Exposure and Toxicity Factors in Health Risk Assessment of Heavy Metal(loid)s in Water
Abstract
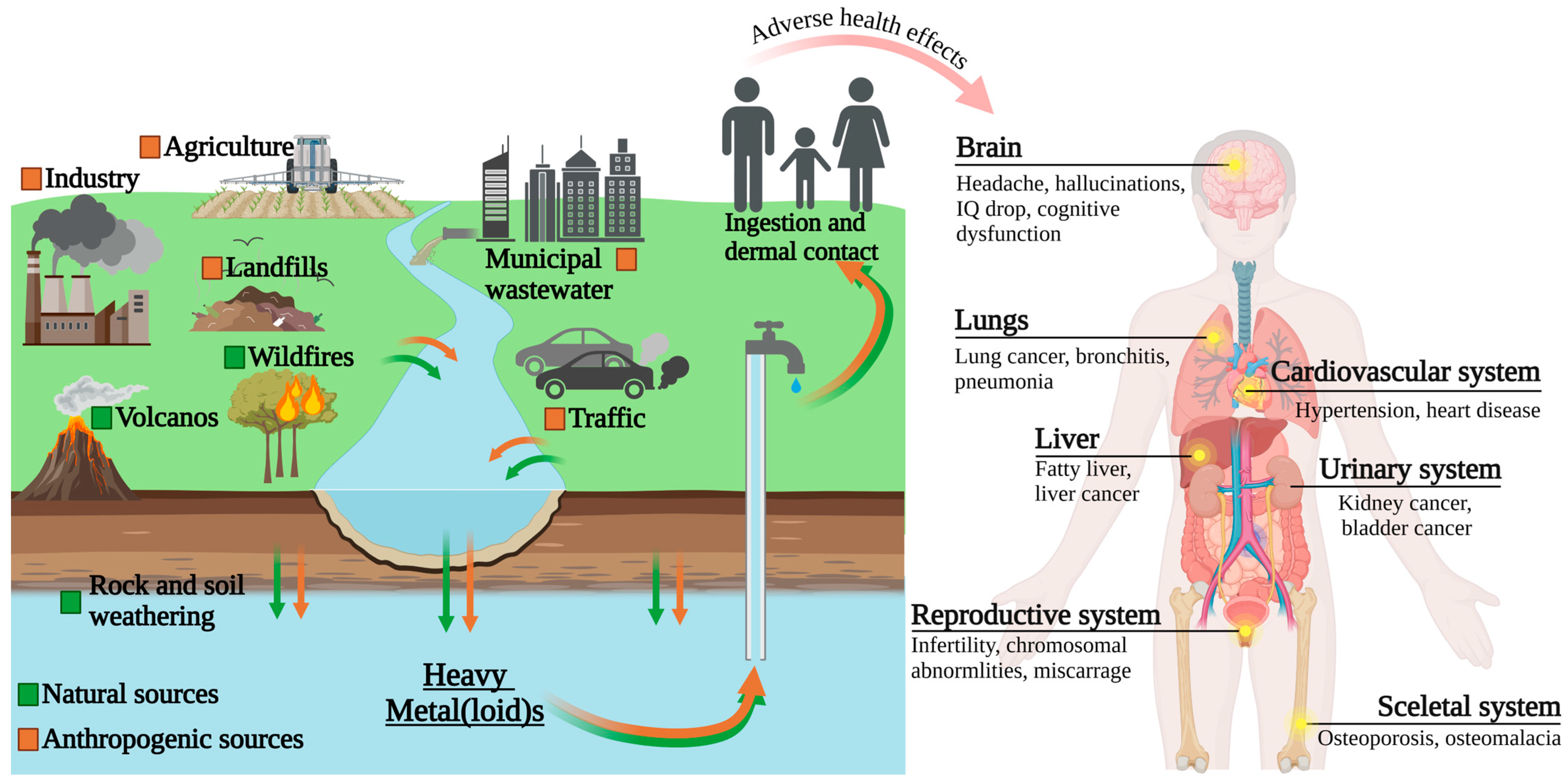
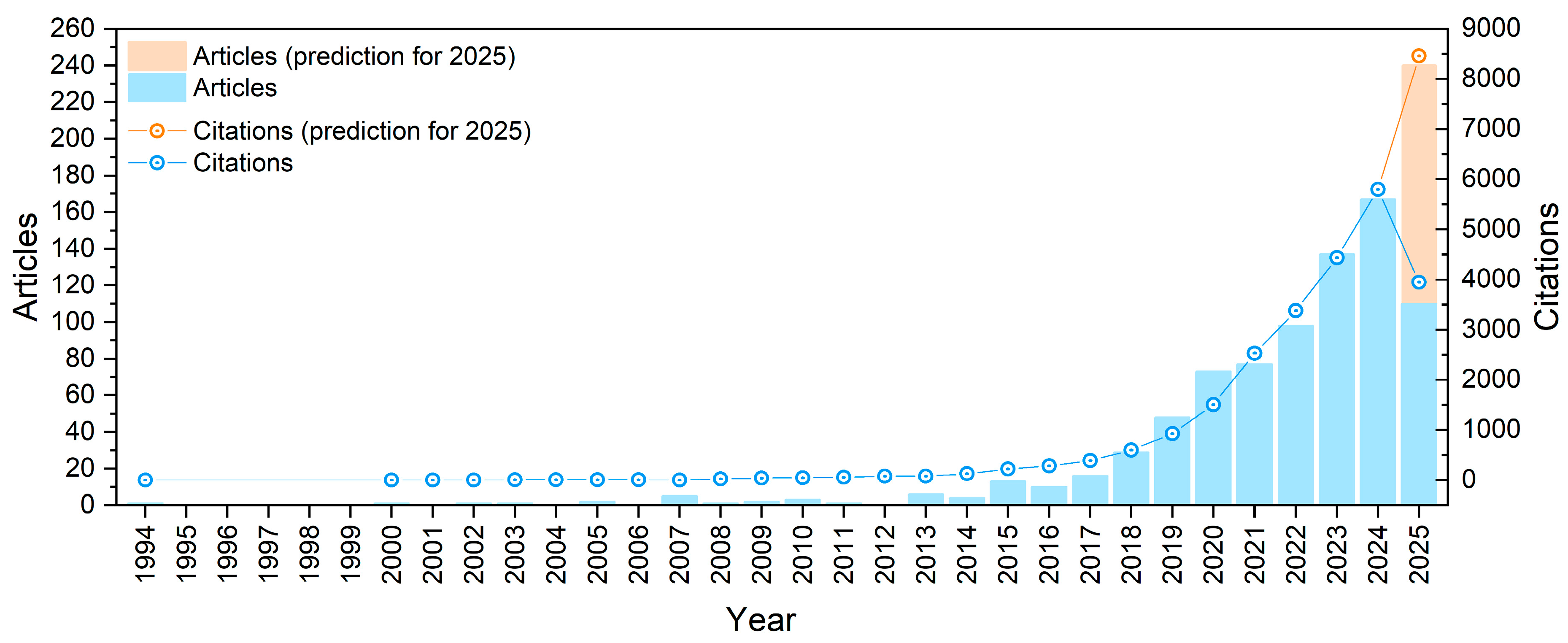
1. Introduction
2. Materials and Methods
2.1. Data Extraction and Categorization
2.2. Health Risk Methodology
3. Exposure and Toxicity Factors
3.1. Ingestion Rate
3.2. Exposure Frequency
3.3. Exposure Duration
3.4. Body Weight
3.5. Averaging Time
3.6. Skin Surface Area
3.7. Exposure Time
3.8. Conversion Factor
3.9. Dermal Permeability Coefficient
3.10. Reference Dose
3.11. Cancer Slope Factor
4. Probabilistic Health Risk Assessment
5. Summary Tables
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abadi, H.T.; Alemayehu, T.; Berhe, B.A. Heavy Metal’s Pollution Health Risk Assessment and Source Appraisal of Groundwater and Surface Water in Irob Catchment, Tigray, Northern Ethiopia. Appl. Water Sci. 2024, 14, 201. [Google Scholar] [CrossRef]
- Vesković, J.; Onjia, A. Influencing Factors of Groundwater 238U, 232Th, 40K, and Rare Earth Element Contamination: Insights from the Two-Dimensional Monte Carlo Simulation of Radiological Risks. Mar. Pollut. Bull. 2025, 213, 117682. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Rahman, A.; Asefa, E.M.; Parveen, M.; Uddin, M.R. Assessing Spatial Distribution of Heavy Metal Contamination in Groundwater and Associated Human Health Risk in the Chittagong Industrial Area, Bangladesh. J. Hazard. Mater. Adv. 2025, 18, 100728. [Google Scholar] [CrossRef]
- Barkat, A.; Bouaicha, F.; Ziad, S.; Mester, T.; Sajtos, Z.; Balla, D.; Makhloufi, I.; Szabó, G. The Integrated Use of Heavy-Metal Pollution Indices and the Assessment of Metallic Health Risks in the Phreatic Groundwater Aquifer—The Case of the Oued Souf Valley in Algeria. Hydrology 2023, 10, 201. [Google Scholar] [CrossRef]
- Ashong, G.W.; Kwaansa-Ansah, E.E.; Ababio, B.A.; Yagra, E.A.; Antwi, G. Pollution Profiling and Quality Assessment of Bonsa River, Tarkwa Nsuaem, Ghana; Toxic Element, Ecotoxicology, Health Risk Assessment, and Multivariate Analysis. Environ. Chall. 2025, 18, 101078. [Google Scholar] [CrossRef]
- Dippong, T.; Resz, M.-A. Water and Sediments Pollution from Iza River (Romania): Influence on Water Quality and Metals Content. Process Saf. Environ. Prot. 2025, 200, 107335. [Google Scholar] [CrossRef]
- Ajala, O.A.; Oke, M.R.; Ajibade, T.F.; Ajibade, F.O.; Adelodun, B.; Ighalo, J.O.; Ajala, M.O.; Kumar, P.; Demissie, H.; Ugya, A.Y.; et al. Concentrations, Bioaccumulation, and Health Risk Assessments of Heavy Metals in Fishes from Nigeria’s Freshwater: A General Overview. Env. Sci. Pollut. Res. 2022, 29, 82660–82680. [Google Scholar] [CrossRef]
- Ray, S.; Vashishth, R. From Water to Plate: Reviewing the Bioaccumulation of Heavy Metals in Fish and Unraveling Human Health Risks in the Food Chain. Emerg. Contam. 2024, 10, 100358. [Google Scholar] [CrossRef]
- Miletić, A.; Lučić, M.; Onjia, A. Exposure Factors in Health Risk Assessment of Heavy Metal(Loid)s in Soil and Sediment. Metals 2023, 13, 1266. [Google Scholar] [CrossRef]
- Parvin, A.; Moniruzzaman, M.; Hossain, M.K.; Saha, B.; Parvin, A.; Suchi, P.D.; Hoque, S. Chemical Speciation and Potential Mobility of Heavy Metals in Organic Matter Amended Soil. Appl. Environ. Soil. Sci. 2022, 2022, 2028860. [Google Scholar] [CrossRef]
- Xu, H.; Han, Q.; Adnan, M.; Li, M.; Wang, M.; Wang, M.; Jiang, F.; Feng, X. Global Environmental Geochemistry and Molecular Speciation of Heavy Metals in Soils and Groundwater from Abandoned Smelting Sites: Analysis of the Contamination Dynamics and Remediation Alternatives in Karst Settings. Toxics 2025, 13, 608. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.N.; Parsai, T. Heavy Metal(Loid) Contamination in Forest Fire Affected Soil and Surface Water: Pollution Indices and Human Health Risk Assessment. Environ. Monit. Assess. 2025, 197, 378. [Google Scholar] [CrossRef]
- Oladimeji, T.E.; Oyedemi, M.; Emetere, M.E.; Agboola, O.; Adeoye, J.B.; Odunlami, O.A. Review on the Impact of Heavy Metals from Industrial Wastewater Effluent and Removal Technologies. Heliyon 2024, 10, e40370. [Google Scholar] [CrossRef]
- Singh, V.; Ahmed, G.; Vedika, S.; Kumar, P.; Chaturvedi, S.K.; Rai, S.N.; Vamanu, E.; Kumar, A. Toxic Heavy Metal Ions Contamination in Water and Their Sustainable Reduction by Eco-Friendly Methods: Isotherms, Thermodynamics and Kinetics Study. Sci. Rep. 2024, 14, 7595. [Google Scholar] [CrossRef]
- Omeka, M.E.; Ezugwu, A.L.; Agbasi, J.C.; Egbueri, J.C.; Abugu, H.O.; Aralu, C.C.; Ucheana, I.A. A Review of the Status, Challenges, Trends, and Prospects of Groundwater Quality Assessment in Nigeria: An Evidence-Based Meta-Analysis Approach. Environ. Sci. Pollut. Res. 2024, 31, 22284–22307. [Google Scholar] [CrossRef]
