Integrated Coagulation–Disinfection Using Aluminium Polychloride and Sodium Hypochlorite for Secondary Wastewater Treatment: Operational Advantages and DBP Mitigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Secondary Effluent Wastewater Quality
2.2. Analytical Methods
2.3. Experimental Procedures
2.3.1. Bench Scale Testing
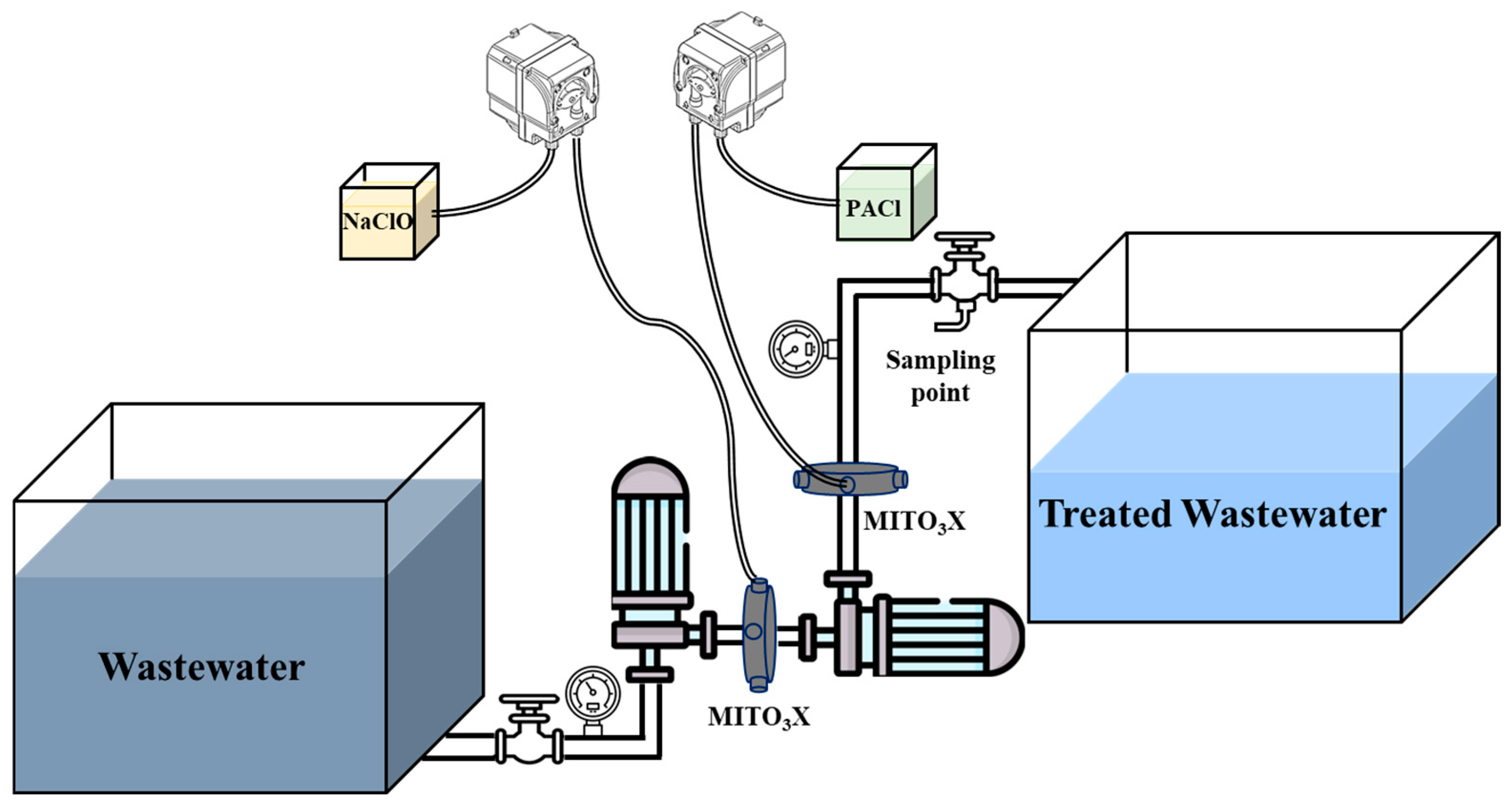
2.3.2. Pilot Scale Testing
3. Results
3.1. Phosphorus Removal
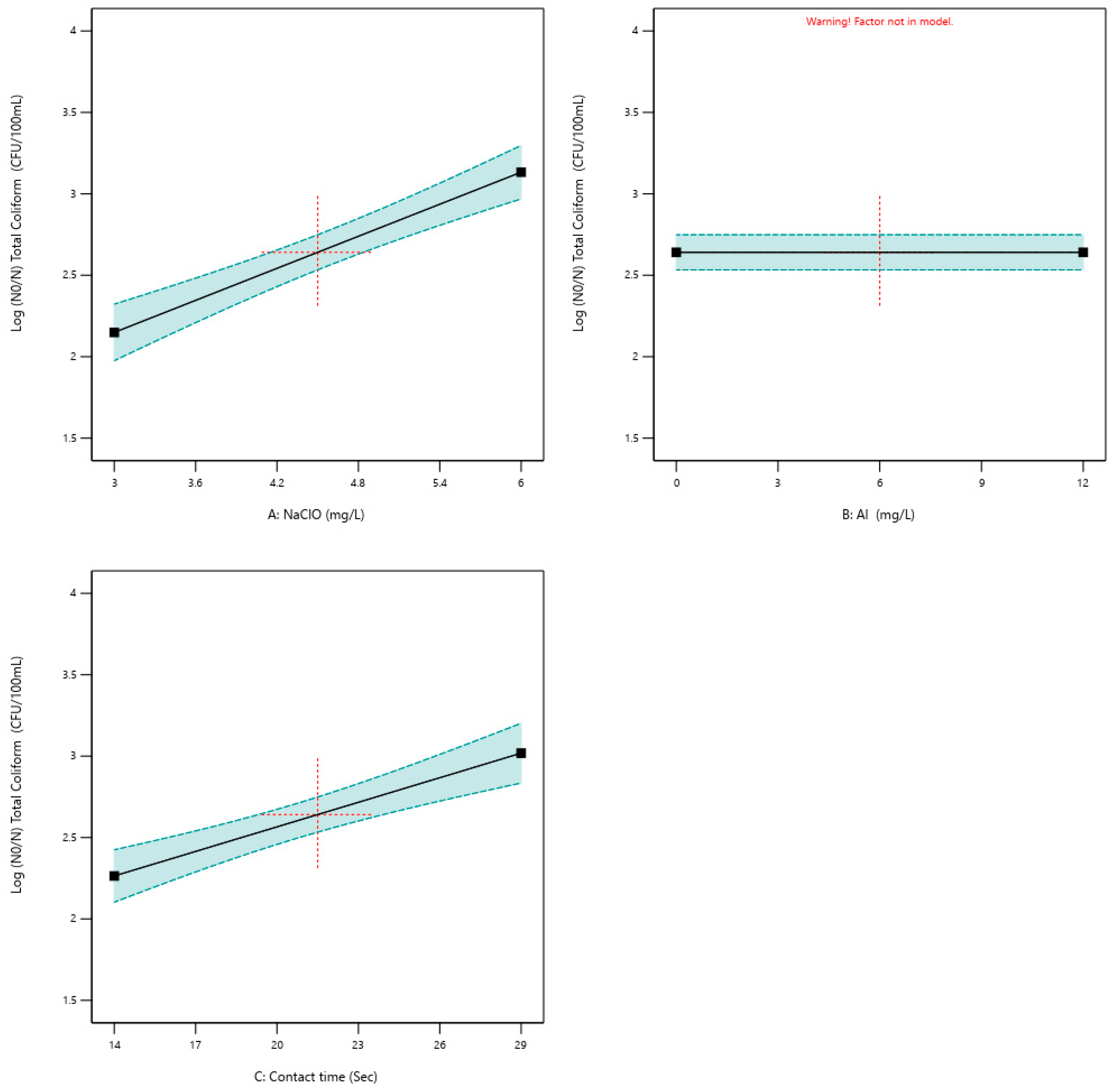
3.2. Microbial Inactivation
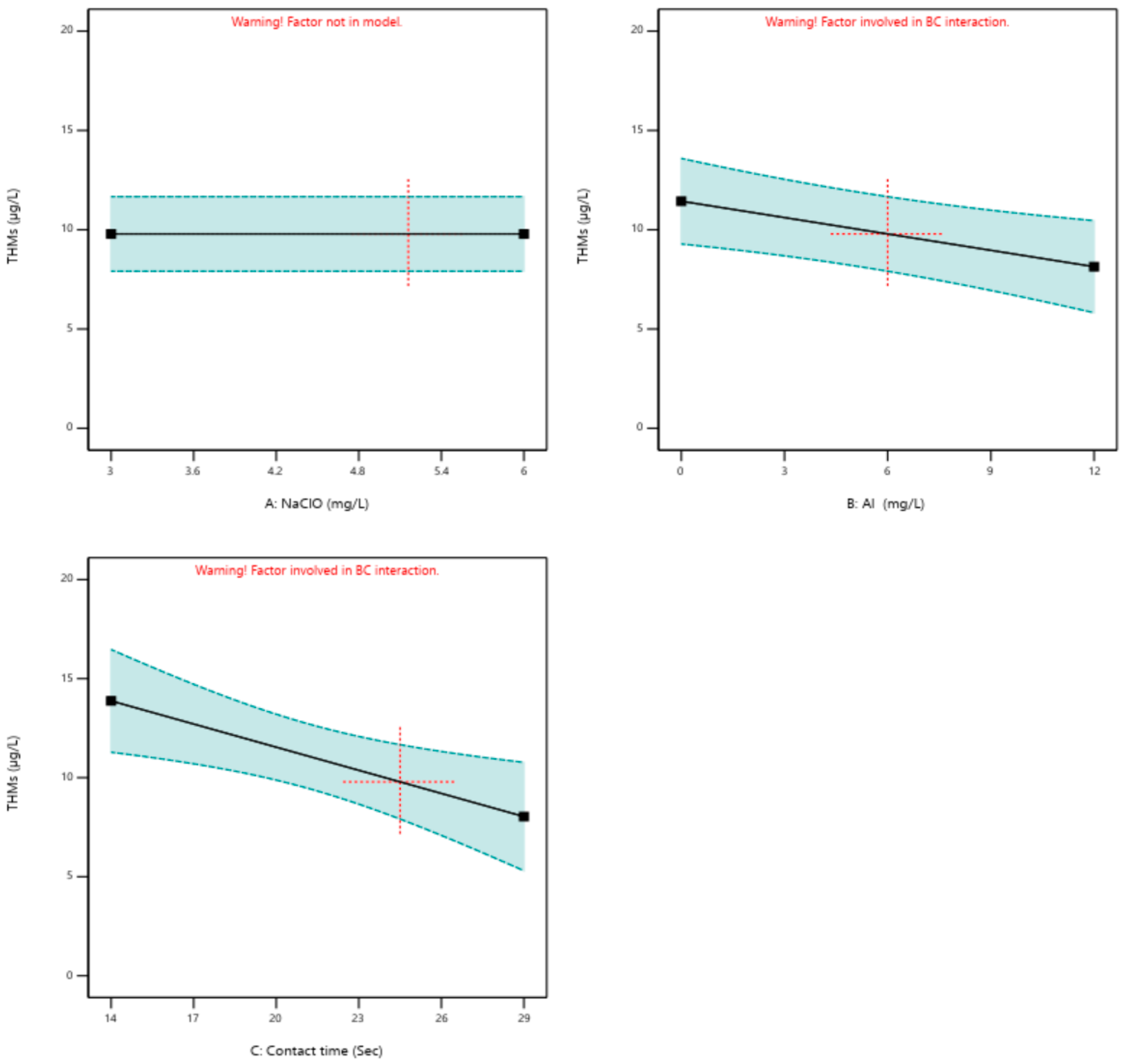
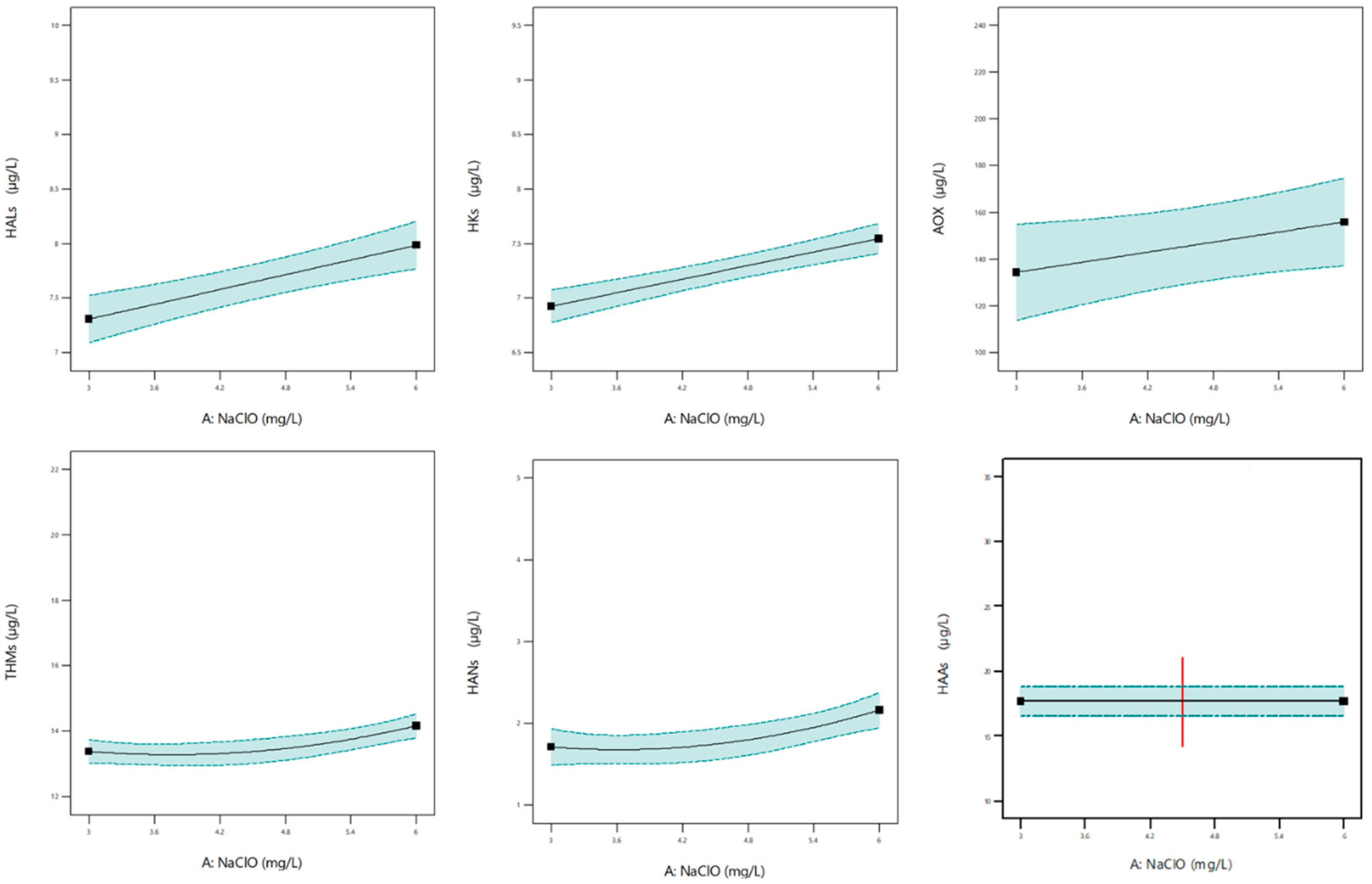
3.3. DBP Formation
- CNaClO and CPACl are initial concentrations of NaClO and PACl (mg/L), respectively.
- t is mixing time (minutes).
- Q represents wastewater flowrate (L/hr).
- CTHMs, CHAAs, CAOX, CHANs, CHALs, and CHKs are the post-treatment concentrations of THMs, HAAs, AOX, HANs, HALs, and HKs, respectively (µg/L).
4. Practical Implications
5. Conclusions
- The integrated approach maintained phosphorus removal (>80%) and microbial inactivation (up to 3-log total coliform reduction), while also lowering disinfection by-product (DBP) formation compared to conventional sequential treatment. For example, haloacetic acids (HAAs) and adsorbable organic halides (AOX) were reduced by ~4–5% with PACl addition under typical operating conditions.
