Abstract
The prevalence and fate of microplastics in macrophytes are insufficiently understood, and data on the abundance of microplastic (MP) accumulation in macrophyte ecosystems are urgently needed to fill this knowledge gap. The main objectives of this study were to quantify and characterize the microplastics that accumulate in macrophytes, associated sediments, and water in Negombo Lagoon, Sri Lanka. The investigation was conducted with specialized sampling and extraction techniques. Microplastics were detected by the fluorescence tagging of polymers using Nile red, and polymer identification was conducted by ATR-µFTIR and ATR-FTIR. This study revealed variations in microplastic abundance across different macrophyte species. Gracilaria sp. had a higher abundance of 9 ± 3 items g−1 wet weight, followed by Chaetomorpha sp. at 8 ± 3 items g−1 wet weight and Halodule pinifolia at 6 ± 1 items g−1 wet weight. The root surfaces of these species had slightly similar levels of abundance. Both blue and transparent microplastics were predominant. Fragments (>47%) were found at a higher percentage in macrophyte samples, but high fiber contents (>60%) were found in associated sediment and water samples. Nevertheless, macrophyte samples contained a higher percentage of fragments in the size range of 50–150 μm. This comprehensive study contributes to expanding our awareness regarding the influence of microplastic pollution on macrophyte ecosystems.
1. Introduction
Humans have created 9200 million metric tons of plastic over the past five decades, and they now end up in landfills, polluting the environment [1,2]. The output of plastic is assumed to double over the next 20 years. In 2023, the world produced 400.3 million metric tons of plastics, and most of them are fossil-based products (90%) [3]. This underscores the urgent need for worldwide initiatives that address the usage of plastic, improve recycling, and support sustainable alternatives [4]. Plastic pollution in coastal ecosystems is caused by land- and ocean-based sources via different pathways. Furthermore, a large quantity of plastic waste is released into coastal ecosystems by wastewater discharges [5] and aquatic waste disposal vessels at sea [6]. Once in the environment, plastic materials can slowly break down and generate a large amount of microplastics. Microplastics (MP) are small plastic particles < 5 mm in size that are derived from both primary and secondary sources [7,8]. Polymers like polypropylene (PP), polyethylene (PE), polypropylene ether (PPE), polyethylene terephthalate (PET), and polyester (PS) are some of the major types that are commonly found in environmental samples [9,10]. These tiny plastic particles have a long residence time, high stability, low potential for fragmentation, and the ability to absorb and function as a vector of other pollutants like heavy metals and pesticides [11,12,13]. Previous studies reported that microplastics function as vectors of hydrophobic organic contaminants (HOCs) and bioaccumulate them in earthworms [14]. Also, nano-microplastics complex with cadmium and bioaccumulate in fish, damaging their gills and liver tissue [15].
Microplastic pollution is a silent threat to coastal lagoons because pollutant loads and concentrations in water, sediments, flora, and fauna can be high, resulting in impacts on biodiversity and ecosystem services, disrupting ecological balance and causing habitat destruction [16,17,18]. Numerous studies have revealed microplastic contamination in fish, crab, shrimp, coastal sediments, and the waters of Sri Lanka [19,20]. Also, some studies provide evidence about microplastic pollution in freshwater ecosystems [21,22,23]. This evidence proves the urgent need to understand the threat of microplastics. Coastal lagoons are sensitive ecosystems around the world that are affected by a number of anthropogenic activities [24] and have a higher potential to accumulate microplastics within this exquisite ecosystem [21]. Coastal lagoons are dynamic ecosystems which are distributed in tropical areas, rich in biodiversity, and consist of large fishery grounds that sustain human community [22]. Negombo Lagoon is one of the most productive estuaries in Sri Lanka, providing a habitat for a diverse range of brackish water fish, crab, and shrimp species, supported by macrophytes and mangrove forests [23,24,25].
The macrophyte ecosystem consists of seagrass and seaweed, performing critical roles in coastal lagoons by providing a habitat and fishery grounds and contributing to marine biodiversity [26]. Both seaweed and seagrass grow together to form patches or larger vegetative beds that support the growth of fish and shrimp species [27,28]. However, recent studies have revealed the concern of microplastic accumulation on these macrophytes, emphasizing the importance of investigating their potential threat to the marine ecosystem [29]. Previous studies have revealed that macrophytes like macroalgae tend to have higher concentrations of microplastics attached to their body surfaces [27,28,30,31,32].
Research communities have already expanded their research areas to cover microplastic contamination in coastal areas and freshwater ecosystems [33,34,35]. Also, they have revealed new research findings about microplastic accumulation in sediments, water, and biota [36,37,38,39,40]. Fish species and marine organisms were highly considered by scientists to address the accumulation of microplastics in their guts and gills [41,42,43,44]. However, a few studies have been conducted on coastal lagoons, and microplastic accumulation in seagrass, seaweed, and coastal aquatic plants has been insufficiently addressed. Sri Lankan coastal areas are highly polluted by plastic litter, and most delicate ecosystems have been devastated by this threat [45,46,47]. Negombo Lagoon is one of the most eye-catching lagoons in Sri Lanka, but unfortunately, it is a lagoon that is highly polluted with plastic and litter and suffers from environmental stress [34,48,49]. Anthropogenic activities and the development of hotels and restaurants on both sides of Negombo Lagoon are prominent reasons for this disaster [50]. Nevertheless, Negombo Lagoon is rich in macrophyte species, yet there has been no study conducted regarding microplastic accumulation in seagrass. But a significant study was conducted regarding the contamination of microplastics in the cultured seaweed of Kappaphycus alvarezii in the north and northwest coasts of Sri Lanka, and that study revealed outstanding evidence about this silent threat [35]. Due to that evidence, in this comprehensive study, we investigated the accumulation of microplastics in macrophytes (macroalgae and seagrass), macrophyte-associated sediments, and water in Negombo Lagoon, Sri Lanka, by investigating the presence, types, and concentrations of microplastics within these important aquatic ecosystems. The current study aims to address the contamination levels of the seagrass species Halodule pinifolia, the macroalgae species Chaetomorpha sp., and Gracilaria sp., which is known as Ceylon moss. Furthermore, this study was conducted to quantify the number of microplastics adhered to the external surfaces of macrophytes, identify differences in contamination between sites, determine whether the roots or leaves accumulate more microplastics on their surfaces, and determine how microplastics in associated water and sediments correspond to what is accumulating on macrophytes in the lagoon to assess the extent of microplastic pollution in these environmental compartments.
2. Materials and Methods
2.1. Study Area
Sri Lanka is an island in the Indian Ocean and has lagoons along 1338 km of its coastline. Forty-five coastal lagoons are currently found in Sri Lanka [23]. According to its distribution, Negombo Lagoon is the largest coastal lagoon in the western coastal zone, and it is characterized by vast diversity and beneficial ecosystems like mangrove and seagrass. According to existing surveys, Negombo Lagoon is the most polluted lagoon in Sri Lanka, and this environmental issue needs to be addressed by further research and mitigation strategies [50,51,52].
2.2. Sampling Location
Investigations were conducted at 9 different sites inside Negombo Lagoon between December 2022 and June 2023 (Figure 1, Table 1). These locations were chosen with attention to the abundance of macrophyte habitat on the lagoon’s east edge. According to the growth conditions and freshwater inlets, these specific macrophyte species have populated on the east edge of Negombo Lagoon for a specific time of the year (from December to May). Chaetomorpha sp. was collected from site 1, site 2, and site 3. Halodule pinifolia was collected from site 4, site 5, and site 6. Gracilaria sp. was collected from site 7, site 8, and site 9. Notably, each site was carefully selected, ensuring the lagoon’s biological processes and distribution patterns of macrophytes could be analyzed. The map was created using ArcGIS 10.8.
