Eco-Friendly Removal of Cationic and Anionic Textile Dyes Using a Low-Cost Natural Tunisian Chert: A Promising Solution for Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Adsorbent
2.2. Chert Deposit
2.3. Point of Zero Charge (pHpzc) Determination
2.4. Adsorbate
2.5. Adsorption Experiment
3. Results
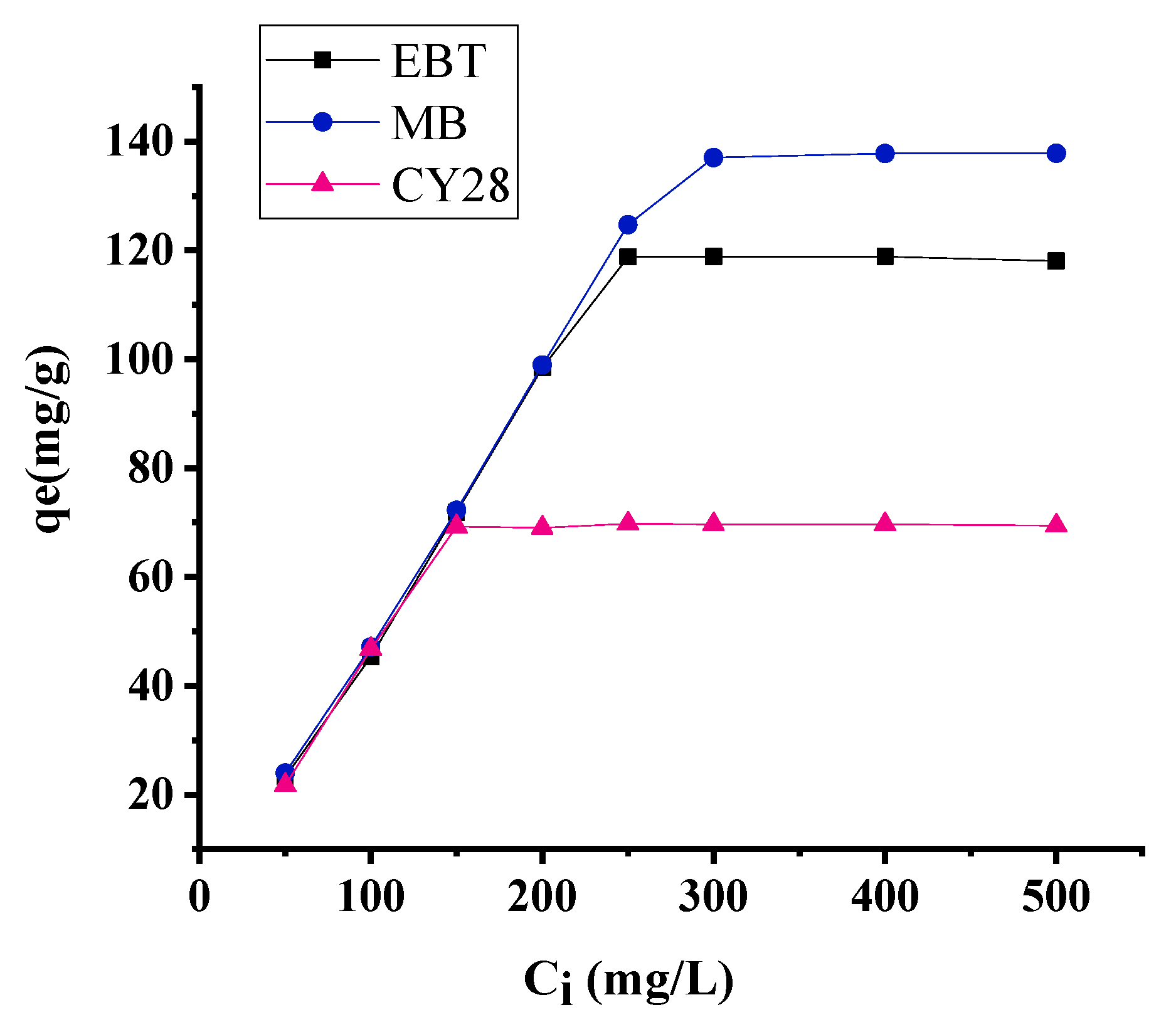
3.1. Effect of Initial Dye Concentration
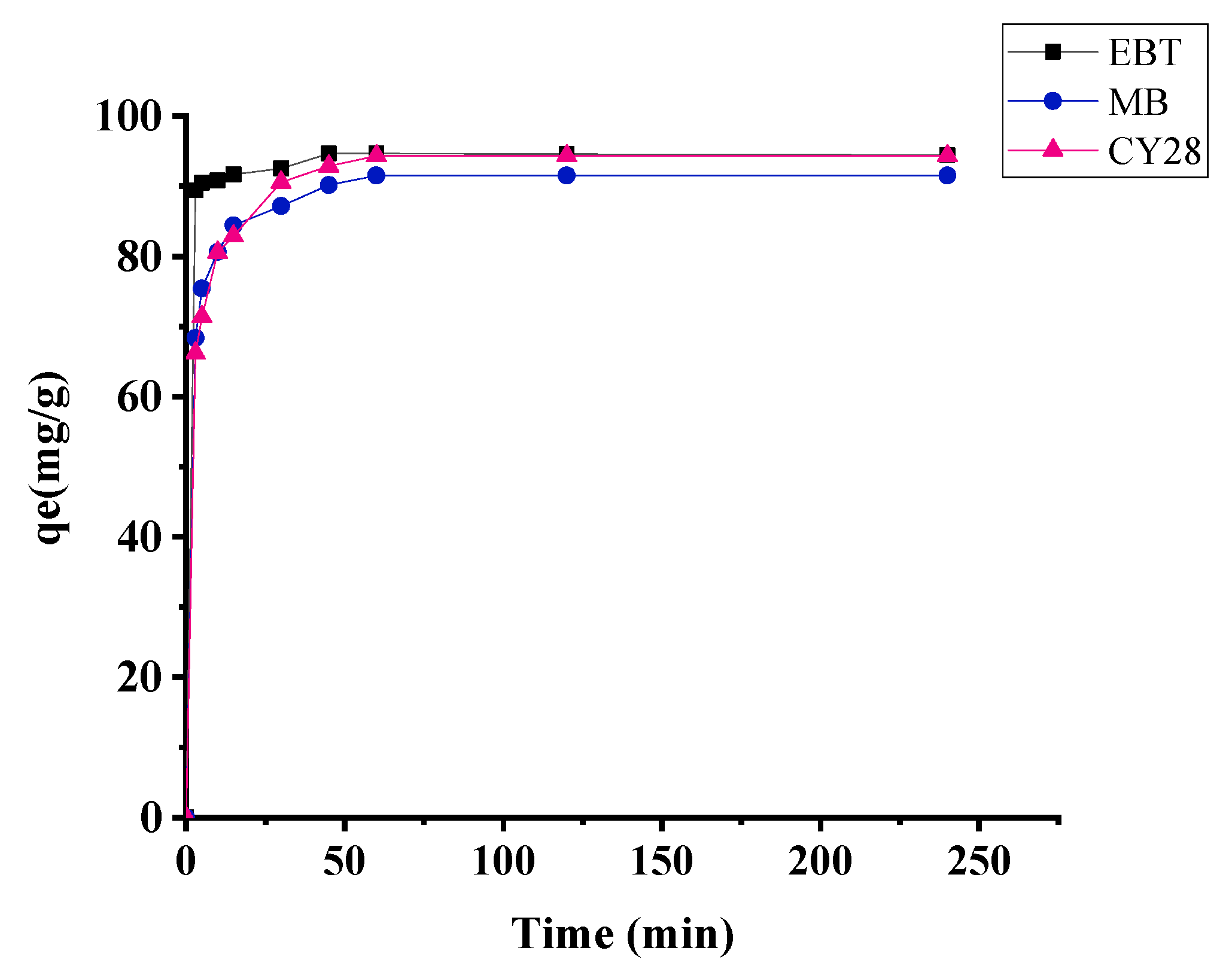
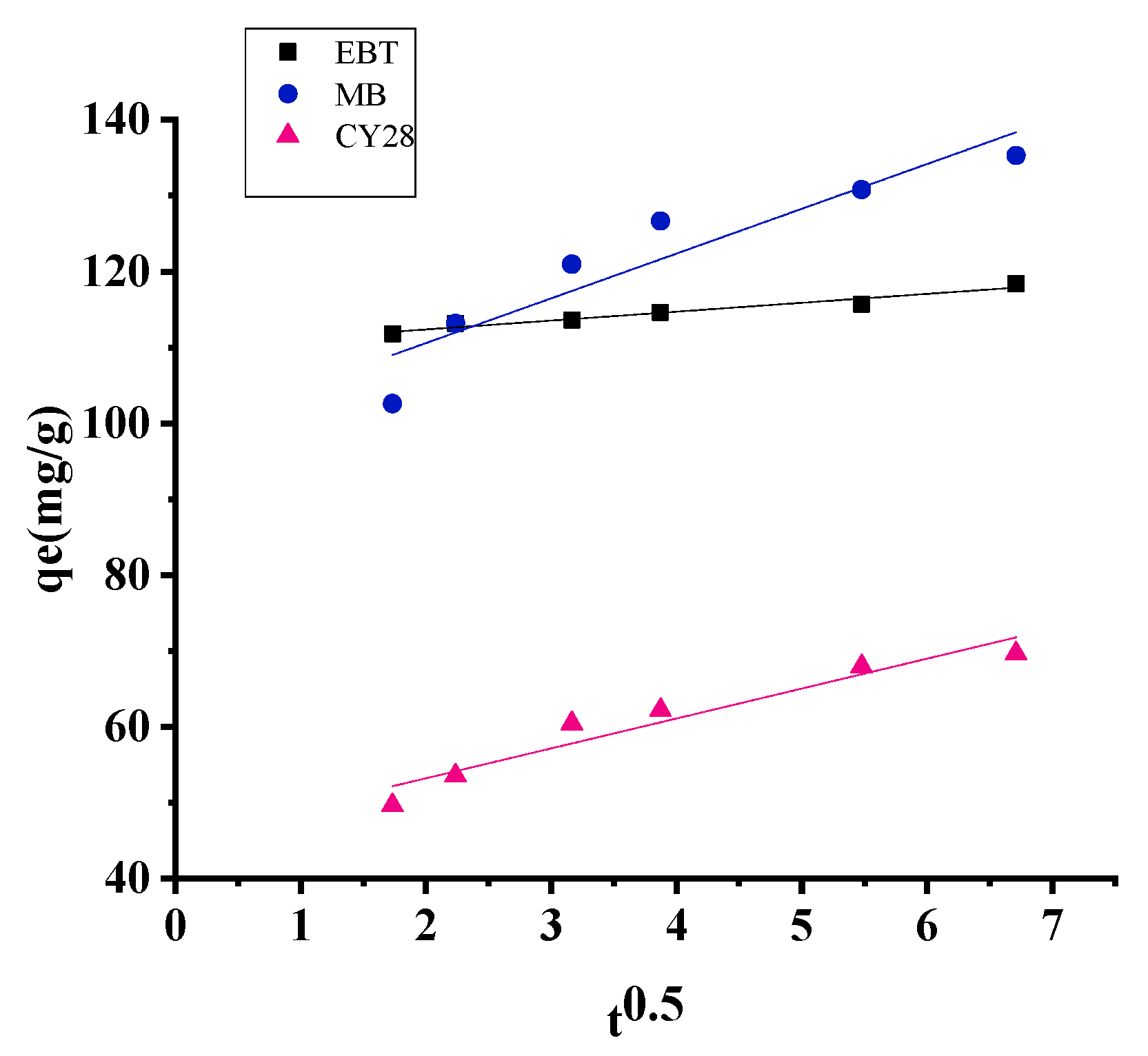
3.2. Effect of Contact Time: Kinetic Study
3.3. Effect of pH
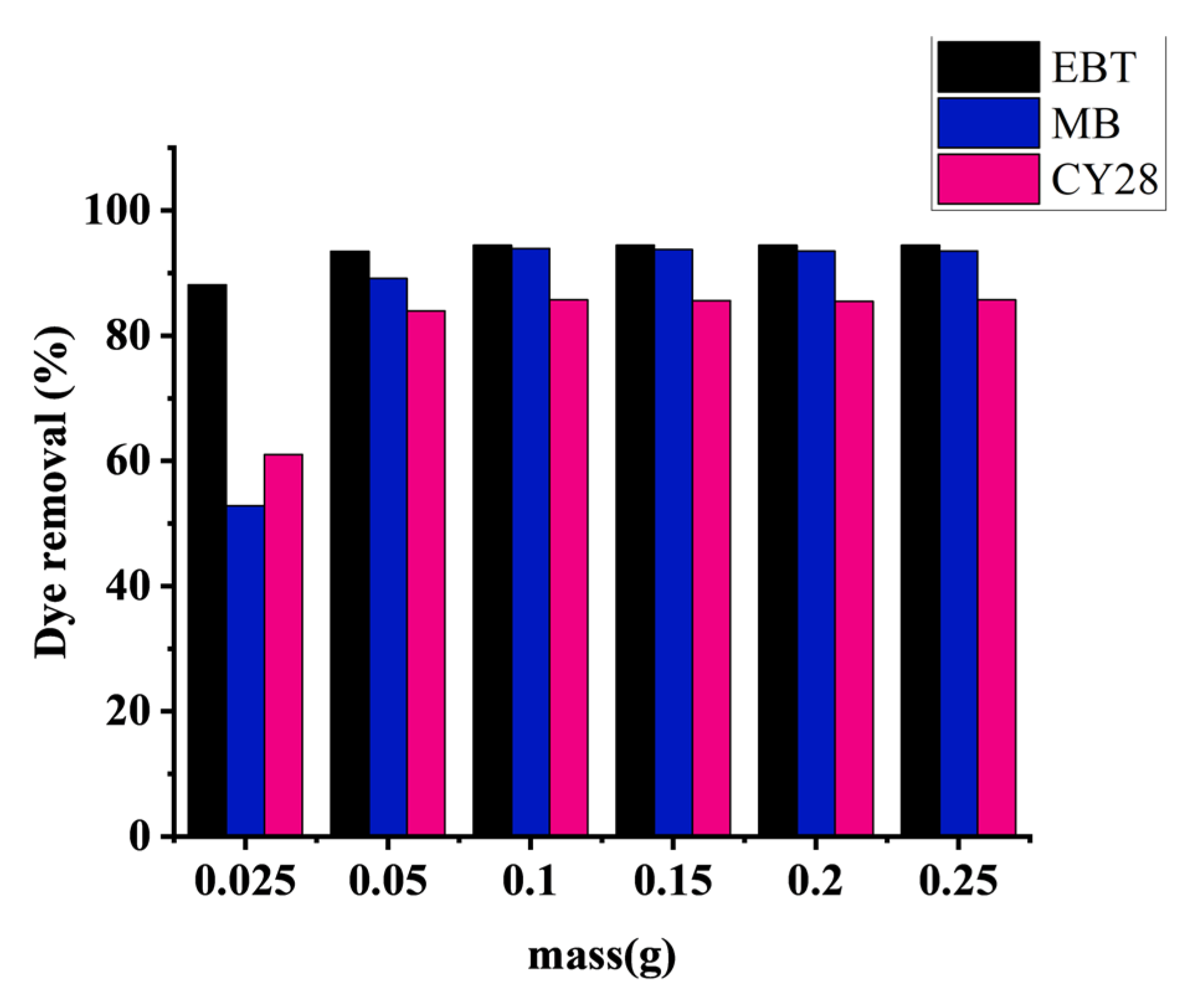
3.4. Effect of Adsorbent Mass
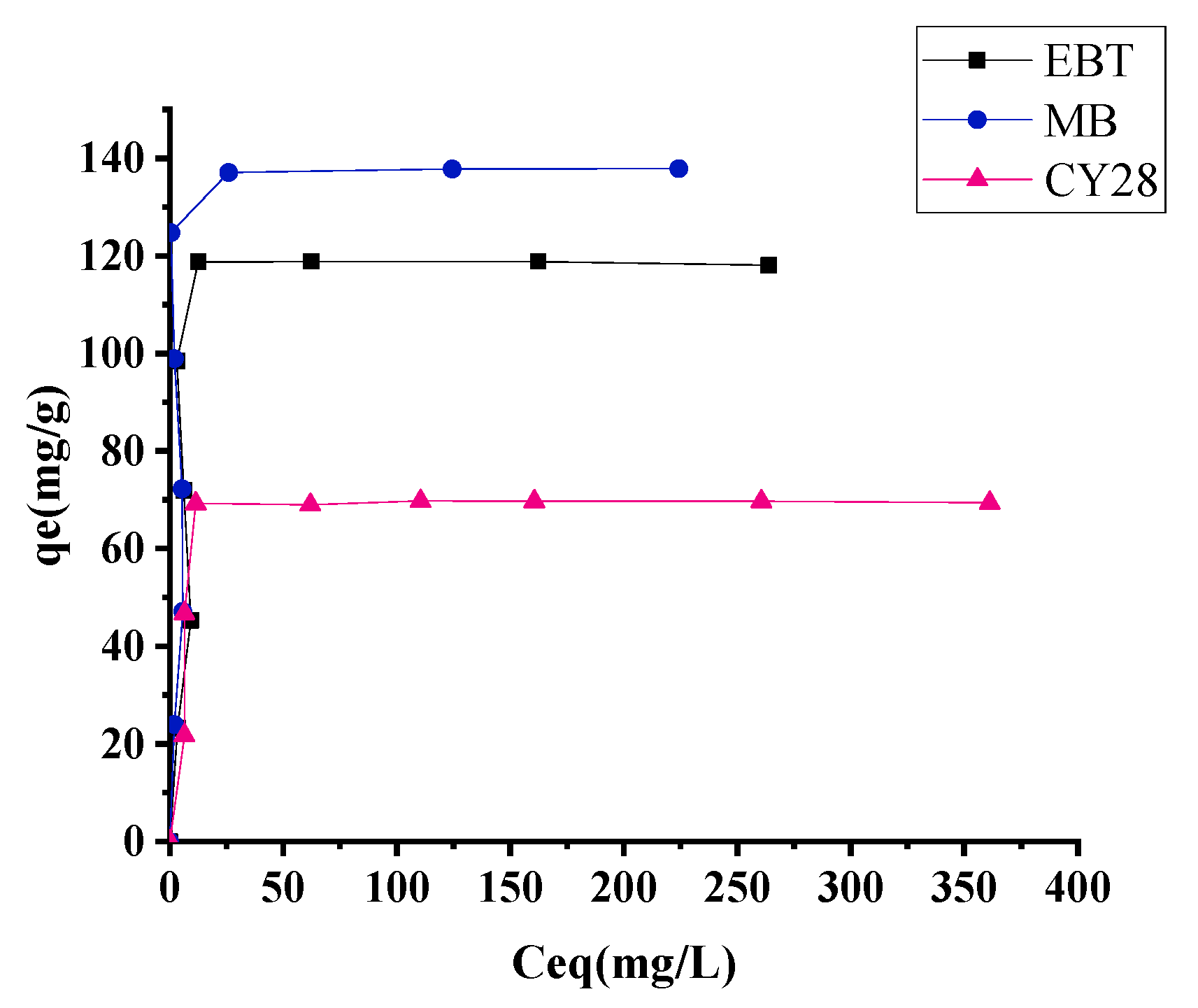
3.5. Adsorption Isotherms
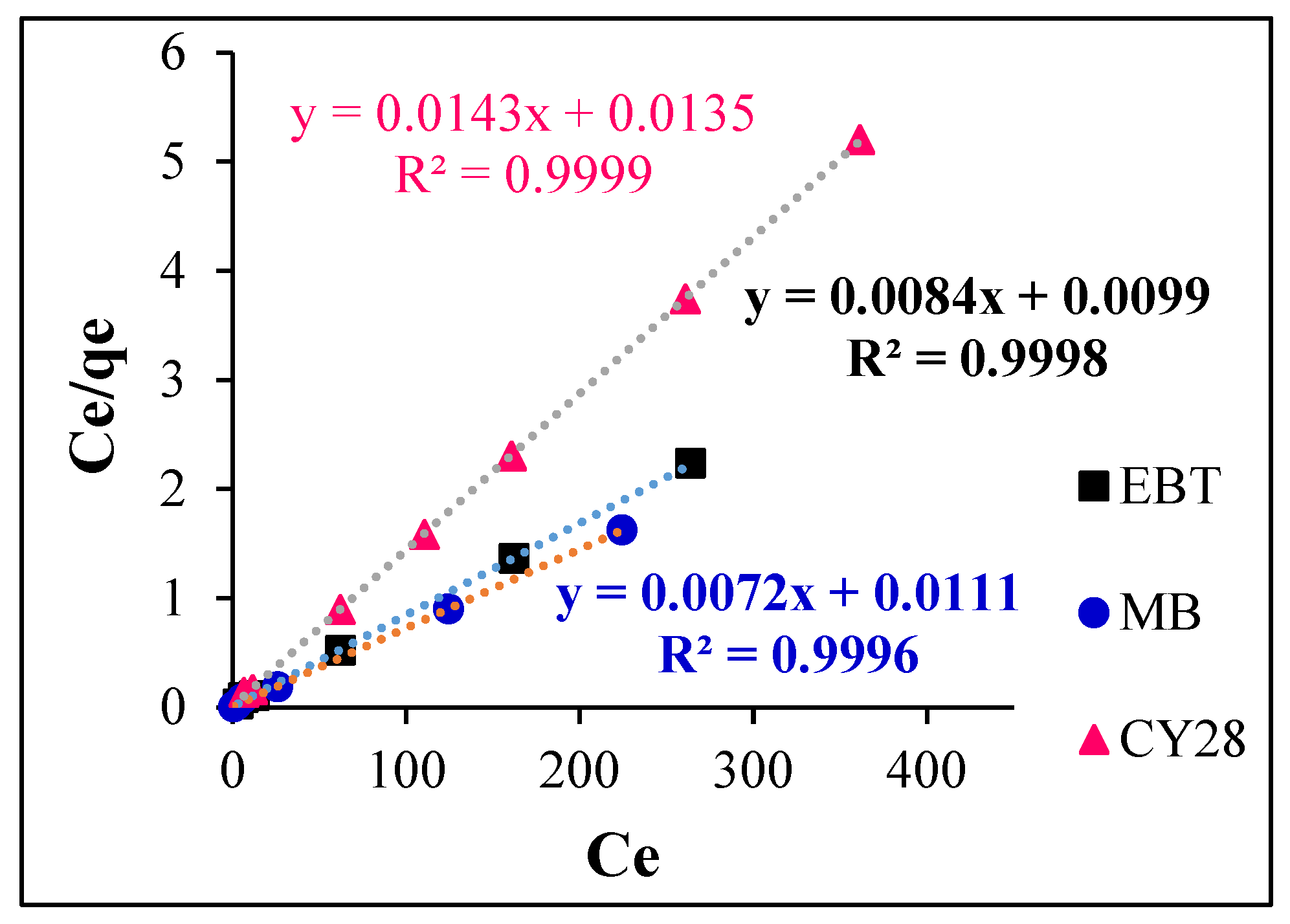
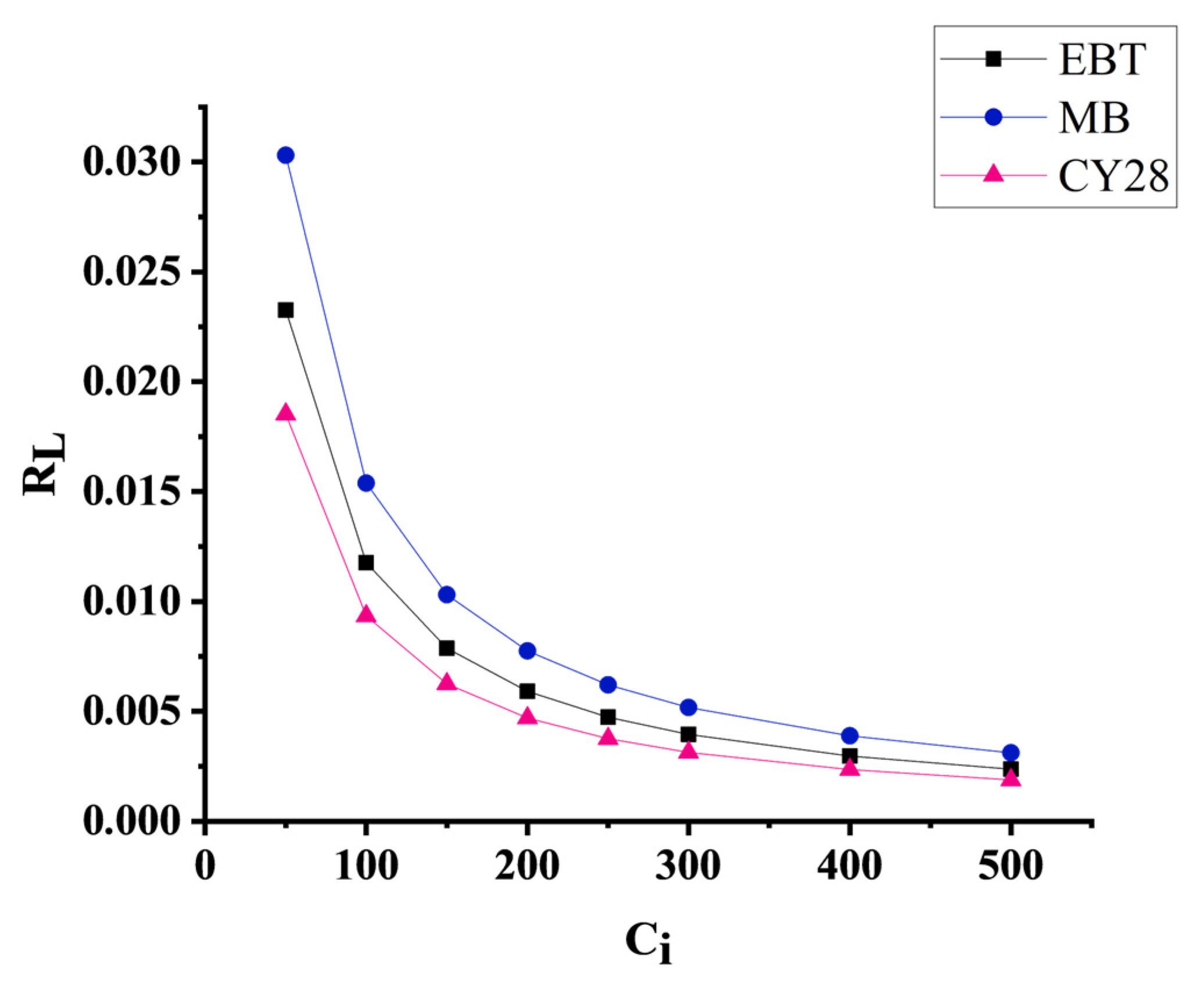
3.5.1. Application of the Langmuir Isotherm Model
3.5.2. Application of the Freundlich Isotherm Model
| Adsorbent | Dyes | Qmax (mg/g) | References |
|---|---|---|---|
| Natural Saudi Red Clay | Methylene Blue (MB) | 50.251 | [22] |
| Raw Clay | 19.194 | [38] | |
| Activated Clay | 36.5 | [38] | |
| Clay | 2.04 | [39] | |
| Kaolin (Algerie) | 52.76 | [40] | |
| Apricot Kernel Shells | 33.67 | [41] | |
| Muscovite (Morocco) | 59.47 | [42] | |
| Zeolite/Fe3O4 | 32.258 | [43] | |
| Natural Clay | 147.71 | [44] | |
| Clinoptilolite | 50 | [45] | |
| Activated Carbon | 232.5 | [46] | |
| Natural Tunisian Chert | 138.88 | Present study | |
| Green Alga | Cationic Yellow 28 (CY28) | 27 | [47] |
| Clinoptilolite | 59 | [48] | |
| Kaolin | 5.71 | [49] | |
| Calcined Eggshells | 28 | [50] | |
| Natural Tunisian Chert | 69.93 | Present study | |
| Bentonite Carbon Composite | Eriochrome Black T (EBT) | 2.899 | [51] |
| Polyvinyl Alcohol/Starch/ZSM-5 Zeolite | 16 | [52] | |
| Raw Montmorillonite | 243.9 | [26] | |
| Orange Peel | 0.667 | [53] | |
| Tea Waste | 151.26 | [54] | |
| Sawdust | 42.49 | [55] | |
| Fava Bean Peels | 5.55 | [56] | |
| Magnetic Composite Carbon | 20.79 | [57] | |
| Doum fruit | 24.68 | [58] | |
| Activated Carbon of Cannabis | 14.025 | [59] | |
| Natural Tunisian Chert | 119.04 | Present study |
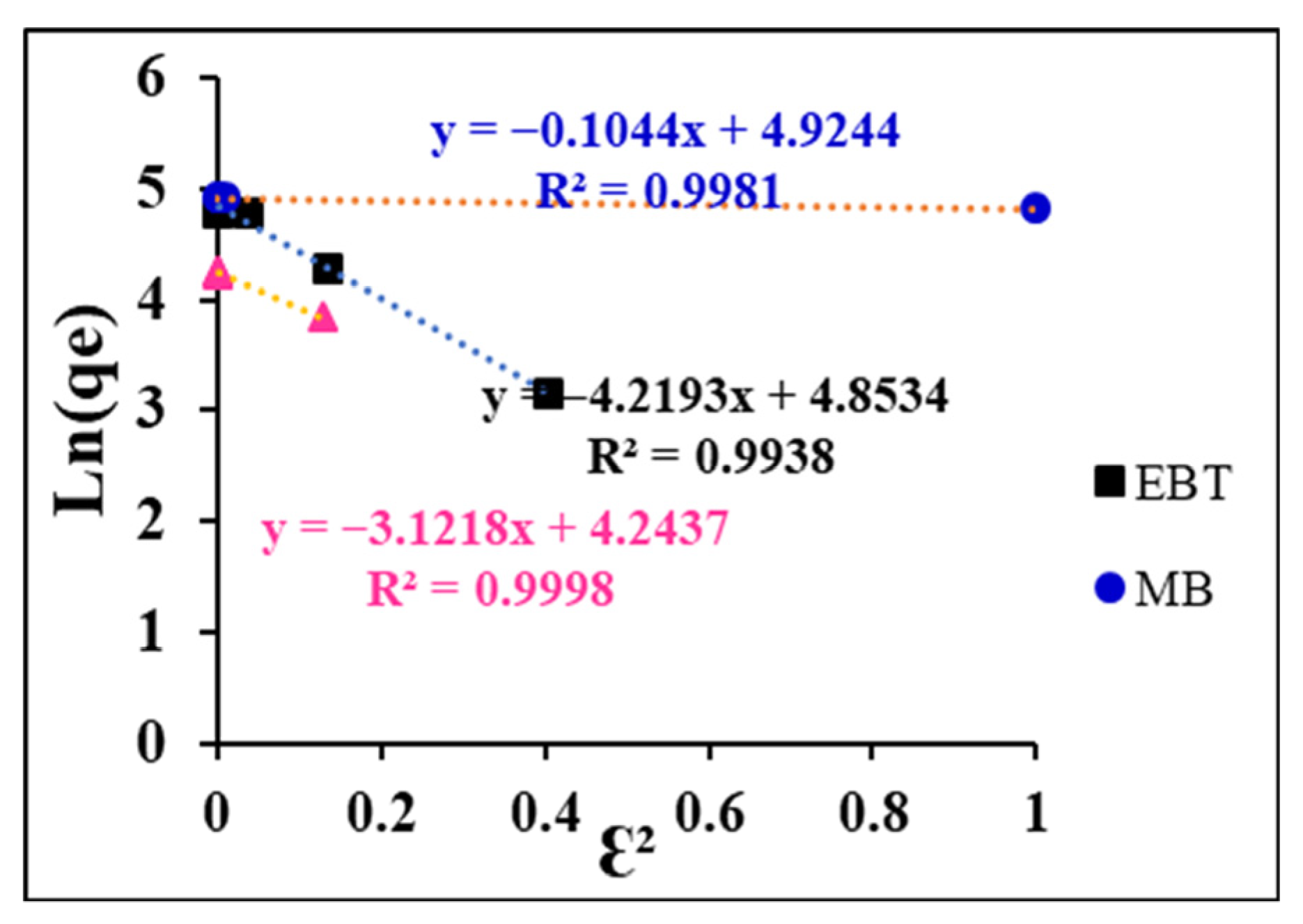
3.5.3. Application of the Dubinin–Radushkevich (D–R) Isotherm Model
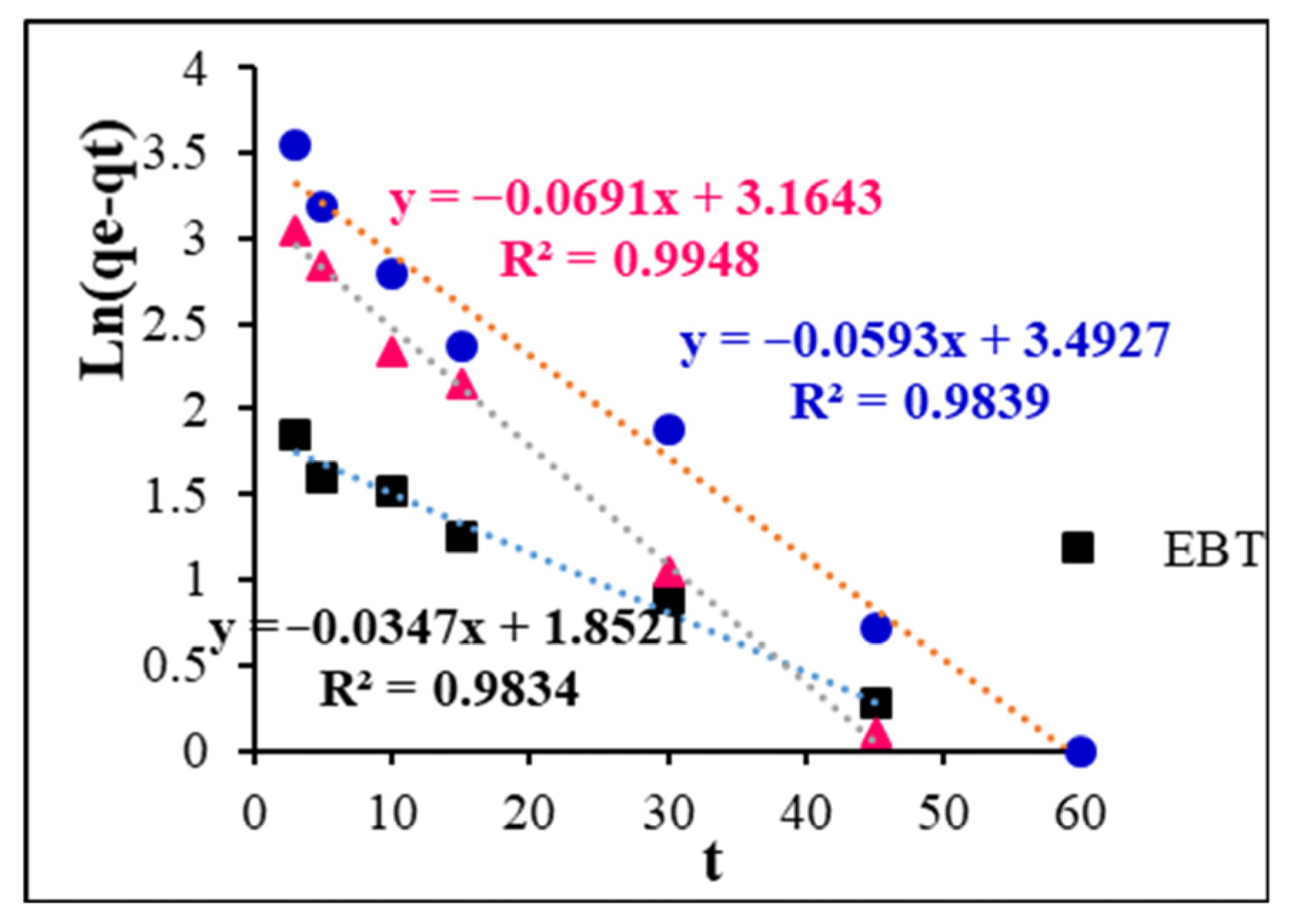
3.6. Kinetics of Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MB | Methylene Blue |
| EBT | Eriochrome Black T |
| CY28 | Cationic Yellow 28 |
References
