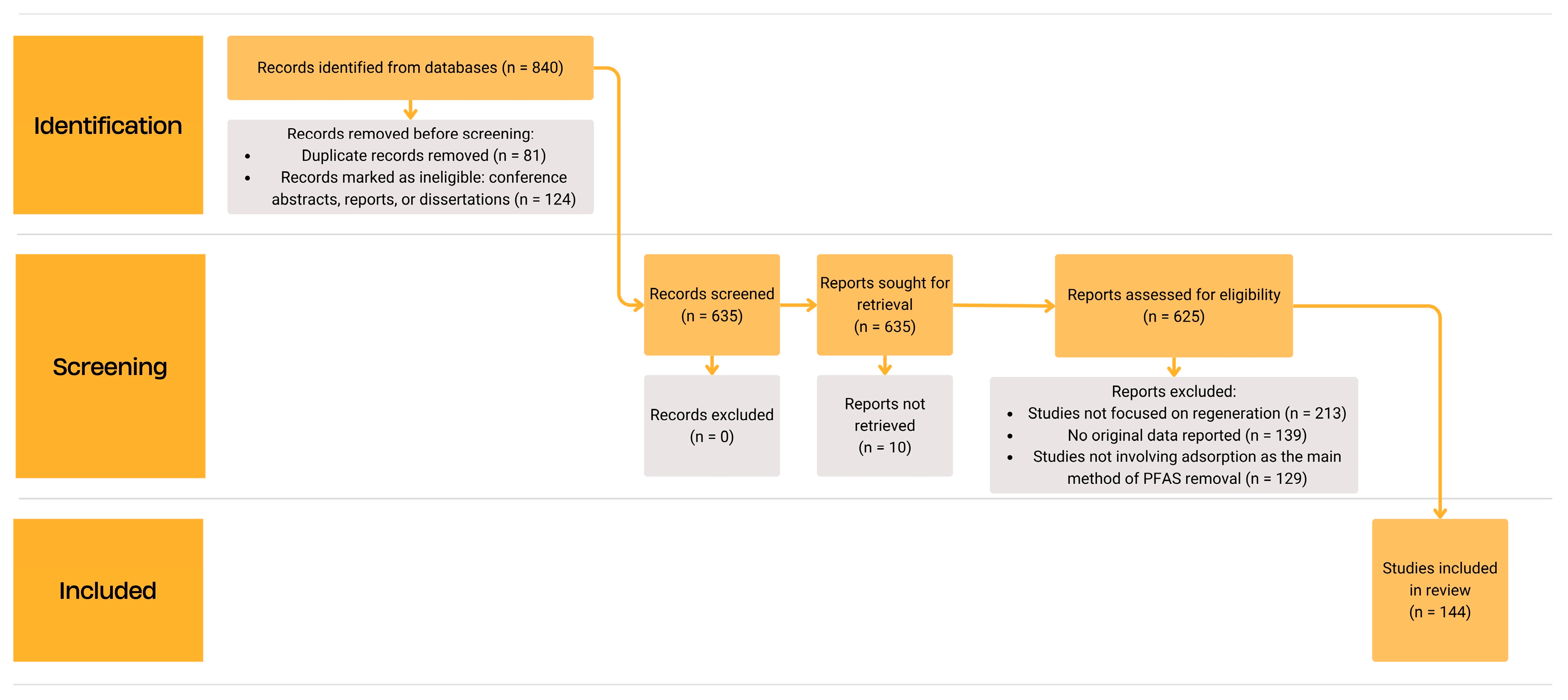
Life After Adsorption: Regeneration, Management, and Sustainability of PFAS Adsorbents in Water Treatment
Abstract
1. Introduction
2. Materials and Methods
3. Policy and Regulatory Context
4. Regeneration and Management of PFAS-Saturated Adsorbents
| Method | Main Advantages | Key Drawbacks |
|---|---|---|
| Thermal treatment [23,25,28,52,55,60,61,132] |
|
|
| Organic solvent-based regeneration [69,76,84,115,119,121,142] |
|
|
| Chemical regeneration (salts, pH/ionic strength shifts, surfactants) [30,104,107,146,147,164] |
|
|
| Emerging and hybrid methods [74,75,87,176,177,180] |
|
|
5. Sustainability of PFAS Adsorbents
6. Research Gaps and Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LCA | Life Cycle Assessment |
| TEA | Techno-Economic Analysis |
| ECHA | European Chemicals Agency |
| SVHCs | Substances of Very High Concern |
| EQSs | Environmental Quality Standards |
| POPs | Persistent organic pollutants |
| REACH | Registration, Evaluation, Authorization, and Restriction of Chemicals |
| FAOLEX | Food, Agriculture, and Renewable Natural Resources Legislation Database |
| PFAS | Per- and polyfluoroalkyl substance |
| PFEAs | Per- and polyfluoroalkyl ether acids |
| PFOS | Perfluorooctane sulfonate |
| PFOA | Perfluorooctanoic acid |
| PFNA | Perfluorononanoic acid |
| PFDA | Perfluorodecanoic acid |
| PFBA | Perfluorobutanoic acid |
| PFBS | Perfluorobutane sulfonate |
| PFHxS | Perfluorohexane sulfonic acid |
| PFHxA | Perfluorohexanoic acid |
References
- Ugrina, M.; Jurić, A. Current Trends and Future Perspectives in the Remediation of Polluted Water, Soil and Air—A Review. Processes 2023, 11, 3270. [Google Scholar] [CrossRef]
- Ugrina, M.; Milojković, J. Advances in Wastewater Treatment, 2024. Energies 2024, 17, 1400. [Google Scholar] [CrossRef]
- European Environment Agency. PFAS Pollution in European Waters. Briefing No. 19/2024. Available online: https://www.eea.europa.eu/en/analysis/publications/pfas-pollution-in-european-waters?utm_source=chatgpt.com&activeTab=81336ca2-2f11-4369-819e-9a74f25a317a (accessed on 12 August 2025).
- European Environment Agency. PFAS Contamination and Soil Remediation (Signal). Available online: https://www.eea.europa.eu/en/european-zero-pollution-dashboards/indicators/pfas-contamination-and-soil-remediation-signal?utm_source=chatgpt.com (accessed on 12 August 2025).
- Cserbik, D.; Casas, M.; Flores, C.; Paraian, A.; Haug, L.S.; Rivas, I.; Bustamante, M.; Dadvand, P.; Sunyer, J.; Vrijheid, M.; et al. Concentrations of per- and polyfluoroalkyl substances (PFAS) in paired tap water and blood samples during pregnancy. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 90–96. [Google Scholar] [CrossRef]
- Uhl, M.; Schoeters, G.; Govarts, E.; Bil, W.; Fletcher, T.; Haug, L.S.; Hoogenboom, R.; Gundacker, C.; Trier, X.; Fernandez, M.F.; et al. PFASs: What can we learn from the European Human Biomonitoring Initiative HBM4EU. Int. J. Hyg. Environ. Health 2023, 250, 114168. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Braun, M.; Groneberg, D.A.; Brüggmann, D. The “forever” per- and polyfluoroalkyl substances (PFAS): A critical accounting of global research on a major threat under changing regulations. Chemosphere 2024, 354, 141694. [Google Scholar] [CrossRef]
- Hussain, H.N.; Jilani, M.I.; Imtiaz, F.; Ahmed, T.; Arshad, M.B.; Mudassar, M.; Sharif, M.N. Advances in the removal of Polyfluoroalkyl Substances (PFAS) from water using destructive and non-destructive methods. Green Anal. Chem. 2025, 12, 100225. [Google Scholar] [CrossRef]
- Sanzana, S.; Fenti, A.; Iovino, P.; Panico, A. A review of PFAS remediation: Separation and degradation technologies for water and wastewater treatment. J. Water Process Eng. 2025, 74, 107793. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Aghaei, M.; Bashardoust, P.; Rezvani Ghalhari, M.; Nayeri, D.; Malekpoor, M.; Sheikhi, S.; Shi, Z. An insight into the environmental and human health impacts of per- and polyfluoroalkyl substances (PFAS): Exploring exposure pathways and their implications. Environ. Sci. Eur. 2025, 37, 81. [Google Scholar] [CrossRef]
- Habib, Z.; Song, M.; Ikram, S.; Zahra, Z. Overview of Per- and Polyfluoroalkyl Substances (PFAS), Their Applications, Sources, and Potential Impacts on Human Health. Pollutants 2024, 4, 136–152. [Google Scholar] [CrossRef]
- European Chemicals Agency. Candidate List of Substances of Very High Concern for Authorisation; ECHA: Helsinki, Finland, 2020. [Google Scholar]
- Jarvis, A.L.; Justice, J.R.; Elias, M.C.; Schnitker, B.; Gallagher, K. Perfluorooctane Sulfonate in US Ambient Surface Waters: A Review of Occurrence in Aquatic Environments and Comparison to Global Concentrations. Environ. Toxicol. Chem. 2021, 40, 2425–2442. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.; Hurst, J.; Miles, J.A.L.; Pancras, T. Per- and Polyfluoroalkyl Substances. In Emerging Contaminants Handbook; Ross, I., Kalve, E., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: New York, NY, USA, 2018; a CRC title, part of the Taylor & Francis imprint, a member of the Taylor & Francis Group, the academic division of T&F Informa, plc, 2018, 2019; pp. 85–262. [Google Scholar]
- United Nations Environment Programme (UNEP). Stockholm Convention on Persistent Organic Pollutants (POPs)—Text and Annexes (Revised in 2023); United Nations Environment Programme (UNEP): Nairobi, Kenya, 2023. [Google Scholar]
- Ackerman Grunfeld, D.; Gilbert, D.; Hou, J.; Jones, A.M.; Lee, M.J.; Kibbey, T.C.G.; O’Carroll, D.M. Underestimated burden of per- and polyfluoroalkyl substances in global surface waters and groundwaters. Nat. Geosci. 2024, 17, 340–346. [Google Scholar] [CrossRef]
- Gebbink, W.A.; van Leeuwen, S.P.J. Environmental contamination and human exposure to PFASs near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in The Netherlands. Environ. Int. 2020, 137, 105583. [Google Scholar] [CrossRef]
