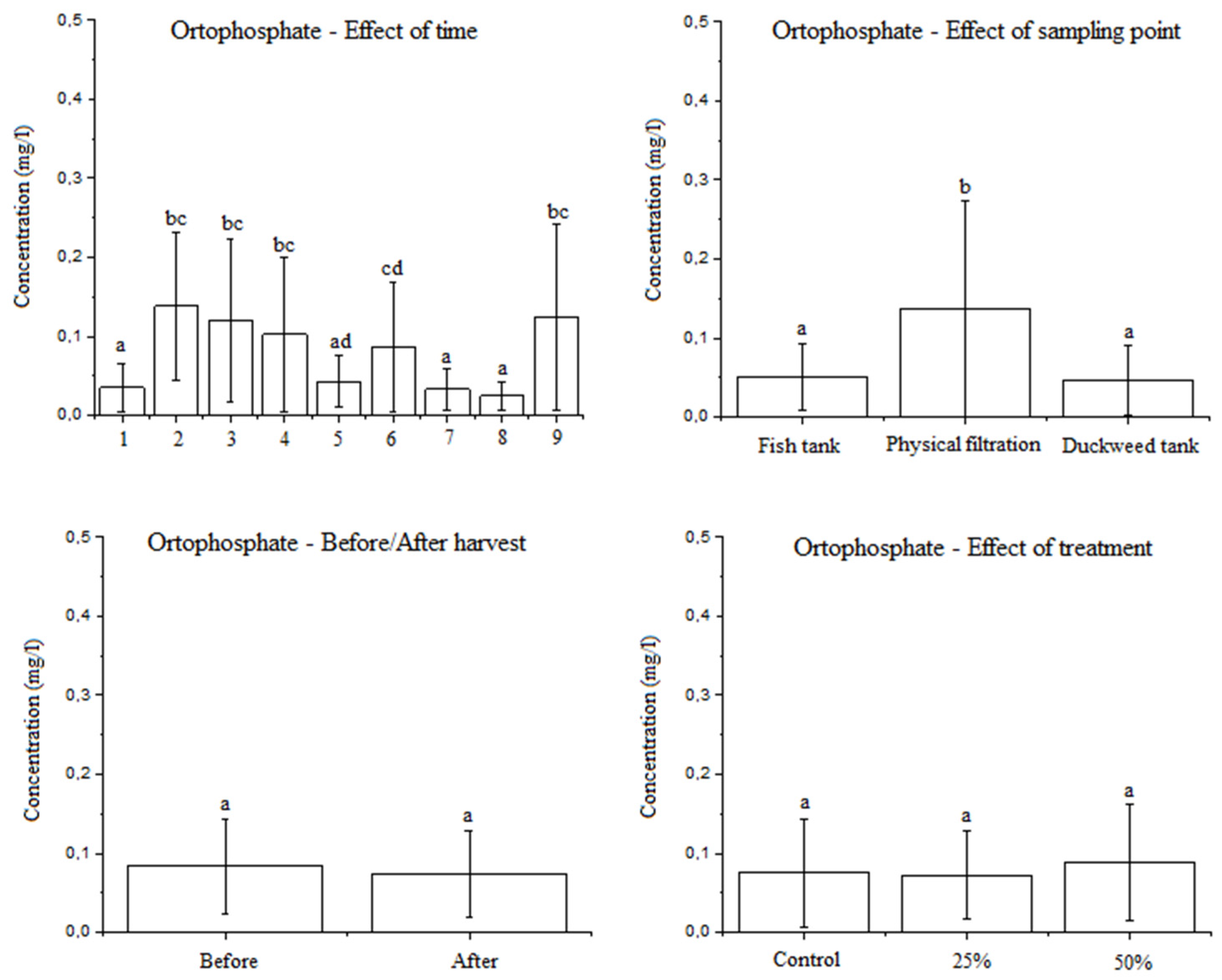
3.2.4. Effect of the Factors Studied on the Orthophosphate Concentration in Water
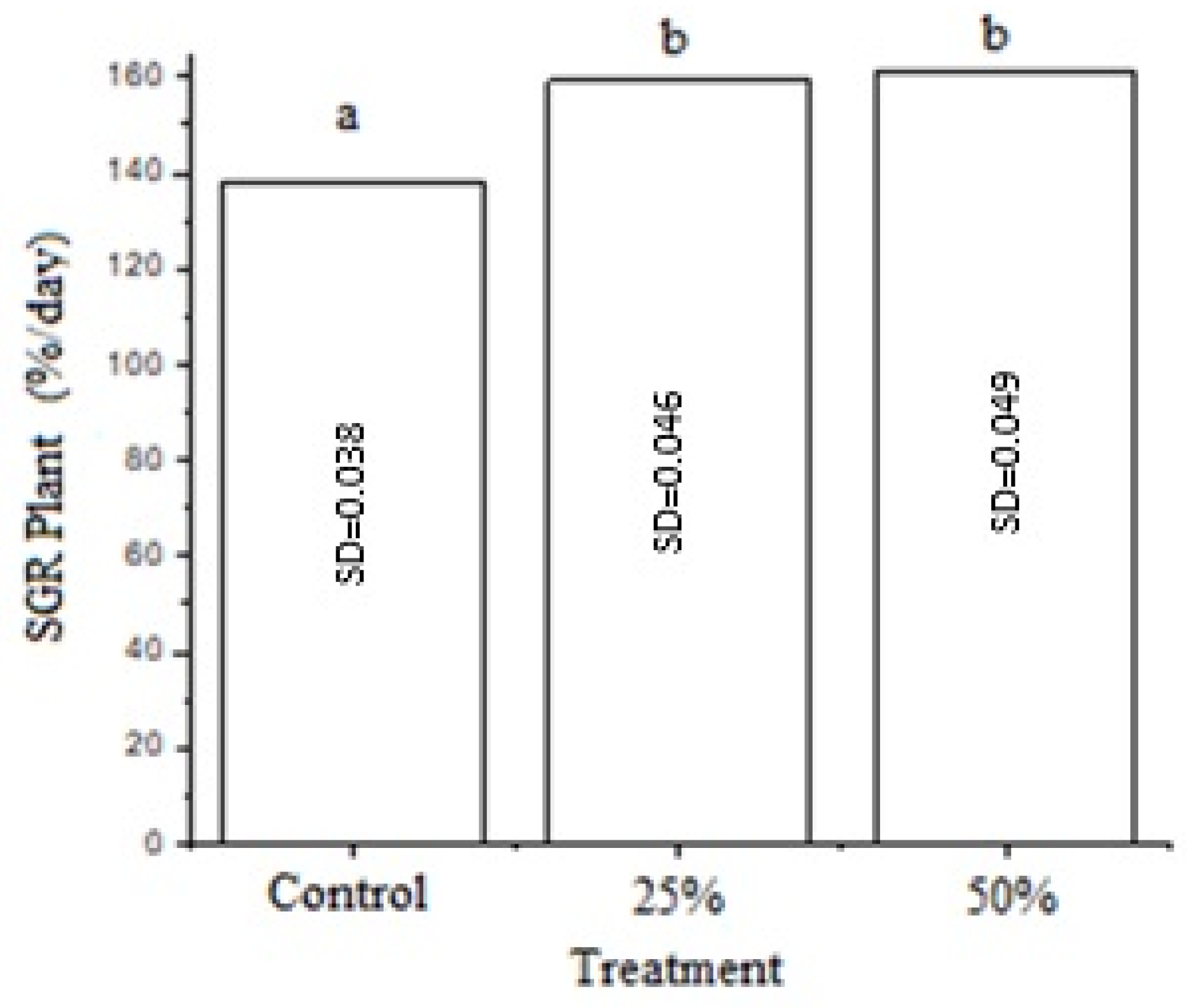
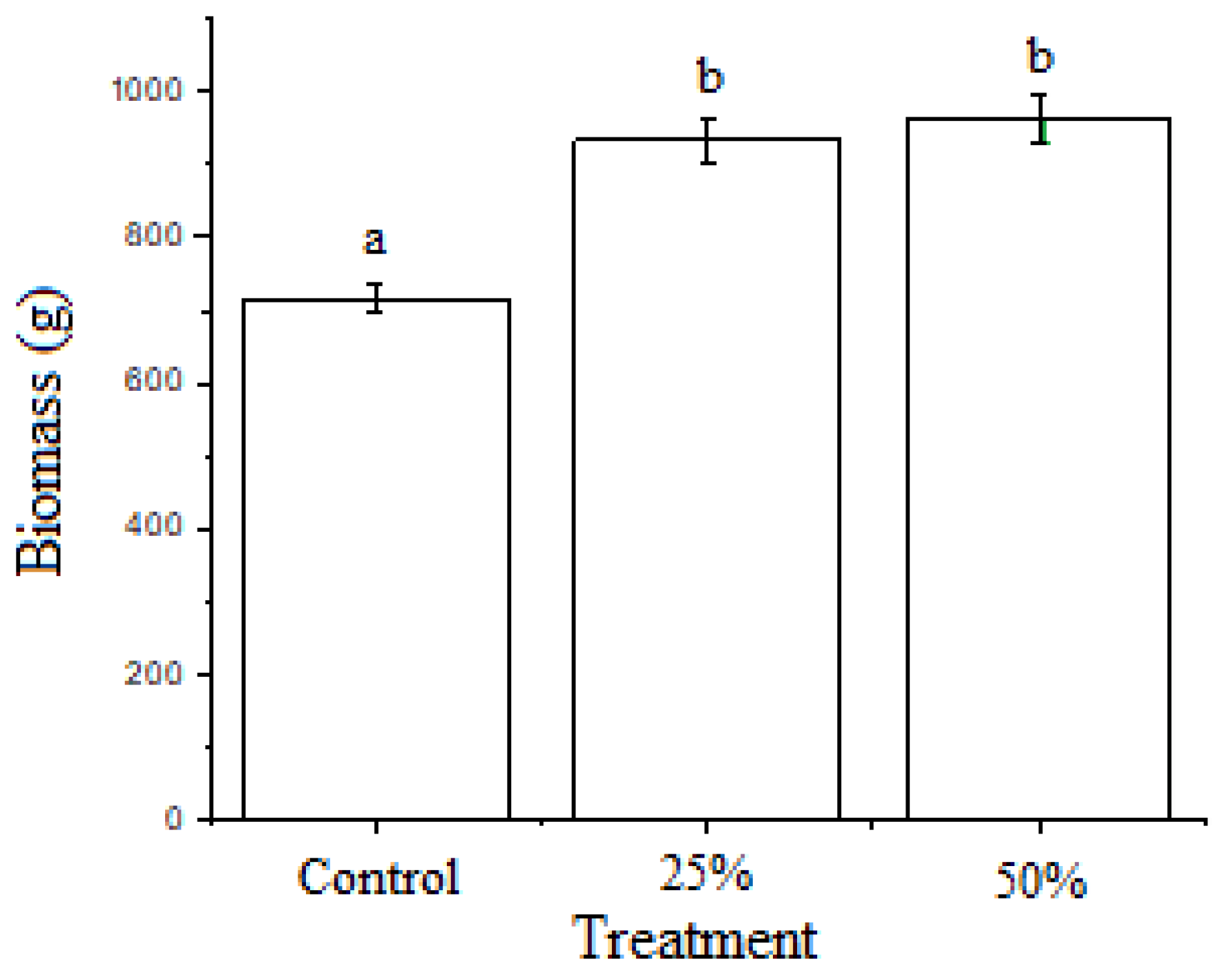
3.3. Growth of Duckweed Biomass
4. Discussion
4.1. Fish Growth and Production Parameters
4.2. The Effect of the Factors Studied on the Ammonia Concentration in Water
4.3. The Effect of the Factors Studied on the Nitrite Concentration in Water
4.4. The Effect of the Factors Studied on the Nitrate Concentration in Water
