Abstract
Shale gas wastewater from hydraulic fracturing poses significant environmental risks due to its high salinity and complex inorganic composition. This study investigates the behavior of major and trace inorganic constituents across a full-scale treatment train in the Sichuan Basin, China. Despite multi-stage processes including equalization, flocculation, flotation, biological reactors, membrane filtration, and clarification, key inorganic species such as Cl, Na, Br, Sr, Li, and B remained largely persistent in the final effluent with values of 13,760, 8811, 70, 95.9, 26.6, and 60.2 mg/L, respectively. Geochemical tracers including Br/Cl (average: 0.0022 mM/mM), Na/Br (average: 125 mg/mg), and Sr/Ca (average: 0.15 mM/mM) ratios, combined with halide endmember mixing models, revealed that salinity primarily originated from highly evaporated formation brines, with limited evidence for halite dissolution or external contamination. Elevated Sr (average: 89.3 mg/L) and Ca (average: 274 mg/L) levels relative to Mg (average: 32 mg/L) suggest significant water–rock interaction. Environmental risk assessments showed that concentrations of several elements in treated effluent greatly exceeded national and international discharge or reuse standards. These findings underscore the limitations of conventional treatment technologies and highlight the urgent need for advanced processes and regulatory frameworks that address the unique challenges of high-TDS (total dissolved solids) unconventional wastewater.
1. Introduction
Unconventional shale gas production via hydraulic fracturing has substantially reshaped global energy strategies over the past two decades, offering enhanced energy security and reduced carbon emissions compared to coal combustion. In China, shale gas development has progressed rapidly, with the Sichuan Basin emerging as a major production hub due to its abundant marine shale reserves, mature infrastructure, and strong governmental support [1]. Proven marine shale gas reserves in the basin exceed 3 × 1012 m3, and annual output has surpassed 25 billion m3 over the past two years, aligning with national goals to increase energy independence and facilitate a transition to cleaner energy sources [1]. Nevertheless, the environmental sustainability of shale gas extraction remains a critical concern, particularly with respect to the generation, treatment, and discharge of large volumes of flowback and produced water (FPW) [2,3,4,5,6,7,8].
Shale gas wastewater is a chemically complex mixture containing both organic and inorganic constituents, including dissolved salts, trace metals, and naturally occurring radionuclides [4,9,10,11,12,13]. Because of this complexity and the typically high TDS, treatment is commonly implemented as a fit-for-purpose train that integrates complementary steps. Front-end clarification via coagulation-flocculation with dissolved air flotation (DAF) and microfiltration/ultrafiltration removes total suspended solids, oil/grease, and colloids but has minimal impact on dissolved salts. Biological processes and membrane bioreactors (MBR) attenuate biodegradable organics and ammonium (NH4+), yet are salinity-sensitive and do not address conservative inorganics. Divalent-ion control by lime or sulfate/carbonate precipitation mitigates calcium/strontium/barium/radium (Ca2+/Sr2+/Ba2+/Ra2+) scaling and naturally occurring radionuclides risks, while sodium/chloride (Na+/Cl−) largely persists. A high-pressure membrane barrier, nanofiltration/reverse osmosis, provides desalination (nanofiltration favoring multivalent rejection; reverse osmosis broad-spectrum) but requires robust pretreatment and anti-scaling at elevated TDS. Electro-driven separations, electrodialysis/electrodialysis reversal, and capacitive deionization/membrane capacitive deionization are typically applied for low- to mid-salinity polishing or selective removal/recovery (e.g., lithium (Li+)), with performance constrained by energy use and monovalent competition. Thermal and hybrid routes, mechanical vapor compression evaporation, membrane distillation, and forward osmosis (FO) hybrids address very high salinity and zero-liquid-discharge/volume-reduction goals but involve trade-offs in scaling control, energy demand, membrane distillation pore-wetting/volatile organic compound permeation, and FO draw reconcentration [3,14,15,16,17].
While regulatory and operational efforts have concentrated on controlling organic pollutants, such as total organic carbon (TOC), biological oxygen demand, and polycyclic aromatic hydrocarbons, comparatively less attention has been paid to the behavior and removal of inorganic constituents (e.g., chloride (Cl), bromide (Br), lithium (Li), boron (B), strontium (Sr), sodium (Na)) that often persist throughout conventional treatment [4,18,19]. These constituents drive salinity in treated effluents and are prioritized as tracers of inorganic persistence and risk in shale gas wastewater because they (1) dominate salinity and scaling potential (Cl−, Na+, Sr2+), (2) signal disinfection-by-product formation risk (Br−), and (3) represent phytotoxic or emerging species relevant to reuse (B, Li+), thus posing risks to aquatic ecosystems, soil structure and agricultural productivity, and human health upon discharge or reuse [5,6,13,20,21,22,23,24]. To avoid redundancy, representative instream benchmarks and their translation to end-of-pipe targets follow the receiving-water method in Section 3.4.
Regionally, cumulative FPW production across major Sichuan Basin shale gas fields (Weiyuan, Changning, Zhaotong, and Luzhou in Sichuan province, and Fuling in Chongqing Municipality) reached 31.3 million m3 during 2012–2022, with 6.3 million m3 in 2022 alone [7]. To achieve the national target of 100 billion m3 of shale gas production by 2030, annual FPW generation is projected to rise to 50–55 million cubic meters [8]. Since 2020, approximately 80–90% of the wastewater has been reused for hydraulic fracturing, 5–10% injected into deep disposal wells, and 5–10% discharged to surface waters following treatment [7]. Effective treatment and management of these volumes are critical to protecting local surface waters essential for both ecological integrity and human use, especially in densely populated and environmentally sensitive watersheds in Chongqing Municipality [4].
Within Chongqing, typical treatment trains combine coagulation–flocculation, DAF, biological reactors, and MBR. These units effectively reduce suspended solids and biodegradable organics but offer limited removal of highly soluble inorganics. For instance, post-treatment concentrations of Cl− and Na often remain above 10,000 mg/L and 6000 mg/L, respectively, far exceeding both national and international effluent standards [14,15,16,17]. Trace elements such as Li and B similarly persist because of conservative transport and the absence of selective removal steps [12]. Although advanced options including reverse osmosis, capacitive deionization, ion exchange, and engineered adsorbents have shown promise for high-salinity industrial wastewaters [3], deployment in shale gas applications in China is still limited by infrastructure constraints, capital and operating costs, and the lack of enforceable regulatory drivers [2]. As a result, a clearer understanding of the fate of inorganic constituents across the full-scale treatment train is essential for identifying critical weaknesses and for guiding more effective and sustainable wastewater management strategies.
This study provides a detailed geochemical evaluation of inorganic constituent behavior in a full-scale shale gas wastewater treatment plant located in Chongqing Municipality, Sichuan Basin, China (Figure 1). Samples from seven sequential treatment units were analyzed to characterize concentration profiles and removal behavior of major and trace inorganics and to identify the geochemical origin of salinity and trace elements. Beyond a descriptive assessment, the study delivers a full-scale, unit-by-unit accounting of conservative inorganic species and couples halide-based endmember mixing with ionic characteristic ratios to disentangle salinity sources and fluid–rock interaction. The objective is to quantify persistence across the treatment train, evaluate compliance against national and international benchmarks, and articulate an implementation-oriented pathway and regulatory framing for inorganic risk control. The insights inform future treatment-system design and support environmentally responsible management of shale-gas wastewater in China and comparable settings.