- Qi, W.; Clark, J.M.; Timme-Laragy, A.R.; Park, Y. Perfluorobutanesulfonic acid (PFBS) potentiates adipogenesis of 3T3-L1 adipocytes. Food Chem. Toxicol. 2018, 120, 340–345. [Google Scholar] [CrossRef]
- Petrović, M.; Šoštarić, T.; Stojanović, M.; Milojković, J.; Mihajlović, M.; Stanojević, M.; Stanković, S. Removal of Pb2+ ions by raw corn silk (Zea mays L.) as a novel biosorbent. J. Taiwan Inst. Chem. Eng. 2016, 58, 407–416. [Google Scholar] [CrossRef]
- Šoštarić, T.D.; Petrović, M.S.; Pastor, F.T.; Lončarević, D.R.; Petrović, J.T.; Milojković, J.V.; Stojanović, M.D. Study of heavy metals biosorption on native and alkali-treated apricot shells and its application in wastewater treatment. J. Mol. Liq. 2018, 259, 340–349. [Google Scholar] [CrossRef]
- Vukojević Medvidović, N.; Nuić, I.; Ugrina, M.; Trgo, M. Evaluation of Natural Zeolite as a Material for Permeable Reactive Barrier for Remediation of Zinc-Contaminated Groundwater Based on Column Study. Water Air Soil Pollut. 2018, 229, 367. [Google Scholar] [CrossRef]
- Ugrina, M.; Jurić, A.; Nuić, I.; Trgo, M. Modeling, Simulation, Optimization, and Experimental Verification of Mercury Removal onto Natural and Sulfur-Impregnated Zeolite Clinoptilolite—Assessment of Feasibility for Remediation of Mercury-Contaminated Soil. Processes 2023, 11, 606. [Google Scholar] [CrossRef]
- Abou-Khalil, C.; Chernysheva, L.; Miller, A.; Abarca-Perez, A.; Peaslee, G.; Herckes, P.; Westerhoff, P.; Doudrick, K. Enhancing the Thermal Mineralization of Perfluorooctanesulfonate on Granular Activated Carbon Using Alkali and Alkaline-Earth Metal Additives. Environ. Sci. Technol. 2024, 58, 11162–11174. [Google Scholar] [CrossRef]
- Shen, Z.; Zhan, L.; Xu, Z. Thermal defluorination behaviors of PFOS, PFOA and PFBS during regeneration of activated carbon by molten salt. Front. Environ. Sci. Eng. 2022, 16, 103. [Google Scholar] [CrossRef]
- Ramos, P.; Schmidt, M.P.; Xuan, R.; Ashworth, D.J. Biochar selection for removal of perfluoroalkyl substances from reclaimed water for agricultural irrigation. Biochar 2025, 7, 56. [Google Scholar] [CrossRef]
- Dong, Q.; Min, X.; Zhang, W.; Zhao, Y.; Wang, Y. Removal of perfluoroalkyl acids and precursors with silylated clay: Efficient adsorption and enhanced reuse. J. Hazard. Mater. 2024, 480, 136202. [Google Scholar] [CrossRef]
- Mancinelli, M.; Martucci, A.; Salani, G.M.; Bianchini, G.; Gigli, L.; Plaisier, J.R.; Colombo, F. High temperature behaviour of Ag-exchanged Y zeolites used for PFAS sequestration from water. Phys. Chem. Chem. Phys. 2023, 25, 20066–20075. [Google Scholar] [CrossRef]
- Martins, A.S.; Zoumpouli, G.A.; Yi, S.; Exposito, A.J.; Wenk, J.; Mattia, D. 3D-printed indium oxide monoliths for PFAS removal. Chem. Eng. J. 2024, 497, 154366. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, L.; Tang, C.; Yang, Y.; Liang, S.; Wang, A.; Xu, J.; Huang, Q.; Lin, H. Broad-spectrum capture of hundreds of per- and polyfluoroalkyl substances from fluorochemical wastewater. Nat. Commun. 2025, 16, 1972. [Google Scholar] [CrossRef]
- Ellis, A.C.; Boyer, T.H.; Strathmann, T.J. Regeneration of conventional and emerging PFAS-selective anion exchange resins used to treat PFAS-contaminated waters. Sep. Purif. Technol. 2025, 355, 129789. [Google Scholar] [CrossRef]
- Wan, H.; Mills, R.; Qu, K.; Hower, J.C.; Mottaleb, M.A.; Bhattacharyya, D.; Xu, Z. Rapid removal of PFOA and PFOS via modified industrial solid waste: Mechanisms and influences of water matrices. Chem. Eng. J. 2022, 433, 133271. [Google Scholar] [CrossRef]
- Sim, D.; Byun, S.; Lee, Y.S.; Kim, J.-O.; Nam, S.Y.; An, A.K.; Jeong, S. Amplified efficacy of short-chain perfluoroalkyl substances removal with nanofiltration-magnetic activated carbon integration. J. Water Process Eng. 2024, 64, 105585. [Google Scholar] [CrossRef]
- Meesters, R.J.W.; Schröder, H.F. Perfluorooctane sulfonate—A quite mobile anionic anthropogenic surfactant, ubiquitously found in the environment. Water Sci. Technol. 2004, 50, 235–242. [Google Scholar] [CrossRef]
- Meyers, J.M.; Street, S.C.; Thompson, S.; Gellman, A.J. Effect of fluorine on the bonding and orientation of perfluoroalkyl ethers on the Cu(111) surface. Langmuir 1996, 12, 1511–1519. [Google Scholar] [CrossRef]
- Banga, R.; Yarwood, J.; Morgan, A.M.; Evans, B.; Kells, J. In-situ FTIR studies of the kinetics and self assembly of alkyl and perfluoroalkyl trichlorosilanes on silicon. Thin Solid Films 1996, 284–285, 261–266. [Google Scholar] [CrossRef]
- Guo, W.; Tong, H.; Luo, D.; Zhao, X.; Long, Q.; Yin, C.; Yong, Y. Adsorption-based removal of PFASs from water: Mechanisms, materials and future perspective. Environ. Chem. 2025, 22, EN25053. [Google Scholar] [CrossRef]
- Moeini, M.; Modaresahmadi, K.; Tran, T.; Reddy, K.R. Sustainability assessment of PFAS adsorbents for groundwater remediation. Mater. Today Proc. 2022, 60, 2209–2216. [Google Scholar] [CrossRef]
- European Union Directive (EU). 2020/2184 of the European Parliament and of the Council on the Quality of Water Intended for Human Consumption. Off. J. Eur. Union 2020, L435, 1–62. [Google Scholar]
- European Union Regulation (EC). No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Off. J. Eur. Union 2006, L396, 1–849. [Google Scholar]
- European Union Directive. 2000/60/EC of the European Parliament and of the Council Establishing a Framework for Community Action in the Field of Water Policy. Off. J. Eur. Union 2000, L327, 1–73. [Google Scholar]
- National Health and Medical Research Council (NHMRC). Updated Australian Drinking Water Guidelines for Per- and Polyfluoroalkyl Substances (PFAS). Canberra Australian Government. 2025. Available online: https://www.nhmrc.gov.au/about-us/news-centre/updated-australian-drinking-water-guidelines (accessed on 23 September 2025).
- Ottawa Government of Canada. Health Canada Drinking Water Screening Values for Per- and Polyfluoroalkyl Substances (PFAS); Ottawa Government of Canada: Ottawa, ON, Canada, 2024.