- Egbueri, J.C.; Agbasi, J.C.; Ezugwu, A.L.; Omeka, M.E.; Ucheana, I.A.; Aralu, C.C.; Abugu, H.O. Metal(Loid)s, Nitrate, Polycyclic Aromatic Hydrocarbons, and Radioactive Contaminants in Nigerian Water Resources: State-of-the-Art of Their Ecological and Health Risk Assessments. Env. Dev. Sustain. 2024, 1–50. [Google Scholar] [CrossRef]
- Kiran; Bharti, R.; Sharma, R. Effect of Heavy Metals: An Overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar] [CrossRef]
- USEPA. User’s Guide: Human Health Risk Assessment 2008; USEPA: Washington, DC, USA, 2008. [Google Scholar]
- USEPA. Exposure Factors Handbook: 2011 Edition; Office of Emergency and Remedial Response, US Environmental Protection Agency: Washington, DC, USA, 2011. [Google Scholar]
- Ghosh, A.; Karmaker, K.D.; Hasan, M.; Rahman, M.; Shimu, N.J.; Islam, M.S.; Hossain, M.S.; Ismail, Z. Trace Element Contamination in Water and Sediment in an Estuarine Ecosystem Connected to the Bay of Bengal: A Preliminary Assessment of Ecological and Human Health Risks. Mar. Pollut. Bull. 2024, 207, 116897. [Google Scholar] [CrossRef]
- Tokatli, C.; Mutlu, E.; Ustaoğlu, F.; Islam, A.R.T.; Muhammad, S. Spatiotemporal Variations, Health Risk Assessment, and Sources of Potentially Toxic Elements in Potamic Water of the Anday Stream Basin (Türkiye), Black Sea Region. Environ. Monit. Assess. 2024, 196, 420. [Google Scholar] [CrossRef]
- Bhat, M.A.; Fan, D.; Nisa, F.U.; Dar, T.; Kumar, A.; Sun, Q.; Li, S.-L.; Mir, R.R. Trace Elements in the Upper Indus River Basin (UIRB) of Western Himalayas: Quantification, Sources Modeling, and Impacts. J. Hazard. Mater. 2024, 476, 135073. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A. Evaluation of Heavy Metal Pollution Index Considering Health Risk in Complete Stretch of Ganga River. Innov. Infrastruct. Solut. 2023, 8, 98. [Google Scholar] [CrossRef]
- Azzam, M.A.; Rizwan Khan, M.; Moustafa Youssef, H. Drinking Water as a Substantial Source of Toxic Alkali, Alkaline and Heavy Metals: Toxicity and Their Implications on Human Health. J. King Saud. Univ.-Sci. 2023, 35, 102761. [Google Scholar] [CrossRef]
- Badeenezhad, A.; Soleimani, H.; Shahsavani, S.; Parseh, I.; Mohammadpour, A.; Azadbakht, O.; Javanmardi, P.; Faraji, H.; Babakrpur Nalosi, K. Comprehensive Health Risk Analysis of Heavy Metal Pollution Using Water Quality Indices and Monte Carlo Simulation in R Software. Sci. Rep. 2023, 13, 15817. [Google Scholar] [CrossRef] [PubMed]
- Chorol, L.; Gupta, S.K. Evaluation of Groundwater Heavy Metal Pollution Index through Analytical Hierarchy Process and Its Health Risk Assessment via Monte Carlo Simulation. Process Saf. Environ. Prot. 2023, 170, 855–864. [Google Scholar] [CrossRef]
- Din, I.U.; Muhammad, S.; Faisal, S.; ur Rehman, I.; Ali, W. Heavy Metal(Loid)s Contamination and Potential Risk Assessment via Groundwater Consumption in the District of Hangu, Pakistan. Environ. Sci. Pollut. Res. 2023, 30, 33808–33818. [Google Scholar] [CrossRef]
- Abiy Andemo, K.; Tilahun, Y.G.; Sorsa, S.S.; Seifu, B.Y. Heavy Metal Contamination in Omo River, Ethiopia: Environmental and Human Health Risks. Environ. Health Insights 2024, 18, 1–13. [Google Scholar] [CrossRef]
- Batool, M.; Toqeer, M.; Shah, M.H. Assessment of Water Quality, Trace Metal Pollution, Source Apportionment and Health Risks in the Groundwater of Chakwal, Pakistan. Environ. Geochem. Health 2023, 45, 4327–4352. [Google Scholar] [CrossRef]
- Vesković, J.; Onjia, A. Two-Dimensional Monte Carlo Simulation Coupled with Multilinear Regression Modeling of Source-Specific Health Risks from Groundwater. J. Hazard. Mater. 2025, 488, 137309. [Google Scholar] [CrossRef]
- Yadav, S.K.; Attry, B.; Shukla, S.; Dutta, S.; Sharma, K.; Rajak, R.; Gupta, A.; Baruah, B.; Ranjan, R.K. Distribution, Toxicity Load and Risk Assessment of Heavy Metals in the Groundwater of Dhemaji, Assam, India. Chemosphere 2024, 358, 141979. [Google Scholar] [CrossRef] [PubMed]
- Nisa, F.U.; Umar, R. Heavy Metal Pollution and Health Risk Assessment Using Deterministic and Monte Carlo Simulation Approaches in the Himalayan Spring and Surface Water Systems of Kulgam District, Kashmir Valley, India. Environ. Earth Sci. 2024, 83, 278. [Google Scholar] [CrossRef]
- Botle, A.; Salgaonkar, S.; Tiwari, R.; Barabde, G. Heavy Metal Pollution in River Ulhas, Maharashtra, India: Unraveling Contamination Dynamics Through Pollution Indices, Multivariate Analysis, and Health Risk Assessment. Int. J. Environ. Res. 2025, 19, 88. [Google Scholar] [CrossRef]
