The Influence of Sewage on the Quantitative and Functional Diversity of Nematode Communities in Constructed Wetlands (VFCW): Analysis of Trophic Relationships Using Canonical Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objects
2.2. Sampling Points and Method
2.3. Performing a Canonical Correspondence Analysis (CCA)
- -
- Physicochemical parameters in the wastewater flowing through the beds, calculated as the average value between the sampling point, pumping station (3), and the wastewater collection point, intermediate well (11): pH, temperature (Temp), dissolved oxygen (DO), alkalinity (Alk), biochemical oxygen demand (BOD5), chemical oxygen demand (COD), total suspended solids (TSS), phosphate phosphorus (P-PO4), phosphorus pentoxide (P2O5), total phosphorus (TP), total nitrogen (TN), ammonium nitrogen (N-NH4), nitrate nitrogen (N-NO3), nitrite nitrogen (N-NO2), chlorides (Cl), iron (Fe);
- -
- Abundance and trophic groups of nematodes inhabiting the deposits.
2.4. Performing a Mann–Whitney–Wilcoxon Test
3. Results and Discussion
3.1. Physicochemical Parameters of Wastewater Flowing Through the Soil-Plant Bed and the Abundance and Trophic Groups of Nematodes Inhabiting This Bed in Jabłonowo-Wypychy 10
3.2. Physicochemical Parameters of Wastewater Flowing Through the Soil-Plant Bed and the Abundance and Trophic Groups of Nematodes Inhabiting This Bed in Rzące 25
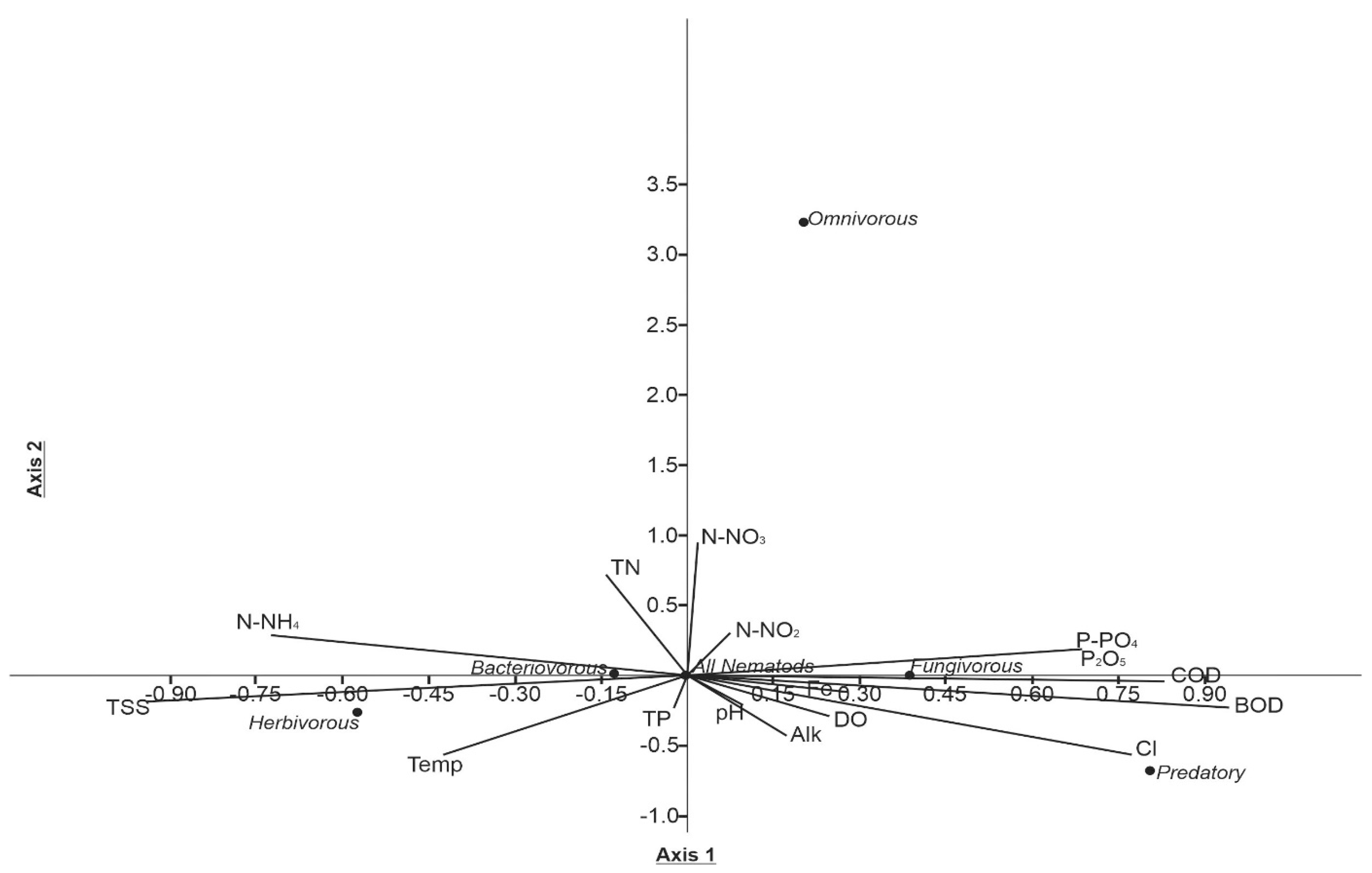
3.3. Canonical Correspondence Analysis (CCA)
