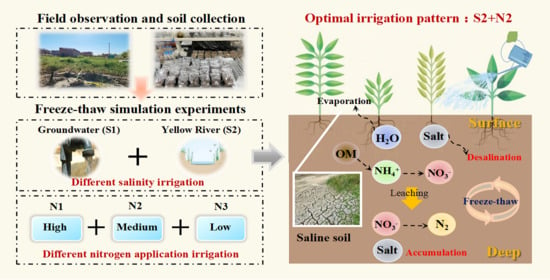
Freeze–Thaw-Driven Dynamics of Soil Water–Salt and Nitrogen: Effects and Implications for Irrigation Management in the Hetao Irrigation District
Abstract
1. Introduction
2. Materials and Methods
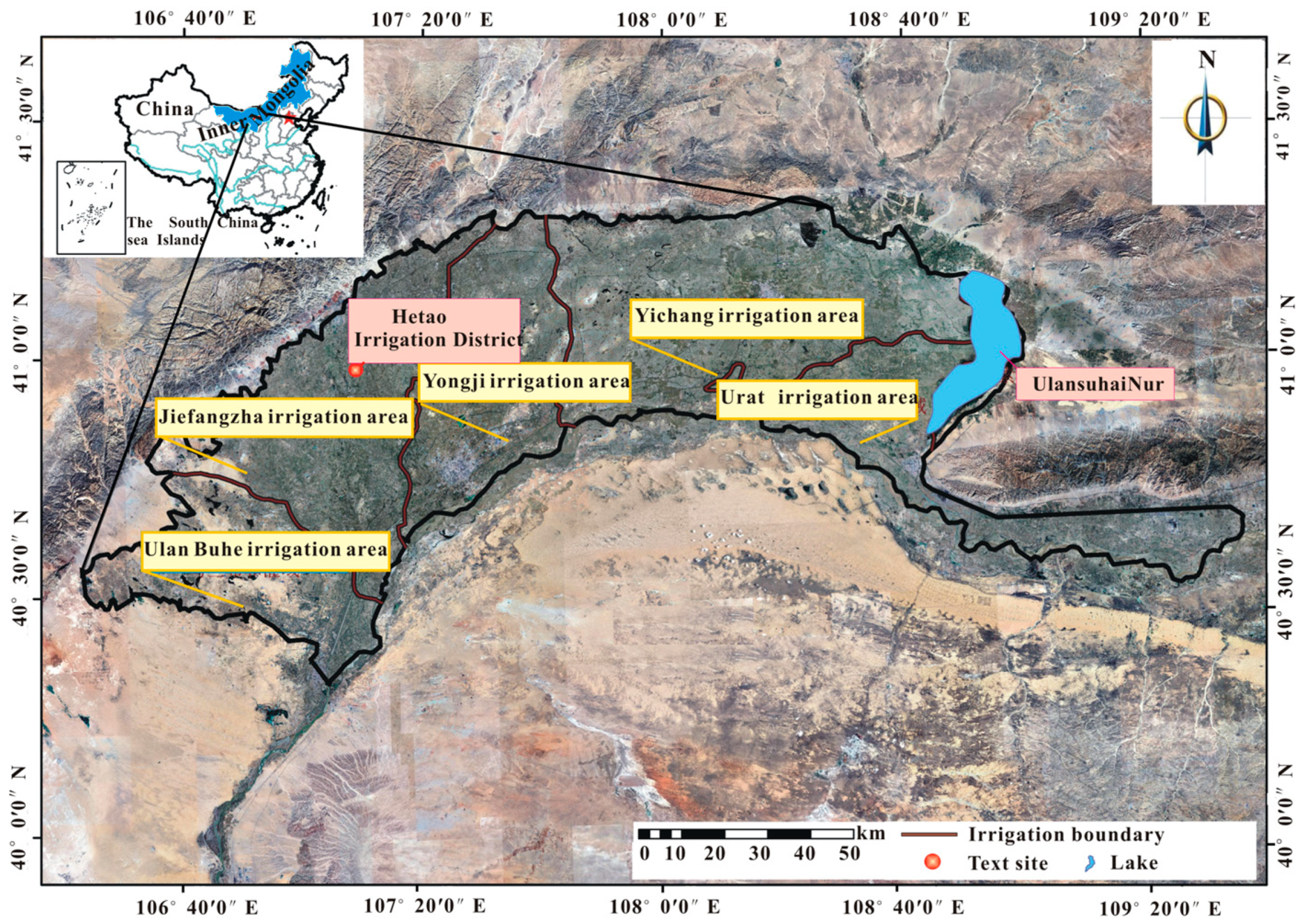
2.1. Study Area
2.2. Methodology
2.2.1. Sample Collection and Analysis
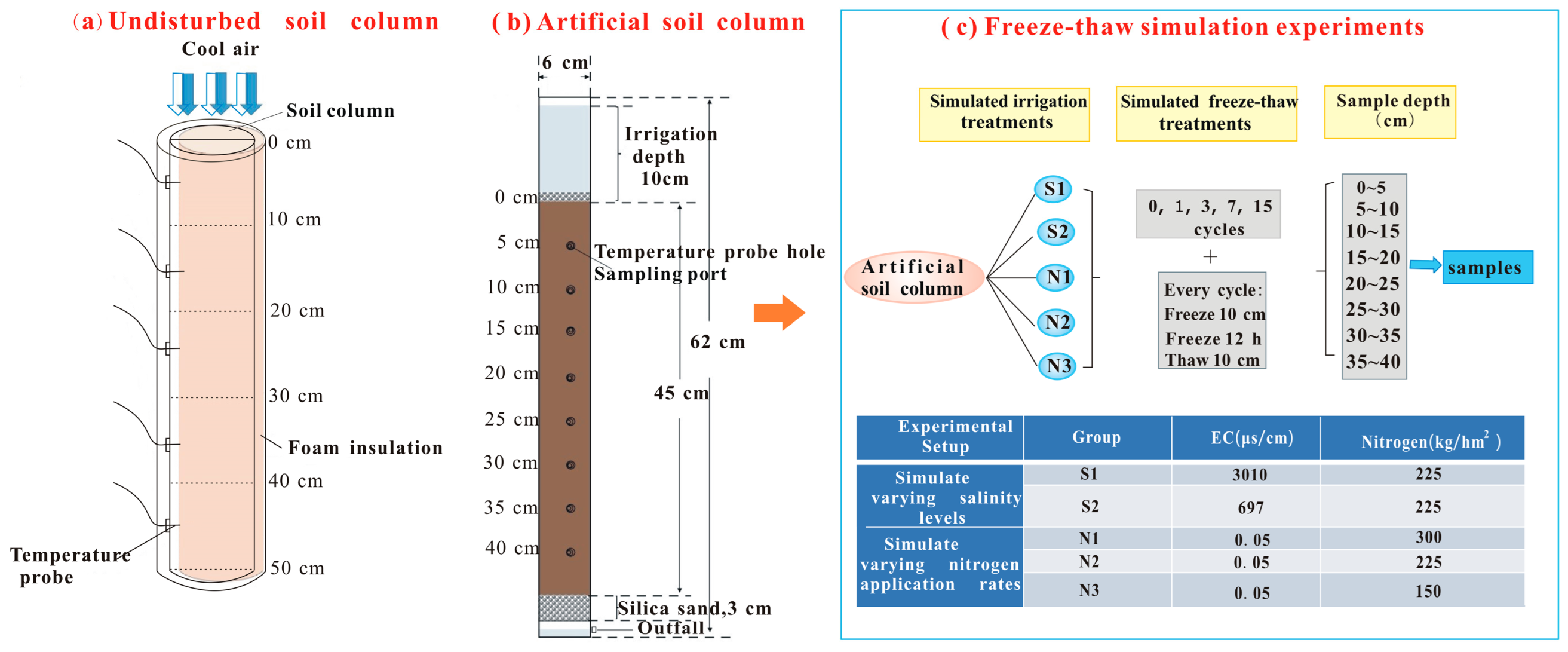
2.2.2. Freeze–Thaw Simulation Experiments
- 1.