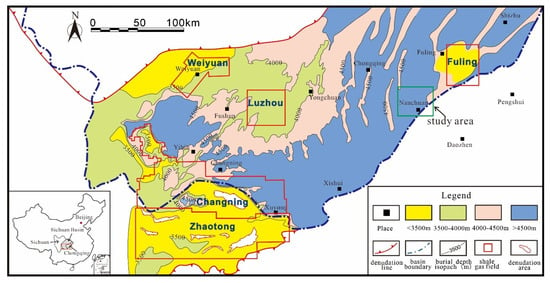
Figure 1.
Location map of the study area in Chongqing, Sichuan Basin, China. The map also shows the major commercial shale gas fields (Weiyuan, Changning, Fuling, Zhaotong and Luzhou) in the Sichuan Basin.
2. Samples and Methods
2.1. Study Site and Sampling Strategy
The shale gas wastewater treatment plant receives a mixture of FPW from multiple nearby shale gas fields and has a designed treatment capacity of 1000 m3/day. The influent exhibited TDS >20 g/L (pH 7.2–8.1), dominated by Cl (typically >10,000 mg/L) and Na (generally >5000 mg/L) with elevated NH4+, Sr, B, and Li. The plant operates under steady conditions and employs a multi-stage treatment system designed primarily to reduce organic pollutants and suspended solids to regulatory limits, including chemical oxygen demand (COD) < 100 mg/L, suspended solids < 70 mg/L, total phosphorus < 0.5 mg/L, and pH between 6 and 9. The complete process consists of the following seven sequential units: (1) flow equalization ponds (two stages, designated as Equalization-1 and Equalization-2); (2) coagulation/flocculation (labeled as Flocculation); (3) DAF (labeled as Flotation); (4) anoxic bioreactor (Anoxic); (5) aerobic bioreactor (Aerobic); (6) MBR; (7) inclined plate clarifier (Clarifiers) for final polishing (Figure 2). The anoxic stage was maintained under near-zero dissolved oxygen to support denitrification, whereas the aerobic tank was controlled at an operator-defined dissolved oxygen (DO) setpoint suitable for nitrification. The membrane bioreactor operated within customary ranges of solids retention time and mixed liquor suspended solids for industrial applications. The coagulation/flocculation step employed an inorganic coagulant together with a polymeric flocculant; exact daily doses during the campaign were not recorded. The post-MBR inclined-plate clarifier serves as a final gravity-based removal of residual fines and a hydraulic buffer to meet permit-oriented suspended solids/turbidity targets prior to discharge. A Fenton oxidation tank appears on the site signage but was not in service during the monitoring campaign and was therefore bypassed and excluded from the sampling train. Residuals are routed to sludge thickening and dewatering prior to off-site disposal. The effluent is managed under the local implementation of national discharge standards (e.g., limits on COD, suspended solids, ammonia, and pH) as displayed on the information board. Unit-by-unit ionic data are listed in Table 1.
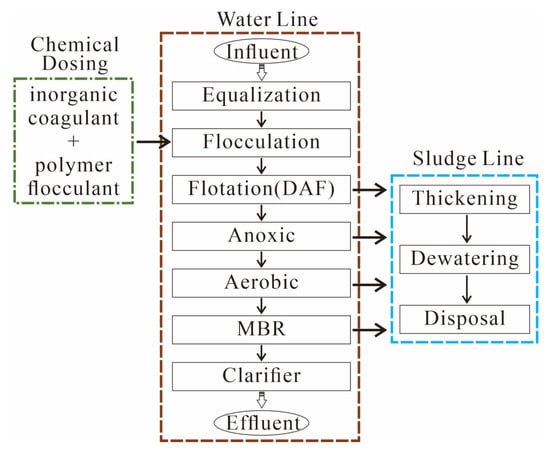
Figure 2.
Full-scale treatment train. Brown square box indicates the water line; blue square box indicates the sludge line. Equalization, flow equalization; Flocculation, coagulation/flocculation; DAF, dissolved air flotation; Anoxic, anoxic biological treatment; Aerobic, aerobic biological treatment; MBR, membrane bioreactor filtration; Clarifier, inclined plate sedimentation. Chemical dosing occurs at the flocculation unit (inorganic coagulant + polymer flocculant). The post-MBR clarifier provides final solids polishing and flow equalization before discharge, complementing biological and membrane steps.

Table 1.
Concentrations of major and trace inorganic constituents and selected ionic ratios across each treatment unit in a full-scale shale gas wastewater plant in Chongqing, Sichuan Basin. CBE is computed from measured species listed; HCO3− and SO42− values are not available and B is not included (neutral speciation).
To evaluate the behavior of inorganic constituents across the treatment train, composite water samples were collected from each of the seven units during steady-state operation. Grab samples were obtained using pre-cleaned polypropylene bottles in accordance with U.S. Geological Survey (USGS) protocols [25]. All samples were filtered using 0.45 μm mixed-cellulose ester (MCE) membranes. Subsamples intended for trace metal analysis were acidified to pH <2 with ultrapure nitric acid (HNO3, Optima grade, Fisher Scientific), while samples for anion analysis were stored unacidified under refrigeration.
2.2. Analytical Methods
Major anions (Cl−, Br−) were analyzed via ion chromatography (Metrohm ECO IC, Herisau, Switzerland), and cations (Na+, K+, Ca2+, Mg2+, NH4+) using a separate IC system (ThermoScientific Aquion-RFIC, Waltham, MA, USA). Trace metals (Li, B, Sr) were measured with ICP-MS (PerkinElmer Elan DRC-e, Cambridge, MA, USA). All samples were diluted in MQ water or 2% HNO3 (v/v, Optima grade, Fisher Scientific) to match instrument calibration ranges before the IC or ICP-MS analyses. Internal standard correction (In and Rh) was used in all samples prior to measurement on the ICP-MS. Calibration was performed using six-point external standards, and analytical accuracy was verified using the NIST SRM 1643f reference material and synthetic mixed standards used in the laboratory. Quality control measures include procedural blanks, duplicate analysis, and reference standards (e.g., Osil Atlantic Seawater). The detection limits of the instrument ensured a precision of ±0.1 mg/L for major elements and ±0.1 μg/L for trace species in all reported concentrations. Relative standard deviations (RSDs) were consistently below 5% for all anions and cations.
2.3. Geochemical Indices and Mixing Models
Salinity sources were interpreted by using halide-based endmember mixing models (log-log Cl-Br) and ionic characteristic ratios (Na/Br, Cl/Br, Mg/Ca, Sr/Ca). The conservative binary (two-endmember) mixing model was calculated between surface freshwater and formation brine, with chloride (Cl) and bromide (Br) treated as conservative tracers under the studied temperature-pH range.
For a conservative species X (Cl or Br), the mass balance for a mixed sample is
where f denotes the brine fraction (0 ≤ f ≤ 1) and C denotes mass concentration. The species-specific estimate is
Endmembers are defined as follows: Cx,freshwater is taken from background surface-water measurements; Cx,brine represents the formation brine water.