3.4. Mann–Whitney–Wilcoxon Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mani, A.; Badola, R.; Kumari, M.; Mishra, V.N.; Thamaga, K.H.; Hasher, F.F.B.; Zhran, M. Watershed Prioritization with Respect to Flood Susceptibility in the Indian Himalayan Region (IHR) Using Geospatial Techniques for Sustainable Water Resource Management. Water 2025, 17, 2039. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Sim, I.; Won, C.; Park, J.; Kim, R. Exploring Urban Water Management Solutions for Mitigating Water Cycle Issues: Application to Bogota, Colombia. Water 2025, 17, 1992. [Google Scholar] [CrossRef]
- Ajtai, I.; Anton, A.; Roba, C.; Botezan, C.; Piștea, I.; Oprea, M.; Baciu, C. Wastewater Impact on Surface Water Quality and Suitability of Water Reuse in Agriculture Using a Comprehensive Methodology Based on PCA and Specific Indices. Water 2025, 17, 2011. [Google Scholar] [CrossRef]
- Christou, A.; Beretsou, V.G.; Iakovides, I.C.; Karaolia, P.; Michael, C.; Benmarhnia, T.; Chefetz, B.; Donner, E.; Gawlik, B.M.; Lee, Y.; et al. Sustainable wastewater reuse for agriculture. Nat. Rev. Earth Environ. 2024, 5, 504–521. [Google Scholar] [CrossRef]
- Muniz, R.N.; Buratto, W.G.; Cardoso, R.; Barros, C.F.d.O.; Nied, A.; Gonzalez, G.V. State-of-the-Art Decarbonization in Sludge Thermal Treatments for Electrical Power Generation Considering Sensors and the Application of Artificial Intelligence. Water 2025, 17, 1946. [Google Scholar] [CrossRef]
- Directive (EU) 2024/3019 of the European Parliament and of the Council of 27 November 2024 Concerning Urban Wastewater Treatment (Recast) (Test with EEA Relevance). Available online: http://data.europa.eu/eli/dir/2024/3019/oj (accessed on 20 October 2025).
