Hydrochemical Characteristics and Evolution Mechanisms of Shallow Groundwater in the Alluvial–Coastal Transition Zone of the Tangshan Plain, China
Abstract
1. Introduction
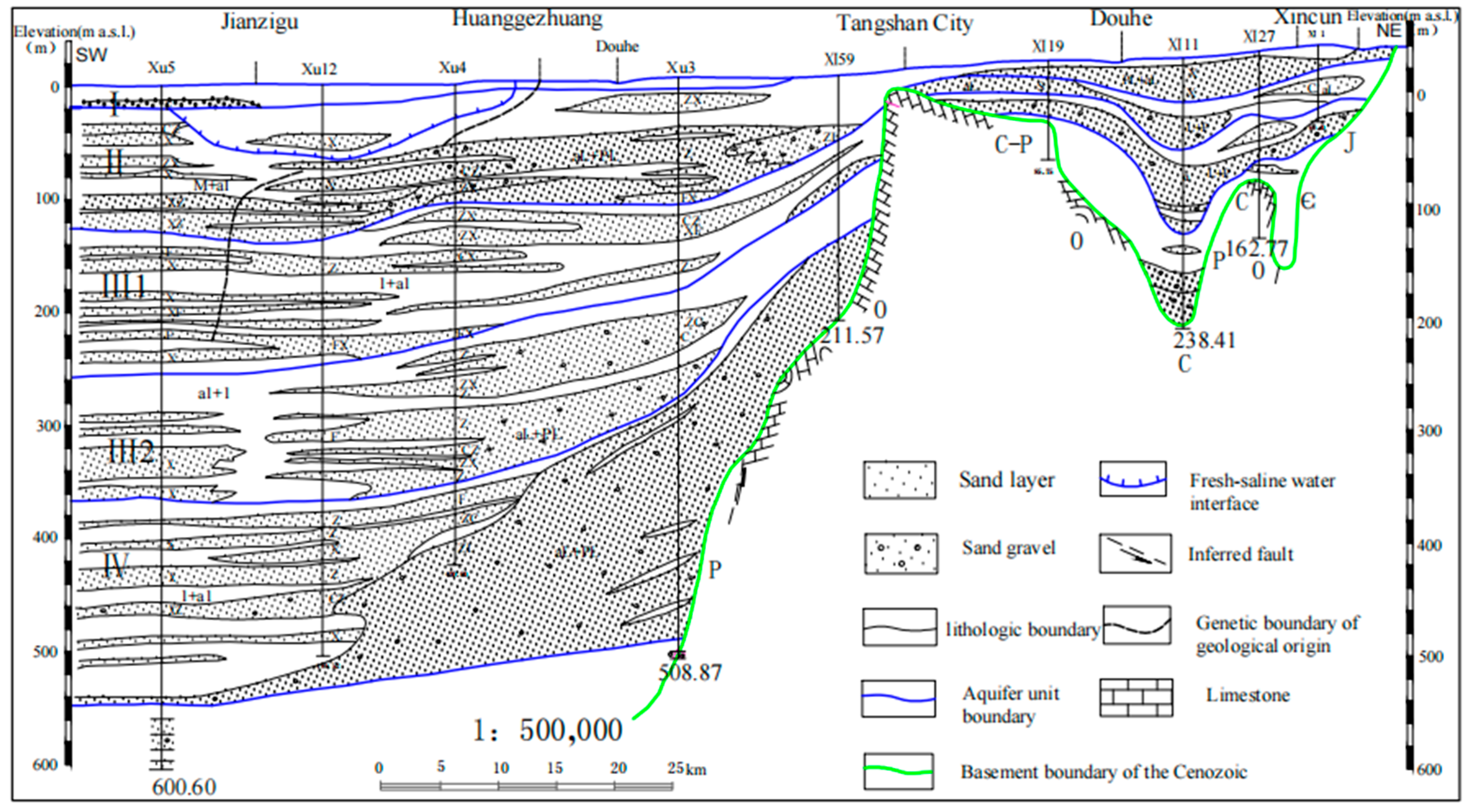
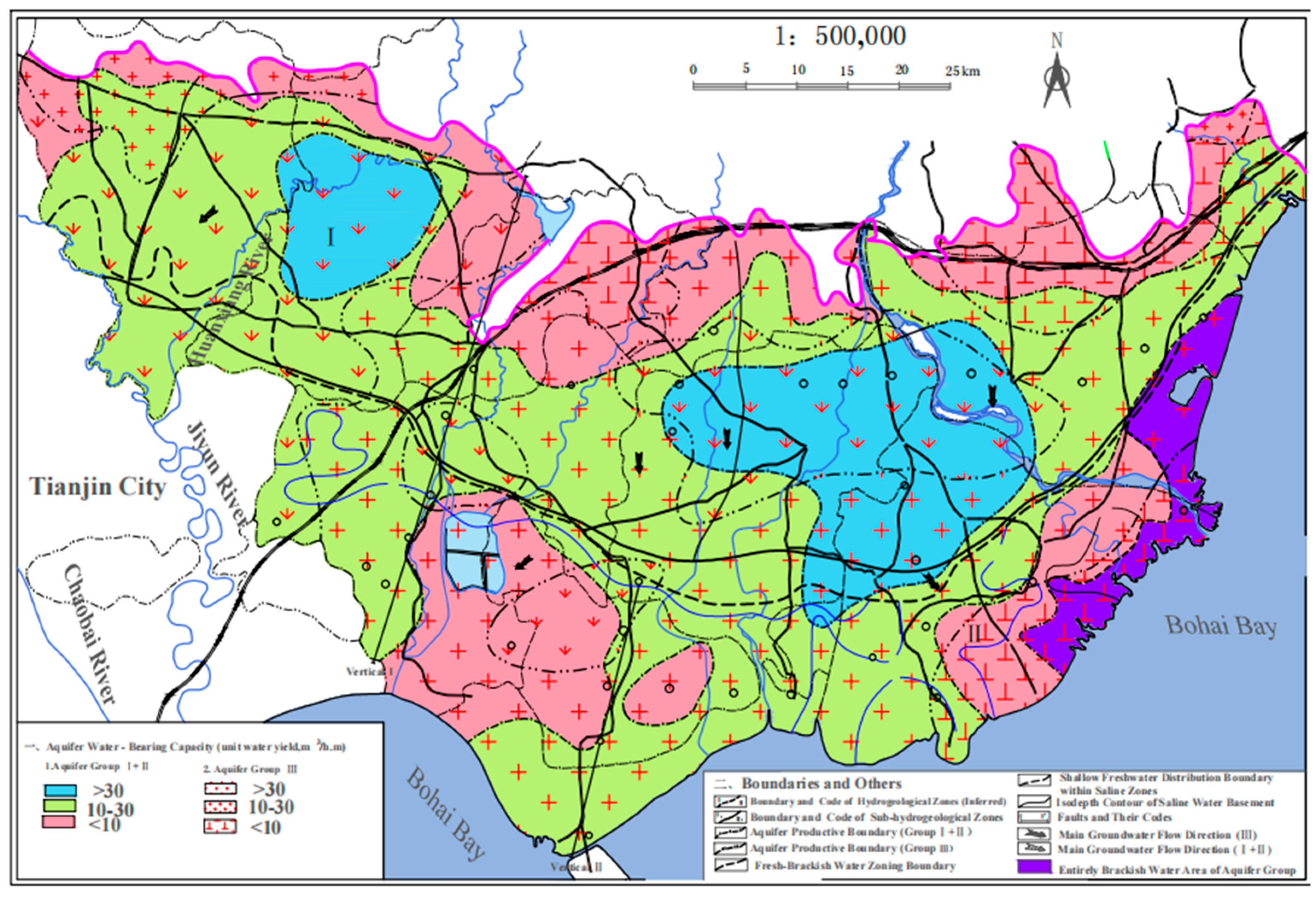
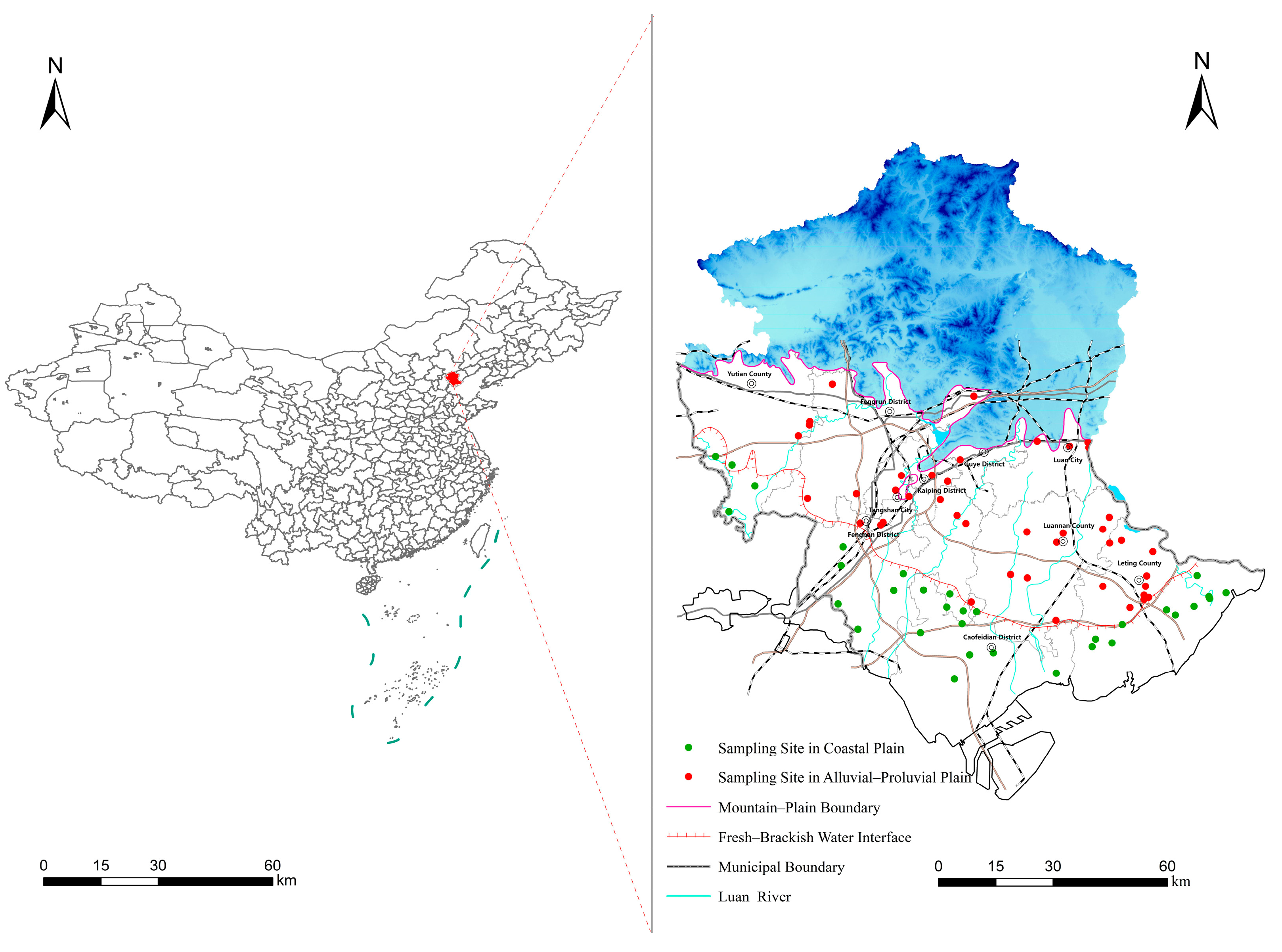
2. Study Area
3. Materials and Methods
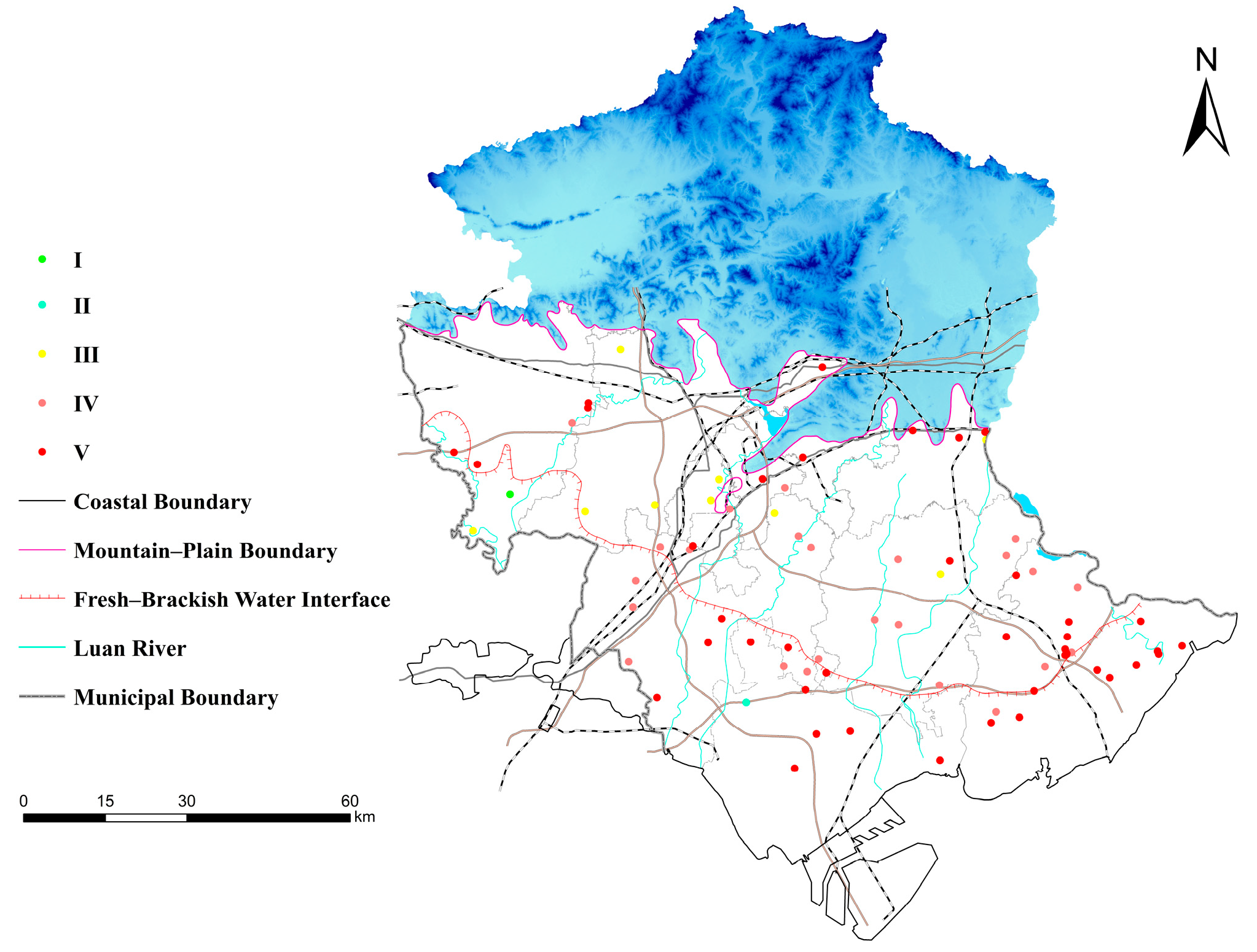
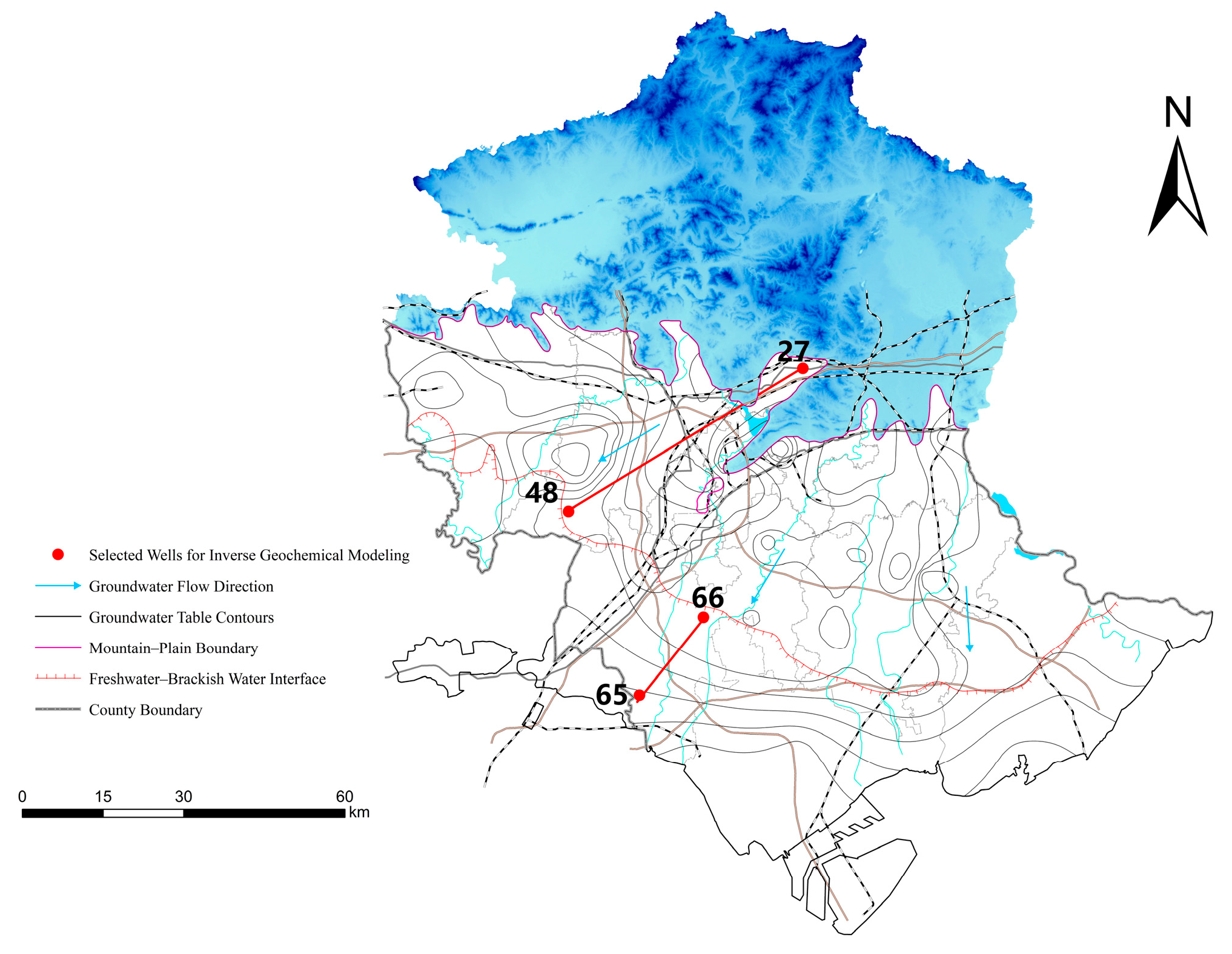
3.1. Sampling Design and Groundwater Sample Collection
3.2. Data Analysis Methods
- (1)
- Data standardization: Due to significant differences in the units and magnitudes of various hydrochemical parameters, directly analyzing the raw data could lead to larger variables dominating the results. To eliminate the effects of differing dimensions and units, all data were standardized using the following formula:
- (2)
- Construction of the correlation matrix. Principal component analysis can be conducted based on either a covariance matrix or a correlation matrix. Considering that the units and magnitudes of groundwater parameters vary significantly, this study adopted a correlation matrix—rather than a covariance matrix—after data standardization to ensure balanced contributions from all variables in the analysis. This approach effectively eliminates the influence of scale differences on PCA results and is more suitable for the structure of the current dataset. The correlation coefficient is calculated using the following formula:
- (3)
- Eigenvalue decomposition. The correlation matrix R was subjected to eigenvalue decomposition to obtain the eigenvalues λj and their corresponding eigenvectors ej:
- (4)
- Determination of the number of principal components. The number of principal components to retain was determined based on the cumulative variance contribution rate, calculated using the following formula:
4. Results and Discussion
4.1. Hydrochemical Characteristics and Origin Identification
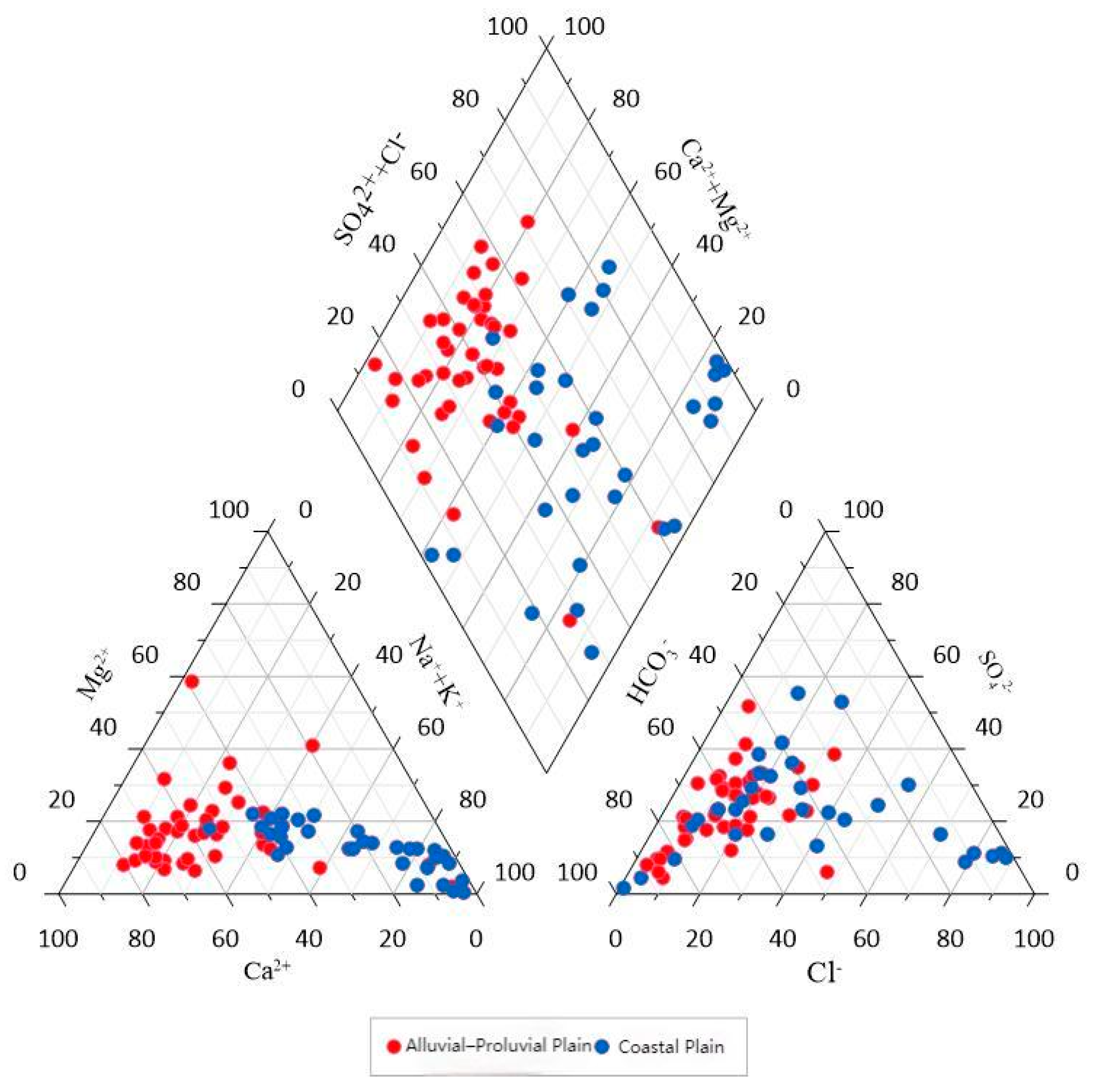
4.1.1. Hydrochemical Characteristics
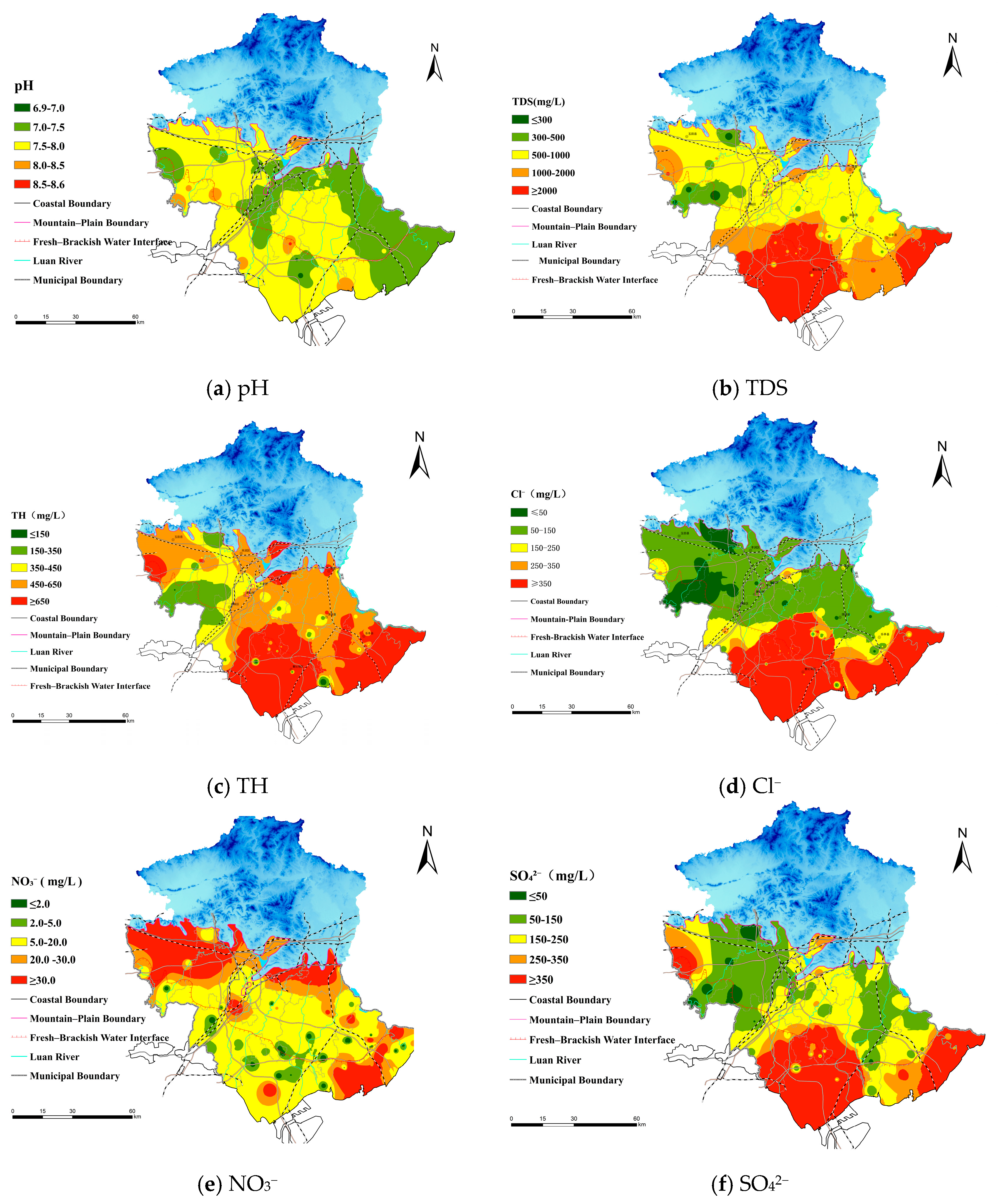
4.1.2. Spatial Trends of Hydrochemical Facies and Indicators
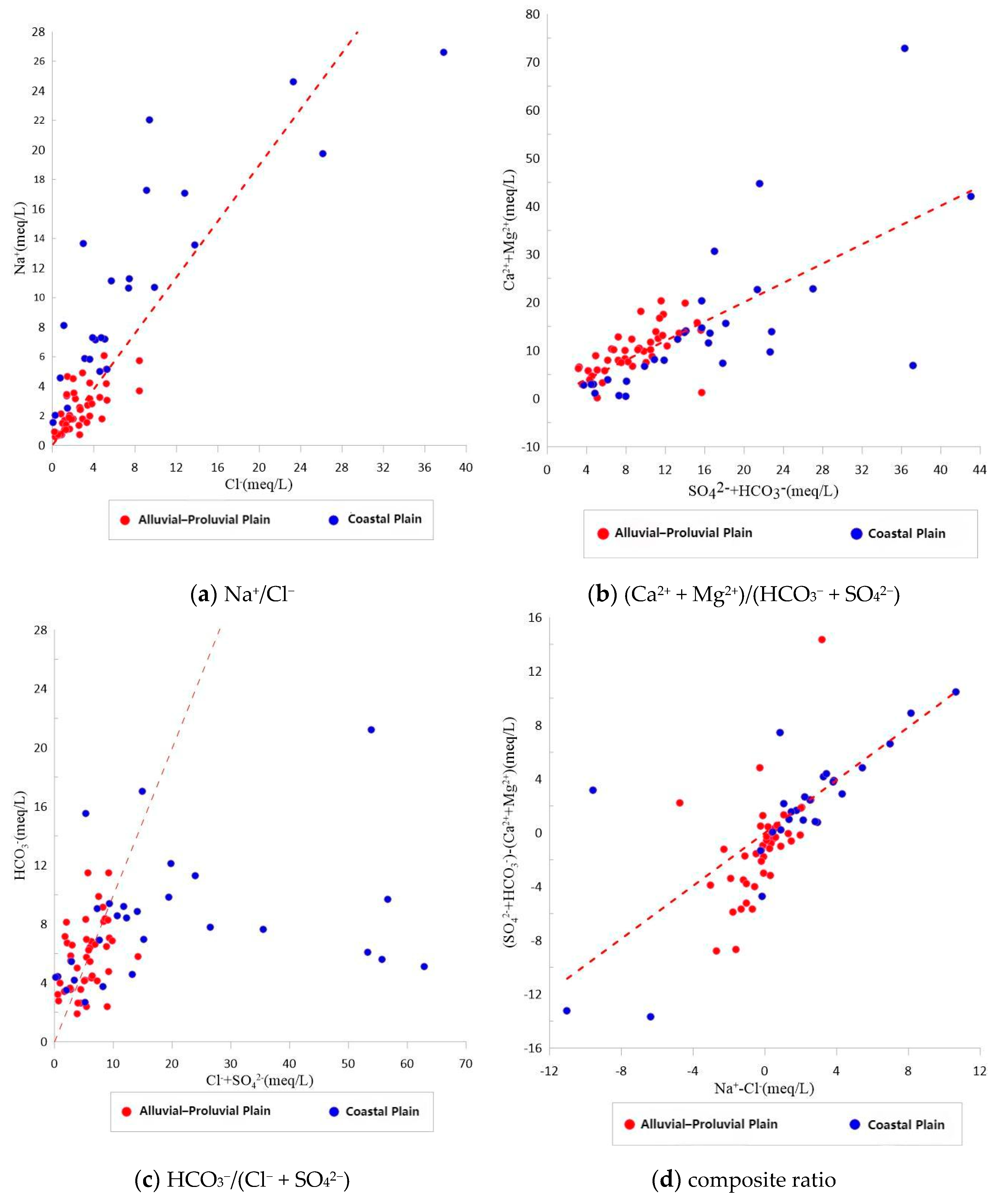
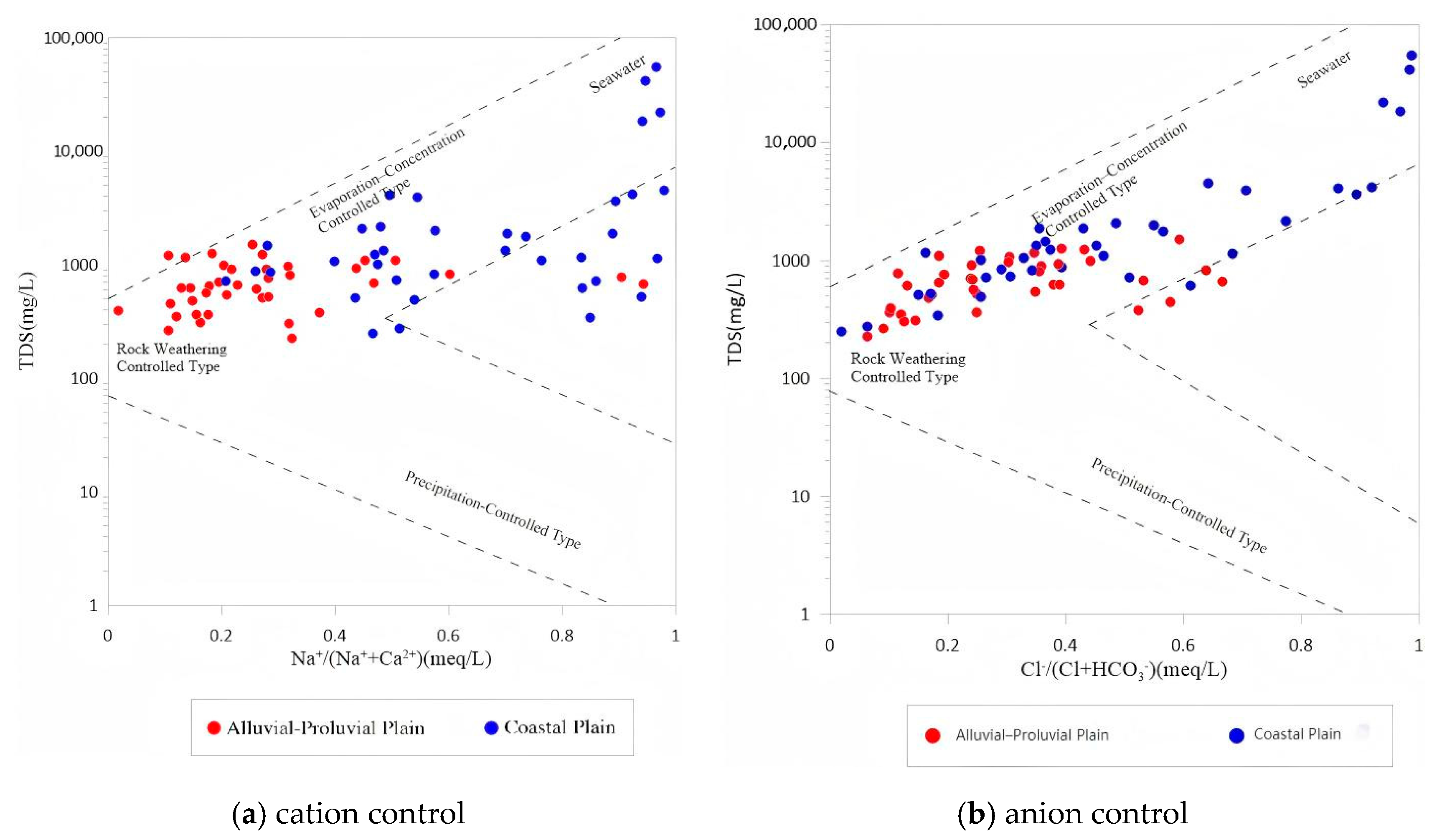
4.1.3. Ionic Ratios and Controlling Mechanisms
4.1.4. Groundwater Quality Classification and Pollution Identification
4.2. Multivariate Statistical Identification of Groundwater Evolution Processes
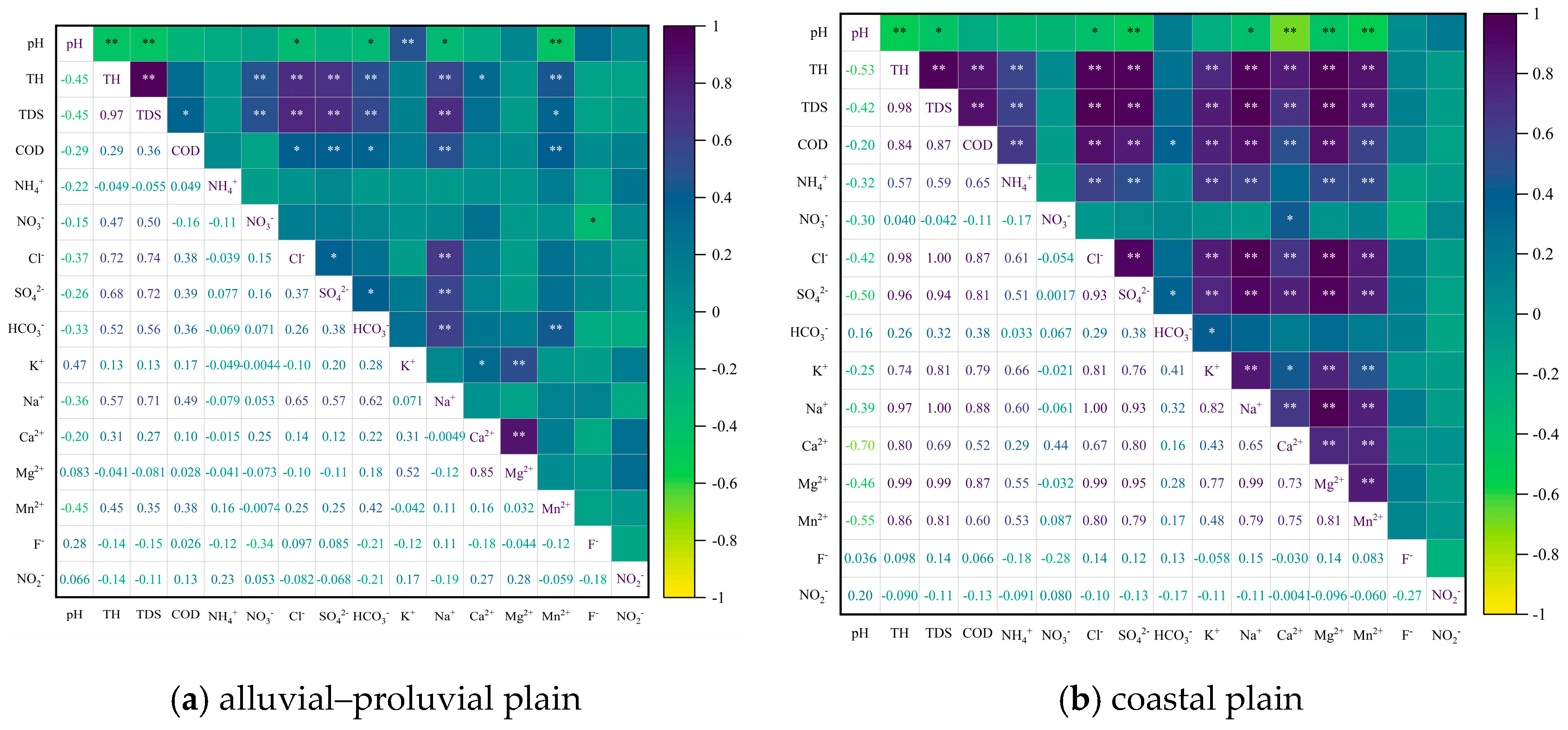
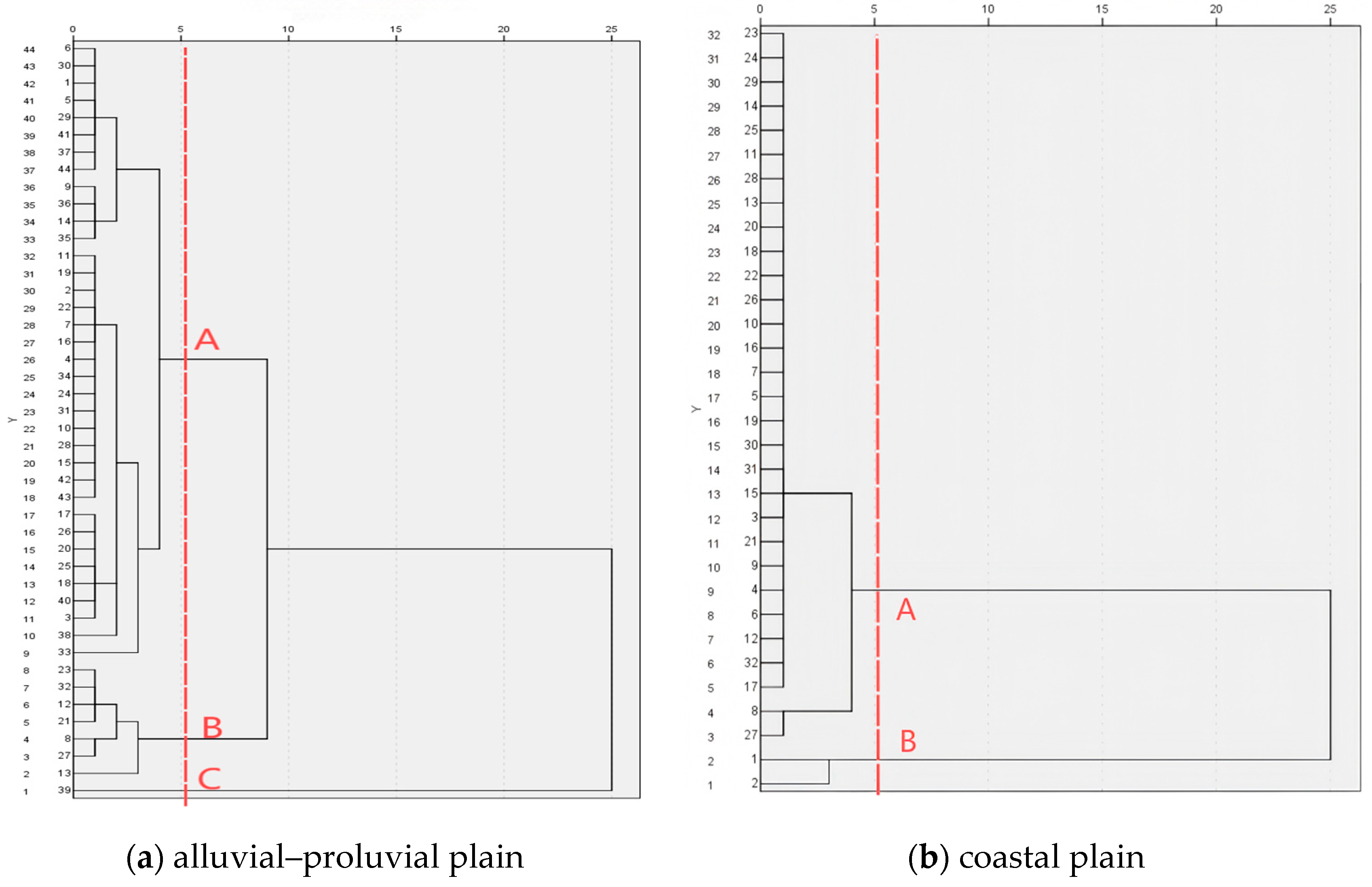
4.2.1. Correlation Analysis and Hierarchical Clustering
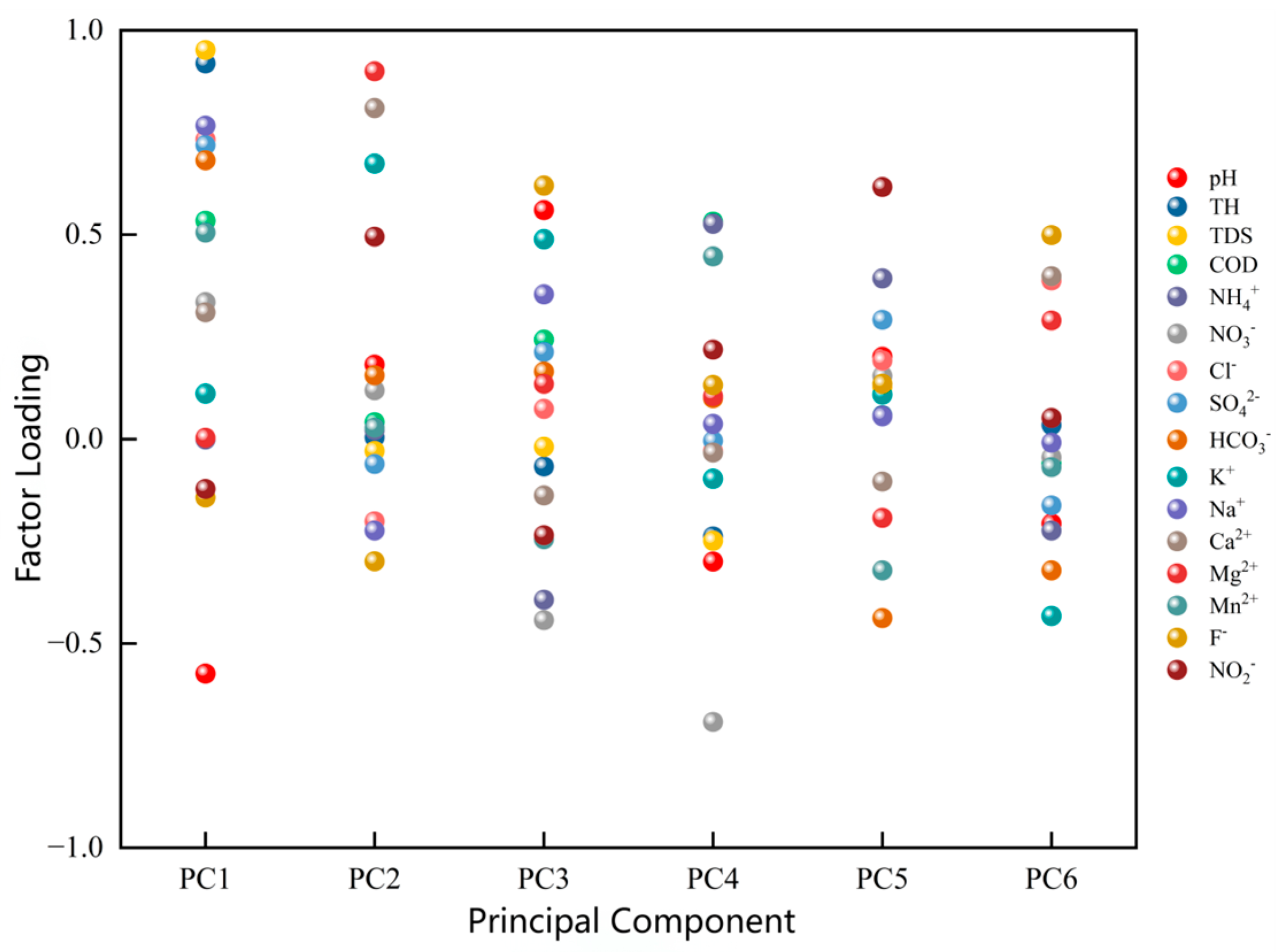
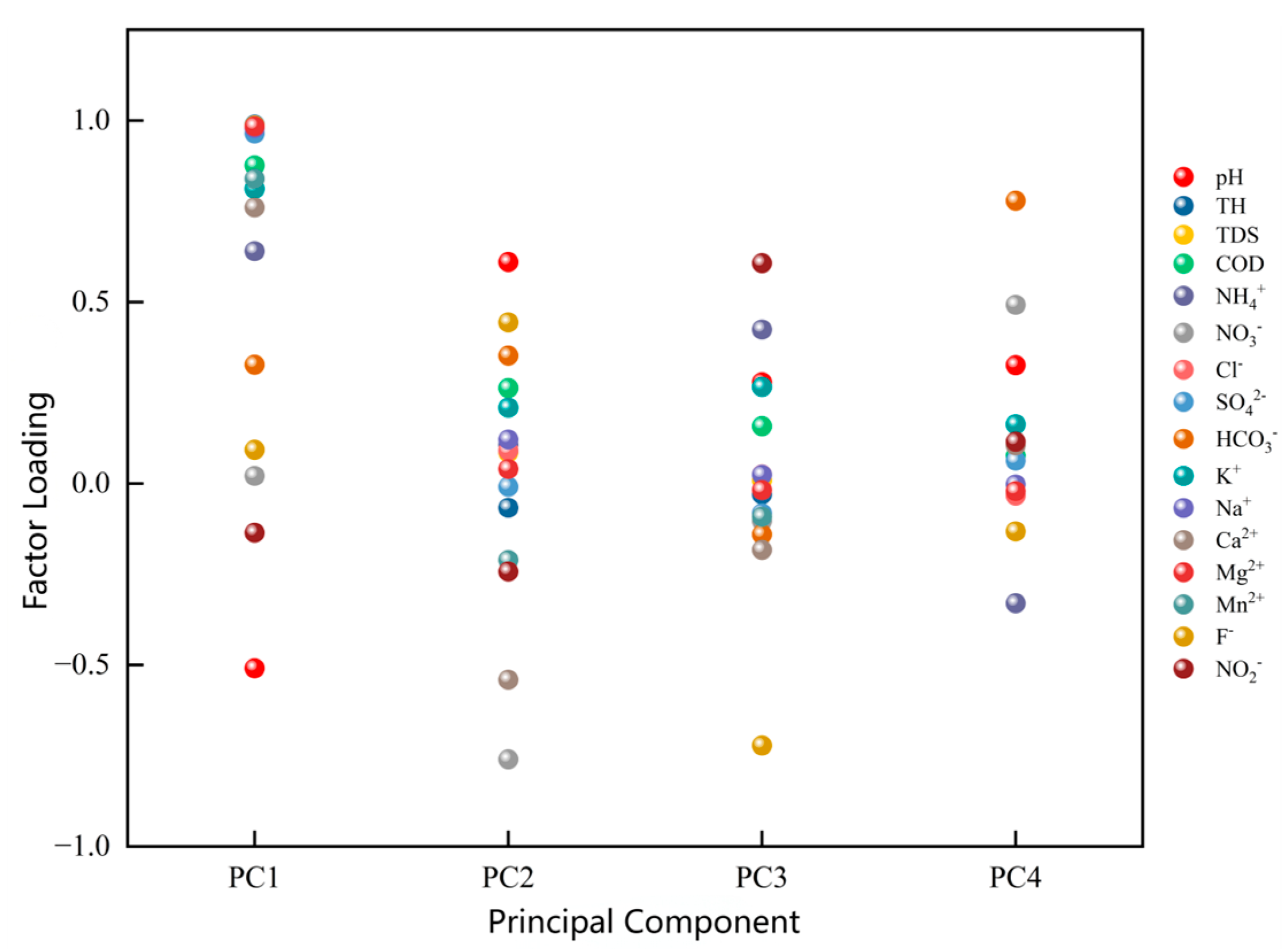
4.2.2. Principal Component Analysis of Dominant Factors
4.3. Inverse Hydrogeochemical Modeling with PHREEQC
4.3.1. Saturation Index Analysis of Mineral Reaction Trends
4.3.2. Inverse Modeling—Identification of Dominant Geochemical Pathways
4.3.3. Conceptual Model of Groundwater Evolution