4.5. The Effect of the Factors Studied on the Orthophosphate Concentration in Water
4.6. Growth of Duckweed Biomass
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, G.; Engle, C.R. Technological advances that led to growth of shrimp, salmon, and tilapia farming. Rev. Fish. Sci. Aquac. 2016, 24, 136–152. [Google Scholar] [CrossRef]
- Safari, R.; Hoseinifar, S.H.; Shabani, A.; Ghafarifarsani, H.; Raissy, M.; Khaleghi, S.R.; Van Doan, H.; Yazici, M.; Rahbar, M.; Nouri, M. Dietary Administration of Green Macroalgae (Ulva intestinalis) on Growth Performance, Serum Immune Parameters, and Gene Expression in Common Carp (Cyprinus carpio). Ann. Anim. Sci. 2025, 25, 317–327. [Google Scholar] [CrossRef]
- Alam, L.; Mokhtar, M.B.; Sumaila, U.R. Climate change impacts on the fisheries and aquaculture sectors with a focus on Malaysia. In Navigating Our Way to Solutions in Marine Conservation; Open Book Publishers: Cambridge, UK, 2025; pp. 75–92. [Google Scholar]
- FAO. FishStat: Global Aquaculture Production 1950–2022, FishstatJ. 2024. Available online: http://www.fao.org/fishery/en/statistics/software/fishstatj (accessed on 29 March 2024).