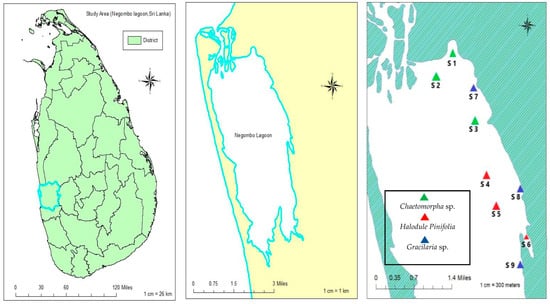
Figure 1.
Overview of sample sites in Negombo Lagoon/S1–S9, Western Province, Sri Lanka (Generated by ArcGIS 10.8; Generated 4 February 2024).

Table 1.
Sampling sites and number of samples collected from each distinct site.
2.3. Sampling Process
2.3.1. Water Sampling
The mean depth of the Negombo Lagoon is 1.2 m, and the lagoon’s east edge is less than 1.0 m [39]. Macrophyte growth can be visibly observed, and the water column depth of the macrophyte bed is less than 50 cm. Three water samples (1 L) were collected by using cleaned glass vessels from each study area. They were stored in glass containers to avoid contamination. The samples were stored at a temperature of 4 °C for further analysis.
2.3.2. Sediment Sampling
Sediment samples were collected from each location, exactly where the macrophyte samples were collected. Surface sediments (0–5 cm depth) were collected with a stainless-steel shovel and small Van Veen grab sampler (1–5 L sampling capacity/15 cm × 30 cm sampling area/type 304 stainless steel). Three samples which contained more than one kilogram (wet weight) were collected from each site. Sediment samples were collected with caution and stored in labeled glass containers.
2.3.3. Macrophyte Sampling
Macrophyte samples were collected from each species (Halodule pinifolia, Chaetomorpha sp., and Gracilaria sp.) inside the macrophyte meadows in Negombo Lagoon using a random sampling strategy. Sites 1, 2, 3, 4, 5, and 6 contain both Halodule pinifolia and Chaetomorpha sp., but Gracilaria sp. was only found at sites 7, 8, and 9. A total of ≈100 g macrophyte samples were collected from each species at each site. Total plant body was collected separately and put into glass containers. Samples were collected using a small steel shovel. Each sample station consisted of a depth of <l.0 m. GPS devices (GPSMAP 78s) (Garmin, KS, USA) were employed during sample collection to record the precise position of each sample. Macrophytes were collected with caution and stored in labeled glass containers. A quadrat (50 cm × 50 cm) was used to collect consistent samples across the study area and used as a sampling frame by identifying all possible sampling units. A few samples were collected separately for the species identification process.
2.4. Contamination and Control During the Sampling Process
Cross-contamination was minimized as much as possible during the sampling process as we recognized the potential impact on the research findings. We utilized glass containers with steel caps to store the macrophyte, sediment, and water samples to ensure minimal contamination.
2.5. Chemicals and Materials
Chemicals used for the research included Milli-Q- water and AR-grade chemicals such as KOH (Loba, Mumbai, India, 85%), NaBr (HiMedia, Mumbai, India, 99.00%), C2H6O (EMSURE, ≥99.9%), and Nile red (Across Organics/Thermo Fisher Scientific, Waltham, MA, USA, 99.00%). Equipment used included Pyrex beakers with various sizes (250 mL, 500 mL, 1000 mL, and 2000 mL), volumetric flasks (500 mL and 1000 mL), watch glasses, a stainless steel spatula, regular glass containers, a glass funnel, a glass dropper, an aluminum tray, aluminum foil, and filter papers (Whatman GF/F filter with 47 mm diameter and 0.7 µm pore size, Whatman cellulose nitrate membrane with 0.2 μm pore size, and regenerated cellulose membrane filter with 47 mm diameter and 0.2 μm pore size).
2.6. A Microplastic Analysis of the Associated Sediment Samples
The sediment samples collected were homogenized and dried at 50 °C for at least five days. Wet sieving was carried out after drying the sediment samples. The sample beaker was cleaned with distilled water to remove any remaining materials before passing 200 g of the sediment sample through a 0.3 mm sieve. Using forceps, visible items that were greater than 5 mm were removed [52]. A 500 mL beaker was labeled after being cleaned and dried. The remaining materials present on the 0.3 mm sieve were transferred to the dried 500 mL beakers. The solids and beakers were dried once more. The dried sediments in the beaker were then mixed with 300 mL of saturated sodium bromide (NaBr; density = 1.55 g mL−1) solution, and the mixture was vigorously stirred for five minutes with a spatula to float out the microplastics and stand for 24 h. The saturated sodium bromide solution was filtered using a regenerated cellulose filter paper (47 mm diameter, 0.2 μm pore size) [40]. The microplastics were separated based on density by leaving the beaker to settle for 24 h. The supernatant of each sample was transferred to a cleaned filtration unit and filtered with a Whatman cellulose nitrate membrane (0.2 μm) [40]. The filtered papers were individually transferred to glass beakers covered with aluminum foil and subjected to alkaline digestion using 30% KOH at 40 °C for 24 h [53]. After digestion, the solution was filtered through a Whatman GF/F filter (47 mm diameter, 0.7 µm pore size) and dyed with Nile red for 2 min [54]. Suspected microplastics were transferred to an anodisc (Whatman, VWR, Leicestershire, UK, with a 25 mm diameter and 0.2 µm pore size) and placed in a drying cabinet for a minimum of 48 h to dry ahead of FTIR polymer identification. Particles that were analyzed by FTIR were also imaged and measured.
2.7. Microplastic Analysis of Water Samples
Water samples taken from macrophyte beds were noticeably clear and free from visible particulates. Direct filtration via a Whatman GF/F filter (0.7 µm pore size) was conducted. Fluorescence tagging of polymers was carried out using Nile red dye. The collected water samples were homogenized by mixing them using a glass rod prior to the analysis. Three water samples (500 mL from one sample) from each site were taken and immediately filtered. The remaining water samples were used to analyze other pollutants like heavy metals, oil, and grease.
2.8. Microplastic Analysis of Macrophyte Samples
A total of 10 macrophyte samples were analyzed from each site, resulting in a combined sample size of 30 (n = 30) for each species present in those sites. Prior to analysis, samples were carefully inspected to collect micro- and macro-plastics that were entrapped on their body surfaces for future analysis. Initially, the collected macrophyte samples were dissected to separate the leaves and roots. The samples were carefully weighed. For each species, 3 g (wet weight) of leaves and 1.5 g (wet weight) of roots were measured. After measuring, each was transferred to separate glass containers. An amount of 100 mL of freshly prepared saturated NaBr solution was added to each container. The samples were subjected to sonication for 10 min, and then the samples were left to undergo 24 h of density separation. The supernatant was filtered through a Whatman filter (GF/F, 0.7 µm), and fluorescence tagging was carried out with Nile red. The remaining residue was further digested using 30% KOH at 40°C for 24 h. After digestion, the solution was filtered again and subjected to fluorescence tagging.
2.9. Quantification of Contamination and Quality Control
Before the analysis, all glassware was cleaned using a laboratory detergent and thoroughly rinsed using distilled water. The glassware used in the analysis was further cleaned by sonication for 20 min using distilled water before analysis. Finally, all the glassware was cleaned again using Milli-Q water. Furthermore, all chemical solutions used in this study were prepared using Milli-Q water and pre-filtered through a regenerated cellulose membrane filter paper with a 47 mm diameter and 0.2 μm pore size [41]. To improve the efficiency and reliability of this study, procedural controls were performed throughout the preparation of sediment, water, and macrophyte samples. They were prepared with every batch of samples and were consistently subjected to each step of the analysis.
2.10. Quality Control in Microplastic Detection and Characterization
The ATR-FTIR (Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy) [Bruker ALPHA] was used for the chemical characterization of microplastics in the range of 400–4000 cm−1 with 112 scans, and the spectra obtained were compared with the Bruker Optics ATR-Polymer Library.