On a log-log Br-Cl plot, conservative two-endmember mixing appears as an approximately linear trend between the two endmember points. Elevated Cl/Br or Na/Br at near-constant Br indicates halite dissolution, whereas patterns in Mg/Ca and Sr/Ca inform water-rock interaction (e.g., Mg depletion accompanied by Sr enrichment). These ICR diagnostics are interpreted in conjunction with process information (Figure 2) and the behavior of divalent cations.
As a quality check, electroneutrality is evaluated using the charge-balance error (CBE)
computed from measured major ions, based on cations such as Na+, K+, Ca2+, Mg2+, NH4+, Li+, Sr2+ and anions including Cl−, Br− (all in meq/L). Boron was not included because it occurs largely as neutral boric acid at pH 7–8. HCO3− and SO42− were not available for all samples and are therefore omitted. Performing the mixing on a molar basis mitigates high-ionic-strength effects; although non-ideal activities can influence cation ratios, the linear halide-mixing assumption remains valid.
3. Results and Discussion
3.1. Persistence of Inorganic Constituents Across the Treatment Train
Despite undergoing a seven-stage full-scale treatment process, shale gas wastewater samples retained high concentrations of major and trace inorganic constituents, with minimal reductions observed across the treatment sequence (Table 1, Figure 3). The influent wastewater was characterized by extremely high TDS (>20,000 mg/L), dominated by chloride (Cl), sodium (Na), and bromide (Br), along with elevated levels of trace inorganic elements such as lithium (Li), boron (B), and strontium (Sr). Among all measured species, chloride (Cl) and sodium (Na) were the most dominant and persistent ions. Cl concentrations ranged from 13,060 to 14,180 mg/L (average: 13,660 mg/L), while Na ranged from 7956 to 8811 mg/L (average: 8449 mg/L) across the treatment stages. These levels are consistent with those reported in FPW from the major commercial shale gas fields in Sichuan Basin [10,14,26,27]. Bromide (Br), a halide of growing regulatory concern, remained between 61 and 73 mg/L (average: 68 mg/L). The source of Br in shale gas wastewater is primarily geological, reflecting its enrichment in evaporatively concentrated formation brines. During fracturing and flowback, formation waters, derived from seawater trapped in sedimentary basins and concentrated via evaporation, are mobilized and diluted by injected fluids. Numerous studies have shown that Br and Cl in flowback waters exhibit a strong linear correlation, indicating that their concentrations are dominantly controlled by mixing between injected fluids and in situ brines, rather than by halite dissolution [9,10,11,12,28].
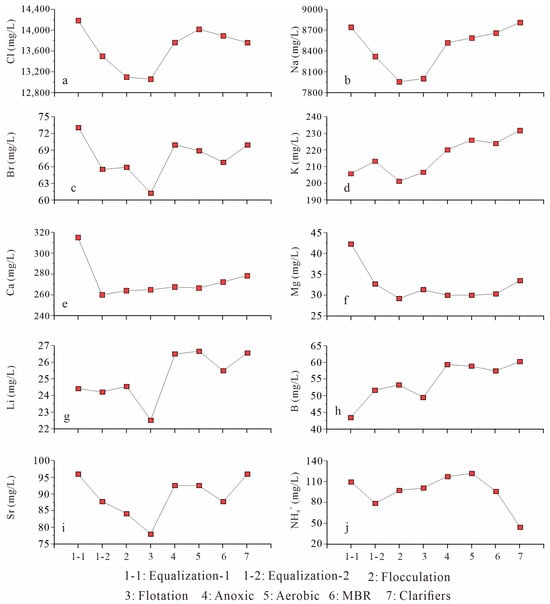
Figure 3.
Concentrations of major and trace inorganic constituents across treatment steps. (a) Cl; (b) Na; (c) Br; (d) K; (e) Ca; (f) Mg; (g) Li; (h) B; (i) Sr; (j) NH4+. Despite extensive treatment, Cl, Na, Br, Sr, B, and Li show minimal reduction, whereas NH4+ displayed a moderate decline across biological treatment stages.
Similarly, Li and B, both classified as highly mobile, conservative solutes, remained essentially unchanged through all stages of the treatment (Table 1, Figure 3g,h). The concentrations of Li (average: 26.6 mg/L) and B (average: 60.2 mg/L) in the final effluent align with typical levels observed in FPW from Sichuan Basin (Li: 20–40 mg/L; B: 30–65 mg/L) [10,14,26,27], yet they remain substantially higher than background groundwater levels (typically <0.1 mg/L for Li and <1 mg/L for B), indicating the ineffectiveness of existing removal mechanisms. Their stability is attributed to the following factors such as lack of reactive sites for biological or physical adsorption, weak complexation under neutral pH and the absence of tailored removal technologies (e.g., boron-selective resins or lithium-sorbents). These findings confirm previous reports of poor removal performance of Li and B in municipal and industrial wastewater treatment plants [29].
Unit-by-unit measurements also corroborate such conclusion. For example, relative to Equalization-2 (pre-dosing), the flocculation/DAF segment produces only minor changes in conservative solutes (e.g., Cl ≈ −3%, Na ≈ −4%, Br ≈ −8%), with B and Li changing by ~4–7% and divalent cations showing small, non-monotonic shifts (e.g., ~−11% of Sr immediately after DAF) that are not sustained downstream (Figure 3). Under high ionic strength and near-neutral pH, these magnitudes are consistent with conservative transport and mixing variability, reflecting the limited leverage of coagulation/DAF on dissolved conservative inorganics.
Collectively, these results demonstrate that while the treatment facility effectively reduces organic constituents (e.g., COD < 100 mg/L, TOC < 15 mg/L), its performance for inorganic constituents is limited. Mechanistically, the poor removal of highly soluble inorganics across coagulation/flotation/biological/MBR units reflects (1) high ionic strength and depressed activity coefficients that weaken electrostatic sorption and complexation; (2) aqueous speciation that disfavors removal at near-neutral pH (e.g., B as boric acid/borate with weak affinity; Li+ as a strongly hydrated monocation with limited surface binding); (3) the absence of ion-selective barriers or alkalinity control required to promote precipitation/co-precipitation. Under such conditions, divalent cations (Sr2+, Ca2+, Mg2+) remain predominantly as hydrated ions, and halides (Cl−, Br−) behave conservatively. In parallel, the extreme salinity and ionic strength typical of shale gas wastewater inhibit microbial activity, limit redox transformations, and destabilize sludge floc formation, further constraining overall performance [30]. Consequently, substantial reductions in Cl−, Na+, Br−, B, Li+, and Sr2+ are unlikely without ion-selective barriers or high-rejection membranes, such as nanofiltration/reverse osmosis, electrodialysis/capacitive deionization, and selective ion exchange or tailored adsorbents, coupled, where relevant, with alkalinity control. These findings underscore the need to modernize treatment trains and to revise regulatory frameworks that currently overlook key inorganic solutes in unconventional wastewater streams [2].
3.2. Variable Behavior of Ammonium and Divalent Cations
Ammonium (NH4+) displayed partial removal across the biological treatment stages, whereas divalent cations, including calcium (Ca2+), magnesium (Mg2+), and strontium (Sr2+), remained largely unchanged throughout the entire process (Table 1, Figure 3). The influent NH4+ concentration was 110 mg/L, decreasing to 44 mg/L after treatment, indicating a removal efficiency of approximately 60%. However, the final effluent concentration still significantly exceeds the Class I discharge standard of 15 mg/L (NH4-N, approximately 19.3 mg/L NH4+) prescribed in the Chinese integrated wastewater discharge standard (GB8978–2002), and is also higher than the standards used in the EU for similar effluents (e.g., 91/271/EEC, 10~15 mg/L total nitrogen) [31,32], indicating suboptimal nitrogen removal under current operating conditions.