- Uy, M.J.; Robles, M.E.; Oh, Y.; Haque, M.T.; Mueca, C.C.; Kim, L.-H. Biodiversity Monitoring in Constructed Wetlands: A Systematic Review of Assessment Methods and Ecosystem Functions. Diversity 2025, 17, 367. [Google Scholar] [CrossRef]
- Khajah, M.; Ahmed, M.E. Performance evaluation of a pilot wetland system for wastewater treatment. J. Eng. Res. 2023, 13, 1387–1395. [Google Scholar] [CrossRef]
- Stefanakis, A.; Akratos Ch, S.; Tsihrintzis, V.A. Vertical Flow Constructed Wetlands. In Eco-Engineering Systems for Wastewater and Sludge Treatment; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780124046122. [Google Scholar] [CrossRef]
- Bagińska, M.; Warężak, T.; Romaniuk, W.; Kozacki, D.; Skibko, Z.; Borusiewicz, A.; Dąbrowski, J. Soil Nematodes as an Indicator of the Efficiency of Hydrophytic Treatment Plants with Vertical Wastewater Flow. Sustainability 2025, 17, 1329. [Google Scholar] [CrossRef]
- Furmanczyk, E.M.; Kozacki, D.; Ourry, M.; Bickel, S.; Olimi, E.; Masquelier, S.; Turci, S.; Bohr, A.; Maisel, H.; D’Avino, L.; et al. An Analysis of Soil Nematode Communities Across Diverse Horticultural Cropping Systems. Soil Syst. 2025, 9, 77. [Google Scholar] [CrossRef]
- Atira, L.S.; Kakouli-Duarte, T. Implications of Fertilisation on Soil Nematode Community Structure and Nematode-Mediated Nutrient Cycling. Crops 2025, 5, 50. [Google Scholar] [CrossRef]
- Muan, W.; Lin, Y.; Shang, Y.; Bai, M.; Li, L.; Li, X.; Deng, P.; Riaz, L.; Guo, Y.; Lu, J. Fate and Removal of Oxytetracycline and Antibiotic Resistance Genes in Vertical-Flow Constructed Wetland with Different Substrates. Water 2025, 17, 1412. [Google Scholar] [CrossRef]
- ISO 5667-10:2020; Water Quality—Sampling. Part 10: Guidance on Sampling of Wastewater. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/70934.html (accessed on 20 October 2025).
- ISO 5815-1:2019; Water Quality—Determination of Biochemical Oxygen Demand After n Days (BODn). Part 1: Dilution and Seeding Method with Allylthiourea Addition. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/69058.html (accessed on 20 October 2025).
- EN 872:1996; Water Quality. Determination of Suspended Solids. Method by Filtration Through Glass Fibre Filters. European Committee for Standardization: Brussels, Belgium, 1996. Available online: https://standards.iteh.ai/catalog/standards/cen/c5e6df29-c5d6-4f5c-8b7d-6f8d9def0921/en-872-1996 (accessed on 20 October 2025).
- EPA Method 350.1; Determination of Ammonia Nitrogen by Semi-Automated Colorimetry. U.S. Environmental Protection Agency: Washington, DC, USA, 1993. Available online: https://www.epa.gov/esam/epa-method-3501-determination-ammonia-nitrogen-semi-automated-colorimetry (accessed on 20 October 2025).
- ISO 7150-1:1984; Water Quality—Determination of Ammonium—Part 1: Manual Spectrometric Method. ISO: Geneva, Switzerland, 1984. Available online: https://www.iso.org/standard/13742.html (accessed on 20 October 2025).
- DIN 38406-5:1983-10; Deutsche Einheitsverfahren zur Wasser—Abwasser—Und Schlammuntersuchung; Kationen (Gruppe E); Bestimmung des Ammonium—Stickstoffs (E5). German Institute for Standardisation: Berlin, Germany, 1983. Available online: https://www.dinmedia.de/de/norm/din-38406-5/1061756 (accessed on 20 October 2025).
- EPA Method 354.1. Nitrogen, Nitrite (Spectrophotometric). Available online: https://www.nemi.gov/methods/method_summary/5777/ (accessed on 20 October 2025).
- APHA-4500 NO2-B; Nitrogen (Amonia). American Public Health Association: Washington, DC, USA, 1992. Available online: https://www.nemi.gov/methods/method_summary/7415/ (accessed on 20 October 2025).
- DIN EN 26777; Water Quality; Determination of Nitrite; Molecular Absorption Spectrometric Method (ISO 6777:1984); German Version EN 26777:1993. German Institute for Standardisation: Berlin, Germany, 1993. Available online: https://www.en-standard.eu/din-en-26777-water-quality-determination-of-nitrite-molecular-absorption-spectrometric-method-iso-6777-1984-german-version-en-26777-1993/?srsltid=AfmBOoqvM6Wusnwg16eSn5WIT7qadPyWtWWNiPUAfcV5e575yKlt_sZo (accessed on 20 October 2025).
