Limitation of Phytoplankton Primary Production by Biogenic Elements in the Coastal Waters of the Azov-Black Sea Basin as a Natural Factor in Conditioning the Marine Environment
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zaitsev, Y.P. The Bluest in the World; United Nations: New York, NY, USA, 1998; 142p. (In Russian) [Google Scholar]
- Gulin, S.B.; Mirzoeva, N.Y.; Egorov, V.N.; Polikarpov, G.G.; Sidorov, I.G.; Proskurnin, V.Y. Secondary radioactive contamination of the Black Sea after Chernobyl accident: Current levels, pathways and trends. Mar. Chem. 2012, 92, 1–18. [Google Scholar] [CrossRef]
- Zagranichny, K.A. On the issue of sources and volumes of oil components entering the Black Sea. Eng. Bull. Don 2014, 28, 80–92. (In Russian) [Google Scholar]
- Yunev, O.A.; Konovalov, S.K.; Velikova, V. Anthropogenic Eutrophication in the Pelagic Zone of the Black Sea: Long-Term Trends, Mechanisms, Consequences; Kovalevsky, A.O., Ed.; GEOS; Institute of Biology of the Southern Seas of the Russian Academy of Sciences: Sevastopol, Russia; Marine Hydrophysical Institute of the Russian Academy of Sciences: Moscow, Russia, 2019; 164p. (In Russian) [Google Scholar]
- Soloveva, O.; Tikhonova, E.; Alyomov, S.; Mirzoeva, N.; Skuratovskaya, E.; Matishov, G.; Egorov, V. Distribution and Genesis of Aliphatic Hydrocarbons in Bottom Sediments of Coastal Water Areas of the Crimea (the Black and Azov Seas). Water 2024, 16, 2395. [Google Scholar] [CrossRef]
- Finenko, Z.Z.; Churilova, T.Y.; Suslin, V.V. Assessment of phytoplankton biomass and primary products in the black sea from satellite data. In Fishing Resources of the Black and Azov Seas; Yeremeev, V.N., Gaevskaya, A.V., Shulman, G.E., Zagorodnaya, Y.A., Eds.; NAS of Ukraine, Institute of Biology of the Southern Seas named after A. O. Kovalevsky: Sevastopol, Russia; EKOSI-Hydrophysika Publ.: Sevastopol, Russia, 2011; pp. 237–248. (In Russian) [Google Scholar]
- Stelmakh, L.V. Patterns of Phytoplankton Growth and Its Consumption by Microzooplankton in the Black Sea: Abstract of the Dissertation. Ph.D. Thesis, A.O. Kovalevsky Institute of Marine Biological Research, Sevastopol, Russia, 2017; 42p. (In Russian). [Google Scholar]