- MA-HAL. 2023 Annual Report 15–18. 2024. Available online: https://ma-hal.hu/2025/02/07/eves-jelentes/ (accessed on 22 September 2025).
- Williams, D.R.; Li, W.; Hughes, M.A.; Gonzalez, S.F.; Vernon, C.; Vidal, M.C.; Jeney, Z.; Jeney, G.; Dixon, P.; McAndrew, B.; et al. Genomic-resources and microarrays for the common carp Cyprinus carpio L. J. Fish Biol. 2008, 72, 2095–2117. [Google Scholar] [CrossRef]
- Mutethya, E.; Yongo, E. A comprehensive review of invasion and ecological impacts of introduced common carp (Cyprinus carpio) in Lake Naivasha, Kenya. Lakes Reserv. Res. Manag. 2021, 26, e12386. [Google Scholar] [CrossRef]
- Skjølstrup, J.; Nielsen, P.H.; Frier, J.O.; McLean, E. Performance characteristics of fluidized bed biofilters in a novel laboratory-scale recirculation system for rainbow trout: Nitrification rates, oxygen consumption and sludge collection. Aquacult. Eng. 1998, 18, 265–276. [Google Scholar] [CrossRef]
- Kuhn, D.D.; Drahos, D.D.; Marsh, L.; Flick, G.J., Jr. Evaluation of nitrifying bacteria product to improve nitrification efficacy in recirculating aquaculture systems. Aquac. Eng. 2010, 43, 78–82. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Factors affecting nitrification in soils. Commun. Soil Sci. Plant Anal. 2008, 39, 1436–1446. [Google Scholar] [CrossRef]
- Stein, L.Y.; Nicol, G.W. Nitrification. Encycl. Life Sci. 2011, 1–9. [Google Scholar] [CrossRef]
- Septriono, W.A.; Indrian, F.; Khairunnisa, S.; Gultom, E.R. Penggunaan Mikroorganisme Akuatik pada Proses Nitrifikasi di Tambak Udang (Litopeneaus vannamei). MAIYAH 2023, 2, 233–239. [Google Scholar] [CrossRef]
- Van Kessel, M.A.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Austin, D.; Vazquez-Burney, R.; Dyke, G.; King, T. Nitrification and total nitrogen removal in a super-oxygenated wetland. Sci. Total Environ. 2019, 652, 307–313. [Google Scholar] [CrossRef]
- Prehn, J.; Waul, C.K.; Pedersen, L.F.; Arvin, E. Impact of water boundary layer diffusion on the nitrification rate of submerged biofilter elements from a recirculating aquaculture system. Water Res. 2012, 46, 3516–3524. [Google Scholar] [CrossRef]
- Sarkheil, M.; Safari, O. Phytoremediation of nutrients from water by aquatic floating duckweed (Lemna minor) in rearing of African cichlid (Labidochromis lividus) fingerlings. Environ. Technol. Innov. 2020, 18, 100747. [Google Scholar] [CrossRef]
- Körner, S.; Vermaat, J.E.; Veenstra, S. The capacity of duckweed to treat wastewater: Ecological considerations for a sound design. J. Environ. Qual. 2003, 32, 1583–1590. [Google Scholar] [CrossRef]
- Kreider, A.N.; Fernandez Pulido, C.R.; Bruns, M.A.; Brennan, R.A. Duckweed as an agricultural amendment: Nitrogen mineralization, leaching, and sorghum uptake. J. Environ. Qual. 2019, 48, 469–475. [Google Scholar] [CrossRef]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: Duckweed as a model plant system in the genomics and postgenomics era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef]
- Adhikari, U.; Harrigan, T.; Reinhold, D.M. Use of duckweed-based constructed wetlands for nutrient recovery and pollutant reduction from dairy wastewater. Ecol. Eng. 2015, 78, 6–14. [Google Scholar] [CrossRef]
- Cheng, J.J.; Stomp, A.M. Growing duckweed to recover nutrients from wastewaters and for production of fuel ethanol and animal feed. Clean–Soil Air Water 2009, 37, 17–26. [Google Scholar] [CrossRef]