- DIN 38405-9:2011-09; German Standard Methods for Examination of Water, Wastewater and Sludge—Anions (Group D)—Part 9: Spectrometric Determination of Nitrate (D 9). German Institute for Standardisation: Berlin, Germany, 2011. Available online: https://www.dinmedia.de/en/standard/din-38405-9/144185708 (accessed on 20 October 2025).
- ISO 11905-1:1997; Water Quality—Determination of Nitrogen. Part 1: Method Using Oxidative Digestion with Peroxodisulfate. ISO: Geneva, Switzerland, 1997. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:11905:-1:ed-1:v1:en (accessed on 20 October 2025).
- Method 365.3: Phosphorous, All Forms (Colorimetric, Ascorbic Acid, Two Reagent). United States Environmental Protection Agency. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/method_365-3_1978.pdf (accessed on 20 October 2025).
- APHA Method 4500-P; Standard Methods for the Examination of Water and Wastewater. American Public Health Association: Washington, DC, USA, 2012. Available online: https://law.resource.org/pub/us/cfr/ibr/002 /apha.method.4500-p.1992.pdf (accessed on 20 October 2025).
- ISO 6878:2004; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. ISO: Geneva, Switzerland, 2004. Available online: https://www.iso.org/standard/36917.html (accessed on 20 October 2025).
- APHA Method 4500-CL; Standard Methods for the Examination of Water and Wastewater. American Public Health Association: Washington, DC, USA, 2011. Available online: https://law.resource.org/pub/us/cfr/ibr/002/apha.method.4500-cl.1992.pdf (accessed on 20 October 2025).
- EPA-NERL: 325.1: Chloride by Automated Colorimetry. Available online: https://www.nemi.gov/methods/method_summary/5771/ (accessed on 20 October 2025).
- ISO 8466-1; Water Quality—Calibration and Evaluation of Analytical Methods—Part 1: Linear Calibration Function. ISO: Geneva, Switzerland, 2021. Available online: https://standards.globalspec.com/std/14480922/8466-1#:~:text=Water%20quality%20%E2%80%94%20Calibration%20and%20evaluation%20of%20analytical,methods%20and%20specifies%20the%20calculation%20of%20analytical%20results (accessed on 20 October 2025).
- DIN 38402.A51; Germen Standard Methods for the Examination of Water, Wastewater and Sludge—General Information (group A)—A51: Calibration of analytical methods—Linear calibration (A51). German Institute for Standardisation: Berlin, Germany, 1986. Available online: https://www.iso.org/standard/77139.html (accessed on 20 October 2025).
- Southey, J.F. Laboratory Methods for Work with Plant and Soil Nematodes; Ministry of Agriculture, Fisheries and Food; Her Majesty’s Stationery Office: Berkeley, CA, USA, 1986. [Google Scholar]
- McSorley, R.; Frederick, J.J. Effect of extraction method on perceived composition of the soil nematode community. Appl. Soil Ecoogy 2004, 27, 55–63. [Google Scholar] [CrossRef]