- Undisturbed soil column experiments for freeze–thaw cycles
- 2.
- Artificial soil column experiments for freeze–thaw and irrigation practices
2.2.3. Data and Statistical Analysis
3. Results and Discussions
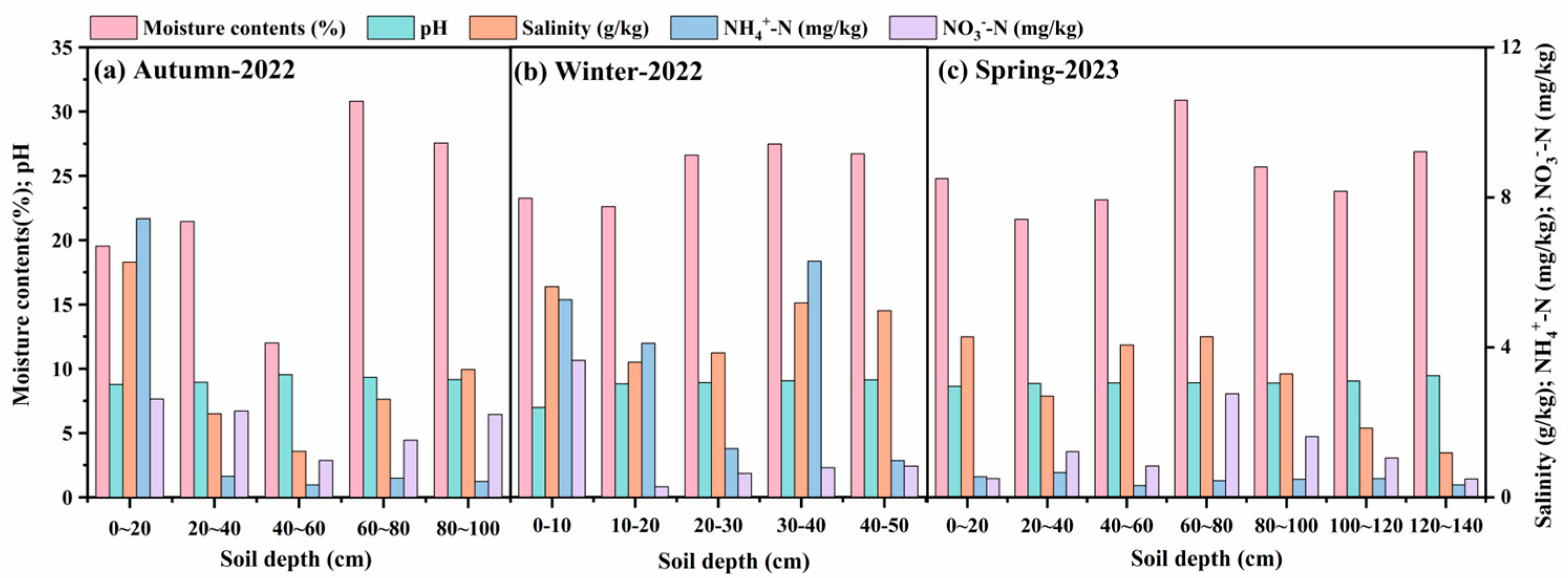
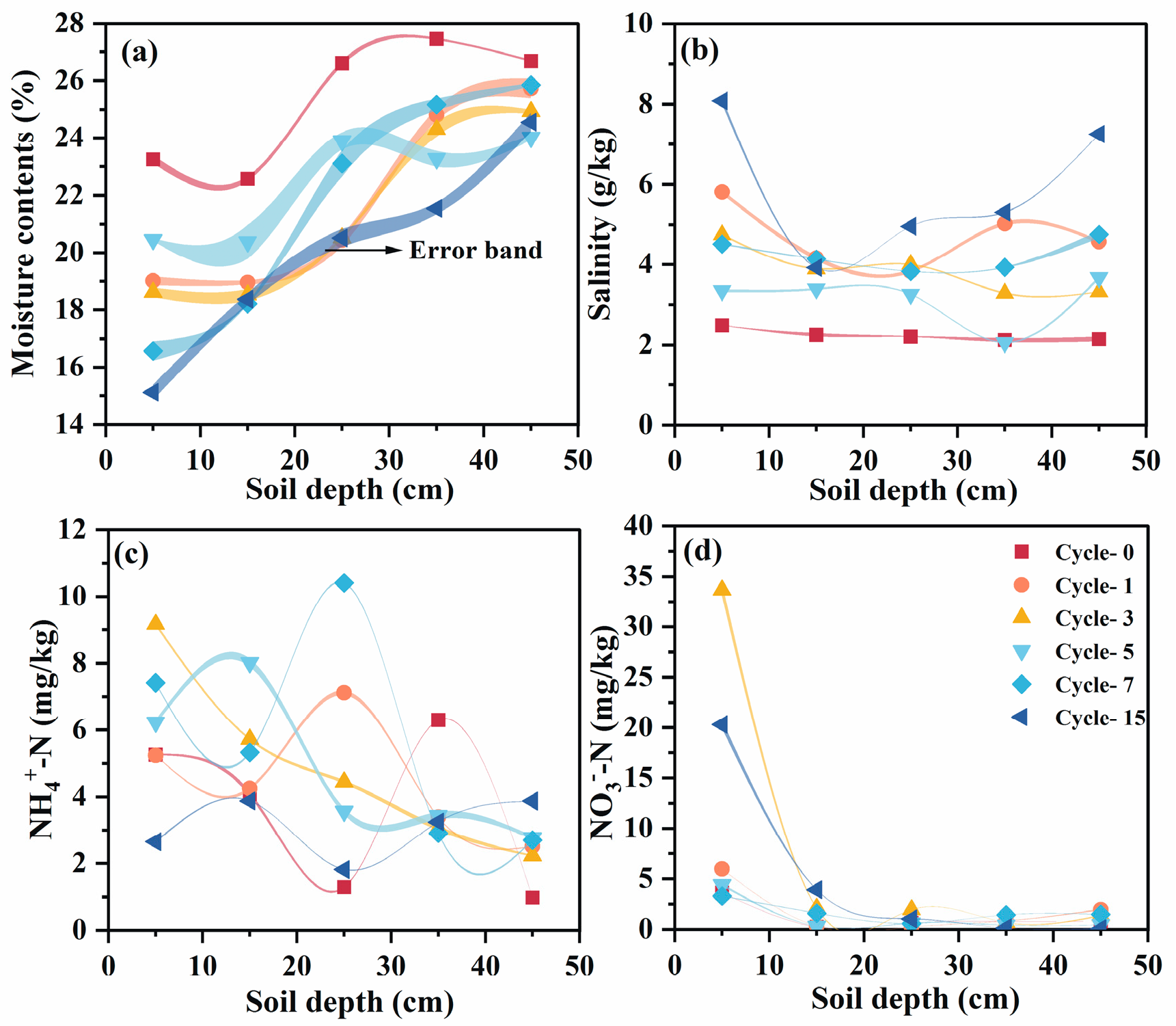
3.1. Effects of Seasonal Freeze–Thaw Processes on Soil Properties
3.2. Distribution Pattern of Water–Salt–Nitrogen Under Simulated Freeze–Thaw Cycles
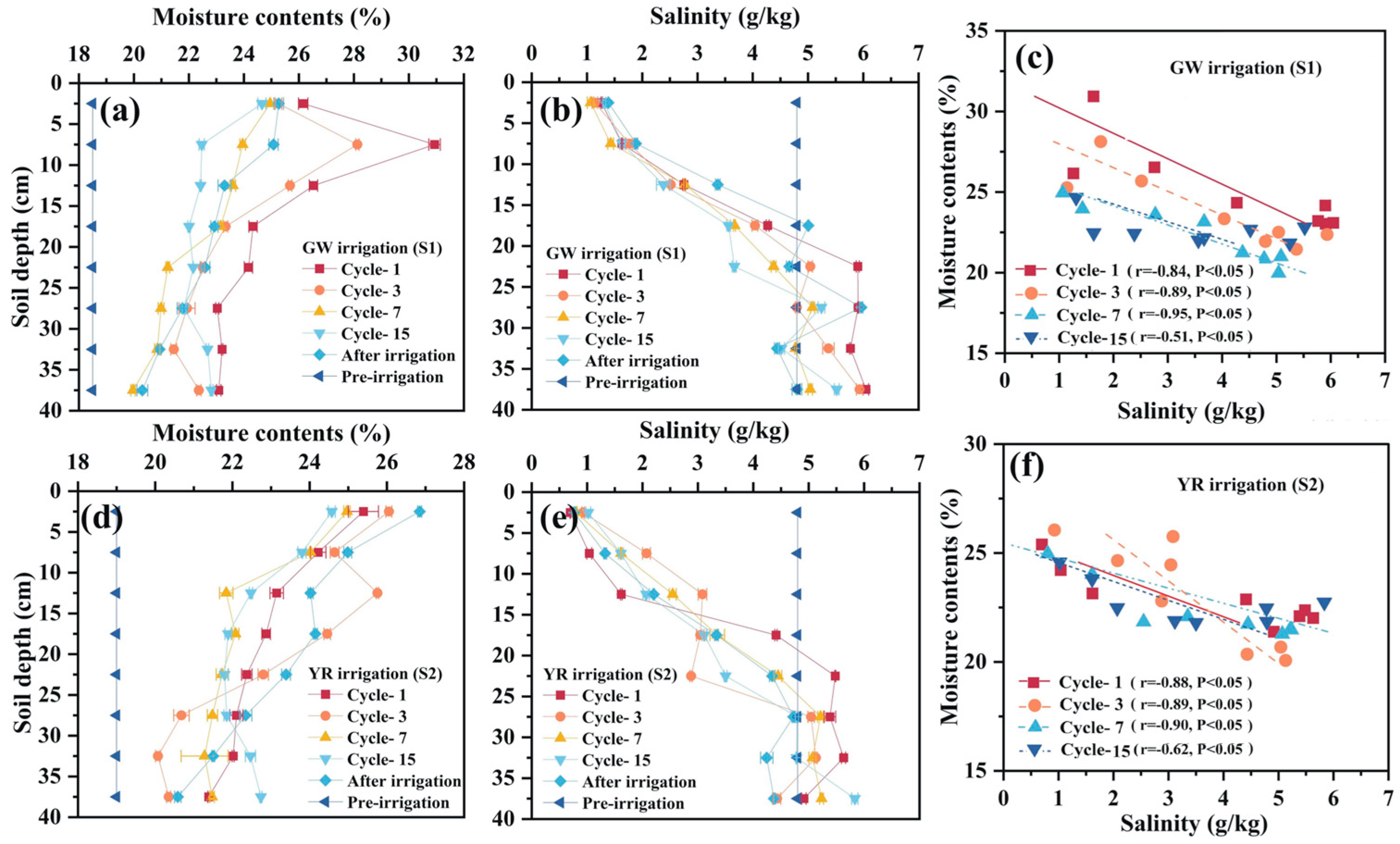
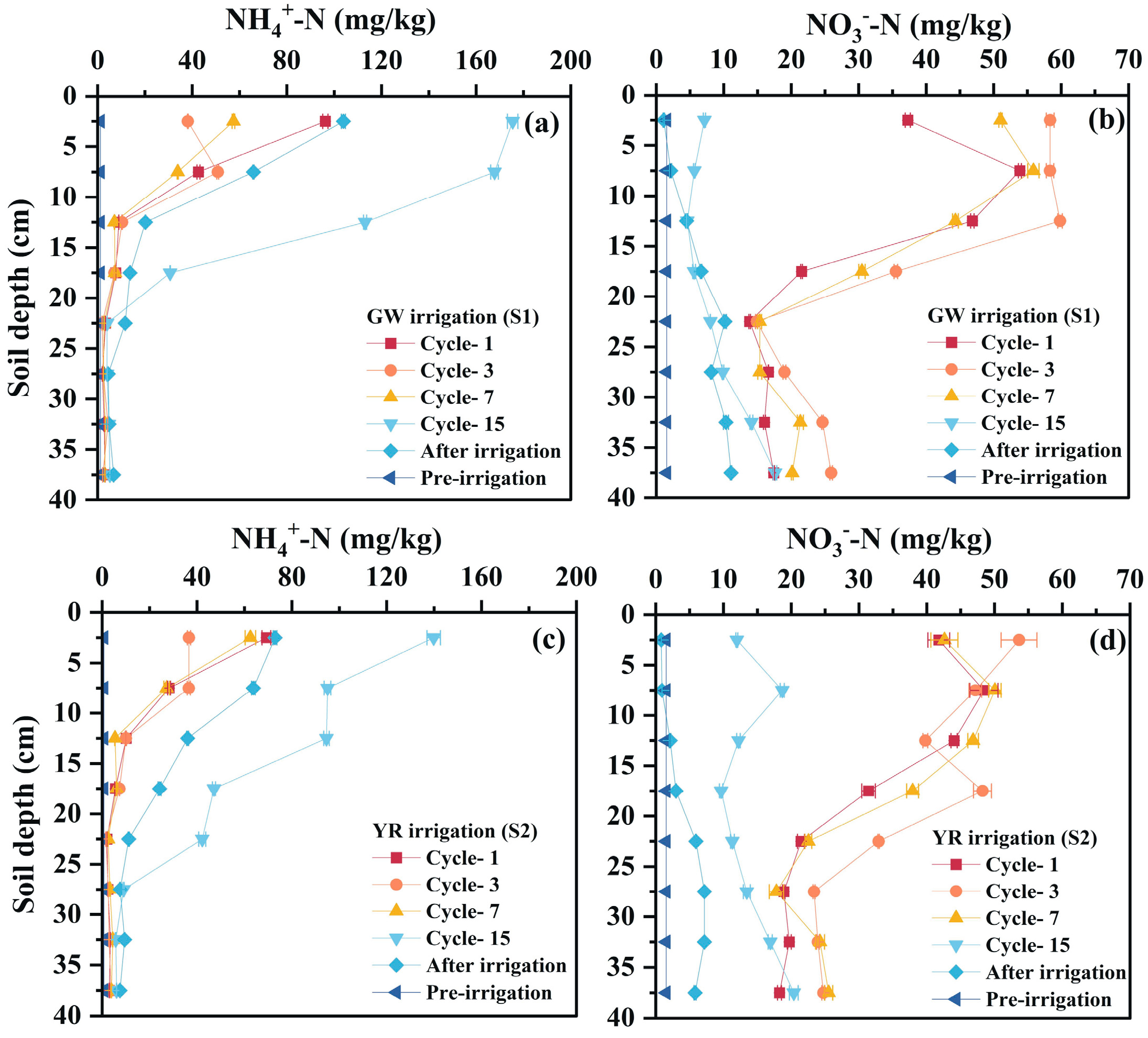
3.3. Water–Salt–Nitrogen Dynamics Driven by Irrigation in Freeze–Thaw Cycling Systems
3.3.1. Freeze-Thaw Driven Water and Salt Transport
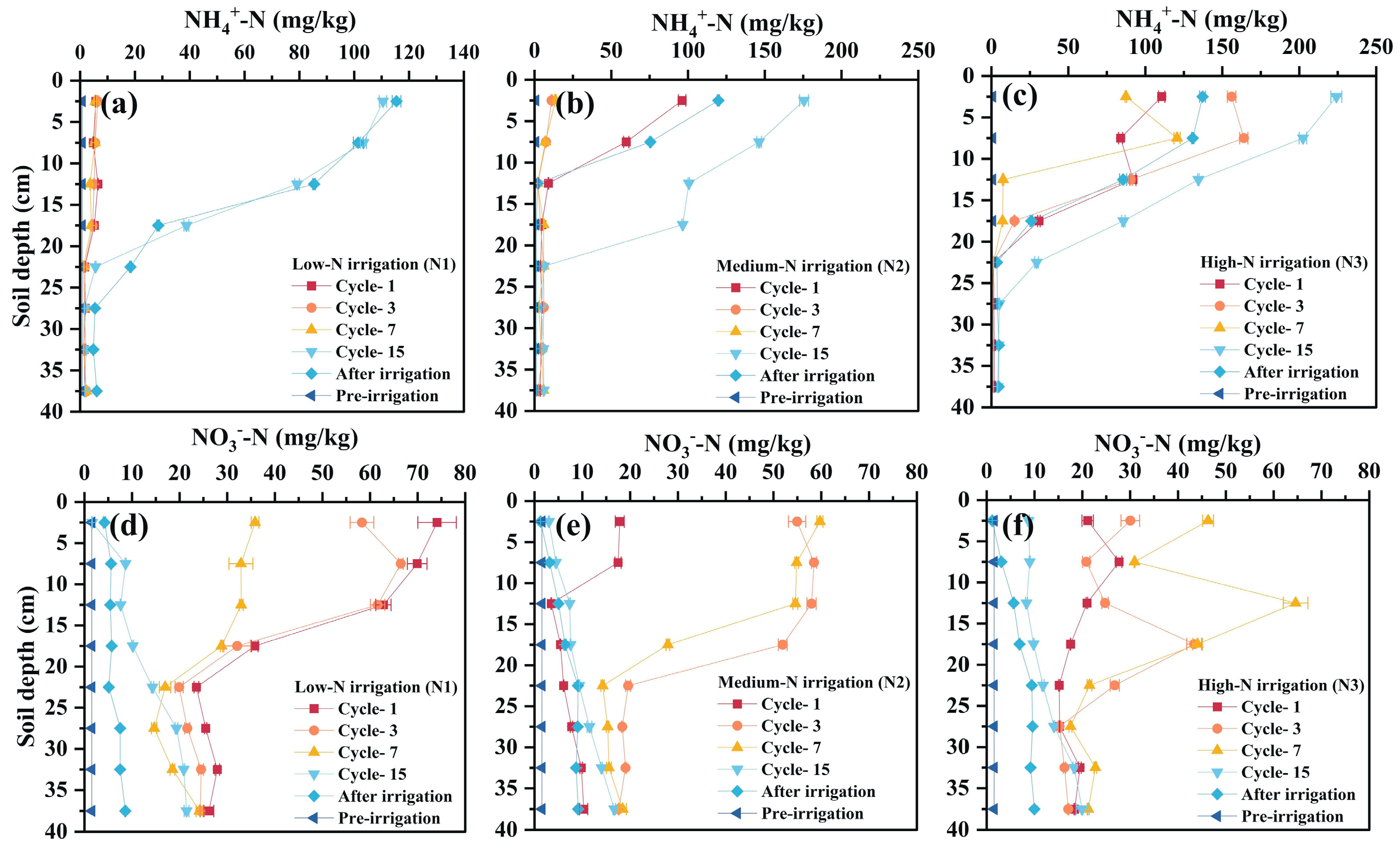
3.3.2. Nitrogen Transport
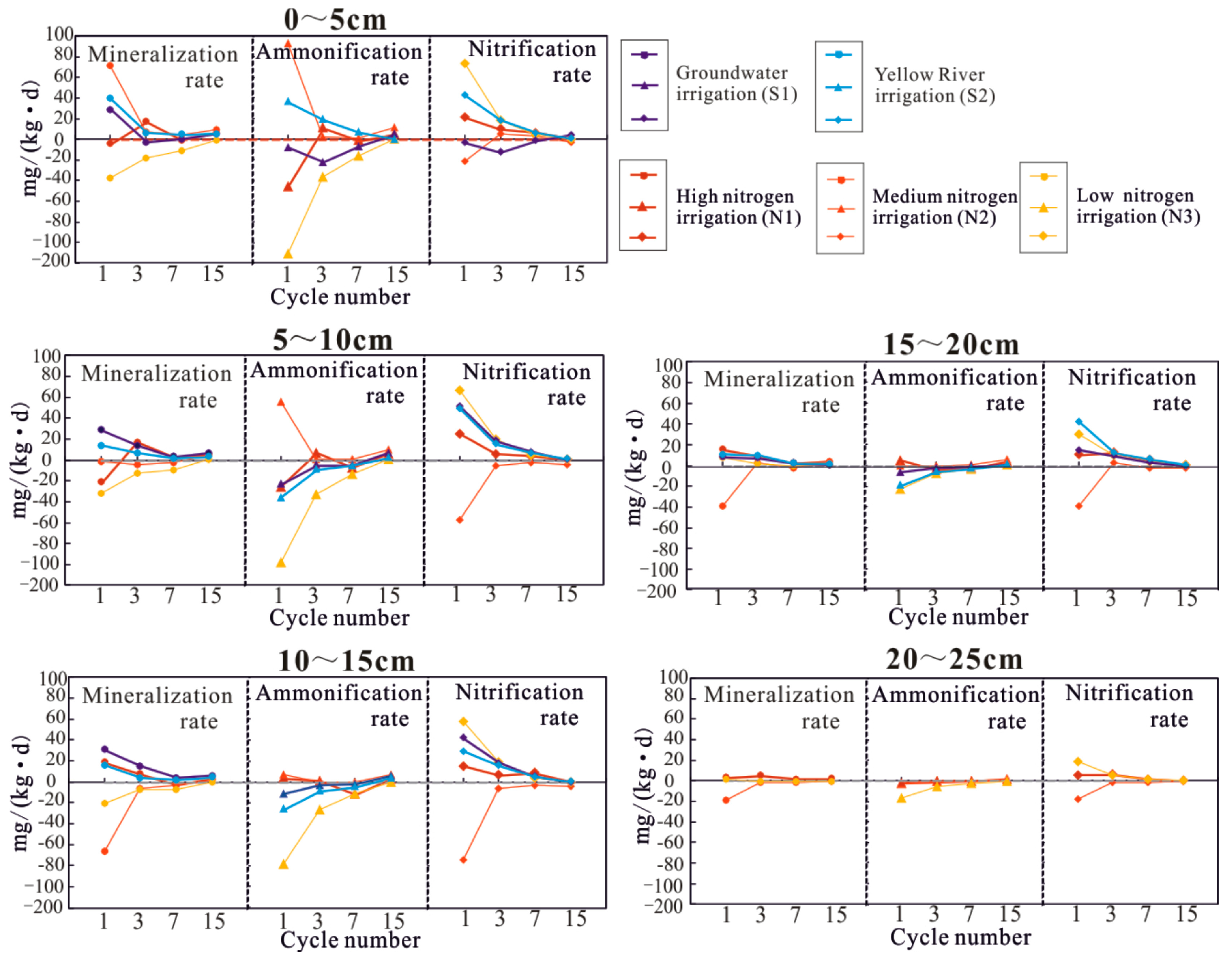
3.4. Response Mechanism of Water–Salt–Nitrogen Migration to Freeze–Thaw Cycles and Irrigation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koponen, H.T.; Martikainen, P.J. Soil water content and freezing temperature affect freeze-thaw related N2O production in organic soil. Nutr. Cycl. Agroecosyst. 2004, 69, 213–219. [Google Scholar] [CrossRef]
- Nie, S.Y.; Jia, X.; Zou, Y.C.; Bian, J.M. Effects of freeze-thaw cycles on soil nitrogen transformation in improved saline soils from an irrigated area in Northeast China. Water 2024, 16, 653. [Google Scholar] [CrossRef]
- Han, Z.M.; Deng, M.W.; Yuan, A.Q.; Wang, J.H.; Li, H.; Ma, J.C. Vertical variation of a black soil’s properties in response to freeze-thaw cycles and its links to shift of microbial community structure. Sci. Total Environ. 2018, 625, 106–113. [Google Scholar] [CrossRef]