- Zeeshan, M.; Tabraiz, S.; Hashmi, S.I.; Iqbal, A.; Dittmann, D.; Abbas, Z.; MacLeod, C.L.; Ruhl, A.S. A comprehensive overview on the occurrence and removal of per- and polyfluoroalkyl substances through adsorption and biodegradation. Bioresour. Technol. Rep. 2025, 29, 102077. [Google Scholar] [CrossRef]
- Pervez, M.N.; Ilango, A.K.; Jiang, T.; Talukder, M.E.; Ehsan, M.N.; Cai, Y.; Liang, Y. PFAS in the textile industry: Sources, fate, detection, and pathways toward sustainable remediation and regulation. Chem. Eng. J. 2025, 522, 168183. [Google Scholar] [CrossRef]
- Vakili, M.; Cagnetta, G.; Deng, S.; Wang, W.; Gholami, Z.; Gholami, F.; Dastyar, W.; Mojiri, A.; Blaney, L. Regeneration of exhausted adsorbents after PFAS adsorption: A critical review. J. Hazard. Mater. 2024, 471, 134429. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- European Union Directive. 2008/105/EC of the European Parliament and of the Council on Environmental Quality Standards in the Field of Water Policy. Off. J. Eur. Union 2008, L348, 84–97. [Google Scholar]
- European Union Regulation (EU). 2019/1021 of the European Parliament and of the Council on Per-sistent Organic Pollutants (POPs). Off. J. Eur. Union 2019, L169, 45–77. [Google Scholar]
- Ellis, A.C.; Boyer, T.H.; Fang, Y.; Liu, C.J.; Strathmann, T.J. Life cycle assessment and life cycle cost analysis of anion exchange and granular activated carbon systems for remediation of groundwater contaminated by per- and polyfluoroalkyl substances (PFASs). Water Res. 2023, 243, 120324. [Google Scholar] [CrossRef] [PubMed]
- Nandikes, G.; Nguyen, A.H.; Oh, S. Towards net-zero adsorbents: A multi-factor selection approach considering performance, life cycle assessment, and end-of-life scenarios. Front. Environ. Sci. Eng. 2025, 19, 148. [Google Scholar] [CrossRef]
- Deng, J.; Han, J.; Hou, C.; Zhang, Y.; Fang, Y.; Du, W.; Li, M.; Yuan, Y.; Tang, C.; Hu, X. Efficient removal of per- and polyfluoroalkyl substances from biochar composites: Cyclic adsorption and spent regenerant degradation. Chemosphere 2023, 341, 140051. [Google Scholar] [CrossRef]
- Birben, N.C.; Zaker, Y.; Faraji, A.; Gagliano, E.; Falciglia, P.P.; Roccaro, P.; Karanfil, T. The effects of granular activated carbon heating rate and moisture content on defluorination of per- and polyfluoroalkyl substances during microwave regeneration. Water Res. 2025, 282, 123618. [Google Scholar] [CrossRef]
- Ao, W.; Mian, M.M.; Zhang, Q.; Zhou, Z.; Deng, S. Bamboo-Derived Low-Cost Mesoporous Biochar for Efficient Removal of Per- and Polyfluoroalkyl Substances from Contaminated Water. ACS ES&T Water 2024, 4, 2711–2720. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Wang, B.; Chen, W.-G.; Zhuang, S.; Chen, Y.; Liu, H.-J. Carbon-dot-modified polyacrylonitrile fiber as recyclable adsorbent for removing anionic, cationic, and zwitterionic perfluorooctane sulfonates from water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129763. [Google Scholar] [CrossRef]
- Sheehan, N.P.; Ponge, C.A.; Pankratz, A.; Hutchison, J.M.; Laird, B.B.; Nguyen, N.P.; Shiflett, M.B.; Timalsina, D.; Wang, M.Z.; Peltier, E.F. Interference of PFAS sorption on zeolites from natural water characteristics. Chemosphere 2025, 378, 144414. [Google Scholar] [CrossRef]
- Jiang, T.; Pervez, M.N.; Ilango, A.K.; Ravi, Y.K.; Zhang, W.; Feldblyum, J.I.; Yigit, M.V.; Efstathiadis, H.; Liang, Y. Magnetic surfactant-modified clay for enhanced adsorption of mixtures of per- and polyfluoroalkyl substances (PFAS) in snowmelt: Improving practical applicability and efficiency. J. Hazard. Mater. 2024, 471, 134390. [Google Scholar] [CrossRef]
- Huo, J.; Min, X.; Dong, Q.; Xu, S.; Wang, Y. Comparison of Zn–Al and Mg–Al layered double hydroxides for adsorption of perfluorooctanoic acid. Chemosphere 2022, 287, 132297. [Google Scholar] [CrossRef]
- Bhattarai, B.; Muruganandham, M.; Suri, R.P.S. Development of high efficiency silica coated β-cyclodextrin polymeric adsorbent for the removal of emerging contaminants of concern from water. J. Hazard. Mater. 2014, 273, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, X.; Luo, P.; Dai, S.; Zhang, W.; Zhang, Y. Novel Fluorinated Nitrogen-Rich Porous Organic Polymer for Efficient Removal of Perfluorooctanoic Acid from Water. Water 2022, 14, 1010. [Google Scholar] [CrossRef]
- Abdelsamad, A.M.A.; Saeidi, N.; Mackenzie, K. Mesoporous silica nanoparticles for rapid removal of PFOA: Impact of surface functional groups on adsorption efficiency and adsorbent regeneration. Environ. Pollut. 2025, 383, 126796. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Usman, M.; Haderlein, S.; Hanna, K. Carbonaceous materials for adsorptive removal of perfluoroalkyl and polyfluoroalkyl substances (PFAS) and regeneration of spent adsorbents. J. Water Process Eng. 2024, 68, 106498. [Google Scholar] [CrossRef]
- Huang, X.; Huang, J.; Wang, K.; Hao, M.; Geng, M.; Shi, B.; Hu, C. Comparison of perfluoroalkyl substance adsorption performance by inorganic and organic silicon modified activated carbon. Water Res. 2024, 260, 121919. [Google Scholar] [CrossRef]
- Shaikh, M.A.N.; Sarkar, P.; Nawaz, T. PFOA remediation from aqueous media using CTAB impregnated activated carbon: A closed-loop sustainable study with comprehensive selectivity analysis. J. Water Process Eng. 2023, 54, 103965. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarkar, D.; Datta, R.; Deng, Y. Adsorption of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) by aluminum-based drinking water treatment residuals. J. Hazard. Mater. Lett. 2021, 2, 100034. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Y.; Ma, W.; Yan, L.; Li, K.; Lin, S. Surface molecular imprinting on carbon microspheres for fast and selective adsorption of perfluorooctane sulfonate. J. Hazard. Mater. 2018, 348, 29–38. [Google Scholar] [CrossRef]
- Wang, W.; Mi, X.; Shi, H.; Zhang, X.; Zhou, Z.; Li, C.; Zhu, D. Adsorption behaviour and mechanism of the PFOS substitute OBS (sodium p-perfluorous nonenoxybenzene sulfonate) on activated carbon. R. Soc. Open Sci. 2019, 6, 191069. [Google Scholar] [CrossRef]
- Gao, B.; Wu, L.; Li, S.; Yang, J.; Dou, M.; Chang, G.; Li, X. Rapid capture of perfluorooctanoic acid and perfluorooctane sulfonate at environmentally relevant concentrations via the ‘mesh trap’ of triazine-based polymer network: Mechanism and photocatalytic regeneration. J. Hazard. Mater. 2025, 483, 136698. [Google Scholar] [CrossRef]
- Chen, Y.; Georgi, A.; Zhang, W.; Kopinke, F.-D.; Yan, J.; Saeidi, N.; Li, J.; Gu, M.; Chen, M. Mechanistic insights into fast adsorption of perfluoroalkyl substances on carbonate-layered double hydroxides. J. Hazard. Mater. 2021, 408, 124815. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Wang, W.; Shen, X.; Wang, J.; Chen, S.; Yu, X.; Lee, C.T.; Chen, Z.; Gu, C. Highly efficient removal of per- and polyfluoroalkyl substances by extrusion-regenerated aminated polyurethane sponges. Water Res. 2025, 275, 123189. [Google Scholar] [CrossRef]
- Guo, B.; Kan, E.; Zeng, S. Enhanced adsorption of aqueous perfluorooctanoic acid on iron-functionalized biochar: Elucidating the roles of inner-sphere complexation. Sci. Total Environ. 2024, 955, 176926. [Google Scholar] [CrossRef]
- Wang, W.; Maimaiti, A.; Shi, H.; Wu, R.; Wang, R.; Li, Z.; Qi, D.; Yu, G.; Deng, S. Adsorption behavior and mechanism of emerging perfluoro-2-propoxypropanoic acid (GenX) on activated carbons and resins. Chem. Eng. J. 2019, 364, 132–138. [Google Scholar] [CrossRef]
- Ranjbar, E.; Ahmadi, F.; Baghdadi, M. Regeneration of perfluorooctane sulfonic acid (PFOS) loaded granular activated carbon using organic/inorganic mixed solutions. Chem. Eng. Sci. 2024, 300, 120623. [Google Scholar] [CrossRef]
- Soker, O.; Tajdini, B.; Abarca-Perez, A.; Wadia, A.; Bellona, C.; Hao, S.; Doudrick, K.; Strathmann, T.J. Reuse of spent granular activated carbon for PFAS removal following hydrothermal alkaline treatment. Water Res. 2025, 283, 123794. [Google Scholar] [CrossRef]
- Trzcinski, A.P.; Harada, K.H. Comparison of perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and perfluorobutane sulfonate (PFBS) removal in a combined adsorption and electrochemical oxidation process. Sci. Total Environ. 2024, 927, 172184. [Google Scholar] [CrossRef] [PubMed]