- Van Bezooijen, J. Methods and Techniques for Nematology; Wageningen University: Wageningen, The Netherlands, 2006. [Google Scholar]
- Aubry, E.; Akanwari, J.; Liang, P.; Ellouze, W.; Gaiero, J.; Sultana, T. Effects of Cover Crops on Nematode Communities in Spinach Production. Int. J. Mol. Sci. 2024, 25, 13366. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T. De nematoden van Nederland. In Een Identificatietabel Voor de in Nederland Aangetroffenen Zoetwater—En Bodembewonende Nematoden; Koninklijke Nederlandse Natuurhistorische Vereniging: Utrecht, The Netherlands, 1988. [Google Scholar]
- Jia, J.; Xia, M.; Zhang, Y.; Tian, S.; Hu, Y.; Zhang, Z.; Zhai, X.; Qu, B.; Hao, L. Effects of Freshwater Restoration on Phytoplankton and Zooplankton Communities in the Yellow River Delta. Water 2025, 17, 2348. [Google Scholar] [CrossRef]
- Saliba, Y.; Bărbulescu, A. A Multi-Method Approach to Analyzing Precipitation Series and Their Change Points in Semi-Arid Climates: The Case of Dobrogea. Water 2025, 17, 391. [Google Scholar] [CrossRef]
- Song, G.; Xu, P.; Zhu, Y.; Abate, A.R.; Mi, W.; Bi, Y. Effects of Massive Use of Disinfectants on the Plankton Communities in Lakes from Wuhan. Water 2023, 15, 3875. [Google Scholar] [CrossRef]
- Wiranegara, P.; Sunardi, S.; Sumiarsa, D.; Juahir, H. Characteristics and Changes in Water Quality Based on Climate and Hydrology Effects in the Cirata Reservoir. Water 2023, 15, 3132. [Google Scholar] [CrossRef]
- Barka, E.; Koukoura, A.; Statiris, E.; Seintos, T.; Stasinakis, A.S.; Mamais, D.; Malamis, S.; Noutsopoulos, C. The Fate of Contaminants of Emerging Concern in an Upflow Anaerobic Sludge Blanket Reactor Coupled with Constructed Wetlands for Decentralized Domestic Wastewater Treatment. Molecules 2025, 30, 2671. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.; Afzal, S.; Nesar, H.; Imran, Z.; Ahmad, W. Impact of metal polluted sewage water on soil nematode assemblages in agricultural settings of Aligarh, India. Soil Ecol. Lett. 2024, 6, 230193. [Google Scholar] [CrossRef]


| Name of Association | Type of Standard |
|---|---|
| NH4+ | EPA Method 350.1 [18]; ISO 7150-1:1984 [19]; DIN 38406-5:1983-10 [20] Error margins: ±1.0 mg [mgN-NH4·dm−3] |
| NO2− | EPA Method 354.1 [21]; APHA-4500 NO2− B [22]; DIN EN 26777 [23] Error margins: ±0.0028 mg [mgN-NO2·dm−3] |
| NO3− | DIN 38405-9 [24] Error margins: ±0.13 mg [mgN-NO3·dm−3] |
| TN | ISO 11905-1:1997 [25]; DIN 38405-9 [24] Error margins: ±1.1 mg [mgN·dm−3] |
| TP | EPA Method 365.3 [26]; APHA Method 4500-P [27]; ISO 6878:2004 [28] Error margins: ±0.09 mg [mgP·dm−3] |
| Cl− | APHA Method 4500-Cl [29]; EPA-NERL: 325.1 [30] Error margins: ±2.8 mg [mgCl·dm−3] |
| Fe | ISO 8466-1 [31]; DIN 38402 A51 [32] Error margins: ±0.016 mg [mgFe·dm−3] |
| St. | Av. | SD | CV [%] | ||||
|---|---|---|---|---|---|---|---|
| pH | [-] | 7.1 | 7.2 | 7.2 | 7.2 | 0 | 0 |
