Abstract
Mineral water content strongly depends on the geologic layer characteristics. Therefore, the aim of the present study is to make a comparison between two renowned mineral water sources in Romania, Borsec and Tusnad. Two public springs were selected from each location: Boldizsar (about 6600 L/day) and Lazar (about 500 L/day) from Borsec and Mikes (about 5000 L/day) and Young’s spring (about 600 L/day) from Tusnad. All investigated springs are naturally carbonated. Water properties were measured in situ and in laboratory for the collected samples; the results found that Borsec mineral water has a pH of about 7.5, while Tusnad mineral water is slightly acid (pH = 6.5). TDS strongly depends on the spring’s flow (for instance, Boldizsar has a TDS of about 900 mg/L, while Lazar has a TDS of about 1529 mg/L due to its high mineralization, while Young’s spring has a TDS of 165 mg/L due to its low mineralization, although it has low flow). Borsec mineral water has a lower salinity of about 1.22 PSU, while Tusnad water has a salinity of about 2 PSU, caused by a high amount of Na and Fe ions. Mineral waters dissolve ions from the geological layers, which react with carbonic acid during drying, generating specific crystallized compounds. The crystallized matter was investigated using XRD coupled with mineralogical optical microscopy (MOM); their microstructural features were observed using SEM coupled with elemental spectroscopy. Borsec water generates mainly Ca, Mg, and Na minerals like calcite, aragonite, pseudo-dolomite, natron, and traces of halite. Tusnad mineral waters have significant amounts of Ca, but also have Fe and much more Cl, since calcite and aragonite are mixed up with large amounts of halite and iron compounds. It looks like the presence of iron ions in the Tusnad mineral water collected from Mikes and Young’s spring explains the acidic pH. All these aspects are useful for further investigation regarding specific therapeutic purposes like chronic colitis and biliary lithiasis symptom amelioration (Boldizsar), chronic colitis, and enterocolitis symptoms (Lazar). Tusnad waters, like the water from Mikes spring, are recommended for anemia and neurasthenia, while Young’s spring is recommended for renal lithiasis amelioration.
1. Introduction
The deeper water deposit interaction with geological layers causes the dissolving of hydro-soluble minerals like carbonates or evaporites, generating mineral waters which also might contain rare earth elements [1,2]. The excessive presence of evaporitic deposits interacting with water generates brines with different concentrations depending on the local geologic conditions [3,4]. Mineral waters are marketed as bottled water, representing a wide share of the worldwide bottled water consumption. For instance, Parag et al. report that 443 billion liters of bottled water were consumed worldwide in 2020 according to Statista [5]. Following this path, we found that Statista reported the consumption of about 39.44 billion liters in China and 38.34 billion liters in United States of America, followed by Mexico with 31.15 billion liters of consumed bottled water [6]. The increased worldwide demand for mineral water and the huge amount bottled yearly raise a great interest regarding their mineralization and its complex implication in drinking behavior and possible therapeutic effects.
Novel approaches have appeared regarding the mineral content within mineral water and geothermal water that imply the extraction of a powder to be used further for specific purposes. For instance, the famous Onsen geothermal waters from Japan [7] are now transformed into a mineral powder by Le Furo Company and used as a powder for mineral baths [8]. One of our previous studies found a similar possibility for collecting mineral powder from Borsec (Romania) public springs through water evaporation, finding that it could be used in a similar manner for personalized baths [9].
Natural carbogaseous mineral waters are formed by the interaction of volcanic gaseous emissions, which interact with the mineral water formed in contact with the geological layers [10,11]. The rock fissures generated by tectonic movements facilitate the movement of deeper mineralized water and volcanic gases to rich upper layers, merging with other aquifers and increasing their mineral content and carbogaseous characteristics [10]. The literature data reveal that CO2, N2, and CH4 are the gases dissolved into the natural carbogaseous waters [11].
Romania has several locations with geothermal and/or mineral water, due to the geological conditions of the Carpathian Mountains and their sub mountain areas. Two locations, Borsec and Tusnad, are renowned for their natural carbogaseous waters for drinking and spa treatments. Both are situated in the Eastern Carpathians (Romania), with a distance of about 100 km. Borsec is situated on the eastern side of Giurgeului Mountain and Tusnad is situated on the western side of the Ciomadu Mare Mountain (a former volcano).
A geological survey found two main aquifers in the Borsec depression: the first occurs at 65 m depth and is formed on a compact dark dolomitic base, with the upper layers containing gray clay, shale, and sand layers; the second aquifer occurs at 60 m depth and is formed on a dolomitic limestone substrate, with the upper layers containing a green shale (chlorite) intercalation with sand and dolomitic limestone [12].
The literature data indicate that the Ciomadu Mare Mountain and Tusnad Bath geologies were investigated by Lazar and Arghiz in 1964 and by Screiber in 1972 [13,14]. The local volcanic activity in the Pliocene era had three or four massive eruptions which raised the local mountain peaks, especially Ciomadu Mare Mountain, causing large deposits of volcanic ash and andesitic lava. The later eruptions involved a more viscous andesitic lava, which intercalated within the sedimentary formations [14]. Unfortunately, there are no available public data regarding the aquifer situation, but several carbogaseous mineral water springs were captured by specific drillings and are publicly available at the Tusnad Bath touristic station. The diffuse emission of CO2 within the Ciomadu Mare Mountain cone’s respective Sf. Ana crater has a variable flux ranging from 2 to 90 g/m2 [15]. The literature indicates carbon dioxide sources from the endogeneous degradation of carbonaceous rocks [16] or from the magma gases trespassing through the tectonic regions and their subsequent fractures [17]. However, the gaseous emissions related to the Sf. Ana crater contain 97.69% CO2, followed by small quantities of nitrogen, methane, oxygen, argon, and helium [18]. These aspects are related to the carbogaseous behavior of the mineral waters for the public springs at the Tusnad Bath touristic station.
Carbonated rocks within sedimentary layers in both Borsec and Tusnad contain calcite and dolomite, having a water solubility of 0.013 g/L [19,20] and being able to release Ca2+ and Mg2+ ions. On the other hand, some investigations regarding dispersed particulate matter in the Sf. Ana volcanic lake revealed a significant amount of biotite and hornblende mixed with feldspar and muscovite [21]. Such clay minerals might be able to release iron ions when in contact with water through the reduction of Fe (III) within their structure via a complex mechanism [22]. In consequence, it is expected that the mineral water formed in the Tusnad Bath has an increased iron content compared to the Borsec mineral waters.
Considering the progress achieved by Le Furo Company with Onsen water, there remains a lack of information regarding the crystallization behavior of Borsec and Tusnad mineral waters. Therefore, the aim of the current research is to compare the crystallization processes of Borsec and Tusnad mineral waters.
Therefore, a comparison between some relevant springs from Borsec and Tusnad is of great interest to reveal their mineralization as a consequence of water evaporation. Since the spring flow influences the mineral concentration, it should be considered as the main parameter for selecting the springs. Therefore, the study should compare a high-flow spring from Borsec with a similar one from Tusnad, with the same comparison for a low-flow spring. Our study indicates that dissolved CO2 interacts directly with dissolved ions, facilitating the precipitation of crystallization germs, which further form crystalline powders [9]. Therefore, the physicochemical properties of the collected water samples must be measured, and their values must be correlated with the crystallization process. Mineralogical optical microscopy (MOM) effectuated in cross-polarized light allows for a proper view of each crystalline particle and ensures a good fit with the X-ray diffraction (XRD) results. The morphological aspects are optimally observed by Scanning Electron Microscopy (SEM) coupled with Energy Dispersive Spectroscopy (EDS), which allows the measuring of the elemental composition of certain microscopic sites and also allows the viewing of an elemental distribution map. Finally, the obtained microstructural data are useful for the identification and potential application of the obtained powder for further treatments.
2. Materials and Methods
2.1. Water Sample Collection and Storage
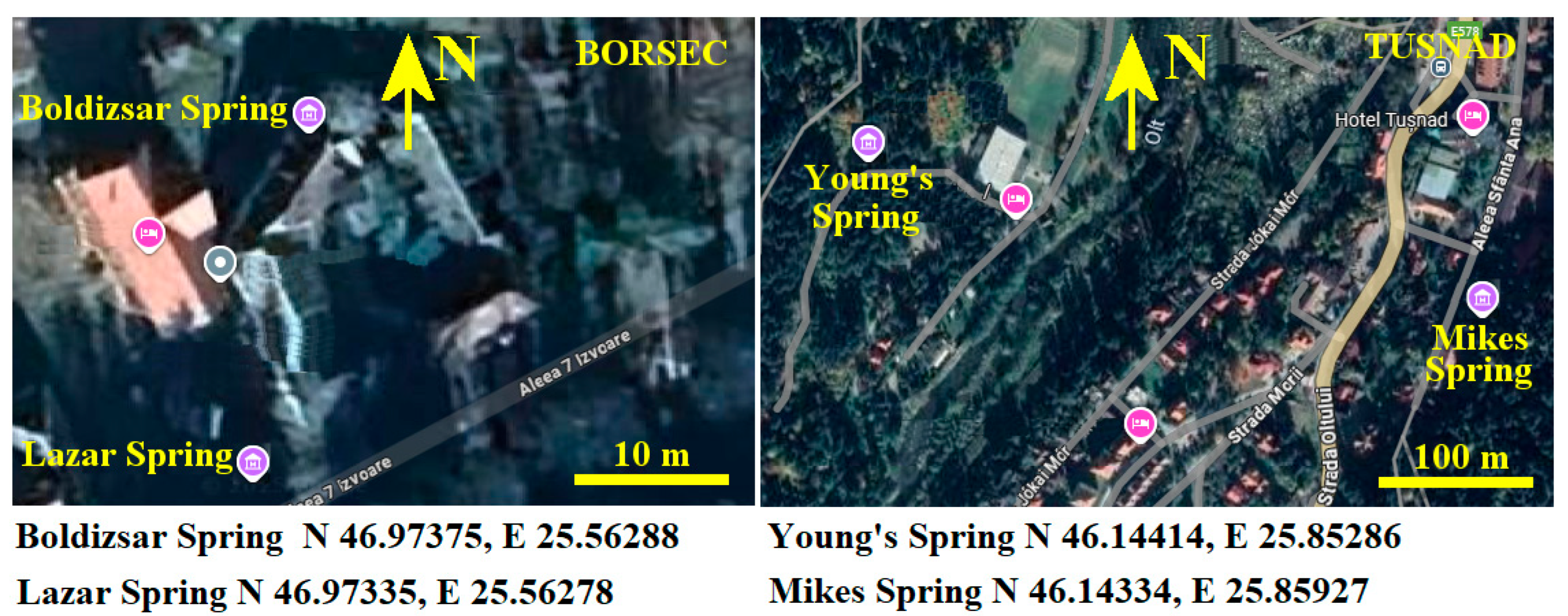
The selected springs, having a flow of about 5000–6000 L/day, are Boldizsar in Borsec and Mikes in Tusnad. The selected springs having a low flow (e.g., about 500 L/day) are Lazar in Borsec and Young’s in Tusnad. Their position is marked on the maps displayed in Figure 1.
