Abstract
Two Indian Ocean surgeon fish Paracanthurus hepatus individuals were observed near Saint-Raphaël (Provence, France, north-western Mediterranean Sea) in the late summer of 2024 in Posidonia oceanica seagrass and reef habitats. This species is very popular among aquarium hobbyists in Europe, and a growing number of mega-yachts, such as those which moor in the Saint-Raphaël marina, have seawater aquariums on board. Accidental or deliberate release from one such aquarium is the most probable origin of these individuals. The first individual was speared and the second one was no longer sighted after a September storm. Their establishment is unlikely; however, in the future, with the warming of Mediterranean waters and the rapid increase in the number of mega-yachts, this could change. Yacht owners and their staff should be informed of the risk posed by aquarium discharges.
1. Introduction
The surgeon fish Paracanthurus hepatus (Linnaeus, 1766) (Acanthuridae: Perciformes) is native to the Indo-Pacific Ocean, e.g., the Philippines, Indonesia, southern Japan, northern Australia, New Caledonia, the Samoa Islands, Sri Lanka, and eastern Africa [1,2,3]. It has not been reported in the Red Sea [4,5]. In its native area, it thrives between (2) 10 and 40 m of depth [1,2,3]. A single individual was photographed underwater in Israeli waters in the Mediterranean Sea, in 2015 [5,6]. In the Indo-Pacific, P. hepatus thrives 1–3 m above the seabed, in areas where the currents are perceptible, and feeds on planktonic invertebrates and benthic algae [1,5,7,8].
The Mediterranean Sea is a hotspot of marine epsilon species diversity, with 12,000 to 17,000 taxa [9,10,11]. It is also a hotspot of marine biological invasions [11,12,13,14,15]: more than 1000 non-indigenous species (NIS), including 188 teleosts, have been reported [5,16,17]. Among these NIS, at least 751 species are definitely established [17], i.e., they are self-reproducing without human assistance [17,18,19,20].
The main pathways of NIS arrival in the Mediterranean Sea are the Suez Canal, shellfish aquaculture, fouling on ship hulls, and ballast waters [21,22,23,24,25,26,27]. Escape from aquariums is regarded as a minor pathway illustrated by the well-known case of the green seaweed Caulerpa taxifolia, likely released from the public marine aquarium of Monaco [28,29,30].
Biological invasions are regarded worldwide as a major threat to biodiversity [31,32,33,34]. In the Mediterranean Sea, they constitute one of the ‘big three’, together with coastal development and overexploitation by fisheries; at the moment, the impact of biological invasions is greater than that of global warming [35,36,37,38].
Here, we report the in situ observation of two individuals of Paracanthurus hepatus in summer 2024 in eastern Provence (France, north-western Mediterranean).
2. Materials and Methods
The first individual (individual No. 1) was observed opportunistically in July 2024 by snorkeling during a spearfishing trip below the Dramont semaphore, near Île d’Or (municipality of Saint-Raphaël; 43°24.561′ N, 6°50.767′ E) (Figure 1); it was speared (Figure 2). A second individual (individual No. 2) was observed (43°24.666′ N, 06°51.402′ E) from 14 July to 5 September 2024, during scuba dives organized almost every day with the specific aim of searching for and observing individual No. 2 and other possible individuals.

Figure 1.
Cape Dramont, with the semaphore (white). In the foreground, the Île d’Or Island. Photo © Vincent Mouny, courtesy of the author.
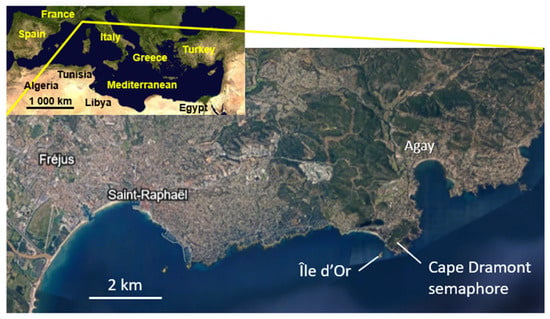
Figure 2.
A map of the Mediterranean Sea (top left) and of the study area: Saint-Raphaël and Cape Dramont. The map is from Google Earth®.
Saint-Raphaël is a seaside tourist resort with a population of 35,000, with four marinas. The main marina, the Vieux Port, harbors a few fishing vessels and more than 260 recreational boats, including ~50 belonging to the mega-yacht category (over 30 m long).