MP identification using ATR-µFTIR (Attenuated Total Reflectance–Micro Fourier Transform Infrared Spectroscopy) (a Lumos II, Bruker, UK) was performed under a liquid-nitrogen-cooled MCT detector (Mercury Cadmium Telluride detector). Thirty-two scans were collected in reflectance mode in the range of 4000–500 cm−1 at a resolution of 4 cm−1. Polymer identification was verified by the percentage match score (above 60%) against polymer libraries (complete ATR-FTIR-library, vol. 1–4; Bruker Optics ATR-Polymer Library; IR-Spectra of Polymers, Diamond-ATR; Geranium-AT and IR-Spectra of Additives, Diamond-ATR).
Filter papers were visually examined under a Leica MZ10F with a GXCAM-U3PRO-20 camera (Wickham brook, Suffolk, UK) attached to a microscope. Suspected plastics were transferred to a Whatman Anodisc (VWR, Leicestershire, UK) with a 25 mm diameter and 0.2 µm pore size for analysis with FTIR. The anodisc was placed in a drying cabinet (100L S/S, LTE, Manchester, UK) at 40 °C for a minimum of 24 h [40,41].
Microplastics were observed and detected under a stereomicroscope (Euromax stereoblue SB.1902-P, Euromex, Duiven, The Netherlands) with magnifications of 10× and 20× directly on the net surface. The fluorescence staining method using Nile red dye was employed to increase the visualization of microplastics.
2.11. Statistical Analysis
To quantify the microplastic abundance, raw microplastic counts were blank-corrected. Data were evaluated for differences across types of macrophyte species (i.e., Chaetomorpha sp., Halodule pinifolia, and Gracilaria sp.) and differences between corresponding sites (i.e., site 1, site 2, etc.). The Kruskal–Wallis test (IBM SPSS 20) with α = 0.05 was used to assess a significant difference in microplastic abundance in the associated sediments and water of each macrophyte growing site. A paired t-test (α = 0.05) was conducted to analyze the significant difference in microplastic abundance between root surfaces and leaf surfaces of each species to obtain a clear understanding of the microplastics that are attached to the plant structure. A graphical analysis of FTIR spectra was carried out and statistically analyzed using Origin Software (Origin 9.3 (2023)). Statistical analysis was indicated for comparative information about the study.
3. Results
3.1. Procedure Controls and Blank Correction
Blank samples were designed by subjecting them to the entire experimental procedure to ensure that the results are not influenced by external factors or contaminants. Three blanks were conducted with each batch of samples (sediment, water, and macrophyte). Each contaminated microplastic content in the blank samples corresponding to each batch was subtracted from the total microplastic content of a particular sample. As an example, the blank controls of site 1 sediment samples contained 5 ± 1 particles per kg.
3.2. Microplastics in Water
The concentration of microplastics in the water in the macrophyte meadows ranged from 24 ± 7 items L−1 to 42 ± 14 items L−1. The mean concentration of each location is shown in Figure 2. The abundance of floating microplastics was not significantly different between each site according to the Kruskal–Wallis test (p = 0.76). The size of most measured particles laid in the range between 1000 µm and 2000 µm. The microplastics in the water samples contained a diverse range of colors, including shades of black, blue, green, purple, red, transparent, and yellow. Blue (48.44%) and transparent (38.44%) particles were the most dominant. The analyzed water samples contained a diversity of shapes, including fragments, fibers, microbeads, filaments, and foam (Figure 3). According to the graphical analysis, fibers (>60%) emerged as the dominant microplastic shape category within each of the examined sites.
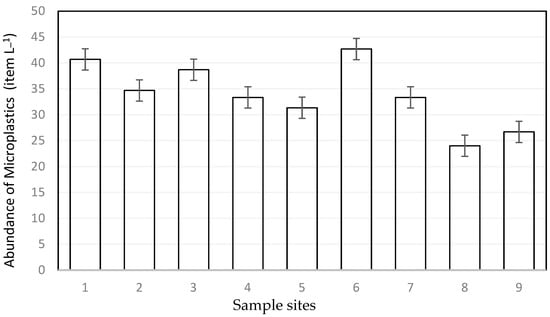
Figure 2.
Number of items in water samples of each site (n = 27) in terms of items/L ± SE.
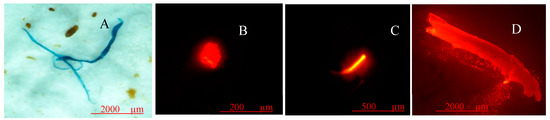
Figure 3.
Examples of microplastics found in water samples. (A) Blue fiber, (B) transparent fragment, (C) red fiber, (D) transparent fragment.
3.3. Microplastics in Associated Sediment
The concentration of microplastics in the associated sediment of macrophyte meadows ranged from 228 ± 51 to 412 ± 80 items per kg (Figure 4). The abundance of microplastics present in sediment samples was not significantly different at each site (p = 0.54). The size range of most measured particles (40%) in the sediments is from 1000 to 2000 µm. Throughout the investigation, a wide range of colors were observed within the microplastics found in the sediments, such as black, blue, green, orange, purple, red, transparent, and yellow. Transparent (40.78%) and blue (44.33%) particles showed a higher abundance compared to the other colors. The analyzed sediment samples contained various shapes, including fragments, fibers, microbeads, filaments, and foam as the predominant forms (Figure 5). Overall, the data reveal a consistent pattern of fiber and fragment microplastics being the dominant shapes across these sites. The fiber percentages vary from 38.91% to 49.22%, and the fragment percentages range from about 40.89% to 50.90%.
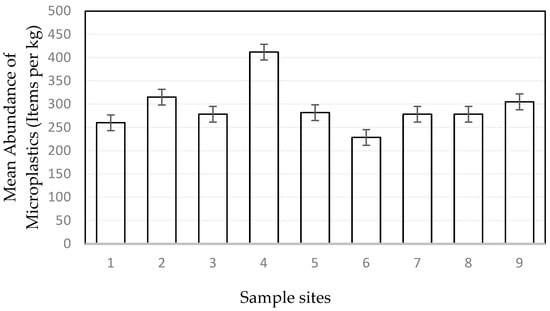
Figure 4.
Number of items in sediment samples of each site (n = 27) in terms of item per kg ± SE.
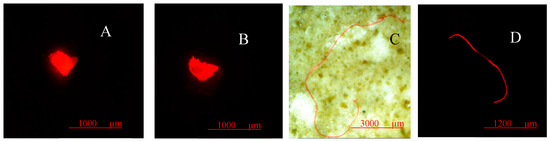
Figure 5.
Examples of microplastics found in sediment samples. (A) Transparent fragment, (B) transparent fragment, (C) red fiber, (D) orange fiber.
3.4. Microplastic in Macrophytes
The Kruskal–Wallis test results (Figure 6) reveal a significant difference in microplastic abundance (items g−1) on the leaf surfaces (p < 0.05) of these species. This implies that microplastic attachment is influenced by the leaf structure of each species. Based on these findings of the study, the abundance of microplastics on leaf surfaces followed a distinct pattern: Gracilaria sp. > Chaetomorpha sp. > Halodule pinifolia. However, there were no statistically significant differences in the microplastic abundance between their root surfaces (p = 0.95).
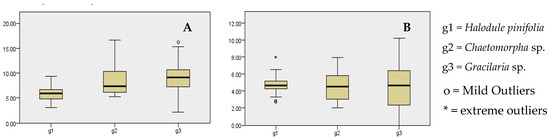
Figure 6.
(A) Independent samples Kruskal–Wallis test for microplastic abundance of leaf surfaces. (B) Independent samples Kruskal–Wallis test for microplastic abundance of root surfaces.
A total of 45 items below 5 mm were analyzed using ATR-µFTIR, corresponding to about 5.7% of the extracted particles from both the roots and leaves. According to the analysis, 29% were confirmed to be synthetic polymers, and 18% were identified as natural polymers (natural plant materials like cellulose and cotton). The main synthetic polymers identified using ATR-µFTIR were rayon (37%), polystyrene (PES) (10%), polyethylene (PE) (5%), polypropylene (PP-blend) (11%), and polymer coatings/epoxy resins (5%) (Figure 7).