| Temp | [°C] | 18.6 | 19.7 | 20.1 | 19.5 | 1 | 5 |
| DO | [mgO2·dm−3] | 0.3 | 2.5 | 1.1 | 1.3 | 1 | 77 |
| Alk | [mmol·dm−3] | 9.5 | 12.0 | 8.6 | 10.0 | 2 | 20 |
| BOD | [mgO2·dm−3] | 25.5 | 150.5 | 77.0 | 84.3 | 63 | 75 |
| COD | [mg·dm−3] | 399.6 | 303.4 | 211.7 | 304.9 | 94 | 31 |
| TSS | [mg·dm−3] | 14.0 | 120.0 | 3031.0 | 1055.0 | 1712 | 162 |
| P-PO4 | [mgP-PO4·dm−3] | 6.2 | 23.1 | 0.5 | 9.9 | 12 | 121 |
| P2O5 | [mgP2O5·dm−3] | 14.2 | 53.1 | 1.0 | 22.8 | 27 | 118 |
| TP | [mgP·dm−3] | 20.3 | 25.3 | 12.5 | 19.4 | 6 | 31 |
| TN | [mgN·dm−3] | 138.3 | 145.8 | 111.7 | 131.9 | 18 | 14 |
| N-NH4 | [mgN-NH4·dm−3] | 67.1 | 132.8 | 6.6 | 68.8 | 63 | 92 |
| N-NO2 | [mgN-NO2·dm−3] | 1.5 | 0.5 | 2.1 | 1.4 | 1 | 71 |
| Cl | [mgCl·dm−3] | 227.4 | 240.9 | 129.9 | 199.4 | 61 | 31 |
| Fe | [mgFe·dm−3] | 0.1 | 0.1 | 0.1 | 0.1 | 0 | 0 |
| N-NO3 | [mgN-NO3·dm−3] | 12.2 | 7.6 | 26.7 | 15.5 | 10 | 64 |
| St. | Nematode Abundance | Bacterivor. | Pred. | Fungivor. | Herbivor. | Omnivor. |
|---|---|---|---|---|---|---|
| [N·m−2] | ||||||
| 567,000.0 | 294,840.0 | 0.0 | 215,460.0 | 28,350.0 | 28,350.0 | |
| 1,466,000.0 | 1,143,480.0 | 0.0 | 249,220.0 | 73,300.0 | 0.0 | |
| 1,161,000.0 | 777,870.0 | 0.0 | 255,420.0 | 69,660.0 | 58,050.0 | |
| Av. | 1,064,666.7 | 738,730.0 | 0.0 | 240,033.3 | 57,103.3 | 28,800.0 |
| SD | 457,176 | 425,672 | 0 | 21,506 | 24,968 | 29,028 |
| St. | Av. | SD | CV [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | [-] | 7.7 | 7.1 | 7.3 | 7.3 | 7.4 | 7.4 | 0 | 0 |
| Temp | [°C] | 4.8 | 18.0 | 19.4 | 5.3 | 19.0 | 13.3 | 8 | 60 |
| DO | [mgO2·dm−3] | 1.9 | 2.1 | 1.4 | 1.5 | 1.7 | 1.7 | 0 | 0 |
| Alk | [mmol·dm−3] | 10.2 | 10.1 | 173.0 | 2.4 | 10.3 | 41.2 | 74 | 180 |
| BOD | [mgO2·dm−3] | 102.0 | 78.5 | 97.5 | 68.0 | 30.0 | 75.2 | 29 | 38 |
| COD | [mg·dm−3] | 323.4 | 338.7 | 142.5 | 190.9 | 61.7 | 211.4 | 119 | 56 |
| TSS | [mg·dm−3] | 65.0 | 22.0 | 52.5 | 55.0 | 511.5 | 141.2 | 208 | 147 |
| P-PO4 | [mgP-PO4·dm−3] | 5.3 | 4.3 | 9.3 | 7.2 | 0.2 | 5.3 | 3 | 57 |
| P2O5 | [mgP2O5·dm−3] | 12.3 | 10.0 | 21.5 | 16.7 | 0.7 | 12.2 | 8 | 65 |
| TP | [mgP·dm−3] | 6.1 | 9.7 | 9.7 | 7.5 | 7.9 | 8.2 | 2 | 24 |
| TN | [mgN·dm−3] | 58.7 | 65.0 | 73.9 | 81.1 | 66.2 | 69.0 | 9 | 13 |
| N-NH4 | [mgN-NH4·dm−3] | 0.8 | 6.0 | 0.9 | 11.8 | 2.1 | 4.3 | 5 | 116 |
| N-NO3 | [mgN-NO3·dm−3] | 0.1 | 10.2 | 0.1 | 4.8 | 2.3 | 3.5 | 4 | 114 |
| N-NO2 | [mgN-NO2·dm−3] | 140.2 | 142.4 | 175.9 | 64.1 | 45.1 | 113.5 | 56 | 49 |
| Cl | [mgCl·dm−3] | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0 | 0 |
| Fe | [mgFe·dm−3] | 0.8 | 6.0 | 0.9 | 11.8 | 2.1 | 4.3 | 5 | 116 |
| St. | Nematode Abundance | Bacterivor. | Pred. | Fungivor. | Herbivor. | Omnivor. |
|---|---|---|---|---|---|---|
| [N·m−2] | ||||||
| 375,000.0 | 236,250.0 | 11,250.0 | 131,250.0 | 0.0 | 0.0 | |
| 783,000.0 | 477,630.0 | 0.0 | 289,710.0 | 15,660.0 | 0.0 | |