- Wang, M.Q.; Zhu, Y.; Zhao, T.X.; Cui, L.H.; Mao, W.; Ye, M.; Wu, J.W.; Yang, J.Z. Chemical characteristics of salt migration in frozen soils during the freezing-thawing period. J. Hydrol. 2021, 606, 127403. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, W.; Feng, W.J.; Xiao, D.H.; Hou, X. Reconstruction of soil particle composition during freeze-thaw cycling: A review. Pedosphere 2016, 26, 167–179. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, J.W.; Guo, C.Y.; Zhang, J.F.; Wang, X.; Liu, Y.W.; Zhao, H.; Zhang, R. Effects of freezing-thawing processes on net nitrogen mineralization in salinized farmland soil. Agronomy 2022, 12, 2986. [Google Scholar] [CrossRef]
- Herrmann, A.; Witter, E. Sources of C and N contributing to the flush in mineralization upon freeze-thaw cycles in soils. Soil Biol. Biochem. 2002, 34, 1495–1505. [Google Scholar] [CrossRef]
- Koponen, H.T.; Jaakkola, T.; Keinänen-Toivola, M.M.; Kaipainen, S.; Tuomainen, J.; Servomaa, K.; Martikainen, P.J. Microbial communities, biomass, and activities in soils as affected by freeze thaw cycles. Soil Biol. Biochem. 2006, 38, 1861–1871. [Google Scholar] [CrossRef]
- Zhou, M.H.; Butterbach-Bahl, K.; Vereecken, H.; Brüggemann, N. A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Glob. Change Biol. 2017, 23, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.P.; Cui, Z.L.; Vitousek, P.M.; Zhang, F.S. Integrated soil–crop system management for food security. Proc. Natl. Acad. Sci. USA 2011, 108, 6399–6404. [Google Scholar] [CrossRef] [PubMed]
- Larson, L.; Kiemnec, G.; Johnson, D. Influence of freeze-thaw cycle on silt loam soil in sagebrush steppe of northeastern oregon. Rangel. Ecol. Manag. 2019, 72, 69–72. [Google Scholar] [CrossRef]
