Analysis of Driving Factors of Groundwater Chemical Characteristics at Different Depths and Health Effects of Nitrate Exposure in Zhengzhou City, China
Abstract
1. Introduction
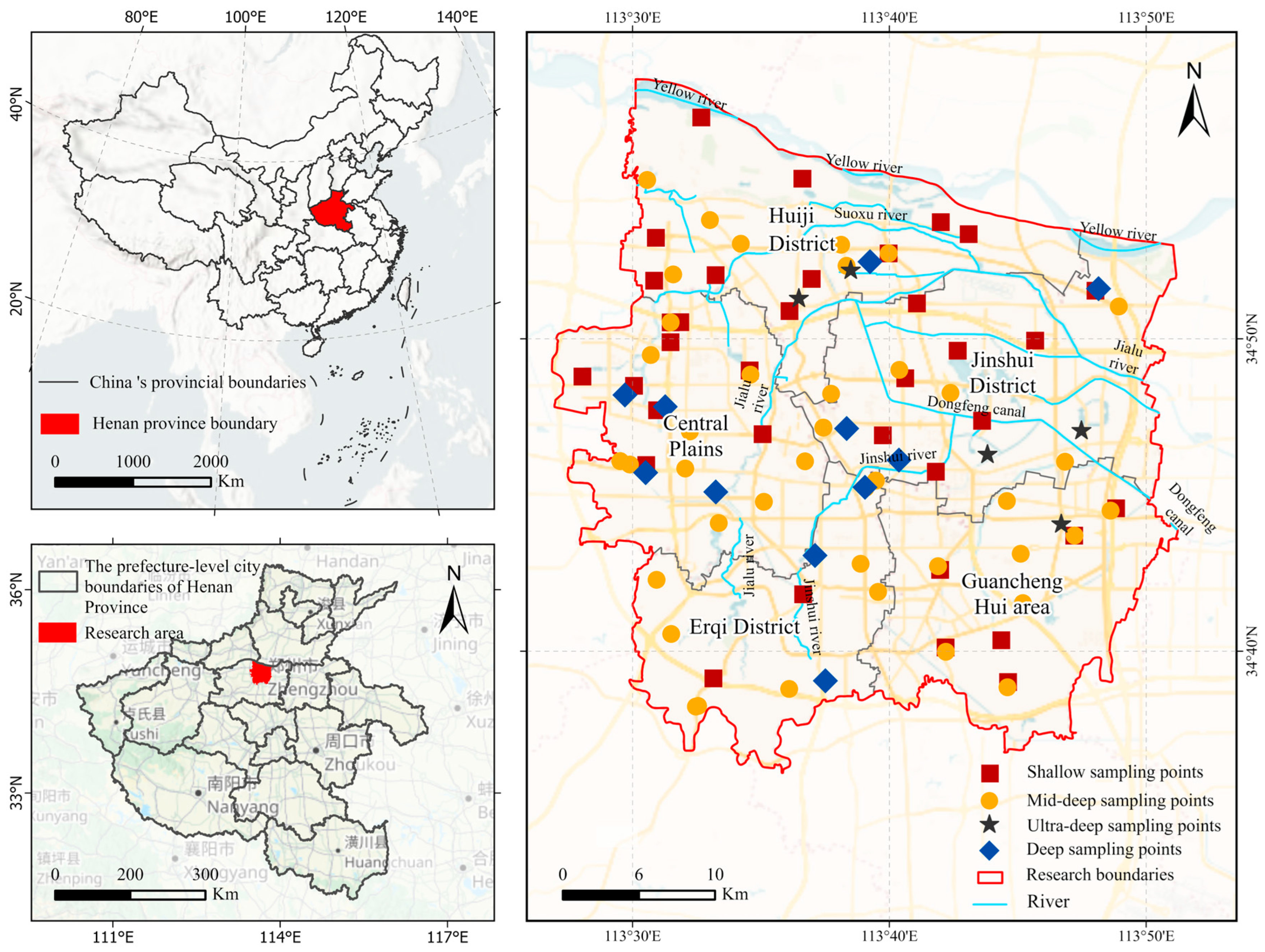
2. Study Region
3. Materials and Methods
3.1. Groundwater Sampling and Analysis
3.2. Human Health Risk Assessment
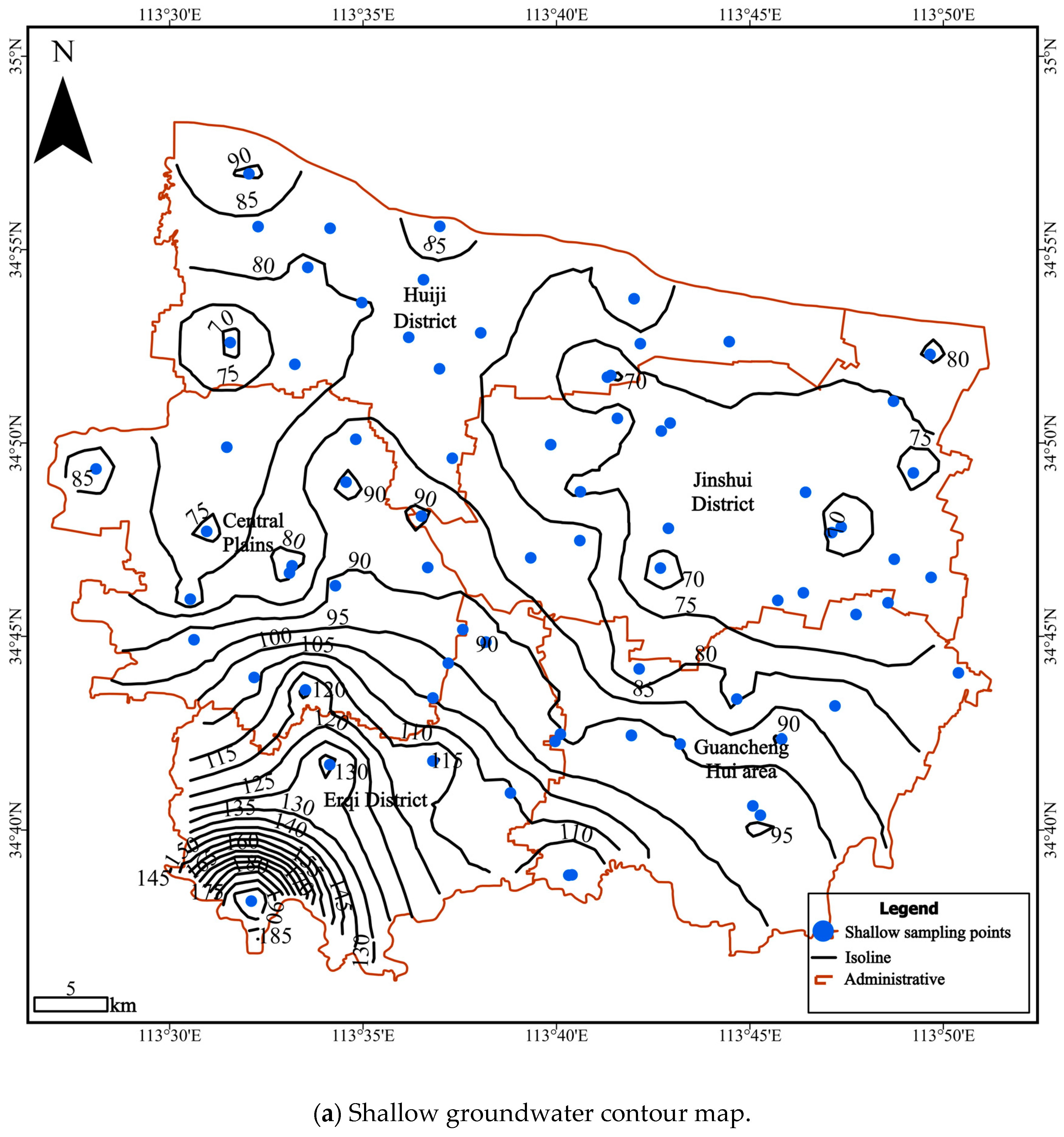
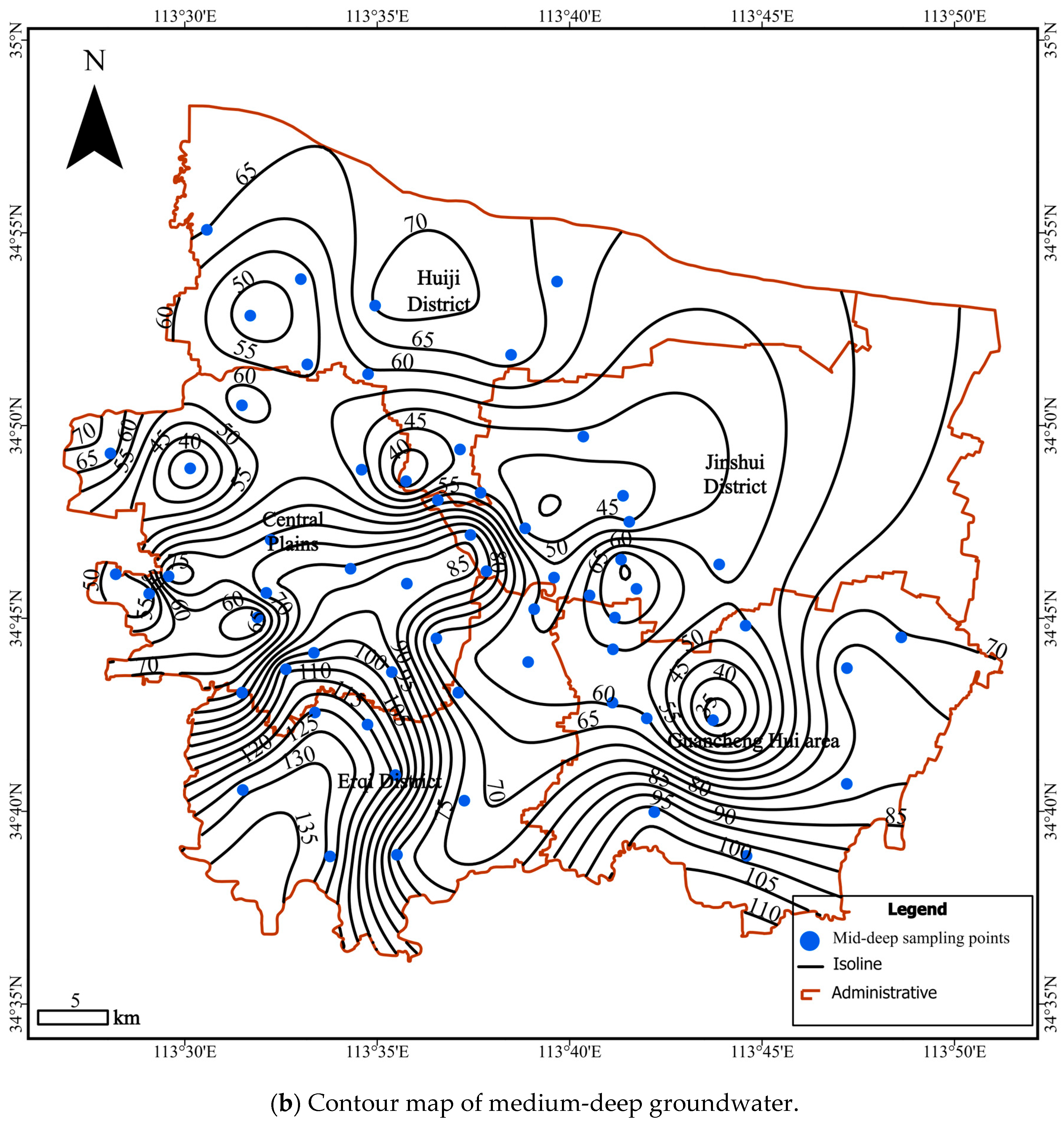
