Comparison of Eukaryotic Community Structures Across Different Habitat Types in the Nearshore Waters of Ma’an Archipelago Based on Environmental DNA Technology
Abstract
1. Introduction
2. Materials and Methods
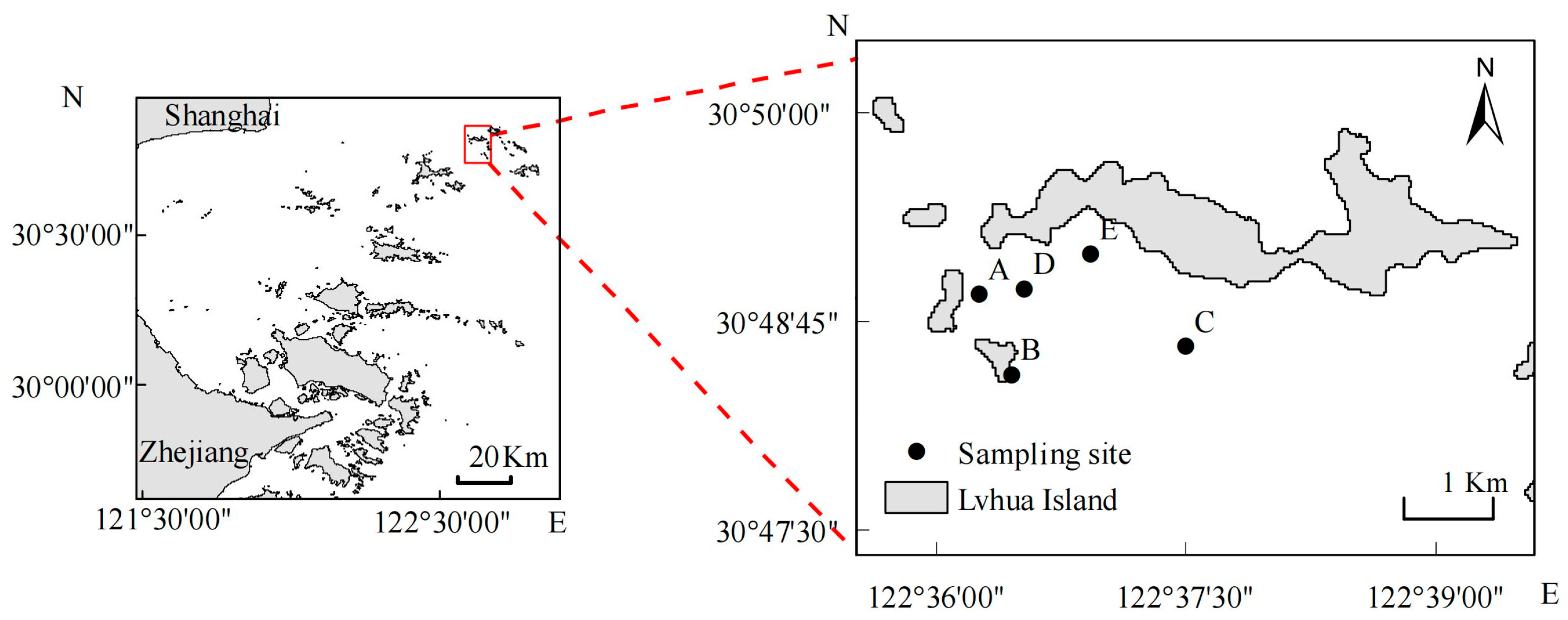
2.1. Study Area and Sample Collection
2.2. 18S rRNA Gene Amplicon Sequencing and Bioinformatics Analysis
2.2.1. DNA Extraction
2.2.2. 18S rRNA Gene Amplification by PCR
2.2.3. 18S Gene Library Construction, Quantification, and Sequencing
2.2.4. Sequence Processing, OTU Clustering, Representative Tags Alignment and Biological Classification
2.3. Data Statistics and Analysis
2.3.1. Biodiversity Indices
2.3.2. Community Structure and Environmental Factors
3. Results
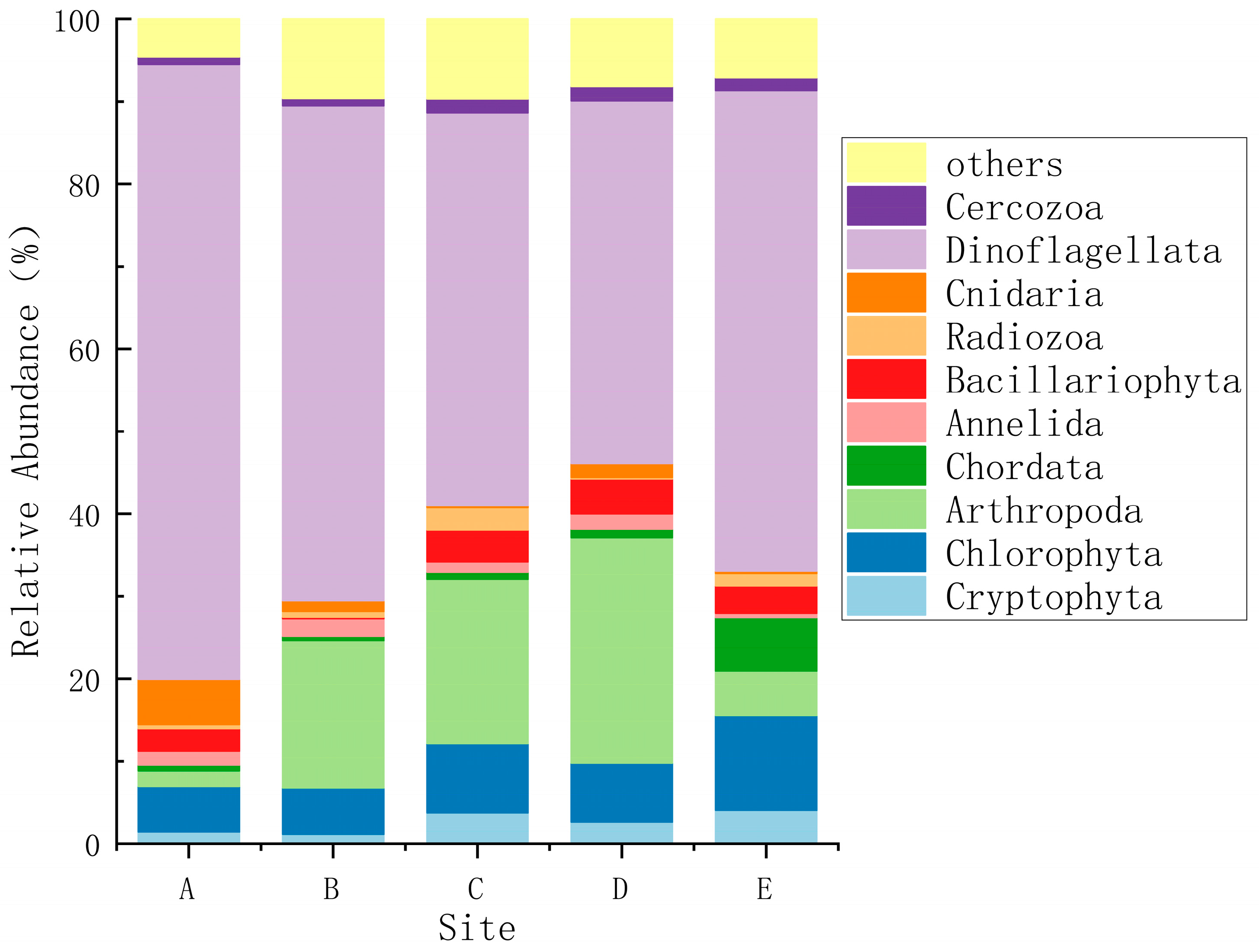
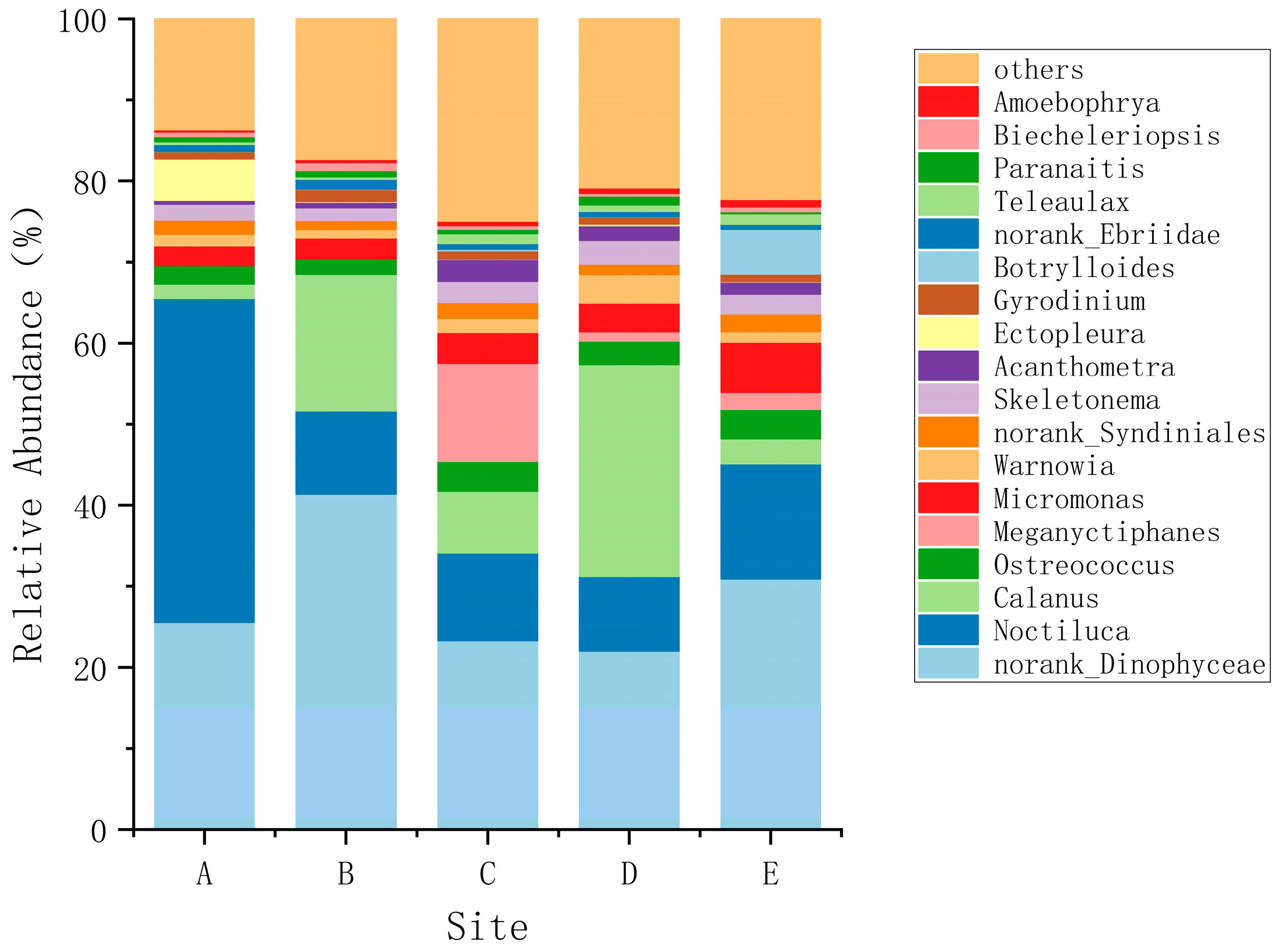
3.1. Species Composition and Dominant Species
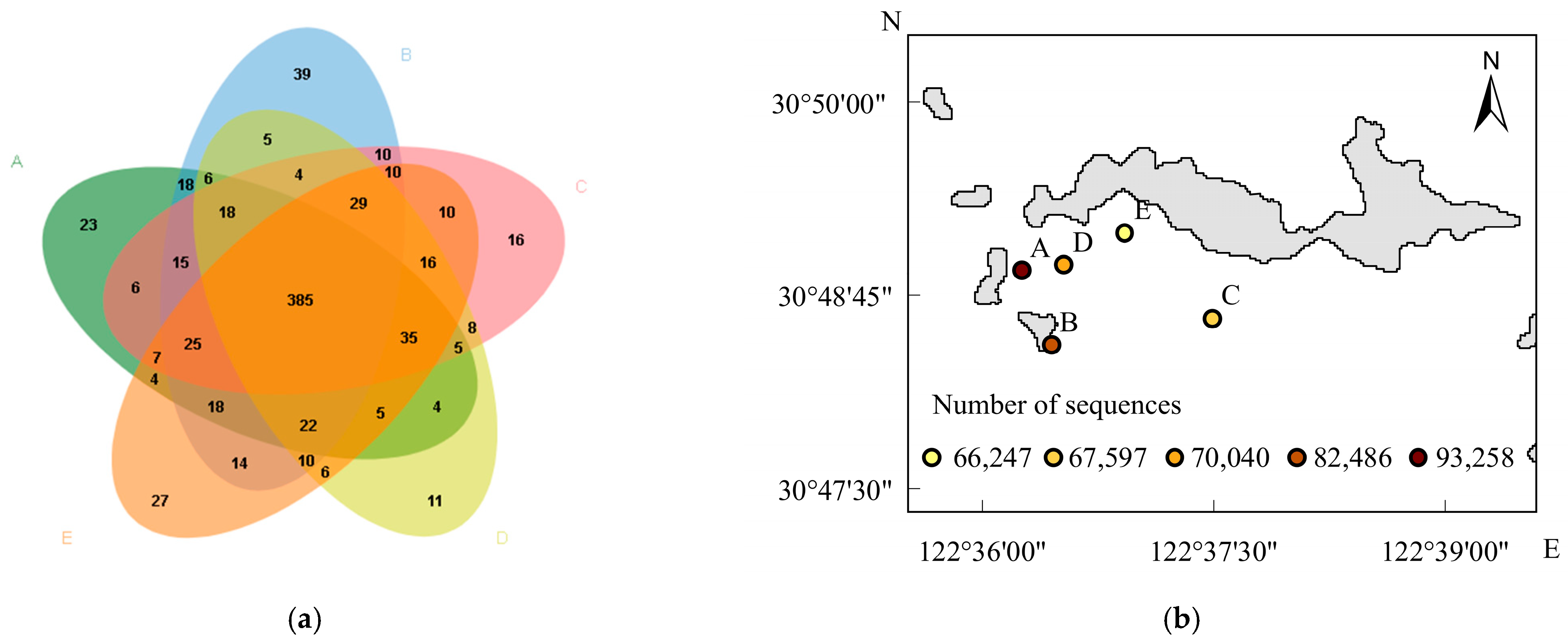
3.2. Biodiversity Characteristics
3.3. Relationship Between Community Composition and Environmental Factors
4. Discussion
4.1. Habitat Specificity of Species and Dominant Species Composition
4.2. Driving Factors of Community Structure Differences
4.3. Regulatory Role of Environmental Factors on Community Structure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Hu, F.; Xiao, K.; Chen, X.; Ye, S.; Sun, L.; Qin, S. Progress in research on delineation of the extent and boundaries of coastal zone. J. Appl. Oceanogr. 2025, 44, 15–20. [Google Scholar] [CrossRef]
- Gao, W.; Song, Q.; Zhang, H.; Wang, S. Selection of priority protected areas based on ecosystem service functions and habitat suitability evaluation for protected animals: A case study of the Sanjiangyuan Region. J. Ecol. Environ. 2024, 33, 1318–1328. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Chong-Seng, K.M.; Huchery, C.; Januchowski-Hartley, F.A.; Nash, K.L. Coral reef community composition in the context of disturbance history on the Great Barrier Reef, Australia. PLoS ONE 2014, 9, e101204. [Google Scholar] [CrossRef]
- Trindade-Santos, I.; Moyes, F.; Magurran, A.E. Global change in the functional diversity of marine fisheries exploitation over the past 65 years. Proc. R. Soc. B 2020, 287, 20200889. [Google Scholar] [CrossRef]