- Pham, L.T.; Tran, Y.T.H.; Tran, T.T.; Bui, H.M.; Le, L.T.; Dao, S.T.; Nguyen, D.T. Ecological and Human Health Risk Assessments of Cyanotoxins and Heavy Metals in a Drinking Water Supply Reservoir. J. Water Health 2023, 21, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.K.; Gope, M.; Pobi, K.K.; Saha, S.; Gupta, S.; Bhattacharjee, R.R.; Nayek, S. Geostatistical Appraisal of Water Quality, Contamination, Source Distribution of Potentially Toxic Elements (PTEs) in the Lower Stretches of Subarnarekha River (Odisha), India, and Health Risk Assessment by Monte Carlo Simulation Approach. Environ. Geochem. Health 2024, 46, 42. [Google Scholar] [CrossRef]
- Dogra, S.; Sharma, K.; Singh, N. Water Quality and Health Risk Assessment of Heavy Metals in Groundwater of Ranbir Singh Pura Tehsil of Jammu and Kashmir, India. Environ. Monit. Assess. 2023, 195, 1026. [Google Scholar] [CrossRef]
- Vetrimurugan, E.; Brindha, K.; Elango, L.; Ndwandwe, O.M. Human Exposure Risk to Heavy Metals through Groundwater Used for Drinking in an Intensively Irrigated River Delta. Appl. Water Sci. 2017, 7, 3267–3280. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, S. Statistical and Geospatial Assessment of Trace and Toxic Elements Distribution in Ground and Surface Water of Northern Parts of the Indo-Gangetic Plains: Source Identification and Health Risk Assessment. Chemosphere 2024, 364, 142990. [Google Scholar] [CrossRef]
- Eziz, M.; Sidikjan, N.; Zhong, Q.; Rixit, A.; Li, X. Distribution, Pollution Levels, and Health Risk Assessment of Heavy Metals in Groundwater in the Main Pepper Production Area of China. Open Geosci. 2023, 15, 20220491. [Google Scholar] [CrossRef]
- Nemati, B.; Fallahizadeh, S.; Mostafaei, G.; Miranzadeh, M.B. Health Risk Assessment of Toxic Elements in Kashan Drinking Water Reservoirs Using Monte Carlo Simulation and Sensitivity Analysis. Sci. Rep. 2025, 15, 17806. [Google Scholar] [CrossRef]
- Subuddhi, S.P.; Kansal, A.; Pandey, P.; Ghoshal, T.; Singhal, N. Impact Examination of the Lockdown on the Status of the Heavy Metal Pollution Index and Health Risk of Ganga River Water Quality. Environ. Eng. Res. 2023, 28, 220507. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Enitan, A.M.; Mutileni, N.; Odiyo, J.O. Evaluation of Water Quality and Human Risk Assessment Due to Heavy Metals in Groundwater around Muledane Area of Vhembe District, Limpopo Province, South Africa. Chem. Cent. J. 2018, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Guddeti, S.S.; Kurakalva, R.M. Potential Toxic Element Contamination and Non-Carcinogenic Risk Assessment of Groundwater from Rapidly Growing Urban Areas in Telangana, India. Environ. Sci. Pollut. Res. 2024, 31, 40269–40284. [Google Scholar] [CrossRef]
- Tanwar, D.; Tyagi, S.; Sarma, K. Seasonal and Spatial Investigation of Groundwater Contamination of Potential Toxic Elements (PTEs) and Associated Health Risks of Southern Region of Delhi, India. Chemosphere 2025, 378, 144398. [Google Scholar] [CrossRef]
- Bharat, A.P.; Singh, A.K.; Mahato, M.K. Heavy Metal Geochemistry and Toxicity Assessment of Water Environment from Ib Valley Coalfield, India: Implications to Contaminant Source Apportionment and Human Health Risks. Chemosphere 2024, 352, 141452. [Google Scholar] [CrossRef]
- Ayejoto, D.A.; Egbueri, J.C. Human Health Risk Assessment of Nitrate and Heavy Metals in Urban Groundwater in Southeast Nigeria. Ecol. Front. 2024, 44, 60–72. [Google Scholar] [CrossRef]
- Badmus, G.O.; Ajiboye, Y.; Ogungbemi, O.S.; Jita, A.P.; Adenuga, O.A. Assessment of Groundwater Quality, Irrigation Suitability, and Health Risks from Radon and Heavy Metals near Ilokun Dumpsite, Ado-Ekiti, Nigeria. J. Water Health 2024, 22, 1725–1742. [Google Scholar] [CrossRef]
- Barnett, A.N.; Irum, A.; Nabi, D.; Khawar, M.I.; Arshad, M. Occurrence of Some Heavy Metals in Drinking Water, Vegetables, and Urine in Rawalpindi and Islamabad, Pakistan—Human Health Risk Assessment. Int. J. Environ. Res. 2025, 19, 10. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, S.K. Application of Monte Carlo Simulation for Carcinogenic and Non-Carcinogenic Risks Assessment through Multi-Exposure Pathways of Heavy Metals of River Water and Sediment, India. Environ. Geochem. Health 2023, 45, 3465–3486. [Google Scholar] [CrossRef] [PubMed]
- Preonty, N.-E.-J.; Hassan, M.N.; Reza, A.H.M.S.; Rasel, M.I.A.; Mahim, M.M.A.; Jannat, M.F.T. Pollution and Health Risk Assessment of Heavy Metals in Surface Water of the Industrial Region in Gazipur, Bangladesh. Environ. Chem. Ecotoxicol. 2025, 7, 527–538. [Google Scholar] [CrossRef]
- Aslani, R.; Esmaeili, S.; Akbari, M.E.; Molaee-Aghaee, E.; Sadighara, P.; Nazmara, S.; Mahmoudi, B. Determination of Heavy Metals, Nitrate and Nitrite in Mineral and Drinking Bottled Water in Tehran, Iran: A Health Risk Assessment by Monte-Carlo Simulation Method. Heliyon 2024, 10, e40714. [Google Scholar] [CrossRef] [PubMed]
- Bu, C.; Li, X.; Li, Q.; Li, L.; Wu, P. Spatiotemporal Distributions, Sources, and Health Risks of Heavy Metals in an Acid Mine Drainage (AMD)-Contaminated Karst River in Southwest China. Appl. Water Sci. 2024, 14, 251. [Google Scholar] [CrossRef]