- Zhou, Y.; Stepanenko, A.; Kishchenko, O.; Xu, J.; Borisjuk, N. Duckweeds for phytoremediation of polluted water. Plants 2023, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Baig, M.A. Effect of nutrient concentration and pH on growth and nutrient removal efficiency of duckweed (Lemna minor) from natural solid waste leachate. Int. J. Health Med. 2016, 1, 1–7. [Google Scholar]
- Gupta, C.; Prakash, D. Duckweed: An effective tool for phyto-remediation. Toxicol. Environ. Chem. 2013, 95, 1256–1266. [Google Scholar] [CrossRef]
- Xu, J.; Shen, G. Growing duckweed in swine wastewater for nutrient recovery and biomass production. Bioresour. Technol. 2011, 102, 848–853. [Google Scholar] [CrossRef]
- Shen, M.; Yin, Z.; Xia, D.; Zhao, Q.; Kang, Y. Combination of heterotrophic nitrifying bacterium and duckweed (Lemna gibba L.) enhances ammonium nitrogen removal efficiency in aquaculture water via mutual growth promotion. J. Gen. Appl. Microbiol. 2019, 65, 151–160. [Google Scholar] [CrossRef]
- Molnár, P.I.; Bak, H.; Ifj Lévai, F.; Bársony, P.; Antalovics, M.; Fehér, M. A békalencse (Lemna minor), mint biológiai szűrő integrálása a hibrid afrikai harcsa (Clarias gariepinus x Heterobranchus longifilis) ivadéknevelési technológiájába. Anim. Welf. Etol. Tartástechnológia 2025, 21, 44–54. [Google Scholar] [CrossRef]
- Al-Qutob, M.A.; Nashashibi, T.S. Duckweed Lemna minor (Liliopsida, Lemnaceae) as a natural biofilter in brackish and fresh closed recirculating systems. Aquac. Aquar. Conserv. Legis. 2012, 5, 380–392. [Google Scholar]
- Chaudhary, E.; Sharma, P. Duckweed as ecofriendly tool for phytoremediation. Int. J. Sci. Res. 2014, 3, 1615–1617. [Google Scholar]
- Ozengin, N.; Elmaci, A. Performance of Duckweed (Lemna minor L.) on different types of wastewater treatment. J. Environ. Biol. 2007, 28, 307–314. [Google Scholar]
- Pasos-Panqueva, J.; Baker, A.; Camargo-Valero, M.A. Unravelling the impact of light, temperature and nutrient dynamics on duckweed growth: A meta-analysis study. J. Environ. Manag. 2024, 366, 121721. [Google Scholar] [CrossRef]
- Akyüz, A.; Ersus, S. Optimization of Hoagland solution macro-elements as a culture media, for increasing protein content of duckweeds (Lemna minor). Food Chem. 2024, 453, 139647. [Google Scholar] [CrossRef] [PubMed]
- Goopy, J.P.; Murray, P.J. A review on the role of duckweed in nutrient reclamation and as a source of animal feed. Asian-Australas. J. Anim. Sci. 2003, 16, 297–305. [Google Scholar] [CrossRef]
- Landesman, L.; Fedler, C.; Duan, R. Plant nutrient phytoremediation using duckweed. Eutrophication Causes Conseq. Control 2010, 341–354. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Sree, K.S.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; et al. Nutritional value of the duckweed species of the genus Wolffia (Lemnaceae) as human food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef]
- Husain, S.O.; Abed, Z.; Raju, Y.K. Investigating the Potential of Aquaponics to Increase Food Production and Reduce Environmental Impact. In Proceedings of the SHS Web of Conferences, Bekasi, Indonesia, 29 September 2025; EDP Sciences: Les Ulis, France, 2025; Volume 216, p. 01038. [Google Scholar] [CrossRef]
- Karimanzira, D.; Keesman, K.J.; Kloas, W.; Baganz, D.; Rauschenbach, T. Dynamic modeling of the INAPRO aquaponic system. Aquac. Eng. 2016, 75, 29–45. [Google Scholar] [CrossRef]
- Krastanova, M.; Sirakov, I.; Ivanova-Kirilova, S.; Yarkov, D.; Orozova, P. Aquaponic systems: Biological and technological parameters. Biotechnol. Biotechnol. Equip. 2022, 36, 305–316. [Google Scholar] [CrossRef]