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Rodrigues, F.H.A.; Paulino, A.T.; Martins, A.F.; Fajardo, A.R. Recent Advances on Composite Hydrogels Designed for the Remediation of Dye-Contaminated Water and Wastewater: A Review. J. Clean. Prod. 2021, 284, 124703. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Ballou, I.; Naja, J.; Bakher, Z.; Kholtei, S. Adsorption of Eriochrome Black T on Pseudo Boehmite and Gamma Alumina Synthesized from Drinking Water Treatment Sludge: A Waste-to-Recycling Approach. Recycling 2024, 9, 49. [Google Scholar] [CrossRef]
- Rápó, E.; Posta, K.; Csavdári, A.; Vincze, B.É.; Mara, G.; Kovács, G.; Haddidi, I.; Tonk, S. Performance Comparison of Eichhornia Crassipes and Salvinia Natans on Azo-Dye (Eriochrome Black t) Phytoremediation. Crystals 2020, 10, 565. [Google Scholar] [CrossRef]
- De, I.; Pahuja, M.; ud din Wani, H.M.; Dey, A.; Dube, T.; Ghosh, R.; Kankan, N.; Mishra, J.; Panda, J.J.; Maruyama, T.; et al. In-Vitro Toxicity Assessment of a Textile Dye Eriochrome Black T and Its Nano-Photocatalytic Degradation through an Innovative Approach Using Mf-NGr-CNTs-SnO2 Heterostructures. Ecotoxicol. Environ. Saf. 2022, 243, 113985. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Sukla, R.K.; Pradhan, A.; Sahoo, S.K.; Rath, J. Removal of Eriochrome Black T Dye Using Plant Based Bio-Adsorbent: A Review. Lett. Appl. NanoBioScience 2024, 13, 73. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene Blue Dye: Toxicity and Potential Elimination Technology from Wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on Recent Advances of Carbon Based Adsorbent for Methylene Blue Removal from Waste Water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; El-Sayyad, G.S.; Saad, F.A.; Shah, R.K.; Abdelrahman, E.A. Efficient Removal of Basic Yellow 28 Dye from Water Using Facilely Synthesized ZnO and Mg3B2O6 Nanostructures. Sci. Rep. 2024, 14, 26181. [Google Scholar] [CrossRef]
- Mahjoubi, N.; Araissi, M.; Elaloui, E. Comparative Adsorption Study of Cationic Yellow 28 and Anionic Congo Red Dyes Using Tunisian Natural Chert. Euro-Mediterr. J. Environ. Integr. 2024, 10, 63–77. [Google Scholar] [CrossRef]
- Saad, M.E.K.; Mnasri, N.; Mhamdi, M.; Chafik, T.; Elaloui, E.; Moussaoui, Y. Removal of Methylene Blue onto Mineral Matrices. Desalin. Water Treat. 2015, 56, 2773–2780. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, M.; Li, X.; Ning, J.; Zhou, Z.; Li, G. Efficient Adsorption of Methylene Blue from Aqueous Solution by Graphene Oxide Modified Persimmon Tannins. Mater. Sci. Eng. C 2020, 108, 110196. [Google Scholar] [CrossRef]