- Singh, B.P.; Samanta, P.; Choudhury, M.; Gupta, P.; Chadha, U.; Eticha, T.K. Physicochemical and Heavy Metal Pollution Level in Hindon River Ecosystem: An Implication to Public Health Risk Assessment. Environ. Qual. Manag. 2024, 34, e22263. [Google Scholar] [CrossRef]
- Shakil, S.; Arooj, A.; Fatima, S.; Sadef, Y. Geochemical Distribution and Environmental Risk Assessment of Trace Metals in Groundwater Released from E-Waste Management Activities in Lahore, Pakistan. Environ. Geochem. Health 2023, 45, 3699–3714. [Google Scholar] [CrossRef]
- Aslam, H.; Hashmi, A.; Khan, I.; Ahmad, S.; Umar, R. Deciphering Effects of Coal Fly Ash on Hydrochemistry and Heavy Metal(Loid)s Occurrence in Surface and Groundwater: Implications for Environmental Impacts and Management. Water Air Soil. Pollut. 2024, 235, 640. [Google Scholar] [CrossRef]
- Younesi Baneh, P.; Ahmadi, B.; Salehzadeh, H.; Mohammadi, H.; Shahmoradi, B.; Ghaderi, B. Assessment of Heavy Metal Contamination in Groundwater of Rural Areas of Kurdistan Province Iran: A Comprehensive Study. Heliyon 2024, 10, e39833. [Google Scholar] [CrossRef]
- Phan, K.; Kim, K.-W. Health Risk Assessment of Trace Elements in the Tonle Sap Great Lake and the Tonle Sap River in Cambodia during the Rainy Season. J. Water Health 2023, 21, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Alani, R.A.; Nwude, D.O.; Bello, I.I.; Okolie, C.J.; Akinrinade, O.E. Levels and Health Risks of Heavy Metals and Organochlorine Pesticide Residues in Soil and Drinking Water of Flood-Prone Residential Area of Lagos, Nigeria. Water Air Soil. Pollut. 2023, 234, 783. [Google Scholar] [CrossRef]
- Vesković, J.; Onjia, A. Environmental Implications of the Soil-to-Groundwater Migration of Heavy Metals in Mining Area Hotspots. Metals 2024, 14, 719. [Google Scholar] [CrossRef]
- Vesković, J.; Bulatović, S.; Miletić, A.; Tadić, T.; Marković, B.; Nastasović, A.; Onjia, A. Source-Specific Probabilistic Health Risk Assessment of Potentially Toxic Elements in Groundwater of a Copper Mining and Smelter Area. Stoch. Env. Res. Risk Assess. 2024, 38, 1597–1612. [Google Scholar] [CrossRef]
- Salami, I.R.S.; Thufailah, N.A.; Fahimah, N.; Roosmini, D. Health Risk Assessment of Physicochemical and Heavy Metals Exposures of the Usage of Shallow Groundwater Located at the Proximity to Citarum River, Indonesia. Case Stud. Chem. Environ. Eng. 2025, 11, 101153. [Google Scholar] [CrossRef]
- Uddin, M.G.; Imran, M.H.; Sajib, A.M.; Hasan, M.A.; Diganta, M.T.M.; Dabrowski, T.; Olbert, A.I.; Moniruzzaman, M. Assessment of Human Health Risk from Potentially Toxic Elements and Predicting Groundwater Contamination Using Machine Learning Approaches. J. Contam. Hydrol. 2024, 261, 104307. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Y.; You, S.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Huang, L.; Li, F. Spatial Distribution, Health Risk Assessment and Statistical Source Identification of the Trace Elements in Surface Water from the Xiangjiang River, China. Environ. Sci. Pollut. Res. 2015, 22, 9400–9412. [Google Scholar] [CrossRef]
- Bhat, M.A.; Janaszek, A. Delving into River Health: Unveiling Microplastic Intrusion and Heavy Metal Contamination in Freshwater. Discov. Environ. 2024, 2, 61. [Google Scholar] [CrossRef]
- Avigliano, E.; Schenone, N.F. Human Health Risk Assessment and Environmental Distribution of Trace Elements, Glyphosate, Fecal Coliform and Total Coliform in Atlantic Rainforest Mountain Rivers (South America). Microchem. J. 2015, 122, 149–158. [Google Scholar] [CrossRef]
- Latif, M.; Nasim, I.; Ahmad, M.; Nawaz, R.; Tahir, A.; Irshad, M.A.; Al-Mutairi, A.A.; Irfan, A.; Al-Hussain, S.A.; Zaki, M.E.A. Human Health Risk Assessment of Drinking Water Using Heavy Metal Pollution Index: A GIS-Based Investigation in Mega City. Appl. Water Sci. 2025, 15, 12. [Google Scholar] [CrossRef]
- Dzinjalamala, G.D.; Kaonga, C.C.; Kumwenda, S.; Kosamu, I.B.M.; Thulu, F.G.D.; Chitete-Mawenda, U.; Makwinja, R.; Kanyerere, T.; Sakugawa, H. Human Health Risk Assessment of Microbial Contamination and Trace Metals in Water and Soils of Chileka Township, Blantyre, Malawi. Discov. Environ. 2024, 2, 62. [Google Scholar] [CrossRef]
- Niknejad, H.; Ala, A.; Ahmadi, F.; Mahmoodi, H.; Saeedi, R.; Gholami-Borujeni, F.; Abtahi, M. Carcinogenic and Non-Carcinogenic Risk Assessment of Exposure to Trace Elements in Groundwater Resources of Sari City, Iran. J. Water Health 2023, 21, 501–513. [Google Scholar] [CrossRef]
- Etikala, B.; Vangala, S.; Madhav, S. Groundwater Geochemistry Using Modified Integrated Water Quality Index (IWQI) and Health Indices with Special Emphasis on Nitrates and Heavy Metals in Southern Parts of Tirupati, South India. Environ. Geochem. Health 2024, 46, 465. [Google Scholar] [CrossRef] [PubMed]