- Ersan, G.; Ersan, M.S.; Perreault, F.; Garcia-Segura, S. Enabling in situ electro-regeneration systems for PFOA-laden spent activated carbon adsorbents reuse. J. Environ. Chem. Eng. 2023, 11, 111369. [Google Scholar] [CrossRef]
- Chaudhary, A.; Usman, M.; Mašek, O.; Haderlein, S.; Hanna, K. Simultaneous removal of PFAS and cadmium from different water matrices using regenerable carbonaceous adsorbents. J. Water Process Eng. 2025, 74, 107875. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Kosaka, K.; Asami, M.; Matsui, Y. Maximum desorption of perfluoroalkyl substances adsorbed on granular activated carbon used in full-scale drinking water treatment plants. Water Res. 2024, 254, 121396. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Fang, X.; Maimaiti, A.; Yu, G.; Deng, S. Efficient removal of perfluorinated compounds from water using a regenerable magnetic activated carbon. Chemosphere 2019, 224, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Sasi, P.C.; Alinezhad, A.; Yao, B.; Kubátová, A.; Golovko, S.A.; Golovko, M.Y.; Xiao, F. Effect of granular activated carbon and other porous materials on thermal decomposition of per- and polyfluoroalkyl substances: Mechanisms and implications for water purification. Water Res. 2021, 200, 117271. [Google Scholar] [CrossRef]
- Zang, L.; Zhu, C.; Zhang, M.; Shu, Y.; Liu, X.; Wang, Z. Comparative analysis of enhanced adsorption and thermal decomposition of oil-borne PFAS using CeO2 nanoparticles and activated carbon. J. Hazard. Mater. Adv. 2024, 16, 100469. [Google Scholar] [CrossRef]
- Sonmez Baghirzade, B.; Zhang, Y.; Reuther, J.F.; Saleh, N.B.; Venkatesan, A.K.; Apul, O.G. Thermal Regeneration of Spent Granular Activated Carbon Presents an Opportunity to Break the Forever PFAS Cycle. Environ. Sci. Technol. 2021, 55, 5608–5619. [Google Scholar] [CrossRef]
- Soker, O.; Hao, S.; Trewyn, B.G.; Higgins, C.P.; Strathmann, T.J. Application of Hydrothermal Alkaline Treatment to Spent Granular Activated Carbon: Destruction of Adsorbed PFASs and Adsorbent Regeneration. Environ. Sci. Technol. Lett. 2023, 10, 425–430. [Google Scholar] [CrossRef]
- Umeh, A.C.; Hassan, M.; Egbuatu, M.; Zeng, Z.; Al Amin, M.; Samarasinghe, C.; Naidu, R. Multicomponent PFAS sorption and desorption in common commercial adsorbents: Kinetics, isotherm, adsorbent dose, pH, and index ion and ionic strength effects. Sci. Total Environ. 2023, 904, 166568. [Google Scholar] [CrossRef]
- Kwak, Y.; Oh, M.; Nam, C. Enhanced removal of short-chain perfluoroalkyl substance using aminated waste-derived sorbent. J. Water Process Eng. 2025, 74, 107829. [Google Scholar] [CrossRef]
- Li, R.; Alomari, S.; Stanton, R.; Wasson, M.C.; Islamoglu, T.; Farha, O.K.; Holsen, T.M.; Thagard, S.M.; Trivedi, D.J.; Wriedt, M. Efficient Removal of Per- and Polyfluoroalkyl Substances from Water with Zirconium-Based Metal–Organic Frameworks. Chem. Mater. 2021, 33, 3276–3285. [Google Scholar] [CrossRef]
- Gagliano, E.; Falciglia, P.P.; Zaker, Y.; Karanfil, T.; Roccaro, P. Microwave regeneration of granular activated carbon saturated with PFAS. Water Res. 2021, 198, 117121. [Google Scholar] [CrossRef]
- Shrestha, B.; Ezazi, M.; Ajayan, S.; Kwon, G. Reversible adsorption and desorption of PFAS on inexpensive graphite adsorbents via alternating electric field. RSC Adv. 2021, 11, 34652–34659. [Google Scholar] [CrossRef]
- Deng, S.; Nie, Y.; Du, Z.; Huang, Q.; Meng, P.; Wang, B.; Huang, J.; Yu, G. Enhanced adsorption of perfluorooctane sulfonate and perfluorooctanoate by bamboo-derived granular activated carbon. J. Hazard. Mater. 2015, 282, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Deng, S.; Chen, Y.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Removal of perfluorinated carboxylates from washing wastewater of perfluorooctanesulfonyl fluoride using activated carbons and resins. J. Hazard. Mater. 2015, 286, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Chularueangaksorn, P.; Tanaka, S.; Fujii, S.; Kunacheva, C. Adsorption of perfluorooctanoic acid (PFOA) onto anion exchange resin, non-ion exchange resin, and granular-activated carbon by batch and column. Desalin. Water Treat. 2014, 52, 6542–6548. [Google Scholar] [CrossRef]
- Freilinger, J.; Back, J.O.; Plangger, R.; Schottenberger, H.; Huck, C.W.; Rupprich, M.; Bakry, R. Development of a fluorophilic ion-exchange material with dual binding mechanism for solid-phase extraction of PFAS. J. Hazard. Mater. Lett. 2025, 6, 100158. [Google Scholar] [CrossRef]
- He, J.; Gomeniuc, A.; Olshansky, Y.; Hatton, J.; Abrell, L.; Field, J.A.; Chorover, J.; Sierra-Alvarez, R. Enhanced removal of per- and polyfluoroalkyl substances by crosslinked polyaniline polymers. Chem. Eng. J. 2022, 446, 137246. [Google Scholar] [CrossRef]
- Ilango, A.K.; Arathala, P.; Musah, R.A.; Liang, Y. Experimental and density functional theory investigation of surface-modified biopolymer for improved adsorption of mixtures of per- and polyfluoroalkyl substances in water. Water Res. 2024, 255, 121458. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, X.; Wang, K.; Wang, X.; Zhang, L.; Jing, H.; Gao, R.; Wang, S. Fabrication of tailored imprinted layer and cladded layer on magnetic nanomaterials with enhanced specificity for recognition of PFOS. Anal. Chim. Acta 2025, 1349, 343799. [Google Scholar] [CrossRef]
- Wan, H.; Fang, F.; Shi, K.; Yi, Z.; Lei, L.; Li, S.; Mills, R.; Bhattacharyya, D.; Xu, Z. pH-Swing membrane adsorption of perfluoroalkyl substances: Anion-exchange brushes and role of water chemistry. Sep. Purif. Technol. 2024, 329, 124800. [Google Scholar] [CrossRef]
- Cao, F.; Wang, L.; Yao, Y.; Wu, F.; Sun, H.; Lu, S. Synthesis and application of a highly selective molecularly imprinted adsorbent based on multi-walled carbon nanotubes for selective removal of perfluorooctanoic acid. Environ. Sci. Water Res. Technol. 2018, 4, 689–700. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Z.; Zhang, X.; Wimmer, A.; Shi, E.; Qin, Y.; Zhao, X.; Zhou, B.; Li, L. Rapid and efficient removal of organic micropollutants from environmental water using a magnetic nanoparticles-attached fluorographene-based sorbent. Chem. Eng. J. 2018, 343, 61–68. [Google Scholar] [CrossRef]
- Steigerwald, J.M.; Peng, S.; Ray, J.R. Novel Perfluorooctanesulfonate-Imprinted Polymer Immobilized on Spent Coffee Grounds Biochar for Selective Removal of Perfluoroalkyl Acids in Synthetic Wastewater. ACS ES&T Eng. 2023, 3, 520–532. [Google Scholar] [CrossRef]
- Gao, P.; Cui, J.; Deng, Y. Direct regeneration of ion exchange resins with sulfate radical-based advanced oxidation for enabling a cyclic adsorption—Regeneration treatment approach to aqueous perfluorooctanoic acid (PFOA). Chem. Eng. J. 2021, 405, 126698. [Google Scholar] [CrossRef]
- Xie, R.; Beckman, M.T.; Almquist, C.B.; Berberich, J.A.; Danielson, N.D. Fixed-bed adsorption of perfluorooctanoic acid from water by a polyamine-functionalized polychlorotrifluoroethylene-ethylene polymer coated on activated carbon. J. Environ. Chem. Eng. 2024, 12, 113001. [Google Scholar] [CrossRef]
- Fang, F.; Chen, S.; Shi, K.; Xu, S.; Yi, Z.; Lei, L.; Zhuang, L.; Wan, H.; Xu, Z. Hydrophilic membranes for effective removal of PFAS from water: Anti-fouling, durability, and reusability. Sep. Purif. Technol. 2024, 348, 127379. [Google Scholar] [CrossRef]
- Park, M.; Daniels, K.D.; Wu, S.; Ziska, A.D.; Snyder, S.A. Magnetic ion-exchange (MIEX) resin for perfluorinated alkylsubstance (PFAS) removal in groundwater: Roles of atomic charges for adsorption. Water Res. 2020, 181, 115897. [Google Scholar] [CrossRef]
- Shu, H.-H.; Shi, L.-Q.; Zhang, H.-Q.; Liu, Y.; Han, S.-L.; Rao, J.-Y.; Liu, C.-M. Synthesis of furan-based cationic biopolyamides and their removal abilities for perfluoroalkyl and polyfluoroalkyl substances. Chem. Eng. J. 2025, 507, 160306. [Google Scholar] [CrossRef]
- Shahrokhi, R.; Hubbe, M.A.; Park, J. Comparative assessment of activated carbon and anion exchange resin for short- and long-chain per- and poly-fluoroalkyl substances sorption: Insight into performance and mechanism. J. Water Process Eng. 2024, 64, 105703. [Google Scholar] [CrossRef]
