Fabrication of MoS2@Fe3O4 Magnetic Catalysts with Photo-Fenton Reaction for Enhancing Tetracycline Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Catalyst Synthesis
2.3. Characterization Method
2.4. Degradation Performance Evaluation
3. Results
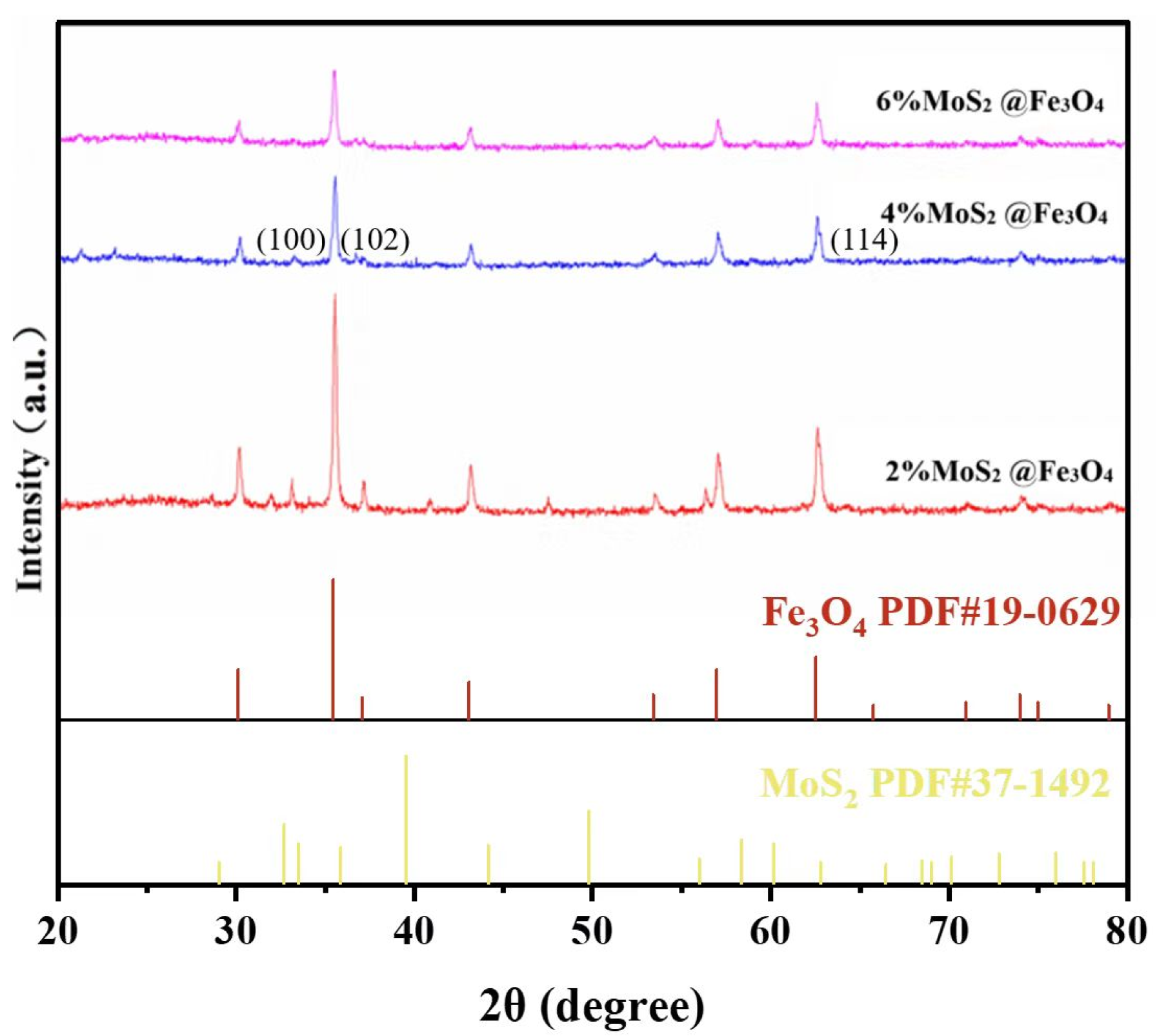
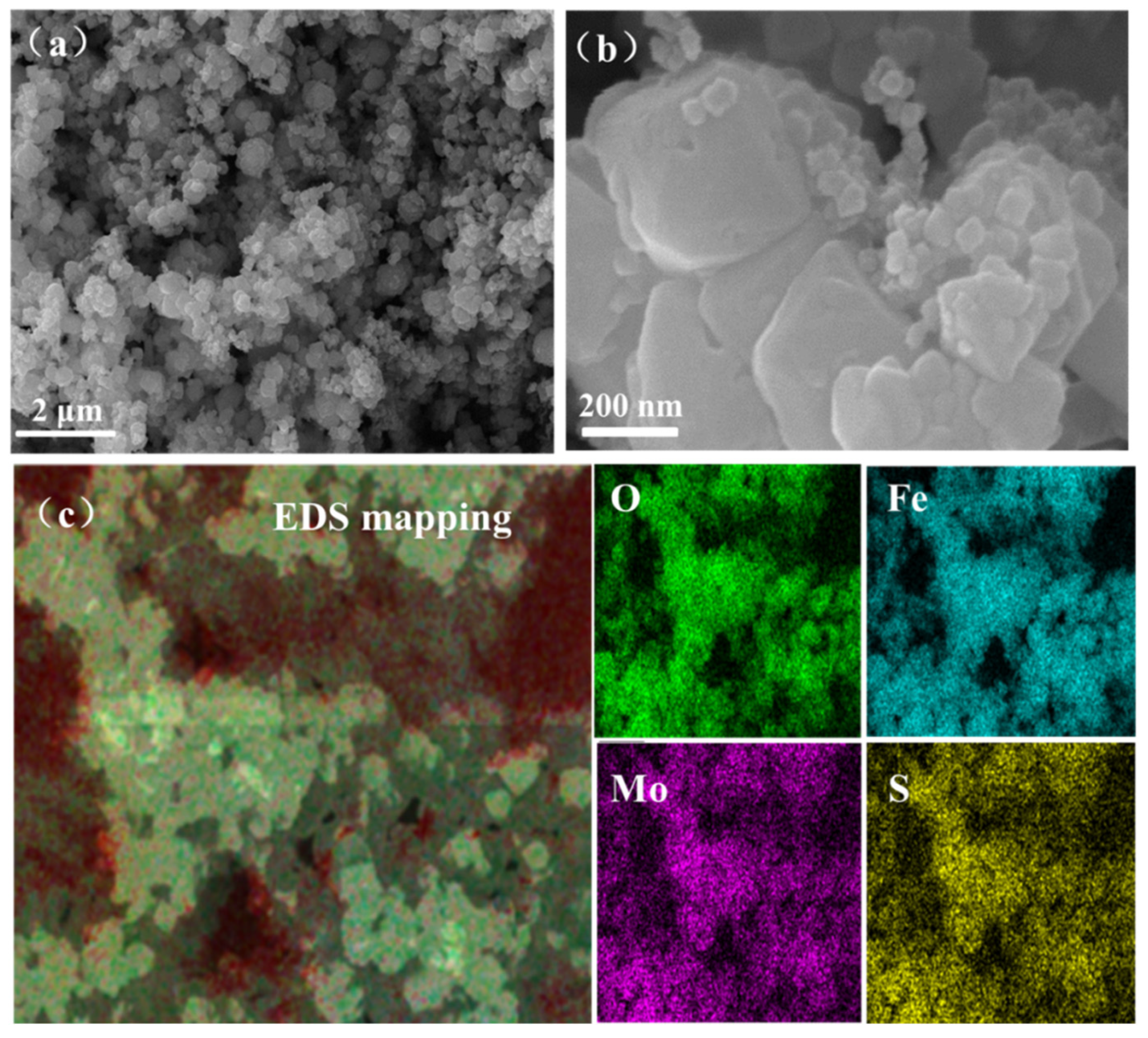
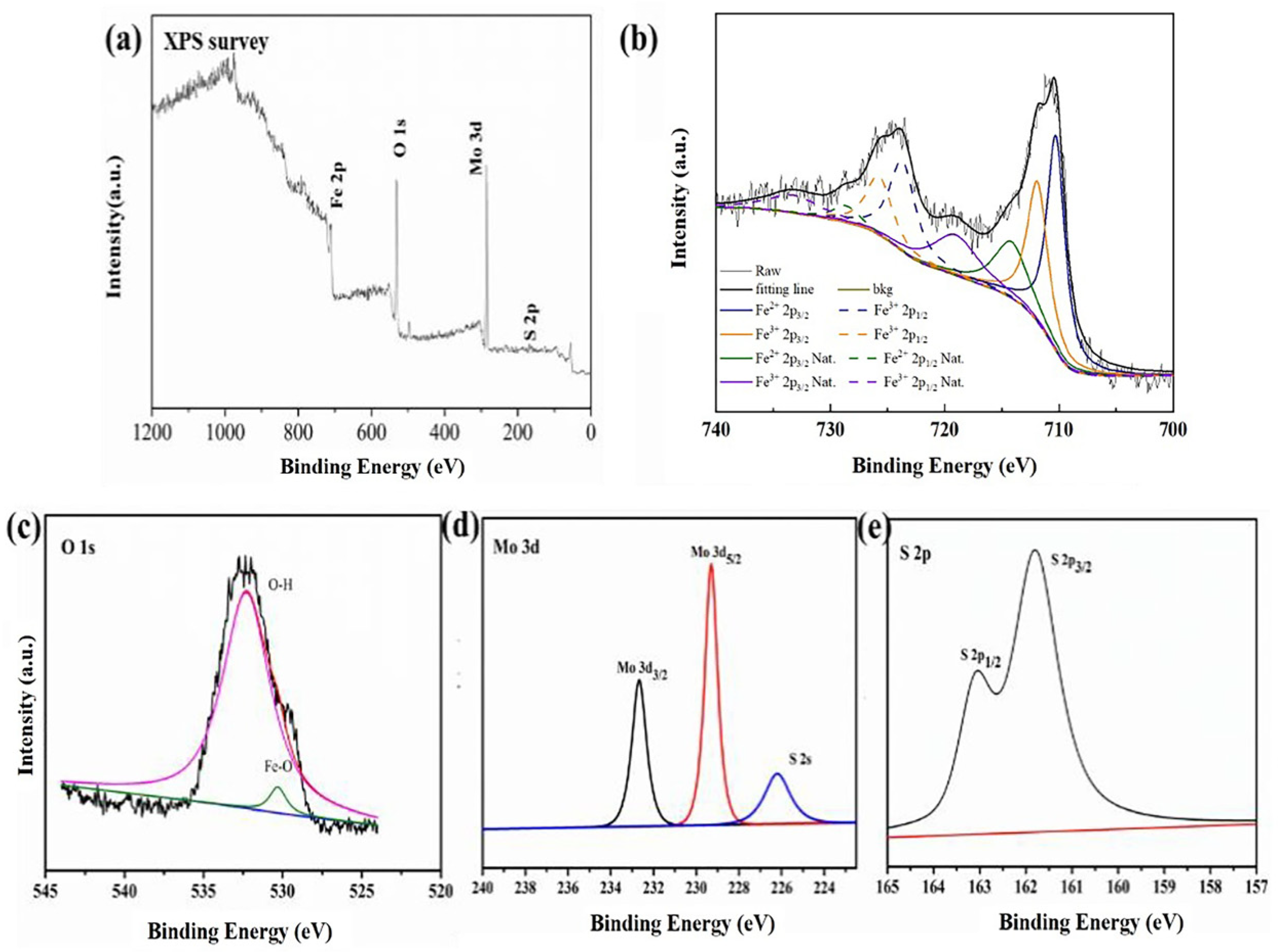
3.1. Characterization
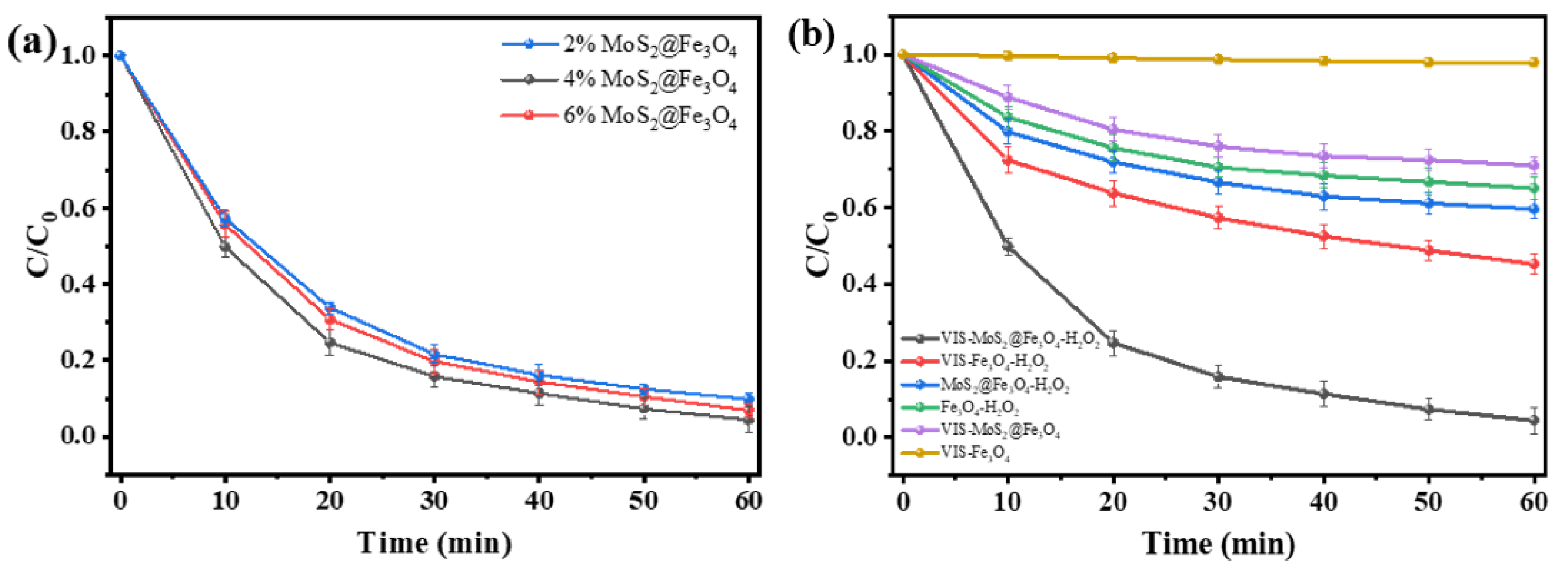
3.2. Evaluation of MoS2@Fe3O4 Photo-Fenton Catalytic Activity
3.2.1. Photo-Fenton Degradation Performance
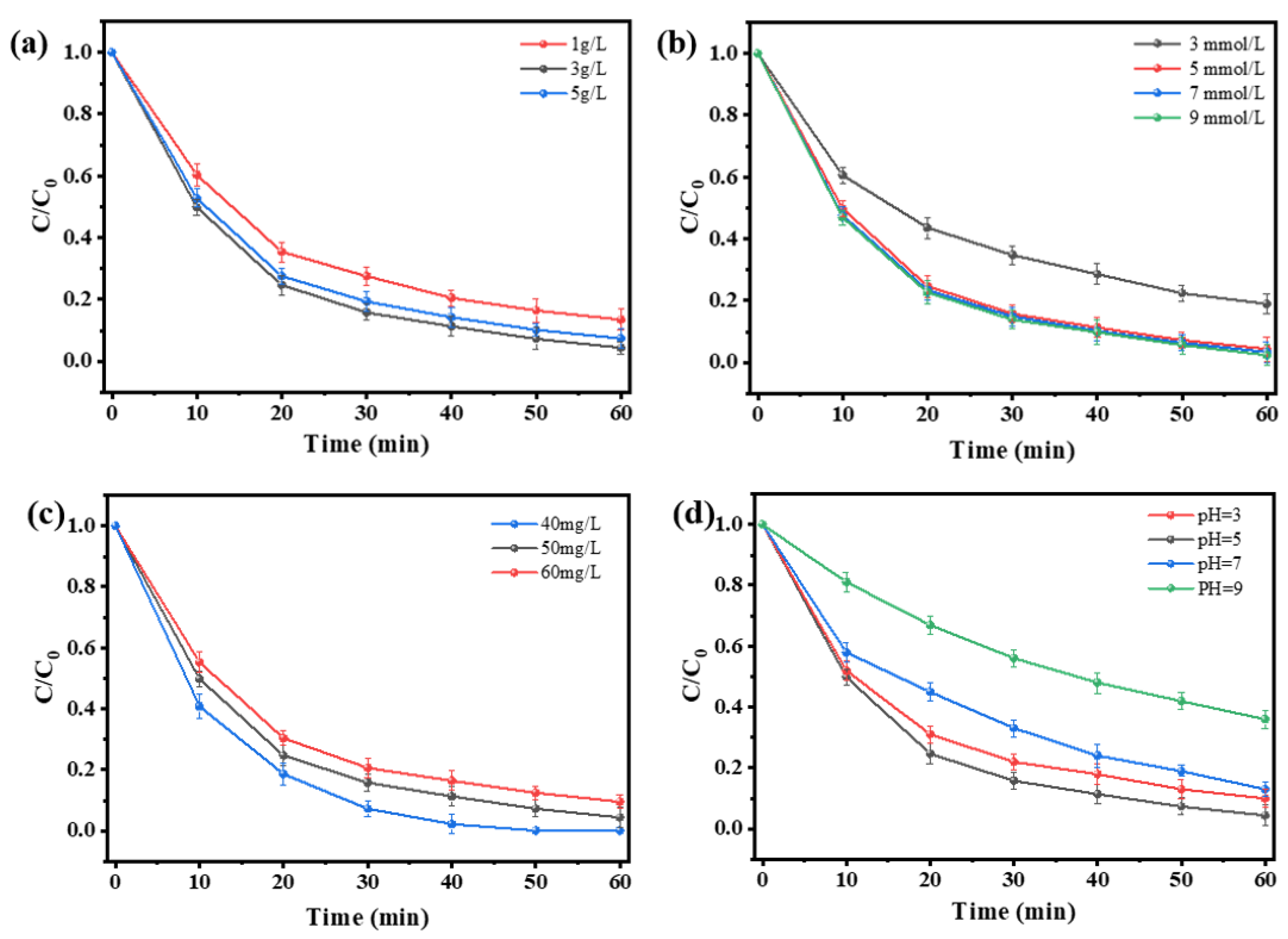
3.2.2. Different Influencing Factors
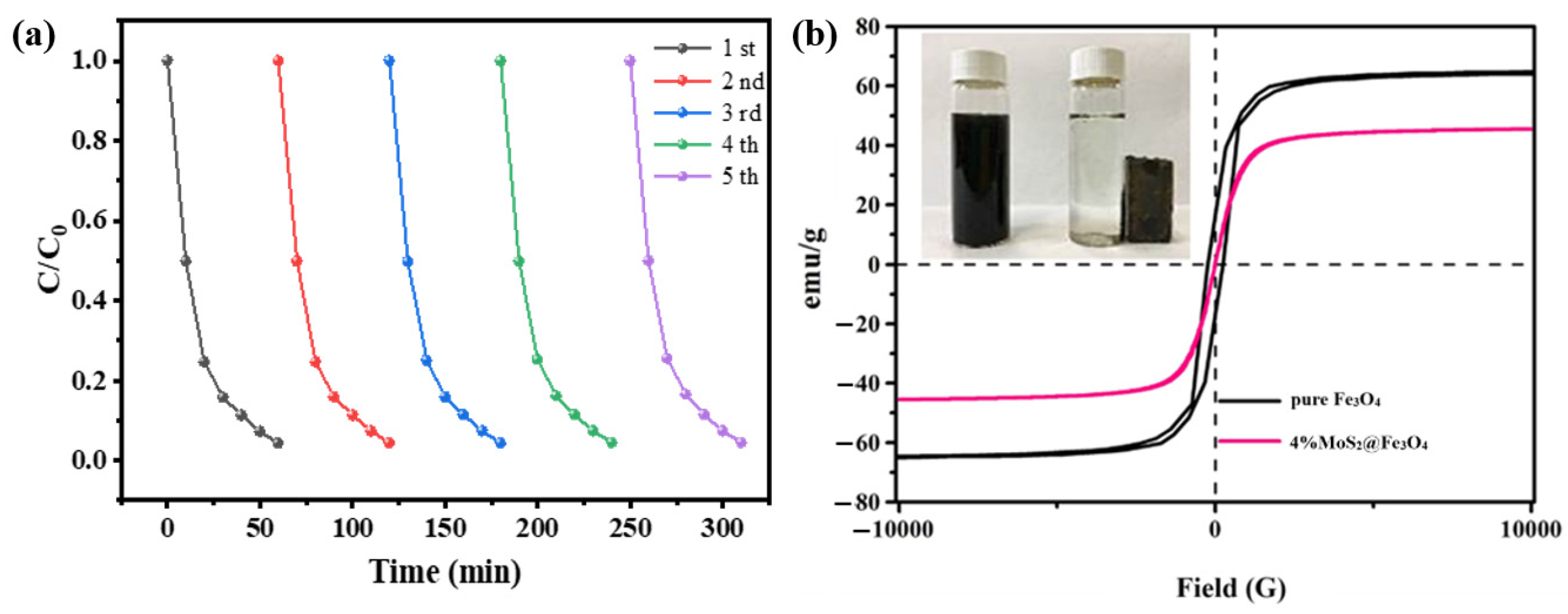
3.2.3. Stability and Recyclability of MoS2@Fe3O4
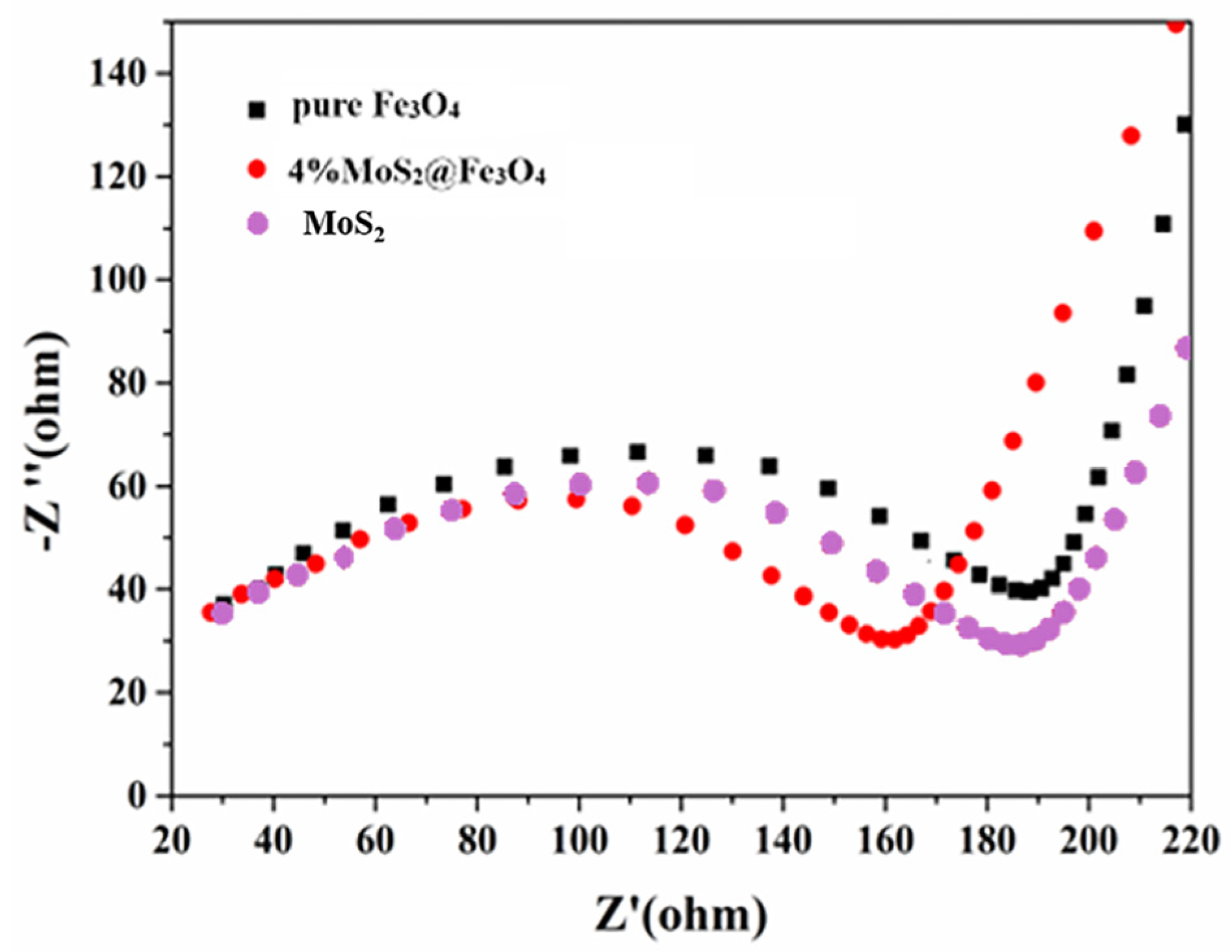
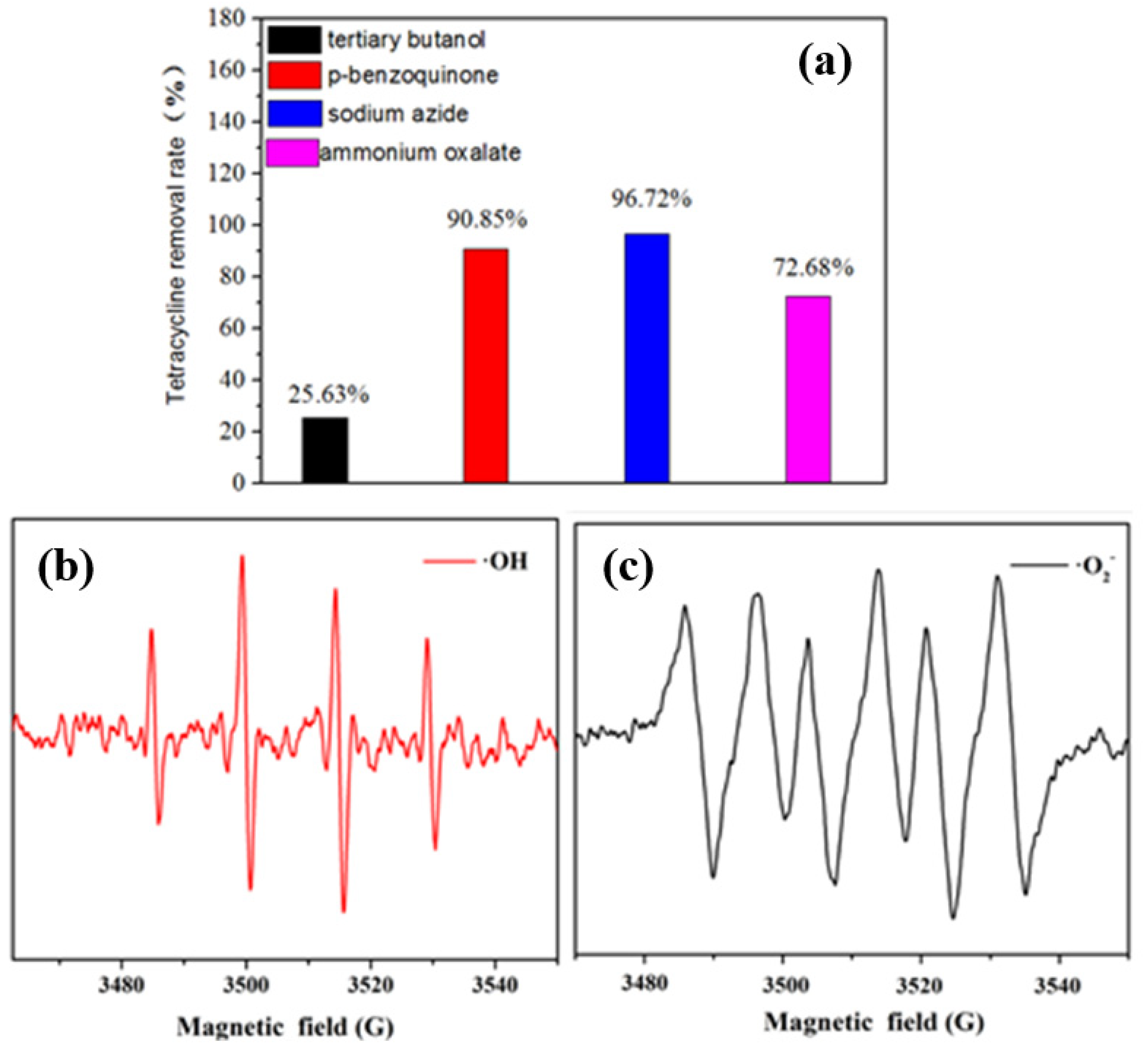
3.3. Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Xu, B.; Wang, S.; Li, X.; Wang, C.; Liu, B.; Han, F.; Xu, Y.; Yu, P.; Sun, Y. Tetracycline degradation by peroxymonosulfate activated with CoNx active sites: Performance and activation mechanism, Chem. Eng. J. 2022, 431, 133477. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, H.; Kang, L.; Gao, Z.; Ren, F. Fe-based metalorganic frameworks as Fenton-like catalysts for highly efficient degradation of tetracycline hydrochloride over a wide pH range: Acceleration of Fe (II)/Fe (III) cycle under visible light irradiation. Appl. Catal. B Environ. 2020, 263, 118282. [Google Scholar] [CrossRef]