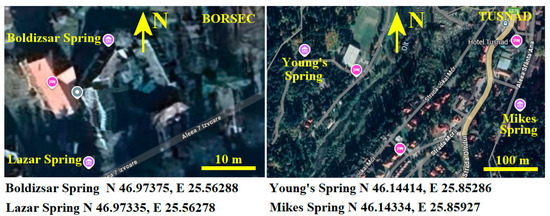
Figure 1.
Investigated spring positioning on Borsec and Tusnad maps [Google Maps 2025]; the precise GPS position of each spring is displayed below maps.
Each of the selected springs has displayed at its entrance the chemical composition determined by the National Mineral Water Company of Romania [23]; these values are centralized in Table 1. The specific values for Young’s spring were not made public, and therefore the specific values cannot be displayed in Table 1.

Table 1.
Spring mineral composition according to the public declaration displayed nearby spring.
The sampling campaign was held on 21–22 April 2025 starting in Borsec and finishing in Tusnad. The water samples were collected directly from the spring by filling glass vials of 1 L equipped with a metallic cap, being properly labeled with the sample’s name. Three individual samples were collected for each spring.
They were stored in a refrigerating container at 4 °C immediately after collection and transported to the laboratory where they were tested. The vials were gently brought to room temperature before measuring the water properties.
Glass slides for microscopy were prepared by deposing about three drops on the fresh surface of the lamella, followed by a primary inspection after 6 h to reveal the incipient stage of crystallization, followed by the final stage after 24 h when the water is completely evaporated and the crystallized matter is completely formed.
Larger amounts of water samples were placed in Petri dishes for longer-time crystallization in order to obtain thicker crystallized deposits, which were inspected microscopically before their erasing to obtain powder for XRD and SEM–EDX investigations.
2.2. Investigation Methods
Water physicochemical properties like pH, electrical conductivity, total dissolved solids (TDS), and salinity were measured with a HANNA HI 9829 multi parameter measuring device (Hanna Instruments Co., Bedfordshire, UK). Turbidity was measured with a nephelometric turbidimeter Hanna 93703 (Hanna Instruments Co., Bedfordshire, UK) with an accuracy of 0–1000 FTU (NTU) and a resolution of ±5% F.S. Turbidity was measured immediately after opening the sample’s vial; since the reading value was influenced by the CO2 bubbles, the measurement was repeated after complete degassing of the sample. Total chloride was measured using photo-colorimeter detection performed on an HI 96711 (Hanna Instruments Co., Bedfordshire, UK) device having a resolution of 0.01 mg/L. Each parameter was measured in triplicate (for each collected sample), and the mean values were calculated along with the standard deviation. The values were statistically analyzed by Anova, followed by a Tukey post hoc test, at a significance level a = 0.05 using Origin 6.0 software, which was used for the graphical representations (Microcal Co., Amherst, MA, USA).
Mineralogical optical microscopy (MOM) was effectuated under cross-polarized light inspection on a Laboval 2 microscope (Carl Zeiss, Oberkochen, Germany) having a digital image capture system with resolution of 10 MPx (Samsung Co., Hangul, Republic of Korea).
X-ray diffraction (XRD) was performed on a Bruker D8 Advance, produced by Bruker Company (Karlsruhe, Germany), diffractometer having a Bragg–Brentano system. It was operated using Cu kα monochromatic radiation (1.540562 Å) in the range of 10° to 80° at a speed of 1°/min. The XRD peak identification was effectuated with Match 1.0 software produced by the Crystal Impact Company (Bonn, Germany) using the PDF 2.0 database.
Scanning Electron Microscopy (SEM) was performed with a Hitachi SU8230 Scanning Electron Microscope (SEM) (Hitachi Co., Tokyo, Japan) operated in high vacuum mode at an acceleration voltage of 30 kV. The Energy Dispersive Spectroscopy (EDS) was performed with an X-Max 1160 detector (Oxford Instruments, Oxford, UK) installed on the SEM microscope. The sample surfaces were coated with a thin layer of Au for ensuring a proper electrical conduction; this component was subtracted from the elemental analysis.
3. Results
3.1. Water Sample Properties
The key role parameters of the water sample mineralization are the spring’s water flow and its dissolved mineral amounts. It was observed that the higher flux of fresh water infiltrating into the aquifer dilutes the dissolved mineral concentration, affecting the water properties. Thus, a spring with a high flow is supported by larger amounts of fresh-water infiltrations, and subsequently has a milder mineralization compared to the springs with a lower flow, such as our previous observation in Borsec regarding the Boldizsar and Lazar springs [9].
This supposition is assumed as a basic hypothesis for the current investigation. Therefore, electrical conductivity of the water samples is affected by their total dissolved solids (TDS) and salinity. Moreover, the total chloride amount is a quantitative measure of the dissolved halite (sodium chloride crystallized in cubic system). The mineral types and their dissolution influence the water samples’ pH values, indicating certain characteristics of the source area.
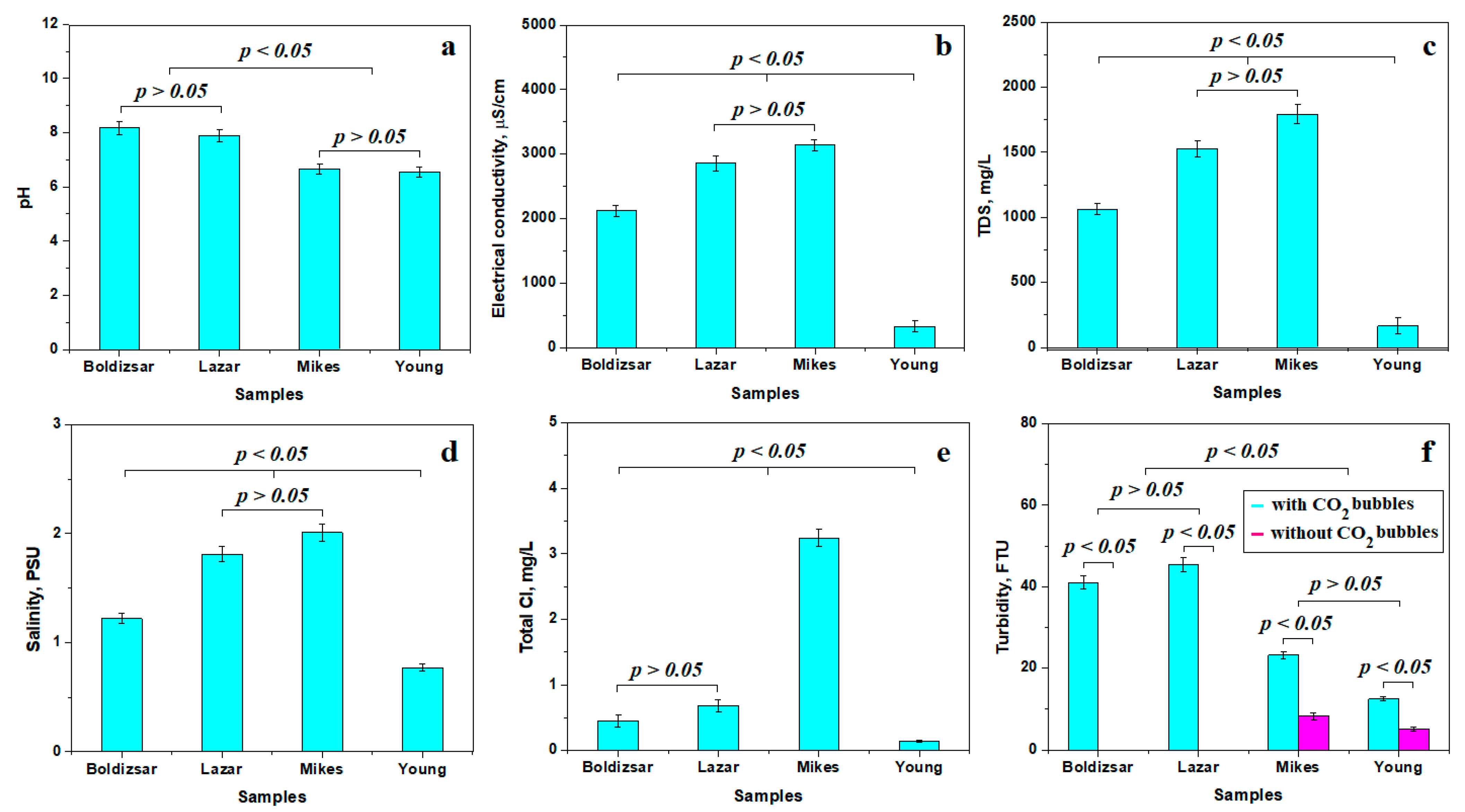
The variation in pH reveals two distinct statistical groups, as shown in Figure 2a. The first group is formed by the slightly alkaline waters collected from the Boldizsar and Lazar springs from Borsec. The second relevant statistical group is formed by the waters collected from Mikes and Young’s spring in Tusnad, having a slightly acidic pH. Although their values are very close to the neutral pH of 7, the observed differences are statistically representative. It should be further noted that Borsec and Tusnad waters have a different chemistry, induced by the specific dissolved minerals.