- Bench-scale tests confirmed that microbial inactivation was primarily driven by NaClO dose, with limited contribution from PACl. In contrast, pilot-scale experiments indicated a modest but consistent synergistic effect, where PACl addition improved coliform reduction under high-mixing conditions.
- DBP formation (THMs, HAAs, AOX, HANs, HALs, HKs) was influenced by both NaClO dose and mixing conditions. Pilot-scale operation provided more stable outcomes, with optimized mixing improving DBP control and suggesting potential for more energy-efficient operation.
- While these results highlight operational and environmental advantages of integration, the magnitude of DBP reductions was modest and the study did not directly assess DBP toxicity or long-term system reliability. Future research should address these limitations to confirm the broader health and sustainability benefits.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, J.L.; Ika, A.R.; Tseng, C.C. Effect of In-Situ Formed Al Hydrates through Long-Term Aging on Enhanced Particle Destabilization by PACl Coagulation. J. Environ. Sci. 2020, 92, 200–210. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Qu, J.; Ni, J.; Chow, C.W.K. Enhanced Coagulation for High Alkalinity and Micro-Polluted Water: The Third Way through Coagulant Optimization. Water Res. 2008, 42, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, Z.; Wang, M.; Yuan, Z.; Jin, L. Response Surface Methodology Approach to Optimize Parameters for Coagulation Process Using Polyaluminum Chloride (PAC). Water 2024, 16, 1470. [Google Scholar] [CrossRef]
- Wei, N.; Zhang, Z.; Liu, D.; Wu, Y.; Wang, J.; Wang, Q. Coagulation Behavior of Polyaluminum Chloride: Effects of pH and Coagulant Dosage. Chin. J. Chem. Eng. 2015, 23, 1041–1046. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Li, A.; Yang, H. Enhanced Coagulation of Low-Turbidity Micro-Polluted Surface Water: Properties and Optimization. J. Environ. Manag. 2019, 233, 739–747. [Google Scholar] [CrossRef]
- Sharafi, K.; Fazlzadeh, M.; Pirsaheb, M.; Moradi, M.; Azari, A.; Sharafi, H.; Dindarloo, K.; Ghafari, H.R. NaDCC) and Sodium Hypochlorite (NaOCL): Modeling, Optimization and Comparative Analysis. Desalination Water Treat. 2017, 66, 221–228. [Google Scholar] [CrossRef]
- Nunes, N.B.; Reis, J.O.D.; Castro, V.S.; Machado, M.A.M.; Cunha-Neto, A.D.; Figueiredo, E.E.D.S. Optimizing the Antimicrobial Activity of Sodium Hypochlorite (NaClO) over Exposure Time for the Control of Salmonella Spp. In Vitro. Antibiotics 2024, 13, 68. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, C.; Liu, H.; Zhao, X.; Qu, J. Role of Aluminum Speciation in the Removal of Disinfection Byproduct Precursors by a Coagulation Process. Environ. Sci. Technol. 2008, 42, 5752–5758. [Google Scholar] [CrossRef]
- Precious Sibiya, N.; Rathilal, S.; Kweinor Tetteh, E. Coagulation Treatment of Wastewater: Kinetics and Natural Coagulant Evaluation. Molecules 2021, 26, 698. [Google Scholar] [CrossRef]
- Smith, S.; Takács, I.; Murthy, S.; Daigger, G.T.; Szabó, A. Phosphate Complexation Model and Its Implications for Chemical Phosphorus Removal. Water Environ. Res. 2008, 80, 428–438. [Google Scholar] [CrossRef]