- Zeng, Z.; Ye, S.; Wu, H.; Xiao, R.; Zeng, G.; Liang, J.; Zhang, C.; Yu, J.; Fang, Y.; Song, B. Research on the Sustainable Efficacy of G-MoS2 Decorated Biochar Nanocomposites for Removing Tetracycline Hydrochloride from Antibiotic-Polluted Aqueous Solution. Sci. Total Environ. 2019, 648, 206–217. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Li, Y.; Wang, C.; Kuang, S.; Ren, P.; Xie, B. Persulfate oxidation of tetracycline, antibiotic resistant bacteria, and resistance genes activated by Fe doped biochar catalysts: Synergy of radical and non-radical processes. Chem. Eng. J. 2023, 464, 142558. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, Q.; Ren, Y.; Wang, Y.; Zhu, D.; Wang, J. Radiometric fluorescent sensor based on europium (III)-functionalized covalent organic framework for selective and sensitive detection of tetracycline. Chem. Eng. J. 2023, 465, 142819. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Duan, L.; Zhu, D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, M.; Ashrafi, S.D.; Taghavi, K.; Joubani, M.N.; Jaafari, J. Evaluation of tetracycline removal by adsorption method using magnetic iron oxide nanoparticles (Fe3O4) and clinoptilolite from aqueous solutions. J. Mol. Liq. 2022, 356, 119040. [Google Scholar] [CrossRef]
- Shan, H.; Si, Y.; Yu, J.; Ding, B. Facile access to highly flexible and mesoporous structured silica fibrous membranes for tetracyclines removal. Chem. Eng. J. 2021, 417, 129211. [Google Scholar] [CrossRef]
- Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Effects of carbon source, nitrogen source, and natural algal powder-derived carbon source on biodegradation of tetracycline (TEC). Bioresour. Technol. 2019, 288, 121567. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, H.; Keung Wong, P.; Wu, Y.; Rittmann, B. Biodegradation of tetracycline using hybrid material (UCPs-TiO2) coupled with biofilms under visible light. Bioresour. Technol. 2021, 323, 124638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiang, Z.; Zhu, W.; Yao, W.; Tang, S.; Yang, Z.; Wang, J.; Duan, J.; Ma, C.; Tan, R. BiPO4 nanorod/graphene composite heterojunctions for photocatalytic degradation of tetracycline hydrochloride. ACS Appl. Nano Mater. 2021, 4, 8680–8689. [Google Scholar] [CrossRef]
- Zhi, D.; Wang, J.; Zhou, Y.; Luo, Z.; Sun, Y.; Wan, Z.; Luo, L.; Tsang, D.C.W.; Dionysiou, D.D. Development of ozonation and reactive electrochemical membrane coupled process: Enhanced tetracycline mineralization and toxicity reduction. Chem. Eng. J. 2020, 383, 123149. [Google Scholar] [CrossRef]
- Li, X.; Cui, K.; Guo, Z.; Yang, T.; Cao, Y.; Xiang, Y.; Chen, H.; Xi, M. Heterogeneous Fenton-like degradation of tetracyclines using porous magnetic chitosan microspheres as an efficient catalyst compared with two preparation methods. Chem. Eng. J. 2020, 379, 122324. [Google Scholar] [CrossRef]