The taxonomic identification of the sighted specimens was carried out using the available literature, e.g., [1,2,3].
3. Results and Discussion
Individual No. 1 was speared in July 2024 at around 5 m of depth above a reef covered with small-size macroalgae (Figure 3). It was ~20 cm long.

Figure 3.
Individual No.1 of Paracanthurus hepatus, spearfished in July 2024 below the Dramont semaphore. The arrow mark is visible on the back of the head. Photo © Éric Visquis, courtesy of the author.
Individual No. 2 was tracked by scuba diving for around twenty days between mid-August and early September 2024. It is plausible to assume that it was the same individual at each sighting, with the same size and at the same site. Its length was estimated to be 20 cm. It was relatively sedentary, between 1 and 5 m deep, remaining during the study period within a perimeter 40 m in diameter, above a Posidonia oceanica (Linnaeus) Delile seagrass meadow, a Mediterranean endemic species, or a rocky reef (Figure 4 and Figure 5). During observations, the fish did not seem to be feeding. It showed no sign of fear, allowing itself to be approached (and photographed) from 1 m away. Tracking stopped due to severe weather. Afterward, the fish was not seen again: it could have moved, been fished, or died due to stormy conditions or low temperatures. Summer storms mix the heated sea surface water with deeper cold water, resulting in the rapid and severe cooling of the sea surface water [39,40,41,42].

Figure 4.
Individual No. 2 of Paracanthurus hepatus swimming above a rocky reef. The white spots correspond to Padina pavonica (Linnaeus) Thivy, a benthic brown macroalga very common in the Mediterranean Sea. Photo © Viviane Monneray.

Figure 5.
Individual No. 2 of Paracanthurus hepatus swimming above a rocky reef (foreground) and a meadow of Posidonia oceanica, a seagrass endemic to the Mediterranean Sea (background). Photo © Viviane Monneray.
Paracanthurus hepatus is very popular among aquarium hobbyists, is one of the most widely traded species of ornamental fish worldwide, and is one of the top ten species imported into the European Union [43]. The individual photographed in Israel in 2016 was probably released from a public or private aquarium: a Lessepsian origin (i.e., a migration from the Red Sea to the Mediterranean through the Suez Canal) has been excluded by Marcelli et al., as the species does not occur in the Red Sea [5,6]. The sighting we report here is the second one in the Mediterranean Sea and the first one in the western Mediterranean.
The release of NIS from aquariums is not infrequent [29,30,44,45,46]. The green macroalga Halimeda incrassata (J. Ellis) J.V. Lamouroux, which has recently invaded soft substrate habitats of Mallorca (Balearic Islands, Spain), is commonly used as an ornament in seawater aquariums [47,48]. The reports of the teleosts Abudefduf vaigiensis (Quoy and Gaimard, 1825) in Italy [49], Pomacanthus asfur (Forsskål, 1775) in Malta [50], Zebrasoma xanthurum (Blyth, 1852) in Sardinia [51], and Heteropriacanthus cruentatus (Lacepède, 1801) in Lebanon [52], also likely correspond to escape or deliberate release from aquariums. In the Florida coral reefs, 16 species of exotic teleosts occur that were likely released by marine aquariums, including the invasive lionfish Pterois volitans which escaped from the the Biscayne Bay Aquarium during Hurricane Andrew [53,54].
In most cases, discharge from an aquarium is no more than a highly probable hypothesis. A unique case is that of an aquarium hobbyist from the Athens region (Greece), recounted by Apostolis Ladopoulos, the owner of an aquarium shop (in Zenetos et al. [55]). This hobbyist bought a specimen of the snapper Lutjanus sebae (Cuvier, 1816) from him, a fish that does not occur in the Mediterranean Sea; as the snapper was growing quickly, and it was feeding on the other species in his aquarium, he decided to release it into the sea, at Lagonissi, in October 2009 (Gulf of Saronikos, Greece). He used to visit ‘his’ snapper, which stayed for some time near the release site; then, he lost sight of it. In December 2010, in the Gulf of Saronikos, an individual of Lutjanus sebae was caught by a spearfisher. There is no absolute proof that it was the same individual, but the probability is very high [55].
A growing number of mega-yachts, such as those that moor in the Saint-Raphaël marinas, have seawater aquariums on board; the risk that, during their maintenance or cleaning, species from warm seas are accidentally or deliberately released into the sea, must be considered [51,56]. This is undoubtedly the most probable origin of the two individuals of P. hepatus that we have observed near Saint-Raphaël.