- Matishov, G.G.; Povazhny, V.V.; Berdnikov, S.V.; Moses, V.J.; Gitelson, A.A. Assessment of chlorophyll a concentration and primary production in the Sea of Azov using satellite data. Rep. Acad. Sci. 2010, 432, 563–566. (In Russian) [Google Scholar]
- Berdnikov, S.V.; Dashkevich, L.V.; Kulygin, V.V.; Povazhny, V.V. Evaluation of the primary production of the Sea of Azov according to remote sensing data. Sci. S. Russ. 2018, 14, 55–65. (In Russian) [Google Scholar]
- Berdnikov, S.V.; Kleshchenkov, A.V.; Kreneva, K.V.; Glushchenko, G.Y.; Dashkevich, L.V.; Kulygin, V.V.; Karasyuk, V.S.; Saprykin, V.V.; Bulysheva, N.I. The results of marine scientific research at the Southern Scientific Center of the Russian Academy of Sciences (SSC RAS) in the Sea of Azov in 2003–2018. Part 2: Hydrobiology. Aquat. Bioresour. Habitat 2019, 2, 7–23. (In Russian) [Google Scholar]
- Filatova, T.B. The relationship of the regime of biogenic substances of the Sea of Azov with hydrometeorological factors // Izvestiya vuzov. The North Caucasus region. Nat. Sci. 2010, 157, 107–111. (In Russian) [Google Scholar]
- Mikhailenko, A.; Fedorov, Y.; Dotsenko, I.; Solodko, D. Water quality formation factors in the Azov Sea. Int. Multidiscip. Sci. GeoConf. SGEM 2020, 20, 871–878. [Google Scholar]
- Ragueneau, O.; Lancelot, C.; Egorov, V.N.; Vervulimmeren, J.; Cociasu, A.; Deliat, G.; Krastev, A.; Daoub, N.; Rousseau, V.; Popovichev, V.; et al. Biogeochemical transformations of inorganic nutrients in the mixing zone between the Danube river and the north-western Black Sea. Estuar. Coast. Shelf Sci. 2002, 54, 321–336. [Google Scholar] [CrossRef]
- Becquevort, S.; Bouvier, T.; Lancelot, C.; Deliat, G.; Egorov, V.N.; Popovichev, V. The seasonal modulation of organic matter utilization by bacteria in the Danube-Black Sea mixing zone. Estuar. Coast. Shelf Sci. 2002, 54, 337–354. [Google Scholar] [CrossRef]
- Popovichev, V.N.; Egorov, V.N. Phosphorus exchange of natural suspended matter in the estuaries of the Danube and Chorokh rivers. Mar. Ecol. J. 2003, 2, 83–97. (In Russian) [Google Scholar]
- Egorov, V.N.; Bobko, N.I.; Marchenko, Y.-G.; Sadogursky, S.E. The content of biogenic elements and the limitation of primary phytoplankton production in the estuary of the Vodopadnaya River (Southern coast of Crimea). Environ. Saf. Coast. Offshore Zones Sea 2021, 3, 37–51. [Google Scholar]