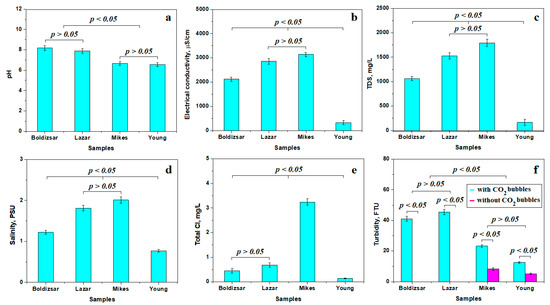
Figure 2.
Variation in the water properties mean values: (a) pH, (b) electrical conductivity, (c) total dissolved solids, (d) salinity, (e) total Cl, and (f) turbidity.
Electrical conductivity, Figure 2b, reveals very interesting values that form three different statistical groups. The first group is formed by the Boldizsar sample, having a mean electrical conductivity of 2121 μS/cm; this is an increased value compared to the regular drinking water, but it is still moderate compared to the second statistical group including the Lazar and Mikes samples. These water samples have a higher electrical conductivity of about 3000 μS/cm. This is a puzzling finding, since the Lazar spring has a low flow and the Mikes spring has a high flow. Finally, the third relevant statistical group formed by Young’s spring, with a lower electrical conductivity of about 329 μS/cm, is a more puzzling result. At first sight, it looks like the Tusnad waters have an opposite behavior to the Borsec waters regarding the mineralization behavior dependence on the spring’s flow. This mysterious reverted behavior could be explained by the other specific physicochemical property variations.
The total dissolved solids (TDS) is a quantitative value summing all dissolved minerals like carbonate rocks and evaporites. It cannot show the difference between dissolved matter, but clearly indicates the mineralization level of the investigated waters. Figure 2c reveals a moderate mineralization within the Boldizsar spring due to its high flow, making it a statistical relevant group. Lazar and Mikes springs have higher TDS values, indicating an increased mineralization in the second statistically relevant group. These high values are due to the low flow of the Lazar spring, but indicate a large amount of hydro-soluble salt dissolved in the Mikes spring regardless of its high flow. Young’s spring has the lowest TDS, indicating a low mineralization despite its reduced flow.
Salinity values, shown in Figure 2d, are complementary to TDS, revealing the presence of dissolved salts, including carbonates and halite, without an ability to make a difference. Still, three relevant statistical groups are evidenced for water salinity: the first one, having moderate salinity, consists of Boldizsar spring; the group having a high salinity contains Lazar and Mikes springs; and the third group, with a low salinity, contains Young’s spring.
The HCO3− anion influences the samples’ salinity by its interaction with other species, facilitating carbonate precipitation, but the lack of a proper measuring kit during this stage of research left us relying on the public declaration in Table 1.
The total chloride is strictly related to dissolved chlorides such as NaCl, since it is natural water which was not treated by chlorination. Thus, the explanation starts to be revealed in Figure 2e. The first statistical group contains water samples collected from Boldizsar and Lazar springs, having a moderate amount of Cl. The second relevant statistical group contains only the water collected from Mikes spring, which has a very high amount of chloride. On the other hand, the Young’s spring water has a very low chloride amount, forming the third relevant statistical group. Significant differences were observed between these three statistical groups, p < 0.05. This clearly indicates an uneven availability of halite deposits within Mikes and Young’s springs that might indicate their sourcing in different geological layers.
Gas bubbles within the carbogaseous waters scatter the incident light beam of the nephelometric sensor for measuring turbidity, increasing its value [24,25]. Therefore, the water tested from Borsec has an increased turbidity of about 40 FTU in its initial state and a turbidity below the detection limit after complete degassing, revealing a significant statistical difference between the initial and final state, p < 0.05, as shown in Figure 2f. Both the Boldizsar and Lazar springs feature similar values and behavior, and form a relevant statistical group. The Tusnad samples have a lower turbidity of about 15–20 FTU in their initial state, which decreases significantly after the complete removal of CO2 bubbles to about 5.15 to 8.24 FTU. The statistical difference between the initial and final state of turbidity is significant, p < 0.05, and also indicates that the remnant turbidity is generated by solid micro-dispersoids within both Mikes and Young’s waters. Both springs investigated from Tusnad have the same turbidity behavior, representing a distinct statistical group. The turbidity difference between Borsec and Tusnad is evidenced by the statistical analysis value p < 0.05. Finally, the solid dispersoids within Tusnad water might be responsible for the slightly acidic pH.
3.2. Mineral Crystallization Assessement
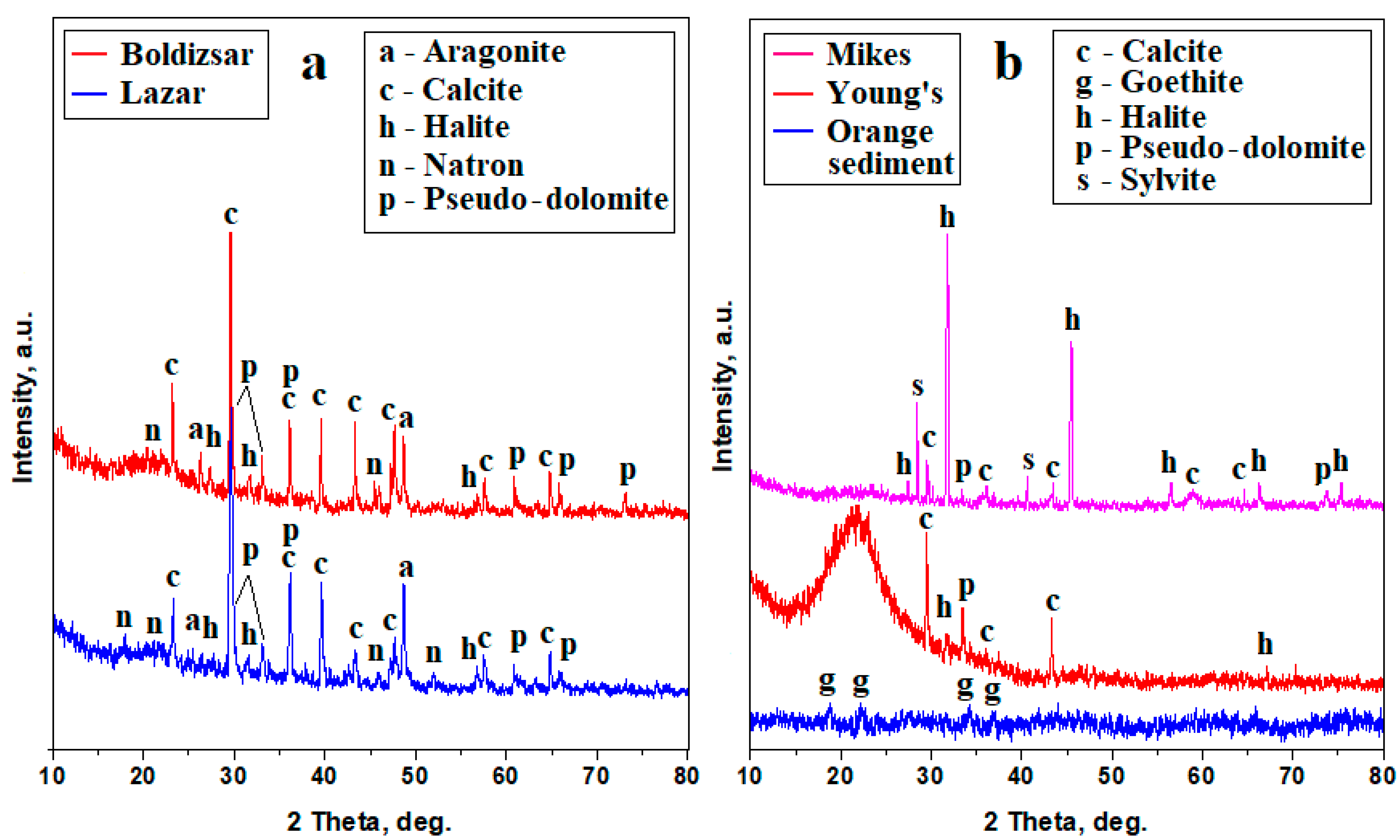
The crystallized powders obtained by natural drying of the water samples were subjected to an XRD investigation. The Borsec samples, shown in Figure 3a, reveal well-developed diffraction peaks with narrow aspects and intensities depending on the amount of each mineral corresponding to a high crystalline characteristic. The mineralization within both Borsec springs is dominated by calcite, pseudo-dolomite, and aragonite, while natron and halite have low intensities and appear as traces.
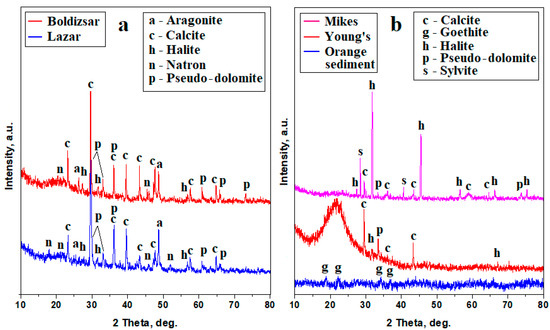
Figure 3.
XRD patterns for the powders obtained from mineral water samples: (a) Borsec and (b) Tusnad.