4. Results and Discussion
4.1. Groundwater Chemistry
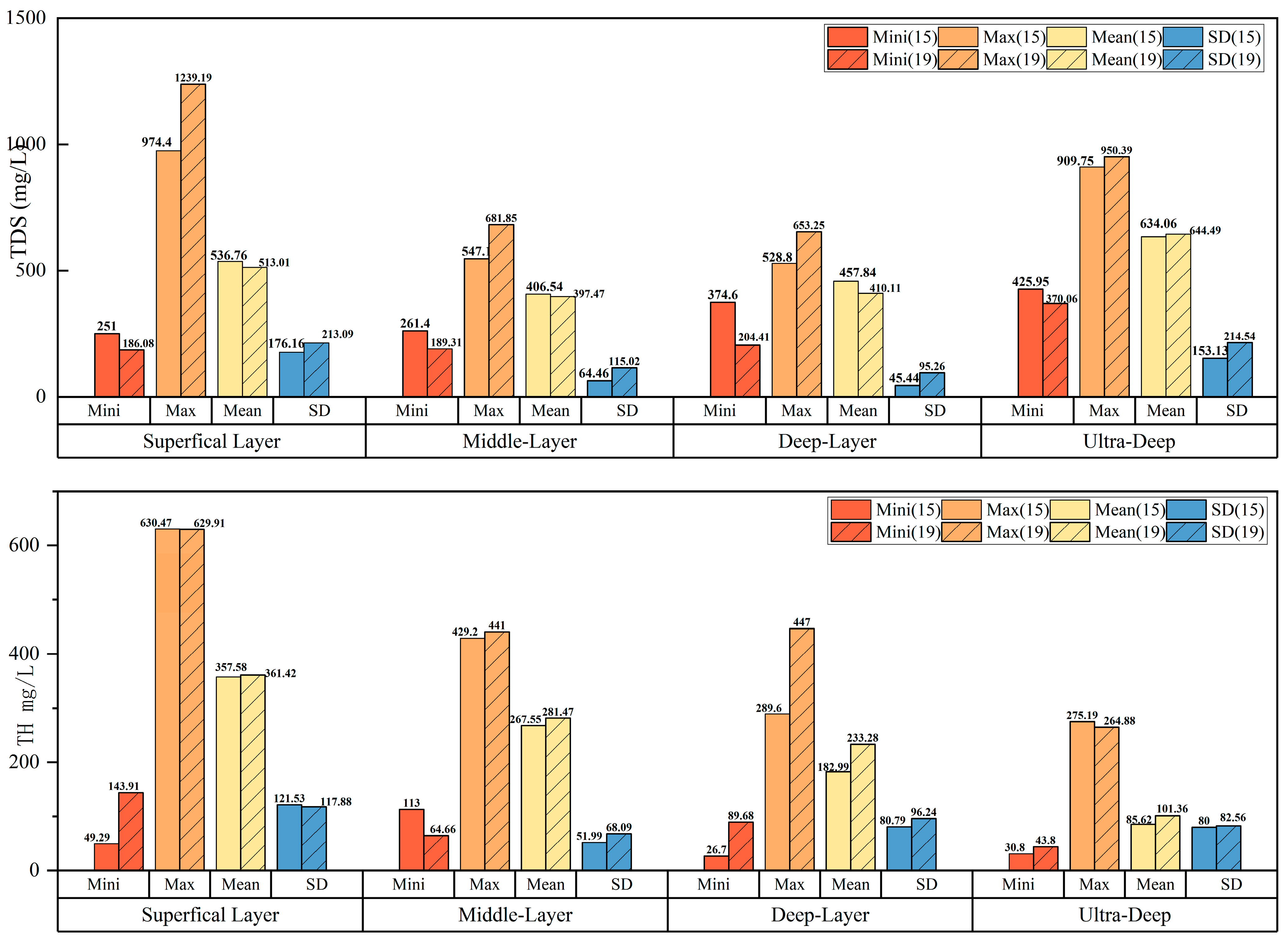
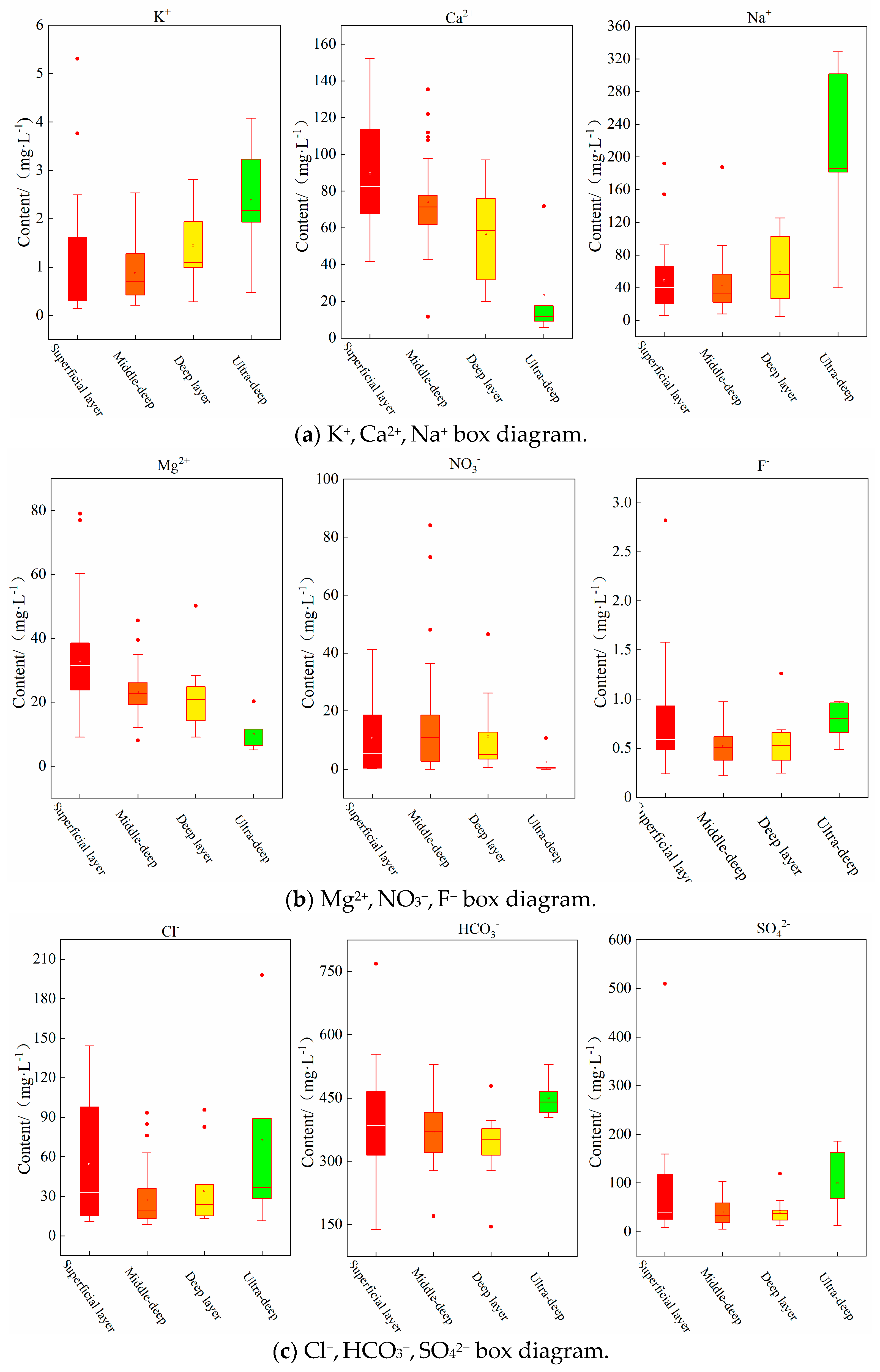
4.1.1. Descriptive Statistics
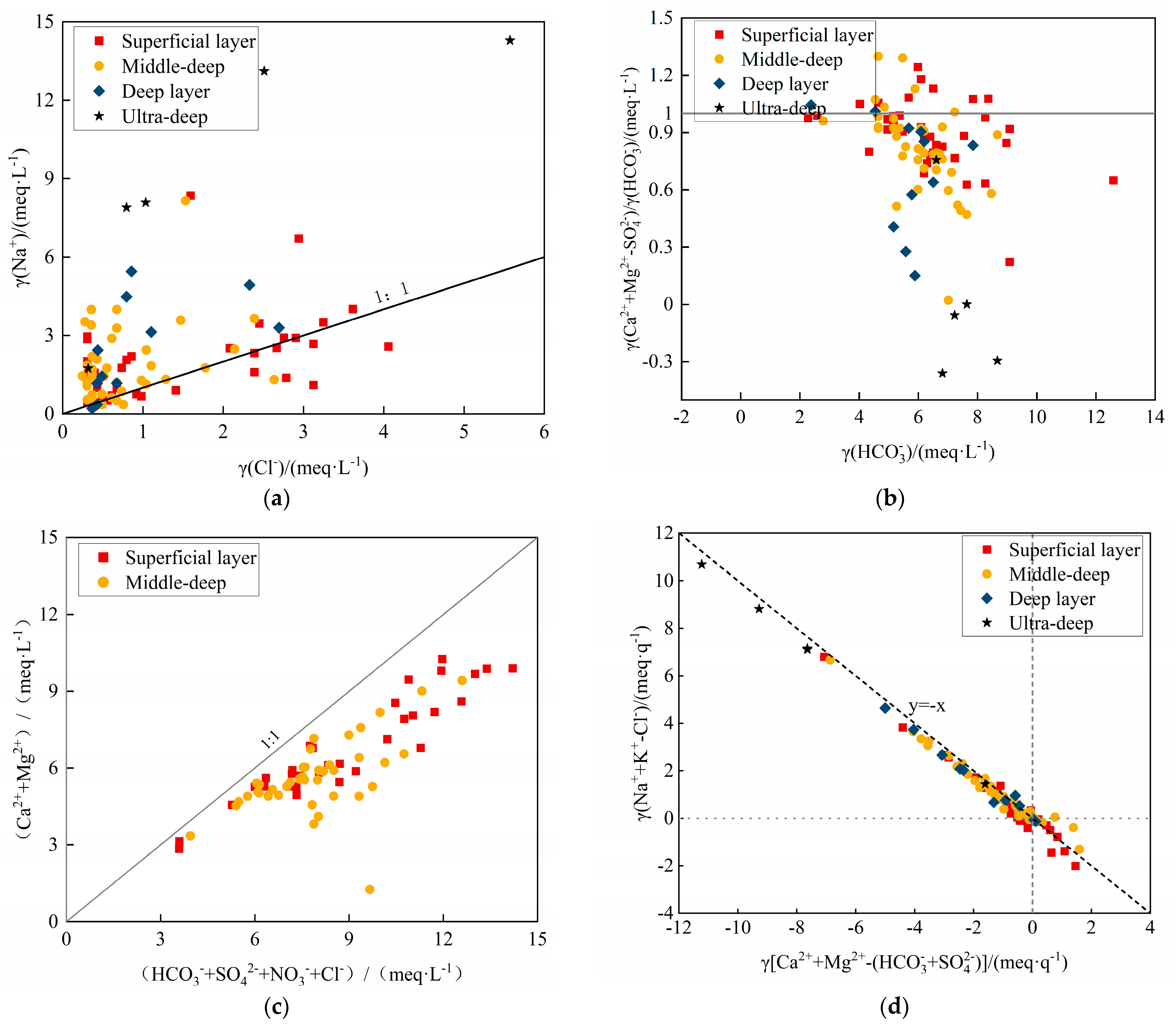
4.1.2. Major Cations and Anions Analysis
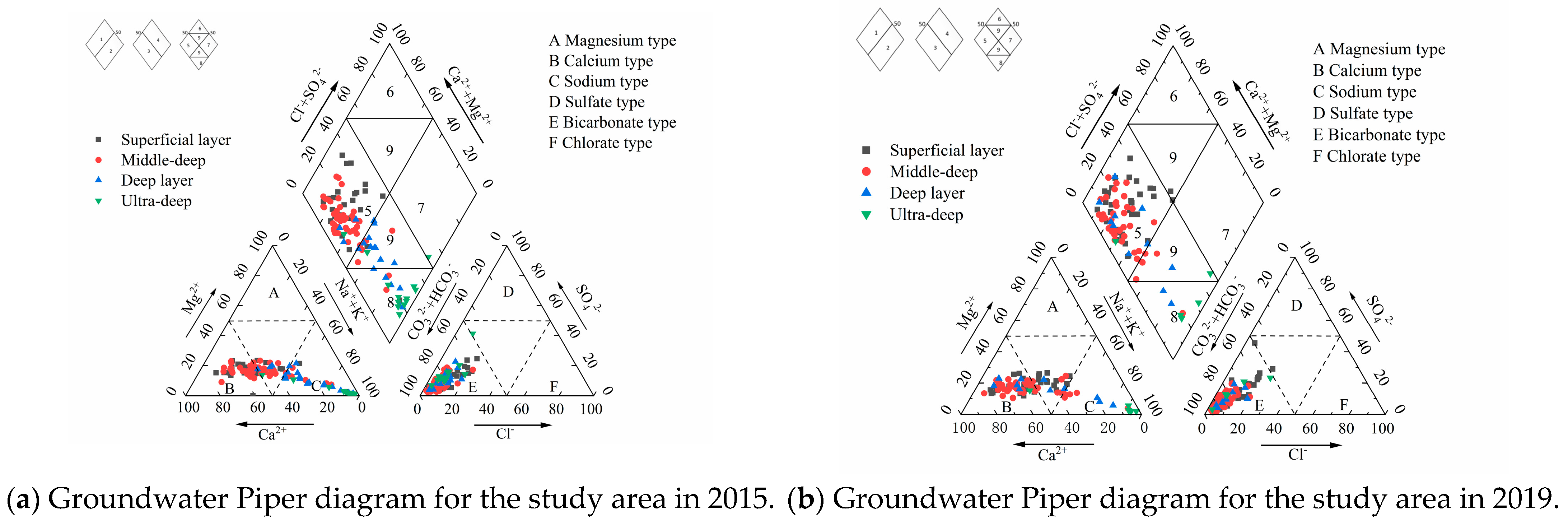
4.1.3. Hydrochemical Types
4.2. Hydrochemical Formation
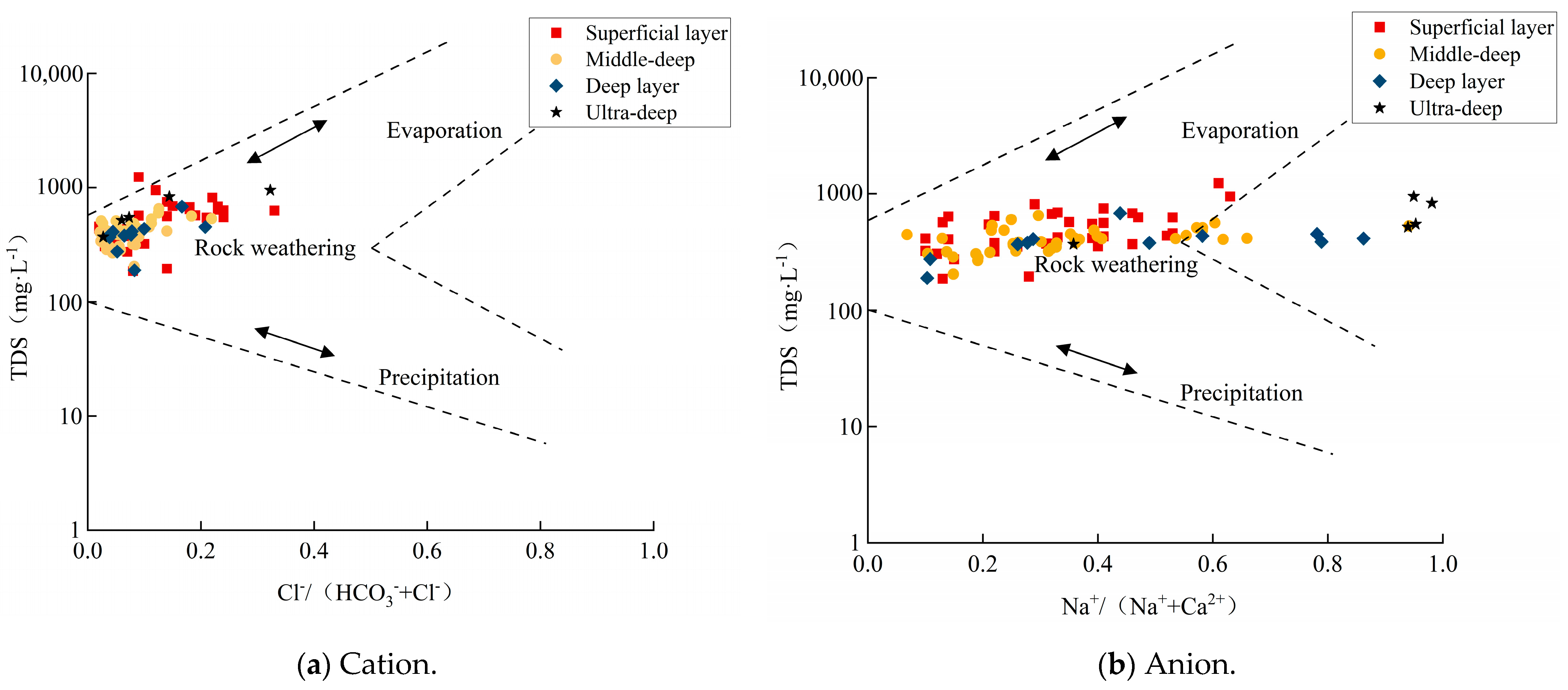
4.2.1. Solubilisation