- Boum-Nkot, S.N.; Nlend, B.; Komba, D.; Ndondo, G.R.N.; Bello, M.; Fongoh, E.J.; Ntamak-Nida, M.-J.; Etame, J. Hydrochemistry and Assessment of Heavy Metals Groundwater Contamination in an Industrialized City of Sub-Saharan Africa (Douala, Cameroon). Implication on Human Health. HydroResearch 2023, 6, 52–64. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, S.; Shi, Y.; Wang, C.; Li, B.; Li, Y.; Wu, S. Heavy Metals in Food Crops, Soil, and Water in the Lihe River Watershed of the Taihu Region and Their Potential Health Risks When Ingested. Sci. Total Environ. 2018, 615, 141–149. [Google Scholar] [CrossRef]
- Maliehe, T.S.; Mavingo, N.; Selepe, T.N.; Masoko, P.; Mashao, F.M.; Nyamutswa, N. Quantitative Assessment of Human Health Risks Associated with Heavy Metal and Bacterial Pollution in Groundwater from Mankweng in Limpopo Province, South Africa. Int. J. Environ. Res. Public. Health 2024, 11, 1489. [Google Scholar] [CrossRef]
- Tajwar, M.; Rahman, M.; Shreya, S.S.; Sakib, N.; Gazi, M.Y.; Hasan, M.; Samm-A, A.; Zahid, A. Is the Groundwater of Dhaka City, Bangladesh Contaminated with Naturally Occurring Potential Toxic Elements? Front. Environ. Sci. 2025, 12, 1514154. [Google Scholar] [CrossRef]
- Shuaibu, A.; Kalin, R.M.; Phoenix, V.; Banda, L.C.; Lawal, I.M. Potentially Toxic Elements Source Identification and Associated Health Risks in the Groundwater of Transboundary Komadugu-Yobe Basin, Lake Chad Region: An Integrated Approach Using Chemometric Analysis and Index-Based Models. J. Hydrol. Reg. Stud. 2025, 59, 102439. [Google Scholar] [CrossRef]
- Silva-Gigante, M.; Hinojosa-Reyes, L.; Bazzan-Dessuy, M.; Rosas-Castor, J.M.; Torres-Gaytán, D.E.; Quero-Jiménez, P.C.; Caballero-Quintero, A.; Guzmán-Mar, J.L. Traces of the Past: Assessing the Impact of Potentially Toxic Elements from an Abandoned Mine on Groundwater and Agricultural Soil in San Luis Potosí, México. Environ. Monit. Assess. 2024, 196, 1015. [Google Scholar] [CrossRef]
- Narwal, N.; Katyal, D.; Malik, A. Evaluation of Contaminated Groundwater for Excessive Heavy Metal Presence and Its Further Assessment of the Potential Risk to Public Health. Water Environ. Res. 2024, 96, e11115. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.M.; Hossen, M.A.; Zuthi, M.F.R.; Mullick, M.R.A.; Hasan, S.M.F.; Khan, F.; Das, T. Exploration of Trace Elements in Groundwater and Associated Human Health Risk in Chattogram City of Bangladesh. Heliyon 2024, 10, e35738. [Google Scholar] [CrossRef]
- Ololade, I.A.; Apata, A.O.; Oladoja, N.A.; Oloyede, O.J.; Ololade, O.O.; Asanga, O.P.; Oloye, F.F. Occurrence, Seasonal Distribution and Probabilistic Source-Specific Health Risk Assessment of Dissolved Trace Metals in Southwestern Rivers, Nigeria. Sci. Total Environ. 2025, 963, 178342. [Google Scholar] [CrossRef]
- Shams, M.; Tavakkoli Nezhad, N.; Dehghan, A.; Alidadi, H.; Paydar, M.; Mohammadi, A.A.; Zarei, A. Heavy Metals Exposure, Carcinogenic and Non-Carcinogenic Human Health Risks Assessment of Groundwater around Mines in Joghatai, Iran. Int. J. Environ. Anal. Chem. 2022, 102, 1884–1899. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Van Nguyen, N.; Van Thai, N. Seasonal Assessment of Ecological and Human Health Risks of Trace Metals in the Saigon River Surface Water, Vietnam. Clean-Soil. Air Water 2023, 51, 2300042. [Google Scholar] [CrossRef]
- Subramaniyan, A.; Elango, L. Evaluating Health Risks from the Release of Trace Elements to Groundwater by Rock-Water Interaction in a Weathered Gneissic Aquifer. Environ. Sci. Pollut. Res. 2024, 31, 18962–18981. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, M.; Shi, Y.; Wu, Y.; Li, M.; Li, Q.; Zhao, Y.; Hua, J. Heavy Metal Pollution in Aquatic Systems of the Hunhe River Basin, China: Source Apportionment, Ecological and Health Risk Assessments. Process Saf. Environ. Prot. 2025, 201, 107478. [Google Scholar] [CrossRef]
- Ali, W.; Muhammad, S.; Tokatli, C.; Ahmad, A.; Luyuan, Z. Toxicological Risk Assessment and Sources of Heavy Metal(Loid)s Using Compositional Data Analysis, Astore River Basin, Himalaya. Mar. Pollut. Bull. 2025, 217, 118065. [Google Scholar] [CrossRef]
- Ncibi, K.; Hamed, Y.; Hadji, R.; Busico, G.; Benmarce, K.; Missaoui, R.; Wederni, K. Hydrogeochemical Characteristics and Health Risk Assessment of Potentially Toxic Elements in Groundwater and Their Relationship with the Ecosystem: Case Study in Tunisia. Environ. Sci. Pollut. Res. 2023, 30, 40031–40048. [Google Scholar] [CrossRef]
- Choudhury, T.R.; Moniruzzaman, M.; Anonna, T.A.; Asad, H.A.-; Samanta, P.; Islam, F. Evaluation of Heavy Metal Contamination in Soil, Water, and Fish in an Industrial Zone in Bangladesh: Ecological and Potential Health Risk. Reg. Stud. Mar. Sci. 2025, 86, 104162. [Google Scholar] [CrossRef]