- Gsell, A.S.; Scharfenberger, U.; Özkundakci, D.; Walters, A.; Hansson, L.; Janssen, A.; Nõges, P.; Reid, P.; Schindler, D. Evaluating early-warning indicators of critical transitions in natural aquatic ecosystems. Proc. Natl. Acad. Sci. USA 2016, 113, E8089–E8095. [Google Scholar] [CrossRef]
- Astles, K.L. Linking risk factors to risk treatment in ecological risk assessment of marine biodiversity. ICES J. Mar. Sci. 2015, 72, 1116–1132. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, S.; Chen, P. Research progress and prospects on benefit assessment of marine ranching. South China Fish. Sci. 2024, 20, 1–13. [Google Scholar] [CrossRef]
- Lotze, H.K.; Guest, H.; O’Leary, J.; Tuda, A.; Wallace, D. Public perceptions of marine threats and protection from around the world. Ocean Coast. Manag. 2018, 152, 14–22. [Google Scholar] [CrossRef]
- Pieretti, N.; Danovaro, R. Acoustic indexes for marine biodiversity trends and ecosystem health. Philos. Trans. R. Soc. B 2020, 375, 20190447. [Google Scholar] [CrossRef] [PubMed]
- Lurgi, M.; López, B.C.; Montoya, J.M. Novel communities from climate change. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
- Isbell, F.; Gonzalez, A.; Loreau, M.; Cowles, J.; Díaz, S.; Hector, A.; Mace, G.; Wardle, D.; O’Connor, M.; Duffy, J.; et al. Linking the influence and dependence of people on biodiversity across scales. Nature 2017, 546, 65–72. [Google Scholar] [CrossRef]
- Amaral Camara Lima, M.; Bergamo, T.F.; Ward, R.D.; Joyce, C.B. A review of seagrass ecosystem services: Providing nature-based solutions for a changing world. Hydrobiologia 2023, 850, 2655–2670. [Google Scholar] [CrossRef]
- Bennett, N.J.; Blythe, J.; White, C.S.; Campero, C. Blue growth and blue justice: Ten risks and solutions for the ocean economy. Mar. Policy 2021, 125, 104387. [Google Scholar] [CrossRef]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019, 17, e00547. [Google Scholar] [CrossRef]
- Shan, X.; Li, M.; Wang, W. Application of Environmental DNA Technology in Aquatic Ecosystem. Prog. Fish. Sci. 2018, 39, 23–29. [Google Scholar] [CrossRef]
- Couton, M.; Hürlemann, S.; Studer, A.; Alther, R.; Altermatt, F. Groundwater environmental DNA metabarcoding reveals hidden diversity and reflects land-use and geology. Mol. Ecol. 2023, 32, 3497–3512. [Google Scholar] [CrossRef]