4.4. Groundwater Management Implications
- (1)
- Priority Monitoring Zones: In low-lying coastal areas, TDS, Cl−, Na+, and SO42− are markedly enriched, forming salinization hotspots. PHREEQC simulations indicate that halite and gypsum dissolution are the dominant mechanisms driving mineralization. These areas should be designated as priority monitoring zones, where real-time monitoring of electrical conductivity and salinity should be implemented, alongside strict regulation of irrigation and domestic water quality.
- (2)
- Pollution Control Zones: In the transitional alluvial–coastal zone, Class IV groundwater exhibits elevated exceedance rates of NO3− and Mn. PCA results suggest that agricultural fertilization, sewage leakage, and organic inputs are the major sources. These areas should be designated as pollution control zones, with measures such as precision fertilization, improved wastewater treatment, and controlled groundwater abstraction to mitigate non-point source pollution pressure.
- (3)
- High-Quality Groundwater Protection Zones: In the alluvial fan apex and northern recharge areas, groundwater is predominantly HCO3−–Ca-type freshwater of high quality, primarily controlled by natural carbonate weathering. These zones should be designated as groundwater protection areas, with strict restrictions on intensive groundwater extraction and high-risk land use.
- (4)
- Salinization Boundary Early Warning: Along the groundwater flow path, hydrochemical types evolve from HCO3−–Ca to Cl−–Na, with both Gibbs diagrams and PHREEQC simulations revealing a transition from “carbonate control” to “evaporation concentration/evaporite dissolution.” Monitoring transects should be established in the freshwater–saline water transition belt, with emphasis on tracking electrical conductivity and the Na+/Cl− ratio to dynamically identify seawater intrusion boundaries.
- (5)
- Trace Element Risk Management: PHREEQC simulations indicate that glauconite and other Mn- and Al-bearing minerals dissolve along the alluvial fan margins, suggesting a risk of trace element mobilization. Long-term monitoring of trace metals and nitrogen species is recommended to prevent environmental and health hazards. Recent studies in tropical natural wetlands have also confirmed that the spatial–seasonal distribution of trace metals is jointly regulated by DOC, salinity, and pH, underscoring the universal necessity of mechanism-based identification and sustained monitoring [90].
5. Conclusions
- (1)
- The hydrochemical types of groundwater exhibit clear geomorphological differentiation. Groundwater in the alluvial–proluvial plain is dominated by the HCO3−–Ca type with low TDS, mainly controlled by natural recharge and carbonate weathering. In contrast, the coastal plain is characterized by Cl−–Na-type high-mineralized water with elevated TDS, primarily influenced by seawater intrusion, evaporation concentration, and evaporite dissolution.
- (2)
- The spatial distribution of key hydrochemical parameters (TDS, Cl−, Na+, SO42−, NO3−) follows a pattern of “fresh in the north, saline in the south; better quality in the fanhead than in the coastal area.” Ion ratio analysis reveals that the alluvial zone is mainly affected by carbonate reactions and cation exchange, while the coastal zone shows evidence of evaporite dissolution and mixed water influences, forming a transition belt from freshwater to saline water.
- (3)
- Multivariate statistical analyses indicate that groundwater chemistry is controlled by multiple interacting factors, including enhanced mineralization, carbonate weathering, evaporation, redox conditions, and anthropogenic inputs. Natural geochemical processes dominate in the alluvial plain, though some areas also show signs of nitrate pollution. In the coastal plain, hydrochemical evolution is governed by a combination of natural processes and human activities.
- (4)
- PHREEQC inverse modeling quantitatively identified key geochemical reactions along representative flow paths. In the alluvial path, CO2 degassing drives dolomite precipitation and gypsum dissolution, forming an oxidizing open system regulated by carbonate buffering. In the coastal path, continuous halite dissolution dominates, while CO2 degassing and carbonate reactions become subdued, reflecting a high-TDS, closed-system water body.