The incomplete removal of ammonium may stem from several operational constraints. First, short hydraulic retention time (HRT) and low DO levels in the aerobic stage could limit complete nitrification. Second, high concentrations of chloride and salinity have been shown to exert osmotic stress on nitrifying bacteria, inhibiting the activity of nitrosomonas and nitrobacter species [33]. Additionally, the presence of co-contaminants such as heavy metals (e.g., Fe, Ni, Se) may exert toxicity to microbial consortia, further impairing the biological conversion pathways [34]. These results suggest that optimization of bioreactor conditions, such as increasing aeration, bioaugmentation with halotolerant strains, or sequential batch operation, may enhance NH4+ removal efficiency under high-salinity conditions typical of shale wastewater.
In contrast to the relatively dynamic behavior of ammonium, divalent cations such as calcium (Ca2+), magnesium (Mg2+), and strontium (Sr2+) showed remarkable stability across the entire treatment train (Figure 3). Ca concentrations ranged from 260 to 315 mg/L (average: 274 mg/L), Mg from 29 to 42 mg/L (average: 32 mg/L), and Sr from 77.9 to 95.9 mg/L (average: 89.3 mg/L), with no statistically significant decrease observed (p > 0.05). The limited change in Sr2+/Ca2+/Mg2+ across the train is consistent with undersaturation (on average conditions) with respect to relevant mineral phases under near-neutral pH and high ionic strength, and with the lack of alkalinity management. Co-precipitation or incorporation into carbonates/sulfates is therefore kinetically and thermodynamically disfavored in this setting. Conversely, NH4+ shows partial removal consistent with salinity-inhibited nitrification and potential hydraulic/oxygen constraints; improving nitrifier resilience (e.g., halotolerant consortia) and DO/HRT control could improve NH4+ attenuation but would not address conservative inorganics.
This mechanistic interpretation is consistent with prior studies showing that conventional biological or coagulation-based systems do not effectively remove divalent metals from high-TDS brines [2,28,35]. The coexistence of elevated Ca2+ and Sr2+ further increases the risk of scaling in downstream infrastructure, particularly in membrane units or discharge pipelines, via precipitation of sparingly soluble salts (e.g., SrCO3, CaSO4) under conducive conditions.
From a geochemical perspective, the consistent divalent-cation profiles support the interpretation that these elements are primarily inherited from formation brines rather than anthropogenic additives or surface-water sources. Their conservative behavior, together with the only partial removal of NH4+, highlights the limitations of conventional systems in handling both reactive and conservative inorganics. Therefore, a shift toward element-specific removal strategies, such as nanofiltration/reverse osmosis, electrodialysis/capacitive deionization, or selective ion exchange (in tandem with alkalinity control where precipitation/co-precipitation is intended), is essential for this group of recalcitrant solutes.
3.3. Geochemical Indicators of Salinity Sources and Rock-Water Interaction
The high concentrations of chloride, sodium, and bromide in the shale gas wastewater effluent, combined with their conservative behavior and low removal efficiency, necessitate geochemical evaluation of their sources and transport pathways. These constituents do not undergo sorption, redox transformation, biological uptake, or chemical precipitation under ambient treatment conditions [9,35,36]. Furthermore, conventional unit operations such as coagulation-flocculation, flotation, and biological reactors are not designed to target ions with high solubility and low affinity for solid surfaces.
To evaluate the origin and evolution of salinity in the wastewater, halide-based mixing models and geochemical ratios (Br/Cl, Na/Br) are employed, along with divalent ion signatures (Mg/Ca, Sr/Ca). The log-log Cl vs. Br plot (Figure 4a) displays a strong linear relationship across all samples, including influent, intermediate, and effluent waters. The data fall precisely along a binary dilution line between a freshwater endmember (Cl = 50 mg/L, Br = 0.23 mg/L) and an evaporated seawater endmember representing ~13.2 fold evaporation (Cl = 182,000 mg/L, Br = 849 mg/L) [36]. Quantitatively, their position corresponds to ~7.5% formation brine in the mixture, strongly supporting a model in which observed halide concentrations are governed by mixing between injected freshwater and deeply sourced formation brine, rather than by halite dissolution or anthropogenic chemical inputs. This interpretation is further supported by the Br/Cl molar ratios (Table 1), which show remarkable consistency across all treated wastewater samples (0.0021–0.0023), closely matching the range observed in seawater with an evaporation degree of 13–15-fold [36]. This strongly indicates a conservative mixing regime with little to no fractionation or selective removal during processing. Furthermore, the absence of significant deviation from the Br-Cl seawater trend reinforces the conservative behavior of these halides throughout the treatment process. These interpretations are corroborated by field data from the Weiyuan, Fuling, and Changning shale gas fields, where FPW exhibit similarly elevated Br/Cl ratios and high TDS values. Studies of FPW from the Sichuan Basin and Marcellus shale have shown that Br and Cl concentrations maintain a strong linear correlation over time, indicating that halide evolution is largely conservative and controlled by fluid mixing with in situ brines [5,9,10].
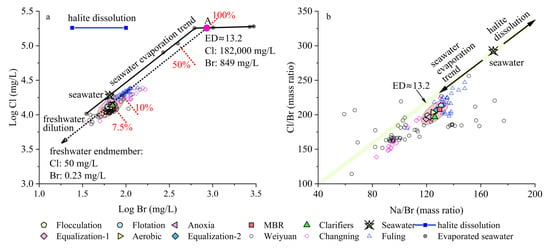
Figure 4.
(a) Log-log plot of Cl vs. Br concentrations showing the mixing trajectory between an evaporated seawater endmember (evaporation degree (ED)≈ 13.2-fold; Cl = 182,000 mg/L; Br = 849 mg/L, Point A) [36] and freshwater (Cl = 50 mg/L, Br = 0.23 mg/L). Treated wastewater samples (red ellipse) fall along the dilution line, corresponding to ~7.5% brine contribution. The halite dissolution trend is shown for comparison. (b) Na/Br vs. Cl/Br plot illustrating that all samples follow the seawater evaporation trend (green band) [36], with no deviation toward halite dissolution, further confirming that salinity is inherited from evaporated marine brines. FPW data from Weiyuan, Changning and Fuling shale gas fields are also shown for comparison [10,14,26].
The Na/Br vs. Cl/Br plot (Figure 4b) further confirms the dominance of marine evaporative brine as the salinity source. All samples cluster tightly along the seawater evaporation trend, consistent with an evaporation degree near 13.2-fold, and show no systematic shift toward the halite dissolution trajectory, which would yield disproportionate increases in Cl without corresponding Br enrichment. Such a shift would typically generate Cl/Br ratios >300 and Na/Br ratios >200, values not observed in any of the samples. The Cl/Br ratios in this study (194–213) fall well within the expected range for evaporated seawater and suggest that halite dissolution played only a minor role, if any, in determining the halide composition of these wastewaters. These observations align with previous studies from other shale plays such as the Marcellus, Barnett, and Sichuan basins, where strong Br-Cl linearity has been used to infer formation water dominance and rule out anthropogenic or halite-derived Br sources [5,9,10].