- Triassi, M.; Cerino, P.; Montuori, P.; Pizzolante, A.; Trama, U.; Nicodemo, F.; D’Auria, J.L.; De Vita, S.; De Rosa, E.; Limone, A. Heavy Metals in Groundwater of Southern Italy: Occurrence and Potential Adverse Effects on the Environment and Human Health. Int. J. Environ. Res. Public. Health 2023, 20, 1693. [Google Scholar] [CrossRef] [PubMed]
- Bortey-Sam, N.; Nakayama, S.M.M.; Ikenaka, Y.; Akoto, O.; Baidoo, E.; Mizukawa, H.; Ishizuka, M. Health Risk Assessment of Heavy Metals and Metalloid in Drinking Water from Communities near Gold Mines in Tarkwa, Ghana. Environ. Monit. Assess. 2015, 187, 397. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, P.; Xu, Y.; Yuan, Y.; Hui, K.; Su, J.; Tan, W. Seasonal Variations in Health Risks Associated with Nitrates and Heavy Metals in Groundwater: A Case Study of Typical Regions along the Riverside Plain in China. Process Saf. Environ. Prot. 2025, 196, 106949. [Google Scholar] [CrossRef]
- Sharafi, S.; Salehi, F. Comprehensive Assessment of Heavy Metal (HMs) Contamination and Associated Health Risks in Agricultural Soils and Groundwater Proximal to Industrial Sites. Sci. Rep. 2025, 15, 7518. [Google Scholar] [CrossRef]
- Adewumi, A.J.; Laniyan, T.A. Contamination, Ecological, and Human Health Risks of Heavy Metals in Water from a Pb-Zn-F Mining Area, North Eastern Nigeria. J. Water Health 2023, 21, 1470–1488. [Google Scholar] [CrossRef]
- Fardullah, M.; Islam, M.S.; Sarkar, M.A.A.S.U.; Rahman, M.R.; Akther, K.; Hossain, M.T.; Robel, F.N. Heavy Metal Contamination and Risk Assessments in the Surface Water of Tanguar Haor, Bangladesh. Geosyst. Geoenviron. 2025, 4, 100399. [Google Scholar] [CrossRef]
- Sikakwe, G.U.; Nwachukwu, N.A.; Igwe, B.N. Detection of Human Health Risks Resulting from Contamination of Borehole Drinking Water with Harmful Volatile Organic Chemicals and Potentially Toxic Elements around Petroleum Products Dispensing Outlets. J. Hazard. Mater. 2025, 494, 138508. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, N.; Liu, Z.; Sun, Y.; Lu, N. Characteristics and Risk Assessment of Potentially Toxic Elements Pollution in River Water and Sediment in Typical Gold Mining Areas of Northwest China. Sci. Rep. 2024, 14, 12715. [Google Scholar] [CrossRef]
- Ihenetu, S.C.; Jude, C.A.; Nwadiogbu, J.O.; Enyoh, C.E. Groundwater Quality Assessment for Irrigation and Drinking Water: Health Risks from Heavy Metals and PAHs in the Niger Delta, Nigeria. Aqua Water Infrastruct. Ecosyst. Soc. 2024, 73, 2013–2029. [Google Scholar] [CrossRef]
- Mandal, R.R.; Raj, D. Distribution and Probabilistic Human Health and Ecological Risk Assessment of Potentially Toxic Elements in Water, Sediments and Aquatic Plants: A Comprehensive Study to Understand the Impacts of Active Coal Mine on the Lentic Ecosystem. Environ. Geochem. Health 2025, 47, 286. [Google Scholar] [CrossRef] [PubMed]
- Vesković, J.; Miletić, A.; Lučić, M.; Onjia, A. Appraisal of Contamination, Hydrogeochemistry, and Monte Carlo Simulation of Health Risks of Groundwater in a Lithium-Rich Ore Area. Env. Geochem. Health 2024, 46, 468. [Google Scholar] [CrossRef]
- Barzegar, R.; Asghari Moghaddam, A.; Adamowski, J.; Nazemi, A.H. Assessing the Potential Origins and Human Health Risks of Trace Elements in Groundwater: A Case Study in the Khoy Plain, Iran. Env. Geochem. Health 2019, 41, 981–1002. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, R.; Kuang, W.; Lin, C.; Sun, X.; Chen, J.; Pan, Z. Characterization, Source Identification, Risk Assessment of Potentially Toxic Elements (PTEs) in the Surface Water and Sediment of the Beibu Gulf, China. Mar. Pollut. Bull. 2023, 191, 114905. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Yang, S.; Zou, L.; Torres-Martínez, J.A.; Zheng, Y.; Hu, Q.; Zhang, Y. Appraisal of Potential Toxic Elements Pollution, Sources Apportionment, and Health Risks in Groundwater from a Coastal Area of SE China. J. Environ. Manag. 2025, 377, 124691. [Google Scholar] [CrossRef]
- Mohammadpour, A.; Emadi, Z.; Samaei, M.R.; Ravindra, K.; Hosseini, S.M.; Amin, M.; Samiei, M.; Mohammadi, L.; Khaksefidi, R.; Zarei, A.a.; et al. The Concentration of Potentially Toxic Elements (PTEs) in Drinking Water from Shiraz, Iran: A Health Risk Assessment of Samples. Environ. Sci. Pollut. Res. 2023, 30, 23295–23311. [Google Scholar] [CrossRef]
- Yang, S.; Xie, Z.; Wei, D.; Tao, L.; Chen, Q.; Uddin, M.G.; Wang, Y.; Wang, Y.; Zhang, Y. Quantitative Source Apportionment and Health Risk Assessment of Potentially Toxic Elements from the Surface Water and Groundwater in a Typical Coal-Mining Area. Phys. Chem. Earth 2025, 140, 104008. [Google Scholar] [CrossRef]