- Egorov, V.N.; Bobko, N.I.; Marchenko, Y.G.; Sadogursky, S.E. Content of nutrients and limitation of phytoplankton primary production in the specially protected natural area “Cape Martyan” (Black Sea). Mar. Biol. J. 2021, 6, 19–30. (In Russian) [Google Scholar] [CrossRef]
- Egorov, V.N.; Popovichev, V.N.; Gulin, S.B.; Bobko, N.I.; Rodionova, N.Y.; Tsarina, T.V.; Marchenko, Y.G. The influence of phytoplankton primary production on the cycle of biogenic elements in the coastal waters off Sevastopol, Black Sea. Russ. J. Mar. Biol. 2018, 44, 240–247. [Google Scholar] [CrossRef]
- Dobrolyubsky, O.K. Microelements and Life; Molodaya Gvardiya: Moscow, Russia, 1956; 124p. (In Russian) [Google Scholar]
- Turn, G.A.; Joshi, G.H.V.; Chauhan, V.D.; Rao, P.S. Effect of metal ions on the growth of Sargassum swartzii. Indian J. Mar. Sci. 1982, 11, 338. [Google Scholar]
- Sapozhnikov, V.V.; Metreveli, M.P. Stoichiometric model of organic matter-the basis for quantitative study of productive and destructive processes in the ocean. Proc. VNIRO 2015, 155, 135–145. (In Russian) [Google Scholar]
- Cooper, L.H.N. On the ratio of nitrogen to phosphorus in the Sea. J. Mar. Biol. Ass. 1937, 22, 177–182. [Google Scholar] [CrossRef]
- Cooper, L.H.N. Redifinition of the anomaly in the nitrate-phosphate ratio. Mar. Biol. Ass. 1938, 23, 171–178. [Google Scholar] [CrossRef]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221. [Google Scholar]
- Ilyichev, V.G.; Semin, V.L. Nitrogen “diabetes” of the Sea of Azov. Nature 2006, 11, 19–24. (In Russian) [Google Scholar]
- Hutchinson, D. Limnology; Mir: Moscow, Russia, 1969; 591p. (In Russian) [Google Scholar]
- Zilov, E.A. Hydrobiology and Aquatic Ecology (Organization, Functioning and Pollution of Aquatic Ecosystems); Publishing House of the Irkutsk State University: Irkutsk, Russia, 2009; 147p. (In Russian) [Google Scholar]
- Patton, A.E. Energy and Kinetics of Biochemical Processes; Mir Publ.: Moscow, Russia, 1968; 159p. (In Russian) [Google Scholar]
- Rubin, A.B. Biophysics; Higher school Publ.: Moscow, Russia, 1987; 319p. (In Russian) [Google Scholar]