Beyond halides, trace element ratios such as Li/Cl and B/Cl provide compelling evidence of active mobilization via geochemical reactions rather than mere concentration through evaporation. The Li/Cl and B/Cl ratios in treated wastewater (Li/Cl: 8.79–9.86 µM/mM; B/Cl: 10.03–14.36 µM/mM), which are comparable to those of flowback and produced water from Weiyuan, Changning, and Fuling shale gas fields (Figure 5a), are orders of magnitude higher than those in modern seawater (Li/Cl: 0.05 µM/mM; B/Cl: 0.86 µM/mM), indicating non-conservative behavior [10,12,14,26,36].
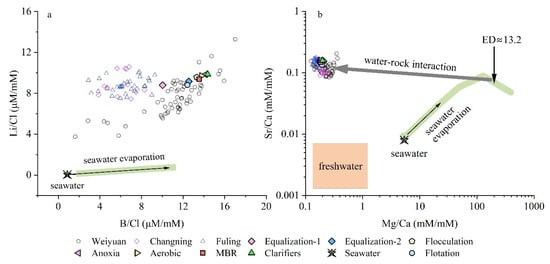
Figure 5.
(a) Li/Cl vs. B/Cl molar ratios showing strong enrichment relative to seawater and its evaporation trend (green arrow), suggesting mobilization of Li and B during water-shale interaction and clay mineral alteration. (b) Sr/Ca vs. Mg/Ca molar ratios plotted on log-log scale. Treated wastewater samples deviate from the seawater evaporation path, showing Sr enrichment and Mg depletion, indicative of water-rock interaction such as dolomitization. Seawater evaporation trend data are according to the Yellow Sea evaporation data [37]. Orange field shows the composition of freshwater from Sichuan Basin [10]. FPW data from Weiyuan, Changning, and Fuling shale gas fields are also shown for comparison [10,14,26].
This enrichment in lithium (Li) and boron (B) reflects the interaction between injected fracturing fluids and host shales, leading to the mobilization of these elements from mineral surfaces. Similar patterns have been observed in FPW from North American shale formations, such as the Marcellus and Fayetteville shales, where high B/Cl and Li/Cl ratios have been linked to the exchange of Li and B between pore fluids and illite-smectite clays [12]. The preferential release of 6Li and 10B due to cation exchange results in lower δ7Li and δ11B values in FPW, making it distinguishable from the produced waters [12]. In the context of shale gas wastewater treatment, these dissolved B (total, dominantly as boric acid/borate) and Li+ ions remain relatively unaffected by conventional processes such as flocculation, flotation, and biological processes in this plant. The persistence of Li and B throughout the treatment process raises concerns regarding their potential release into surface waters, as high B and Li contents have been detected downstream of wastewater discharge points in previous studies [12]. Consequently, when comparing samples from different treatment steps with raw flowback and produced waters, the B/Cl and Li/Cl ratios remain largely unchanged (Figure 5a), suggesting that specialized ion-exchange resins or advanced sorbents would be required to achieve meaningful removal [38].
To further trace the origin and geochemical transformation of shale gas wastewater, the behavior of divalent cation ratios Mg/Ca vs. Sr/Ca is examined (Figure 5b). These ratios are sensitive indicators of both evaporation-driven brine concentration and subsurface water–rock interaction, particularly involving carbonate and silicate phases. The plot clearly shows that seawater and its evaporation trajectory (green arrow) follow a systematic trend of rising Mg/Ca with relatively stable Sr/Ca ratios until an evaporation degree (ED) of approximately 13.2-fold, where Mg/Ca nearly peaks. This endpoint (annotated as ED ≈ 13.2-fold in Figure 5b) reflects the inferred geochemical characteristics of the deep formation brine, consistent with the halide-based mixing model (see Figure 4). In contrast, freshwaters from Sichuan Basin, as defined by datasets from Ni et al. [10], occupy a low-Mg/Ca, low-Sr/Ca region at the bottom left. Notably, all treated wastewater samples and the field flowback and produced water samples (Weiyuan, Fuling, Changning) plot above and to the left of the ED ≈ 13.2-fold endmember, which is characterized by relatively high Sr/Ca ratios but significantly low Mg/Ca ratios compared to seawater and its evaporative trajectory. This dual signal of Sr and Ca enrichment and Mg depletion deviates from a purely physical evaporation pathway and instead suggests both diagenetic processes in deep formations and extended water-rock interactions with shale.
The relative enrichment in Ca and depletion in Mg typically indicates dolomitization or other carbonate transformations whereby dissolved magnesium is incorporated into dolomite (CaMg(CO3)2) or precipitated in associated mineral phases, while calcium is released or remains in the fluid, thereby reducing Mg/Ca [10,11,39]. Such reactions deplete Mg in the fluid, driving the Mg/Ca ratio lower relative to marine waters. Meanwhile, the enrichment in Sr suggests the mobilization of strontium from shale minerals—including feldspars, carbonates, and clay minerals—during prolonged contact times at elevated temperatures and pressures [40,41,42]. These processes lead to flowback and produced waters that plot along a corridor of high Sr/Ca and low Mg/Ca relative to modern seawater-a hallmark of deep formation brines and FPW that have undergone extensive water-rock interaction.
From a wastewater treatment perspective, the Sr/Ca and Mg/Ca ratios in the plant’s effluents show limited departure from the raw FPW composition, highlighting the fact that the standard treatment processes in this plant generally do not target or effectively remove these dissolved cations. While flocculation and flotation can reduce suspended solids, and biological treatments remove organic matter, the calcium and magnesium content largely persist in the aqueous phase. Consequently, samples from different process steps remain clustered in the high-Ca, low-Mg domain, with elevated Sr/Ca, mirroring the original brine signature. This observation aligns with domestic and international reports showing that these conventional treatments have minimal influence on major cation ratios unless specialized processes (e.g., ion exchange, advanced sorption, or membrane desalination) are implemented [2,43].
Although sampling targeted the aqueous phase, residuals are generated from coagulation/flotation and biological treatment. Because dissolved salts dominate the matrix, most halides and alkali/alkaline–earth metals reside primarily in solution; however, minor incorporation into solids cannot be excluded. A facility-wide mass balance will therefore require coordinated co-sampling of waste sludges and dewatering filtrates; this limitation is acknowledged and identified as a priority for future work. Therefore, charge-balance errors (CBE) were calculated from measured major ions (Na+, K+, Ca2+, Mg2+, NH4+, Li+, Sr2+ versus Cl−, Br−). Across treatment units, CBEs are small (≈1.0–3.2%; Table 1), indicating internally consistent analyses; a slight positive bias is consistent with modest contributions from unmeasured anions. The results are characteristic of Na–Cl–dominated, high-TDS brines [35].
3.4. Environmental and Regulatory Implications
The treated shale gas wastewater effluent exhibited consistently high concentrations of inorganic solutes, raising urgent environmental and regulatory concerns. Despite effective removal of organic pollutants (e.g., COD < 100 mg/L and TOC < 15 mg/L), major ions (Cl−, Na+, Br−) and trace elements (Li, B, Sr) remained largely unaffected by conventional treatment, due to their high aqueous stability, conservative behavior, and the lack of ion-targeted removal mechanisms.