- Nie, M.; Li, Y.; He, J.; Xie, C.; Wu, Z.; Sun, B.; Zhang, K.; Kong, L.; Liu, J. Degradation of tetracycline in water using Fe3O4 nanospheres as Fenton-like catalysts: Kinetics, mechanisms and pathways. New J. Chem. 2020, 44, 2847–2857. [Google Scholar] [CrossRef]
- Qin, G.; Song, X.; Chen, Q.; He, W.; Yang, J.; Li, Y.; Zhang, Y.; Wang, J.; Dionysiou, D.D. Novel durable and recyclable Cu@MoS2/polyacrylamide/copper alginate hydrogel photo-Fenton-like catalyst with enhanced and self-regenerable adsorption and degradation of high concentration tetracycline. Appl. Catal. B Environ. Energy 2024, 344, 123640. [Google Scholar] [CrossRef]
- Lai, C.; Shi, X.; Li, L.; Cheng, M.; Liu, X.; Liu, S.; Li, B.; Yi, H.; Qin, L.; Zhang, M.; et al. Enhancing iron redox cycling for promoting heterogeneous Fenton performance: A review. Sci. Total Environ. 2021, 775, 145850. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.H.; Chen, Y.T.; Liu, Z.Q.; Huang, C.; Li, Y.Q.; Kong, D.Z.; Shen, W.; Tang, S. Modification and application of Fe3O4 nanozymes in analytical chemistry: A review. Chin. Chem. Lett. 2023, 34, 107820. [Google Scholar] [CrossRef]
- Dhiman, P.; Sharma, J.; Kumar, A.; Sharma, G.; Dawi, E.A. Photocatalytic degradation of malachite green by waste derived bio-char impregnated Bi5O7Br/Fe3O4 magnetic composite. Opt. Materials. 2023, 146, 114568. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, L.; Liu, L.; Li, J.; Wang, S.; Znad, H.; Liu, S. Magnetic ZnO @ Fe3O4 Composite for self-generated H2O2 toward photo-Fenton-l`ike oxidation of nitrophenol. Compos. Part B 2020, 200, 108345. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, B. Environmental applications of 2D molybdenum disulfide (MoS2) nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef]
- Li, T.; Yin, W.; Zhang, P.; Zhao, X.; Wei, R.; Zhou, W.; Tu, X. Dual heterojunctions and sulfur vacancies of AgInS2/rGO/MoS2 co-induced photocatalytic degradation of tetracycline hydrochloride. Colloids Surf. A Physicochem. Eng. Asp. 2023, 667, 131396. [Google Scholar] [CrossRef]
- Peng, J.; Weng, J. Enhanced peroxidase-like activity of MoS2/graphene oxide hybrid with light irradiation for glucose detection. Biosens. Bioelectron. 2017, 89, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.N.; Zhang, Q.; Shah, S.N.A.; Khan, M.; Uchiyama, K.; Lin, J.M. MoS2-quantum dot triggered reactive oxygen species generation and depletion: Responsible for enhanced chemiluminescence. Chem. Sci. 2019, 10, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Y.; Li, Y.Y.; Zang, C.J.; Ma, X.J.; Zhao, B.; Chen, F. Piezoelectric field-promoted heterogeneous sono-Fenton performance of MoS2/α-Fe2O3 heterojunction structure. Appl. Surf. Sci. 2020, 534, 147499. [Google Scholar] [CrossRef]
- Luo, H.W.; Cheng, Y.; Zeng, Y.F.; Luo, K.; Pan, X.L. Enhanced decomposition of H2O2 by molybdenum disulfide in a Fenton-like process for abatement of organic micropollutants. Sci. Total Environ. 2020, 732, 139335. [Google Scholar] [CrossRef]