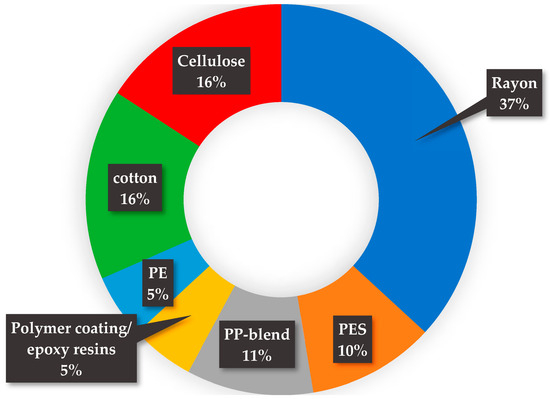
Figure 7.
Summary of ATR-µFTIR data of synthetic polymers found in macrophyte samples.
3.4.1. Microplastics Entangled in Plant Bodies
The quantitative assessment of large microplastics entangled in Macrophytes was conducted by using ATR-FTIR. Various polymer kinds were identified in different percentages within each macrophyte meadow. Diverse types of polymer materials, ranging from epoxy resin to polyethylene (PE), including high-density polyethylene (HD-PE) and low-density polyethylene (LD-PE), polypropylene (PP), polystyrene (PS), olefin-based thermoplastics (TPO), and ethylene butyl acrylate, were found during the investigation (Figure 8). Polypropylene (PP) was found in each site, representing 44.67% of the total. Different combinations of shape categories, such as fibers, films, foams, pellets, and fragments, were observed. Fibers (38%) were found in high concentrations in all sites.
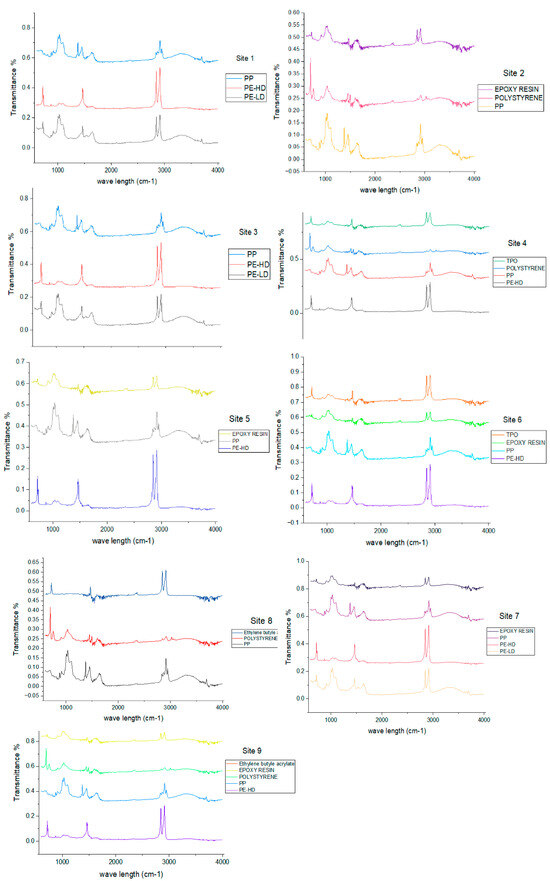
Figure 8.
FTIR spectra of each polymer found in each site during investigation.
3.4.2. Microplastics in Chaetomorpha sp.
There were significant differences in the mean abundance of Chaetomorpha sp. leaf and root surfaces according to the paired t-test analysis (p < 0.05). The mean abundances of the leaf surface and root surface of Chaetomorpha are 8 ± 3 item g−1 wet weight and 5 ± 2 item g−1 wet weight. The blank controls of the leaf samples and root samples contained 1.53 ± 0.57 item g−1 wet weight and 1.23 ± 0.43 item g−1 wet weight. Based on the statistical analysis, it is evident that the most dominant size range of microplastics present on both surfaces was 50–150 µm (leaf surface, 47.79%; root surface, 57.78%). Fragments (leaf surface, 49.56%; root surface, 65.19%) were the most prevalent types of microplastics on both the leaf and root surfaces. A considerable number of blue-colored microplastics were found on both the leaf (45.13%) and root (42.22%) surfaces (Figure 9).

Figure 9.
Examples of microplastics found in Chaetomorpha sp. (A) Blue fiber, (B) blue film, (C) green fiber, (D) transparent fiber.
3.4.3. Microplastics in Halodule pinifolia
The paired t-test analysis revealed a statistically significant difference in the mean microplastic abundance between the leaf and root surfaces of Halodule pinifolia (p < 0.05). The mean abundances of the leaf surface and root surface of Halodule pinifolia are 6 ± 1 item g−1 wet weight and 5 ± 1 item g−1 wet weight. The leaf surface showed a higher abundance compared to the root surface of Halodule pinifolia. There were variations in the color, shape, and size distributions of microplastics in the leaves and roots of Halodule pinifolia (Figure 10). For example, the blue color dominated the leaf surfaces (72.36%), but it was significantly less prevalent on the root surfaces (34.86%). On the other hand, the transparent color was more prevalent on the root surfaces (45.41%) compared to the leaf surfaces (17.08%). Fragments were the most predominant microplastic shape on both the leaf (47.52%) and root surfaces (55.50%) of Halodule pinifolia. The size range of 50–150 µm was notably predominant on both surfaces (Figure 12).
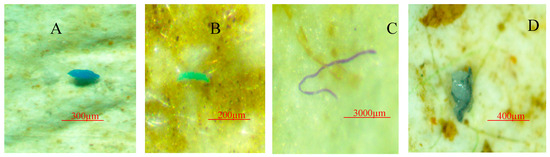
Figure 10.
Examples of microplastics found in Halodule pinifolia. (A) Blue fragment, (B) green fragment, (C) purple fiber, (D) transparent foam.
3.4.4. Microplastics in Gracilaria sp.
According to the paired t-test analysis, there is a significant difference in the mean microplastic abundance between the leaf and root surfaces of Gracilaria sp. (p < 0.05). The leaf surfaces exhibited a higher microplastic abundance than the root surfaces. The mean abundances of leaf surfaces and root surfaces of Gracilaria are 9 ± 3 item g−1 wet weight and 5 ± 3 item g−1 wet weight. Gracilaria sp. was revealed to have a distinct pattern of color, shape, and size distributions on the leaf and root surfaces. The blue color was prevalent on both the leaf (53.87%) and root (32.23%) surfaces, and a considerable number of transparent microplastics were found on the root surfaces (58.21%) compared to the leaf surfaces (32.42%) (Figure 11). Like other species, fragments were the most abundant type of microplastics found on both the leaf (47.91%) and root surfaces (57.32%) of Gracilaria sp. The size range of 50–150 µm was highly dominant in both surfaces (Figure 12).
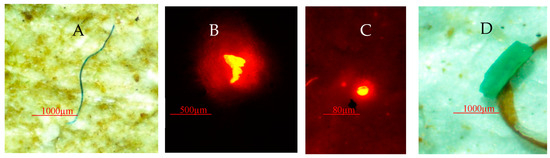
Figure 11.
Examples of microplastics found in Gracilaria sp. (A) Blue fiber, (B) transparent foam, (C) transparent microbead, (D) green fragment.