- Sarkar, A.; Karri, S.D. Human Health Risk Assessment of Selected Heavy Metals in Tanker Water: A Study from Kudlu, Bengaluru. Chin. J. Anal. Chem. 2023, 51, 100337. [Google Scholar] [CrossRef]
- Şener, Ş. Groundwater Quality, Heavy Metal Pollution, and Health Risk Assessment Using Geospatial Techniques and Index Methods in Eber Wetland and Surroundings (Afyonkarahisar/Turkey). Environ. Sci. Pollut. Res. 2023, 30, 51387–51411. [Google Scholar] [CrossRef]
- Vesković, J.; Bulatović, S.; Ražić, S.; Lučić, M.; Miletić, A.; Nastasović, A.; Onjia, A. Arsenic-contaminated Groundwater of the Western Banat (Pannonian Basin): Hydrogeochemical Appraisal, Pollution Source Apportionment, and Monte Carlo Simulation of Source-specific Health Risks. Water Environ. Res. 2024, 96, e11087. [Google Scholar] [CrossRef]
- Khan, Y.K.; Toqeer, M.; Shah, M.H. Characterization, Source Apportionment and Health Risk Assessment of Trace Metals in Groundwater of Metropolitan Area in Lahore, Pakistan. Expo. Health 2023, 15, 915–931. [Google Scholar] [CrossRef]
- Reghais, A.; Drouiche, A.; Zahi, F.; Ewuzie, U.; Debieche, T.-H.; Drias, T. Risk Assessment of Potentially Toxic Elements and Mapping of Groundwater Pollution Indices Using Soft Computer Models in an Agricultural Area, Northeast Algeria. J. Hazard. Mater. 2025, 491, 137991. [Google Scholar] [CrossRef] [PubMed]
- Sany, S.R.; Deb, S.R.; Ahmed, F.; Nayem, M.A.A.; Ashikuzzaman, A.K.M.; Numanbakth, M.A.A. Evaluation of Groundwater Quality and Potential Health Risks in the Tengratila Gas Field Blowout Region, Bangladesh: An in-Depth Analysis Utilizing Multivariate Statistics, Heavy Metal Indices and Monte Carlo Simulation. J. Hazard. Mater. 2025, 490, 137744. [Google Scholar] [CrossRef]
- Kapoor, K.; Kumar, S.; Vishwakarma, D.K.; Obaidullah, A.J.; Yadav, K.K. Health Risk Assessment of Heavy Metals in Groundwater Sources: Carcinogenic and Non-Carcinogenic Evaluation. J. Water Health 2024, 22, 1972–1987. [Google Scholar] [CrossRef]
- Turdiyeva, K.; Lee, W. Comparative Analysis and Human Health Risk Assessment of Contamination with Heavy Metals of Central Asian Rivers. Heliyon 2023, 9, e17112. [Google Scholar] [CrossRef]
- Vesković, J.; Deršek-Timotić, I.; Lučić, M.; Miletić, A.; Đolić, M.; Ražić, S.; Onjia, A. Entropy-Weighted Water Quality Index, Hydrogeochemistry, and Monte Carlo Simulation of Source-Specific Health Risks of Groundwater in the Morava River Plain (Serbia). Mar. Pollut. Bull. 2024, 201, 116277. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Meng, J.; Wang, S.; Xiao, S.; Wang, H.; Sun, X.; Wu, P. Source Apportionment, Source-Specific Health Risks, and Control Factors of Heavy Metals in Water Bodies of a Typical Karst Basin in Southwestern China. PLoS ONE 2024, 19, e0309142. [Google Scholar] [CrossRef]
- Jha, S.; Sinha, S.; Mahadevappa, P.; Hazra, S.; Sarkar, S. Assessing Water Quality and Human Health Risk near Coal Mines and Industrial Area of Singrauli, India: Special Emphasis on Toxic Elements. Environ. Geochem. Health 2024, 46, 449. [Google Scholar] [CrossRef]
- Sridhar, D.; Parimalarenganayaki, S. Evaluation of Human and Ecological Health Risks Associated with the Potentially Toxic Heavy Metals in Groundwater of Vellore City, Tamil Nadu, India. J. Hazard. Mater. Adv. 2024, 16, 100497. [Google Scholar] [CrossRef]
- Sun, L.; Liu, T.; Duan, L.; Tong, X.; Zhang, W.; Cui, H.; Wang, Z.; Zheng, G. Spatial and Temporal Distribution Characteristics and Risk Assessment of Heavy Metals in Groundwater of Pingshuo Mining Area. Environ. Geochem. Health 2024, 46, 141. [Google Scholar] [CrossRef]
- Kouassi, K.M.; Kinimo, K.C.; Yao, K.M.; Coulibaly, A.S. Water Physicochemical Characteristics and Health Risk Assessment of Trace Metals in River Mouths Along a Tropical Coastline of Gulf Guinea, West Africa. Chem. Afr. 2024, 7, 1497–1507. [Google Scholar] [CrossRef]
- Vesković, J.; Onjia, A. Identification of Priority Sources of Potentially Hazardous Elements from Public Drinking Water Fountains in Zaječar/East Serbia. J. Environ. Sci. 2026, 159, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Du, Y.; Li, Y.; Deng, Y.; Tao, Y.; Ma, T. Determination of High-Risk Factors and Related Spatially Influencing Variables of Heavy Metals in Groundwater. J. Environ. Manag. 2024, 358, 120853. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.; Liu, S.; Bi, Z.; Jiang, M.; Zhang, M.; Cai, H.; Wang, X. Risk Assessment of Heavy Metal Contamination in Air, Water, Soil, and Crops Adjacent to a Spent Lead-Acid Battery Storage Facility. J. Mater. Cycles Waste Manag. 2025, 27, 1557–1569. [Google Scholar] [CrossRef]
- Vig, N.; Ravindra, K.; Mor, S. Heavy Metal Pollution Assessment of Groundwater and Associated Health Risks around Coal Thermal Power Plant, Punjab, India. Int. J. Environ. Sci. Technol. 2023, 20, 6259–6274. [Google Scholar] [CrossRef]