- Rakocy, J.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic production of tilapia and basil: Comparing a batch and staggered cropping system. In Proceedings of the South Pacific Soilless Culture Conference-SPSCC 648, Palmerston North, New Zealand, 10–13 February 2003; pp. 63–69. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Schmautz, Z.; Sambo, P.; Komives, T.; Borin, M.; Junge, R. Vegetable intercropping in a small-scale aquaponic system. Agronomy 2017, 7, 63. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, S.; Zhang, M.; Liu, F.; Cheng, L. Effects of multi-plant harvesting on nitrogen removal and recovery in constructed wetlands. Chemosphere 2024, 353, 141550. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Z.; Xiao, R.; Zhang, S.; Liu, F.; Xiang, Z. Absorption capacity of nitrogen and phosphorus of aquatic plants and harvest management research. J. Grassl. Sci. 2013, 22, 294–299. [Google Scholar]
- Jabłońska, E.; Winkowska, M.; Wiśniewska, M.; Geurts, J.; Zak, D.; Kotowski, W. Impact of vegetation harvesting on nutrient removal and plant biomass quality in wetland buffer zones. Hydrobiologia 2021, 848, 3273–3289. [Google Scholar] [CrossRef]
- Okada, K.; Vymazal, J. The effect of aboveground biomass harvesting on nutrients removal in a constructed wetland treating municipal sewage. Ecol. Eng. 2023, 190, 106918. [Google Scholar] [CrossRef]
- Irhayyim, T.; Fehér, M.; Lelesz, J.; Bercsényi, M.; Bársony, P. Nutrient removal efficiency and growth of watercress (Nasturtium officinale) under different harvesting regimes in integrated recirculating aquaponic systems for rearing common carp (Cyprinus carpio L.). Water 2020, 12, 1419. [Google Scholar] [CrossRef]
- Molnár, P.; Lelesz, J.É.; Kertész, A.; Bereczki, G.; Fehér, M. Preliminary results of the combined production of duckweed Spirodela polyrhiza and common carp (Cyprinus carpio) in an aquaponic system. Acta Agrar. Debreceniensis 2023, 1, 83–89. [Google Scholar] [CrossRef]
- Zhou, Y.; Kishchenko, O.; Stepanenko, A.; Chen, G.; Wang, W.; Zhou, J.; Oan, C.; Borisjuk, N. The dynamics of NO3− and NH4+ uptake in duckweed are coordinated with the expression of major nitrogen assimilation genes. Plants 2021, 11, 11. [Google Scholar] [CrossRef]
- Hamid, S.H.A.; Lananan, F.; Noor, N.A.M.; Endut, A. Physical filtration of nutrients utilizing gravel-based and lightweight expanded clay aggregate (LECA) as growing media in aquaponic recirculation system (ARS). Aquac. Eng. 2022, 98, 102261. [Google Scholar] [CrossRef]
- Mlih, R.; Bydalek, F.; Klumpp, E.; Yaghi, N.; Bol, R.; Wenk, J. Light-expanded clay aggregate (LECA) as a substrate in constructed wetlands—A review. Ecol. Eng. 2020, 148, 105783. [Google Scholar] [CrossRef]
- Schumacher, G.; Sekoulov, I. Polishing of secondary effluent by an algal biofilm process. Water Sci. Technol. 2002, 46, 83–90. [Google Scholar] [CrossRef]
- Caicedo, J.R.; van Der Steen, N.P.; Arce, O.; Gijzen, H.J. Effect of total ammonia nitrogen concentration and pH on growth rates of duckweed (Spirodela polyrrhiza). Water Res. 2000, 34, 3829–3835. [Google Scholar] [CrossRef]
- Paolacci, S.; Stejskal, V.; Toner, D.; Jansen, M.A. Wastewater valorization in an integrated multitrophic aquaculture system; assessing nutrient removal and biomass production by duckweed species. Environ. Pollut. 2022, 302, 119059. [Google Scholar] [CrossRef]
- Goolish, E.M.; Adelman, I.R. Effects of ration size and temperature on the growth of juvenile common carp (Cyprinus carpio L.). Aquaculture 1984, 36, 27–35. [Google Scholar] [CrossRef]