- Benali, Y.; Mabrouki, N.; Agougui, H.; Jabli, M.; Majdoub, H.; Predoi, D.; Ciobanu, S.; Iconaru, S.L.; Ţălu, Ş.; Boughzala, K. A New Porous Composite Hydroxyapatite/Chitosan/Microcrystalline-Cellulose: Synthesis, Characterization and Application to the Adsorption of Eriochrome Black T. Polym. Bull. 2024, 81, 16875–16902. [Google Scholar] [CrossRef]
- Maamria, J.; Soli, J.; Coperaa, C.; Bonnet, P.; Elaloui, E. Preparation and Physicochemical Properties Studies of SnS2 and SnS/SnS2 Nanoparticles and Use as Efficient Photocatalysts for the Degradation of Rhodamine B. Chem. Afr. 2024, 7, 5339–5354. [Google Scholar] [CrossRef]
- Karam, A.; Bakhoum, E.S.; Zaher, K. Coagulation/Flocculation Process for Textile Mill Effluent Treatment: Experimental and Numerical Perspectives. Int. J. Sustain. Eng. 2021, 14, 983–995. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.d.C.B.S.M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- Gayathri, P.V.; Yesodharan, S.; Yesodharan, E.P. Microwave/Persulphate Assisted ZnO Mediated Photocatalysis (MW/PS/UV/ZnO) as an Efficient Advanced Oxidation Process for the Removal of RhB Dye Pollutant from Water. J. Environ. Chem. Eng. 2019, 7, 103122. [Google Scholar] [CrossRef]
- Kamati, S.N.; Yan, J.; Fan, J. A Review on Progresses in Reactive Dye-Containing Wastewater Treatment. Water Pract. Technol. 2024, 19, 2712–2733. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Removal of a Cationic Dye from Aqueous Solution by Natural Clay; Elsevier: Amsterdam, The Netherlands, 2018; Volume 6, ISBN 2126737403. [Google Scholar]
- Larbi, H.; Abdelkader, H.; Belkhir, D.; Asma, M.A. Thermo-Kinetic Study of Adsorption of Methylene Blue in Aqueous Solutions Using Algerian Illite Natural and Activated Mineral Clay. Defect. Diffus. Forum 2021, 406, 348–363. [Google Scholar] [CrossRef]
- Khan, M.I. Adsorption of Methylene Blue onto Natural Saudi Red Clay: Isotherms, Kinetics and Thermodynamic Studies. Mater. Res. Express 2020, 7, 055507. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A. Diatomite Chemical Activation for Effective Adsorption of Methylene Blue Dye from Model Textile Wastewater. Int. J. Environ. Sci. Dev. 2021, 12, 23–28. [Google Scholar] [CrossRef]