- Gao, X.; Han, G.; Liu, J.; Zhang, S.; Zeng, J. Heavy Metal Accumulation and Source Apportionment in Urban River under Ecological Restoration: Relationships with Land Use and Risk Assessment Based on Monte Carlo Simulation. ACS Earth Space Chem. 2023, 7, 642–652. [Google Scholar] [CrossRef]
- Chowdhury, A.N.; Naher, S.; Likhon, M.N.A.; Hassan, J.; Fariha, Z.N.; Hasan, M.R.; Apon, T.D.; Bhuiyan, M.A.H.; Bhuiyan, M.M.U. Heavy Metal (Pb, Cd and Cr) Contamination and Human Health Risk Assessment of Groundwater in Kuakata, Southern Coastal Region of Bangladesh. Geosyst. Geoenviron. 2025, 4, 100325. [Google Scholar] [CrossRef]
- Vesković, J.; Sentić, M.; Onjia, A. Hydrogeochemical Facies and Health Hazards of Fluoride and Nitrate in Groundwater of a Lithium Ore Deposit Basin. Metals 2024, 14, 1062. [Google Scholar] [CrossRef]
- Peng, S.; Xiao, X.; Zou, H.; Yang, Z.; Ahmad, U.M.; Zhao, Y.; Chen, H.; Li, G.; Liu, G.; Duan, X.; et al. Levels, Origins and Probabilistic Health Risk Appraisal for Trace Elements in Drinking Water from Lhasa, Tibet. Environ. Geochem. Health 2023, 45, 3405–3421. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Wang, M.; Wang, M.; Ma, W.; Yang, S.; Zhang, F.; Jia, L.; Liu, T. Pronounced Transition of Heavy Metal Pollution Sources in Chinese Agricultural Surface Waters: The Rising Prominence of Non-Point Source Pollution. iScience 2025, 28, 112524. [Google Scholar] [CrossRef]
- Kumar, S.; Maurya, N.S. Analysis of Heavy Metal Contamination in Groundwater and Associated Probabilistic Human Health Risk Assessment Using Monte Carlo Simulation: A Case Study in Gaya, Bihar. J. Water Health 2025, 23, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, A.; Motamed-Jahromi, M.; Soleimani, H.; Dehbandi, R.; Doost, Z.E.; Samaei, M.R.; Derakhshan, Z.; Renella, G.; Mahvi, A.H. Trace Elements Human Health Risk Assessment by Monte Carlo Probabilistic Method in Drinking Water of Shiraz, Iran. Int. J. Environ. Sci. Technol. 2023, 20, 3775–3788. [Google Scholar] [CrossRef]



| Parameter | Abbreviation | Unit | Population Group | Probability Distribution | |
|---|---|---|---|---|---|
| Adults | Children | ||||
| Heavy metal(loid) concentration | C | mg/L | / | / | Lognormal |
| Ingestion rate | IR | L/day | 2 | 1 | Normal |
| Exposure frequency | EF | days/year | 365 | 365 | Triangular |
| Exposure duration | ED | years | 30 | 6 | Point |
| Body weight | BW | kg | 70 | 15 | Lognormal |
| Averaging time | AT | days | 10,950 nc | 2190 nc | Point |
| 25,550 c | 25,550 c | ||||
| Skin surface area | SA | cm2 | 18,000 | 6600 | Lognormal |
| Exposure time | ET | h/day | 0.58 | 1 | Lognormal |
| Conversion factor | CF | L/cm3 | 0.001 | Point | |
| Heavy Metal(loid) | Dermal Permeability Coefficient (Kp), cm/h | Reference Dose (RfD), mg/(kg × day) | Probability Distribution | |
|---|---|---|---|---|
| Ingestion Route | Dermal Route | |||
| Cd | 1.0 × 10−3 | 5.0 × 10−4 | 2.5 × 10−5 | Point |
| Cr(VI) | 2.0 × 10−3 | 3.0 × 10−3 | 7.5 × 10−5 | |
| Hg | 1.0 × 10−3 | 3.0 × 10−4 | 2.1 × 10−5 | |
| Pb | 1.0 × 10−4 | 3.5 × 10−3 | 4.2 × 10−4 | |
| Al | 1.0 × 10−3 | 1.0 × 100 | - | |
| Zn | 6.0 × 10−4 | 3.0 × 10−1 | 6.0 × 10−2 | |
| Cu | 1.0 × 10−3 | 4.0 × 10−2 | 1.2 × 10−2 | |
| Ni | 2.0 × 10−4 | 2.0 × 10−2 | 5.4 × 10−3 | |
| Sb | 1.0 × 10−3 | 4.0 × 10−4 | - | |
| V | 1.0 × 10−3 | 5.04 × 10−3 | - | |
| Mn | 1.0 × 10−3 | 1.40 × 10−1 | 9.6 × 10−4 | |
| Fe | 1.0 × 10−3 | 7.0 × 10−1 | 1.4 × 10−1 | |
| As | 1.0 × 10−3 | 3.0 × 10−4 | 1.23 × 10−4 | |
| Co | 4.0 × 10−4 | 3.0 × 10−4 | 1.60 × 10−2 | |
| Se | 1.0 × 10−3 | 5.0 × 10−3 | - | |
| Sr | 1.0 × 10−3 | 6.0 × 10−1 | - | |
| Ba | 1.0 × 10−3 | 2.0 × 10−1 | - | |
| Sn | 1.0 × 10−3 | 6.0 × 10−1 | - | |
| Mo | 1.0 × 10−3 | 5.0 × 10−3 | - | |
| Heavy Metal(loid) | Cancer Slope Factor (SF), (kg × day)/mg | Probability Distribution | |
|---|---|---|---|
| Ingestion Route | Dermal Route | ||
| Cr(VI) | 0.5 | 20 | Point |
| As | 1.5 | 3.66 | |
| Cd | 6.1 | 6.1 | |
| Pb | 8.5 × 10−3 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesković, J.; Onjia, A. Exposure and Toxicity Factors in Health Risk Assessment of Heavy Metal(loid)s in Water. Water 2025, 17, 2901. https://doi.org/10.3390/w17192901
Vesković J, Onjia A. Exposure and Toxicity Factors in Health Risk Assessment of Heavy Metal(loid)s in Water. Water. 2025; 17(19):2901. https://doi.org/10.3390/w17192901
Chicago/Turabian StyleVesković, Jelena, and Antonije Onjia. 2025. "Exposure and Toxicity Factors in Health Risk Assessment of Heavy Metal(loid)s in Water" Water 17, no. 19: 2901. https://doi.org/10.3390/w17192901
APA StyleVesković, J., & Onjia, A. (2025). Exposure and Toxicity Factors in Health Risk Assessment of Heavy Metal(loid)s in Water. Water, 17(19), 2901. https://doi.org/10.3390/w17192901