Chloride and sodium concentrations exceeded 13,000 and 8000 mg/L, respectively, far surpassing regulatory thresholds for surface water (250 mg/L Cl in China’s GB3838–2022) and posing serious environmental concerns such as saline intrusion into water bodies, soil salinization, and systemic ecological imbalance [15]. For example, high concentrations of chloride (Cl−) and sodium (Na+) can elevate TDS and osmotic pressure, stressing freshwater organisms and potentially reducing aquatic biodiversity [44]. When such highly saline effluents enter soils, Na+ can displace exchangeable Ca2+ and Mg2+, weakening soil aggregate stability and leading to sodification, decreased permeability, and impaired agricultural productivity [21]. Infiltration of such high-salinity fluids into aquifers can cause long-term degradation of groundwater quality, threatening drinking water resources and requiring costly remediation [4]. Elevated salt levels can also modify microbial community composition and function, affecting nutrient cycling and organic matter decomposition, thus triggering cascading effects across trophic levels, with possible reductions in ecosystem resilience and stability [20].
Bromide, while not itself acutely toxic, persisted at 61–73 mg/L and is a well-known precursor to carcinogenic disinfection by-products (DBPs), including brominated trihalomethanes and haloacetic acids [4]. The persistence of Br in treated effluent, combined with its transformation under chlorination, presents a latent but serious risk to downstream drinking water sources [4,6]. The lack of bromide-specific discharge limits in most national and international standards results in unregulated DBPs formation potential and elevated public health risk if these waters enter drinking water supply chains.
Trace elements also present unique challenges. Lithium (~26.6 mg/L) and boron (~60.2 mg/L) are not removed by biological or physicochemical processes and have documented ecological effects. B is phytotoxic and its regulatory limits in drinking water vary across jurisdictions (e.g., 2.4 mg/L by WHO, 1 mg/L by China, 1.5 mg/L by EU, see Table 2) [16,17,31], while Li, although unregulated, is emerging as a constituent of concern due to its developmental and endocrine-disrupting effects in aquatic organisms [45,46] (Table 2). Strontium (~90 mg/L), a divalent metal with strong geogenic signals, contributes to potential bone toxicity and is resistant to removal unless advanced membrane or exchange technologies are applied [47,48].

Table 2.
Comparison of final treated effluent quality with national and international water quality standards and guidelines [15,16,17,49,50,51,52]. Values are instream benchmarks used for screening and context (Section 3.4); they are not end-of-pipe discharge limits.
To connect effluent chemistry to receiving-water protection, a conservative mixing screen is applied:
where Cdown represents the downstream concentration, Qe and Ce are plant effluent discharge flow and effluent concentration, and Qr and Cr are receiving-water flow and background concentration. Assuming the river contributes negligible background concentration (Cr ≈ 0), the minimum dilution needed is the effluent-to-benchmark ratio of Qr/Qe ≥ Ce/C*, where C* is a protective reference value for context (used here strictly for screening, not as a discharge limit). Using measured final-stage values from this study (Table 1) yields order-of-magnitude dilution ratios of Ce(Cl−) ≈ 1.38 × 104 mg/L against a 250 mg/L context, i.e., Qr/Qe ≳ 55; and Ce(Na+) ≈ 8.81 × 103 mg/L against a 200 mg/L context, i.e., Qr/Qe ≳ 44. For a plant discharge of 1000 m3/d, the minimum steady receiving-water flow to reach those contextual values would thus exceed (4.4–5.4) × 104 m3/d, before accounting for non-zero background, incomplete lateral/vertical mixing, or site-specific mixing-zone allowances. This screening result substantiates that conservative inorganics require either (i) a desalination barrier prior to discharge or (ii) very large assimilative capacity to achieve comparable dilution in the near field.
A comparison between final effluent concentrations and national/international standards/guidelines highlights major compliance gaps (Table 2) [15,16,17,49,50,51,52]. While current frameworks effectively limit organic parameters (COD, TOC, NH4+), they often neglect conservative inorganics. For example, Cl− and Na+ in the final effluent exceed typical drinking-water guideline values by tens-fold (Chinese and WHO benchmarks) [16,17]; B and Li+ remain within ecotoxicologically significant ranges; Br− is omitted entirely from most discharge frameworks, despite being indirectly regulated through DBP limits; and Sr2+ is present at levels raising concerns for chronic exposure. However, it is emphasized that stable Sr isotopes are non-radioactive; radiological risks in oil-and-gas wastewaters are primarily associated with radium isotopes (226Ra, 228Ra) and gross α/β activity, which were not analyzed in this dataset and should be part of future monitoring. In contrast, Sr2+ in this study is a chemical concern (scaling and potential bone effects at elevated exposure) that persists without ion-selective treatment. These gaps reveal a mismatch between what is routinely monitored and what poses long-term risk, especially for treated waters intended for reuse or for discharge into sensitive receiving waters.
To close the identified gaps, regulatory and permitting frameworks should explicitly require a desalination barrier and selective polishing for conservative inorganics and embed these functions in compliance pathways. A two-pillar approach is recommended: (i) constituent scope—routinely monitor and regulate Cl−, Na+, Br−, B, Li+, and Sr2+ (and, in future monitoring, 226Ra/228Ra and gross α/β); (ii) limit derivation—combine technology-based effluent limits with receiving-water-based limits derived from site-specific mixing and use classifications, including explicit control of disinfection-by-product (DBP) precursors where Br− is present.
Building on this framing, a phased engineering strategy is appropriate for the existing configuration: (1) Optimize current operations—maintain anoxic near-zero DO for denitrification; set aerobic DO for nitrification with adequate HRT; manage alkalinity; and tune coagulation/DAF via jar tests (coagulant/polymer selection). (2) Add a desalination barrier, deploy tight nanofiltration/reverse osmosis downstream of biological polishing with pretreatment to control Ca/Sr scaling. (3) Apply selective polishing on low-TDS streams, such as boron-selective ion exchange and lithium-sorbents where recovery is intended, or electrodialysis for monovalent trimming. This staged pathway explains where desalination/polishing becomes necessary (based on unit-by-unit chemistry) and why organics-centric trains underperform for conservative ions.
Practical rollout is governed by (1) scaling control (co-precipitation risks from Ca/Sr and associated antiscalant/softening needs), (2) energetics (pressure- vs. electro- vs. thermal-driven trade-offs as functions of feed TDS and target recovery), (3) concentrate/regenerant management under existing permits, and (4) site-specific techno-economic assessment reflecting local electricity price, chemical costs, and sludge/brine handling. Adoption at scale has been constrained by energy/maintenance costs, fouling/scaling risk, and the absence of clear regulatory drivers; accordingly, policy should expand frameworks to include inorganic tracers and bioaccumulative metals, enforce technology-based effluent limits beyond organic-carbon metrics, fund pilot-scale demonstrations with techno-economic assessment under local water-quality and cost conditions, and require site-specific risk assessments and long-term monitoring (including receiving-water hydrodynamics) for unconventional wastewater discharges. Aligning these measures with fit-for-purpose infrastructure provides a practicable path to compliance and risk reduction for high-salinity shale-gas wastewaters.