- Saad, A.; Mills, R.; Wan, H.; Mottaleb, M.A.; Ormsbee, L.; Bhattacharyya, D. Thermo-responsive adsorption-desorption of perfluoroorganics from water using PNIPAm hydrogels and pore functionalized membranes. J. Memb. Sci. 2020, 599, 117821. [Google Scholar] [CrossRef] [PubMed]
- Zeidabadi, F.A.; Esfahani, E.B.; McBeath, S.T.; Mohseni, M. Managing PFAS exhausted Ion-exchange resins through effective regeneration/electrochemical process. Water Res. 2024, 255, 121529. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, R.; Xu, X.; Li, Y.; Wang, N.; Leiviskä, T. Sorption/desorption and degradation of long- and short-chain PFAS by anion exchange resin and UV/sulfite system. Environ. Pollut. 2024, 361, 124847. [Google Scholar] [CrossRef]
- Shaikh, M.A.N.; Nawaz, T. Efficient removal of short- and long-chain perfluoroalkyl carboxylate acids from surface water matrices using a quaternary ammonium functionalized adsorbent derived from waste Karanja shells. Environ. Sci. Water Res. Technol. 2025, 11, 2002–2016. [Google Scholar] [CrossRef]
- Min, X.; Wang, Y. Enhanced adsorption of short-chain perfluorobutanoic acid by functionalized periodic mesoporous organosilica: Performance and mechanisms. J. Hazard. Mater. 2023, 449, 131047. [Google Scholar] [CrossRef] [PubMed]
- Aly, Y.H.; McInnis, D.P.; Lombardo, S.M.; Arnold, W.A.; Pennell, K.D.; Hatton, J.; Simcik, M.F. Enhanced adsorption of perfluoro alkyl substances for in situ remediation. Environ. Sci. Water Res. Technol. 2019, 5, 1867–1875. [Google Scholar] [CrossRef]
- Min, X.; Huo, J.; Dong, Q.; Xu, S.; Wang, Y. Enhanced sorption of perfluorooctanoic acid with organically functionalized layered double hydroxide. Chem. Eng. J. 2022, 446, 137019. [Google Scholar] [CrossRef]
- Kim, H.-H.; Koster van Groos, P.G.; Zhao, Y.; Pham, A.L.-T. Removal of PFAS by hydrotalcite: Adsorption mechanisms, effect of adsorbent aging, and thermal regeneration. Water Res. 2024, 260, 121925. [Google Scholar] [CrossRef]
- Mahpishanian, S.; Zhou, M.; Foudazi, R. Magnetic amino-functionalized graphene oxide nanocomposite for PFAS removal from water. Environ. Sci. Adv. 2024, 3, 1698–1713. [Google Scholar] [CrossRef]
- Xian, A.; Wei, C.; Tang, Z.; Zhang, Y.; Wang, Q.; Han, Z.; Song, X. Fluorinated LDHs for selective sorption of PFOS: Unveiling the roles of increased hydrophobicity and F-F interactions. Sep. Purif. Technol. 2025, 362, 131851. [Google Scholar] [CrossRef]
- Mahanty, B.; Saawarn, B.; Mahto, B.; Hussain, S.; Hait, S. Efficient removal of perfluorooctanoic acid from aqueous matrices using cationic surfactant functionalized graphene oxide nanocomposite: RSM and ANN modeling, and adsorption behaviour. J. Water Process Eng. 2024, 68, 106448. [Google Scholar] [CrossRef]
- Sahu, O. Remediation of perfluorooctanoic acid (PFOA) with nano ceramic clay: Synthesis, characterization, scale-up and regenerations. Environ. Pollut. 2023, 322, 121241. [Google Scholar] [CrossRef]
- Nie, Z.; Sui, C.; Xie, X.; Liu, H.; Chen, Y.; Ni, S.-Q.; Cai, B.; Kong, L.; Zhan, J. Bio-inspired interfacial micro-electric field of FeOCl nanosheets for rapid adsorption of perfluorocarboxylic acids (PFCAs). Sep. Purif. Technol. 2023, 327, 124980. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Yu, Y.; Wang, F.; Yin, W.; Liu, Y.; Ye, P.; Gong, Y. Synergistic adsorption and photocatalytic degradation of perfluorooctanoic acid in aqueous solution by a regenerable biochar-titania nanotube composite. RSC Adv. 2025, 15, 14917–14928. [Google Scholar] [CrossRef] [PubMed]
- Borui, W.; Xiangyangcun; Zhong, M.; Yang, H. Comparative Assessment of Short-Chain PFAS Removal Using MIP-202: An Eco-Friendly, High-Performance, Fast-Recoverable, and Economic Adsorbent for Water Treatment. Water Air Soil Pollut. 2024, 235, 612. [Google Scholar] [CrossRef]
- Arana Juve, J.-M.; Li, F.; Zhu, Y.; Liu, W.; Ottosen, L.D.M.; Zhao, D.; Wei, Z. Concentrate and degrade PFOA with a photo-regenerable composite of In-doped TNTs@AC. Chemosphere 2022, 300, 134495. [Google Scholar] [CrossRef] [PubMed]
- Yousif, M.; Zhang, M.; Hussain, B.; Khan, T.; Hu, W.; Li, M.; Zhuang, X.; Wang, Z.; Wang, H.; Lin, T. Effective PFAS removal from water using vinyltrimethoxysilane-modified polyethyleneimine-aramid-banana nanocellulose aerogels. Sep. Purif. Technol. 2025, 375, 133667. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Bhattarai, B.; Suri, R.P.S. Environmentally Friendly β-Cyclodextrin–Ionic Liquid Polyurethane-Modified Magnetic Sorbent for the Removal of PFOA, PFOS, and Cr(VI) from Water. ACS Sustain. Chem. Eng. 2017, 5, 9223–9232. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Zhang, Y.; Mamadiev, M. Facile and efficient synthesis of polyacrylonitrile-based functional fibers and its sorption properties of perfluorooctane sulfonate and perfluorooctanoate. J. Mol. Liq. 2017, 241, 1013–1022. [Google Scholar] [CrossRef]
- Huang, J.; Fu, K.; Liu, H.; Zhang, J.; Luo, J. Unveiling the Differential Intensity of Fluorous Active Sites Toward Selective Polyfluoroalkyl Substance Removal: Insights into Adsorption and Desorption Trade-Offs. Environ. Sci. Technol. 2025, 59, 10650–10661. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, T.; Wang, Y.; Wei, X.; Yue, Z.; Bai, D.; Zhao, W.; Zhang, S.; Zhang, W. Synthesis of Olefin-Linked high stability covalent organic frameworks for the removal of perfluoroalkyl acids from water. Sep. Purif. Technol. 2024, 338, 126476. [Google Scholar] [CrossRef]
- Liu, T.; Gu, Y.; Xing, D.Y.; Dong, W.; Wu, X. Rapid and high-capacity adsorption of PFOS and PFOA by regenerable ammoniated magnetic particle. Environ. Sci. Pollut. Res. 2018, 25, 13813–13822. [Google Scholar] [CrossRef]
- Li, F.; Wei, Z.; He, K.; Blaney, L.; Cheng, X.; Xu, T.; Liu, W.; Zhao, D. A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst. Water Res. 2020, 185, 116219. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Wang, B.; Zhuang, S.; Chen, Y.; Wei, Y.-P. Polyacrylonitrile fiber functionalized with fluorous hyperbranched polyethylenimine for selective removal of perfluorooctane sulfonate (PFOS) in firefighting wastewaters. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126539. [Google Scholar] [CrossRef]
- Wan, H.; Fang, F.; Shi, K.; Yi, Z.; Zeng, L.; Bhattacharyya, D.; Tang, K.; Xu, Z. Dual-functional adsorptive membranes for PFAS removal: Mechanism, CFD simulation, and selective enrichment. Chem. Eng. J. 2024, 500, 156095. [Google Scholar] [CrossRef]
- Ren, Z.; Bergmann, U.; Uwayezu, J.N.; Carabante, I.; Kumpiene, J.; Lejon, T.; Leiviskä, T. Combination of adsorption/desorption and photocatalytic reduction processes for PFOA removal from water by using an aminated biosorbent and a UV/sulfite system. Environ. Res. 2023, 228, 115930. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Dong, J.; Li, Y.; Zhang, W.; Li, Y. Adsorption of low-concentration perfluorooctanoic acid on corn stover-based lignin amine by synergy of electrostatic and hydrophobic interactions. New J. Chem. 2025, 49, 8553–8563. [Google Scholar] [CrossRef]
- Zhang, Q.; Mian, M.M.; Zhang, A.; Zhou, L.; Du, R.; Ao, W.; Yu, G.; Deng, S. Catalytic Degradation of Hexafluoropropylene Oxide Trimeric Acid during the Hydrothermal Regeneration of Spent Activated Carbon. ACS ES&T Eng. 2024, 4, 1391–1400. [Google Scholar] [CrossRef]
- Rodrigo, P.M.; Navarathna, C.; Pham, M.T.H.; McClain, S.J.; Stokes, S.; Zhang, X.; Perez, F.; Gunatilake, S.R.; Karunanayake, A.G.; Anderson, R.; et al. Batch and fixed bed sorption of low to moderate concentrations of aqueous per- and poly-fluoroalkyl substances (PFAS) on Douglas fir biochar and its Fe3O4 hybrids. Chemosphere 2022, 308, 136155. [Google Scholar] [CrossRef]
- Sun, Y.; Angelotti, B.; Brooks, M.; Dowbiggin, B.; Evans, P.J.; Devins, B.; Wang, Z.-W. A pilot-scale investigation of disinfection by-product precursors and trace organic removal mechanisms in ozone-biologically activated carbon treatment for potable reuse. Chemosphere 2018, 210, 539–549. [Google Scholar] [CrossRef]