Fortunately, these tropical fish do not seem likely to establish themselves in the Mediterranean, for several reasons. Although they survive there in the summer, winter temperatures are less favorable for them, especially in the north-western Mediterranean. In addition, the founding population is too small (Propagule Pressure Hypothesis). Their low genetic diversity is not favorable for long-term establishment, although some species have successfully naturalized from a very few founding individuals or with low genetic diversity (e.g., [57,58,59,60,61]). As far as P. hepatus is concerned, it is a gonochoric species, with males and females; during spawning, individuals gather in small numbers and take on a paler color; males chase females and, when a couple forms, the partners suddenly rise near the sea surface, releasing their gametes into the water before descending to the bottom [62]. We may presume that P. hepatus will not be successful in spawning and establishing in the north-western Mediterranean, like many other NIS (see, e.g., [17,63,64,65,66]).
However, in the future, the risk of tropical teleosts released from aquariums on board mega-yachts successfully establishing themselves in the Mediterranean Sea will increase. Firstly, the sea surface temperature is increasing, sometimes at a faster pace than elsewhere in the world, making conditions in these habitats closer to those of their native area [67,68]. Secondly, the Mediterranean harbors between half and a third of the world fleet of yachts over 24 m long, likely to be equipped with on-board marine aquariums [69,70]; in addition, the number of yachts over 30 m in length is rapidly growing worldwide: 966 in 1988 vs. 4950 in 2018 [71].
It would be useful if yacht owners and their staff were made aware of the risk posed by aquarium discharges. This could be the role of harbor masters and should constitute a criterion for the award of the ‘Blue Flag’ to coastal municipalities in Europe. The Blue Flag is an ecolabel awarded to beaches and marinas based on 27 specific criteria for beaches and 16 specific criteria for marinas. The requirements cover four aspects: water quality, environmental education and information, environmental management, and safety and services. The Blue Flag Campaign is owned and run by the independent non-profit organization Foundation for Environmental Education (FEE) [72,73,74].
4. Conclusions
Two individuals of the Indian Ocean surgeon fish Paracanthurus hepatus were observed near Saint-Raphaël in the north-western Mediterranean Sea. The first one was spearfished, and the second was observed by scuba divers for around 20 days. The most probable hypothesis is that they were accidentally or deliberately released from a marine aquarium on board a mega-yacht, such as those which moor in the Saint-Raphaël marinas.
The released population is probably too small, and winter temperatures are too cold, for this tropical species to be established in the north-western Mediterranean Sea. However, this could change with the ongoing warming of the Mediterranean.
Yacht owners and their staff should be made aware of the risk posed by aquarium discharges. They should be informed of this risk by harbor masters, and the provision of this information should constitute a criterion for the award of the ‘Blue Flag’ label to coastal municipalities.
Author Contributions
Conceptualization, C.-F.B.; investigation, C.R., M.V., O.D., and V.M.; original draft preparation, C.-F.B.; writing, review and editing, C.-F.B., C.R., M.V., O.D., and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to the Club de plongée d’Agay, to which they belong. They also thank Michèle Perret-Boudouresque, for bibliographical assistance, four anonymous reviewers for their valuable comments, and Michael Paul, a native English speaker, for proofreading the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lieske, E.; Myers, R.F. Collins Pocket Guide to Coral Reef Fishes, Indo-Pacific and Caribbean; Harper Collins Publishers: New York, NY, USA, 1994. [Google Scholar]
- Randall, J.E.; Allen, G.R.; Steen, R.C. Fishes of the Great Barrier Reef and Coral Sea; University of Hawaii Press: Honolulu, HI, USA, 1998. [Google Scholar]
- Pauly, D.; Froese, R. (Eds.) Paracanthurus hepatus. In Fish Base; World Wide Web Electronic Publication, 2024; Version (06/2024); Available online: www.fishbase.org (accessed on 15 October 2024).