- Mahjoubi, N.; Araissi, M.; Mhamdi, M.; Elaloui, E. Valorisation, Characterisation and Application of Natural Materials (Zeolite and Chert) as Adsorbents for the Removal of Chromium(III) from an Aqueous Solution. Water. Air. Soil. Pollut. 2022, 233, 246. [Google Scholar] [CrossRef]
- Bouchmila, I.; Bejaoui Kefi, B.; Souissi, R.; Abdellaoui, M. Purification, Characterization and Application of Cherty Rocks as Sorbent for Separation and Preconcentration of Rare Earths. J. Mater. Res. Technol. 2019, 8, 2910–2923. [Google Scholar] [CrossRef]
- Rashidi, R.; Omidi Khaniabadi, Y.; Ghaderpoori, M. Adsorption of Eriochrome Black-T from Aqueous Environment by Raw Montmorillonite. Int. J. Environ. Anal. Chem. 2023, 103, 2601–2615. [Google Scholar] [CrossRef]
- Zeydouni, G.; Kianizadeh, M.; Omidi Khaniabadi, Y.; Nourmoradi, H.; Esmaeili, S.; Mohammadi, M.J.; Rashidi, R. Eriochrme Black-T Removal from Aqueous Environment by Surfactant Modified Clay: Equilibrium, Kinetic, Isotherm, and Thermodynamic Studies. Toxin Rev. 2019, 38, 307–317. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Chitosan-Clay Composite as Highly Effective and Low-Cost Adsorbent for Batch and Fixed-Bed Adsorption of Methylene Blue. Chem. Eng. J. 2014, 237, 352–361. [Google Scholar] [CrossRef]
- Islem, C. Adsorptive/Desorptive Potential of Cationic Basic Yellow 28 (By28) Dyes onto Natural Untreated Clay from Aqueous Phase. Open Access J. Sci. 2018, 2, 74–79. [Google Scholar] [CrossRef]
- Djomgoue, P.; Siewe, M.; Djoufac, E.; Kenfack, P.; Njopwouo, D. Surface Modification of Cameroonian Magnetite Rich Clay with Eriochrome Black T. Application for Adsorption of Nickel in Aqueous Solution. Appl. Surf. Sci. 2012, 258, 7470–7479. [Google Scholar] [CrossRef]
- Rehman, M.U.; Manan, A.; Uzair, M.; Khan, A.S.; Ullah, A.; Ahmad, A.S.; Wazir, A.H.; Qazi, I.; Khan, M.A. Physicochemical Characterization of Pakistani Clay for Adsorption of Methylene Blue: Kinetic, Isotherm and Thermodynamic Study. Mater. Chem. Phys. 2021, 269, 124722. [Google Scholar] [CrossRef]
- El Kassimi, A.; Achour, Y.; El Himri, M.; Laamari, R.; El Haddad, M. Removal of Two Cationic Dyes from Aqueous Solutions by Adsorption onto Local Clay: Experimental and Theoretical Study Using DFT Method. Int. J. Environ. Anal. Chem. 2023, 103, 1223–1244. [Google Scholar] [CrossRef]
- Neag, E.; Török, A.I.; Tanaselia, C.; Aschilean, I.; Senila, M. Kinetics and Equilibrium Studies for the Removal of Mn and Fe from Binary Metal Solution Systems Using a Romanian Thermally Activated Natural Zeolite. Water 2020, 12, 1614. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Nikkar, H.; Mahmoodi, N.M.; Markazi, M.; Menger, F.M. The Sorption of Cationic Dyes onto Kaolin: Kinetic, Isotherm and Thermodynamic Studies. Desalination 2011, 266, 274–280. [Google Scholar] [CrossRef]