| 539,000.0 | 334,180.0 | 16,170.0 | 167,090.0 | 21,560.0 | 0.0 | |
| 240,000.0 | 168,000.0 | 0.0 | 64,800.0 | 0.0 | 7200.0 | |
| 1,092,000.0 | 884,520.0 | 0.0 | 141,960.0 | 65,520.0 | 0.0 | |
| Av. | 605,800.0 | 420,116.0 | 5484.0 | 158,962.0 | 20,548.0 | 1440.0 |
| SD | 338,870 | 284,488 | 7708 | 82,287 | 26,888 | 3220 |
| Ordinal Number | Physicochemical Parameter | Parameter “W” | Parameter “p-Value” | Statistical Significance |
|---|---|---|---|---|
| 1. | Alkalinity (Alk) | 23 | 0.283 | absence |
| 2. | Biochemical oxygen demand (BOD) | 10 | 0.3677 | absence |
| 3. | Chlorides (Cl) | 24 | 0.2141 | absence |
| 4. | Chemical oxygen demand (COD) | 16 | 1 | absence |
| 5. | Iron (Fe) | 4 | 0.0448 | absence |
| 6. | Ammonium nitrogen (N-NH4) | 19 | 0.6706 | absence |
| 7. | Nitrite nitrogen (N-NO2) | 18 | 0.7763 | absence |
| 8. | Nitrate nitrogen (N-NO3) | 27.5 | 0.060 | absence |
| 9. | Total nitrogen (TN) | 22 | 0.3677 | absence |
| 10. | Dissolved oxygen (DO) | 7 | 0.1453 | absence |
| 11. | Phosphorus pentoxide (P2O5) | 18 | 0.8081 | absence |
| 12. | pH reaction | 16.5 | 1 | absence |
| 13. | Phosphate phosphorus (P-PO4) | 21.5 | 0.3949 | absence |
| 14. | Total phosphorus (TP) | 24 | 0.2141 | absence |
| 15. | Total suspended solids (TSS) | 10 | 0.3677 | absence |
| 16. | Temperature (Temp) | 23 | 0.2828 | absence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagińska, M.; Warężak, T.; Romaniuk, W.; Kozacki, D.; Skibko, Z.; Borusiewicz, A.; Dąbrowski, J. The Influence of Sewage on the Quantitative and Functional Diversity of Nematode Communities in Constructed Wetlands (VFCW): Analysis of Trophic Relationships Using Canonical Methods. Water 2025, 17, 3044. https://doi.org/10.3390/w17213044
Bagińska M, Warężak T, Romaniuk W, Kozacki D, Skibko Z, Borusiewicz A, Dąbrowski J. The Influence of Sewage on the Quantitative and Functional Diversity of Nematode Communities in Constructed Wetlands (VFCW): Analysis of Trophic Relationships Using Canonical Methods. Water. 2025; 17(21):3044. https://doi.org/10.3390/w17213044
Chicago/Turabian StyleBagińska, Magdalena, Tomasz Warężak, Wacław Romaniuk, Dawid Kozacki, Zbigniew Skibko, Andrzej Borusiewicz, and Jarosław Dąbrowski. 2025. "The Influence of Sewage on the Quantitative and Functional Diversity of Nematode Communities in Constructed Wetlands (VFCW): Analysis of Trophic Relationships Using Canonical Methods" Water 17, no. 21: 3044. https://doi.org/10.3390/w17213044
APA StyleBagińska, M., Warężak, T., Romaniuk, W., Kozacki, D., Skibko, Z., Borusiewicz, A., & Dąbrowski, J. (2025). The Influence of Sewage on the Quantitative and Functional Diversity of Nematode Communities in Constructed Wetlands (VFCW): Analysis of Trophic Relationships Using Canonical Methods. Water, 17(21), 3044. https://doi.org/10.3390/w17213044