- Golani, D.; Bogorodsky, S.V. The fishes of the Red Sea—Reappraisal and updated checklist. Zootaxa 2010, 2462, 1–135. [Google Scholar] [CrossRef]
- Golani, D.; Azzurro, E.; Dulčić, J.; Massutí, E.; Orsi-Relini, L. Atlas of Exotic Fishes in the Mediterranean Sea, 2nd ed.; Entirely Revised, Edition; CIESM Publishers: Paris, France; Toulouse, Monaco, 2021. [Google Scholar]
- Marcelli, M.; Dayan, A.R.; Langeneck, J. Finding Dory: First record of Paracanthurus hepatus (Perciformes: Acanthuridae) in the Mediterranean Sea. Mar. Biodiv. 2016, 47, 599–602. [Google Scholar] [CrossRef]
- Winterbottom, R.; McLennan, D.A. Cladogram versatility: Evolution and biogeography of Acanthuroid fishes. Evolution 1993, 47, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L. Do You Know Where Your Aquarium Fish Come From? National Geographic: Burbank, CA, USA, 2014; Available online: https://www.nationalgeographic.com/animals/article/140718-aquarium-fish-source-sustainability-animals-ocean-science. (accessed on 16 October 2024).
- Boudouresque, C.F. Marine biodiversity in the Mediterranean: Status of species, populations and communities. Sci. Rep. Port-Cros Natl. Park. 2004, 20, 97–146. [Google Scholar]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Katsanevakis, S.; Gatto, F.; Zenetos, A.; Cardoso, A.C. How many marine aliens in Europe? Manag. Biol. Inv. 2013, 4, 37–42. [Google Scholar] [CrossRef]
- Galil, B.S. A sea under siege—Alien species in the Mediterranean. Biol. Inv. 2000, 2, 177–186. [Google Scholar] [CrossRef]
- Galil, B.S. Alien species in the Mediterranean Sea—Which, when, where, why? Hydrobiologia 2008, 606, 105–116. [Google Scholar] [CrossRef]
- Schaffelke, B.; Smith, J.E.; Hewitt, C.D. Introduced macroalgae—A growing concern. J. Appl. Phycol. 2006, 18, 529–541. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Perret-Boudouresque, M.; Verlaque, M. Donor and recipient regions for exotic species of marine macrophytes: A case of unidirectional flow, the Mediterranean Sea. Rapp. Comm. Int. Mer. Médit. 2016, 41, 426. [Google Scholar]
- Zenetos, A.; Gofas, S.; Verlaque, M.; Cinar, M.E.; García Raso, J.E.; Bianchi, C.N.; Morri, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C.; et al. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution. Mediterr. Mar. Sci. 2010, 11, 381–493. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; López-Garcia, E.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošik, V.; Wilson, J.R.U.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, C.F.; Verlaque, M. An overview of species introduction and invasion processes in marine and coastal lagoon habitats. Cah. Biol. Mar. 2012, 53, 309–317. [Google Scholar]
- Richardson, D.M.; Pyšek, P.; Rejmanek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Methuen publ. 1958; University of Chicago Press: Chicago, IL, USA, 2000; Reprinted. [Google Scholar]
- Verlaque, M.; Boudouresque, C.F.; Mineur, F. Oyster transfers as a vector for marine species introductions: A realistic approach based on the macrophytes. In Impact of mariculture on coastal ecosystems. CIESM Workshop Monogr. 2007, 32, 39–47. [Google Scholar]
- Calvo, S.; Tomasello, A.; Di Maida, G.; Pirrotta, M.; Buia, M.C.; Cinelli, F.; Cormaci, M.; Furnari, G.; Giaccone, G.; Luzzu, F.; et al. Seagrasses along the Sicilian coasts. Chem. Ecol. 2010, 26, 249–266. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Poursanidis, D.; Yokes, M.B.; Mačić, V.; Beqiraj, S.; Kashta, L.; Sghaier, R.; Zakhama-Sriaeb, R.; Benamer, I.; Bitar, G.; et al. Twelve years after the first report of the crab Percnon gibbesi (H. Milne-Edwards, 1853) in the Mediterranean: Current distribution and invasion rates. J. Biol. Res.-Thessalon. 2011, 16, 224–236. [Google Scholar]
- Petrocelli, A.; Antolić, B.; Bolognini, L.; Cecere, E.; Cvitković, I.; Despalatović, M.; Falace, A.; Finotto, S.; Iveša, L.; Mačić, V.; et al. Port baseline biological surveys and seaweed bioinvasion in port areas: What’s the matter in the Adriatic Sea? Mar. Pollut. Bull. 2019, 147, 98–116. [Google Scholar] [CrossRef]