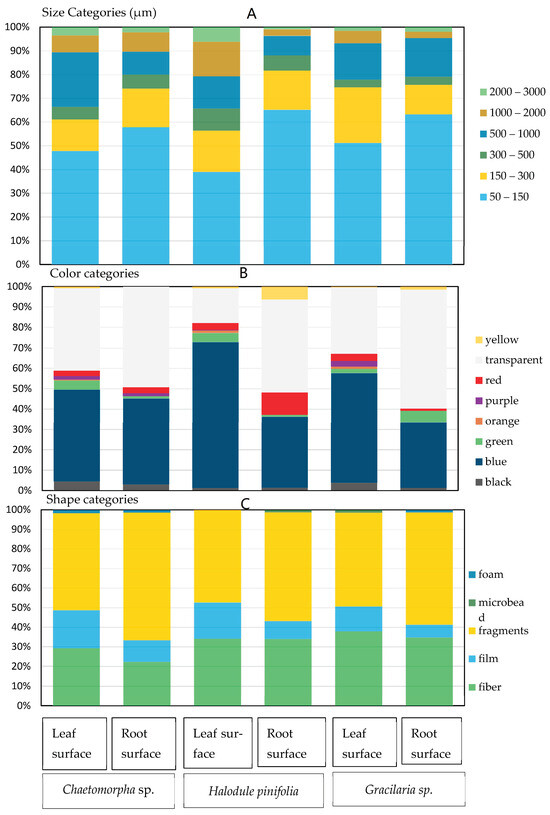
Figure 12.
Physical descriptions of microplastics found in macrophytes by size (A), color (B), and shape (C).
4. Discussions
4.1. Validity of Method and Limitations
The comprehensive methodology used in this study was adapted to prevent cross-contamination caused by airborne microfibers and maintain the integrity of the research findings. Precautions were taken because airborne microplastics were observed in atmospheric fallouts in indoor and outdoor environments according to previous studies [55,56]. Submerged macrophytes consisted of biofilms made from polysaccharide materials, which created a microenvironment in the leaf blades [57]. Careful extraction was conducted to extract these kinds of polymeric substances from the macrophyte samples. Polymer identification was carried out using ATR-FTIR, but it was restricted to particles larger than 1 mm [58] in size. But ATR-µFTIR appears to be more sensitive for the smallest particles, which are <6 µm [58]. The Nile red fluorescence staining technique was an accurate, fast, and cost-effective method to detect and visualize microplastic particles in water, sediment, and biota samples, and it was also the easiest way to map microplastics on a large scale [59].
4.2. Microplastics in Sediments, Water, and Macrophytes
Over the past 20 years, severe changes have occurred in lagoons along the Western Province coastal zone of Sri Lanka due to urban development and population pressure, unplanned tourist activities, the establishment of infrastructure facilities, and shrimp farm ponds [60,61,62]. The change in population density caused highly negative impacts on Negombo Lagoon [63]. Negombo Lagoon consists of a few canals representing sewage outflow sources through rivers and storm drains [64]. Most plastic materials entering the lagoon originate from land-based sources and are transported by wastewater drainage into coastal ecosystems [29,65]. Both macrophyte-associated water and sediments were contaminated by microplastics due to effluent from hotels, runoff outflows, and sewage transport pathways within Negombo Lagoon.
Data on the occurrence and abundance of microplastics in the water, sediments, and macrophytes of Negombo Lagoon are limited, and there is an urgent need to fill this knowledge gap by conducting research and monitoring baseline data. The above comprehensive analysis of microplastics in macrophytes, sediments, and water in Negombo Lagoon offers significant awareness to the community about the plastic pollution across several ecosystem components. The abundance of microplastics in associated waters of macrophyte meadows ranged from 24.00 ± 7.21 to 42.67 ± 14.74 items L−1. The results suggest that the concentration of floating microplastics did not significantly differ (p = 0.76) between each site. The concentration of microplastics in the associated sediment of macrophyte meadows ranged from 228.33 ± 51.32 to 411.67 ± 80.21 items per kg. The abundance of microplastics present in the associated sediment samples was not significantly different between each site (p = 0.54).
The findings of this study indicate widespread contamination of microplastics in the specific macrophyte species which were studied. Halodule pinifolia, Chaetomorpha sp., and Gracilaria sp. were found to be contaminated with microplastics. The results demonstrate significant differences in microplastic abundance between the leaf and root surfaces across all three species. Specifically, the leaf surfaces of the macrophytes exhibited higher microplastic abundance than the root surfaces. Regarding species, the abundance of microplastics (p < 0.05) on the leaf surfaces varied as follows: Gracilaria sp. > Chaetomorpha sp. > Halodule pinifolia. But there were no statistically significant differences in the microplastic abundance between their root surfaces (p = 0.95). These macrophytes’ leaf surfaces comprise biofilms made from expo polysaccharide materials [57]. These materials on leaf surfaces function as a glue for the plastic particles available in the water column [35]. The important fact that is responsible for microplastic contamination on root surfaces is that the roots of seagrass and seaweed function as traps for particulate matter, which also includes microplastics [66]. These microplastics can accumulate in aquatic organisms, including filter feeders and smaller organisms at lower trophic levels [67]. The smaller microplastics on macrophyte surfaces may function as vectors for the transport of microplastics through the food web [14,15,68].
Large amounts of microplastic particles in the 1000–2000 µm size range were discovered in the sediment and water samples (>40%). A significant amount of microplastics in the 50–150 µm size range were found on both surfaces of macrophyte species (>40%). Both fragments and fibers were highly observed in all three sample compartments. Fibers had a lower settling velocity than other shapes of microplastics [69] and a higher fraction of fibers in macrophyte meadows compared to the others. Fragments also exhibited a higher percentage, and this may be a result of the physical forces of water movement [70]. It increases the breakdown of macro-plastic waste and fishing gear. Blue and transparent microplastics were most dominant across species, sediments, and water [71]. Blue microplastics commonly originate from various consumer products, indicating human-related sources of pollution [46]. However, industry experts are using pigments as light-shielding agents, which may delay photodegradation. Since blue has a greater ability to shield light, it is used in most plastic products. Blue-colored particles might remain in the environment for a longer time because they delay photodegradation [72]. Transparent microplastics could result from the breakdown of larger plastic items. Notably, transparent microplastics exhibited a higher abundance, which might indicate a growing output of certain polyethylene bags or degradation processes of transparent plastic granules [73]. Manufacturers have specific colors that they use when constructing fishing gear, and they widely use blue and white colors because they are related to the behavior of marine organisms [74]. The fish industry in Negombo Lagoon is outstanding. Most fishers use fishing gear, and this gear might be one source of microplastics [75]. These findings underline the complexity of microplastic sources and pathways in the ecosystem [73].
Several types of larger polymer materials entangle macrophyte bodies. They range from epoxy resin to polyethylene (PE), polypropylene (PP), polystyrene (PS), olefin-based thermoplastics (TPO), and ethylene butyl acrylate. The dominant polymer type was PP ingested at the nanoscale, and according to previous studies, it has been highly recorded in surface waters of the western coast of Sri Lanka [64]. Toothpaste products were identified as the most commonly used (95.8%) personal care product in Sri Lanka, and they are one of the major sources of microplastics as they consist of acrylate copolymers, PE, and PP [76]. The main polymers adsorbed on leaf and root surfaces were rayon (17%), polystyrene (PS) (4%), polyethylene (PE) (4%), polypropylene (PP-blend) (2%), and polymer coatings/epoxy resins (2%). Rayon was the most dominant polymer found on submerged macrophytes. In a previous study, rayon was found as the most dominant polymer (62.2%) in deep sea sediments and the biota of the Bay of Bengal and the coast of Sri Lanka [77,78]. Not only that, but the most coastal environmental compartments (sediments, water, fish, etc.) also reported a higher percentage of rayon in South Asian coastal areas [56,78].
These microplastics can undergo fracturing into nanoplastics [79]. Currently, these nanoplastics are a widely emerging pollutant in our world. They are small enough to penetrate through biological barriers and enter the cells of organisms [80]. A few studies have already revealed the presence of microplastics and nanoplastics in cardiovascular systems [74]. They have investigated the potential cytotoxic and genotoxic effects caused by nanoplastic particles like polystyrene in peripheral human blood cells [74,81]. A very recent study revealed microplastic and nanoplastic particles in human placental tissues, providing some data to support further research on human diseases caused by them [36]. Also, newly emerging airborne micro- and nanoplastic particles were found in lung tissues, and they pose some difficulties in the respiratory system [21]. It was proven that employees who work in the plastic, rubber, and flock industries have some health problems related to the respiratory system, which may lead to pulmonary fibrosis or even carcinogenesis.