- Egorov, V.N. Theory of Radioisotopic and Chemical Homeostasis of Marine Ecosystems; Springer: Cham, Switzerland, 2021; 320p. [Google Scholar]
- Hillebrand, H.; Steinert, G.; Boersma, M.; Malzahn, A.; Meunier, C.L.; Plum Ch Ptacnik, R. Goldman revisited: Faster-growing phytoplankton has lower N: P and lower stoichiometric flexibility. Limnol. Oceanogr. 2013, 58, 2076–2088. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, J.Y.; Han, B.P.; Naselli-Flores, L. The effects of absolute and relative nutrient concentrations (N/P) on phytoplankton in a subtropical reservoir. Ecol. Indic. 2020, 115, 106466. [Google Scholar] [CrossRef]
- Mathew, T.; Prakash, S.; Baliarsingh, S.K.; Samanta, A.; Lakshmi, R.S.; Lotliker, A.A.; Chatterjee, A.; Nair, T.B. Response of phytoplankton biomass to nutrient stoichiometry in coastal waters of the western Bay of Bengal. Ecol. Indic. 2021, 131, 108–119. [Google Scholar] [CrossRef]
- de Fommervault, O.P.; Migon, C.; D’Ortenzio, F.; d’Alcalà, M.R.; Coppola, L. Temporal variability of nutrient concentrations in the northwestern Mediterranean sea (DYFAMED time-series station). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2015, 100, 1–12. [Google Scholar] [CrossRef]
- Trommer, G.; Leynaert, A.; Klein, C.; Naegelen, A.; Beker, B. Phytoplankton phosphorus limitation in a North Atlantic coastal ecosystem not predicted by nutrient load. J. Plankton Res. 2013, 35, 1207–1219. [Google Scholar] [CrossRef]
- Howarth, R.W.; Marino, R. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnol. Oceanogr. 2006, 1, 364–376. [Google Scholar] [CrossRef]
- Ngatia, L.; Grace, J.M., III; Moriasi, D. Nitrogen and phosphorus eutrophication in marine. In Monitoring of Marine Pollution; Intech Open: London, UK, 2019; pp. 77–93. [Google Scholar]
- Grasshoff, K. (Ed.) Methods of Seawater Analysis; Verlag Chemie: Weinheim, Germany; New York, NY, USA, 1976; 317p. [Google Scholar]
- Sapozhnikov, V.V. (Ed.) Manual for the Chemical Analysis of Marine and Fresh Waters in the Environmental Monitoring of Fisheries Reservoirs and Prospective Fishing Areas of the World Ocean; VNIRO Publishing House: Moscow, Russia, 2003; 202p. (In Russian) [Google Scholar]