- Lyu, H.; Li, H.; Zhang, P.; Cheng, C.T.; Zhang, H.; Wu, S.S.; Su, X.S. Response mechanism of groundwater dynamics to freeze-thaw process in seasonally frozen soil areas: A comprehensive analysis from site to regional scale. J. Hydrol. 2023, 625, 129861. [Google Scholar] [CrossRef]
- Oztas, T.; Fayetorbay, F. Effect of freezing and thawing processes on soil aggregate stability. Catena 2003, 52, 1–8. [Google Scholar] [CrossRef]
- Hou, R.; Li, T.; Fu, Q.; Liu, D.; Li, M.; Zhou, Z.Q.; Yan, J.W.; Zhang, S. Research on the distribution of soil water, heat, salt and their response mechanisms under freezing conditions. Soil Tillage Res. 2020, 196, 104486. [Google Scholar] [CrossRef]
- Li, R.; Shi, H.; Flerchinger, G.N.; Akae, T.; Wang, C.S. Simulation of freezing and thawing soils in Inner Mongolia Hetao Irrigation District, China. Geoderma 2012, 173–174, 28–33. [Google Scholar] [CrossRef]
- Wang, G.; Shi, H.B.; Li, X.Y.; Yan, J.W.; Miao, Q.F.; Li, Z.; Akae, T. A study on water and salt transport, and balance analysis in sand dune-wasteland-lake systems of Hetao oases, upper reaches of the Yellow River Basin. Water 2020, 12, 3454. [Google Scholar] [CrossRef]
- Zhou, W.M.; Qing, S.J.; Liu, J.S.; Dai, L.M. Effects of temperature and freeze-thaw on soil nitrogen mineralization in typical calamagrostis angustifolia wetlands in Sanjiang Plain. J. Agro-Environ. Sci. 2011, 30, 806–811. [Google Scholar]
- Bao, T.; Xu, X.; Jia, G.; Billesbach, D.; Sullivan, R. Much stronger tundra methane emissions during autumn freeze than spring thaw. Glob. Change Biol. 2020, 27, 376–387. [Google Scholar] [CrossRef]