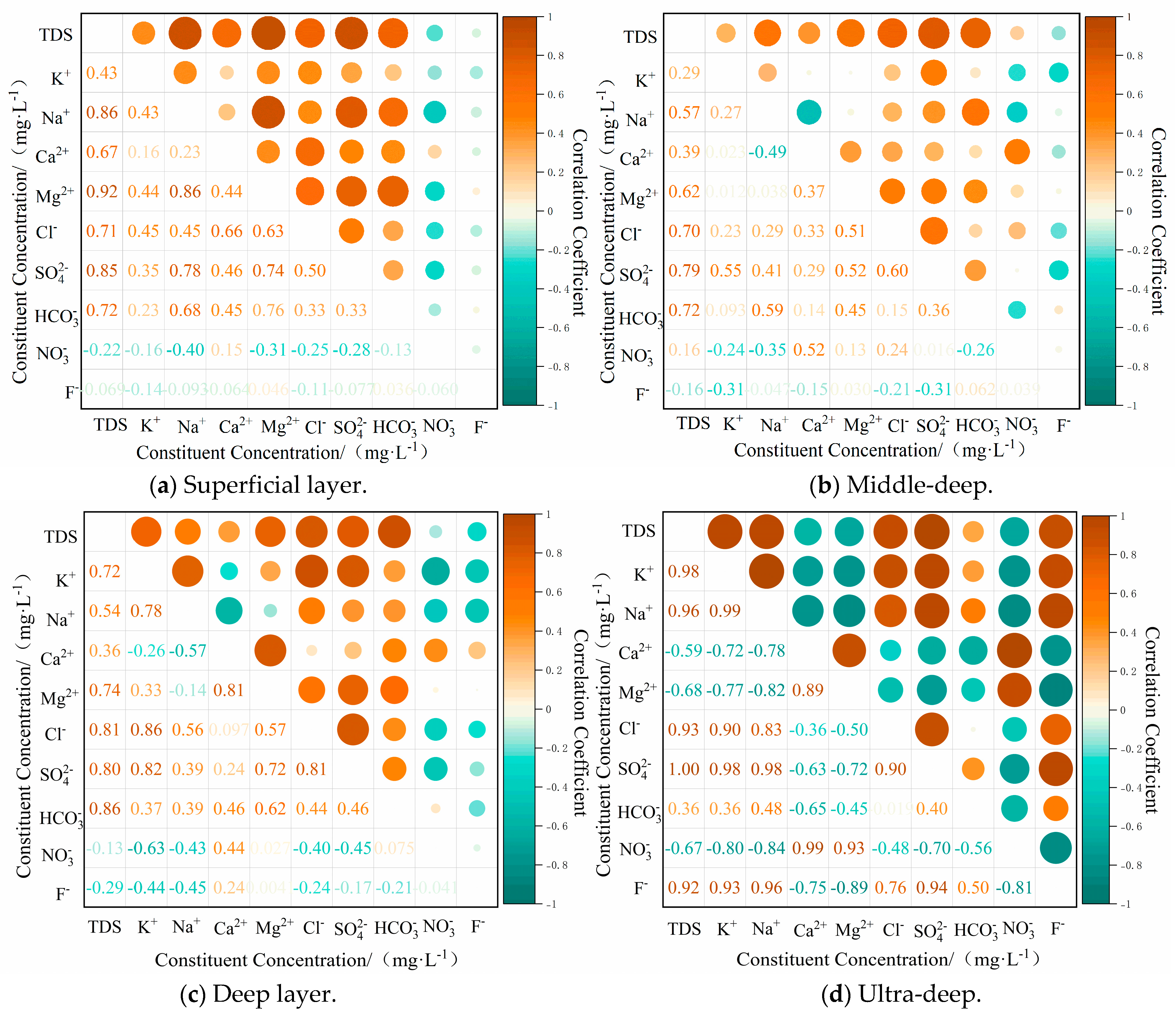
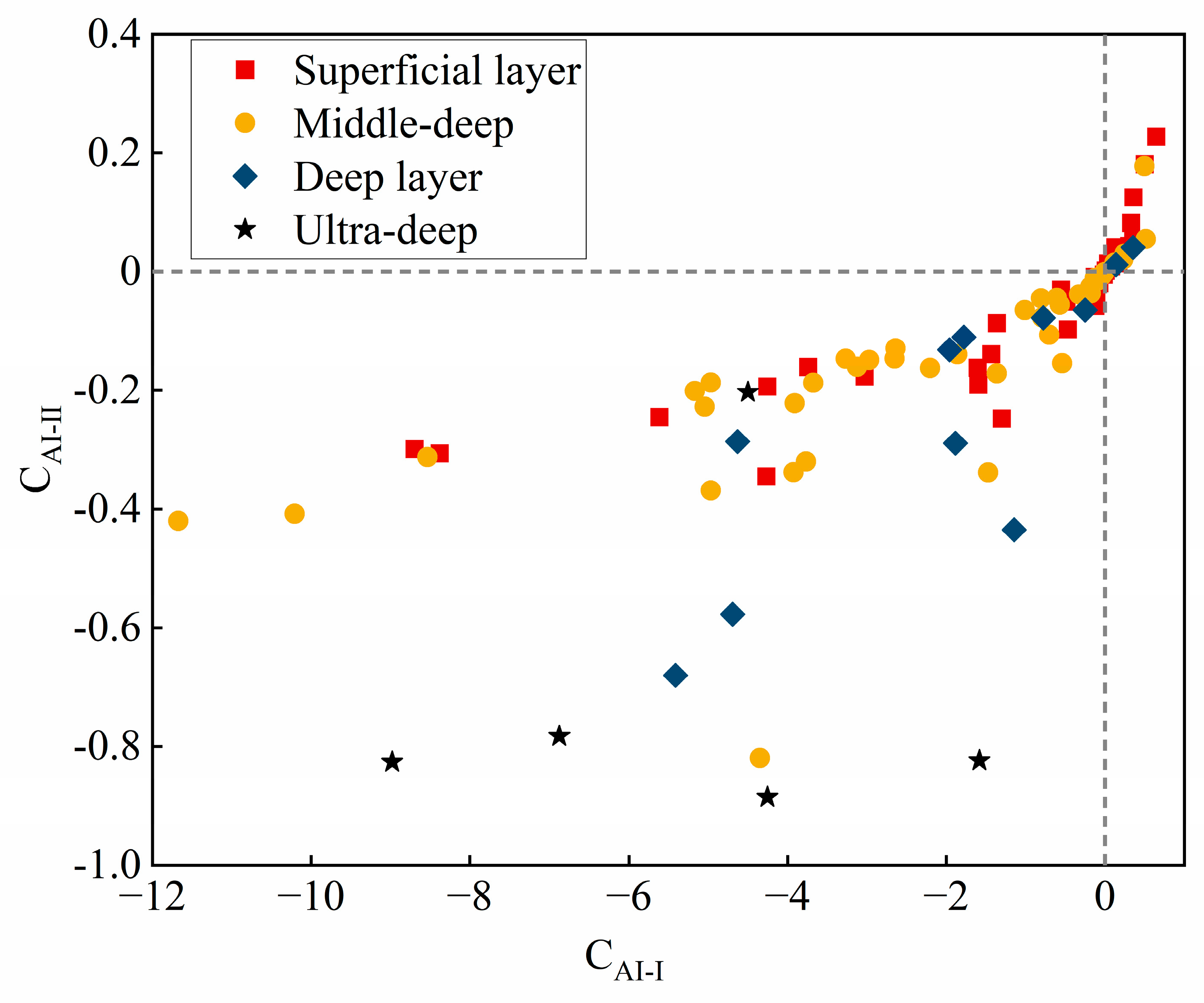
4.2.2. Cationic Alternating Adsorption
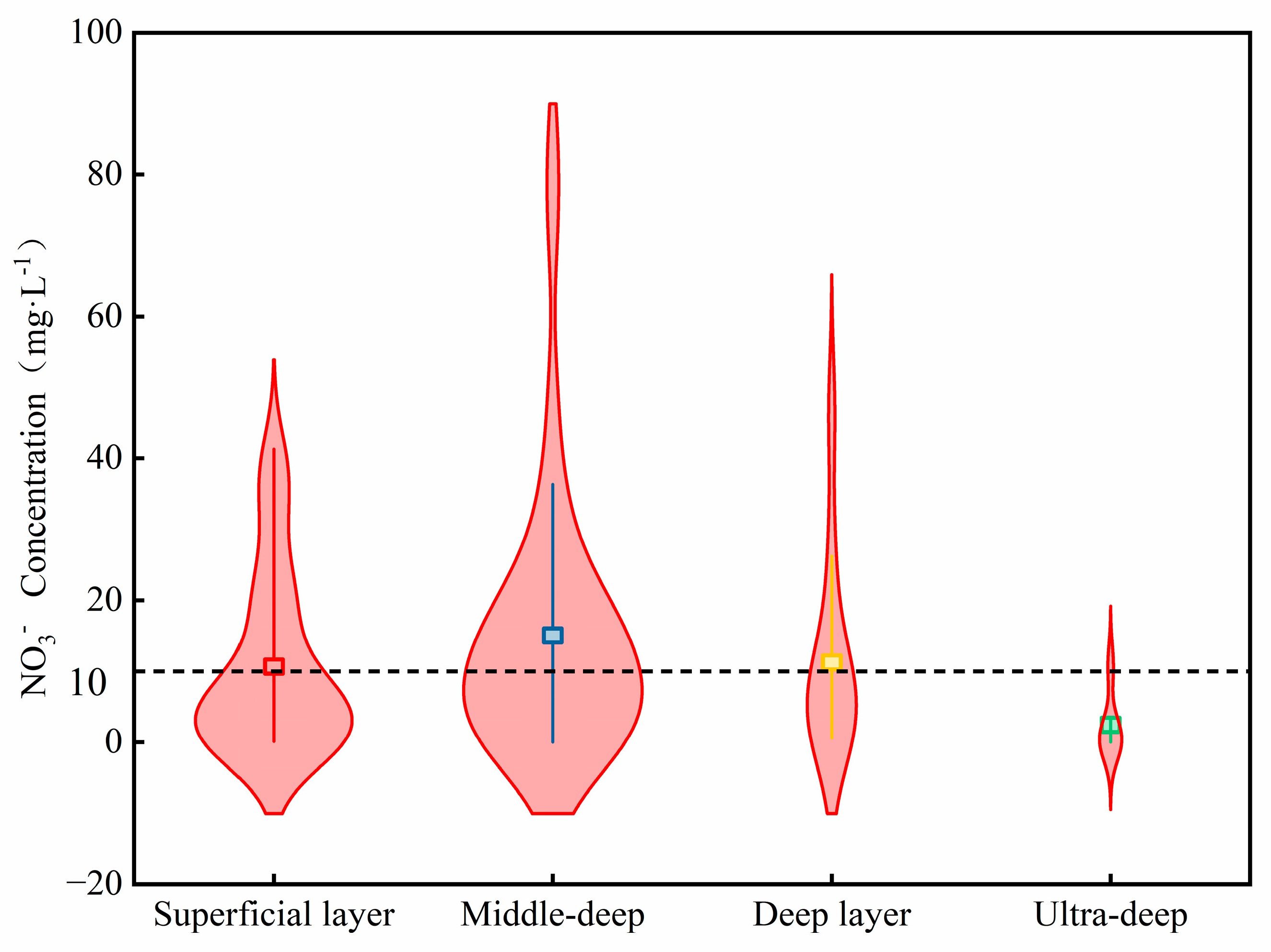
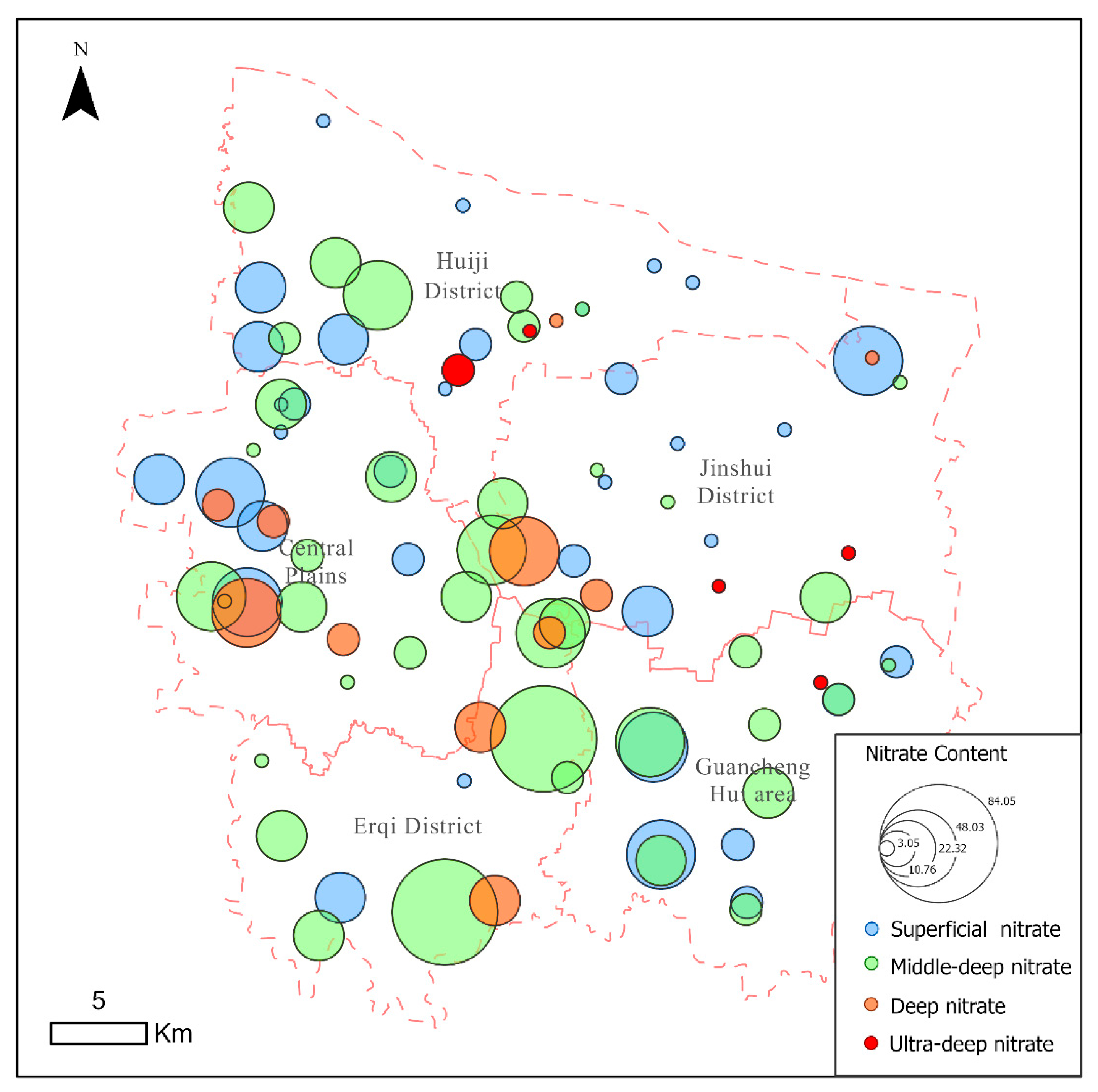
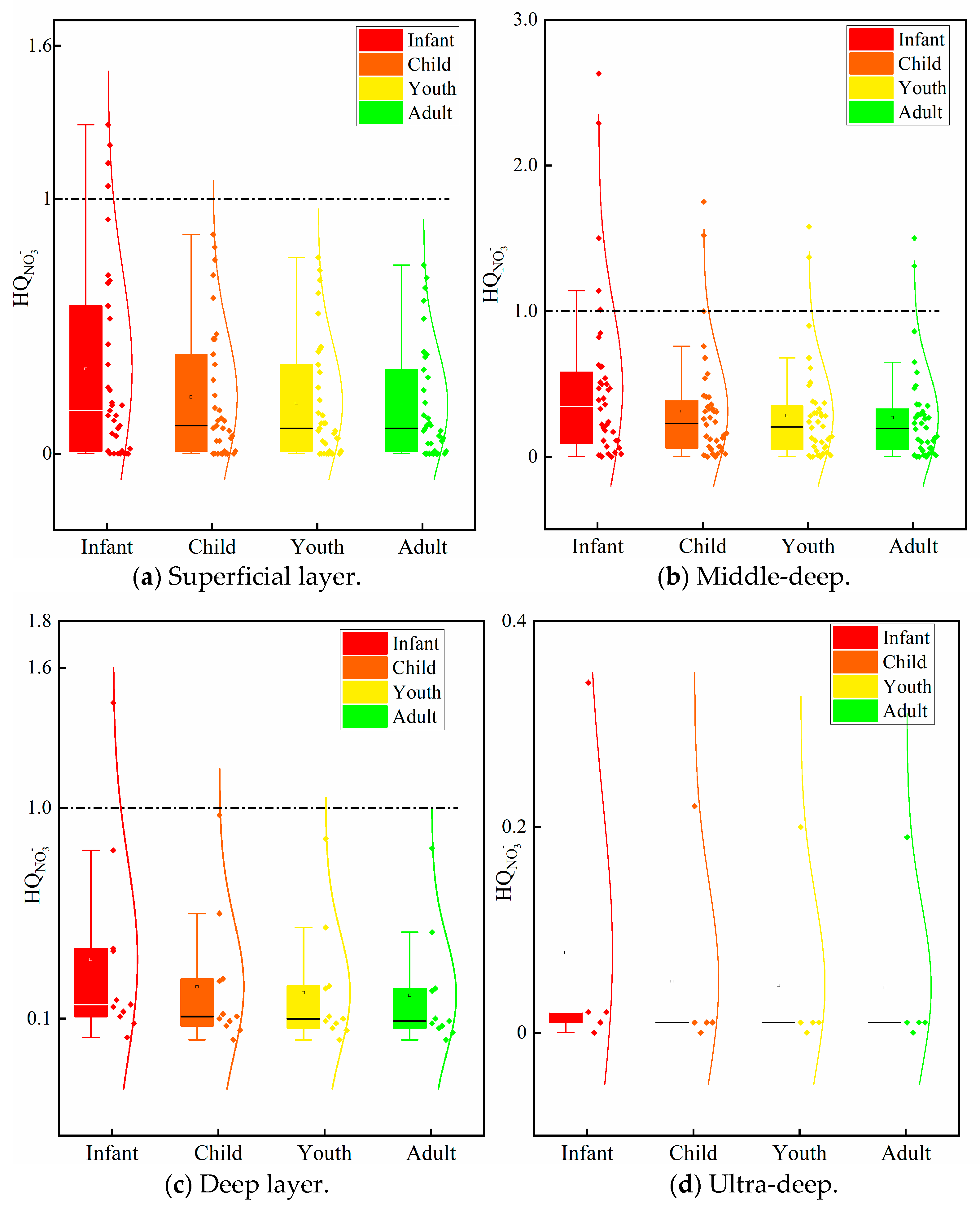
4.3. Health Risk Assessment of Nitrate in Groundwater
5. Conclusions
- (1)
- The major cation concentrations in the shallow groundwater and the medium-deep groundwater are similar, with Ca2+ > Na+ > Mg2+ > K+, while those in the deep groundwater and the ultra-deep groundwater are approximately Na+ > Ca2+ > Mg2+ > K+. In the deep groundwater, Na+ and Ca2+ account for over 40% of the total dissolved solids, and in the ultra-deep groundwater, the Na+ content reaches as high as 85%. The percentage of Na+ increases gradually with the burial depth of the sampling points, while the percentage of Ca2+ decreases in a similar, gradual fashion. The predominant controlling factor in this phenomenon is cation exchange. The primary anion concentrations in the groundwater at varying burial depths are as follows: HCO3− > SO42− > Cl− > NO3− > F−. HCO3− has been found to account for 70~82% of the total anion concentration, thus establishing itself as the dominant anion in the study area’s groundwater.