The Tusnad samples, shown in Figure 3b, also reveal well-developed peaks with narrow aspects and strong intensities corresponding to the crystallized minerals, but also have some nanocrystaline components, most likely related to the pre-existing dispersoids detected by the remnant turbidity. Thus, the Tusnad springs have a divergent dominance of minerals: the Mikes spring is dominated by halite, followed by sylvite, calcite, and pseudo-dolomite as accompanying minerals. On the other hand, Young’s spring is dominated by calcite as a main mineral, followed by pseudo-dolomite, and having only small traces of halite. A small amount of amorphous or nanocrystaline components influence the baseline of the Mikes XRD pattern in Figure 3b and significantly increased that of Young’s spring by forming of an amorphous bump ranging from about 18° to 22°. These nanocrystaline features are convergent with the observations for the orange sediment precipitated out of the Ciomadu Mare Mountain slope beside the Mikes spring location. Its XRD pattern shows broadened peaks of goethite, indicating the presence of a nanostructural iron hydroxide which corresponds to the amorphous–nanocrystaline features observed in the Mikes and Young’s springs. The iron hydroxide nanoparticles are about 50 nm according to the Scherrer formula, which was applied to the relevant XRD peaks.
The precise amount of each mineral formed by the water evaporation can be calculated with the Relative Intensity Ratio (RIR) method, which correlates the corundum factor of each identified mineral with the relative intensities of its specific diffraction peaks [26]. The RIR method was applied to the XRD patterns in Figure 3, and the obtained values are centralized in Table 2.

Table 2.
XRD data correlation with MOM observations.
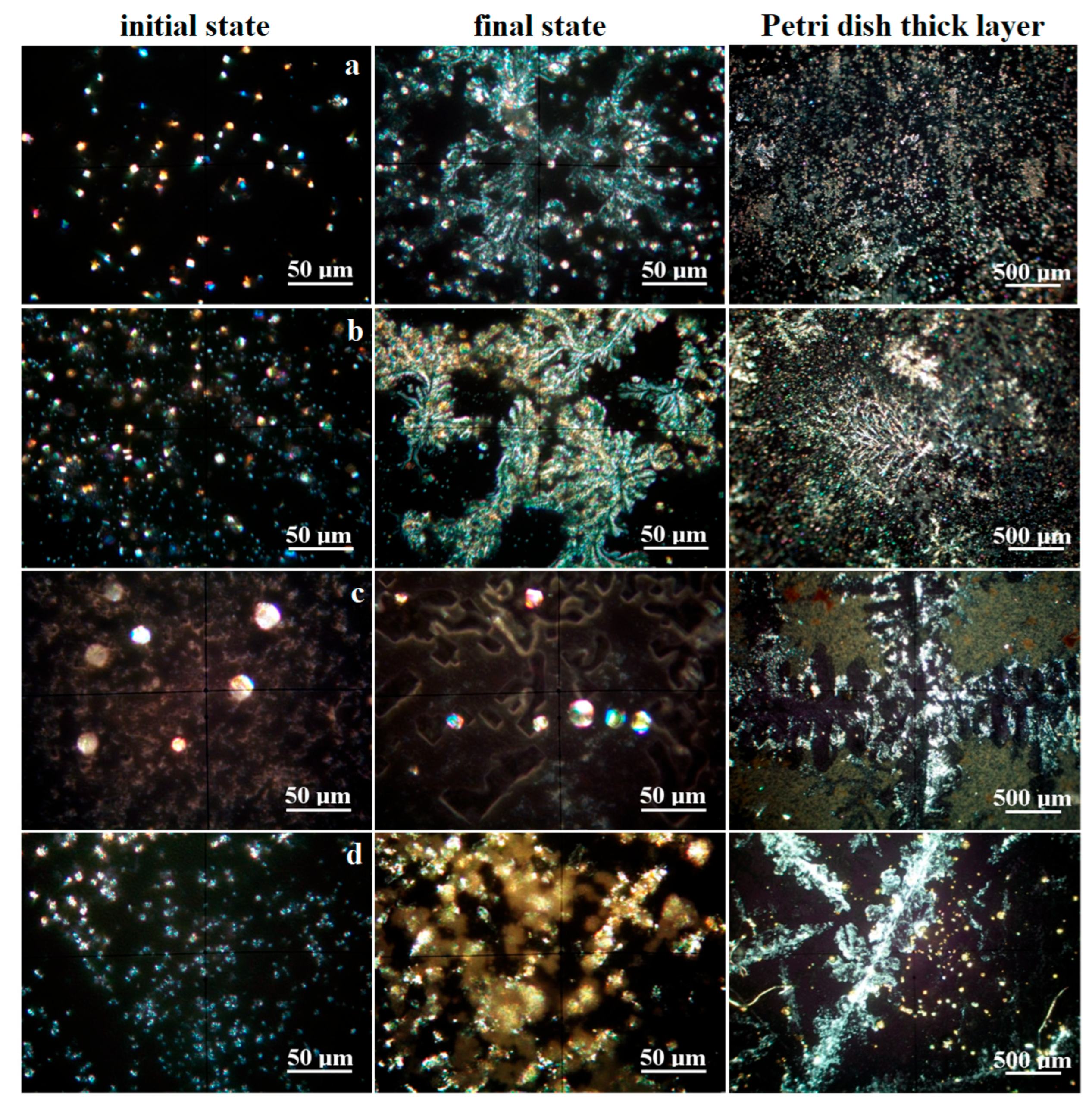
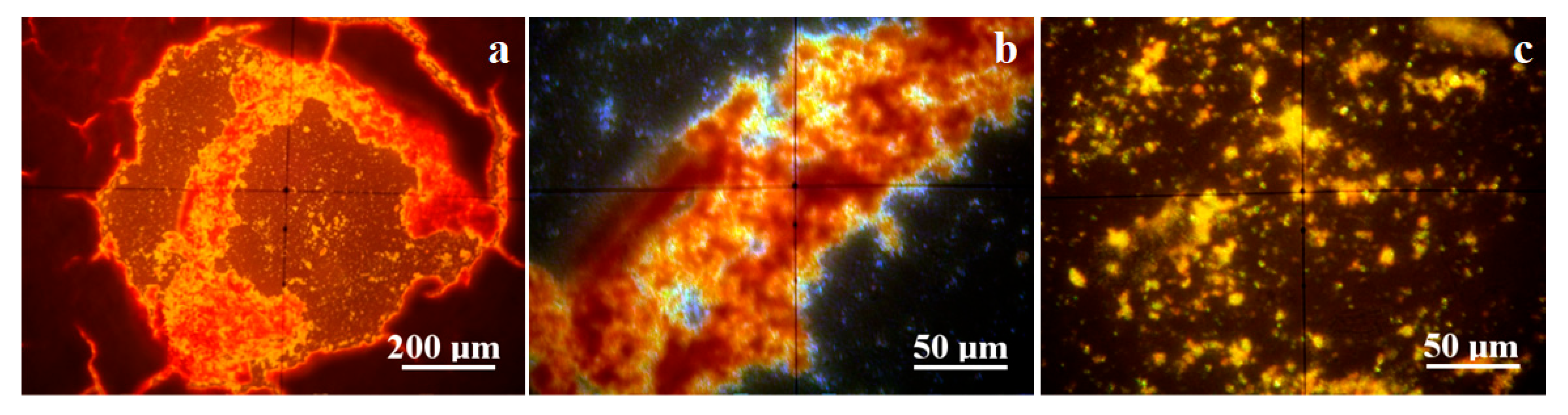
The crystallization process was monitored by mineralogical optical microscopy (MOM) under cross-polarized light, revealing the specific colors of each mineral. It was observed on the water drops spread onto glass slides. The initial crystallization state becomes visible after 12 h, revealing well-formed crystals which continue growing until the final stage at about 24 h. On the other hand, the minerals formed on the glass slide are not enough for the XRD investigation; therefore, the long-term crystallization of about 30 mL of water was conducted in Petri dishes for about 7 days. The obtained MOM images are presented in Figure 4. The size range of each mineral’s crystals was measured in the final stage of crystallization on the glass slide, and the values are centralized in Table 2.
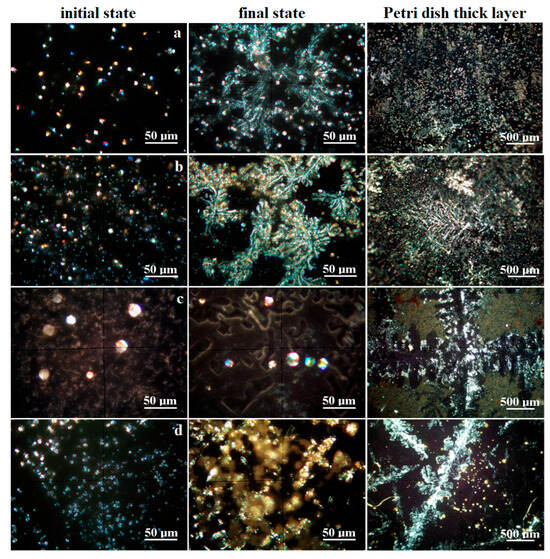
Figure 4.
Crystallization process evidenced by mineralogical optical microscopy for the investigated water samples: (a) Boldizsar, (b) Lazar, (c) Mikes, and (d) Young’s spring.
Boldizsar water has a turbidity below the detection limit; thus, the crystallization process occurs via homogeneous germination. CO2 reacts with dissolved ions within the water samples, causing the precipitation of carbonates, which grow as well-individualized particles of calcite (predominantly white, having pseudo-hexagonal shapes), aragonite (rhombic particles colored in blue–magenta depending on the face position of the optical axis of microscope), and pseudo-dolomite (predominantly yellow with granular aspect, having elongation tendency to columnar bodies), as shown in Figure 4a.
The limited solubility of carbonate into water facilitates its crystallization during the initial state when sodium chloride remains dissolved. The carbonate mineral crystallization ends when the concentration reaches its supersaturated level. The formed calcite, pseudo-dolomite, and aragonite crystals self-assemble into dendritic structures sustained by the remnant saline solution. Several prismatic yellow crystals of natron are observed as a consequence of the direct reaction between Na ions and CO2. The dissolved sodium chloride crystallizes at the end, due to its high hydro-solubility, and forms thin crusts that develop beside the carbonate dendrites, giving a blue hue over their sides as observed in the final stage in Figure 4a. The long-time crystallization in Petri dishes allows for the formation of thicker carbonate dendrite deposits embedded in the remnant halite.