- Monbet, P.; McKelvie, I.D. Phosphates. In Handbook of Water Analysis; CRC Press: Boca Raton, FL, USA, 2007; pp. 233–266. [Google Scholar]
- Partchevskaya, D.S. Statistics for Radioecologists (A Practical Guide to Statistics and Experimental Planning in Radioecology); Naukova dumka: Kyiv, Ukraine, 1969; 115p. (In Russian) [Google Scholar]
- Vernadsky, V.I. Chemical Structure of the Earth’s Biosphere and Its Environment; Nauka Publ.: Moscow, Russia, 1965; 374p. (In Russian) [Google Scholar]
- Egorov, V.N.; Popovichev, V.N.; Burlakova, Z.P.; Krupatkina, D.K.; Kovalenko, T.P.; Alexandrov, B.G. Mathematical model of the biosedimentation function of the ecosystem of the photic layer of the Western chalistatics of the Black Sea. In Molismology of the Black Sea; Naukova Dumka: Kiev, Ukraine, 1992; pp. 50–62. (In Russian) [Google Scholar]
- Polikarpov, G.G.; Egorov, V.N. Marine Dynamic Radiochemoecology; Energoatomizdat: Moscow, Russia, 1986; 176p. (In Russian) [Google Scholar]
- Egorov, V.N.; Zesenko, A.Y.; Parkhomenko, A.V.; Finenko, Z.Z. Mathematical description of the kinetics of mineral phosphorus metabolism by unicellular algae. Hydrobiol. J. 1982, 18, 45–50. (In Russian) [Google Scholar]
- Brunel, L.; Dauta, A.; Guerri, M.M. Croissance algae: Validation d’un modele a stock l’aide de donnees experimentales. Ann. Limnol. 1982, 18, 91–99. [Google Scholar] [CrossRef]
- Giammatteo, P.A.; Schindler, J.E.; Waldron, M.C.; Speziale, B.J.; Freedman, M.L.; Zimmerman, M.J. Use of equilibrium programs in predicting phosphorus availability. In Environmental Biogeochemistry; Halberg, R., Ed.; Ecol Bull: Stockholm, Sweden, 1983; pp. 491–501. [Google Scholar]
- Michaelis, L.; Menten, M.L. Die kinetik der invertinwirkung. Biochem. Z. 1913, 49, 333–369. [Google Scholar]
- Burmaster, D.E.; Chisholm, S.W. A comparison of two methods for measuring phosphate uptake by Monochrysis lutheri Droop grown in continuous culture. J. Experim. Marine Ecol. 1979, 39, 187–202. [Google Scholar] [CrossRef]
- Jacques, M. Recherches Sur la Croissance des Cultures Bactériennes. Ph.D. Thesis, Université de Paris, Paris, France, 1942; pp. 1–210. [Google Scholar]
- Dugdale, R.C. Nutrient limitation in the sea: Dynamics, identification and significance. Limnol. Oceanogr. 1967, 12, 685–695. [Google Scholar] [CrossRef]