- Chang, Q.; Zhu, L.; Yu, C.; Tang, H.; He, Q.T. Synthesis and properties of magnetic and luminescent Fe3O4/SiO2/Dye/SiO2 nanoparticles. J. Luminescent. 2008, 128, 1890–1895. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, Y.; Feng, J.; Wang, J. Heterogeneous electro-Fenton oxidation of azo dye methyl orange catalyzed by magnetic Fe3O4 nanoparticles. Water Sci. Technol. 2016, 74, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, V.; Kondal, N.; Prashant; Dhiman, P.; Kumari, A.; Sharma, R. Bryophyllum Pinnatum leaf extract mediated MoS2/ZnO nanocomposite for robust photocatalysis applications. J. Mol. Struct. 2024, 1318, 139196. [Google Scholar] [CrossRef]
- Li, G.; Gu, B.; Luo, Y.; Fan, G.; Yu, X. Architecture engineering of Fe/Fe2O3@MoS2 enables highly efficient tetracycline remediation via peroxymonosulfate activation: Critical roles of adsorption capacity and redox cycle regulation. J. Environ. Manag. 2024, 353, 120210. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, H.; Liu, Z.; Liu, L.; Zeng, Z.; Yang, X.; Wang, H.; Du, J.; Zheng, B.; Guo, Y. Composites of Fe3O4, Ag, and Au Nanoparticles for Dye Degradation and Selective Detection of Glutathione. ACS Appl. Nano Mater. 2023, 6, 11531–11540. [Google Scholar] [CrossRef]