An extensive approach is required to reduce microplastic contamination in Negombo Lagoon. To accomplish this, an effective waste management strategy must be put in place with the help of the central government. These systems must include proper waste and sewage disposal, recycling, and a reduction in single-use plastic [76]. Community involvement is highly required, and awareness campaigns can support behavioral change among people [82]. If these tactics are effectively practiced in Negombo Lagoon, it will eventually protect marine ecosystems and human health.
5. Conclusions
This study addressed, for the first time, the occurrence, abundance, and characterization of microplastics in macrophytes, associated sediments, and water in Sri Lanka. A comprehensive methodology was used based on sampling, extraction, and detection through the fluorescence tagging of polymers using Nile red and polymer identification with ATR-µFTIR and ATR-FTIR. The results of this study reveal a widespread occurrence of microplastics, which were detected in associated water, sediment, and macrophyte samples.
This study revealed variations in microplastic abundance across different macrophyte species, with Gracilaria sp. exhibiting the highest density of 9 ± 3 items g−1 wet weight, followed by Chaetomorpha sp. at 8 ± 3 items g−1 wet weight and Halodule pinifolia at 6 ± 1 items g−1 wet weight. Interestingly, root surfaces showed similar abundance levels. No significant differences were observed in the microplastic content between sediment and water samples across the nine study sites. Both blue and transparent microplastics were predominant, with macrophyte samples containing a higher percentage of fragments in the 50–150 μm range. Sediment and water samples, on the other hand, exhibited a higher percentage of microplastics ranging from 1000 to 2000 μm in size.
Macrophytes are ecologically significant plants that help to maintain healthy ecosystems. On the other hand, seagrass can filter pollutants from water and make them clear. They also aid in the sedimentation of pollutants by extending their roots into sediments to form a network [83]. In a future study, we will aim to analyze the nanoplastic absorption of submerged seaweed and seagrass through their leaves and roots. Further monitoring of the accumulation of micro and nano particles through food webs is required to identify the potential impacts on human health. A better understating of polymers and colors helps to define the correct source of the microplastic’s origin. Most plastic materials enter into coastal ecosystems by anthropogenic activities and land-based sources like wastewater canals. Releasing sources need to be identified for the mitigation process. Investigating various plant and animal species from different ecosystems would provide a clear image of microplastic accumulation in the environment.
Author Contributions
Conceptualization, A.A.D.A. and D.S.M.D.S.; Data Curation, R.M.L.I.R. and A.R.M.; Formal Analysis, R.M.L.I.R., A.R.M. and A.B.; Funding Acquisition, D.S.M.D.S.; Investigation, R.M.L.I.R.; Methodology, R.M.L.I.R., A.R.M. and A.B.; Project Administration, D.S.M.D.S., A.A.D.A., D.B.S., C.R., A.A.D.A., D.S.M.D.S., A.R.M., A.B., D.B.S., C.R. and S.R.C.N.K.N.; Software, R.M.L.I.R. and A.R.M.; Supervision, D.S.M.D.S. and A.A.D.A.; Validation, R.M.L.I.R., A.R.M. and A.B.; Visualization, R.M.L.I.R.; Writing—Original Draft, R.M.L.I.R.; Writing—Review and Editing, A.A.D.A., D.S.M.D.S., A.R.M., A.B., D.B.S. and C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially assisted by the University of Kelaniya [Grant number RP/03/02/06/02/2021] and the Centre for Environment, Fisheries and Aquaculture Science [Cefas] under the Ocean Country Partnership Programme [OCPP] of the Blue Planet Fund, UK.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors acknowledge the National Aquatic Resources Research and Development Agency [NARA], Sri Lanka, for providing laboratory facilities and technical assistance. Susantha Udagedara provided guidance in the macrophyte identification process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 1700782. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.L.; Wang, S.; Jambeck, J.R. The Chinese import ban and its impact on global plastic waste trade. Sci. Adv. 2018, 4, 131. [Google Scholar] [CrossRef]
- Europe Plastics. Circular Economy for Plastics—A European Overview; Plastics The Facts; AISBL: Brussels, Belgium, 2024. [Google Scholar]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Rochman, C.M. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Thushari, G.; Suchana, C.; Amararatne, Y. Coastal debris analysis in beaches of Chonburi Province, eastern of Thailand as implications for coastal conservation. Mar. Pollut. Bullatin 2017, 116, 121–129. [Google Scholar] [CrossRef]
- Bhuyan, M.S.; Venkatramanan, S.; Selvam, S.; Szabo, S.; Hossain, M.M.; Rashed-Un-Nabi, M.; Paramasivam, C.R.; Jonathan, M.P.; Islam, M.S. Plastics in marine ecosystem: A review of their sources and pollution conduits. Reg. Stud. Mar. Sci. 2021, 41, 101539. [Google Scholar]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bullatin 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Horton, A.; Walton, A.; Spurgeon, D.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Shim, W.; Hong, S.; Eo, S. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Athukorala, D.; Estoque, R.; Murayama, Y.; Matsushita, B. Impacts of urbanization on the Muthurajawela Marsh and Negombo Lagoon, Sri Lanka: Implications for landscape planning towards a sustainable urban wetland ecosystem. Remote Sens. 2021, 13, 316. [Google Scholar] [CrossRef]
- Okoye, C.O.; Addey, C.I.; Oderinde, O.; Okoro, J.O.; Uwamungu, J.Y.; Ikechukwu, C.K.; Okeke, E.S.; Ejeromedoghene, O.; Odii, E.C. Toxic chemicals and persistent organic pollutants associated with micro-and nanoplastics pollution. Chem. Eng. J. Adv. 2022, 11, 100310. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–882. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, E.; Singh, S.; Pandey, A.; Bhargava, P.C. Micro-and nano-plastics (MNPs) as emerging pollutant in ground water: Environmental impact, potential risks, limitations and way forward towards sustainable management. Chem. Eng. J. 2023, 141568, 459. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Wang, Q.; Sun, Y. Microplastics as a vector for HOC bioaccumulation in earthworm Eisenia fetida in soil: Importance of chemical diffusion and particle size. Environ. Sci. Technol. 2020, 54, 12154–12163. [Google Scholar] [CrossRef]
- Wang, S.; Xie, S.; Zhang, C.; Pan, Z.; Sun, D.; Zhou, A.; Xu, G.; Zou, J. Interactions effects of nano-microplastics and heavy metals in hybrid snakehead (Channa maculata♀× Channa argus♂). Fish Shellfish. Immunol. 2022, 124, 74–81. [Google Scholar] [CrossRef]
- Espinosa-Díaz, L.F.; Zapata-Rey, Y.T.; Ibarra-Gutierrez, K.; Bernal, C.A. Spatial and temporal changes of dissolved oxygen in waters of the Pajarales complex, Ciénaga Grande de Santa Marta: Two decades of monitoring. Sci. Total Environ. 2021, 785, 147203. [Google Scholar] [CrossRef]
- Martínez-Megías, C.; Rico, A. Biodiversity impacts by multiple anthropogenic stressors in Mediterranean coastal wetlands. Sci. Total Environ. 2022, 818, 151712. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Ordóñez, O.; Saldarriaga-Vélez, J.F.; Espinosa-Díaz, L.F.; Patiño, A.D.; Cusba, J.; Canals, M.; Mejía-Esquivia, K.; Fragozo-Velásquez, L.; Sáenz-Arias, S.; Córdoba-Meza, T.; et al. Microplastic pollution in water, sediments and commercial fish species from Ciénaga Grande de Santa Marta lagoon complex, Colombian Caribbean. Sci. Total Environ. 2022, 829, 154643. [Google Scholar] [CrossRef] [PubMed]
- Sarijan, S.; Azman, S.; Said, M.; Jamal, M.H. Microplastics in freshwater ecosystems: A recent review of occurrence, analysis, potential impacts, and research needs. Environ. Sci. Pollut. Res. 2021, 28, 1341–1356. [Google Scholar] [CrossRef]
- Wicramaarachchi, W.D.; Azmy, S.K.; Amarathunga, A. Comparison of water quality status of Dutch channel and identification of effect of dredging. In Water Resource Research in Sri Lanka; NARA: Peradeniya, Sri Lanka, 2010; pp. 125–135. [Google Scholar]
- Facciolà, A.; Visalli, G.; Pruiti Ciarello, M.; Di Pietro, A. Newly emerging airborne pollutants: Current knowledge of health impact of micro and nanoplastics. Int. J. Environ. Res. Public Health 2021, 18, 2997. [Google Scholar] [CrossRef]
- Mahapatro, D.; Panigrahy, R.; Panda, S. Coastal Lagoon: Present Status and Future Challenges. Int. J. Mar. Sci. 2013, 3, 178–186. [Google Scholar] [CrossRef]
- Silva, E.; Katupotha, J.; Amarasinghe, O.; Manthrithilake, H.; Ariyaratna, R. Lagoons of Sri Lanka: From the Origins to the Present; IWMI: Colombo, Sri Lanka, 2013. [Google Scholar]
- Jayasiri, H.B.; Haputhantri, S.K. Negombo Lagoon, National Aquatic Resources Research and Development Agency (NARA), Crow Island, Colombo 15, Sri Lanka 2015. Available online: http://www.nara.ac.lk/ (accessed on 9 October 2024).