4. Conclusions
This study presents a detailed assessment of inorganic constituents across a full-scale shale gas wastewater treatment plant in the Sichuan Basin. Despite sequential treatment through seven units, key inorganic solutes including Cl−, Br−, Na+, Sr2+, Li+, and B persisted at high concentrations in the final effluent. For instance, Cl remained >13,000 mg/L, Sr exceeded 70 mg/L, and Br exceeded 60 mg/L, far above environmental thresholds or in absence of enforceable discharge limits. Geochemical modeling indicates that the source of salinity is dominantly from evaporated marine brines (~13× seawater), with little contribution from halite dissolution. Elemental ratios (e.g., high Sr/Ca and B/Cl) and Mg depletion suggest strong fluid–rock interactions, including cation exchange with clays and dolomitization. The full-scale data show that organics-focused trains provide limited control of conservative inorganic solutes. Particularly, strontium (Sr2+) behaves as a chemically persistent divalent cation relevant to scaling and potential chronic exposure, whereas radiological risks in shale gas wastewaters are typically associated with radium isotopes (226Ra/228Ra), which were not measured here and warrant future monitoring. Effective risk reduction therefore requires a desalination barrier (nanofiltration/reverse osmosis) with selective polishing (e.g., ion exchange or electro-driven separations) for targeted species; the regulatory framework should explicitly recognize these constituents and align limits with receiving-water protection.
Author Contributions
Y.N.: Conceptualization; Funding acquisition; Data curation; Writing—original draft; Writing—review and editing; Visualization. Y.Z., C.M.: Validation; Investigation; Funding acquisition. L.Y., J.S.: Formal analysis; Methodology; Data curation. J.Z., Q.Z., M.D.: Data curation; Formal analysis. J.C.: Investigation; Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This study is funded by the National Key Research and Development Projects of China (Grant No. 2019YFC1805505) and the open project of the Key Laboratory of Shale Gas Exploration, Ministry of Natural Resources (Chongqing Institute of Geology and Mineral Resources) (Grant No. KLSGE-202301).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Jinxing Dai from the PetroChina Research Institute of Petroleum Exploration and Development for helpful discussion.
Conflicts of Interest
Author Chun Meng was also employed by the Chongqing Huadi Resources and Environment Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CBE: charge balance error; COD: chemical oxygen demand; DAF: dissolved air flotation; DBP: disinfection-by-product; DO: dissolved oxygen; ED: evaporation degree; FPW: flowback and produced water; FO: forward osmosis; HRT: hydraulic retention time; MBR: membrane bioreactors; TDS: total dissolved solids; TOC: total organic carbon.
References
- Zou, C.; Zhao, Q.; Wang, H.; Xiong, W.; Dong, D.; Yu, R. Principal characteristics of marine shale gas, and the theory and technology of its exploration and development in China. Nat. Gas Ind. B 2023, 10, 1–13. [Google Scholar] [CrossRef]
- Zhao, T. Treatment technology of shale gas fracturing flowback fluid: A mini review. Front. Energy Res. 2023, 11, 1245552. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, D.; Tsang, D.C.W.; Wang, L.; Ok, Y.S.; Feng, Y. A critical review of risks, characteristics, and treatment strategies for potentially toxic elements in wastewater from shale gas extraction. Environ. Int. 2019, 125, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Vengosh, A.; Jackson, R.B.; Warner, N.; Darrah, T.H.; Kondash, A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 2014, 48, 8334–8348. [Google Scholar] [CrossRef] [PubMed]
- Warner, N.R.; Jackson, R.B.; Darrah, T.H.; Osborn, S.G.; Down, A.; Zhao, K.; White, A.; Vengosh, A. Geochemical evidence for possible natural migration of Marcellus Formation brine to shallow aquifers in Pennsylvania. Proc. Natl. Acad. Sci. USA 2012, 109, 11961–11966. [Google Scholar] [CrossRef]
- Parker, K.M.; Zeng, T.; Harkness, J.; Vengosh, A.; Mitch, W.A. Enhanced formation of disinfection byproducts in shale gas wastewater-impacted drinking water supplies. Environ. Sci. Technol. 2014, 48, 11161–11169. [Google Scholar] [CrossRef]
- Gao, J.; Zou, C.; Zhang, X.; Guo, W.; Yu, R.; Ni, Y.; Liu, D.; Kang, L.; Liu, Y.; Kondash, A.; et al. The water footprint of hydraulic fracturing for shale gas extraction in China. Sci. Total Environ. 2024, 907, 168135. [Google Scholar] [CrossRef]
- Zou, C.; Ni, Y.; Li, J.; Kondash, A.; Coyte, R.; Lauer, N.; Cui, H.; Liao, F.; Vengosh, A. The water footprint of hydraulic fracturing in Sichuan Basin, China. Sci. Total Environ. 2018, 630, 349–356. [Google Scholar] [CrossRef]
- Haluszczak, L.O.; Rose, A.W.; Kump, L.R. Geochemical evaluation of flowback brine from Marcellus gas wells in Pennsylvania, USA. Appl. Geochem. 2013, 28, 55–61. [Google Scholar] [CrossRef]
- Ni, Y.; Zou, C.; Cui, H.; Li, J.; Lauer, N.E.; Harkness, J.S.; Kondash, A.J.; Coyte, R.M.; Dwyer, G.S.; Liu, D.; et al. The origin of flowback and produced waters from Sichuan Basin, China. Environ. Sci. Technol. 2018, 52, 14519–14527. [Google Scholar] [CrossRef] [PubMed]
- Rowan, E.; Engle, M.; Kraemer, T.; Schroeder, K.; Hammack, R.; Doughten, M. Geochemical and isotopic evolution of water produced from Middle Devonian Marcellus shale gas wells, Appalachian Basin, Pennsylvania. AAPG Bull. 2015, 99, 181–206. [Google Scholar] [CrossRef]
- Warner, N.R.; Darrah, T.H.; Jackson, R.B.; Millot, R.; Kloppmann, W.; Vengosh, A. New tracers identify hydraulic fracturing fluids and accidental releases from oil and gas operations. Environ. Sci. Technol. 2014, 48, 12552–12560. [Google Scholar] [CrossRef] [PubMed]
- Harkness, J.S.; Dwyer, G.S.; Warner, N.R.; Parker, K.M.; Mitch, W.A.; Vengosh, A. Iodide, bromide, and ammonium in hydraulic fracturing and oil and gas wastewaters: Environmental implications. Environ. Sci. Technol. 2015, 49, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yao, L.; Sui, J.; Chen, J.; Liu, F.; Wang, F.; Zhu, G.; Vengosh, A. Shale gas wastewater geochemistry and impact on the quality of surface water in Sichuan Basin. Sci. Total Environ. 2022, 851, 158371. [Google Scholar] [CrossRef]
- GB3838-2002; Environmental Quality Standards for Surface Water. State Environmental Protection Administration, State Administration of Quality Supervision, Inspection and Quarantine: Beijing, China, 2002.
- GB5749-2022; Standards for Drinking Water Quality. Ministry of Health of People’s Republic of China, Standardization Administration of China: Beijing, China, 2022.