- Yamamoto, S.; Masuda, R.; Sato, Y.; Sado, T.; Araki, H.; Kondoh, M.; Minamoto, T.; Miya, M. Environmental DNA metabarcoding reveals local fish communities in a species-rich coastal sea. Sci. Rep. 2017, 7, 40368. [Google Scholar] [CrossRef]
- Shu, L.; Chen, S.; Li, P.; Li, X.; Song, R.; Zhang, X.; Li, C.; Li, Y. Environmental DNA metabarcoding reflects fish DNA dynamics in lentic ecosystems: A case study of freshwater ponds. Fishes 2022, 7, 257. [Google Scholar] [CrossRef]
- Ramírez-Amaro, S.; Bassitta, M.; Picornell, A.; Ramon, C.; Terrasa, B. Environmental DNA: State-of-the-art of its application for fisheries assessment in marine environments. Front. Mar. Sci. 2022, 9, 1004674. [Google Scholar] [CrossRef]
- Sun, J.; Song, Y.; Shi, Y.; Zhai, J.; Yan, W. Progress of marine biodiversity studies in China seas in the past decade. Biodivers. Sci. 2022, 30, 22526. [Google Scholar] [CrossRef]
- Zhang, Z.; Bi, M.; Wu, H. Challenges and Development Strategies of Coastal and Marine Tourism in China. China Ecotourism 2021, 11, 536–547. [Google Scholar]
- Xia, Y.; Zhang, S.; Zhang, S.; Wang, K.; Xu, P.; Li, N.; Li, X. Decomposition and sedimentation of two species of macro algae detritus at the Gouqi Island seaweed beds. Acta Fish. Sin. 2023, 47, 039307-1–039307-10. [Google Scholar]
- Yang, G.; Lin, J.; Zhang, S.; Li, N. Study on Offshore Transport of Organic Detritus in Seaweed Field Based on Lagrange Method. Prog. Fish. Sci. 2022, 43, 49–60. [Google Scholar]
- Zhang, S.; Liu, S.; Zhou, X.; Wang, Z.; Wang, K. Ecological Functions of Large seaweed habitats and their application in Marine ranches. Acta Fish. Sin. 2019, 43, 2004–2014. [Google Scholar]
- Zhang, G.; Sun, J. State of environmental pollution in the Bohai Sea, China: A review. Environ. Chem. 2023, 42, 918–930. [Google Scholar]
- Lu, X.; Wang, Y.; Zhang, S. Effects of mariculture on benthic microbial communities in the Yellow Sea, China. Mar. Pollut. Bull. 2018, 135, 845–853. [Google Scholar]
- Stadler, J.; Klotz, S.; Brandl, R.; Knapp, S. Species richness and phylogenetic structure in plant communities: 20 years of succession. Web Ecol. 2017, 17, 37–46. [Google Scholar] [CrossRef]