- Prakash, T.G.; Weerasingha, A.; Withanage, A.; Kusuminda, T. Mangrove diversity in Muthurajawela and Negombo lagoon wetland complex, Sri Lanka: Insights for conservation and management. Wildlanka 2017, 5, 99–106. [Google Scholar]
- Sadaf, N.; Amrit, K.M.; Janardhana, R.; Gauhar, M. Coastal macrophytes as bioindicators of trace metals in the Asia’s largest lagoon ecosystem. Mar. Pollut. Bull. 2022, 178, 113576. [Google Scholar] [CrossRef]
- Norderhaug, K.M. Use of red algae as hosts by kelp-associated amphipods. Mar. Biol. 2004, 144, 225–230. [Google Scholar] [CrossRef]
- Hartvig, C.; Kjell, M.N.; Fredriksen, S. Macrophytes as habitat for fauna. Mar. Ecol. Prog. Ser. 2009, 396, 221–233. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Zhang, T.; Ma, C.; Shi, H. Microplastics in the commercial seaweed nori. J. Hazard. Mater. 2020, 388, 122060. [Google Scholar] [CrossRef] [PubMed]
- Saley, A.; Smart, A.; Bezerra, M.; Burnham, T.; Capece, L.; Lima, L.; Morgan, S. Microplastic accumulation and biomagnification in a coastal marine reserve situated in a sparsely populated area. Mar. Pollut. Bull. 2019, 146, 54–59. [Google Scholar] [CrossRef]
- Syeiqido Sora Datu, S.A. Microplastic in Cymodocea rotundata Seagrass Blades. Int. J. Environ. Agric. Biotechnol. IJEAB 2019, 4, 1758–1761. [Google Scholar] [CrossRef]
- Lorenzo, C.; Nicastro, K.R.; Zardi, G.I.; Santos, C.B. Species-specific plastic accumulation in the sediment and canopy of coastal vegetated habitats. Sci. Total Environ. 2020, 723, 138018. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, T.; Wang, J.; Huang, W.; Wang, R.; Xu, J.; Gao, G. Spatio-temporal features of microplastics pollution in macroalgae growing in an important mariculture area, China. Sci. Total Environ. 2020, 719, 137490. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, C.; Weerasinghe, K.; Piyadasa, R.; Pathirana, S. Salinity, pH and Turbidity changes of water in the Negombo lagoon. 2014. In Proceedings of the Annual Research Symposium 2013 ‘ Higher Education Through the State Sector of Sri Lanka in a Competitive Knowledge Economy’ of University of Colombo, Sri Lanka, Abstract Volume, Colombo, Sri Lanka, 3–4 October 2013; (Pg 21). ISBN 978-955-0460-48-9. [Google Scholar]
- Sfriso, A.; Tomio, Y.; Juhmani, A.; Sfriso, A.; Munari, C.; Mistri, M. Macrophytes: A temporary sink for microplastics in transitional water systems. Water 2021, 13, 3032. [Google Scholar] [CrossRef]
- Ragusa, A.; Matta, M.; Cristiano, L.; Matassa, R.; Battaglione, E.; Svelato, A.; Catalano, P. Deeply in plasticenta: Presence of microplastics in the intracellular compartment of human placentas. Int. J. Environ. Res. Public Health 2022, 19, 11593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, J.; Liu, D.; Sun, Z.; Tang, R.; Ma, X.; Feng, Z. Loading microplastics by two related macroalgae in a sea area where gold and green tides occur simultaneously. Sci. Total Environ. 2022, 814, 152809. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshani, K.; Karunasena, G.; Jayasuriya, S. Construction safety assessment framework for developing countries: A case study of Sri Lanka. 2013. Available online: http://dl.lib.mrt.ac.lk/handle/123/10115 (accessed on 26 June 2014).
- Bakir, A.; Desender, M.; Wilkinson, T.; Van Hoytema, N.; Amos, R.; Airahui, S.; Maes, T. Occurrence and abundance of meso and microplastics in sediment, surface waters, and marine biota from the South Pacific region. Mar. Pollut. Bull. 2020, 160, 111572. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Doran, D.; Silburn, B.; Russell, J.; Archer-Rand, S.; Barry, J.; Nicolaus, E.E. A spatial and temporal assessment of microplastics in seafloor sediments: A case study for the UK. Front. Mar. Sci. 2023, 9, 1093815. [Google Scholar] [CrossRef]
- Roch, S.; Brinker, A. Rapid and efficient method for the detection of microplastic in the gastrointestinal tract of fishes. Environ. Sci. Technol. 2017, 51, 4522–4530. [Google Scholar] [CrossRef] [PubMed]
- McGoran, A.R.; Clark, P.F.; Morritt, D.J.E.P. Presence of microplastic in the digestive tracts of European flounder, Platichthys flesus, and European smelt, Osmerus eperlanus, from the River Thames. Environ. Pollut. 2017, 220, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Gurjar, U.R.; Xavier, M.; Nayak, B.B.; Ramteke, K.; Deshmukhe, G.; Jaiswar, A.K.; Shukla, S.P. Microplastics in shrimps: A study from the trawling grounds of northeastern part of Arabian Sea. Environ. Sci. Pollut. Res. 2021, 28, 48494–48504. [Google Scholar] [CrossRef] [PubMed]
- Phaksopa, J.; Sukhsangchan, R.; Keawsang, R.; Tanapivattanakul, K.; Thamrongnawasawat, T.; Worachananant, S.; Sreesamran, P. Presence and characterization of microplastics in coastal fish around the eastern coast of Thailand. Sustainability 2021, 13, 13110. [Google Scholar] [CrossRef]
- Akanyange, S.; Zhang, Y.; Zhao, X.; Adom-Asamoah, G.; Ature, A.; Anning, C.; Crittenden, J. A holistic assessment of microplastic ubiquitousness: Pathway for source identification in the environment. Sustain. Prod. Consum. 2022, 33, 113–145. [Google Scholar] [CrossRef]
- Dambrosio, A.; Cometa, S.; Capuozzo, F.; Ceci, E.; Derosa, M.; Quaglia, N.C. Occurrence and Characterization of Microplastics in Commercial Mussels (Mytilus galloprovincialis) from Apulia Region (Italy). Foods 2023, 12, 1495. [Google Scholar] [CrossRef] [PubMed]
- Manage, P.; Liyanage, G.; Abinaiyan, I.; Madusanka, D.; Bandara, K.R. Pollution levels in Sri Lanka’s west-south coastal waters: Making progress toward a cleaner environment. Reg. Stud. Mar. Sci. 2022, 51, 102193. [Google Scholar] [CrossRef]
- Azmy, S.; Weerasekara, S.; Hettige, N.; Wickramaratne, C.; Amarathunga, D. Rapid Assessment Survey to Determine Current Status of Water Quality in Puttalam Lagoon, Giant’s Tank and Akurala Water Bodies. In Proceedings of the International Symposium on Water Quality and Human Health: Challenges Ahead, Post Graduate Institute of Science, University of Peradeniya, Kandy, Sri Lanka, 22–23 March 2012. [Google Scholar]
- Kader, S.; Raimi, M.; Spalevic, V.; Iyingiala, A.; Bukola, R.; Jaufer, L.; Butt, T. A concise study on essential parameters for the sustainability of Lagoon waters in terms of scientific literature. Turk. J. Agric. For. 2023, 47, 288–307. [Google Scholar] [CrossRef]
- Katupotha, K. Anthropogenic impacts on urban coastal lagoons in the Western and North-western coastal zones of Sri Lanka. In Proceedings of the International Forestry and Environment Symposium, University of Sri Jayawardhanapura, Nugegoda, Sri Lanka, 20 December 2012. [Google Scholar]