- Droop, M.R. The nutrient status of algae cells in continuous culture. J. Mar. Biol Assoc. 1974, 2, 541–555. [Google Scholar]
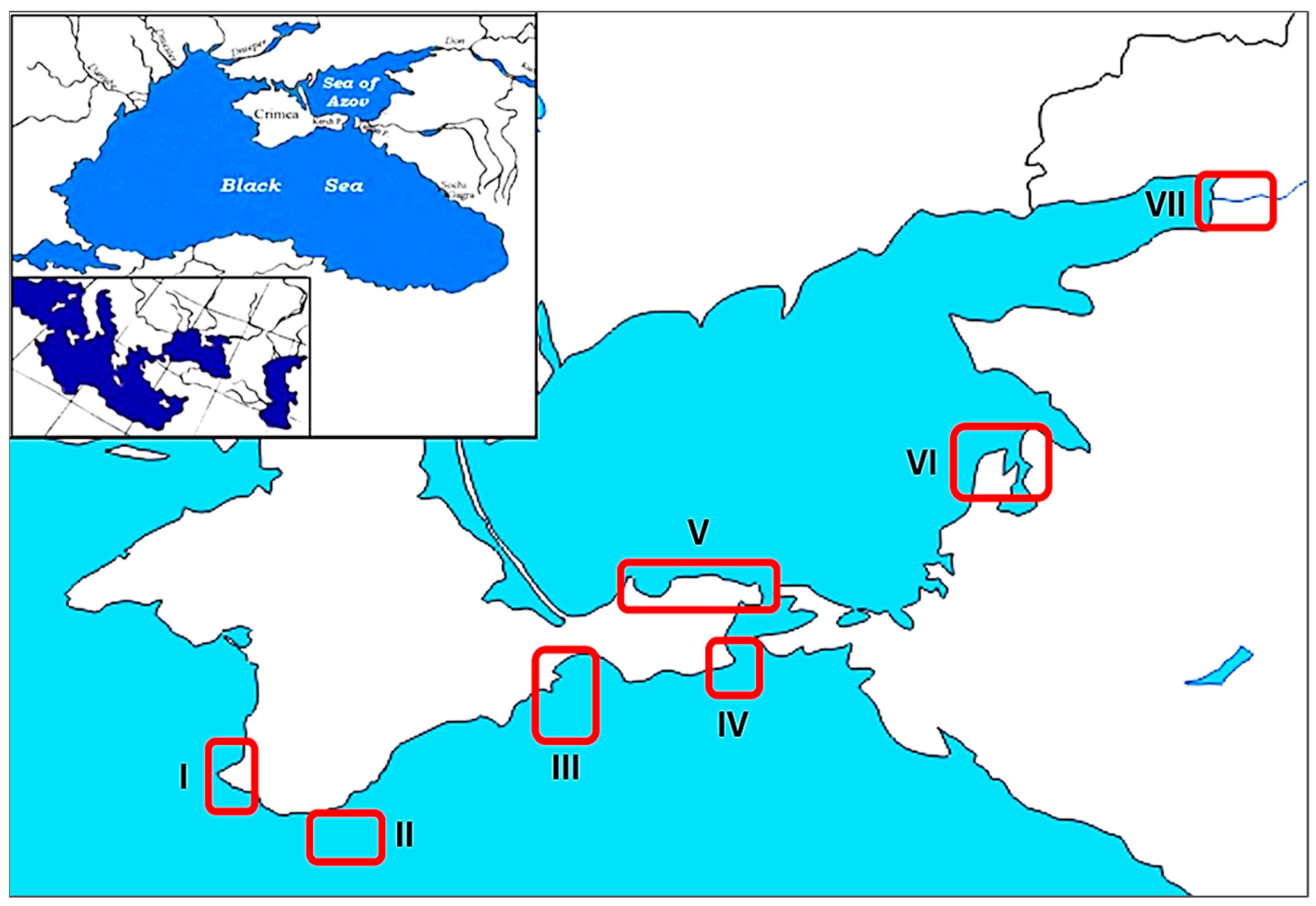
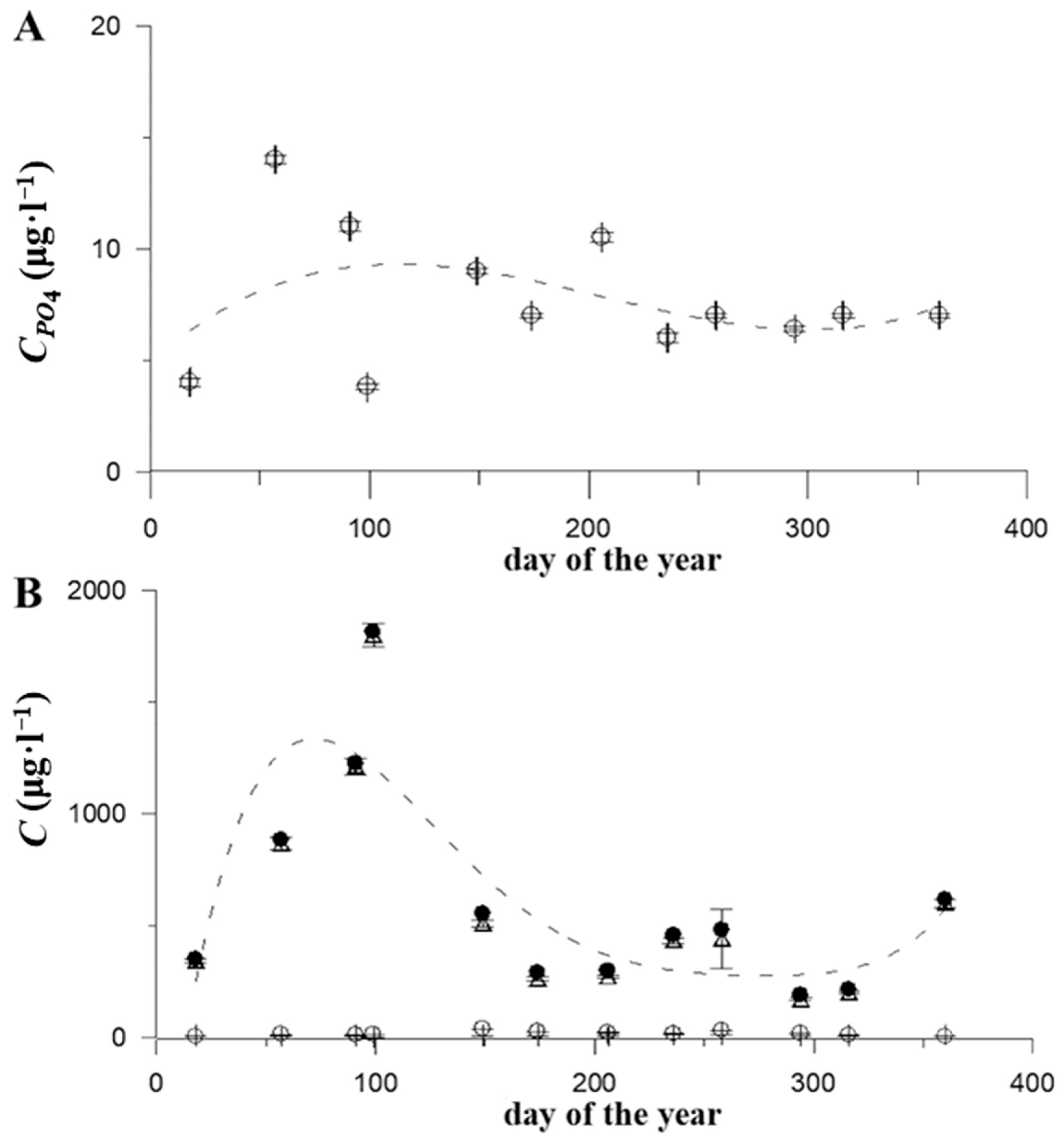
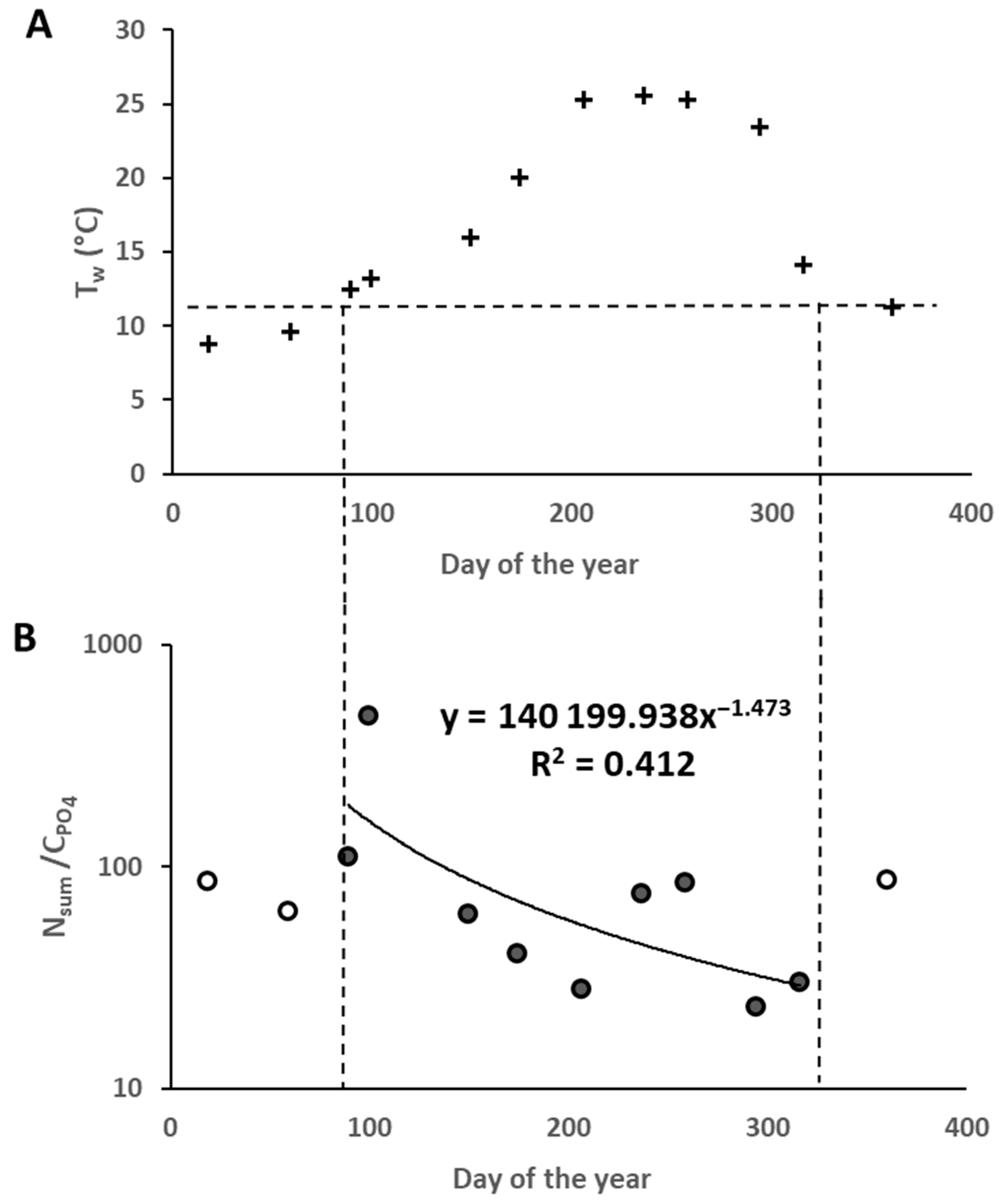
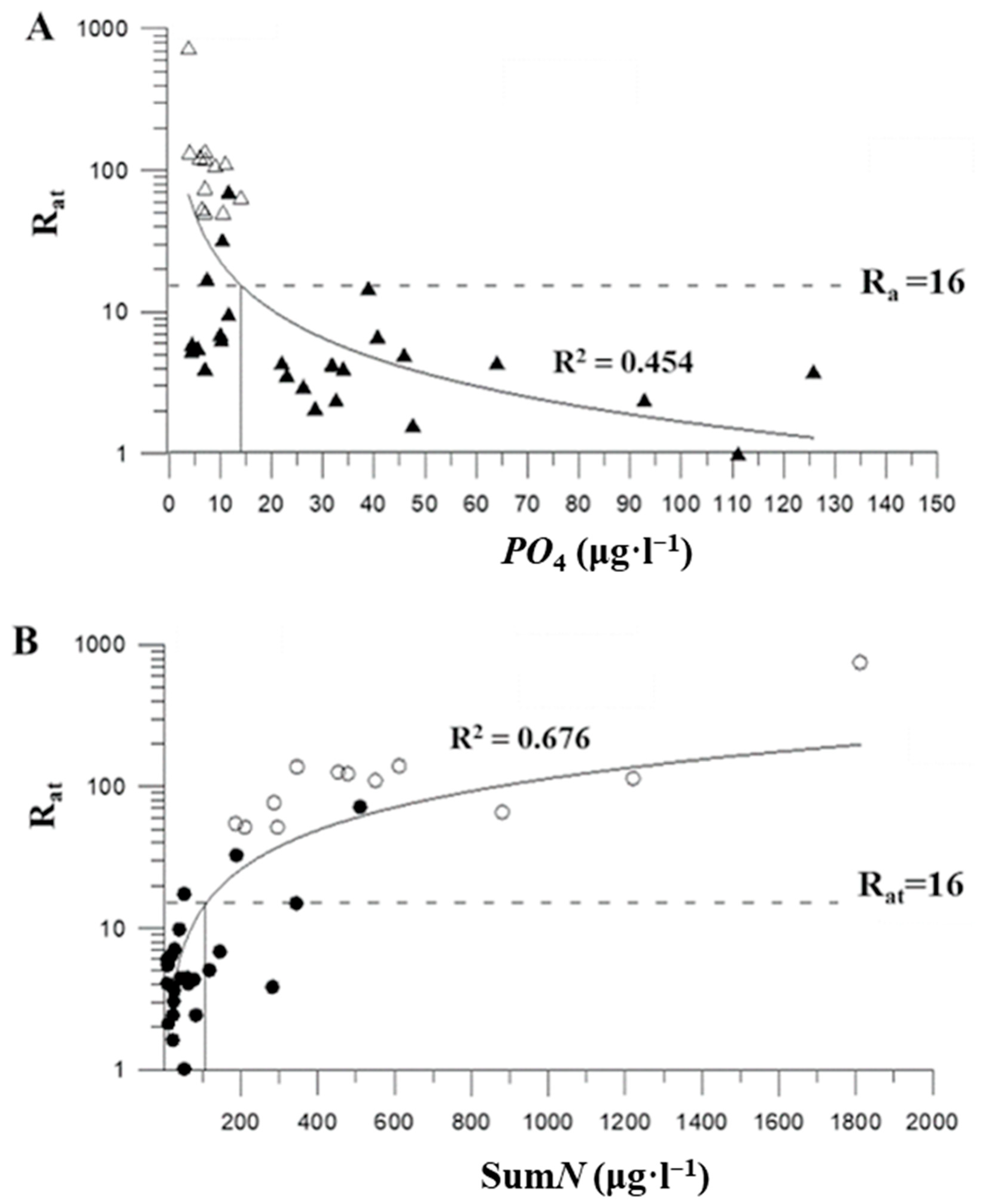
| N | Date | Region | Coordinates | Day Number of the Year | Water Temperature, °C | NH4 ± SD, μg·L−1 | NO2 ± SD, μg·L−1 | NO3 ± SD, μg·L−1 | Nsum, μg·L−1 | PO4 ± SD, μg·L−1 | S‰ | pH | Rat ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 April 2023 | I | 44.59597° N 33.46977° E | 99 | 13.2 | 12.0 ± 0.5 | 0.9 ± 0.01 | 1800 ± 54 | 1813 | 3.8 ± 0.1 | - | - | 742 ± 98.43 |
| 2 | 28 May 2023 | I | 44.59597° N 33.46977° E | 149 | 16.0 | 36.7 ± 1.8 | 5.6 ± 0.10 | 510 ± 15 | 552 | 9.0 ± 0.1 | - | - | 109 ± 5.61 |
| 3 | 24 June 2023 | I | 44.59597° N 33.46977° E | 174 | 20.0 | 24.0 ± 1.1 | 4.3 ± 0.20 | 260 ± 8 | 288 | 7.0 ± 0.1 | - | - | 76 ± 4.83 |
| 4 | 24 July 2023 | I | 44.59597° N 33.46977° E | 206 | 25.3 | 20.0 ± 1.0 | 7.3 ± 0.10 | 270 ± 8 | 297 | 10.5 ± 0.2 | - | - | 51 ± 2.00 |
| 5 | 23 August 2023 | I | 44.59597° N 33.46977° E | 236 | 25.6 | 14.0 ± 0.7 | 8.0 ± 0.10 | 433 ± 13 | 455 | 6.0 ± 0.2 | - | - | 125 ± 9.80 |
| 6 | 14 September 2023 | I | 44.59597° N 33.46977° E | 258 | 25.3 | 30.0 ± 1.4 | 9.8 ± 0.10 | 440 ± 132 | 480 | 7.0 ± 0.1 | - | - | 122 ± 8.10 |
| 7 | 20 October 2023 | I | 44.59597° N 33.46977° E | 294 | 23.4 | 16.0 ± 0.8 | 2.2 ± 0.01 | 170 ± 5 | 188 | 6.4 ± 0.1 | - | - | 54 ± 3.59 |
| 8 | 22 November 2023 | I | 44.59597° N 33.46977° E | 316 | 14.1 | 7.4 ± 0.4 | 4.4 ± 0.10 | 200 ± 6 | 212 | 7.0 ± 0.1 | - | - | 51 ± 3.00 |
| 9 | 25 December 2023 | I | 44.59597° N 33.46977° E | 360 | 11.3 | 2.0 ± 0.1 | 7.4 ± 0.10 | 600 ± 18 | 614 | 7.0 ± 0.1 | - | - | 138 ± 9.05 |
| 10 | 18 January 2024 | I | 44.59597° N 33.46977° E | 18 | 8.8 | 2.0 ± 0.1 | 4.8 ± 0.07 | 342 ± 10 | 348 | 4.0 ± 0.2 | - | - | 136 ± 16.18 |
| 11 | 28 February 2024 | I | 44.59597° N 33.46977° E | 59 | 9.6 | 12.0 ± 0.6 | 3.1 ± 0.10 | 867 ± 26 | 882 | 14.0 ± 0.2 | - | - | 65 ± 3.25 |
| 12 | 31 March 2024 | I | 44.59597° N 33.46977° E | 89 | 12.5 | 10.0 ± 0.5 | 3.6 ± 0.15 | 1210 ± 36 | 1223 | 11.0 ± 0.2 | - | - | 113 ± 7.51 |