- (2)
- The analysis of water chemistry indicates the presence of 12 distinct types of shallow groundwater chemistry within the designated study area. The HCO3-Ca type is identified as the predominant chemistry, accounting for 26% of the total, and is primarily distributed in the southern and central regions of the study area. The investigation revealed eight distinct types of groundwater chemistry in the medium-deep layer, primarily HCO3-Ca·Mg type, accounting for 42%, predominantly distributed in the southwestern, northwestern, and southern parts of Putian Township; followed by the HCO3-Ca type, accounting for 25%, primarily distributed in the central-southern part of the study area. The chemical composition of the deep groundwater is characterised by seven distinct water types, predominantly the HCO3-Ca·Mg type, accounting for 36% of the total, and predominantly distributed in the central, southwestern, and southern regions. The investigation revealed that the ultra-deep groundwater predominantly exhibits the HCO3-Na type, accounting for 60%, and is distributed throughout the northeastern part of the study area.
- (3)
- The chemical components present in the groundwater of the study area are of the rock-weathering type, with the predominant source of these components being the weathering of carbonate rocks. The predominant factors that govern the process of chemical component formation include leaching, cation exchange, counter-cation exchange, and mixing.
- (4)
- The potential non-carcinogenic risks associated with the medium-to-deep groundwater are higher than those of the shallow, deep, and ultra-deep groundwater. The mid-deep groundwater has the potential to pose risks to all four categories of people, while the shallow and deep groundwater only pose potential risks to infants. It is therefore recommended that Zhengzhou prioritise restricting agricultural non-point source pollution inputs into the mid-deep aquifer recharge areas (e.g., controlling nitrogen fertiliser application rates, establishing buffer zones), installing nitrate real-time monitoring wells in sensitive areas, and focusing particularly on vulnerable groups in water supply management.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheng, D.; Meng, X.; Wen, X.; Wu, J.; Yu, H.; Wu, M.; Zhou, T. Hydrochemical characteristics, quality and health risk assessment of nitrate enriched coastal groundwater in northern China. J. Clean. Prod. 2023, 403, 136872. [Google Scholar] [CrossRef]
- Frappart, F.; Merwade, V.M. Groundwater systems worldwide. Front. Earth Sci. 2022, 10, 1097789. [Google Scholar] [CrossRef]
- Cao, T.; Han, D.; Song, X.; Trolle, D. Subsurface hydrological processes and groundwater residence time in a coastal alluvium aquifer: Evidence from environmental tracers (δ18O, δ2H, CFCs, 3H) combined with hydrochemistry. Sci. Total Environ. 2020, 743, 140684. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xiao, C.; Liang, X.; Wei, H. Response of groundwater chemical characteristics to land use types and health risk assessment of nitrate in semi-arid areas: A case study of Shuangliao City, Northeast China. Ecotoxicol. Environ. Saf. 2022, 236, 113473. [Google Scholar] [CrossRef]
- Vadiati, M.; Adamowski, J.; Beynaghi, A. A brief overview of trends in groundwater research: Progress towards sustainability? J. Environ. Manag. 2018, 223, 849–851. [Google Scholar] [CrossRef]
- Yin, Z.; Luo, Q.; Wu, J.; Xu, S.; Wu, J. Identification of the long-term variations of groundwater and their governing factors based on hydrochemical and isotopic data in a river basin. J. Hydrol. 2021, 592, 125604. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Q.; Xie, C.; Lu, X. Source identification and health risks of nitrate contamination in shallow groundwater: A case study in Subei Lake basin. Environ. Sci. Pollut. Res. Int. 2023, 30, 13660–13670. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, Z.; Zhang, Y.; Sun, Z.; Sun, T.; Fan, H.; Wu, B.; Li, M.; Qian, L. Hydrochemical evaluation of groundwater quality and human health risk assessment of nitrate in the largest peninsula of China based on high-density sampling: A case study of Weifang. J. Clean. Prod. 2021, 322, 129164. [Google Scholar] [CrossRef]
- Pandey, S.; Mohapatra, G.; Arora, R. Groundwater quality, human health risks and major driving factors in arid and semi-arid regions of Rajasthan, India. J. Clean. Prod. 2023, 427, 139149. [Google Scholar] [CrossRef]
- Liu, R.; Xie, X.; Hou, Q.; Han, D.; Song, J.; Huang, G. Spatial distribution, sources, and human health risk assessment of elevated nitrate levels in groundwater of an agriculture-dominant coastal area in Hainan Island, China. J. Hydrol. 2024, 634, 131088. [Google Scholar] [CrossRef]
- Adimalla, N. Spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India. Hum. Ecol. Risk Assess. Int. J. 2019, 26, 310–334. [Google Scholar] [CrossRef]
- Wang, D.; Li, P.; Yang, N.; Yang, C.; Zhou, Y.; Li, J. Distribution, sources and main controlling factors of nitrate in a typical intensive agricultural region, northwestern China: Vertical profile perspectives. Environ. Res. 2023, 237, 116911. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Feng, Q.; Zhu, M.; Shu, H.; Liu, W.; Yang, L.; Yin, Z.; Zhang, C. Source apportionment of nitrates in different aquifers in an arid region, northwestern China. J. Clean. Prod. 2022, 374, 133969. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, Y.; Guo, X.; Han, J.; Ma, C.; Zhang, X. Current status and future challenges of groundwater vulnerability assessment: A bibliometric analysis. J. Hydrol. 2022, 615, 128694. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Q.; Peng, W.; Liu, X. Source apportionment and natural background levels of major ions in shallow groundwater using multivariate statistical method: A case study in Huaibei Plain, China. J. Environ. Manag. 2022, 301, 113806. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, C.; Lei, X.; Wang, H.; Zhang, X. Distribution of Nitrate Content in Groundwater and Evaluation of Potential Health Risks: A Case Study of Rural Areas in Northern China. Int. J. Environ. Res. Public Health 2020, 17, 9390. [Google Scholar] [CrossRef]
- Fenech, C.; Rock, L.; Nolan, K.; Tobin, J.; Morrissey, A. The potential for a suite of isotope and chemical markers to differentiate sources of nitrate contamination: A review. Water Res. 2012, 46, 2023–2041. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hao, Q.; Zhang, Y.; Zhu, Y.; Yin, S.; Qin, L.; Li, X. Investigating sources, driving forces and potential health risks of nitrate and fluoride in groundwater of a typical alluvial fan plain. Sci. Total Environ. 2022, 802, 149909. [Google Scholar] [CrossRef]
- Adimalla, N.; Qian, H. Groundwater chemistry, distribution and potential health risk appraisal of nitrate enriched groundwater: A case study from the semi-urban region of South India. Ecotoxicol. Environ. Saf. 2021, 207, 111277. [Google Scholar] [CrossRef] [PubMed]
- Amiri, V.; Sohrabi, N.; Li, P.; Amiri, F. Groundwater Quality for Drinking and Non-Carcinogenic Risk of Nitrate in Urban and Rural Areas of Fereidan, Iran. Expo. Health 2022, 15, 807–823. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Liu, Z.; Li, C.; Niu, Y.; Jiang, X. Seasonal nitrate input drives the spatiotemporal variability of regional surface water-groundwater interactions, nitrate sources and transformations. J. Hydrol. 2025, 655, 132973. [Google Scholar] [CrossRef]
- Abu, M.; Egbueri, J.C.; Agbasi, J.C. Kriging-interpolated mapping and predictive modeling of groundwater F− and NO3− contamination with chemometric and health risk assessments in Ghana’s Birimian Province. Environ. Geochem. Health 2025, 47, 165. [Google Scholar] [CrossRef]
- Abascal, E.; Gomez-Coma, L.; Ortiz, I.; Ortiz, A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2022, 810, 152233. [Google Scholar] [CrossRef]
- Yu, G.; Wang, J.; Liu, L.; Li, Y.; Zhang, Y.; Wang, S. The analysis of groundwater nitrate pollution and health risk assessment in rural areas of Yantai, China. BMC Public Health 2020, 20, 437. [Google Scholar] [CrossRef] [PubMed]
- Sahraoui, H.; Fakir, Y.; Bouimouass, H.; Tweed, S.; Leblanc, M.; Benaddi, R.; Chehbouni, A. Spatial and depth distribution of salinity and nitrate in a depleted alluvial aquifer (Haouz plain, Morocco). J. Hydrol. Reg. Stud. 2025, 57, 102143. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, C.; Zhang, D.; Ma, Y.; Huang, P. Integrating the impact of large-scale hydraulic engineering with a sustainable groundwater development strategy: A case study of Zhengzhou City, China. Sci. Total Environ. 2022, 838, 156579. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Wang, Z.; Zhang, T.; Liu, Z.; Ma, Y.; Xiong, Y.; An, F. Assessment and Prediction of Groundwater Vulnerability Based on Land Use Change—A Case Study of the Central Urban Area of Zhengzhou. Water 2024, 16, 3716. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, M.; Wang, S.; Fu, Y.; Lu, J. Effects of extreme heavy rainfall on groundwater in different layers-Taking Zhengzhou 7 · 20 heavy rainstorm as an example. Groundwater 2024, 46, 79–84. (In Chinese) [Google Scholar] [CrossRef]
- Wu, B.; Guo, X.; Jia, X.; Guo, L.; Tang, H.; Liu, S. Vulnerability assessment of shallow groundwater in the central urban area of Zhengzhou. Environ. Prot. Sci. 2022, 48, 135–139+144. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Li, Y.; Liu, Y.; Li, C.; Kong, X.; Zhang, Y. Impacts of River Bank Filtration on Groundwater Hydrogeochemistry in the Upper of Hutuo River Alluvial Plain, North China. Water 2023, 15, 1343. [Google Scholar] [CrossRef]
- Duvva, L.K.; Panga, K.K.; Dhakate, R.; Himabindu, V. Health risk assessment of nitrate and fluoride toxicity in groundwater contamination in the semi-arid area of Medchal, South India. Appl. Water Sci. 2021, 12, 11. [Google Scholar] [CrossRef]
- Adimalla, N. Controlling factors and mechanism of groundwater quality variation in semiarid region of South India: An approach of water quality index (WQI) and health risk assessment (HRA). Environ. Geochem. Health 2020, 42, 1725–1752. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liang, X.; Gong, Y.; Kang, Z.; Jin, H. Health risk assessment of nitrate pollution in shallow groundwater: A case study in Changchun New District, China. Houille Blanche 2019, 105, 45–58. [Google Scholar] [CrossRef]
- Adimalla, N.; Wu, J. Groundwater quality and associated health risks in a semi-arid region of south India: Implication to sustainable groundwater management. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 191–216. [Google Scholar] [CrossRef]
- Adimalla, N.; Li, P.; Qian, H. Evaluation of groundwater contamination for fluoride and nitrate in semi-arid region of Nirmal Province, South India: A special emphasis on human health risk assessment (HHRA). Hum. Ecol. Risk Assess. Int. J. 2018, 25, 1107–1124. [Google Scholar] [CrossRef]
- Gan, L.; Huang, G.; Pei, L.; Gan, Y.; Liu, C.; Yang, M.; Han, D.; Song, J. Distributions, origins, and health-risk assessment of nitrate in groundwater in typical alluvial-pluvial fans, North China Plain. Environ. Sci. Pollut. Res. Int. 2022, 29, 17031–17048. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P. Appraisal of shallow groundwater quality with human health risk assessment in different seasons in rural areas of the Guanzhong Plain (China). Environ. Res. 2021, 207, 112210. [Google Scholar] [CrossRef] [PubMed]
- GB/T 14848—2017; Groundwater Quality Standard. China Standards Press: Beijing, China, 2017.