- Crawford, C.; Quinn, B.; Crawford, C.; Quinn, B. Microplastic identification techniques. Microplast. Pollut. 2017, 219–267. [Google Scholar]
- Enders, K.; Lenz, R.; Beer, S.; Stedmon, C. Extraction of microplastic from biota: Recommended acidic digestion destroys common plastic polymers. ICES J. Mar. Sci. 2017, 74, 326–331. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [PubMed]
- Enyoh, C.; Verla, A.; Verla, E.; Ibe, F.; Amaobi, C.E. Airborne microplastics: A review study on method for analysis, occurrence, movement and risks. Environ. Monit. Assess. 2019, 191, 668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Zhu, F.; Zhou, S. Airborne microplastics: A review on the occurrence, migration and risks to humans. Bull. Environ. Contam. Toxicol. 2021, 107, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.M.; Hong, W.; Liu, K.; Yan, S.; Song, W.; Zhang, J. Temporal variations in reactive oxygen species in biofilms of submerged macrophytes: The key role of microbial metabolism mediated by oxygen fluctuations. J. Hazard. Mater. 2024, 461, 132542. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.O.; Ahmed, A.E.; Yasser, M.S.; Diaa, K.; Tarik, B. Detection of Sub-20 μm Microplastic Particles by Attenuated Total Reflection Fourier Transform Infrared Spectroscopy and Comparison with Raman Spectroscopy. ACS Omega 2023, 8, 10335–10341. [Google Scholar] [CrossRef]
- Shruti, V.; Pérez-Guevara, F.; Roy, P.; Kutralam-Muniasamy, G. Analyzing microplastics with Nile Red: Emerging trends, challenges, and prospects. J. Hazard. Mater. 2022, 423, 127171. [Google Scholar] [CrossRef] [PubMed]
- Balasuriya, A. Coastal area management: Biodiversity and ecological sustainability in Sri Lankan perspective. In Biodiversity and Climate Change Adaptation in Tropical Islands; Academic Press: Cambridge, MA, USA, 2018; pp. 701–724. [Google Scholar]
- Bandara, H.M.; Ratnayake, I. Coastal Land Uses for Tourism in Sri Lanka: Conflicts and Planning Efforts. Sabaragamuwa Univ. J. 2015, 14, 41. [Google Scholar] [CrossRef][Green Version]
- Kodippili, A.A.; Amarathunga, A.A.; Piyathilaka, M.A.; Maddumage, M.D.; Narangoda, S.R.; Jayasekara, H.N.; Weerasekara, K. Assessment of Surface Water Pollution Status in the Mirissa and Beruwala Fishery Harbors with Reference to Anthropogenic Activities; Sustainable Fisheries for Economic Prosperity and Food Security, NARA Scientific Session: Colombo, Sri Lanka, 2023. [Google Scholar]
- Athawuda, A.; Jayasiri, H.; Thushari, G.; Guruge, K.P. Quantification and morphological characterization of plastic litter (0.30–100 mm) in surface waters of off Colombo, west coast of Sri Lanka. Environ. Monit. Assess. 2020, 192, 509. [Google Scholar] [CrossRef] [PubMed]
- Amarathunga, A.A.; Gunawardhana, M.K.; Hitinayake, H.M.; Jayawardana, G.D. Anthropogenic impacts on vegetation diversity and spatial floral composition of the swamp forest associated with the tropical river basin. J. Environ. Prof. Sri Lanka 2016, 5, 23–40. [Google Scholar] [CrossRef]
- Li, W.; Tse, H.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiao, X.; Xu, C.; Perianen, Y.; Hu, J.; Holmer, M. Seagrass beds acting as a trap of microplastics-emerging hotspot in the coastal region? Environ. Pollut. 2020, 257, 113450. [Google Scholar] [CrossRef] [PubMed]
- Au, S.; Lee, C.; Weinstein, J.; van den Hurk, P.; Klaine, S. Trophic transfer of microplastics in aquatic ecosystems: Identifying critical research needs. Integr. Environ. Assess. Manag. 2017, 13, 505–509. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Khatmullina, L.; Isachenko, I. Settling velocity of microplastic particles of regular shapes. Mar. Pollut. Bull. 2017, 114, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Enfrin, M.; Lee, J.; Gibert, Y.; Basheer, F.; Kong, L.; Dumée, L.F. Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces. J. Hazard. Mater. 2020, 384, 121393. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Yee Leung, K.M.; Wu, F. Color: An important but overlooked factor for plastic photoaging and microplastic formation. Environ. Sci. Technol. 2022, 56, 9161–9163. [Google Scholar] [CrossRef] [PubMed]
- Asamoah, B.; Kanyathare, B.; Roussey, M.; Peiponen, K. A prototype of a portable optical sensor for the detection of transparent and translucent microplastics in freshwater. Chemosphere 2019, 231, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.; Dubey, R.; Samarth, R.; Shubham, S.; Chowdhury, P.; Kumawat, M.; Kumar, M. The biological effects of polystyrene nanoplastics on human peripheral blood lymphocytes. Nanomaterials 2022, 12, 1632. [Google Scholar] [CrossRef] [PubMed]
- Nawalage, N.S.; Bellanthudawa, B.K. Synthetic polymers in personal care and cosmetics products (PCCPs) as a source of microplastic (MP) pollution. Mar. Pollut. Bull. 2022, 182, 113927. [Google Scholar] [CrossRef] [PubMed]
- Nijamdeen, T.; Atugoda, T.; Kumara, P.; Gunasekara, A.; Vithanage, M. Status of Particulate Marine Plastics in Sri Lanka: Research Gaps and Policy Needs. In Particulate Plastics in Terrestrial and Aquatic Environments; CRC Press: Boca Raton, FL, USA, 2020; pp. 297–326. [Google Scholar]
- Qi, H.; Li, H.; Meng, X.; Peng, L.; Zheng, H.; Wang, L.; Cai, M. Fate of microplastics in deep-sea sediments and its influencing factors: Evidence from the Eastern Indian Ocean. Sci. Total Environ. 2022, 828, 154266. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Liu, K.; Zhu, L.; Song, Z.; Li, D. Atmospheric microplastic over the South China Sea and East Indian Ocean: Abundance, distribution and source. J. Hazard. Mater. 2020, 389, 121846. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.; Xu, E.; Farner, J.; Tufenkji, N. Separation and analysis of microplastics and nanoplastics in complex environmental samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef]
- Ng, E.L.; Lwanga, E.H.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Lett, Z.; Hall, A.; Skidmore, S.; Alves, N. Environmental microplastic and nanoplastic: Exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. 2021, 291, 118190. [Google Scholar] [CrossRef] [PubMed]
- Kibria, M.; Masuk, N.; Safayet, R.; Nguyen, H.; Mourshed, M. Plastic waste: Challenges and opportunities to mitigate pollution and effective management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D. Microplastics in seagrass ecosystems: A review of fate and impacts. Res. Ecol. 2024, 6, 41–53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).