- Elijah, O.; Nwabanne, J.T. Adsorption Studies on the Removal of Eriochrome Black-T from Aqueous Solution Using Nteje Clay. SOP Trans. Appl. Chem. 2014, 1, 14–25. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Kazeem, T.S.; Zubair, M.; Al-Harthi, M. Bentonite-Layered Double Hydroxide Composite for Enhanced Aqueous Adsorption of Eriochrome Black T. Appl. Clay Sci. 2018, 161, 23–34. [Google Scholar] [CrossRef]
- Gauden, P.; Terzyk, A.; Kowalczyk, P.; Aranovich, G.; Donohue, M.; Cwiertnia, M.; Furmaniak, S.; Rychlicki, G. Giles’ Classification of Solute Adsorption Isotherms for Binary Non-Electrolyte Solutions via Lattice DFT Supported by Experimental Sorption Data from Aqueous Solutions on Carbonaceous Materials. Carbon Materials: Theory and Practice; Research Signpost: Kerala, India, 2008; pp. 517–570. [Google Scholar]
- Sadki, H.; Ziat, K.; Saidi, M. Adsorption d’un Colorant Cationique d’un Milieu Aqueux Sur Une Argile Locale Activée (Adsorption of Dyes on Activated Local Clay in Aqueous Solution). Environ. Sci. 2014, 5, 2060–2065. [Google Scholar]
- Teğin, İ.; Saka, C. Chemical and Thermal Activation of Clay Sample for Improvement Adsorption Capacity of Methylene Blue. Int. J. Environ. Anal. Chem. 2021, 103, 4503–4514. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from Aqueous Solutions by Adsorption on Kaolin: Kinetic and Equilibrium Studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Namal, O.O.; Kalipci, E. Adsorption Kinetics of Methylene Blue Removal from Aqueous Solutions Using Potassium Hydroxide (KOH) Modified Apricot Kernel Shells. Int. J. Environ. Anal. Chem. 2020, 100, 1549–1565. [Google Scholar] [CrossRef]
- Amrhar, O.; Berisha, A.; El Gana, L.; Nassali, H.; Elyoubi, S.M. Removal of Methylene Blue Dye by Adsorption onto Natural Muscovite Clay: Experimental, Theoretical and Computational Investigation. Int. J. Environ. Anal. Chem. 2023, 103, 2419–2444. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Rendo, D.; Suherman, S. Removal of Methylene Blue Dye in Water by Using Recoverable Natural Zeolite/Fe3O4 Adsorbent. Glob. Nest J. 2021, 23, 119–126. [Google Scholar] [CrossRef]