- Ji, Y.; Wu, J.; Wang, Y.; Elumalai, V.; Subramani, T. Seasonal Variation of Drinking Water Quality and Human Health Risk Assessment in Hancheng City of Guanzhong Plain, China. Expo. Health 2020, 12, 469–485. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, H.; Ren, W.; Wang, H.; Liu, F.; Yang, F. Hydrogeochemical characterization and quality assessment of groundwater based on integrated-weight water quality index in a concentrated urban area. J. Clean. Prod. 2020, 260, 121006. [Google Scholar] [CrossRef]
- Su, C.; Nie, s.; Zou, S.; Zhao, G.; Luo, F.; Huang, Q.; Ba, J.; Li, X.; Liang, J.; Yang, Y. Hydrochemical characteristics and genesis analysis of strontium-rich groundwater in Xintian, Hunan. Mod. Geol. 2018, 32, 554–564. (In Chinese) [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Li, C.; Gao, Z.; Chen, S. An investigation into the hydrochemistry, quality and risk to human health of groundwater in the central region of Shandong Province, North China. J. Clean. Prod. 2021, 282, 125416. [Google Scholar] [CrossRef]
- Wei, M.; Wu, J.; Li, W.; Zhang, Q.; Su, F.; Wang, Y. Groundwater Geochemistry and its Impacts on Groundwater Arsenic Enrichment, Variation, and Health Risks in Yongning County, Yinchuan Plain of Northwest China. Expo. Health 2021, 14, 219–238. [Google Scholar] [CrossRef]
- Marghade, D.; Malpe, D.B.; Duraisamy, K.; Patil, P.D.; Li, P. Hydrogeochemical evaluation, suitability, and health risk assessment of groundwater in the watershed of Godavari basin, Maharashtra, Central India. Environ. Sci. Pollut. Res. Int. 2021, 28, 18471–18494. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, A.; Ren, J.; Qian, H.; Hou, K. Multi-isotope tracer for identifying nitrate sources in shallow groundwater in a large irrigation area, China. J. Environ. Manag. 2025, 376, 124424. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Q.; Lin, Y.; Fang, Y.; Qian, H.; Liu, R.; Ma, H. Hydrogeochemical Characteristics and Quality Assessment of Groundwater in an Irrigated Region, Northwest China. Water 2019, 11, 96. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Zhang, Y.; Nie, B. analysis of chemical characteristics and controlling factors of groundwater in alluvial-proluvial fan of Jianjiang River. Environ. Sci. 2019, 40, 3089–3098. (In Chinese) [Google Scholar] [CrossRef]
- Liu, C.; Liu, F.; Zhen, P.; Guo, X.; Chai, H.; Guo, Y. Groundwater hydrochemical change characteristics and controlling factors of typical pressure mining area in Hebei. Environ. Sci. 2025, 46, 2193–2205. (In Chinese) [Google Scholar] [CrossRef]
- GB 5749-2022; Sanitary Standards for Drinking Water. State Administration for Market Regulation and Standardization Administration of China: Beijing, China, 2022.
- Wei, X.; Zhou, Y.; Ran, L.; Chen, M.; Zou, J.; Fan, Z.; Fu, Y. Sources and Transformation of Nitrate in Shallow Groundwater in the Three Gorges Reservoir Area: Hydrogeochemistry and Isotopes. Water 2024, 16, 3299. [Google Scholar] [CrossRef]
- World Health Organization (WHO), Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization (WHO): Geneva, Switzerland, 2022.
- Adimalla, N. Groundwater Quality for Drinking and Irrigation Purposes and Potential Health Risks Assessment: A Case Study from Semi-Arid Region of South India. Expo. Health 2018, 11, 109–123. [Google Scholar] [CrossRef]
- Gugulothu, S.; Subba Rao, N.; Das, R.; Duvva, L.K.; Dhakate, R. Judging the sources of inferior groundwater quality and health risk problems through intake of groundwater nitrate and fluoride from a rural part of Telangana, India. Environ. Sci. Pollut. Res. Int. 2022, 29, 49070–49091. [Google Scholar] [CrossRef] [PubMed]
- Adimalla, N.; Qian, H.; Nandan, M.J. Groundwater chemistry integrating the pollution index of groundwater and evaluation of potential human health risk: A case study from hard rock terrain of south India. Ecotoxicol. Environ. Saf. 2020, 206, 111217. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; You, M.; Liu, G.; Dong, Z. Distribution and potential health risk of nitrate in centralized groundwater sources of Wanbei Plain, Central China. J. Water Supply Res. Technol. Aqua 2021, 70, 684–695. [Google Scholar] [CrossRef]

| Aquifer Type | Burial Depth (m) | Lithological Characteristics | Aquifer Properties | Thickness (m) | Set of Streams |
|---|---|---|---|---|---|
| Superficial layer | 45~55 | It is dominated by fine sand, containing a clay lens, and locally visible sand–gravel layer. | Diving or Confined water | 25~45 | Flowing from southwest to northeast |
| Middle-deep | 80~350 | It is mainly composed of medium-coarse sand and gravel-bearing medium sand, with brown–red clay layers between layers. | Confined water | 21~41 | From southwest and west to northeast and east |
| Deep layer | 350~800 | Dense sand gravel and brown yellow clay are interbedded, and the degree of cementation is high. | Confined water | 34~140 | From southwest to northeast |
| Ultra-deep | 800~1200 | It is mainly composed of thick glutenite, containing calcareous cement and weak permeability. | Confined water | 86~170 | From west to east or from east to south |
| Parameters | Infant | Child | Youth | Adult |
|---|---|---|---|---|
| RfD (mg·kg−1·d−1) | 1.6 | 1.6 | 1.6 | 1.6 |
| IR (L·d−1) | 0.5 | 1.0 | 1.5 | 2 |
| BW (kg) | 10 | 30 | 50 | 70 |
| ED (a) | 1 | 6 | 12 | 30 |
| EF (d·a−1) | 365 | 365 | 365 | 365 |
| Aquifer Type | Parameters | PH | EC/μs·cm−1 | Ion Concentration/(mg·L−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | HCO3− | Cl− | SO42− | NO3− | F− | TDS | TH | ||||
| Superficial Layer | Minimum | 7.01 | 452.00 | 14.69 | 0.22 | 10.69 | 0.00 | 8.80 | 146.50 | 10.14 | 0.00 | 0.31 | 251.00 | 49.29 |
| Maximum | 8.09 | 1451.00 | 164.70 | 15.77 | 176.00 | 70.32 | 158.90 | 775.40 | 141.80 | 100.10 | 1.22 | 974.40 | 630.47 | |
| Mean | 7.52 | 804.08 | 57.38 | 1.95 | 93.13 | 29.86 | 66.49 | 417.16 | 58.75 | 20.02 | 0.58 | 536.76 | 357.58 | |
| SD | 0.28 | 231.27 | 37.00 | 3.31 | 36.67 | 14.04 | 40.79 | 125.33 | 40.19 | 26.49 | 0.23 | 176.16 | 121.53 | |
| CV | 3.72% | 28.76% | 64.48% | 169.68% | 39.37% | 47.02% | 61.35% | 30.04% | 68.40% | 132.30% | 38.78% | 32.82% | 33.99% | |