- Heery, E.C.; Bishop, M.J.; Critchley, L.P.; Bugnot, A.B.; Airoldi, L.; Mayer-Pinto, M.; Sheehan, E.V.; Coleman, R.A.; Loke, L.H.L.; Johnston, E.L.; et al. Identifying the consequences of ocean sprawl for sedimentary habitats. J. Exp. Mar. Biol. Ecol. 2017, 492, 31–48. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, M.; Li, L.; Fang, T.; Li, W. Risk Assessment of Heavy metals in Sediments near the Luhua Offshore Bulk Cargo Unloading Platform. J. Shanghai Ocean Univ. 2015, 24, 249–255. [Google Scholar]
- Yang, H. Construction of marine ranching in China: Reviews and prospects. J. Fish. China 2016, 40, 1133–1140. [Google Scholar] [CrossRef]
- Li, X. Research and Prospect of Marine Macrobenthic Biodiversity in China: A Case Study of the Yellow Sea. Biodiversity 2011, 19, 676–684. [Google Scholar]
- Jiang, Z.; Dong, C.; Zhan, P.; Zhao, J.; Zhao, C.; Tang, F.; Han, Y. Community structure and biodiversity of zooplankton in Kai Lake, Daxing. J. Dalian Ocean Univ. 2003, 18, 292–295. [Google Scholar]
- Fu, H.; Zhang, Y.; Huang, S.; Chu, L.; Yan, Y.; Ge, Y. Seasonal dynamics and construction mechanism of benthic animal community structure in Hui River. Resour. Environ. Yangtze River Basin 2024, 33, 1239–1249. [Google Scholar]
- Zhang, W.; Qi, Y. Randomized Test and Calculation Software for Significance of Biodiversity and uniformity (English). Biodiversity 2002, 10, 431–437. [Google Scholar]
- Sala-Pérez, M.; Lattuada, M.; Flecker, R.; Anesio, A.; Leroy, S.A. Dinoflagellate cyst assemblages as indicators of environmental conditions and shipping activities in coastal areas of the Black and Caspian Seas. Reg. Stud. Mar. Sci. 2020, 39, 101472. [Google Scholar] [CrossRef]
- Xu, Z. Annual Variations of Noctiluca scintillans in the Yangtze River Estuary and the Trend of Water eutrophication. Oceanol. Limnol. 2009, 40, 793–798. [Google Scholar]
- Weiher, E.; Keddy, P.A. The assembly of experimental wetland plant communities. Oikos 1995, 73, 323–335. [Google Scholar] [CrossRef]
- Zhou, M.; Shen, Z.; Yu, R. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Cont. Shelf Res. 2008, 28, 1483–1489. [Google Scholar] [CrossRef]
- Chen, B.; Dong, D.; Qin, X.; Xu, M.; Chen, X.; Shi, M. Relationship between the occurrence of red tides and the dynamic response mechanisms in the northern Beibu Gulf. J. Coast. Res. 2019, 93, 185–193. [Google Scholar] [CrossRef]
- Su, J. Research on Red Tides in China. Bull. Chin. Acad. Sci. 2001, 16, 340–342. [Google Scholar]