- El-Habacha, M.; Dabagh, A.; Lagdali, S.; Miyah, Y.; Mahmoudy, G.; Sinan, F.; Chiban, M.; Iaich, S.; Zerbet, M. An Efficient and Adsorption of Methylene Blue Dye on a Natural Clay Surface: Modeling and Equilibrium Studies. Environ. Sci. Pollut. Res. 2023, 31, 62065–62079. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption Characteristics for the Removal of a Toxic Dye, Tartrazine from Aqueous Solutions by a Low Cost Agricultural by-Product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of Low-Cost Adsorbents for Dye Removal—A Review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Yener, J.; Kopac, T.; Dogu, G.; Dogu, T. Adsorption of Basic Yellow 28 from Aqueous Solutions with Clinoptilolite and Amberlite. J. Colloid. Interface Sci. 2006, 294, 255–264. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Alene, A.N. A Comparative Study of Acidic, Basic, and Reactive Dyes Adsorption from Aqueous Solution onto Kaolin Adsorbent: Effect of Operating Parameters, Isotherms, Kinetics, and Thermodynamics. Emerg. Contam. 2022, 8, 59–74. [Google Scholar] [CrossRef]
- Slimani, R.; El Ouahabi, I.; Abidi, F.; El Haddad, M.; Regti, A.; Laamari, M.R.; El Antri, S.; Lazar, S. Calcined Eggshells as a New Biosorbent to Remove Basic Dye from Aqueous Solutions: Thermodynamics, Kinetics, Isotherms and Error Analysis. J. Taiwan. Inst. Chem. Eng. 2014, 45, 1578–1587. [Google Scholar] [CrossRef]
- El-Dars, F.M.S.E.; Ibrahim, H.M.; Farag, H.A.B.; Abdelwahhab, M.Z.; Shalabi, M.E.H. Adsorption kinetics of bromophenol blue and eriochrome black t using bentonite carbon composite material. Int. J. Sci. Eng. Res. 2015, 6, 679–688. [Google Scholar]
- Radoor, S.; Karayil, J.; Parameswaranpillai, J.; Siengchin, S. Adsorption Study of Anionic Dye, Eriochrome Black T from Aqueous Medium Using Polyvinyl Alcohol/Starch/ZSM-5 Zeolite Membrane. J. Polym. Environ. 2020, 28, 2631–2643. [Google Scholar] [CrossRef]
- Salman Taghried, A.; Ali Mayasa, I. Eriochrome Black T Dye Adsorption onto Natural and Modified Orange Peel. Res. J. Chem. Environ. 2019, 23, 155–169. [Google Scholar]
- Bansal, M.; Patnala, P.K.; Dugmore, T. Adsorption of Eriochrome Black-T(EBT) Using Tea Waste as a Low Cost Adsorbent by Batch Studies: A Green Approach for Dye Effluent Treatments. Curr. Res. Green. Sustain. Chem. 2020, 3, 100036. [Google Scholar] [CrossRef]
- Akhouairi, S.; Ouachtak, H. Natural Sawdust as Adsorbent for the Eriochrome Black T Dye Removal from Natural Sawdust as Adsorbent for the Eriochrome Black T Dye Removal from Aqueous Solution. Water Air Soil Pollut 2019, 30, 181. [Google Scholar] [CrossRef]