| 13 | 7 August 2024 | II | 44.32834° N 34.13334° E | - | 26.0 | 10.5 ± 0.5 | 1.48 ± 0.02 | 500.0 ± 15 | 512 | 11.6 ± 0.2 | 17.87 ± 0.01 | - | 71.0 ± 2.68 |
| 14 | 24 July 2024 | III | 44.35334° N 35.76167° E | - | 26.0 | 12.2 ± 0.6 | 0.81 ± 0.01 | 177.0 ± 5 | 190 | 10.4 ± 0.2 | 17.69 ± 0.01 | 32.4 ± 1.13 | |
| 15 | 31 July 2024 | III | 44.96547° N 35.39734° E | - | 26.0 | 13.0 ± 0.6 | 0.44 ± 0.01 | 28.0 ± 1 | 41 | 11.6 ± 0.2 | 17.51 ± 0.01 | - | 9.7 ± 0.04 |
| 16 | 20 August 2024 | IV | 45.06189° N 36.32576° E | - | - | 11.6 ± 0.6 | 2.0 ± 0.03 | 40.5 ± 1 | 54 | 7.4 ± 0.1 | 17.00 ± 0.01 | - | 17.2 ± 0.57 |
| 17 | 26 April 2024 | V | 45.37202° N 36.02468° E | - | - | 8.9 ± 0.4 | 3.8 ± 0.06 | 52.4 ± 2 | 65 | 34.0 ± 0.5 | - | 7.90 | 4.0 ± 0.07 |
| 18 | 26 April 2024 | V | 45.37202° N 36.02468° E | - | - | 7.3 ± 0.4 | 2.5 ± 0.04 | 34.7 ± 1 | 45 | 22.0 ± 0.3 | - | 8.15 | 4.4 ± 0.10 |
| 19 | 26 April 2024 | V | 45.37202° N 36.02468° E | - | - | 10.0 ± 0.5 | 3.2 ± 0.05 | 14.5 ± 1 | 28 | 23.0 ± 0.3 | - | 8.17 | 3.6 ± 0.12 |
| 20 | 30 July 2024 | V | 45.47090° N 36.35680° E | - | - | 48.3 ± 2.3 | 4.00 ± 0.06 | 10.0 ± 1 | 62 | 64.0 ± 0.9 | 14.25 ± 0.01 | 7.90 | 4.4 ± 0.04 |
| 21 | 1 August 2024 | V | 45.47146° N 35.83910° E | - | - | 25.9 ± 1.2 | 3.03 ± 0.05 | 56.0 ± 1 | 85 | 92.8 ± 1.4 | 14.58 ± 0.01 | 7.90 | 2.4 ± 0.03 |
| 22 | 20 August 2024 | V | 45.43143° N 36.59767° E | - | - | 12.4 ± 0.6 | 2.7 ± 0.04 | 331.0 ± 10 | 346 | 38.9 ± 0.6 | 14.65 ± 0.01 | - | 14.8 ± 0.08 |
| 23 | 20 August 2024 | V | 45.47038° N 36.31504° E | - | - | 13.0 ± 0.6 | 1.9 ± 0.03 | 132.0 ± 4 | 147 | 40.7 ± 0.6 | 14.29 ± 0.10 | - | 6.74 ± 0.03 |
| 24 | 20 August 2024 | V | 45.47189° N 36.24817° E | - | - | 15.1 ± 0.7 | 3.3 ± 0.05 | 93.5 ± 3 | 120 | 45.9 ± 0.7 | 14.20 ± 0.01 | - | 5.0 ± 0.04 |
| 25 | 12 July 2024 | VI | 47.07845° N 39.30465° E | - | - | 6.0 ± 0.3 | 31.8 ± 0.48 | 246.2 ± 7 | 284 | 125.8 ± 3.6 | - | - | 3.8 ± 0.02 |
| 26 | 9 September 2024 | VI | 46.15524° N 38.26916° E | - | - | 7.0 ± 0.3 | 6.5 ± 0.10 | 18.0 ± 1 | 25 | 47.6 ± 0.7 | 13.42 ± 0.01 | - | 1.6 ± 0.08 |
| 27 | 10 September 2024 | VI | 45.86233° N 37.89013° E | - | - | 8.0 ± 0.4 | 5.9 ± 0.20 | 7.1 ± 1 | 21 | 10.2 ± 0.2 | 13.73 ± 0.01 | - | 6.4 ± 0.12 |