- van der Loos, L.M.; Bafort, Q.; Bosch, S.; Ballesteros, E.; Barbara, I.; Bercibar, E.; Blanfuné, A.; Bogaert, K.; Bouckenooghe, S.; Boudouresque, C.F.; et al. Non-indigenous seaweeds in the Northeast Atlantic Ocean, the Mediterranean Sea and Macaronesia: A critical synthesis of diversity, spatial and temporal patterns. Eur. J. Phycol. 2024, 59, 127–156. [Google Scholar] [CrossRef]
- Por, F.D. Lessepsian Migrations. The Influx of Red Sea Biota into the Mediterranean by Way of the Suez Canal; Springer publ.: Berlin, Germany, 1978. [Google Scholar]
- Meinesz, A.; Hesse, B. Introduction et invasion de l’algue tropicale Caulerpa taxifolia en Méditerranée nord-occidentale. Oceanol. Acta 1991, 14, 415–426. [Google Scholar]
- Jousson, O.; Pawlowski, J.; Zaninetti, L.; Meinesz, A.; Boudouresque, C.F. Molecular evidence for the aquarium origin of the green alga Caulerpa taxifolia introduced to the Mediterranean Sea. Mar. Ecol. Prog. Ser. 1998, 172, 275–280. [Google Scholar] [CrossRef]
- Meinesz, A.; Boudouresque, C.F. Sur l’origine de Caulerpa taxifolia en Méditerranée. C. R. Acad. Sci. Paris Life Sci. 1996, 319, 603–613. [Google Scholar]
- Schmitz, D.C.; Simberloff, D. Biological invasions: A growing threat. Issues Sci. Technol. 1997, 13, 33–40. [Google Scholar]
- Canning-Clode, J. General introduction—Aquatic and terrestrial biological invasions in the 21st century. In Biological Invasions in Changing Ecosystems. Vectors, Ecological Impacts, Management and Predictions; Canning-Clode, J., Ed.; De Gruyter Publ.: Warsaw, Poland; Berlin, Germany, 2002; pp. 13–20. [Google Scholar]
- Johnson, C.R.; Chapman, A.R.O. Seaweed invasions: Introduction and scope. Bot. Mar. 2007, 50, 321–325. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E.M. The ravages of guns, nets and bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Tiralongo, F.; Hall-Spencer, J.M.; Giovos, I.; Kleitou, P. Editorial: Biological invasions in the Mediterranean Sea. Front. Mar. Sci. 2022, 9, 1016168. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Blanfuné, A.; Fernandez, C.; Lejeusne, C.; Pérez, T.; Ruitton, S.; Thibault, D.; Thibaut, T.; Verlaque, M. Marine Biodiversity—Warming vs. biological invasions and overfishing in the Mediterranean Sea: Take care, ‘One train can hide another’. MOJ Ecol. Environ. Sci. 2017, 2, 172–183. [Google Scholar] [CrossRef]
- Corrales, X.; Ofir, E.; Coll, M.; Goren, M.; Edelist, D.; Heymans, J.J.; Gal, G. Modeling the role and impact of alien species and fisheries on the Israeli marine continental shelf ecosystem. J. Mar. Syst. 2017, 170, 88–102. [Google Scholar] [CrossRef]
- Rindi, F.; Gavio, B.; Díaz-Tapia, P.; Di Camillo, C.G. Long-term changes in the benthic macroalgal flora of a coastal area affected by urban impacts (Cornero Riviera, Mediterranean Sea). Biodiv. Conserv. 2020, 29, 2275–2295. [Google Scholar] [CrossRef]
- Millot, C.; Wald, L. The effect of Mistral wind on the Ligurian current near Provence. Oceanol. Acta 1980, 3, 399–402. [Google Scholar]
- Millot, C.; Taupier-Letage, I. Circulation of the Mediterranean Sea. Handb. Environ. Chem. 2005, 5, 29–66. [Google Scholar]
- Bensoussan, N.; Romano, J.C.; Harmelin, J.G.; Garrabou, J. High resolution characterization of northwest Mediterranean coastal waters thermal regime: To better understand responses of benthic communities to climate change. Estuar. Coast. Shelf Sci. 2010, 87, 431–441. [Google Scholar] [CrossRef]
- Ragone, F.; Meli, A.; Napoli, A.; Pasquero, C. Ocean surface anomalies after strong winds in the Western Mediterranean Sea. J. Mar. Sci. Engin. 2019, 7, 182. [Google Scholar] [CrossRef]
- Wabnitz, C.; Taylor, M.; Green, E.; Razak, T. From Ocean to Aquarium; UNEP-WCMC: Cambridge, UK, 2003. [Google Scholar]
- Boudouresque, C.F.; Meinesz, A.; Ribera, M.A.; Ballesteros, E. Spread of the green alga Caulerpa taxifolia (Caulerpales, Chlorophyta) in the Mediterranean: Possible consequences of a major ecological event. Sci. Mar. 1995, 59 (Suppl. 1), 21–29. [Google Scholar]