- Wang, T.; Shan, S.; Yang, C.; Wu, Z.; Chen, Z. Effects of the manure collection equipment on growth performance and nitrogen and phosphorus budgets of Cyprinus carpio var. specularis cage culture in reservoir. J. Fish. China 2016, 40, 218–224. [Google Scholar] [CrossRef]
- Hillebrand, H.; Bennett, D.M.; Cadotte, M.W. Consequences of dominance: A review of evenness effects on local and regional ecosystem processes. Ecology 2008, 89, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef]
- Chou, L.M. Enhancing Marine Biodiversity with Artificial Structures. In Sustainable Engineering Technologies and Architectures; AIP Publishing LLC: Melville, NY, USA, 2021. [Google Scholar]
- Beaugrand, G.; Edwards, M.; Legendre, L. Marine biodiversity, ecosystem functioning, and carbon cycles. Proc. Natl. Acad. Sci. USA 2010, 107, 10120–10124. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Lefcheck, J.S.; Byrnes, J.E.K.; Cardinale, B.J.; Duffy, J.E.; Griffin, J.N. Marine biodiversity and ecosystem functioning: What’s known and what’s next? Oikos 2015, 124, 252–265. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Smith, R.S.; Johnston, E.L.; Clark, G.F. The role of habitat complexity in community development is mediated by resource availability. PLoS ONE 2014, 9, e102920. [Google Scholar] [CrossRef]
- Fowler, A.M.; Jørgensen, A.M.; Svendsen, J.C.; Macreadie, P.I.; Jones, D.O.; Boon, A.R.; Booth, D.J.; Brabant, R.; Callahan, E.; Claisse, J.T.; et al. Environmental benefits of leaving offshore infrastructure in the ocean. Front. Ecol. Environ. 2018, 16, 571–578. [Google Scholar] [CrossRef]
- Smaal, A.C.; Ferreira, J.G.; Grant, J.; Petersen, J.K.; Strand, Ø. Goods and Services of Marine Bivalves; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Ysebaert, T.; Walles, B.; Haner, J.; Hancock, B. Habitat modification and coastal protection by ecosystem-engineering reef-building bivalves. In Goods and Services of Marine Bivalves; Springer International Publishing: Cham, Switzerland, 2018; pp. 253–273. [Google Scholar]
- Koivisto, M.E.; Westerbom, M. Habitat structure and complexity as determinants of biodiversity in blue mussel beds on sublittoral rocky shores. Mar. Biol. 2010, 157, 1463–1474. [Google Scholar] [CrossRef]