- Aumeier, B.M.; Georgi, A.; Saeidi, N.; Sigmund, G. Is sorption technology fit for the removal of persistent and mobile organic contaminants from water? Sci. Total Environ. 2023, 880, 163343. [Google Scholar] [CrossRef]
- Ramos, P.; Singh Kalra, S.; Johnson, N.W.; Khor, C.M.; Borthakur, A.; Cranmer, B.; Dooley, G.; Mohanty, S.K.; Jassby, D.; Blotevogel, J.; et al. Enhanced removal of per- and polyfluoroalkyl substances in complex matrices by polyDADMAC-coated regenerable granular activated carbon. Environ. Pollut. 2022, 294, 118603. [Google Scholar] [CrossRef]
- Xing, D.Y.; Chen, Y.; Zhu, J.; Liu, T. Fabrication of hydrolytically stable magnetic core-shell aminosilane nanocomposite for the adsorption of PFOS and PFOA. Chemosphere 2020, 251, 126384. [Google Scholar] [CrossRef]
- Naim Shaikh, M.A.; Nawaz, T. Highly Efficient Cationic Surfactant Functionalized Alginate Hydrogel for Perfluorooctanoic Acid Adsorption: Optimization through Response Surface Methodology and Performance Evaluation for Aqueous Media. ACS ES&T Water 2024, 4, 3078–3088. [Google Scholar] [CrossRef]
- Usman, M.; Ahmed, A.; Yu, B.; Rafiq, M.; Ji, Z.; Shen, Y.; Cong, H. Installation of synergetic binding sites in β-Cyclodextrin-Bipyridine ionic liquid based magnetic sorbent for simultaneous removal of anionic PFAS and Cr (Ⅵ) in water matrix. Sep. Purif. Technol. 2024, 335, 126190. [Google Scholar] [CrossRef]
- Wang, T.; Wu, J.; Hu, T.; Wang, C.; Li, S.; Li, Z.; Chen, J. Mechanistic insights into adsorption-desorption of PFOA on biochars: Effects of biomass feedstock and pyrolysis temperature, and implication of desorption hysteresis. Sci. Total Environ. 2024, 957, 177668. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, M.; Zhang, S.; Jiang, W.; Xiu, T.; Yang, S.; Kang, M.; Dongye, Z.; Li, Z.; Wang, L. Microwave synthesis of metal-organic frameworks absorbents (DUT-5-2) for the removal of PFOS and PFOA from aqueous solutions. Microporous Mesoporous Mater. 2022, 333, 111740. [Google Scholar] [CrossRef]
- Song, X.-L.; Zhu, S.-Q.; Liu, Y.-Q.; Wang, D.-D.; Wang, X.-S.; Lv, H.; Wang, P.-P.; Li, L.; Li, F.; Wang, J.-L.; et al. Novel graphene oxide hollow microspheres adsorbent for efficient extraction of perfluoroalkyl substances in environmental water samples. Microchem. J. 2025, 215, 114368. [Google Scholar] [CrossRef]
- Rittner, T.; Staudt, K.; Boßmann, B.; Kautenburger, R.; Ruthes, J.G.A.; Kay, C.W.M.; Presser, V.; Beck, H.P.; Gallei, M. Polyelectrolyte metallopolymer particles for efficient PFAS capture and release. Desalination 2025, 613, 119018. [Google Scholar] [CrossRef]
- Salimi Torkamani, Z.; Shahin, M.S.; Baghdadi, M. Preparation of high-capacity adsorbent for removing perfluorooctane sulfonic acid (PFOS) from aquatic solutions using expanded graphite-modified with iron/triethylenetetramine. J. Water Process Eng. 2024, 68, 106299. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Ma, S.; Zhang, Q. Adsorption of per- and polyfluoroalkyl substances (PFAS) from water with porous organic polymers. Chemosphere 2024, 346, 140600. [Google Scholar] [CrossRef] [PubMed]
- Butzlaff, A.H.; Mezgebe, B.; Collins, A.; Lin, Z.-W.; Lassalle-Vega, D.; Harmody, I.M.; Coronell, O.; Leibfarth, F.A.; Dichtel, W.R.; Nadagouda, M.; et al. Comparative evaluation of PFAS-selective adsorbents in hard-to-treat residual waste streams. Chem. Eng. J. 2025, 511, 161983. [Google Scholar] [CrossRef]
- Tan, X.; Dewapriya, P.; Prasad, P.; Chang, Y.; Huang, X.; Wang, Y.; Gong, X.; Hopkins, T.E.; Fu, C.; Thomas, K.V.; et al. Efficient Removal of Perfluorinated Chemicals from Contaminated Water Sources Using Magnetic Fluorinated Polymer Sorbents. Angew. Chem. Int. Ed. 2022, 61, e202213071. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, X.; Wang, X.; Song, Y.; Wang, W.; Zhang, M.; Kong, D.; Chen, Z.; Gu, C. Efficient separation of per- and polyfluoroalkyl substances (PFAS) from water by aminated polyacrylamide hydrogel foam. Chem. Eng. J. 2024, 501, 157833. [Google Scholar] [CrossRef]
- Zaggia, A.; Conte, L.; Falletti, L.; Fant, M.; Chiorboli, A. Use of strong anion exchange resins for the removal of perfluoroalkylated substances from contaminated drinking water in batch and continuous pilot plants. Water Res. 2016, 91, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Lynch, R.; Solomon, J.; Weaver, J.D.; May, A.R. Development of novel fluor mop materials for remediation of perfluoroalkyl substances (PFAS) from groundwater. J. Hazard. Mater. 2023, 448, 130853. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, S.; Jiang, X.; Yu, G.; Deng, S. Fluorinated quaternary ammonium covalent organic frameworks for selective and efficient removal of typical per- and polyfluoroalkyl substances. Chem. Eng. J. 2023, 474, 145629. [Google Scholar] [CrossRef]
- Wang, W.-R.; Chen, P.-Y.; Deng, J.; Chen, Y.; Liu, H.-J. Carbon-dot hydrogels as superior carbonaceous adsorbents for removing perfluorooctane sulfonate from water. Chem. Eng. J. 2022, 435, 135021. [Google Scholar] [CrossRef]
- Niu, B.; Yu, M.; Sun, C.; Wang, L.; Niu, Y.; Huang, H.; Zheng, Y. A Comparative Study for Removal of Perfluorooctanoic Acid Using Three Kinds of N-polymer Functionalized Calotropis Gigantea Fiber. J. Nat. Fibers 2022, 19, 2119–2128. [Google Scholar] [CrossRef]
- Zhang, Z.; Joudiazar, S.; Satpathy, A.; Fernando, E.; Rahmati, R.; Kim, J.; de Falco, G.; Datta, R.; Sarkar, D. Removal of Per- and Polyfluoroalkyl Substances Using Commercially Available Sorbents. Materials 2025, 18, 1299. [Google Scholar] [CrossRef] [PubMed]
- Kassar, C.; Graham, C.; Boyer, T.H. Removal of perfluoroalkyl acids and common drinking water contaminants by weak-base anion exchange resins: Impacts of solution pH and resin properties. Water Res. X 2022, 17, 100159. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, X.; Xiang, H.; Wang, X.; Luo, X.; Fu, J.; Fan, J. TPU/PEI nanofibers for the efficient removal of PFOA and its analogs: Effect of pH, humic acid and co-existing ions, mechanism, and regenerative effects. J. Environ. Chem. Eng. 2024, 12, 113210. [Google Scholar] [CrossRef]
- Olshansky, Y.; Gomeniuc, A.; Chorover, J.; Abrell, L.; Field, J.A.; Hatton, J.; He, J.; Sierra-Alvarez, R. Tailored Polyanilines Are High-Affinity Adsorbents for Per- and Polyfluoroalkyl Substances. ACS ES&T Water 2022, 2, 1402–1410. [Google Scholar] [CrossRef]
- Pala, J.; Le, T.; Kasula, M.; Rabbani Esfahani, M. Systematic investigation of PFOS adsorption from water by Metal Organic Frameworks, Activated Carbon, Metal Organic Framework@Activated Carbon, and functionalized Metal Organic Frameworks. Sep. Purif. Technol. 2023, 309, 123025. [Google Scholar] [CrossRef]
- Elanchezhiyan, S.S.D.; Muthu Prabhu, S.; Han, J.; Kim, Y.M.; Yoon, Y.; Park, C.M. Synthesis and characterization of novel magnetic Zr-MnFe2O4@rGO nanohybrid for efficient removal of PFOA and PFOS from aqueous solutions. Appl. Surf. Sci. 2020, 528, 146579. [Google Scholar] [CrossRef]
- Fang, Y.; Meng, P.; Schaefer, C.; Knappe, D.R.U. Removal and destruction of perfluoroalkyl ether carboxylic acids (PFECAs) in an anion exchange resin and electrochemical oxidation treatment train. Water Res. 2023, 230, 119522. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Sun, M. Ion exchange removal and resin regeneration to treat per- and polyfluoroalkyl ether acids and other emerging PFAS in drinking water. Water Res. 2021, 207, 117781. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, Q.; Yang, M.; Wang, Y.; Liu, N.; Zhang, X. Magnetic ion exchange resin for effective removal of perfluorooctanoate from water: Study of a response surface methodology and adsorption performances. Environ. Sci. Pollut. Res. 2018, 25, 29267–29278. [Google Scholar] [CrossRef]
- Dixit, F.; Barbeau, B.; Mostafavi, S.G.; Mohseni, M. PFAS and DOM removal using an organic scavenger and PFAS-specific resin: Trade-off between regeneration and faster kinetics. Sci. Total Environ. 2021, 754, 142107. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.C.; Liu, C.J.; Fang, Y.; Boyer, T.H.; Schaefer, C.E.; Higgins, C.P.; Strathmann, T.J. Pilot study comparison of regenerable and emerging single-use anion exchange resins for treatment of groundwater contaminated by per- and polyfluoroalkyl substances (PFASs). Water Res. 2022, 223, 119019. [Google Scholar] [CrossRef]