- Pang, X.; Fu, S.J.; Zhang, Y.G. Acclimation temperature alters the relationship between growth and swimming performance among juvenile common carp (Cyprinus carpio). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 199, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Oyugi, D.O.; Cucherousset, J.; Baker, D.J.; Britton, J.R. Effects of temperature on the foraging and growth rate of juvenile common carp, Cyprinus carpio. J. Therm. Biol. 2012, 37, 89–94. [Google Scholar] [CrossRef]
- Porath, D.; Pollock, J. Ammonia stripping by duckweed and its feasibility in circulating aquaculture. Aquat. Bot. 1982, 13, 125–131. [Google Scholar] [CrossRef]
- Tian, X.; Fang, Y.; Jin, Y.; Yi, Z.; Li, J.; Du, A.; He, K.; Huang, Y.; Zhao, H. Ammonium detoxification mechanism of ammonium-tolerant duckweed (Landoltia punctata) revealed by carbon and nitrogen metabolism under ammonium stress. Environ. Pollut. 2021, 277, 116834. [Google Scholar] [CrossRef] [PubMed]
- Gazzarrini, S.; Lejay, L.; Gojon, A.; Ninnemann, O.; Frommer, W.B.; von Wirén, N. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 1999, 11, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Maneepong, S. Nutrient dynamics of an aquaponic system in Southern Thailand. J. Agric. Sci. 2019, 11, 57–65. [Google Scholar] [CrossRef]
- Neidoni, D.G.; Dragalina, M.; Nicorescu, V.; Izabela, A. Lemna minor L. and pistia stratiotes L. in the accumulation of total phosphorus from the water. In Proceedings of the 25th International Symposium on Analytical and Environmental Problems, Szeged, Ungaria, 7–8 October 2019. [Google Scholar]
- Huang, X.; Dalsgaard, J.; Aalto, S.L.; Lund, I.; Pedersen, P.B. Influence of dietary phosphorus on orthophosphate accumulation in recirculating aquaculture systems with rainbow trout (Oncorhynchus mykiss). Aquac. Eng. 2023, 103, 102363. [Google Scholar] [CrossRef]
- Ardiansyah, A.; Fotedar, R. The abundance and diversity of heterotrophic bacteria as a function of harvesting frequency of duckweed (Lemna minor L.) in recirculating aquaculture systems. Lett. Appl. Microbiol. 2016, 63, 53–59. [Google Scholar] [CrossRef]
- Xu, J.; Shen, G. Effects of harvest regime and water depth on nutrient recovery from swine wastewater by growing Spirodela oligorrhiza. Water Environ. Res. 2011, 83, 2049–2056. [Google Scholar] [CrossRef]
- Coughlan, N.E.; Walsh, É.; Bolger, P.; Burnell, G.; O’Leary, N.; O’Mahoney, M.; Paolacci, S.; Wall, D.; Jansen, M.A. Duckweed bioreactors: Challenges and opportunities for large-scale indoor cultivation of Lemnaceae. J. Clean. Prod. 2022, 336, 130285. [Google Scholar] [CrossRef]
- Petersen, F.; Demann, J.; von Salzen, J.; Olfs, H.W.; Westendarp, H.; Wolf, P.; Appenroth, K.J.; Ulbrich, A. Re-circulating indoor vertical farm: Technicalities of an automated duckweed biomass production system and protein feed product quality evaluation. J. Clean. Prod. 2022, 380, 134894. [Google Scholar] [CrossRef]
- Leng, R.A.; Stambolie, J.H.; Bell, R. Duckweed—A potential high-protein feed resource for domestic animals and fish. Livest. Res. Rural Dev. 1995, 7, 36. [Google Scholar]
- Cui, W.; Cheng, J.J. Growing duckweed for biofuel production: A review. Plant Biol. 2015, 17, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Guo, W.; Yang, J.; Zhao, X.; Chen, Y.; Yao, L.; Hou, H. Enhanced biomass production and pollutant removal by duckweed in mixotrophic conditions. Bioresour. Technol. 2020, 317, 124029. [Google Scholar] [CrossRef]