- Bryndza, H.E.; Tam, W. Monomeric Metal Hydroxides, Alkoxides, and Amides of the Late Transition Metals: Synthesis, Reactions, and Thermochemistry. Chem. Rev. 1988, 88, 1163–1188. [Google Scholar] [CrossRef]
- Plewa, M.J.; Richardson, S.D. Disinfection By-Products in Drinking Water, Recycled Water and Wastewater: Formation, Detection, Toxicity and Health Effects: Preface. J. Environ. Sci. 2017, 58, 1. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, H.; Hu, C.; Qu, J.; Wang, D.; Li, J. Removal of Disinfection By-Products Precursors by Polyaluminum Chloride Coagulation Coupled with Chlorination. Sep. Purif. Technol. 2008, 62, 464–469. [Google Scholar] [CrossRef]
- Ma, M.; Liu, R.; Liu, H.; Qu, J.; Jefferson, W. Effects and Mechanisms of Pre-Chlorination on Microcystis aeruginosa Removal by Alum Coagulation: Significance of the Released Intracellular Organic Matter. Sep. Purif. Technol. 2012, 86, 19–25. [Google Scholar] [CrossRef]
- Chow, C.W.K.; Van Leeuwen, J.A.; Fabris, R.; Drikas, M. Optimised Coagulation Using Aluminium Sulfate for the Removal of Dissolved Organic Carbon. Desalination 2009, 245, 120–134. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Choo, K.-H.; Pramanik, S.K.; Suja, F.; Jegatheesan, V. Comparisons between Biological Filtration and Coagulation Processes for the Removal of Dissolved Organic Nitrogen and Disinfection By-Products Precursors. Int. Biodeterior. Biodegrad. 2015, 104, 164–169. [Google Scholar] [CrossRef]
- Shahid, A.; Khan, A.Z.; Malik, S.; Liu, C.-G.; Mehmood, M.A.; Syafiuddin, A.; Wang, N.; Zhu, H.; Boopathy, R. Advances in Green Technologies for the Removal of Effluent Organic Matter from the Urban Wastewater. Curr. Pollut. Rep. 2021, 7, 463–475. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Chu, Y.; Gao, B.; Yue, Q.; Yang, Z.; Li, Q. Reduction of Organic Matter and Trihalomethane Formation Potential in Reclaimed Water from Treated Municipal Wastewater by Coagulation and Adsorption. Chem. Eng. J. 2013, 223, 696–703. [Google Scholar] [CrossRef]
- Ding, S.; Chu, W.; Bond, T.; Cao, Z.; Xu, B.; Gao, N. Contribution of Amide-Based Coagulant Polyacrylamide as Precursors of Haloacetamides and Other Disinfection by-Products. Chem. Eng. J. 2018, 350, 356–363. [Google Scholar] [CrossRef]
- Fallah, N.; Bell, K.; Mao, T.; Hofmann, R.; Bossoni, G.E.B.; Santoro, D.; Mele, G. Chemical Disinfection of Secondary Municipal Wastewater Effluents: Optimizing CT Dose and Tailing Effects through High-intensity Mixing. Water Environ. Res. 2025, 97, e70066. [Google Scholar] [CrossRef]
- Ghernaout, D. Enhanced Coagulation: Promising Findings and Challenges. OALib 2020, 7, 1–19. [Google Scholar] [CrossRef]
- Hahn, H.; Hoffman, E.; Odegaard, H. Chemical Water and Wastewater Treatment VIII. Water Intell. Online 2015, 4, 9781780402840. [Google Scholar] [CrossRef]
- Wang, H.; Yu, L.-Q.; Chen, S.-N.; Liu, M.; Fan, N.-S.; Huang, B.-C.; Jin, R.-C. Coagulation Enhanced High-Rate Contact-Stabilization Process for Pretreatment of Municipal Wastewater: Simultaneous Organic Capture and Phosphorus Removal. Sep. Purif. Technol. 2022, 298, 121669. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Zhang, G.; Liu, W.; Xu, J.; Tian, Y.; Wang, Y.; Xie, X.; Peng, Z.; Li, A.; et al. Efficient Treatment of the Starch Wastewater by Enhanced Flocculation–Coagulation of Environmentally Benign Materials. Sep. Purif. Technol. 2023, 307, 122788. [Google Scholar] [CrossRef]