- Chularueangaksorn, P.; Tanaka, S.; Fujii, S.; Kunacheva, C. Regeneration and reusability of anion exchange resin used in perfluorooctane sulfonate removal by batch experiments. J. Appl. Polym. Sci. 2013, 130, 884–890. [Google Scholar] [CrossRef]
- Riegel, M.; Haist-Gulde, B.; Sacher, F. Sorptive removal of short-chain perfluoroalkyl substances (PFAS) during drinking water treatment using activated carbon and anion exchanger. Environ. Sci. Eur. 2023, 35, 12. [Google Scholar] [CrossRef]
- Khazdooz, L.; Zarei, A.; Abbaspourrad, A. Synthesis of an anion exchange resin for enhanced PFAS adsorption in water treatment. RSC Appl. Polym. 2025, 3, 885–896. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Li, L.; Xu, L. Removal of perfluorooctanoic acid from water with economical mesoporous melamine-formaldehyde resin microsphere. Chem. Eng. J. 2017, 320, 501–509. [Google Scholar] [CrossRef]
- Cagnetta, G.; Yin, Z.; Qiu, W.; Vakili, M. Mechanochemical Synthesis of Cross-Linked Chitosan and Its Application as Adsorbent for Removal of Per- and Polyfluoroalkyl Substances from Simulated Electroplating Wastewater. Materials 2024, 17, 3006. [Google Scholar] [CrossRef]
- Tamanna, T.; Mahon, P.J.; Hockings, R.K.; Alam, H.; Raymond, M.; Smith, C.; Clarke, C.; Yu, A. Ion Exchange MIEX® GOLD Resin as a Promising Sorbent for the Removal of PFAS Compounds. Appl. Sci. 2023, 13, 6263. [Google Scholar] [CrossRef]
- Deng, J.; Fang, Y.; Hou, C.; Zhang, Y.; Li, M.; Han, J.; Du, W.; Tang, C.; Hu, X. Ultrasonic assisted activation of persulfate for the treatment of spent porous biochar: Degradation of adsorbed PFOA and adsorbent regeneration. J. Environ. Chem. Eng. 2023, 11, 111146. [Google Scholar] [CrossRef]
- Wang, B.; Lee, L.S.; Wei, C.; Fu, H.; Zheng, S.; Xu, Z.; Zhu, D. Covalent triazine-based framework: A promising adsorbent for removal of perfluoroalkyl acids from aqueous solution. Environ. Pollut. 2016, 216, 884–892. [Google Scholar] [CrossRef]
- Niaz, W.; Zhang, D.; Ahmad, Z.; Shen, N.; Haider, W.; Ali, I.; Usman, M.; Majid, A.; Javaid, S.F.; Amjed, M.A.; et al. Efficient removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) from aqueous solution using modified biochar: Preparation, performance, and mechanistic insights. J. Environ. Chem. Eng. 2024, 12, 114894. [Google Scholar] [CrossRef]
- Teng, B.; Zhao, Z.; Wu, J.; Xia, L.; Wang, Y.; Wang, H.; Yemele, O.M.; Adnan, M. Study on the effect of alginate coated Ca-Fe bimetallic composite biochar microspheres on the removal of short-chain PFAS. Environ. Res. 2025, 285, 122395. [Google Scholar] [CrossRef]
- Hussain, F.A.; Janisse, S.E.; Heffern, M.C.; Kinyua, M.; Velázquez, J.M. Adsorption of perfluorooctanoic acid from water by pH-modulated Brönsted acid and base sites in mesoporous hafnium oxide ceramics. iScience 2022, 25, 104138. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Xu, X.; Zhu, L. Response of methanogenic granules enhanced by magnetite to ammonia stress. Water Res. 2022, 212, 118123. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Guo, Z.; Wang, R.; Yang, L.; Cao, Y.; Wang, H. A novel approach for treating acid mine drainage by forming schwertmannite driven by a combination of biooxidation and electroreduction before lime neutralization. Water Res. 2022, 221, 118748. [Google Scholar] [CrossRef]
- Mukherjee, P.; Sathiyan, K.; Zidki, T.; Nadagouda, M.N.; Sharma, V.K. Electrochemical degradation of per- and poly-fluoroalkyl substances in the presence of natural organic matter. Sep. Purif. Technol. 2023, 325, 124639. [Google Scholar] [CrossRef]
- Li, G.; Dunlap, J.; Wang, Y.; Huang, Q.; Li, K. Environmental Life Cycle Assessment (LCA) of Treating PFASs with Ion Exchange and Electrochemical Oxidation Technology. ACS ES&T Water 2022, 2, 1555–1564. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, T.; Zhao, D.; Li, F.; Liu, W.; Wang, B.; An, B. Adsorption and solid-phase photocatalytic degradation of perfluorooctane sulfonate in water using gallium-doped carbon-modified titanate nanotubes. Chem. Eng. J. 2021, 421, 129676. [Google Scholar] [CrossRef]
- Xu, T.; Ji, H.; Gu, Y.; Tong, T.; Xia, Y.; Zhang, L.; Zhao, D. Enhanced adsorption and photocatalytic degradation of perfluorooctanoic acid in water using iron (hydr)oxides/carbon sphere composite. Chem. Eng. J. 2020, 388, 124230. [Google Scholar] [CrossRef]
- Beyioku, O.E.; Gilboa, A.; Ronen, A. PFAS adsorption and desorption on functionalized surfaces: A QCM and kinetic modeling approach. Sep. Purif. Technol. 2025, 372, 133457. [Google Scholar] [CrossRef]
- Sun, H.; Cannon, F.S.; He, X. Effective removal of perfluorooctanoate from groundwater using quaternary nitrogen-grafted granular activated carbon. J. Water Process Eng. 2020, 37, 101416. [Google Scholar] [CrossRef]
- Murray, C.C.; Safulko, A.; Vatankhah, H.; Liu, C.J.; Tajdini, B.; Marshall, R.E.; Bellona, C. PFAS adsorbent selection: The role of adsorbent use rate, water quality, and cost. J. Hazard. Mater. 2023, 454, 131481. [Google Scholar] [CrossRef]
- Roman, J.B.; Kemperman, A.J.B.; van der Meer, W.G.J.; Wood, J.A. Assessing the viability of bio-based adsorbents for PFAS removal. Chem. Eng. Sci. 2025, 306, 121215. [Google Scholar] [CrossRef]

| PFAS | Typical Sources/Uses | Occurrence in Environment |
|---|---|---|
| PFOS (Perfluorooctane sulfonate) [10,13,14,15] | Formerly used in firefighting foams (AFFFs), stain- and water-resistant coatings for textiles, leather and paper, metal plating, and pesticides. Phased out in EU by 2010 under the Stockholm Convention on Persistent Organic Pollutants (listed as a persistent organic pollutant) but still present in legacy materials. | Widespread in EU surface waters, often exceeding quality standards. High persistence and bioaccumulation in rivers, lakes, sediments and biota (fish, wildlife), especially near airports and industrial sites. |
| PFOA (Perfluorooctanoic acid) [10,14,15,16,17] | Processing aid in fluoropolymer manufacturing (e.g., Teflon/PTFE), non-stick cookware, waterproof textiles and coatings, and some AFFFs. Listed under the Stockholm Convention in 2019; production and use in the EU have ceased, with industry substituting alternative PFASs (e.g., GenX). | Common in EU groundwater and surface water near chemical plants and former production areas. Persists and travels long distances; frequently detected in drinking water and food. |
| PFHxS (Perfluorohexane sulfonate) [10,14,15] | Used in some AFFFs, textiles and leather coatings; also an impurity in PFOS products. PFHxS and its salts were added to the Stockholm Convention in 2022 due to their extreme persistence (notably long human half-life) and bioaccumulative properties. | Found with PFOS at contaminated sites (airports, fire training areas). Detected in EU groundwater and human serum; mobile in water and accumulates in top predators. |
| PFNA (Perfluorononanoic acid) [14,16] | Surfactant/processing aid in some fluoropolymers (e.g., to polymerise vinylidene fluoride for PVDF plastic); also a degradation product of other PFASs. Limited EU production but present via imports and precursors breakdown. | Detected at lower levels than PFOS/PFOA but still notable in EU water and wildlife. Long-chain and bioaccumulative; contributes to dietary PFAS exposure. |
| PFBS (Perfluorobutane sulfonate) [14,16,18] | PFOS replacement used in water- and stain-resistant coatings for textiles, carpets, and paper, and as an industrial surfactant. It yields a much lower bioaccumulation potential in humans. | Increasingly detected in EU rivers and groundwater, especially near textile and waste facilities. Highly mobile and can reach drinking water despite lower bioaccumulation. |
| GenX (Hexafluoropropylene oxide dimer acid, HFPO-DA) [14,15,16,17] | PFOA replacement in fluoropolymer production (e.g., making Teflon-like coatings). Used as processing emulsifier. Came into use in the EU after PFOA was phased out. GenX was added to the EU Substances of Very High Concern list in 2019. | High levels found downstream of manufacturing plants; also detected in groundwater, rainwater and crops. Persistent but less bioaccumulative than PFOA. |