- Liu, M.H.; Feng, F.L.; Cai, T.J.; Tang, S.J. Soil microbial community response differently to the frequency and strength of freeze-thaw events in a larix gmelinii forest in the Daxing’an Mountains, China. Front. Microbiol. 2020, 11, 1164. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.W.; Zhang, S.J.; Li, Y.G.; Wu, N.; Zhou, X.B.; Yin, B.F.; Zhang, Y.M. Differentiate responses of soil nutrient levels and enzymatic activities to freeze-thawing cycles in different layers of moss-dominated biocrusts in a temperate desert. Front. Plant Sci. 2023, 14, 1137754. [Google Scholar] [CrossRef] [PubMed]
- Han, H.T. Engineering Mechanical Properties of Saline Soil and Rice Husk Ash Solidified Saline Soil Under Freeze-Thaw Cycles. Master’s Thesis, Lanzhou University of Technology, Lanzhou, China, 2020. [Google Scholar]
- Wu, M.S.; Huang, J.S.; Tan, X.; Wu, J. Water, salt and heat influences on carbon and nitrogen dynamics in seasonally frozen soils in Hetao Irrigation District, Inner Mongolia, China. Pedosphere 2019, 29, 632–641. [Google Scholar] [CrossRef]
- Li, H.F.; Wang, J.; Liu, H.; Miao, H.L. Quantitative analysis of temporal and spatial variations of soil salinization and groundwater depth along the Yellow River saline-alkali land. Sustainability 2022, 14, 6967. [Google Scholar] [CrossRef]
- Wang, E.Q.; Chen, J.F.; Liu, L.; Cui, L.H.; Xue, J.; Ren, J.M.; Du, Q. Effect of soil texture on water and salt transport in freeze-thaw soil in the shallow groundwater area. Water 2023, 15, 2587. [Google Scholar] [CrossRef]
- Phogat, V.; Skewes, M.A.; McCarthy, M.G.; Cox, J.W.; Simunek, J.; Petrie, P.R. Evaluation of crop coefficients, water productivity, and water balance components for wine grapes irrigated at different deficit levels by a sub-surface drip. Agric. Water Manag. 2017, 180, 22–34. [Google Scholar] [CrossRef]