The Lazar spring also has a turbidity below the detection limit, facilitating a homogeneous crystallization following the path described for the Boldizsar spring. Its early stage of crystallization is characterized by a denser precipitation of crystallization germs, leading to a larger number of well-individualized crystals of calcite, pseudo-dolomite, and aragonite, followed by traces of natron, shown in Figure 4b. The dissolved sodium chloride amount is too small for initializing crystals at this stage. The carbonate dendrites developed over the final stage are better-defined in the case of the Lazar spring because of its higher mineral content. Their sides also have a blue hue caused by the formation of halite crusts crystallized from the remnant liquid during the end stage. The better-defined shape of the carbonate dendrites superimpose the long-time crystallization pattern in the Petri dishes, being clearly visible and defined.
The Mikes spring from Tusnad Bath has a TDS and salinity similar to the Lazar spring in Borsec, forming a relevant statistical group in the matter of values. The difference is given by the increased level of chloride. The fact is sustained by the crystallization process, as shown in Figure 4c. The initial stage causes a rapid crystallization of calcite and pseudo-dolomite because of their limited hydro-solubility, which occurs on the heterogeneous germination grains favored by the remnant turbidity of the sample given by the solid dispersoids. The calcite crystals have a pseudo-hexagonal shape and small sizes, while the pseudo-dolomite crystals are sparse, with bigger sizes and a granular shape. These different crystallizations are caused by the higher salinity of the sample, which promotes carbonate clustering instead of dendritic disposal. This fact is further evidenced by the coalescence of calcite and pseudo-dolomite crystals in micro crystalline clusters, surrounded by the halite and sylvite crystals with well-individualized shapes as squares, rectangles, and less octahedral forms. The long-term crystallization promotes an even harder segregation of the halite/sylvite dendrites, embedding at their borders calcite and pseudo-dolomite crystals. Several brown clusters of about 50–100 μm are observed beside the other discussed crystals. These belong to the iron hydroxide nanoparticles.
Young’s spring, Figure 4d, has a prominent carbonated characteristic based on the white calcite and slightly white–yellow pseudo-dolomite crystals formed during the early stage. These are most likely promoted by heterogeneous germs consisting in micro-dispersoids related to the remnant turbidity. The carbonate minerals end their crystallization, since the supersaturated limit is achieved by the water evaporation, while sodium chloride remains dissolved. This influences the coalescence of carbonate crystals, which do not have enough mobility to form well-structured dendrites, facilitating the appearance of rounded micro-clusters of about 25–40 μm. The halite crystallizes as thin crusts at the end of the final stage, consisting of a pale blue hue beside the fine carbonate formations. The long-term crystallization in the Petri dish allows carbonate crystals such as calcite and pseudo-dolomite to re-organize into dendritic shapes, surrounded by the halite crusts. Some bright yellow spots are observed as distinct segregations. Their color is insensitive to their position in relation to optical axis of the microscope, proving their nanocrystaline nature. Their color indicates most likely a mixture of iron hydroxide with sulfur traces. Such an assumption requires further investigations. First of all, we found a fine orange sediment carried out from the Ciomadu Mare slope on the back of Mikes spring through a minor water flow (below 5 L/day). Its XRD pattern reveals merely an amorphous state, with some broadened peaks for goethite, shown in Figure 3b. The dried powder was inspected by MOM, as shown in Figure 5.

Figure 5.
Mineralogical optical microscopy aspect of the iron orange sediment: (a) overall aspect, (b) microstructural detail, and (c) individualized clusters of goethite nanoparticles.
The drying process led to a compact scale crust, shown in Figure 5a, having a uniform brown nuance specific to iron oxides and hydroxides. The excoriation of central scales allows for the observation of a thin layer of constituent particles still attached onto the glass slide. They have a fine granular matter colored brown–orange, indicating the massive presence of iron hydroxide (goethite). The higher-magnification image in Figure 5b clearly shows that in fact they are clusters of even more fine particles. Therefore, a re-dispersion in ultra-pure water, followed by a magnetic agitation for 30 min at 4000 rpm and adsorption onto a fresh glass slide, allows for a better dispersion of these clusters, as shown in Figure 5c. They feature a uniform orange nuance insensitive to their relative position to the optical axis of the microscope. This clearly indicates that the observed particles are fine clusters of goethite nanoparticles, which ensure the uniform color without maximums and extinctions. This fine mineral matter is insoluble in water [27], causing the observed remnant turbidity in Mikes and Young’s spring water samples and corresponding to the MOM observations in Figure 4c,d after long-time crystallization.
3.3. Microstructural Observation and Elemental Spectroscopy
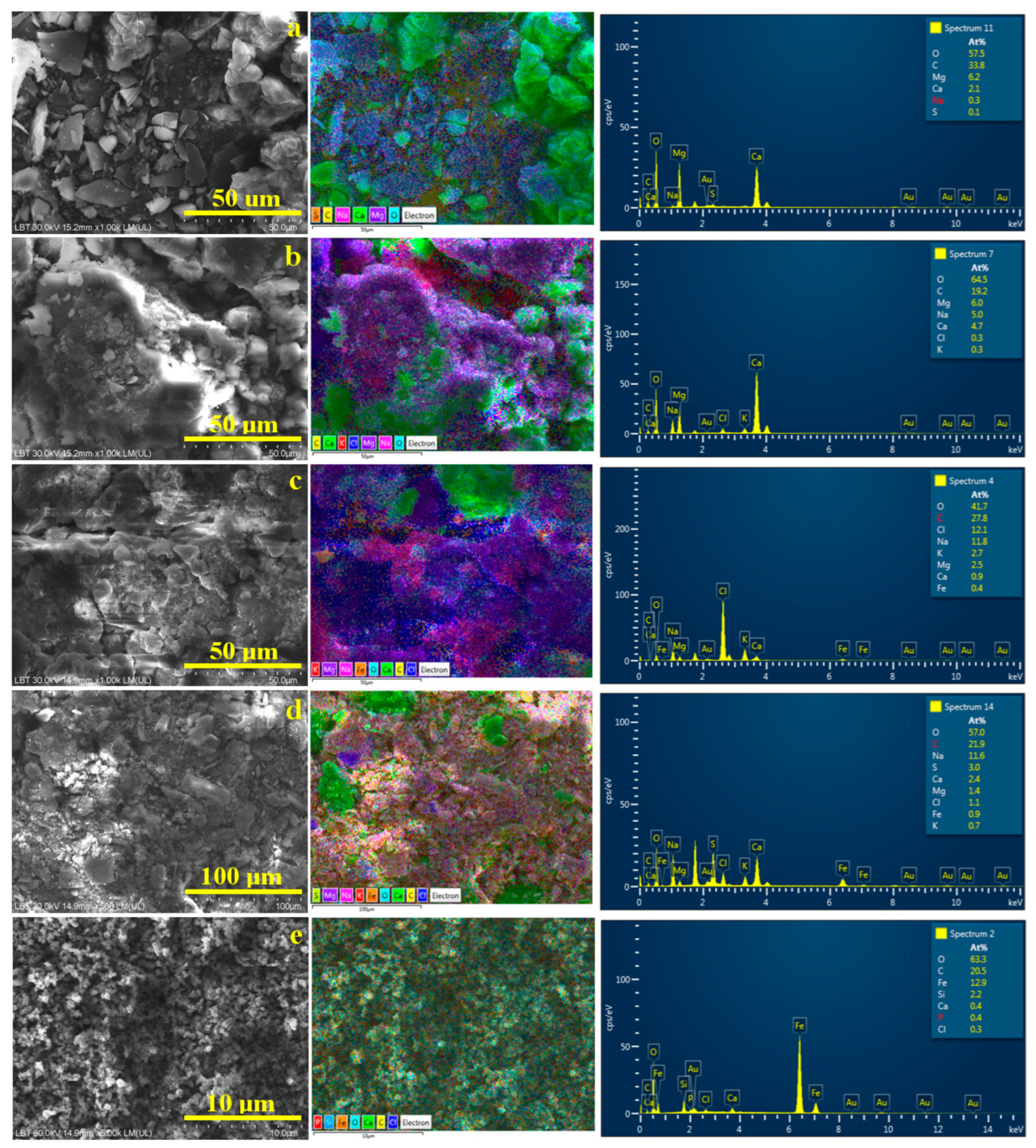
The SEM investigation of the powder samples, which were previously subjected to XRD, allows for the correlation of the morphology with the element distribution. Thus, the Mikes powder is predominantly granular and derived from the broken pseudo-hexagonal crystals of calcite, rhombic aragonite, and granular–elongated crystals of pseudo-dolomite, as shown in Figure 6a. The elemental map clearly shows the calcite and aragonite crystals in a pale green color (due to the green label of Ca, turquoise label of O, and yellow label of C). Pseudo-dolomite has a violet nuance because the Mg label and acicular natron slivers appear in pink nuances because of the Na label color. Minor traces of sulfur are observed as a segregation at the carbonate crystals borders. A halite crust was not detected in the observed micro-site for the Mikes sample; therefore, Cl is missing from the elemental composition.
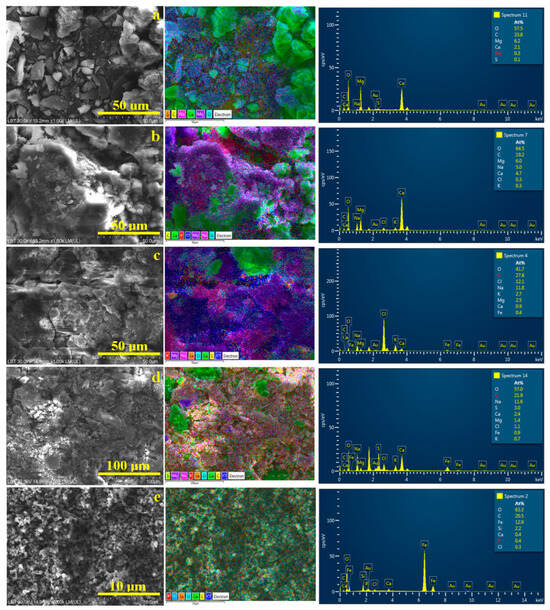
Figure 6.