| Regulation | Summary of Key PFAS-Related Aspects | Practical Implications |
|---|---|---|
| Drinking Water Directive (2020/2184) [38] |
| Adsorbent systems must achieve very low PFAS residual concentrations; regeneration or disposal methods must ensure compliance with strict limits. |
| Water Framework Directive (2000/60/EC) [40] |
| Indirectly drives reduction in PFASs in aquatic environments; promotes safe disposal to prevent leaching. |
| Environmental Quality Standards Directive (2008/105/EC) [47] |
| Encourages end-of-life management methods that prevent PFAS release to meet EQS values. |
| REACH Regulation (1907/2006) [39] |
| May restrict use of certain adsorbents if they contain or degrade to PFASs; impacts choice of regeneration chemicals. |
| ECHA SVHCs Candidate List [12] |
| Identifies high-risk PFASs to be prioritised in treatment and end-of-life strategies. |
| POPs Regulation (2019/1021) [48] |
| Disposal and destruction strategies must eliminate PFASs to meet POPs requirements; limits landfill disposal options. |
| Category | Adsorbent | Chemical Structure | Functional Groups | Main Adsorption Mechanisms | Adsorption Efficiency |
|---|---|---|---|---|---|
| Carbon-based | Biochar loaded with sulfobetaine–acrylamide copolymer [51] | Wood-derived biochar (BET 22.8 m2/g) coated with SBMA–acrylamide copolymer (BET 167.3 m2/g). | Quaternary ammonium groups (SBMA), –SO3− groups, amide (–CONH2) groups from polyacrylamide, N- and S-containing groups on biochar surface. | Combined hydrophobic interaction (fluorocarbon chain–polymer), electrostatic attraction of anionic PFASs to cationic groups, pore diffusion and complexation. | ~265–635 mg/g |
| Bituminous granular activated carbon (Filtrasorb 400, F400) [52] | Microporous bituminous coal-based GAC commonly used in water treatment plants; BET area ~1000 m2/g. | Surface oxygen-containing groups; high microporosity; PFASs trapped in pores with 65–70% moisture content. | Primarily hydrophobic adsorption of long-chain PFASs; electrostatic interactions for short-chain PFASs; pore-filling and aggregation inside micropores. | ~0.2 mg/g | |
| Bamboo-derived mesoporous biochar [53] | Highly porous carbon with micropores (0.6–1.3 nm) and mesopores (2–4 nm); SBET 1085 m2/g, pore volume 0.670 cm3/g, O/C 0.09, H/C 0.27. | Predominantly aromatic carbon with reduced oxygen-containing groups (C–O, C=O); some oxygen groups reintroduced during steam activation; very low surface polarity; near-neutral zeta potential at pH 7.5. | Hydrophobic interactions (favouring long-chain PFASs), electrostatic attraction for anionic PFASs, pore-filling (pores ~1–3× PFOS size), enrichment at air bubbles on the surface. | ~640 mg/g | |
| Polymeric | Carbon-dot-modified polyacrylonitrile fibre (PAN-g-CD) [54] | Polyacrylonitrile fibre grafted with amine-functionalised carbon dots (CDs)—fibrous, low surface area (15–16 m2 g−1), average pore radius ~1.6 nm. | Zwitterionic with amine groups (pH-responsive), carboxylate groups, anchored carbon dots providing hydrophobic domains. | Combined electrostatic attraction/repulsion depending on PFOS derivative head group; hydrophobic interactions between CDs and fluorocarbon chains; chemisorption on energetically heterogeneous surface. | ~640–1055 mg/g |
| Anion exchange resin (AER) [29] | Strong-base anion exchange resin with quaternary ammonium functional groups on a cross-linked polystyrene matrix (gel-type). | Quaternary ammonium groups (Cl− form initially); hydrophobic polymer backbone. | Ion exchange (replacement of Cl− with PFAS anions), hydrophobic interactions, semi-micellar adsorption in presence of long-chain PFASs, enhanced by pre-treatment to remove competing anions. | ~775 mg/g | |
| Mineral-based | Calcined beta zeolite (BEA) [55] | Ion exchange (replacement of Cl− with PFAS anions), hydrophobic interactions, semi-micellar adsorption in presence of long-chain PFASs, enhanced by pre-treatment to remove competing anions. | Bronsted-acid sites (Si–O(H)–Al) and Lewis-acid sites inside micropores; hydrophilic external surface, internal hydrophobic channels; converted to hydrogen form after calcination at 550 °C. | Size-selective molecular sieving; electrostatic interactions with cations; hydrophobic adsorption inside pores; minimal effect of pH 6–9 and natural organic matter at high loading. | ~100 mg/g |
| Magnetic surfactant-modified clay (MMC) [56] | Montmorillonite (2:1 layered aluminosilicate) intercalated with CTAC, then co-precipitated with Fe2+/Fe3+ to form magnetite nanoparticles; particle size ~3.6 µm; BET surface area 48 m2/g. | Quaternary ammonium groups from CTAC impart positive charge; Fe–O groups from magnetite; Si–O framework from clay. | Electrostatic attraction of anionic PFASs to positively charged CTAC-modified clay; hydrophobic interactions between PFAS fluorocarbon chains and CTAC tails; ion exchange and complexation with Fe3+ sites. | ~35 mg/g | |
| Zn–Al LDH (nitrate intercalated) [57] | Layered double hydroxides with brucite-like positively charged layers and exchangeable nitrate anions; Zn/Al ratio 3:1 and Mg/Al ratio 3:1. | Abundant hydroxyl groups within LDH layers; interlayer nitrate ions; high positive surface charge (pHpzc ~10 for Zn–Al). | Mainly electrostatic interactions between positively charged layers and anionic PFASs; fast uptake (Zn–Al LDH removes ~95% PFOA in 1 h); enhanced affinity with longer chain PFASs. | ~625 mg/g | |
| Emerging/hybrid | β-Cyclodextrin polymer coated on silica [58] | Silica particles (40 × 100 mesh) coated with β-cyclodextrin crosslinked by hexamethylene diisocyanate (HMDI) in DMSO; ~32% w/w polymer loading; core–shell morphology. | β-CD with abundant hydroxyl groups; carbamate linkages from HMDI; silanol groups from silica. | Inclusion complexation (β-CD cavity trapping PFOA), hydrophobic interactions and hydrogen bonding between PFASs and β-CD hydroxyl rims; host–guest complexation stabilised by hydrogen bonding. | ~0.3 mg/g |
| POP-4F (fluorine and amine porous organic polymer) [59] | Mainly electrostatic interactions between positively charged layers and anionic PFASs; fast uptake (Zn–Al LDH removes ~95% PFOA in 1 h); enhanced affinity with longer-chain PFASs. | Triazine nitrogen, secondary amines (protonated at pH < 6), fluorine atoms providing hydrophobic domains. | Strong electrostatic attraction between protonated amines and deprotonated PFOA carboxylate; hydrophobic interactions from fluorinated framework; rapid uptake due to high surface area. | ~100 mg/g | |
| AMSN (mesoporous silica nanoparticles APTES only) [60] | Dendritic mesoporous silica nanoparticles (average 177 nm) with ~14.4 nm pores; functionalised solely with APTES. | Protonated amine groups (–NH3+ at pH < 9) on silica surface; silanol groups partly capped. | Predominantly electrostatic attraction to anionic PFASs; limited hydrophobic interaction compared to OAMSN; slower kinetics. | ~80 mg/g |
| Adsorbents | Main Findings |
|---|---|
| Granular activated carbon vs. ion exchange resin [37] |
|
| Strong-base anion exchange resin [49] |
|
| Reusable ion exchange resin vs. single-use resin [164] |
|
| Granular activated carbon, ion exchange resin, and emerging adsorbents [184] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrunik, M.; Smol, M. Life After Adsorption: Regeneration, Management, and Sustainability of PFAS Adsorbents in Water Treatment. Water 2025, 17, 2813. https://doi.org/10.3390/w17192813
Andrunik M, Smol M. Life After Adsorption: Regeneration, Management, and Sustainability of PFAS Adsorbents in Water Treatment. Water. 2025; 17(19):2813. https://doi.org/10.3390/w17192813
Chicago/Turabian StyleAndrunik, Magdalena, and Marzena Smol. 2025. "Life After Adsorption: Regeneration, Management, and Sustainability of PFAS Adsorbents in Water Treatment" Water 17, no. 19: 2813. https://doi.org/10.3390/w17192813
APA StyleAndrunik, M., & Smol, M. (2025). Life After Adsorption: Regeneration, Management, and Sustainability of PFAS Adsorbents in Water Treatment. Water, 17(19), 2813. https://doi.org/10.3390/w17192813