- Degraer, S.; Carey, D.A.; Coolen, J.W.P.; Hutchison, Z.L.; Kerckhof, F.; Rumes, B.; Vanaverbeke, J. Offshore wind farm artificial reefs affect ecosystem structure and functioning. Oceanography 2020, 33, 48–57. [Google Scholar] [CrossRef]
- Crawford, C.M.; Macleod, C.K.A.; Mitchell, I.M. Effects of shellfish farming on the benthic environment. Aquaculture 2003, 224, 117–140. [Google Scholar] [CrossRef]
- Gissi, E.; Manea, E.; Mazaris, A.D.; Fraschetti, S.; Almpanidou, V.; Bevilacqua, S.; Coll, M.; Guarnieri, G.; Lloret-Lloret, E.; Pascual, M.; et al. A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 2021, 755, 142564. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.A.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef]
- Fu, S.-f.; Wu, H.-y.; Cai, X.-q. Determination of the Benthic Biodiversity Protection Targets in Xiamen Sea Area. J. Ecol. Rural Environ. 2021, 37, 1264–1270. [Google Scholar] [CrossRef]
- Christie, H.; Norderhaug, K.M.; Fredriksen, S. Macrophytes as habitat for fauna. Mar. Ecol. Prog. Ser. 2009, 396, 221–233. [Google Scholar] [CrossRef]
- Thompson, R.M.; Brose, U.; Dunne, J.A.; Hall, R.O.; Hladyz, S.; Kitching, R.L.; Martinez, N.D.; Rantala, H.; Romanuk, T.N.; Stouffer, D.B.; et al. Food webs: Reconciling the structure and function of biodiversity. Trends Ecol. Evol. 2012, 27, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Read, P.; Fernandes, T. Management of environmental impacts of marine aquaculture in Europe. Aquaculture 2003, 226, 139–163. [Google Scholar] [CrossRef]
- Glibert, P.M.; Icarus Allen, J.; Artioli, Y.; Beusen, A.; Bouwman, L.; Harle, J.; Holmes, R.; Holt, J. Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: Projections based on model analysis. Glob. Change Biol. 2014, 20, 3845–3858. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, J.J.; Bruno, J.F.; Duffy, J.E. Understanding the effects of marine biodiversity on communities and ecosystems. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 739–766. [Google Scholar] [CrossRef]
- Adams, T.P.; Miller, R.G.; Aleynik, D.; Burrows, M.T. Offshore marine renewable energy devices as stepping stones across biogeographical boundaries. J. Appl. Ecol. 2014, 51, 330–338. [Google Scholar] [CrossRef]
- Forrest, B.M.; Keeley, N.B.; Hopkins, G.A.; Webb, S.C.; Clement, D.M. Bivalve aquaculture in estuaries: Review and synthesis of oyster cultivation effects. Aquaculture 2009, 298, 1–15. [Google Scholar] [CrossRef]