SEM investigation of the powder crystallized from the water samples: (a) Boldizsar, (b) Lazar, (c) Mikes, (d) Young’s spring and (e) iron orange sediment. EDS elemental map and elemental spectrum are displayed on the right side of each SEM image.
The Lazar powder has a more crusty shape because of the slightly increased amount of halite which embeds well in the carbonate crystals having various shapes derived from the pseudo-hexagonal and rhombic habits of the crystals prior to fracturing. Thus, calcite and aragonite particles (green nuance) and pseudo-dolomite particles (violet nuance) are embedded throughout the halite crust, appearing in a dark blue nuance specific to the Cl label, shown in Figure 6b. Some local concentrations of natron particles are observed beside the calcite and pseudo-dolomite. The mineral disposal in the elemental map confirms the MOM observation made for the long-term crystallization deposits within the Lazar sample. Overall, the SEM investigation proves a congruent crystallization within the Borsec springs (e.g., Boldizsar and Lazar), respecting the initial assumption of the higher mineralization due to the lower flow of the Lazar spring.
XRD and MOM observations reveal a higher content of dissolved chlorides (e.g., halite and sylvite) in the Tusnad waters compared to the Borsec ones. Thus, the mineralization changes affect the sample’s microstructure. Figure 6c shows fragments of compact crusts formed by the synergistic crystallization of halite and sylvite (deep-blue alternating with reddish areas in the elemental map as consequence of Na and K labels). The formed crusts confirm the presence of evaporitic dendrites observed in Figure 4c after long-term crystallization. The carbonate crystals have a predominantly clustered shape induced by the high concentration of brine during water evaporation. Both the calcium carbonate crystals (green color) and pseudo-dolomite (violet color) are predominantly rounded, ranging from about 10 to 25 μm, in good agreement with the MOM observations. Fe deposits are slightly difficult to observe, partly because of their action as crystallization germs for carbonate minerals and the local clustering process of the remnant turbid particles. For instance, such clusters are visible on the upper left side the elemental map in Figure 6c, having an orange nuance induced by the Fe label.
Young’s spring has a lower flow, and thus it is expected to have a greater amount of dissolved minerals; but the results looks different. The SEM image in Figure 6d reveals a dense mixture of broken crystals and less evidence of embedding crusts. The larger formations (15–20 μm) have broken borders intersected at specific angles of pseudo-hexagonal crystallization, representing calcite particles (green nuance in elemental map), while the pseudo-dolomite particles are predominantly broken into fine fractions of about 10–15 μm, only a few being about 25 μm. The iron and sulfur combined labels give a light orange nuance in the elemental map, which is well dispersed among the broken pseudo-dolomite particles, indicating their action as heterogeneous germination seeds. Still, some small clusters of iron and sulfur are observed within the finest particles, having a light-orange nuance and sizes of about 5–10 μm, in good agreement with the MOM observations.
The elemental map in Figure 6d reveals some irregular deep-blue lines at the crystals’ borders corresponding to the halite crust traces formed during the final stage of crystallization. The elemental compositions of the investigated micro-sites in Figure 6 are displayed in Table 3. They are expressed in atomic percents for a better correlation with the minerals’ stoichiometric chemical formulas.

Table 3.
The elemental composition of the crystalline powders.
All samples are dominated by oxygen as the main element because of their oxide nature, followed by carbon due to the high amount of carbonate minerals. The chloride and potassium amounts are strongly related to evaporitic minerals like halite and sylvite. The investigated springs’ elemental composition comparisons reveal the dominance of carbonate minerals in the Boldizsar and Lazar springs, proving to be the main characteristic of the Borsec area. Tusnad waters are characterized by an increased amount of evaporitic minerals like halite and sylvite diminishing carbonate mineral amounts. Another specific footprint of the Tusnad waters proves to be iron dispersoids, which are completely lacking in the Borsec waters. Another elemental difference is observed between the Mikes and Young’s springs, mainly regarding the Cal and Cl amount, which suggests a mineralization source from different aquifers affected by different geological layers.
Finally, the iron dispersoids observed in the orange sediment were analyzed using SEM, as shown in Figure 6d. They are formed by very small clusters with a rounded shape and sizes ranging from 125 to 300 nm, surrounding fine micro particles of about 1–3 μm. The elemental map reveals that these micro particles are SiO2 (having a pale blue nuance induced by the specific labels of Si and O) and calcite particles with a pale green nuance. These are too few (perhaps below 1% of the total weight of the sample), becoming undetectable by the XRD investigation. The nano-clusters are made of iron, tightly surrounding the other mineral particles within the sediment. The traces of Cl and P most likely occur from some intercalation of volcanic rock particles, along with the detected SiO2 exuded from the mountain slope by the weak water flow bringing them to the surface. These sample characteristics are important because both Mikes and Young’s spring feature such dispersoids in their waters, being a characteristic of the local mineralization.
4. Discussion
The initial assumption that a spring’s flow is directly responsible for the water mineralization is partly rejected based on the obtained results. At first sight, a slower flow of the spring would keep an increased mineralization, while a high flow supported by larger infiltrations of top water would decrease the mineralization grade, a fact already observed in our previous study regarding the Borsec public springs [9]. Thus, the Boldizsar and Lazar spring behavior sustains this assumption, but it is contradicted by the Tusnad springs having the opposite behavior. Young’s spring, having a slower flow, has a lower mineralization than the Mikes spring, which has a high flow. Moreover, this contradiction is highlighted by the Lazar and Mikes springs forming statistically relevant groups for electrical conductivity, TDS, and salinity in Figure 2b–d.
The first clue resolving the contradiction is given by the total Cl amount, which forms two distinct relevant groups for the Tusnad springs: Mikes represents the highest level and Young’s spring the lowest level, while the Borsec springs form another statistically relevant group with a mild Cl amount. This indicates the different mineralization within Tusnad compared to Borsec springs.
XRD coupled with MOM gives a complete overview regarding all samples’ mineralization, observations confirmed by SEM coupled with EDS elemental analysis. It was found that there is a complex behavior of mineral crystallization as a consequence of water evaporation. First of all, CO2 dissolved in water samples generates carbonic acid, which reacts with the dissolved mineral ions, especially with Ca, Mg, and Na, causing the precipitation of CaCO3 crystallized as calcite and aragonite, along with magnesian calcite CaMg(CaCO3)2, while sodium carbonate precipitates and crystallizes as natron [28,29]. Magnesian calcite has the same chemical formula as dolomite, but the crystal structure cannot be replicated by a crystallization from an aqueous solution because of the incapacity of ensuring successively ordered crystal planes of Mg and Ca within the crystal lattice [30,31]. Both Ca and Mg atoms take random places within the magnesian calcite lattice, named suggestive pseudo-dolomite [31,32]. Tsuchiya et al. report the successful crystallization of dolomite from concentrated brine by a slow injection of fine CO2 bubbles [33]. It looks like simply degassing through CO2 bubbles within our samples is not enough to ensure the proper dolomitization of Ca and Mg ions. We found that carbonates precipitate and grow in the first stage of crystallization due to their limited aqueous solubility.
Evaporitic minerals like halite and sylvite have a high aqueous solubility, and therefore the supersaturated solution forms only at the final stage of crystallization [34,35]. We found that low amounts of halite have a limited influence on the carbonate crystallization, being limited to crust formation beside the calcite–pseudo-dolomite dendrites during the final stage of crystallization (Boldizsar, Lazar, and Young’s spring). Higher amounts of evaporites (e.g., halite and sylvite) have a greater influence on the carbonate crystallization, causing their clusterization perhaps due to the increased superficial tension caused by the considerably greater density of the solution. Thus, the Tusnad water samples are subjected to different crystallization conditions compared to the Borsec samples.
Another major difference is given by the remnant turbidity observed in the Mikes and Young’s springs, which proves to be the fine dispersion of nanostructural iron hydroxide (e.g., goethite), partly accompanied with sulfur traces. These solid nanoparticles act as heterogeneous crystallization germs, promoting the growth of carbonate minerals. The literature data show that calcium carbonate crusts are involved with goethite in the corrosion of mild steel pipes at pH = 8.8, a fact favored by the increased temperature of geothermal water [36]. Some of our previous observations reveal nano iron hydroxide recrystallization in a ferrous conglomerate, with calcite occurring in a carwash slurry [37]. Other research reports the sequential precipitation of goethite–calcite nano-composites mediated by Ca(OH)2 [38]; the solution pH depends on the calcium hydroxide concentration. Overall, the literature data sustain the goethite affinity for calcite being able to act as heterogeneous germination centers.
Papp and Nitoi reveal that the Tusnad mineralization of the geothermal and mineral water within the area is caused by an intense underground circulation of water through the Neogene fault system, occurring in magmatites [39]. The observation is in good agreement with the other literature data [13,14]. These magmatites are represented by andesite deposits in close contact with the underground water. Andesite is known for its rich content in plagioclase, pyroxenes, and hornblende [40,41], explaining the rich iron content of water from Tusnad. Since hornblende is a complex mineral with the conventional formula (Ca,Na)2−3(Mg,Fe,Al)5(Al,Si)8O22(OH,F)2, this explains the presence of almost all identified dissolved elements within the Mikes and Young’s springs. Their reaction with carbonic acid induced by CO2 explains the crystallization of the carbonate minerals without pre-existing sedimentary rocks like dolomite substrate, as in Borsec. The higher amount of chloride found in the Mikes spring and significant level in Young’s spring would require water contact with some evaporitic sedimentary deposit interlocked with magmatic bursts. Unfortunately, we assume that this is most likely possible, but we did not find any reference to confirm this assumption.