| 28 | 10 September 2024 | VI | 46.16491° N 38.24925° E | - | - | 8.0 ± 0.4 | 4.9 ± 0.10 | 21.6 ± 1 | 35 | 32.6 ± 0.5 | 13.42 ± 0.01 | - | 2.4 ± 0.10 |
| 29 | 13 September 2024 | VI | 46.27268° N 38.28073° E | - | - | 10.0 ± 0.5 | 2.4 ± 0.04 | 14.0 ± 1 | 26 | 26.2 ± 0.4 | 13.28 ± 0.01 | - | 3.0 ± 0.11 |
| 30 | 13 September 2024 | VI | 46.27223° N 38.40142° E | - | - | 7.0 ± 0.3 | 2.0 ± 0.03 | 20.0 ± 1 | 30 | 10.0 ± 0.3 | 37.76 ± 0.01 | - | 7.0 ± 0.09 |
| 31 | 11 July 2024 | VII | 47.21737° N 39.80865° E | - | - | 6.0 ± 0.3 | 11.8 ± 0.18 | 36.2 ± 1 | 54 | 111.0 ± 1.7 | - | - | 1.0 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorov, V.; Skuratovskaya, E.; Matishov, G.; Stepanyan, O.; Gorbunov, R.; Mirzoeva, N.; Tikhonova, E.; Alyomov, S.; Bobko, N.; Marchenko, Y.; et al. Limitation of Phytoplankton Primary Production by Biogenic Elements in the Coastal Waters of the Azov-Black Sea Basin as a Natural Factor in Conditioning the Marine Environment. Water 2025, 17, 2968. https://doi.org/10.3390/w17202968
Egorov V, Skuratovskaya E, Matishov G, Stepanyan O, Gorbunov R, Mirzoeva N, Tikhonova E, Alyomov S, Bobko N, Marchenko Y, et al. Limitation of Phytoplankton Primary Production by Biogenic Elements in the Coastal Waters of the Azov-Black Sea Basin as a Natural Factor in Conditioning the Marine Environment. Water. 2025; 17(20):2968. https://doi.org/10.3390/w17202968
Chicago/Turabian StyleEgorov, Viktor, Ekaterina Skuratovskaya, Gennady Matishov, Oleg Stepanyan, Roman Gorbunov, Natalia Mirzoeva, Elena Tikhonova, Sergey Alyomov, Nikolay Bobko, Yulia Marchenko, and et al. 2025. "Limitation of Phytoplankton Primary Production by Biogenic Elements in the Coastal Waters of the Azov-Black Sea Basin as a Natural Factor in Conditioning the Marine Environment" Water 17, no. 20: 2968. https://doi.org/10.3390/w17202968
APA StyleEgorov, V., Skuratovskaya, E., Matishov, G., Stepanyan, O., Gorbunov, R., Mirzoeva, N., Tikhonova, E., Alyomov, S., Bobko, N., Marchenko, Y., & Soloveva, O. (2025). Limitation of Phytoplankton Primary Production by Biogenic Elements in the Coastal Waters of the Azov-Black Sea Basin as a Natural Factor in Conditioning the Marine Environment. Water, 17(20), 2968. https://doi.org/10.3390/w17202968