- Nahali, L.; Miyah, Y.; Mejbar, F.; Benjelloun, M.; Assila, O.; Fahoul, Y.; Nenov, V.; Zerrouq, F. Assessment of Brilliant Green and Eriochrome Black T Dyes Adsorption onto Fava Bean Peels: Kinetics, Isotherms and Regeneration Study. Desalin. Water Treat. 2022, 245, 255–269. [Google Scholar] [CrossRef]
- Fajarwati, F.I.; Anugrahwati, M.; Yanti, I.; Safitri, R.A.; Yeni; Yuanita, E. Adsorption Study of Methylene Blue and Eriochrome Black T Dyes on Activated Carbon and Magnetic Carbon Composite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 599, 012025. [Google Scholar] [CrossRef]
- Abdel-Khalek, A.A.; Abdel-Hafeez, M.M.; Mohamed, R.A.; Gabrail, E.H. Insights into Removal of Eriochrome Black-T Dye from Aqueous Solution by Doum Fruit as a Natural Dsorbent. Egypt. J. Chem. 2022, 65, 189–199. [Google Scholar] [CrossRef]
- El Mansouri, F.; Pelaz, G.; Morán, A.; Da Silva, J.C.G.E.; Cacciola, F.; El Farissi, H.; Tayeq, H.; Zerrouk, M.H.; Brigui, J. Efficient Removal of Eriochrome Black T Dye Using Activated Carbon of Waste Hemp (Cannabis Sativa L.) Grown in Northern Morocco Enhanced by New Mathematical Models. Separations 2022, 9, 283. [Google Scholar] [CrossRef]
- Mahajan, T.; Paikaray, S.; Mahajan, P. Applicability of the Equilibrium Adsorption Isotherms and the Statistical Tools on to Them: A Case Study for the Adsorption of Fluoride onto Mg-Fe-CO3LDH. J. Phys. Conf. Ser. 2023, 2603, 012056. [Google Scholar] [CrossRef]
- Ouzani, A.; Zouambia, Y.; Maachou, H.; Krea, M.; Assadi, A.A.; Khezami, L.; Benguerba, Y.; Zhang, J.; Amrane, A.; Elfalleh, W.; et al. Sustainable Removal of Basic Fuchsine and Methylene Blue Dyes Using Chicken Bone Biomass: Thermodynamics, Kinetics, and Insights from Experimental Studies and Decision Tree with Least Squares Boosting Predictive Modeling. Water 2025, 17, 1053. [Google Scholar] [CrossRef]
- Haggag, E.S.A.; Tohamy, H.A.S.; Mahmoud, A.E.D.; El-Sakhawy, M.; Salem, A.R. Acrylamide/Ethyl Cellulose Schiff Base Hydrogel for Uranium Capture from Aqueous Solutions Using Response Surface Methodology. Int. J. Biol. Macromol. 2025, 321, 145666. [Google Scholar] [CrossRef]




| Name | Eriochrome Black T | Methylene Blue | Cationic Yellow 28 |
|---|---|---|---|
| Commercial name | Eriochrome Black T | Methylene Blue | Maxilon Golden Yellow GL 200% |
| Chemical formula | C20H12N3O7SNa | C16H18N3ClS | C21H27N3O5S |