- (5)
- This study developed an integrated analytical framework combining hydrochemical diagrams, statistical recognition, and inverse modeling. It effectively revealed the transformation of groundwater types, spatial distribution of controlling mechanisms, and evolutionary trends. The results provide theoretical support and methodological guidance for identifying transition zones of saline groundwater, understanding the succession of dominant hydrogeochemical processes, and informing regional salinization risk assessment, pollution zoning, and sustainable groundwater management.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hebei Hydrogeological and Engineering Geological Team, Hebei Geological Bureau. Hydrogeological Investigation Report on Irrigation Water Supply in the Eastern Hebei Plain; Hebei Hydrogeological and Engineering Geological Team, Hebei Geological Bureau: Shijiazhuang, China, 1983. (In Chinese)
- Wang, D.C.; Zhang, R.Q.; Shi, Y.H. Fundamentals of Hydrogeology; Geological Publishing House: Beijing, China, 1995; pp. 1–5. (In Chinese)
- Dolan, F.; Lamontagne, J.; Link, R.; Hejazi, M.; Reed, P.; Edmonds, J. Evaluating the economic impact of water scarcity in a changing world. Nat. Commun. 2021, 12, 1915. [Google Scholar] [CrossRef]
- Jia, W.; Yin, L.; Zhang, M.; Zhang, X.; Zhang, J.; Tang, X.; Dong, J. Quantification of groundwater recharge and evapotranspiration along a semi-arid wetland transect using diurnal water table fluctuations. J. Arid Land 2021, 13, 455–469. [Google Scholar] [CrossRef]
- Hebei Institute of Geological Environment Monitoring. Geological Environment Monitoring Report of Tangshan City, Hebei Province; Hebei Institute of Geological Environment Monitoring: Tangshan, China, 2022. (In Chinese) [Google Scholar]
- Wen, S.; Wen, M.; Liang, S.; Pang, G.; Fan, J.; Dong, M.; Wang, Y.; Zhang, J.; Ye, Y. Spatial Distribution and Mechanisms of Groundwater Hardness in the Plain Area of Tangshan City, China. Water 2024, 16, 3627. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Sunkari, E.D.; Abu, M.; Zango, M.S. Geochemical Evolution and Tracing of Groundwater Salinization Using Different Ionic Ratios, Multivariate Statistical and Geochemical Modeling Approaches in a Typical Semi-Arid Basin. J. Contam. Hydrol. 2021, 236, 103742. [Google Scholar] [CrossRef]
- Çelik Güney, M.; Kayaalp, G.-T. A Comparative Evaluation of the Outlier Detection Methods. Black Sea J. Eng. Sci. 2024, 7, 155–159. [Google Scholar] [CrossRef]
- El-Rawy, M.; Fathi, H.; Abdalla, F.; Alshehri, F.; Eldeeb, H. An Integrated Principal Component and Hierarchical Cluster Analysis Approach for Groundwater Quality Assessment in Jazan, Saudi Arabia. Water 2023, 15, 1466. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Guo, S.; Fu, K.; Liao, L.; Xu, Y.; Cheng, S. Groundwater pollution source apportionment using principal component analysis in a multiple land-use area in southwestern China. Environ. Sci. Pollut. Res. 2020, 27, 9000–9011. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, H.; Sun, C.; Li, H.; Gao, Y. Multivariate statistical approaches to identify the major factors governing groundwater quality. Appl. Water Sci. 2018, 8, 215. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, C.; Chao, J. Source apportionment of pollutants in Changtan Reservoir, Zhejiang Province, using an absolute principal component score–multiple linear regression (APCS–MLR) model. J. Ecol. Rural Environ. 2023, 39, 530–539. (In Chinese) [Google Scholar]
- Fentahun, A.; Mechal, A.; Karuppannan, S. Hydrochemistry and quality appraisal of groundwater in Birr River Catchment, Central Blue Nile River Basin, using multivariate techniques and water quality indices. Environ. Monit. Assess. 2023, 195, 655. [Google Scholar] [CrossRef]
- Samadi, J. Modelling hydrogeological parameters to assess groundwater pollution and vulnerability in Kashan aquifer: Novel calibration-validation of multivariate statistical methods and human health risk considerations. Environ. Res. 2022, 211, 113028. [Google Scholar] [CrossRef]
- Saliba, Y.; Bărbulescu, A. Assessing pollution with heavy metals and its impact on population health. Toxics 2025, 13, 52. [Google Scholar] [CrossRef]
- Guo, X.; Zuo, R.; Shan, D.; Cao, Y.; Wang, J.; Teng, Y.; Fu, Q.; Zheng, B. Source apportionment of pollution in groundwater source area using factor analysis and positive matrix factorization methods. Hum. Ecol. Risk Assess. 2017, 23, 1417–1436. [Google Scholar] [CrossRef]
- Zhang, H.; Du, X.Y.; Gao, F.; Zeng, Z.; Cheng, S.Q.; Xu, Y. Groundwater pollution source apportionment using combined PMF model and stable isotope analysis. Environ. Sci. 2022, 43, 4054–4063. (In Chinese) [Google Scholar]
- Zuo, R.; Wei, B.; Wang, J.; Wang, J.S.; Teng, Y.G.; Yang, Y.F.; Dai, N. Identification of pollution sources in groundwater source areas based on multivariate statistical analysis. Hydrogeol. Eng. Geol. 2012, 39, 17–21. (In Chinese) [Google Scholar]
- Ma, R.; Shi, J. Assessing groundwater pollution using fuzzy factor analysis method: A case study of Luoyang city in Henan province. Diqiu Xuebao (Acta Geosci. Sin.) 2011, 32, 611–622. (In Chinese) [Google Scholar]
- Zuo, R.; Chen, X.; Wang, B.; Shan, D.; Yang, J.; Li, X.; Wang, J.; Teng, Y. Pollution risk evaluation of regional groundwater based on sources apportionment of pollution. Water Sci. Technol. Water Supply 2018, 18, 1294–1307. [Google Scholar] [CrossRef]
- Trabelsi, R.; Zouari, K. Coupled geochemical modeling and multivariate statistical analysis approach for the assessment of groundwater quality in irrigated areas: A study from north eastern of Tunisia. Groundw. Sustain. Dev. 2019, 8, 197–206. [Google Scholar] [CrossRef]
- Mudzielwana, R.; Gitari, M.; Akinyemi, S.; Talabi, A.; Ndungu, P. Hydrogeochemical characteristics of arsenic-rich groundwater in Greater Giyani Municipality, Limpopo Province, South Africa. Groundw. Sustain. Dev. 2020, 10, 100336. [Google Scholar] [CrossRef]
- Poetra, R.P.; Adji, T.N.; Santosa, L.W.; Khakhim, N. Hydrogeochemical conditions in groundwater systems with various geomorphological units in Kulonprogo Regency, Java Island, Indonesia. Aquat. Geochem. 2020, 26, 421–454. [Google Scholar] [CrossRef]
- Tziritis, E.; Sachsamanoglou, E.; Güler, C. Evaluating spatiotemporal groundwater quality changes in the Rhodope coastal aquifer system (NE Greece) employing a GIS-assisted hybrid approach of multivariate statistics and inverse geochemical modeling. Sci. Total Environ. 2024, 947, 174676. [Google Scholar] [CrossRef]
- Rao, N.S.; Dinakar, A.; Sun, L. Estimation of groundwater pollution levels and specific ionic sources in the groundwater using a comprehensive approach of geochemical ratios, pollution index of groundwater, unmix model and land use/land cover—A case study. J. Contam. Hydrol. 2022, 248, 103990. [Google Scholar] [CrossRef]
- Quinodoz, F.B.; Cabrera, A.; Blarasin, M.; Matteoda, E.; Pascuini, M.; Prámparo, S.; Boumaiza, L.; Matiatos, I.; Schroeter, G.; Lutri, V. Chemical and isotopic tracers combined with mixing models for tracking nitrate contamination in the Pampa de Pocho aquifer, Argentina. Environ. Res. 2024, 259, 119571. [Google Scholar] [CrossRef] [PubMed]
- Narvaez-Montoya, C.; Mondragón Bonilla, R.; Goldscheider, N.; Mahlknecht, J. Groundwater salinization patterns in the Yucatán Peninsula reveal contamination and vulnerability of the karst aquifer. Commun. Earth Environ. 2025, 6, 256. [Google Scholar] [CrossRef]
- Gomes, O.; Marques, E.; Kütter, V.; Aires, J.; Travi, Y.; Silva-Filho, E. Origin of salinity and hydrogeochemical features of porous aquifers from northeastern Guanabara Bay, Rio de Janeiro, SE-Brazil. J. Hydrol. Reg. Stud. 2019, 25, 100601. [Google Scholar] [CrossRef]
- Nogueira, G.; Stigter, T.; Zhou, Y.; Mussá, F.; Juízo, D. Understanding Groundwater Salinization Mechanisms to secure Freshwater Resources in the Water-Scarce City of Maputo, Mozambique. Sci. Total Environ. 2019, 661, 723–736. [Google Scholar] [CrossRef]
- Xing, L. Hydrochemical Characteristics and Hydrogeochemical Processes of Groundwater Along a Typical Profile in the North China Plain. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2012. (In Chinese). [Google Scholar]
- Wang, H.; Guo, X.; Zhang, Q.; Li, B. Hydrochemical characteristics, evolution, and genesis of groundwater in the Hutuo River Basin. Environ. Chem. 2021, 40, 3838–3845. (In Chinese) [Google Scholar]
- Guo, X.; Wang, H.; Shi, J.; Wang, W. Hydrochemical variation characteristics and evolution model of the groundwater system in Baiyangdian Wetland. Acta Geol. Sin. 2022, 96, 656–672. (In Chinese) [Google Scholar]
- Long, W. Study on Hydrochemical Characteristics and Evolution of Shallow Groundwater in Tongliao Area. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2010. (In Chinese). [Google Scholar]
- Lou, Z.; Jin, A.; Zhu, R.; Cai, X.; Gao, R. Vertical zonation and planar differentiation of groundwater hydrochemical fields in oilfields of the Songliao Basin. Chin. J. Geol. 2006, 41, 392–403. (In Chinese) [Google Scholar]
- Wu, X.; An, Y.; Wei, S.; Yin, D.; Cui, H.; Wang, X.; Wang, Q. Hydrochemical characteristics and evolution of shallow groundwater in the Dingxin Valley of the lower Heihe River. Arid Zone Resour. Environ. 2021, 35, 103–109. (In Chinese) [Google Scholar]
- Chen, M.; Wu, Y.; Gao, D.; Chang, M. Hydrochemical evolution and controlling factors of shallow groundwater in the plain area of Guanghan City. J. Jilin Univ. (Earth Sci. Ed.) 2016, 46, 831–843. (In Chinese) [Google Scholar]
- Sun, Y.; Wang, W.; Duan, L.; Zhang, C.; Wang, Y. Formation and evolutionary mechanisms of shallow groundwater geochemistry in the Guanzhong Basin. Hydrogeol. Eng. Geol. 2014, 41, 29–35. (In Chinese) [Google Scholar]
- Cao, J.; Xu, X.; Yu, H.; Huang, C. Hydrochemical characteristics and evolution of shallow groundwater in the Yellow River Delta. Mar. Sci. 2014, 38, 78–85. (In Chinese) [Google Scholar]
- Zhao, X.; Chen, J.; Tang, C.; Zeng, S.; Lu, G. Hydrochemical characteristics and evolution of groundwater in small catchments of the Pearl River Delta. Ecol. Environ. 2007, 16, 1620–1626. (In Chinese) [Google Scholar]
- Wang, S.; Liu, H.; Yu, Y.; Zhao, W.; Yang, Q.; Liu, J. Evaluation of groundwater sustainability in the arid Hexi Corridor of Northwestern China, using GRACE, GLDAS and measured groundwater data products. Sci. Total Environ. 2020, 705, 135829. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Groundwater Quality Assessment and Hydrogeochemical Evolution Modeling in the Xianyang Section of the Weihe River Basin. Master’s Thesis, Chang’an University, Xi’an, China, 2023. (In Chinese). [Google Scholar]
- Fu, X.; Sun, Y.; Su, J.; Zheng, M.; Xi, B.; Qian, G. Source apportionment of nitrate in groundwater based on hydrochemistry and dual isotopes of nitrogen and oxygen. Chin. Environ. Sci. 2019, 39, 3951–3958. (In Chinese) [Google Scholar]
- Zhang, Z.; Fei, Y.; Guo, C.; Qian, Y.; Li, Y. Regional groundwater pollution assessment in the North China Plain. J. Jilin Univ. (Earth Sci. Ed.) 2012, 42, 1456–1461. (In Chinese) [Google Scholar]
- Liu, Y.; Lu, C.; Ma, R. Source identification and analysis of iron ions in groundwater of the Eastern Hebei Plain. Geol. Surv. Res. 2019, 42, 134–142. (In Chinese) [Google Scholar]
- Ma, F.; Wei, A.; Deng, Q.; Zhao, H. Hydrochemical characteristics and the suitability of groundwater in the coastal region of Tangshan, China. J. Earth Sci. 2014, 25, 1067–1075. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Technical Specification for Groundwater Environmental Monitoring; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2020. (In Chinese)
- Liu, H.; Chen, S.; Hou, M.; He, L. Improved inverse distance weighting method application considering spatial autocorrelation in 3D geological modeling. Earth Sci. Inform. 2020, 13, 619–632. [Google Scholar] [CrossRef]
- Kim, J.; Han, J.; Park, K.; Seok, S. Improved IDW interpolation application using 3D search neighborhoods: Borehole data-based seismic liquefaction hazard assessment and mapping. Appl. Sci. 2022, 12, 11652. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Chen, X.; Mabaire, A. Hydrogeochemical evolution of multilayer aquifers in a massive coalfield. Environ. Earth Sci. 2019, 78, 675. [Google Scholar] [CrossRef]
- Lalumbe, L.; Kanyerere, T. Characterisation of hydro-geochemical processes influencing groundwater quality in rural areas: A case study of Soutpansberg Region, Limpopo Province, South Africa. Water 2022, 14, 1972. [Google Scholar] [CrossRef]
- Rezaei, A.; Hassani, H.; Jabbari, N. Evaluation of groundwater quality and assessment of pollution indices for heavy metals in North of Isfahan Province, Iran. Sustain. Water Resour. Manag. 2019, 5, 491–512. [Google Scholar] [CrossRef]
- Bu, J.; Liu, W.; Pan, Z.; Ling, K. Comparative study of hydrochemical classification based on different hierarchical cluster analysis methods. Int. J. Environ. Res. Public Health 2020, 17, 9515. [Google Scholar] [CrossRef]
- Das, C.R.; Das, S.; Panda, S. Groundwater quality monitoring by correlation, regression and hierarchical clustering analyses using WQI and PAST tools. Groundw. Sustain. Dev. 2022, 16, 100708. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Salhi, N.; Douaoui, A.; Trolard, F.; Bourrié, G. Specific interaction theory versus Pitzer’s model in groundwaters and brines for checking equilibria/non-equilibria with calcite, gypsum, and halite: Application to predict the evolution of solutions concentrated by evaporation in irrigated areas. Environ. Earth Sci. 2019, 78, 196. [Google Scholar] [CrossRef]
- Vasu, D.; Singh, S.K.; Tiwary, P.; Sahu, N.; Ray, S.K.; Butte, P.; Duraisami, V.P. Influence of geochemical processes on hydrochemistry and irrigation suitability of groundwater in part of semi-arid Deccan Plateau, India. Appl. Water Sci. 2017, 7, 3803–3815. [Google Scholar] [CrossRef]
- Selvakumar, S.; Chandrasekar, N.; Srinivas, Y.; Selvam, S.; Kaliraj, S.; Magesh, N.; Venkatramanan, S. Hydrogeochemical processes controlling the groundwater salinity in the coastal aquifers of Southern Tamil Nadu, India. Mar. Pollut. Bull. 2021, 174, 113264. [Google Scholar] [CrossRef] [PubMed]
- Asare, A.; Appiah-Adjei, E.; Ali, B.; Owusu-Nimo, F. Assessment of seawater intrusion using ionic ratios: The case of coastal communities along the Central Region of Ghana. Environ. Earth Sci. 2021, 80, 566. [Google Scholar] [CrossRef]
- Shin, K.; Koh, D.; Jung, H.; Lee, J. The hydrogeochemical characteristics of groundwater subjected to seawater intrusion in the Archipelago, Korea. Water 2020, 12, 1542. [Google Scholar] [CrossRef]
- Seddique, A.; Masuda, H.; Anma, R.; Bhattacharya, P.; Yokoo, Y.; Shimizu, Y. Hydrogeochemical and isotopic signatures for the identification of seawater intrusion in the paleobeach aquifer of Cox’s Bazar city and its surrounding area, south-east Bangladesh. Groundw. Sustain. Dev. 2019, 9, 100215. [Google Scholar] [CrossRef]
- Chen, Q.; Abu-Al-Saud, M.O.; Ayirala, S.C.; AlYousef, A.A. Propagation of mineral dissolution waves driven by cation exchange in low salinity waterflooding. Fuel 2022, 328, 125350. [Google Scholar] [CrossRef]
- Baumann, G.; Vital, M.; Glok-Galli, M.; Grondona, S.; Massone, H.; Martinez, D. Hydrogeochemical modeling and dedolomitization processes in the Patagonian Boulders and Patagonia Formation in the eastern Patagonia, Argentina. Environ. Earth Sci. 2019, 78, 405. [Google Scholar] [CrossRef]
- Telahigue, F.; Souid, F.; Agoubi, B.; Chahlaoui, A.; Kharroubi, A. Hydrogeochemical and isotopic evidence of groundwater salinization in a coastal aquifer: A case study in Jerba Island, southeastern Tunisia. Phys. Chem. Earth 2020, 120, 102886. [Google Scholar] [CrossRef]
- GB/T 14848—2017; Groundwater Quality Standard. Standards Press of China: Beijing, China, 2017.