- Minchin, D.; Gollash, S. Vectors—How exotics get around. In Invasive Aquatic Species of Europe: Distribution, Impacts and Management; Leppäkoski, E., Gollash, S., Olenin, S., Eds.; Kluwer Academic publ.: Dordreicht, Germany, 2002; pp. 183–192. [Google Scholar]
- Woodhouse, K.O.W.; Zuccarello, G.C. Diversity of tropical macroalgae in the New Zealand marine aquarium trade. N. Z. J. Mar. Freshw. Res. 2022, 57, 207–228. [Google Scholar] [CrossRef]
- Alós, J.; Tomas, F.; Terrados, J.; Verbruggen, H.; Ballesteros, E. Fast-spreading green beds of recently introduced Halimeda incrassata invade Mallorca Island (NW Mediterranean Sea). Mar. Ecol. Prog. Ser. 2016, 558, 153–158. [Google Scholar] [CrossRef]
- Verbruggen, H.; Ballesteros, E. An Atlantic origin for the introduced species Halimeda incrassata (Bryopsidales, Chlorophyta). Mediterr. Mar. Sci. 2024, 25, 55–57. [Google Scholar] [CrossRef]
- Vacchi, M.; Chiantore, M.C. Abudefduf vaigiensis (Quoy & Gaimard, 1825): A tropical damselfish in Mediterranean Sea. Biol. Mar. Mediterr. 2000, 7, 841–843. [Google Scholar]
- Karachle, P.K.; Angelidis, A.; Apostolopoulos Gayas, D.; Ballesteros, M.; Bonnici, C.; Brodersen, M.M.; Castriota, L.; Chalari, N.; Cottalorda, J.M.; Crocetta, F.; et al. New Mediterranean Biodiversity Records (March 2016). Mediterr. Mar. Sci. 2016, 17, 230–252. [Google Scholar] [CrossRef]
- Guidetti, P.; Magnani, L.; Navone, A. First record of the acanthurid fish Zebrasoma xanthurum (Blyth, 1852) in the Mediterranean Sea, with some considerations on the risk associated with the aquarium trade. Mediterr. Mar. Sci. 2016, 17, 147–151. [Google Scholar] [CrossRef]
- Badreddine, A.; Bitar, G. First record of Heteropriacanthus cruentatus (Lacepède, 1801) (Chordata: Priacanthidae) in the Mediterranean Sea from the Lebanese waters. J. Black Sea/Mediterr. Environ. 2019, 25, 178–181. [Google Scholar]
- Semmens, B.X.; Buhle, E.R.; Salomon, A.K.; Pattengill-Semmens, C.V. A hotspot of non-native marine fishes: Evidence for the aquarium trade as an invasion pathway. Mar. Ecol. Prog. Ser. 2004, 266, 239–244. [Google Scholar] [CrossRef]
- Gulenç, Z. Marine aquariums and lionfish trade in Turkey. In Lionfish Invasion and Its Management in the Mediterranean Sea; Hüseyinoğlu, M.F., Öztük, B., Eds.; Turkish Marine Research Foundation (TUDAV) Publ.: Istanbul, Turkey, 2018; pp. 88–99. [Google Scholar]
- Zenetos, A.; Apostolopoulos, G.; Crocetta, F. Aquaria kept marine fish species possibly released in the Mediterranean Sea: First confirmation of intentional release in the wild. Acta Ichthyol. Piscat. 2016, 46, 255–262. [Google Scholar] [CrossRef]
- Verlaque, M.; Ballesteros, E.; Blanfuné, A.; Boudouresque, C.F.; Dominici, J.M.; Duriez, O.; Grémillet, D.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; et al. Problèmes liés à l’accueil des super-yachts et des navires de croisière. Sci. Rep. Port-Cros Natl. Park. 2023, 37, 451–495. [Google Scholar]
- Von Holle, B.; Simberloff, D. Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 2005, 86, 3212–3218. [Google Scholar] [CrossRef]
- Golani, D.; Azzurro, E.; Corsini-Foka, M.; Falautano, M.; Andaloro, F.; Bernardi, G. Genetic bottlenecks and successful biological invasions: The case of a recent Lessepsian migrant. Biol. Lett. 2007, 3, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. The role of propagule pressure in biological invasions. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 81–102. [Google Scholar] [CrossRef]
- McDermott, S.M.; Finnoff, D.C. Impact of repeated human introductions and the Allee effect on invasive species spread. Ecol. Modell. 2016, 329, 100–111. [Google Scholar] [CrossRef]
- Bariche, M.; Kleitou, P.; Kalogirou, S.; Bernardi, G. Genetics reveal the identity and origin of the lionfish invasion in the Mediterranean Sea. Sci. Rep. 2017, 7, 6782. [Google Scholar] [CrossRef]
- Le Bris, S.; Mitel, C. Paracanthurus hepatus (Linnaeus, 1766). In Doris, 28/01/2022. Available online: https://doris.ffessm.fr/ref/specie/2219 (accessed on 23 November 2024).