| Molecular weight | 461.38 g/mol | 319.85 g/mol | 433.52 g/mol |
| Class | Azo dye | Polymethine dye | Azo dye |
| Nature | Anionic | Cationic | Cationic |
| λmax (nm) | 550 | 665 | 438 |
| Color type | Brownish black | Blue | Yellow |
| Chemical structure |  | 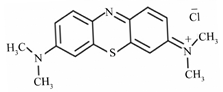 | 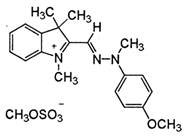 |
| Dyes | Experimental qe (mg/g) | Langmuir Parameters | Freundlich Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | Qmax (mg/g) | KL | RL | R2 | nF | KF | ||
| EBT | 118.76 | 0.9998 | 119.04 | 0.84 | 0.023–0.0023 | 0.37 | 4.74 | 42.10 |
| MB | 137.03 | 0.9996 | 138.88 | 0.64 | 0.030–0.0031 | 0.77 | 2.96 | 28.57 |
| CY28 | 69.25 | 0.9999 | 69.93 | 1.05 | 0.018–0.0018 | 0.45 | 5.92 | 29.39 |
| Dyes | R2 | β (mol2/kJ2) | Qmax (mg/g) | E (kJ/mol) |
|---|---|---|---|---|
| EBT | 0.9938 | 4.219 | 128.17 | 0.34 |
| MB | 0.9981 | 0.104 | 137.88 | 2.18 |
| CY28 | 0.9998 | 3.121 | 69.66 | 0.40 |
| Adsorbent | Experimental qe (mg/g) | Pseudo-First Order | Pseudo-Second Order | Intra-Particle Diffusion | |||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | qe (mg/g) | K1 (min−1) | R2 | qe (mg/g) | K2 (g/mg.min) | R2 | Kid | ||
| EBT | 118.36 | 0.98 | 6.37 | 0.0347 | 0.99 | 117.64 | 0.038 | 0.95 | 1.17 |
| MB | 137.30 | 0.98 | 32.87 | 0.0593 | 0.99 | 138.88 | 0.0069 | 0.87 | 5.90 |
| CY28 | 70.75 | 0.99 | 23.67 | 0.0691 | 0.99 | 70.92 | 0.011 | 0.92 | 3.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahjoubi, N.; Hamdi, R. Eco-Friendly Removal of Cationic and Anionic Textile Dyes Using a Low-Cost Natural Tunisian Chert: A Promising Solution for Wastewater Treatment. Water 2025, 17, 2806. https://doi.org/10.3390/w17192806
Mahjoubi N, Hamdi R. Eco-Friendly Removal of Cationic and Anionic Textile Dyes Using a Low-Cost Natural Tunisian Chert: A Promising Solution for Wastewater Treatment. Water. 2025; 17(19):2806. https://doi.org/10.3390/w17192806
Chicago/Turabian StyleMahjoubi, Najah, and Raghda Hamdi. 2025. "Eco-Friendly Removal of Cationic and Anionic Textile Dyes Using a Low-Cost Natural Tunisian Chert: A Promising Solution for Wastewater Treatment" Water 17, no. 19: 2806. https://doi.org/10.3390/w17192806
APA StyleMahjoubi, N., & Hamdi, R. (2025). Eco-Friendly Removal of Cationic and Anionic Textile Dyes Using a Low-Cost Natural Tunisian Chert: A Promising Solution for Wastewater Treatment. Water, 17(19), 2806. https://doi.org/10.3390/w17192806