- Yahaya, N.; Hamdan, N.H.; Zabidi, A.R.; Mohamad, A.M.; Suhaimi, M.L.H.; Johari, M.A.A.M.; Yahya, H.N.; Yahya, H. Duckweed as a future food: Evidence from metabolite profile, nutritional and microbial analyses. Future Foods 2022, 5, 100128. [Google Scholar] [CrossRef]
- Sońta, M.; Rekiel, A.; Batorska, M. Use of duckweed (Lemna L.) in sustainable livestock production and aquaculture–a review. Ann. Anim. Sci. 2019, 19, 257–271. [Google Scholar] [CrossRef]
- Oláh, V.; Appenroth, K.J.; Lam, E.; Sree, K.S. Sixth international conference on duckweed research and applications presents lemnaceae as a model plant system in the genomics and postgenomics era. Plants 2023, 12, 2134. [Google Scholar] [CrossRef]
- Fiordelmondo, E.; Ceschin, S.; Magi, G.E.; Mariotti, F.; Iaffaldano, N.; Galosi, L.; Roncarati, A. Effects of partial substitution of conventional protein sources with duckweed (Lemna minor) meal in the feeding of rainbow trout (Oncorhynchus mykiss) on growth performances and product quality. Plants 2022, 11, 1220. [Google Scholar] [CrossRef]
- Minich, J.J.; Michael, T.P. A review of using duckweed (Lemnaceae) in fish feeds. Rev. Aquac. 2024, 16, 1212–1228. [Google Scholar] [CrossRef]
- Goswami, R.K.; Sharma, J.; Shrivastav, A.K.; Kumar, G.; Glencross, B.D.; Tocher, D.R.; Chakrabarti, R. Effect of Lemna minor supplemented diets on growth, digestive physiology and expression of fatty acids biosynthesis genes of Cyprinus carpio. Sci. Rep. 2022, 12, 3711. [Google Scholar] [CrossRef]
- Vulpe, C.B.; Toplicean, I.M.; Agachi, B.V.; Datcu, A.D. A Review on Uses of Lemna minor, a Beneficial Plant for Sustainable Water Treatments, in Relation to Bioeconomy Aspects. Water 2025, 17, 1400. [Google Scholar] [CrossRef]
- Stejskal, V.; Paolacci, S.; Toner, D.; Jansen, M.A. A novel multitrophic concept for the cultivation of fish and duckweed: A technical note. J. Clean. Prod. 2022, 366, 132881. [Google Scholar] [CrossRef]


| Control | 25 | 50 | |
|---|---|---|---|
| Average initial weight (g) | 38.39 ± 0.64 | 37.97 ± 0.66 | 38.37 ± 0.67 |
| Average final weight (g) | 64.63 ± 13.75 | 63.31 ± 14.01 | 64.70 ± 11.44 |
| Survival rate (%) | 90.00 ± 10.00 | 96.67 ± 5.77 | 86.67 ± 15.27 |
| SGRFish (%/day) | 0.98 ± 0.032 | 0.90 ± 0.067 | 0.98 ± 0.072 |
| FCR (g) | 0.83 ± 0.13 | 0.89 ± 0.23 | 0.85 ± 0.22 |
| Weight gain (g) | 26.24 ± 4.02 | 25.35 ± 7.22 | 26.33 ± 6.69 |
| Final biomass (g) | 601.67 ± 13.20 | 594.00 ± 114.64 | 554.33 ± 48.81 |
| CV% | 21.36 ± 5.72 | 22.58 ± 6.59 | 17.81 ± 2.09 |
| p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnár, P.I.; Bényi, B.C.; Bársony, P.; Posta, J.; Fehér, M. Investigation of Nutrient Removal Capacity and Growth Rate of Duckweed (Lemna minor) Under Different Harvesting Protocols in Aquaponics. Water 2025, 17, 3203. https://doi.org/10.3390/w17223203
Molnár PI, Bényi BC, Bársony P, Posta J, Fehér M. Investigation of Nutrient Removal Capacity and Growth Rate of Duckweed (Lemna minor) Under Different Harvesting Protocols in Aquaponics. Water. 2025; 17(22):3203. https://doi.org/10.3390/w17223203
Chicago/Turabian StyleMolnár, Péter István, Benedek Csaba Bényi, Péter Bársony, János Posta, and Milán Fehér. 2025. "Investigation of Nutrient Removal Capacity and Growth Rate of Duckweed (Lemna minor) Under Different Harvesting Protocols in Aquaponics" Water 17, no. 22: 3203. https://doi.org/10.3390/w17223203
APA StyleMolnár, P. I., Bényi, B. C., Bársony, P., Posta, J., & Fehér, M. (2025). Investigation of Nutrient Removal Capacity and Growth Rate of Duckweed (Lemna minor) Under Different Harvesting Protocols in Aquaponics. Water, 17(22), 3203. https://doi.org/10.3390/w17223203