- Han, T.; Wei, Y.; Jin, X.; Jiu, H.; Zhang, L.; Sun, Y.; Tian, J.; Shang, R.; Hang, D.; Zhao, R. Hydrothermal self-assembly of α-Fe2O3 nanorings@ graphene aerogel composites for enhanced Li storage performance. J. Mater. Sci. 2019, 54, 7119–7130. [Google Scholar] [CrossRef]
- Li, X.T.; Lv, X.D.; Li, N.; Wu, J.J.; Zheng, Y.Z.; Tao, X. One-step hydrothermal synthesis of high-percentage 1T-phase MoS2 quantum dots for remarkably enhanced visible light-driven photocatalytic H2 evolution. Appl. Catal. B Environ. 2019, 243, 76–85. [Google Scholar] [CrossRef]
- Zhou, Q.; Yao, Z.; Wang, H.; Yang, J.; Zhang, Y.; Qian, L.; Zhang, S. Interface engineering of NixSy@MoS2 heterostructure nanorods as high-efficient electrocatalysts for water splitting. Int. J. Hydrogen Energy 2021, 46, 35077–35087. [Google Scholar] [CrossRef]
- Narewadikar, N.A.; Pedanekar, R.S.; Parale, V.G.; Park, H.H.; Rajpure, K.Y. Spray deposited yttrium incorporated TiO2 photoelectrode for efficient photo electrocatalytic degradation of organic pollutants. J. Rare Earths 2023, 41, 1929–1937. [Google Scholar] [CrossRef]
- Scaria, J.; Nidheesh, P.V. Magnetite−reduced graphene oxide nanocomposite as an efficient heterogeneous Fenton catalyst for the degradation of tetracycline antibiotics. Environ. Sci. Water Res. Technol. 2022, 8, 1261–1276. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Z.; Chen, Y.; Jia, Y.; Wang, Q.; Cheng, H. Heterostructure coal-bearing strata kaolinite/MnFe2O4 composite for activation of peroxydisulfate to efficiently degrade chlortetracycline hydrochloride. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128789. [Google Scholar] [CrossRef]