| Middle-Deep | Minimum | 7.00 | 433.00 | 14.28 | 0.19 | 23.03 | 9.97 | 6.99 | 238.10 | 9.94 | 0.00 | 0.15 | 261.40 | 113.00 |
| Maximum | 8.15 | 813.00 | 148.30 | 3.38 | 108.60 | 37.90 | 110.60 | 476.20 | 87.65 | 91.41 | 1.05 | 547.10 | 429.20 | |
| Mean | 7.51 | 624.83 | 49.71 | 1.09 | 68.68 | 22.83 | 28.45 | 366.91 | 36.81 | 15.04 | 0.46 | 406.54 | 267.55 | |
| SD | 0.34 | 96.98 | 26.96 | 0.71 | 15.91 | 5.39 | 17.02 | 47.31 | 22.77 | 15.53 | 0.19 | 64.46 | 51.99 | |
| CV | 4.53% | 15.52% | 54.24% | 65.59% | 23.16% | 23.62% | 59.84% | 12.90% | 61.86% | 103.29% | 40.39% | 15.86% | 19.43% | |
| Deep-Layer | Minimum | 7.00 | 563.00 | 45.77 | 0.81 | 4.93 | 2.99 | 15.80 | 317.50 | 17.84 | 0.12 | 0.16 | 374.60 | 26.70 |
| Maximum | 8.10 | 796.00 | 181.90 | 3.11 | 70.73 | 38.90 | 87.77 | 432.44 | 114.55 | 38.05 | 0.37 | 528.80 | 289.60 | |
| Mean | 7.32 | 691.84 | 104.40 | 2.13 | 41.38 | 18.85 | 41.96 | 364.37 | 55.64 | 9.15 | 0.25 | 457.84 | 182.99 | |
| SD | 0.31 | 68.26 | 39.59 | 0.63 | 17.95 | 9.75 | 15.82 | 26.69 | 23.87 | 10.41 | 0.07 | 45.44 | 80.79 | |
| CV | 4.23% | 9.87% | 37.92% | 29.44% | 43.38% | 51.75% | 37.70% | 7.33% | 42.90% | 113.74% | 26.48% | 9.92% | 44.15% | |
| Ultra-Deep | Minimum | 7.11 | 590.00 | 55.79 | 1.04 | 7.40 | 1.00 | 15.80 | 354.10 | 33.80 | 0.00 | 0.28 | 425.95 | 30.80 |
| Maximum | 7.76 | 1355.00 | 324.20 | 4.45 | 74.84 | 20.95 | 159.75 | 622.80 | 299.70 | 5.14 | 1.39 | 909.75 | 275.19 | |
| Mean | 7.37 | 954.08 | 214.57 | 2.61 | 21.45 | 7.29 | 55.32 | 465.40 | 96.75 | 1.74 | 0.72 | 634.06 | 85.62 | |
| SD | 0.20 | 230.93 | 76.65 | 0.97 | 21.55 | 6.45 | 35.88 | 77.76 | 66.43 | 1.93 | 0.31 | 153.13 | 80.00 | |
| CV | 2.71% | 24.20% | 35.72% | 37.24% | 100.49% | 88.50% | 64.86% | 16.71% | 68.66% | 110.54% | 43.70% | 24.15% | 93.44% | |
| Aquifer Type | Parameters | PH | EC/μs·cm−1 | Ion Concentration/(mg·L−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | HCO3− | Cl− | SO42− | NO3− | F− | TDS | TH | ||||
| Superficial Layer | Minimum | 7.51 | 271 | 6.14 | 0.14 | 41.76 | 9.12 | 138.54 | 10.87 | 8.89 | 0.10 | 0.24 | 186.08 | 143.91 |
| Maximum | 7.82 | 1832 | 192.10 | 5.31 | 152.02 | 79.02 | 768.27 | 144.06 | 509.80 | 41.28 | 2.82 | 1239.19 | 629.87 | |
| Mean | 7.67 | 781.80 | 48.82 | 1.11 | 89.61 | 32.92 | 393.13 | 54.45 | 77.90 | 10.67 | 0.75 | 513.01 | 361.42 | |
| SD | 0.08 | 317.90 | 38.46 | 1.08 | 29.30 | 15.99 | 117.89 | 42.19 | 88.85 | 12.32 | 0.48 | 213.09 | 117.88 | |
| CV | 1.09% | 40.66% | 78.77% | 97.48% | 32.69% | 48.57% | 29.99% | 77.48% | 114.05% | 115.46% | 64.13% | 41.54% | 32.62% | |
| Middle-Deep | Minimum | 7.62 | 291.00 | 8.02 | 0.21 | 11.69 | 8.10 | 170.03 | 8.70 | 5.15 | 0.00 | 0.22 | 204.41 | 64.66 |
| Maximum | 7.80 | 985.00 | 187.60 | 2.53 | 135.31 | 45.59 | 528.97 | 93.50 | 103.20 | 84.05 | 0.97 | 653.25 | 441.00 | |
| Mean | 7.64 | 631.38 | 43.57 | 0.87 | 74.15 | 23.21 | 370.12 | 27.24 | 40.43 | 4.35 | 0.52 | 410.11 | 281.47 | |
| SD | 0.10 | 155.41 | 33.55 | 0.56 | 22.05 | 7.15 | 69.85 | 21.10 | 25.50 | 18.28 | 0.19 | 95.26 | 68.09 | |
| CV | 1.27% | 24.61% | 77.00% | 64.00% | 29.74% | 30.79% | 18.87% | 77.46% | 63.07% | 121.49% | 36.12% | 23.23% | 24.19% | |
| Deep- Layer | Minimum | 7.53 | 284 | 4.81 | 0.28 | 20.05 | 9.12 | 144.84 | 13.05 | 12.92 | 0.57 | 0.25 | 189.31 | 89.68 |
| Maximum | 7.79 | 1016 | 125.40 | 2.81 | 96.89 | 50.15 | 478.59 | 95.68 | 119.40 | 46.49 | 1.26 | 681.85 | 447.00 | |
| Mean | 7.69 | 642.91 | 58,7 | 1.45 | 56.80 | 21.78 | 341.77 | 34.23 | 41.68 | 11.27 | 0.56 | 397.47 | 233.28 | |
| SD | 0.07 | 183.61 | 40.46 | 0.77 | 23.37 | 10.55 | 78.66 | 27.14 | 27.94 | 13.09 | 0.26 | 115.02 | 96.24 | |
| CV | 0.91% | 28.56% | 68.93% | 52.99% | 41.14% | 48.45% | 23.01% | 79.30% | 67.03% | 116.15% | 46.55% | 28.94% | 41.26% | |
| Ultra-Deep | Minimum | 7.55 | 563.00 | 39.99 | 0.48 | 5.85 | 5.07 | 528.97 | 11.30 | 13.43 | 0.00 | 0.49 | 370.06 | 43.80 |
| Maximum | 7.75 | 1393.00 | 328.60 | 4.08 | 71.83 | 20.26 | 403.02 | 197.88 | 186.60 | 10.76 | 0.97 | 950.39 | 264.88 | |
| Mean | 7.67 | 977.80 | 207.56 | 2.38 | 23.22 | 10.03 | 450.88 | 72.66 | 99.92 | 3.93 | 0.78 | 644.49 | 101.36 | |
| SD | 0.08 | 327.30 | 102.68 | 1.22 | 24.61 | 5.58 | 44.64 | 67.79 | 64.74 | 4.15 | 0.18 | 214.54 | 82.56 | |
| CV | 1.05% | 33.47% | 49.47% | 51.40% | 105.96% | 55.64% | 9.90% | 93.30% | 64.80% | 105.53% | 23.57% | 33.29% | 81.45% | |
| Partition Code | Chemical Characteristics |
|---|---|
| 1 | Alkaline earth metal ions are greater than alkali metal ions. |
| 2 | Alkali metal ions are greater than alkaline earth metal ions. |
| 3 | Weak acid root is greater than strong acid root. |
| 4 | Strong acid root is greater than weak acid root. |
| 5 | Carbonate hardness > 50%. |
| 6 | Non-carbonate hardness > 50%. |
| 7 | Non-carbonate base > 50%. |
| 8 | Carbonate base > 50%. |
| 9 | No pair of anions and cations > 50%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Liu, X.; Zhang, S.; Zhao, G.; Zhi, J.; Jia, L.; Liu, W.; Lin, D. Analysis of Driving Factors of Groundwater Chemical Characteristics at Different Depths and Health Effects of Nitrate Exposure in Zhengzhou City, China. Water 2025, 17, 2851. https://doi.org/10.3390/w17192851
Zhang C, Liu X, Zhang S, Zhao G, Zhi J, Jia L, Liu W, Lin D. Analysis of Driving Factors of Groundwater Chemical Characteristics at Different Depths and Health Effects of Nitrate Exposure in Zhengzhou City, China. Water. 2025; 17(19):2851. https://doi.org/10.3390/w17192851
Chicago/Turabian StyleZhang, Chunyan, Xujing Liu, Shuailing Zhang, Guizhang Zhao, Jingru Zhi, Lulu Jia, Wenhui Liu, and Dantong Lin. 2025. "Analysis of Driving Factors of Groundwater Chemical Characteristics at Different Depths and Health Effects of Nitrate Exposure in Zhengzhou City, China" Water 17, no. 19: 2851. https://doi.org/10.3390/w17192851
APA StyleZhang, C., Liu, X., Zhang, S., Zhao, G., Zhi, J., Jia, L., Liu, W., & Lin, D. (2025). Analysis of Driving Factors of Groundwater Chemical Characteristics at Different Depths and Health Effects of Nitrate Exposure in Zhengzhou City, China. Water, 17(19), 2851. https://doi.org/10.3390/w17192851