- Lu, Z.; Xian, S.; Yao, H. Influence of freeze-thaw cycles in the presence of a supplementary water supply on mechanical properties of compacted soil. Cold Reg. Sci. Technol. 2019, 157, 42–52. [Google Scholar] [CrossRef]
- Zeng, H.B.; Su, C.L.; Xie, X.J.; Pan, H.J.; Ji, Q.N.; Tao, Y.Z. Mechanism of salinization of shallow groundwater in western Hetao Irrigation Area. Earth Sci. Rev. 2021, 46, 2267–2277. [Google Scholar]
- Liu, S.; Huang, Q.; Ren, D.; Xu, X.; Xiong, Y.; Huang, G. Soil evaporation and its impact on salt accumulation in different landscapes under freeze-thaw conditions in an arid seasonal frozen region. Vadose Zone J. 2021, 20, e20098. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Zhao, H.; Li, C.; Mao, J.; Zhang, R.; Liu, J.; Zhao, Q. Ions transport in seasonal frozen farmland soil and its effect on soil salinization chemical properties. Agronomy 2023, 13, 660. [Google Scholar] [CrossRef]
- Su, C.L.; Tao, Y.Z.; Xie, X.J.; Pan, H.J.; Yan, F.G.; Zeng, H.B.; Gao, S.; Huang, H.Z. Novel physical techniques for soil salinization restoration based on gravel: Performance and mechanism. J. Soils Sediments 2023, 23, 1281–1294. [Google Scholar] [CrossRef]
- Cui, L.H.; Zhu, Y.; Zhao, T.X.; Yang, J.Z.; Wu, J.W. Soil ion components and soil salts transport in frozen layer in seasonal freezing thawing areas. Trans. Chin. Soc. Agric. Eng. 2019, 35, 75–82. [Google Scholar]
- ISO 11465:1993; Soil Quality—Determination of Dry Matter and Water Content on a Mass Basis—Gravimetric Method. International Organization for Standardization: Geneva, Switzerland, 1993.