- Hu, C.; Liu, H.; Qu, J.; Wang, Y. Coagulation and Disinfection Efficiency of an Electrochemically Prepared Dual-Function Reagent in Municipal Wastewater. J. Environ. Sci. Health Part A 2006, 41, 2387–2398. [Google Scholar] [CrossRef]
- Song, J.; Wang, J.; Wang, D. Changes in the Structural Characteristics of EfOM during Coagulation by Aluminum Chloride and the Effect on the Formation of Disinfection Byproducts. J. Environ. Manag. 2023, 326, 116850. [Google Scholar] [CrossRef]
- Golfinopoulos, S.K.; Nikolaou, A.D.; Alexakis, D.E. Innovative Approaches for Minimizing Disinfection Byproducts (DBPs) in Water Treatment: Challenges and Trends. Appl. Sci. 2024, 14, 8153. [Google Scholar] [CrossRef]
- Wang, J.; Yue, W.; Wang, Z.; Bai, Y.; Song, J. Removal Effect of Trihalomethanes (THMs) and Halogenated Acetic Acids (HAAs) Precursors in Reclaimed Water by Polyaluminum Chloride (PACl) Coagulation. Water Sci. Technol. 2023, 87, 672–684. [Google Scholar] [CrossRef]
- Wang, P.; Ding, S.; Xiao, R.; An, G.; Fang, C.; Chu, W. Enhanced Coagulation for Mitigation of Disinfection By-Product Precursors: A Review. Adv. Colloid Interface Sci. 2021, 296, 102518. [Google Scholar] [CrossRef]
- Krasner, S.W.; Westerhoff, P.; Chen, B.; Rittmann, B.E.; Nam, S.-N.; Amy, G. Impact of Wastewater Treatment Processes on Organic Carbon, Organic Nitrogen, and DBP Precursors in Effluent Organic Matter. Environ. Sci. Technol. 2009, 43, 2911–2918. [Google Scholar] [CrossRef]
- Lin, J.-L.; Ika, A.R. Minimization of Halogenated DBP Precursors by Enhanced PACl Coagulation: The Impact of Organic Molecule Fraction Changes on DBP Precursors Destabilization with Al Hydrates. Sci. Total Environ. 2020, 703, 134936. [Google Scholar] [CrossRef]
- Kalita, I.; Kamilaris, A.; Havinga, P.; Reva, I. Assessing the Health Impact of Disinfection Byproducts in Drinking Water. ACS EST Water 2024, 4, 1564–1578. [Google Scholar] [CrossRef]
- Luan, X.; Liu, X.; Fang, C.; Chu, W.; Xu, Z. Ecotoxicological Effects of Disinfected Wastewater Effluents: A Short Review of in Vivo Toxicity Bioassays on Aquatic Organisms. Environ. Sci. Water Res. Technol. 2020, 6, 2275–2286. [Google Scholar] [CrossRef]
- Sun, G.; Li, Y. Exposure to DBP Induces the Toxicity in Early Development and Adverse Effects on Cardiac Development in Zebrafish (Danio rerio). Chemosphere 2019, 218, 76–82. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Zhu, L.; Wang, J.; Li, H.; Zhang, Y.; Liu, W.; Gao, J. Oxidative Damage and Genetic Toxicity Induced by DBP in Earthworms (Eisenia fetida). Arch. Environ. Contam. Toxicol. 2018, 74, 527–538. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, J.; Huang, Y.; Shen, G.; Pang, S.; Wang, C.; Li, Y.; Mu, X. Integrated Toxicity Assessment of DEHP and DBP toward Aquatic Ecosystem Based on Multiple Trophic Model Assays. Environ. Sci. Pollut. Res. 2022, 29, 87402–87412. [Google Scholar] [CrossRef]






| Parameters | Units | Number of Samples | Min–Max | Average |
|---|---|---|---|---|
| N-NO2− | mg/L | 5 | 0.005–0.008 | 0.0062 |
| N-NO3− | mg/L | 5 | 31.3–38.2 | 34.8 |