- Chang, P.-H.; Li, Z.; Jiang, W.-T.; Jean, J.-S. Adsorption and intercalation of tetracycline by swelling clay minerals. Appl. Clay Sci. 2009, 46, 27–36. [Google Scholar] [CrossRef]
- Yang, X.; He, J.; Yang, Q.; Jiao, R.; Liao, G.; Wang, D. Cu(I)-doped Fe3O4 nanoparticles/porous composite for enhanced H2O2 oxidation of carbamazepine. J. Colloid Interface Sci. 2019, 551, 16–25. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-L.; Sun, J.-H.; Liu, B.; Chen, Y.-N.; Feng, W. Fabrication of MoS2@Fe3O4 Magnetic Catalysts with Photo-Fenton Reaction for Enhancing Tetracycline Degradation. Water 2025, 17, 235. https://doi.org/10.3390/w17020235
Liu Z-L, Sun J-H, Liu B, Chen Y-N, Feng W. Fabrication of MoS2@Fe3O4 Magnetic Catalysts with Photo-Fenton Reaction for Enhancing Tetracycline Degradation. Water. 2025; 17(2):235. https://doi.org/10.3390/w17020235
Chicago/Turabian StyleLiu, Zong-Lai, Jia-Hong Sun, Bing Liu, Ya-Nan Chen, and Wei Feng. 2025. "Fabrication of MoS2@Fe3O4 Magnetic Catalysts with Photo-Fenton Reaction for Enhancing Tetracycline Degradation" Water 17, no. 2: 235. https://doi.org/10.3390/w17020235
APA StyleLiu, Z.-L., Sun, J.-H., Liu, B., Chen, Y.-N., & Feng, W. (2025). Fabrication of MoS2@Fe3O4 Magnetic Catalysts with Photo-Fenton Reaction for Enhancing Tetracycline Degradation. Water, 17(2), 235. https://doi.org/10.3390/w17020235