- Zhang, J.; Feng, Z.X.; Cui, S.Y. Seasonal variations of soil salinity and soil factors in reclamation area of tidal flats with different salinity levels. Jiangsu J. Agric. Sci. 2017, 33, 836–842. [Google Scholar]
- HJ 634-2012; Soil Quality—Determination of Dry Matter and Water Content—Gravimetric Method. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2012.
- Yu, Z.M.; Ma, X.N.; Xie, Y. Study of main nutrients adsorption on clays in seawater. Oceanol. Limnol. Sin. 1995, 26, 208–214. [Google Scholar]
- Duan, L.; Lu, J.J.; Sun, Y.Q. Adsorption/desorption mechanism of ammonia nitrogen in aerated zone of loess soil. Agric. Res. Arid Areas 2021, 39, 184–193. [Google Scholar]
- Li, H.; Weng, B.S.; Huang, F.Y.; Su, J.Q.; Yang, X.R. pH regulates ammonia-oxidizing bacteria and archaea in paddy soils in Southern China. Appl. Microbiol. Biotechnol. 2015, 99, 6113–6123. [Google Scholar] [CrossRef]
- Guo, J.J.; Ling, N.; Chen, H.; Zhu, C.; Kong, Y.L.; Wang, M.; Shen, Q.R.; Guo, S.W. Distinct drivers of activity, abundance, diversity and composition of ammonia-oxidizers: Evidence from a long-term field experiment. Soil Biol. Biochem. 2017, 115, 403–414. [Google Scholar] [CrossRef]
- Bing, H.; He, P.; Zhang, Y. Cyclic freeze-thaw as a mechanism for water and salt migration in soil. Environ. Earth Sci. 2015, 74, 675–681. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, W.; Bing, H. Impact of freezing and thawing cycles on structure of soils and its mechanism analysis by laboratory testing. Rock Soil Mech. 2015, 36, 1282–1287. [Google Scholar]
- Song, Y.; Yu, X.F.; Zou, Y.C. Progress of freeze-thaw effects on carbon, nitrogen and phosphorus cyclings in soils. Soils Crops 2016, 5, 78–90. [Google Scholar]
- Kong, L.M.; Liang, K.; Peng, L.Y. Experimental study on the influence of specific surface area on the soil-freezing characteristic curve. Rock Soil Mech. 2021, 42, 1883–1893. [Google Scholar]
- Guo, H.M.; Li, X.M.; Xiu, W.; He, W.; Cao, Y.S.; Zhang, D.; Wang, A. Controls of organic matter bioreactivity on arsenic mobility in shallow aquifers of the Hetao Basin, P.R. China J. Hydrol. 2019, 571, 448–459. [Google Scholar] [CrossRef]
- Liang, J.; Chen, H.H.; Xu, J.S. Study on the Form of Ammonium Nitrogen Absorbed in Sand. J. Anhui Agric. Sci. 2011, 39, 1705–1707, 1761. [Google Scholar]
- Callesen, I.; Borken, W.; Kalbitz, K.; Matzner, E. Long-term development of nitrogen fluxes in a coniferous ecosystem: Does soil freezing trigger nitrate leaching. J. Plant Nutr. Soil Sci. 2007, 170, 189–196. [Google Scholar] [CrossRef]
- Liu, J.J.; Ma, W.D.; He, S.; Wu, Z.X.; Li, C. Ammonia-oxidizing microorganisms in acidic soil and their influencing factors: A review. J. Microbiol. China 2023, 50, 413–426. [Google Scholar]
- Dörsch, P.; Palojärvi, A.; Mommertz, S. Overwinter greenhouse gas fluxes in two contrasting agricultural habitats. Nutr. Cycl. Agroecosyst. 2004, 70, 117–133. [Google Scholar] [CrossRef]
- Cao, S.B.; Du, R.; Li, B.K.; Wang, S.Y.; Ren, N.Q.; Peng, Y.Z. Nitrite production from partial-denitrification process fed with low carbon/nitrogen (C/N) domestic wastewater: Performance, kinetics and microbial community. Chem. Eng. J. 2017, 326, 1186–1196. [Google Scholar] [CrossRef]
- Niggemann, K.; Ziegler, M.; Fuentes, R. Influence of freezing directions on ice lens formations in soils. Acta Geotech. 2024, 19, 5781–5798. [Google Scholar] [CrossRef]
- Kurylyk, B.L.; MacQuarrie, K.T.B.; McKenzie, J.M. Climate change impacts on groundwater and soil temperatures in cold and temperate regions: Implications, mathematical theory, and emerging simulation tools. Earth Sci. Rev. 2014, 138, 313–334. [Google Scholar] [CrossRef]
- Hou, R.J.; Qi, Z.Y.; Li, T.X.; Fu, Q.; Meng, F.X.; Liu, D.; Li, Q.L.; Zhao, H.; Yu, P.F. Mechanism of snowmelt infiltration coupled with salt transport in soil amended with carbon-based materials in seasonally frozen areas. Geoderma 2022, 420, 115882. [Google Scholar] [CrossRef]
- Huang, S.B.; Lu, Z.X.; Ye, Z.Y.; Xin, Z.K. An elastoplastic model of frost deformation for the porous rock under freeze-thaw. Eng. Geol. 2020, 278, 105820. [Google Scholar] [CrossRef]
- Wang, H.; Vanapalli, S.K.; Li, X. A unified model for the soil freezing characteristic curve based on pore size distribution and principles of thermodynamics. Water Resour. Res. 2025, 61, e2024WR038715. [Google Scholar] [CrossRef]
- Hao, Y.L.; Song, Y.; Li, X.W.; Li, M.; Wei, X.R.; Guo, S.L.; Hu, Y.X. Progressive melting of surface water and unequal discharge of different DOM components profoundly perturb soil biochemical cycling. Water Res. 2024, 266, 122360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Wang, C.; Xue, R.; Wang, L.J. Effects of salinity on the soil microbial community and soil fertility. J. Integr. Agric. 2019, 18, 1360–1368. [Google Scholar] [CrossRef]
- Zhang, P.P.; Shen, J.L. Effect of brackish water irrigation on the movement of water and salt in salinized soil. Open Geosci. 2022, 14, 404–413. [Google Scholar] [CrossRef]