- Luo, M.; Lu, J.; Shen, X.; Wang, Y.; Xu, Z.; Zhu, J. The Impact of Large-scale Marine Engineering on the Ecological Distribution of Large Benthic Animals in the Surrounding Waters of Yangshan Island. J. Agric. Environ. Sci. 2007, 26, 97–102. [Google Scholar]
- Besemer, K. Biodiversity, community structure and function of biofilms in stream ecosystems. Res. Microbiol. 2015, 166, 774–781. [Google Scholar] [CrossRef]
- Luo, M.; Jian, T.; Wang, Y.; Zhang, H.; Ying, Y.; Zhang, H.; Yang, J. Analysis of Ecological Characteristics and Influencing Factors of Phytoplankton Communities in Spring and Summer in the East China Sea. Mar. Fish. 2022, 44, 589–597. [Google Scholar]
- Wang, Y.; Li, S.; Jiang, Y.; Zhang, S.; Hu, Q.; Shen, T.; Jiao, H. Construction of the Xiangshan Port Marine Fishery Farm and Techniques for the Enhancement and Conservation of Biological Resources. Chin. J. Aquat. Sci. 2019, 43, 1972–1980. [Google Scholar]
- Britton, D.; Schmid, M.; Revill, A.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L.; Mundy, C.N. Seasonal and site-specific variation in the nutritional quality of temperate seaweed assemblages: Implications for grazing invertebrates and the commercial exploitation of seaweeds. J. Appl. Phycol. 2021, 33, 603–616. [Google Scholar] [CrossRef]
- Duffy, J.E. Biodiversity and ecosystem function in the sea. Mar. Ecol. Prog. Ser. 2006, 311, 253–265. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Q.; Liu, C.; Xian, W. Seasonal and spatial variations in fish assemblage in the Yangtze estuary and adjacent waters and their relationship with environmental factors. J. Mar. Sci. Eng. 2022, 10, 1679. [Google Scholar] [CrossRef]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z. Evolution of Bohai Rim Port City Cluster Based on Niche Theory: Pattern Changes and Symbiosis Strategies. Geogr. Geogr. Inf. Sci. 2024, 40, 80. [Google Scholar]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.M.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of biodiversity loss on ocean ecosystem services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef]
- Ortega, A.; Geraldi, N.R.; Alam, I.; Kamau, A.A.; Acinas, S.G.; Logares, R.; Gasol, J.M.; Massana, M.; Krause-Jensen, D.; Duarte, C.M. Important contribution of macroalgae to oceanic carbon sequestration. Nat. Geosci. 2019, 12, 748–754. [Google Scholar] [CrossRef]