| N-NH4+ | mg/L | 5 | 0.05–0.07 | 0.62 |
| Total-N | mg/L | 5 | 25.7–33.9 | 29.98 |
| COD | mg/L | 5 | 22–45 | 33.8 |
| Total-PO43− | mg/L | 5 | 0.38–2.38 | 1.54 |
| Reactive-PO43− | mg/L | 5 | 0.15–1.26 | 0.615 |
| Series. n | NaClO Dose (mg/L) | PACl Dose (mg/L) | Process Time | Outcome |
|---|---|---|---|---|
| 1 | 3 | 0, 3, 6 | 21 s mixing | Soluble phosphorus |
| 2 | 4.5 | 0, 4.5, 9 | 21 s mixing | Soluble phosphorus |
| 3 | 6 | 0, 6, 12 | 21 s mixing | Soluble phosphorus |
| 4 | 3 | 0, 3, 6 | 14, 21, 29 s mixing | Total coliform, Fecal coliform, DBPs |
| 5 | 4.5 | 0, 4.5, 9 | 14, 21, 29 s mixing | Total coliform, Fecal coliform, DBPs |
| 6 | 6 | 0, 6, 12 | 14, 21, 29 s mixing | Total coliform, Fecal coliform, DBPs |
| Parameter | Value |
|---|---|
| Model | Quadratic |
| R2 | 0.67 |
| Adjusted R2 | 0.65 |
| Predicted R2 | 0.60 |
| Std. Dev. | 0.046 |
| Bench Scale | Pilot Scale | |||
|---|---|---|---|---|
| Parameter | Fecal Coliform | Total Coliform | Fecal Coliform | Total Coliform |
| Model | Linear | Linear | Linear | Linear |
| R2 | 0.59 | 0.82 | 0.6 | 0.56 |
| Adjusted R2 | 0.57 | 0.80 | 0.58 | 0.54 |
| Predicted R2 | 0.52 | 0.76 | 0.5 | 0.47 |
| Std. Dev. | 0.35 | 0.26 | 0.12 | 0.23 |
| Bench Scale |
| Pilot Scale |
| Bench Scale | Pilot Scale | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | THMs | HAAs | HALs | HKs | THMs | HAAs | AOX | HANs | HALs | HKs |
| Model | 2FI | 2FI | 2FI | Linear | Quadratic | Quadratic | Quadratic | Quadratic | Quadratic | Quadratic |
| R2 | 0.35 | 0.61 | 0.6 | 0.77 | 0.98 | 0.87 | 0.75 | 0.96 | 0.9 | 0.95 |
| Adjusted R2 | 0.29 | 0.54 | 0.5 | 0.74 | 0.97 | 0.86 | 0.66 | 0.95 | 0.89 | 0.94 |
| Predicted R2 | 0.14 | 0.46 | 0.43 | 0.69 | 0.96 | 0.83 | 0.46 | 0.94 | 0.84 | 0.91 |
| Std. Dev. | 4.01 | 1.63 | 0.14 | 0.1 | 0.41 | 1.85 | 21.11 | 0.24 | 0.26 | 0.18 |
| Bench Scale |
| Pilot Scale |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallah, N.; Bell, K.; Mao, T.; Hofmann, R.; Bossoni, G.E.B.; Santoro, D.; Mele, G. Integrated Coagulation–Disinfection Using Aluminium Polychloride and Sodium Hypochlorite for Secondary Wastewater Treatment: Operational Advantages and DBP Mitigation. Water 2025, 17, 2867. https://doi.org/10.3390/w17192867
Fallah N, Bell K, Mao T, Hofmann R, Bossoni GEB, Santoro D, Mele G. Integrated Coagulation–Disinfection Using Aluminium Polychloride and Sodium Hypochlorite for Secondary Wastewater Treatment: Operational Advantages and DBP Mitigation. Water. 2025; 17(19):2867. https://doi.org/10.3390/w17192867
Chicago/Turabian StyleFallah, Naghmeh, Katherine Bell, Ted Mao, Ronald Hofmann, Gabriela Ellen Barreto Bossoni, Domenico Santoro, and Giuseppe Mele. 2025. "Integrated Coagulation–Disinfection Using Aluminium Polychloride and Sodium Hypochlorite for Secondary Wastewater Treatment: Operational Advantages and DBP Mitigation" Water 17, no. 19: 2867. https://doi.org/10.3390/w17192867
APA StyleFallah, N., Bell, K., Mao, T., Hofmann, R., Bossoni, G. E. B., Santoro, D., & Mele, G. (2025). Integrated Coagulation–Disinfection Using Aluminium Polychloride and Sodium Hypochlorite for Secondary Wastewater Treatment: Operational Advantages and DBP Mitigation. Water, 17(19), 2867. https://doi.org/10.3390/w17192867