- Xaza, A.; Mapoma, H.; Abiye, T.; Clarke, S.; Kanyerere, T. Investigating seawater intrusion in Republic of South Africa’s Heuningnes, Cape Agulhas using hydrogeochemistry and seawater fraction techniques. Water 2023, 15, 12141. [Google Scholar] [CrossRef]
- Nguyen, G.; Huynh, N. Seasonal variations in groundwater quality under different impacts using statistical approaches. Civ. Eng. J. 2023, 9, 537–551. [Google Scholar] [CrossRef]
- Mahaqi, A. Groundwater quality for drinking and agricultural purposes, Saleh Abad (NE Iran): Geochemical and statistical approaches. Carbonates Evaporites 2021, 36, 7. [Google Scholar] [CrossRef]
- Mukate, S.; Panaskar, D.; Wagh, V.; Baker, S. Understanding the influence of industrial and agricultural land uses on groundwater quality in semiarid region of Solapur, India. Environ. Dev. Sustain. 2019, 22, 3207–3238. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Sridhar, S.; Ayyamperumal, R.; Karuppannan, S.; Gopalakrishnan, G.; Chakraborty, M.; Huang, X. Isotopic signatures, hydrochemical and multivariate statistical analysis of seawater intrusion in the coastal aquifers of Chennai and Tiruvallur District, Tamil Nadu, India. Mar. Pollut. Bull. 2021, 174, 113232. [Google Scholar] [CrossRef]
- Rajendiran, T.; Sabarathinam, C.; Chandrasekar, T.; Panda, B.; Mathivanan, M.; Nagappan, G.; Natesan, D.; Ghai, M.; Singh, D.; Alagappan, R. Geochemical variations due to salinization in groundwater along the southeast coast of India. SN Appl. Sci. 2021, 3, 396. [Google Scholar] [CrossRef]
- Hasan, S.; Salem, Z.; Sefelnasr, A. Assessment of hydrogeochemical characteristics and seawater intrusion in coastal aquifers by integrating statistical and graphical techniques: Quaternary aquifer, West Nile Delta, Egypt. Water 2023, 15, 1803. [Google Scholar] [CrossRef]
- Sarma, R.; Singh, S. Assessment of groundwater quality and human health risks of nitrate and fluoride contamination in a rapidly urbanizing region of India. Environ. Sci. Pollut. Res. 2023, 30, 55437–55454. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, P.; Chen, X.; Yang, S.; Zhang, P.; Guo, F. Major ion hydrogeochemistry and health risk of groundwater nitrate in selected rural areas of the Guanzhong Basin, China. Hum. Ecol. Risk Assess. 2023, 29, 701–727. [Google Scholar] [CrossRef]
- Cellone, F.; Carol, E.; Pugliese, I.; Córdoba, J.; Butler, L.; Lamarche, L. Nitrate pollution in dairy farms and its impact on groundwater quality in a sector of the Pampas plain, Argentina. Environ. Earth Sci. 2020, 79, 356. [Google Scholar] [CrossRef]
- Adelana, S.; Heaven, M.; Dresel, P.; Giri, K.; Holmberg, M.; Croatto, G.; Webb, J. Controls on species distribution and biogeochemical cycling in nitrate-contaminated groundwater and surface water, southeastern Australia. Sci. Total Environ. 2020, 726, 138426. [Google Scholar] [CrossRef]
- Abba, S.; Egbueri, J.; Benaafi, M.; Usman, J.; Usman, A.; Aljundi, I. Fluoride and nitrate enrichment in coastal aquifers of the Eastern Province, Saudi Arabia: The influencing factors, toxicity, and human health risks. Chemosphere 2023, 322, 139083. [Google Scholar] [CrossRef]
- Krishan, G.; Bhagwat, A.; Sejwal, P.; Yadav, B.; Kansal, M.; Bradley, A.; Singh, S.; Kumar, M.; Sharma, L.; Muste, M. Assessment of groundwater salinity using principal component analysis (PCA): A case study from Mewat (Nuh), Haryana, India. Environ. Monit. Assess. 2022, 195, 923. [Google Scholar] [CrossRef] [PubMed]
- Alsabti, B.; Sabarathinam, C.; Svv, D. Identification of high nitrate concentration in shallow groundwater of an arid region: A case study of South Kuwait’s Bay. Environ. Monit. Assess. 2022, 195, 781. [Google Scholar] [CrossRef]
- Lu, C.; Cheng, W.; Yin, H.; Li, S.; Zhang, Y.; Dong, F.; Cheng, Y.; Zhang, X. Study on inverse geochemical modeling of hydrochemical characteristics and genesis of groundwater system in coal mine area—A case study of Longwanggou Coal Mine in Ordos Basin. Environ. Sci. Pollut. Res. 2024, 31, 16583–16600. [Google Scholar] [CrossRef]
- Okofo, L.; Bedu-Addo, K.; Martienssen, M. Characterization of groundwater in the ‘Tamnean’ Plutonic Suite aquifers using hydrogeochemical and multivariate statistical evidence: A study in the Garu-Tempane District, Upper East Region of Ghana. Appl. Water Sci. 2022, 12, 63. [Google Scholar] [CrossRef]
- Rajendiran, T.; Sabarathinam, C.; Chandrasekar, T.; Keesari, T.; Senapathi, V.; Sivaraman, P.; Viswanathan, P.; Nagappan, G. Influence of variations in rainfall pattern on the hydrogeochemistry of coastal groundwater—An outcome of periodic observation. Environ. Sci. Pollut. Res. 2019, 26, 29173–29190. [Google Scholar] [CrossRef] [PubMed]
- Gori, F.; Paternoster, M.; Barbieri, M.; Buttitta, D.; Caracausi, A.; Parente, F.; Sulli, A.; Petitta, M. Hydrogeochemical multi-component approach to assess fluids upwelling and mixing in shallow carbonate-evaporitic aquifers (Contursi area, southern Apennines, Italy). J. Hydrol. 2023, 620, 129258. [Google Scholar] [CrossRef]
- Lu, S.; Chen, J.; Zheng, X.; Liang, Y.; Jia, Z.; Li, X. Hydrogeochemical characteristics of karst groundwater in Jinci spring area, north China. Carbonates Evaporites 2020, 35, 10. [Google Scholar] [CrossRef]
- Zilberbrand, M.; Gimburg, A.; Doroshev, A.; Mirlas, V.; Anker, Y. Chemical evolution of runoff in Eastern Mediterranean mountainous karstic terrains. J. Hydrol. 2021, 598, 127388. [Google Scholar] [CrossRef]
- Roy, A.; Keesari, T.; Mohokar, H.; Pant, D.; Sinha, U.; Mendhekar, G. Geochemical evolution of groundwater in hard-rock aquifers of South India using statistical and modelling techniques. Hydrol. Sci. J. 2020, 65, 951–968. [Google Scholar] [CrossRef]
- Jang, E.; Wiese, B.; Pilz, P.; Fischer, S.; Schmidt-Hattenberger, C. Geochemical modeling of CO2 injection and gypsum precipitation at the Ketzin CO2 storage site. Environ. Earth Sci. 2022, 81, 398. [Google Scholar] [CrossRef]
- Reghais, A.; Drouiche, A.; Zahi, F.; Debieche, T. Hydrogeochemical evaluation of the Terminal Complex aquifer system in an arid area: A case study from the Biskra region, north-east Algeria. Environ. Earth Sci. 2023, 82, 471. [Google Scholar] [CrossRef]
- Abdelshafy, M.; Saber, M.; Abdelhaleem, A.; Abdelrazek, S.; Seleem, E. Hydrogeochemical processes and evaluation of groundwater aquifer at Sohag city, Egypt. Sci. Afr. 2019, 6, e00196. [Google Scholar] [CrossRef]
- Qian, K.; Sun, H.; Li, J.; Xie, X. Strontium isotopes as tracers for water–rock interactions of groundwater to delineate iodine enrichment in aquifer of Datong Basin, northern China. Appl. Geochem. 2023, 152, 105783. [Google Scholar] [CrossRef]
- Alkhadher, S.A.A.; Suratman, S.; Mohd Sallan, M.I.B. An evaluation of spatial and seasonal distributions of dissolved trace metals in the water of the natural wetlands. J. Environ. Manag. 2023, 345, 118464. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Minimum (mg/L) | Maximum (mg/L) | Mean (mg/L) | Standard Deviation | Number of Exceedances | Exceedance Rate (%) | Class III Standard (mg/L) |
|---|---|---|---|---|---|---|---|
| pH | 7 | 8.5 | 7.46 | 0.29 | 1 | 2.27% | 6.5 < pH < 8.5 |
| TH | 150.59 | 1008 | 511.86 | 210.14 | 26 | 59.09% | 450 |
| TDS | 226.86 | 1498 | 715.54 | 303.63 | 7 | 15.91% | 1000 |
| COD | 0.25 | 4.2 | 1.18 | 0.93 | 3 | 6.82% | 3 |