- Li, X.B.; Chen, X.Y.; Gao, Y.C.; Yang, J.H.; Ding, W.T.; Azad, N.; Li, J.B.; He, H.L. Freezing induced soil water redistribution: A review and global meta-analysis. J. Hydrol. 2025, 651, 132594. [Google Scholar] [CrossRef]
- Zhou, B.B.; Liang, C.F.; Chen, X.P.; Ye, S.T.; Peng, Y.; Yang, L.; Duan, M.L.; Wang, X.P. Magnetically-treated brackish water affects soil water-salt distribution and the growth of cotton with film mulch drip irrigation in Xinjiang, China. Agric. Water Manag. 2022, 263, 107487. [Google Scholar] [CrossRef]
- Liu, J.J.; Wu, W.X.; Ding, Y.; Shi, D.Z.; Chen, Y.X. Ammonia-oxidizing archaea and their important roles in nitrogen biogeochemical cycling. J. Appl. Ecol. 2010, 21, 2154–2160. [Google Scholar]
- Smith, J.; Wagne-Riddle, C.; Dunfield, K. Season and management related changes in the diversity of nitrifying and denitrify-ing bacteria over winter and spring. Appl. Soil Ecol. 2010, 44, 138–146. [Google Scholar] [CrossRef]
- Xu, J.Z.; Wei, Q.; Bai, W.H.; Li, K.L.; Liu, X.Y.; Li, Y.W. Soil nitrification process under different soil moisture and salinity conditions. J. Drain. Irrig. Mach. Eng. 2018, 36, 909–913. [Google Scholar]
- Zhou, H.; Shi, H.B.; Wen, C.; Wang, W.G.; Su, Y.D.; Yan, Y. Effects of combination of organic and inorganic nitrogen on nitrification and denitrification in two salinized soils. Environ. Sci. Technol. 2021, 42, 5010–5020. [Google Scholar]
- Amador, J.A.; Gorres, J.H.; Savin, M.C. Role of soil water content in the carbon and nitrogen dynamics of Lumbricus terrestris L. burrow soil. Appl. Soil Ecol. 2005, 28, 15–22. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.B.; Wang, D.B.; Lu, M.J.; Fan, Y.J.; Ji, J.J.; Wu, J. Combined effects of carbon source and C/N ratio on the partial denitrification performance: Nitrite accumulation, denitrification kinetic and microbial transition. J. Environ. Chem. Eng. 2024, 12, 113343. [Google Scholar] [CrossRef]
- Sulkava, P.; Huhta, V. Effects of hard frost and freeze-thaw cycles on decomposer communities and N mineralization in boreal forest soil. Appl. Soil Ecol. 2003, 22, 225–239. [Google Scholar] [CrossRef]
- Fu, Q.; Yan, J.W.; Li, Y.; Li, T.X.; Hou, R.J.; Li, D.; Ji, Y. Effects of biochar amendment on nitrogen mineralization in black soil with different moisture contents under freeze-thaw cycles. Geoderma 2019, 353, 459–467. [Google Scholar] [CrossRef]
- Wu, X.K.; Shi, W.M.; Xu, Y.H.; Min, J. Effects of long-term different chemical nitrogen rates on soil nitrogen mineralization and nitrification in greenhouse vegetable field. Soils 2021, 53, 1160–1166. [Google Scholar]
- Chen, S.Y.; Gu, Y.Z.; Liu, F.Y.; Wu, M.H.; Cheng, X.; Yang, P.Z.; Bahadur, A.; Bai, R.Q.; Chen, J.W.; Zhang, M.Y.; et al. Freeze-thaw strength increases microbial stability to enhance diversity-soil multifunctionality relationship. Commun. Earth Environ. 2024, 5, 578. [Google Scholar] [CrossRef]
- Meisner, A.; Snoek, B.L.; Nesme, J.; Dent, E.; Jacquiod, S.; Classen, A.T.; Priemé, A. Soil microbial legacies differ following drying-rewetting and freezing-thawing cycles. ISME J. 2021, 15, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, H.G.; He, X.L.; Lin, E.; Yang, G. Winter irrigation effects on soil moisture, temperature and salinity, and on cotton growth in salinized fields in Northern Xinjiang, China. Sustainability 2020, 12, 7573. [Google Scholar] [CrossRef]
- Guo, G.W.; Tan, X.; He, Y.X.; Guo, J.H.; Yu, J.; Zhuang, W.H.; Liu, C. Transport of heat, water, and salts in freeze-thaw soils under flood irrigation: Experiment and simulation. J. Hydrol. 2025, 652, 132688. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, Y.Q.; Qi, J.Y.; Marek, G.W.; Srinivasan, S.R.; Feng, P.Y.; Hu, K.L.; Liu, D.L.; Chen, Y. Effects of changes in freeze-thaw cycles on soil hydrothermal dynamics and erosion degradation under global warming in the black soil region. Water Resour. Res. 2025, 61, e2024WR038318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, W.; Jiang, J.; Su, C.; Xie, X.; Zhang, Q.; Zhang, C.; Li, Y.; Li, X.; Song, J.; Su, Y. Freeze–Thaw-Driven Dynamics of Soil Water–Salt and Nitrogen: Effects and Implications for Irrigation Management in the Hetao Irrigation District. Water 2025, 17, 2991. https://doi.org/10.3390/w17202991
Ge W, Jiang J, Su C, Xie X, Zhang Q, Zhang C, Li Y, Li X, Song J, Su Y. Freeze–Thaw-Driven Dynamics of Soil Water–Salt and Nitrogen: Effects and Implications for Irrigation Management in the Hetao Irrigation District. Water. 2025; 17(20):2991. https://doi.org/10.3390/w17202991
Chicago/Turabian StyleGe, Weili, Jiaqi Jiang, Chunli Su, Xianjun Xie, Qing Zhang, Chunming Zhang, Yanlong Li, Xin Li, Jiajia Song, and Yinchun Su. 2025. "Freeze–Thaw-Driven Dynamics of Soil Water–Salt and Nitrogen: Effects and Implications for Irrigation Management in the Hetao Irrigation District" Water 17, no. 20: 2991. https://doi.org/10.3390/w17202991
APA StyleGe, W., Jiang, J., Su, C., Xie, X., Zhang, Q., Zhang, C., Li, Y., Li, X., Song, J., & Su, Y. (2025). Freeze–Thaw-Driven Dynamics of Soil Water–Salt and Nitrogen: Effects and Implications for Irrigation Management in the Hetao Irrigation District. Water, 17(20), 2991. https://doi.org/10.3390/w17202991