Overall, the results showed that the crystallization process within the Borsec water is dominated by the carbonate mineral formation started in the first stage; thus, it is classified as carbonated mineral water. The saline characteristics of the Tusnad water strongly influence the carbonate minerals’ crystallization during the first stage and dominate the end stage of crystallization. Nanostructured iron hydroxide particles are another special characteristic of the Tusnad mineralization. Thus, the Mikes spring can be categorized as salted mineral water, while Young’s spring can be categorized as iron-carbonated mineral water. Of course, each of the spring’s source water can be used for its specific balneotherapy. For instance, carbonated mineral waters are recommended for adjusting stomach hyper acidity [42]. These are also efficient for balneology, relieving rheumatic pains by a proper adjustment of the temperature and adapting the cure parameters to the patient’s needs [43,44].
Saline water can be useful for ingestion cures prescribed by therapists for adjusting symptoms related to colitis and acute enterocolitis affection because of the antibacterial effects of saline solutions [45,46]. On the other hand, these waters have a lot of balneologic benefits, alleviating the symptoms of various skin diseases such as fungal infection [47], atopic dermatitis, and psoriasis [48].
Drinking water with a significant iron content is useful for the amelioration of anemia, especially for infants [49]. This fact is confirmed by data from the other literature [50,51]. Thus, Tusnad mineral water proves to contain enough nanostructural iron to be effective in anemia cures under strict medical observation.
The investigated springs have a continuous flow, which is publicly available to anyone without charge. Anyone can enter into the spring’s room and fill a bottle with mineral water. The water which is not taken by the visitors and the bath’s patients is discharged into the adjacent rivers. The increased mineral content of the spilled water affects the mineral equilibrium of the surface water, which might increase its hardness. What if this mineral water had been collected in a buffer tank and subjected to assisted evaporation? This would result in mineral powders like the ones obtained in this research:
- -
- Boldizsar spring: calcite, pseudo-dolomite, aragonite, halite, and natron;
- -
- Lazar spring: calcite, aragonite, pseudo-dolomite, halite, and natron;
- -
- Mikes spring: halite, sylvite, calcite, pseudo-dolomite, and iron nano-dispersoids;
- -
- Young’s spring: calcite, pseudo–dolomite, halite, and iron nano-dispersoids.
Following the example of the Le Furo application of the Onsen mineral waters, our obtained mineral powders might be further used for home cures or balneology treatments under direct medical prescription, having therapeutic benefits and local ecological enhancements by avoiding mineral amounts being spilled in the surface waters. This is a challenge for further research.
The specific parameters depend on each spring’s flow and the degree of mineralization, which could depend on the seasonality and precipitation water infiltration. This is a limitation of the current investigation that will be addressed in the next investigations, which will be focused on each season—spring, summer, autumn, and winter—revealing their influence on crystallization behavior. Also, the iron content influence on the pH value is an implicit function revealed by the measured values, which will be further investigated in regard to the seasonality chemistry of the considered springs.
5. Conclusions
The crystallization process is driven by the formation of carbonate minerals (calcite, aragonite, and pseudo-dolomite) in the early stage, followed by their growth and arrangement into dendritic structures until the supersaturated solution is obtained and the crystallization of evaporitic minerals (e.g., halite and sylvite).
The Boldizsar and Lazar springs in Borsec are predominantly carbonated because of their source on a dolomitic substrate.
The Mikes spring in Tusnad has a high content of evaporitic minerals like halite and sylvite, imposing a salted characteristic. Young’s spring in Tusnad is rich in its mineralization from other geological layers, having less halite, causing a more carbonated characteristic. Both of the investigated Tusnad springs contain iron hydroxide nano-dispersoids, imposing a ferric characteristic.
The Borsec waters form Boldizsar and Lazar springs are predominantly carbonated and are useful for alleviating gastric hyper acidity, while also having balneologic benefits, alleviating rheumatic symptoms.
Tusnad waters could be effective in anemia treatments because of their amount of iron nano-dispersoids. Young’s spring might be a good candidate for this purpose because of its low halite content and moderate carbonates. The high content of halite and sylvite within the Mikes spring make it a good candidate for balenologic purposes for alleviating skin affection symptoms.
The obtained mineral powders are ready to be re-dissolved at a patient’s home for personalized cures under strict medical prescription, or could be used as dietary supplements for adjusting electrolytic balances on hot days.
Author Contributions
Conceptualization, S.E.A. and I.P.; methodology, S.E.A. and I.P.; software, G.B.; validation, S.E.A. and I.P.; formal analysis, I.P.; investigation, S.E.A., L.B.T., G.B., and I.P.; resources, S.E.A.; data curation, I.P.; writing—original draft preparation, S.E.A. and I.P.; writing—review and editing, I.P.; visualization, L.B.T., and I.P.; supervision, S.E.A. and I.P.; project administration, S.E.A. and I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article.
Acknowledgments
The authors gratefully acknowledge the XRD diffractometer maintenance which was supported by the Ministry of Research, Innovation, and Digitization through Program 1-Development of the National Research and Development System, Subprogram 1.2-Institutional Performance-Funding Projects for Excellence in RDI, Contract No. 37PFE/30.12.2021.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EDS | Energy Dispersive Spectroscopy |
| MOM | Mineralogical Optical Microscopy |
| RIR | Relative Intensity Ratio |
| SEM | Scanning Electron Microscopy |
| TDS | Total Dissolved Solids |
| XRD | X-ray Diffraction |
References
- Li, C.; Shan, X.; Li, C.; Hao, S.; Cheng, B.; Lu, C.; Zhao, J.; Wang, X.; Su, Z. Analysis of the Occurrence Conditions and Formation Mechanism of Mineral Water in the Southern Region of Yaoquan Mountain, Wudalianchi. Water 2024, 16, 3130. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Wang, P.; Feng, C.; Cao, S.; Li, J. Unraveling the geochemical behaviors of rare earth elements (REEs) in Chinese drinking natural mineral waters: Environmental and health perspectives. J. Hazard. Mater. 2025, 494, 138731. [Google Scholar] [CrossRef] [PubMed]
- Stiller, M.; Yechieli, Y.; Gavrieli, I. Rates of halite dissolution in natural brines: Dead Sea solutions as a case study. Chem. Geol. 2016, 447, 161–172. [Google Scholar] [CrossRef]
- Bai, H.; Pan, T.; Han, G.; Fan, Q.; Miao, Q.; Bu, H. Hydrochemical Characteristics and Genesis of Sand–Gravel Brine Deposits in the Mahai Basin of the Northern Qinghai–Tibetan Plateau. Water 2024, 16, 3562. [Google Scholar] [CrossRef]
- Parag, Y.; Elimelech, E.; Opher, T. Bottled Water: An Evidence-Based Overview of Economic Viability, Environmental Impact, and Social Equity. Sustainability 2023, 15, 9760. [Google Scholar] [CrossRef]
- Ridder, Consumption Volume of Bottled Water Worldwide 2020, by Leading Countries, Statista, 26 June 2025. Available online: https://www.statista.com/statistics/1307883/consumption-of-bottled-water-worldwide-in-2009/?srsltid=AfmBOorZx9WuHBSGSNXNhDqHqQL2CzCVuNyE742p2ZS1R2klahWaJPW8 (accessed on 14 August 2025).
- Serbulea, M.; Payyappallimana, U. Onsen (hot springs) in Japan—Transforming terrain into healing landscapes. Health Place 2012, 18, 1366–1373. [Google Scholar] [CrossRef]
- Le Furo, Mineral Mist Bath. Available online: https://aquaignis.jp/en/spa_lefuro.php (accessed on 15 August 2025).
- Avram, S.E.; Platon, D.V.; Tudoran, L.B.; Borodi, G.; Petean, I. Mineral Powder Extraction by the Natural Drying of Water from the Public Springs in Borsec. Appl. Sci. 2024, 14, 10806. [Google Scholar] [CrossRef]
- Dewandel, B.; Alazard, M.; Lachassagne, P.; Bailly-Comte, V.; Couëffé, R.; Grataloup, S.; Ladouche, B.; Lanini, S.; Maréchal, J.C.; Wyns, R. Respective roles of the weathering profile and the tectonic fractures in the structure and functioning of crystalline thermo-mineral carbo-gaseous aquifers. J. Hydrol. 2017, 547, 690–707. [Google Scholar] [CrossRef]
- Dupuy, M.; Garel, E.; Chatton, E.; Labasque, T.; Mattei, A.; Santoni, S.; Vergnaud, V.; Aquilina, L.; Huneau, F. Using natural gas content of groundwater to improve the understanding of complex thermo-mineral spring systems. J. Hydrol. 2024, 634, 130956. [Google Scholar] [CrossRef]
- Pricajan, A. Mineral and Thermal Water from Romania (in Romanian: Apele Minerale Si Termale Din Romania); Technical Publishing House: Bucharest, Romania, 1972; 296p. [Google Scholar]
- Gal, E.E. Tusnad Baths Touristic Station and its Surroundings—Touristic Potential and Development Perspectives. Bachelor’s Thesis, Faculty of Economic, Law and Administrative Sciences, Petru Maior University, Targu Mures, Romania, 2008. (In Romanian). [Google Scholar]
- Schreiber, W.E. Geographical classification and genesis of massive Ciomadu. Studia Univ. Babes-Bolyai Geogr. 1972, 1, 15–22. [Google Scholar]
- Frunzeti, N.; Baciu, C. Diffuse CO2 Emission at Sfânta Ana Lake-Filled Crater (Eastern Carpathians, Romania). Procedia Environ. Sci. 2012, 14, 188–194. [Google Scholar] [CrossRef][Green Version]
- Keller, C.K.; Bacon, D.H. Soil respiration and georespiration distinguished by transport analyses of vadose CO2, 13CO2 and 14CO2, Glob. Biogeochem. Cycles 1998, 12, 361–372. [Google Scholar] [CrossRef]
- Giggenbach, W.F.; Sano, Y.; Wakita, H. Isotopic composition of helium and CO2 and CH4 contents in gases produced along the New Zealand part of a convergent plate boundary. Geochim. Cosmochim. Acta 1993, 57, 3427–3455. [Google Scholar] [CrossRef]
- Vaselli, O.; Minissale, A.; Tassi, F.; Magro, G.; Seghedi, I.; Ioane, D.; Szakacs, A. A geochemical traverse across the Eastern Carpathians (Romania): Constraints on the origin and evolution of the mineral water and gas discharges. Chem. Geol. 2002, 182, 637–654. [Google Scholar] [CrossRef]
- Yang, M.; Tan, L.; Batchelor-McAuley, C.; Compton, R.G. The solubility product controls the rate of calcite dissolution in pure water and seawater. Chem. Sci. 2024, 15, 2464–2472. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, Y.; Wang, G.; Li, T.; Liu, F.; Wei, S.; Yan, X.; Gan, H.; Zhang, W. Genesis Mechanisms of Geothermal Resources in Mangkang Geothermal Field, Tibet, China: Evidence from Hydrochemical Characteristics of Geothermal Water. Water 2022, 14, 4041. [Google Scholar] [CrossRef]
- Campean, R.F.; Petean, I.; Baraian, M.; Hosu-Prack, A.G.; Ristoiu, D.; Arghir, G. Mineral particulate matter from the St. ana lake sand related to the water suspensions. Carpathian J. Earth Environ. Sci. 2012, 7, 57–66. [Google Scholar]
- Liu, Y.; Ma, T.; Chen, J.; Peng, Z.; Xiao, C.; Qiu, W.; Liu, R.; Du, Y. Impact of clayey sediment compaction on pore water evolution and the release of iron. Appl. Geochem. 2023, 152, 105635. [Google Scholar] [CrossRef]
- Borsec Public Springs Composition Declaration Displayed on the Entrance Front, National Mineral Water Company of Romania. Available online: https://www.snam.ro/ (accessed on 21 February 2024).