| NH4+ | 0.01 | 13.9 | 0.47 | 2.11 | 5 | 11.36% | 0.5 |
| NO3− | 0 | 109.02 | 20.84 | 25.20 | 14 | 31.82% | 20 |
| CL | 7.54 | 299 | 92.52 | 68.68 | 2 | 4.55% | 250 |
| SO42− | 16.9 | 305 | 141.16 | 79.10 | 4 | 9.09% | 250 |
| HCO3− | 117 | 701 | 350.22 | 145.36 | - | - | - |
| K+ | 0.09 | 64.8 | 4.17 | 11.01 | - | - | - |
| Na+ | 13.2 | 140 | 56.65 | 34.30 | 0 | 0.00% | 200 |
| Ca2+ | 2.99 | 832 | 148.80 | 126.48 | - | - | - |
| Mg2+ | 1.33 | 1246 | 66.10 | 184.00 | 14 | 31.82% | 50 |
| Mn | 0.01 | 7.12 | 0.60 | 1.19 | 24 | 54.55% | 0.1 |
| F | 0.12 | 1.75 | 0.47 | 0.39 | 6 | 13.64% | 1 |
| NO2− | 0.00 | 0.22 | 0.02 | 0.04 | 0 | 0.00% | 1 |
| Parameter | Minimum (mg/L) | Maximum (mg/L) | Mean (mg/L) | Standard Deviation | Number of Exceedances | Exceedance Rate (%) | Class III Standard (mg/L) |
|---|---|---|---|---|---|---|---|
| pH | 6.9 | 8.6 | 7.59 | 0.43 | 1 | 3.13% | 6.5 < pH < 8.5 |
| TH | 32.09 | 10,758 | 1449.98 | 2419.07 | 11 | 34.38% | 450 |
| TDS | 249.86 | 55,600 | 5781.20 | 12,336.21 | 24 | 75.00% | 1000 |
| COD | 0.94 | 55.2 | 6.55 | 11.18 | 15 | 46.88% | 3 |
| NH4+ | 0.005 | 4.7 | 0.32 | 0.98 | 2 | 6.25% | 0.5 |
| NO3− | 0.002 | 118 | 17.93 | 30.76 | 7 | 21.88% | 20 |
| CL | 3 | 32,172 | 2800.75 | 7146.26 | 17 | 53.13% | 250 |
| SO42− | 4.45 | 3617 | 603.98 | 826.68 | 18 | 56.25% | 250 |
| HCO3− | 163 | 1296.06 | 538.84 | 283.23 | - | - | - |
| K+ | 0.35 | 381 | 36.10 | 74.53 | - | - | - |
| Na+ | 35.55 | 18,270 | 1700.81 | 4040.66 | 19 | 59.38% | 200 |
| Ca2+ | 7.52 | 650 | 172.23 | 175.73 | - | - | - |
| Mg2+ | 1.3 | 2271 | 238.42 | 513.33 | 22 | 68.75% | 50 |
| Mn | 0.005 | 4.78 | 0.72 | 1.20 | 15 | 46.88% | 0.1 |
| F | 0.189 | 2.465 | 0.93 | 0.65 | 10 | 31.25% | 1 |
| NO2− | 0.00 | 0.33 | 0.02 | 0.06 | 0 | 0.00% | 1 |
| Parameter | Factor Loadings | |||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |
| pH | −0.574 | 0.182 | 0.56 | −0.3 | 0.201 | −0.207 |
| TH | 0.919 | 0.004 | −0.067 | −0.238 | 0.058 | 0.034 |
| TDS | 0.952 | −0.029 | −0.019 | −0.249 | 0.127 | −0.009 |
| COD | 0.534 | 0.041 | 0.243 | 0.532 | 0.135 | −0.06 |
| NH4+ | −0.001 | 0.022 | −0.393 | 0.527 | 0.393 | −0.224 |
| NO3− | 0.335 | 0.119 | −0.443 | −0.692 | 0.154 | −0.045 |
| Cl− | 0.733 | −0.201 | 0.074 | −0.029 | 0.192 | 0.388 |
| SO42− | 0.719 | −0.061 | 0.213 | −0.004 | 0.292 | −0.162 |
| HCO3− | 0.682 | 0.156 | 0.165 | 0.1 | −0.438 | −0.321 |
| K+ | 0.111 | 0.674 | 0.489 | −0.097 | 0.109 | −0.433 |
| Na+ | 0.767 | −0.224 | 0.354 | 0.037 | 0.056 | −0.009 |
| Ca2+ | 0.31 | 0.81 | −0.138 | −0.033 | −0.104 | 0.398 |
| Mg2+ | 0.003 | 0.9 | 0.135 | 0.105 | −0.193 | 0.29 |
| Mn | 0.505 | 0.028 | −0.245 | 0.447 | −0.321 | −0.069 |
| F− | −0.143 | −0.299 | 0.62 | 0.132 | 0.135 | 0.499 |
| NO2− | −0.122 | 0.495 | −0.235 | 0.219 | 0.617 | 0.052 |
| Eigenvalue | 4.986 | 2.425 | 1.706 | 1.547 | 1.135 | 1.067 |
| Variance Contribution (%) | 31.16 | 15.16 | 10.66 | 9.67 | 7.09 | 6.67 |
| Cumulative Contribution (%) | 31.16 | 46.32 | 56.98 | 66.65 | 73.74 | 80.41 |
| Parameter | Factor Loadings | |||
|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | |
| pH | −0.509 | 0.61 | 0.279 | 0.326 |
| TH | 0.988 | −0.067 | −0.03 | −0.031 |
| TDS | 0.986 | 0.086 | 0.009 | −0.003 |
| COD | 0.876 | 0.263 | 0.158 | 0.076 |
| NH4+ | 0.64 | 0.107 | 0.424 | −0.33 |
| NO3− | 0.021 | −0.76 | −0.103 | 0.492 |
| Cl− | 0.981 | 0.091 | 0.025 | −0.033 |
| SO42− | 0.964 | −0.009 | −0.082 | 0.063 |
| HCO3− | 0.327 | 0.352 | −0.14 | 0.779 |
| K+ | 0.812 | 0.209 | 0.266 | 0.163 |
| Na+ | 0.979 | 0.121 | 0.024 | −0.003 |
| Ca2+ | 0.76 | −0.541 | −0.183 | 0.105 |
| Mg2+ | 0.984 | 0.04 | −0.018 | −0.021 |
| Mn | 0.839 | −0.211 | −0.092 | −0.132 |
| F− | 0.093 | 0.444 | −0.722 | −0.132 |
| NO2− | −0.136 | −0.243 | 0.607 | 0.115 |
| Eigenvalue | 9.277 | 1.827 | 1.324 | 1.162 |
| Variance Contribution (%) | 57.98 | 11.42 | 8.28 | 7.26 |
| Cumulative Contribution (%) | 57.98 | 69.40 | 77.68 | 84.94 |
| Mineral | SI at Starting Point | SI at Endpoint | Change in SI |
|---|---|---|---|
| Calcite | 1.41 | 0.64 | −0.77 |
| Dolomite | 3.19 | 1.19 | −2 |
| Aragonite | 1.26 | 0.5 | −0.76 |
| CO2 (g) | −9.33 | −2.79 | 6.54 |
| Goethite | 5.7 | 6.43 | 0.73 |
| Hematite | 13.4 | 14.87 | 1.47 |
| Fe(OH)3 | −0.2 | 0.54 | 0.74 |
| Gibbsite | 2.15 | −0.28 | −2.43 |
| Gypsum | −2.35 | −2.56 | −0.21 |
| Anhydrite | −1.71 | −2.86 | −1.15 |
| Halite | −6.83 | −8.6 | −1.77 |
| Mineral | SI at Starting Poin | SI at Endpoint | Change in SI |
|---|---|---|---|
| Calcite | 0.89 | 0.91 | 0.02 |
| Dolomite | 2.47 | 2.74 | 0.27 |
| Aragonite | 0.74 | 0.91 | 0.17 |
| CO2 (g) | −2.02 | −2.2 | −0.18 |
| Gypsum | −1.68 | −2.28 | −0.6 |
| Anhydrite | −1.5 | −2.46 | −0.96 |
| Halite | −4.36 | −6.79 | −2.43 |
| Hematite | 13.4 | 16.15 | 2.75 |
| Goethite | 7.06 | 7.07 | 0.01 |
| Fe(OH)3 | −0.2 | 0.06 | 0.26 |
| Gibbsite | 2.15 | 2.9 | 0.75 |
| FeS(ppt) | −71.25 | −75.16 | −3.91 |
| H2S (g) | −74.2 | −82.14 | −7.94 |
| Mineral Phase | Molar Transfer (mol·L−1) | Chemical Formula | Reaction Type | SI at Start | SI at End | ΔSI |
|---|---|---|---|---|---|---|
| CO2 (g) | −2.366 × 103 | CO2 | Degassing | −2.76 | −2.89 | −0.13 |
| Dolomite | −2.517 × 103 | CaMg(CO3)2 | Precipitation | 3.65 | 0.86 | −2.79 |
| Gypsum | 2.169 × 103 | CaSO4·2H2O | Dissolution | −1.37 | −2.67 | −1.3 |
| Halite | −0.8985 × 103 | NaCl | Continuous Dissolution | −6.85 | −8.56 | −1.71 |
| Glauconite | −1.797 × 103 | Mn3Ca2(SiO4)2 | Dissolution | −8.52 | −12.26 | −3.74 |
| Mineral Phase | Molar Transfer (mol·L−1) | Chemical Formula | Reaction Type | SI at Start | SI at End | ΔSI |
|---|---|---|---|---|---|---|
| H2O (g) | −2.502 × 102 | H2O | Degassing | −2.76 | −2.89 | −0.13 |
| CO2 (g) | −1.437 × 10−2 | CO2 | Degassing | 3.65 | 0.86 | −2.79 |
| Aragonite | −5.115 × 10−3 | CaCO3 | Dissolution | −1.37 | −2.67 | −1.3 |
| Dolomite | −1.467 × 10−2 | CaMg(CO3)2 | Continuous Dissolution | −6.85 | −8.56 | −1.71 |
| Halite | 1.425 × 10−2 | NaCl | Dissolution | −8.52 | −12.26 | −3.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, S.; Liang, S.; Pang, G.; Shan, Q.; Ye, Y.; Zhang, J.; Dong, M.; Fu, L.; Wen, M. Hydrochemical Characteristics and Evolution Mechanisms of Shallow Groundwater in the Alluvial–Coastal Transition Zone of the Tangshan Plain, China. Water 2025, 17, 2810. https://doi.org/10.3390/w17192810
Wen S, Liang S, Pang G, Shan Q, Ye Y, Zhang J, Dong M, Fu L, Wen M. Hydrochemical Characteristics and Evolution Mechanisms of Shallow Groundwater in the Alluvial–Coastal Transition Zone of the Tangshan Plain, China. Water. 2025; 17(19):2810. https://doi.org/10.3390/w17192810
Chicago/Turabian StyleWen, Shiyin, Shuang Liang, Guoxing Pang, Qiang Shan, Yingying Ye, Jianan Zhang, Mingqi Dong, Linping Fu, and Meng Wen. 2025. "Hydrochemical Characteristics and Evolution Mechanisms of Shallow Groundwater in the Alluvial–Coastal Transition Zone of the Tangshan Plain, China" Water 17, no. 19: 2810. https://doi.org/10.3390/w17192810
APA StyleWen, S., Liang, S., Pang, G., Shan, Q., Ye, Y., Zhang, J., Dong, M., Fu, L., & Wen, M. (2025). Hydrochemical Characteristics and Evolution Mechanisms of Shallow Groundwater in the Alluvial–Coastal Transition Zone of the Tangshan Plain, China. Water, 17(19), 2810. https://doi.org/10.3390/w17192810