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; García Raso, J.E.; Çinar, M.E.; Almogi-Labin, A.; Ates, A.S.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef]
- Zenetos, A.; Çinar, M.E.; Crocetta, F.; Golani, D.; Rosso, A.; Servello, G.; Shenkar, N.; Turon, S.; Verlaque, M. Uncertainties and validation of alien species catalogues: The Mediterranean as an example. Estuar. Coast. Shelf Sci. 2017, 191, 171–187. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Semroud, R.; Blanfuné, A.; Perret-Boudouresque, M. Sighting of Saccorhiza polyschides (Phaeophyceae, Stramenopiles) in Algeria (Mediterranean Sea): An insight into range expansion routes. Cryptog. Algol. 2020, 41, 31–36. [Google Scholar] [CrossRef]
- Faget, D.; Boudouresque, C.F.; Lejeusne, C. The possible failed pre-Linnaean introduction in the Mediterranean Sea: An archival case study of the brown mussel Perna perna. Divers. 2023, 15, 1072. [Google Scholar] [CrossRef]
- Sicre, M.A.; Jalali, B.; Martrat, B.; Schmidt, S.; Bassetti, M.A.; Kallel, N. Sea surface temperature variability in the North Western Mediterranean Sea (Gulf of Lion) during the Common Era. Earth Planet. Sci. Lett. 2016, 456, 124–133. [Google Scholar] [CrossRef]
- von Schuckmann, K.; Moreira, L.; Cancet, M.; Gues, F.; Autret, E.; Aydogdu, A.; Castrillo, L.; Ciani, D.; Cipollone, A.; Clementi, E.; et al. The state of the ocean in the northeastern Atlantic and adjacent seas. State Planet 2024, 2, 1–32. [Google Scholar] [CrossRef]
- Carreño, A.; Lloret, J. Environmental impacts of increasing leisure boating activity in Mediterranean coastal waters. Ocean Coast. Manag. 2021, 209, 105693. [Google Scholar] [CrossRef]
- Carreño, A.; Hardy, P.Y.; Sánchez, E.; Martínez, E.; Plante, C.; Lloret, J. Safeguarding Marine Protected Area in the Growing Mediterranean Blue Economy. Recommendations for Leisure Boating; PHAROS4MPA projects, Interreg Mediterranean, European Union; WWF-France: Marseille, France, 2019. [Google Scholar]
- Salle, G. Superyachts. Luxe, Calme et Ecocide; Éditions Amsterdam: Paris, France, 2021. [Google Scholar]
- Pencarelli, T.; Splendiani, S.; Fraboni, C. Enhancement of the “Blue Flag” Eco-label in Italy: An empirical analysis. Anatolia 2015, 27, 28–37. [Google Scholar] [CrossRef]
- Sipic, T. Eco-labelling of marine recreation services: The case of Blue Flag price premium in Croatia. J. Ecotour. 2017, 16, 1–23. [Google Scholar] [CrossRef]
- Borrello, P.; Chiesa, S.; Maltese, S.; Silvestri, C.; Scarpato, A. Management of Posidonia oceanica beached accumulations and the ‘ecological beach’ model. Rapp. Comm. Intl. Mer. Méditerr. 2019, 42, 218. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).