- Hsieh, Z.-H.; Fan, C.-H.; Tu, H.-H.; Hu, C.-V.; Li, M.L.; Yeh, C.-K. Acoustic vortex induced bubble cage: An on-demand light guide in turbid media. Ultrasonics 2025, 156, 107761. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.H.; Choe, J.H.; Kim, Y.J. Removal of deposits and improvement of shelf life in CO2 rich mineral water by ozone microbubbles. Qimica Nova 2025, 48, e-20250045. [Google Scholar] [CrossRef]
- Avram, S.E.; Birle, B.V.; Tudoran, L.B.; Borodi, G.; Petean, I. Investigation of Used Water Sediments from Ceramic Tile Fabrication. Water 2024, 16, 1027. [Google Scholar] [CrossRef]
- Villalobos, M.; Cruz-Valladares, A.X. Towards Building a Unified Adsorption Model for Goethite Based on Variable Crystal Face Contributions: III Carbonate Adsorption. Colloids Interfaces 2025, 9, 51. [Google Scholar] [CrossRef]
- Nie, X.; Wang, Z.; Wan, J.; Wang, G.; Li, Y.; Ouyang, S. Competition between homogeneous and heterogeneous crystallization of CaCO3 during water softening. Water Res. 2024, 250, 121061. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Mendes, C.E.; de Melo, L.G.T.C.; Wang, J.; Santos, R.M. Production of Sodium Bicarbonate with Saline Brine and CO2 Co-Utilization: Comparing Modified Solvay Approaches. Crystals 2023, 13, 470. [Google Scholar] [CrossRef]
- Gregg, J.M.; Bish, D.L.; Kaczmarek, S.E.; Machel, H.G. Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: A review. Sedimentology 2015, 62, 1749–1769. [Google Scholar] [CrossRef]
- Land, L.S. Failure to precipitate dolomite at 25 °C from dilute solution despite 1000-fold oversaturation after 32 years. Aquat. Geochem. 1998, 4, 361–368. [Google Scholar] [CrossRef]
- Pimentel, C.; Pina, C.M. Reaction pathways towards the formation of dolomite-analogues at ambient conditions. Geochim. Cosmochim. Acta 2016, 178, 259–267. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Wada, Y.; Hiaki, T.; Onoe, K.; Matsumoto, M. Effects of CO2 fine bubble injection on reactive crystallization of dolomite from concentrated brine. J. Cryst. Growth 2017, 469, 36–41. [Google Scholar] [CrossRef]
- Chruszcz-Lipska, K.; Zelek-Pogudz, S.; Solecka, U.; Solecki, M.L.; Szostak, E.; Zborowski, K.K.; Zając, M. Use of the Far Infrared Spectroscopy for NaCl and KCl Minerals Characterization—A Case Study of Halides from Kłodawa in Poland. Minerals 2022, 12, 1561. [Google Scholar] [CrossRef]
- Petean, I.; Arghir, G.; Câmpean, R.F.; Bărăian, M.; Hosu Prack, A.G. Crystalographyc relations applied to homogeneous cristalization of badenian salt. Acta Tech. Napoc. Ser. Appl. Mech. Math. 2011, 54, 193–200. [Google Scholar]
- Maior, I.; Badea, G.E.; Stănășel, O.D.; Sebeșan, M.; Cojocaru, A.; Petrehele, A.I.G.; Creț, P.; Blidar, C.F. Chemical Composition and Corrosion—Contributions to a Sustainable Use of Geothermal Water. Energies 2025, 18, 3634. [Google Scholar] [CrossRef]
- Avram, S.E.; Filip, M.R.; Barbu Tudoran, L.; Borodi, G.; Petean, I. Investigation of ferrous conglomerate particles found in carwash slurry and their environmental implications. Stud. UBB Chem. 2023, 68, 57–70. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Renard, F.; Chiriac, R.; Findling, N.; Ghanbaja, J.; Toche, F. Sequential precipitation of a new goethite–calcite nanocomposite and its possible application in the removal of toxic ions from polluted water. Chem. Eng. J. 2013, 214, 139–148. [Google Scholar] [CrossRef]
- Papp, D.C.; Niţoi, E. Isotopic composition and origin of mineral and geothermal waters from Tuşnad Băi Spa, Harghita Mountains, Romania. J. Geochem. Explor. 2006, 89, 314–317. [Google Scholar] [CrossRef]
- Kao, N.-C.; Wang, M.-K.; Chiang, P.-N.; Chang, S.-S. Characterization of wheat-rice-stone developed from porphyritic hornblende andesite. Appl. Clay Sci. 2003, 23, 337–346. [Google Scholar] [CrossRef]
- Topaz, A.; Golan, T.; Boneh, Y. Natural fabrics of a hornblende-rich amphibolite: Implications for hornblende crystallographic preferred orientation and seismic anisotropy of the lower crust. Tectonophysics 2023, 865, 230036. [Google Scholar] [CrossRef]
- Linc, R.; Pantea, E.; Șerban, E.; Ciurba, A.-P.; Serban, G. Hydrochemical and Microbiological Investigations and the Therapeutic Potential of Some Mineral Waters from Bihor County, Romania. Sustainability 2023, 15, 15640. [Google Scholar] [CrossRef]
- Romay-Barrero, H.; Herrero-López, J.; Llorente-González, J.A.; Melgar Del Corral, G.; Palomo-Carrión, R.; Martínez-Galán, I. Balneotherapy and health-related quality of life in individuals with Rheumatoid arthritis: An observational study under real clinical practice conditions. Balneo PRM Res. J. 2022, 13, 527. [Google Scholar] [CrossRef]
- Verhagen, A.P.; Bierma-Zeinstra, S.M.; Boers, M.; Cardoso, J.R.; Lambeck, J.; Bie, R.; Vet, H.C. Balneotherapy (or spa therapy) for rheumatoid arthritis. Cochrane Database Syst. Rev. 2015, 11, CD000518. [Google Scholar] [CrossRef]
- Dickson-Gomez, J.; Nyabigambo, A.; Rudd, A.; Ssentongo, J.; Kiconco, A.; Mayega, R.W. Water, Sanitation, and Hygiene Challenges in Informal Settlements in Kampala, Uganda: A Qualitative Study. Int. J. Environ. Res. Public Health 2023, 20, 6181. [Google Scholar] [CrossRef]
- Wangchuk, P.; Yeshi, K.; Ugyen, K.; Dorji, J.; Wangdi, K.; Samten; Tshering, P.; Nugraha, A.S. Water-Based Therapies of Bhutan: Current Practices and the Recorded Clinical Evidence of Balneotherapy. Water 2021, 13, 9. [Google Scholar] [CrossRef]
- Huang, A.; Seité, S.; Adar, T. The use of balneotherapy in dermatology, Clin. Dermatol. 2018, 36, 363–368. [Google Scholar] [CrossRef]
- Milanković, V.; Djuriš, J.; Tubić, A.; Agbaba, J.; Forkapić, S.; Lukić, M. Assessing the safety of thermal mineral water for cosmetic applications: An integrated approach using physicochemical, cheminformatics, and bioinformatics techniques. RSC Adv. 2025, 15, 17755–17775. [Google Scholar] [CrossRef]
- Choudhury, N.; Siddiqua, T.J.; Ahmed, S.M.T.; Haque, M.A.; Ali, M.; Dil Farzana, F.; Naz, F.; Rahman, S.S.; Faruque, A.S.G.; Rahman, S.; et al. Iron content of drinking water is associated with anaemia status among children in high groundwater iron areas in Bangladesh. Trop. Med. Int. Health 2022, 27, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Kenmogne-Tchidjo, J.F.; Vollmer, S. Iron-fortified water: A new approach for reducing iron deficiency anemia in resource-constrained settings. Sci. Rep. 2023, 13, 13565. [Google Scholar] [CrossRef]
- Dutra-de-Oliveira, J.E.; Lamounier, J.A.; de Almeida, C.N.A.; Marchini, J.S. Fortification of Drinking Water to Control Iron- Deficiency Anemia in Preschool Children. Food Nutr. Bull. 2007, 28, 173–180. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).