- Cheng, A.; Lim, W.Y.; Lim, P.E.; Yang Amri, A.; Poong, S.W.; Song, S.L.; Ilham, Z. Marine autotroph-herbivore synergies: Unravelling the roles of macroalgae in marine ecosystem dynamics. Biology 2022, 11, 1209. [Google Scholar] [CrossRef] [PubMed]
- Krumhansl, K.A.; Scheibling, R.E. Production and fate of kelp detritus. Mar. Ecol. Prog. Ser. 2012, 467, 281–302. [Google Scholar] [CrossRef]
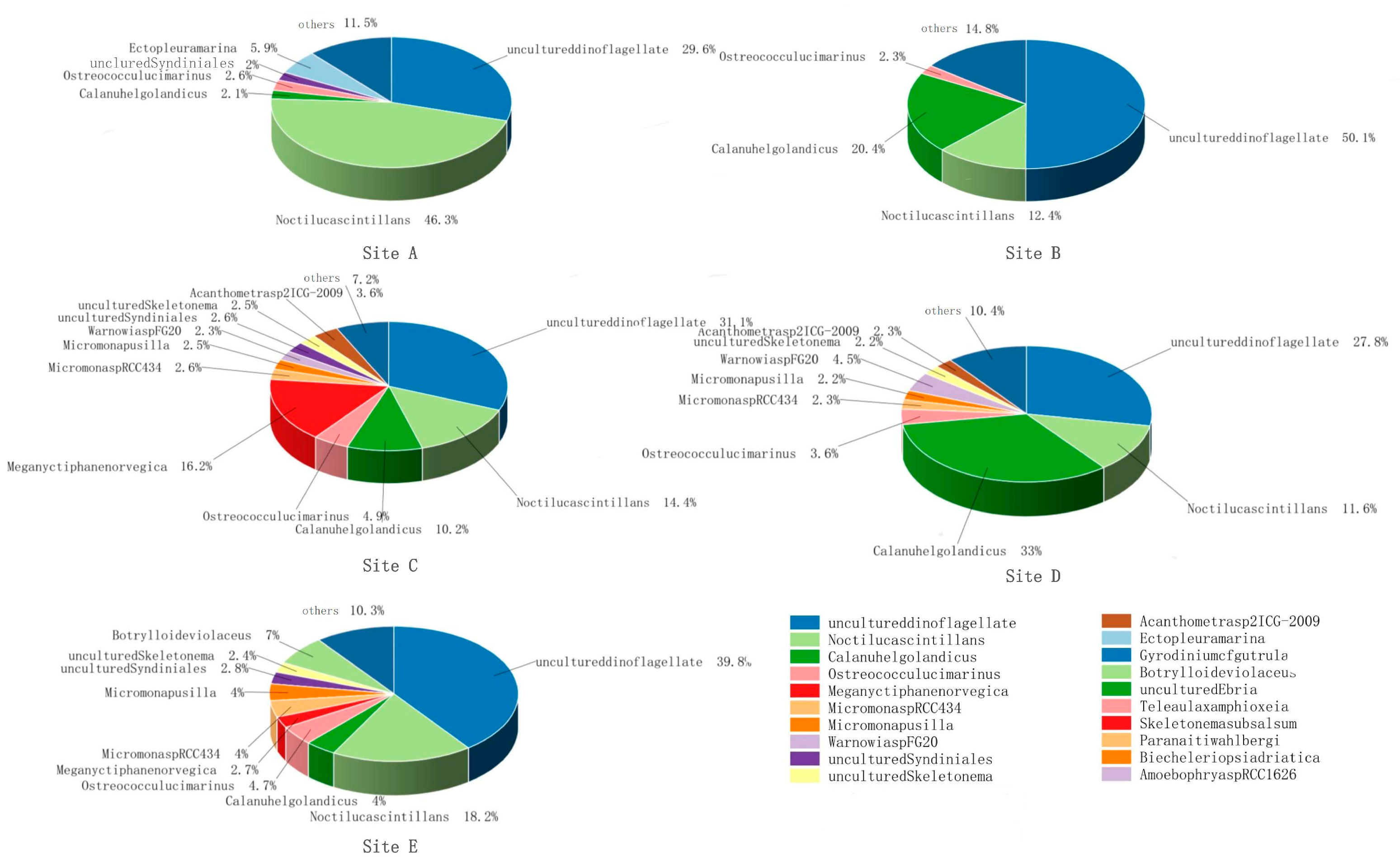
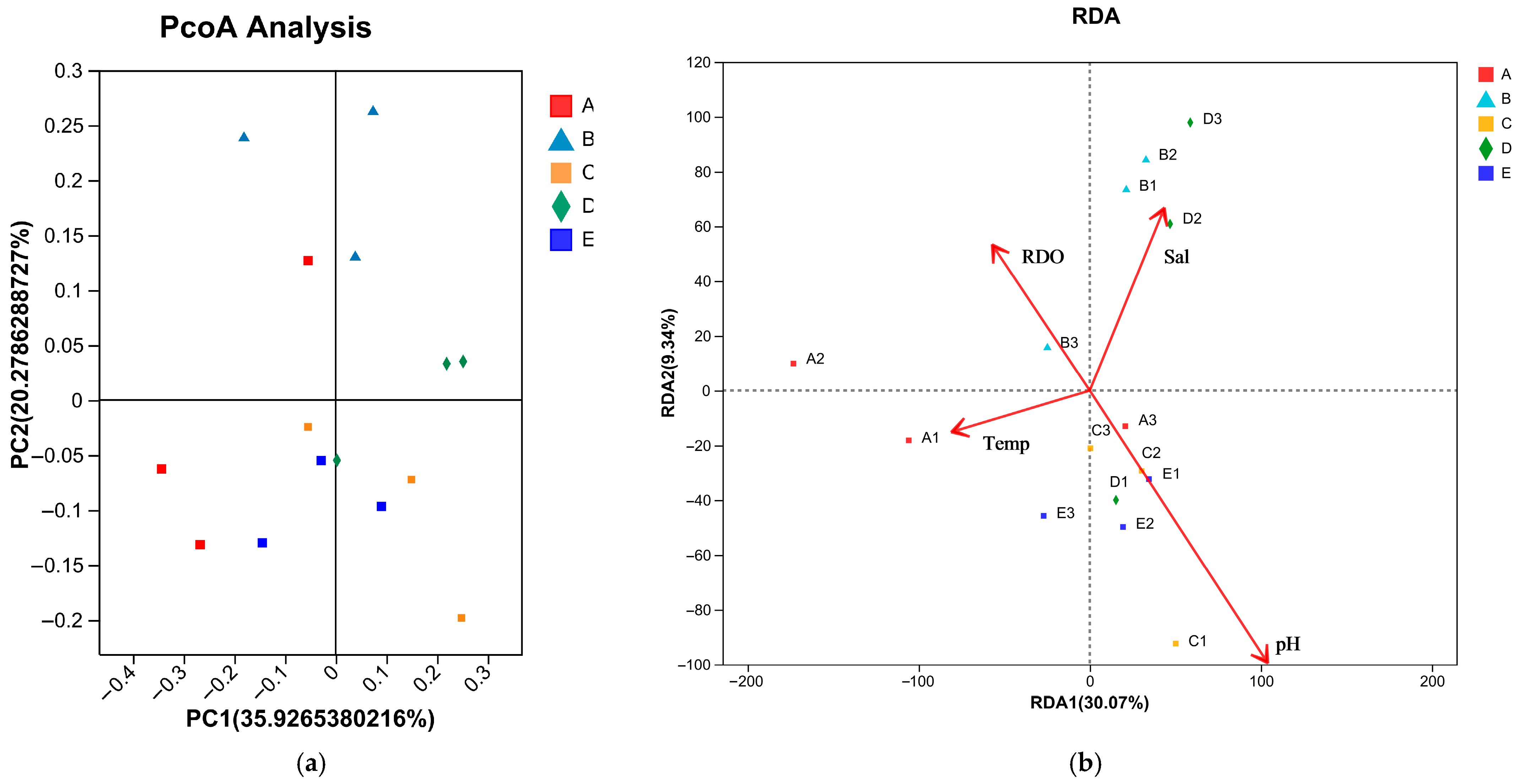
| Site | Shannon | Chao1 | Peilou |
|---|---|---|---|
| A | 2.45 ± 0.50 | 509.09 ± 61.14 | 0.41 ± 0.08 |
| B | 2.57 ± 0.29 | 566.72 ± 31.42 | 0.42 ± 0.05 |
| C | 3.32 ± 0.22 | 539.64 ± 25.50 | 0.54 ± 0.4 |
| D | 3.28 ± 0.28 | 540.42 ± 13.67 | 0.50 ± 0.05 |
| E | 3.22 ± 0.21 | 578.96 ± 10.25 | 0.52 ± 0.03 |
| Site | Temp (°C) | RDO (mg/L) | pH (pH) |
|---|---|---|---|
| A | 21.48 ± 0.65 | 5.67 ± 0.43 | 8.93 ± 0.05 |
| B | 21.48 ± 0.65 | 5.67 ± 0.43 | 8.93 ± 0.05 |
| C | 20.77 ± 0.58 | 4.99 ± 0.46 | 9.1 ± 0.17 |
| D | 20.93 ± 0.52 | 5.35 ± 0.37 | 9.2 ± 0.25 |
| E | 21.22 ± 0.49 | 5.24 ± 0.43 | 9.22 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, A.; Wang, Y.; Zhao, X.; Wang, K. Comparison of Eukaryotic Community Structures Across Different Habitat Types in the Nearshore Waters of Ma’an Archipelago Based on Environmental DNA Technology. Water 2025, 17, 2970. https://doi.org/10.3390/w17202970
Dai A, Wang Y, Zhao X, Wang K. Comparison of Eukaryotic Community Structures Across Different Habitat Types in the Nearshore Waters of Ma’an Archipelago Based on Environmental DNA Technology. Water. 2025; 17(20):2970. https://doi.org/10.3390/w17202970
Chicago/Turabian StyleDai, Anqi, Yuqing Wang, Xu Zhao, and Kai Wang. 2025. "Comparison of Eukaryotic Community Structures Across Different Habitat Types in the Nearshore Waters of Ma’an Archipelago Based on Environmental DNA Technology" Water 17, no. 20: 2970. https://doi.org/10.3390/w17202970
APA StyleDai, A., Wang, Y., Zhao, X., & Wang, K. (2025). Comparison of Eukaryotic Community Structures Across Different Habitat Types in the Nearshore Waters of Ma’an Archipelago Based on Environmental DNA Technology. Water, 17(20), 2970. https://doi.org/10.3390/w17202970