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization (WHO): Geneva, Switzerland, 2017. [Google Scholar]
- Luek, J.L.; Gonsior, M. Organic compounds in hydraulic fracturing fluids and wastewaters: A review. Water Res. 2017, 123, 536–548. [Google Scholar] [CrossRef]
- Consolazio, N.; Hakala, J.A.; Lowry, G.V.; Karamalidis, A.K. Sorption and transformation of biocides from hydraulic fracturing in the Marcellus Shale: A review. Environ. Chem. Lett. 2022, 20, 773–795. [Google Scholar] [CrossRef]
- Golding, L.A.; Kumar, A.; Adams, M.S.; Binet, M.T.; Gregg, A.; King, J.; McKnight, K.S.; Nidumolu, B.; Spadaro, D.A.; Kirby, J.K. The influence of salinity on the chronic toxicity of shale gas flowback wastewater to freshwater organisms. J. Hazard. Mater. 2022, 428, 128219. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Qureshi, A.; Cheraghi, S. Extent and characterisation of salt-affected soils in Iran and strategies for their amelioration and management. Land Degrad. Dev. 2008, 19, 214–227. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Jiang, W.; Sheng, Y.; Wang, G.; Shi, Z.; Liu, F.; Zhang, J.; Chen, D. Cl, Br, B, Li, and noble gases isotopes to study the origin and evolution of deep groundwater in sedimentary basins: A review. Environ. Chem. Lett. 2022, 20, 1497–1528. [Google Scholar] [CrossRef]
- Wu, K.; Cui, W.; Ren, G.; An, J.; Zheng, K.; Zeng, X.; Ouyang, M.; Yu, Z. Organoiodines in effluents of a shale-fracturing wastewater treatment plant. Environ. Chem. Lett. 2023, 21, 1943–1949. [Google Scholar] [CrossRef]
- Tasker, T.L.; Burgos, W.D.; Ajemigbitse, M.A.; Lauer, N.E.; Gusa, A.V.; Kuatbek, M.; May, D.; Landis, J.D.; Alessi, D.S.; Johnsen, A.M.; et al. Accuracy of methods for reporting inorganic element concentrations and radioactivity in oil and gas wastewaters from the Appalachian Basin, U.S. based on an inter-laboratory comparison. Environ. Sci. Process. Impacts 2019, 21, 224–241. [Google Scholar] [CrossRef]
- Gao, J.L.; Zou, C.N.; Li, W.; Ni, Y.Y.; Liao, F.R.; Yao, L.M.; Sui, J.L.; Vengosh, A. Hydrochemistry of flowback water from Changning shale gas field and associated shallow groundwater in Southern Sichuan Basin, China: Implications for the possible impact of shale gas development on groundwater quality. Sci. Total Environ. 2020, 713, 136591. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Zou, C.N.; Cui, H.Y.; Ni, Y.Y.; Liu, J.Q.; Wu, W.; Zhang, L.; Coyte, R.; Kondash, A.; et al. Recycling flowback water for hydraulic fracturing in Sichuan Basin, China: Implications for gas production, water footprint, and water quality of regenerated flowback water. Fuel 2020, 272, 117621. [Google Scholar] [CrossRef]
- Osselin, F.; Nightingale, M.; Hearn, G.; Kloppmann, W.; Gaucher, E.; Clarkson, C.R.; Mayer, B. Quantifying the extent of flowback of hydraulic fracturing fluids using chemical and isotopic tracer approaches. Appl. Geochem. 2018, 93, 20–29. [Google Scholar] [CrossRef]
- Liu, X.; Xu, C.; Chen, P.; Li, K.; Zhou, Q.; Ye, M.; Zhang, L.; Lu, Y. Advances in technologies for boron removal from water: A comprehensive review. Int. J. Environ. Res. Public Health 2022, 19, 10671. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.J.; Luek, J.L.; Tummings, S.S.; McLaughlin, M.C.; Blotevogel, J.; Mouser, P.J. High total dissolved solids in shale gas wastewater inhibit biodegradation of alkyl and nonylphenol ethoxylate surfactants. Sci. Total Environ. 2019, 668, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- European Council. Council Directive of 21 May 1991 concerning urban waste water treatment (91/271/EEC). Off. J. Eur. Union 1991, 135, 40–52. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A01991L0271-20140101 (accessed on 5 July 2025).
- GB8978-2002; Integrated Wastewater Discharge Standard. State Department of Environmental Conservation: Beijing, China, 2002.
- Cortés-Lorenzo, C.; Rodríguez-Díaz, M.; Sipkema, D.; Juárez-Jiménez, B.; Rodelas, B.; Smidt, H.; González-López, J. Effect of salinity on nitrification efficiency and structure of ammonia-oxidizing bacterial communities in a submerged fixed bed bioreactor. Chem. Eng. J. 2015, 266, 233–240. [Google Scholar] [CrossRef]
- Juliastuti, S.; Baeyens, J.; Creemers, C. Inhibition of nitrification by heavy metals and organic compounds: The ISO 9509 test. Environ. Eng. Sci. 2003, 20, 79–90. [Google Scholar] [CrossRef]
- Appelo, C.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; CRC Press: London, UK, 2004; p. 683. [Google Scholar]
- McCaffrey, M.A.; Lazar, B.; Holland, H.D. The evaporation path of seawater and the coprecipitation of Br− and K+ with halite. J. Sediment. Res. 1987, 57, 928–937. [Google Scholar] [CrossRef]
- Chen, Y. Sequence of salt separation and regularity of some trace elements distribution during isothermal evaporation (25 °C) of the Huanghai sea water. Acta Geol. Sin. 1983, 57, 379–390. [Google Scholar]
- Khatoon, R.; Raksasat, R.; Ho, Y.C.; Lim, J.W.; Jumbri, K.; Ho, C.-D.; Chan, Y.J.; Abdelfattah, E.A.; Khoo, K.S. Reviewing advanced treatment of hydrocarbon-contaminated oilfield-produced water with recovery of lithium. Sustainability 2023, 15, 16016. [Google Scholar] [CrossRef]
- Machel, H.-G.; Mountjoy, E.W. Chemistry and Environments of Dolomitization —A Reappraisal. Earth-Sci. Rev. 1986, 23, 175–222. [Google Scholar] [CrossRef]
- Chapman, E.C.; Capo, R.C.; Stewart, B.W.; Kirby, C.S.; Hammack, R.W.; Schroeder, K.T.; Edenborn, H.M. Geochemical and strontium isotope characterization of produced waters from Marcellus shale natural gas extraction. Environ. Sci. Technol. 2012, 46, 3545–3553. [Google Scholar] [CrossRef]
- Phan, T.T.; Hakala, J.A.; Lopano, C.L.; Sharma, S. Rare earth elements and radiogenic strontium isotopes in carbonate minerals reveal diagenetic influence in shales and limestones in the Appalachian Basin. Chem. Geol. 2019, 509, 194–212. [Google Scholar] [CrossRef]
- Huang, T.; Li, Z.; Long, Y.; Zhang, F.; Pang, Z. Role of desorption-adsorption and ion exchange in isotopic and chemical (Li, B, and Sr) evolution of water following water–rock interaction. J. Hydrol. 2022, 610, 127800. [Google Scholar] [CrossRef]
- Olsson, O.; Weichgrebe, D.; Rosenwinkel, K.-H. Hydraulic fracturing wastewater in Germany: Composition, treatment, concerns. Environ. Earth Sci. 2013, 70, 3895–3906. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M.; Kefford, B.J.; Piscart, C.; Prat, N.; Schäfer, R.B.; Schulz, C.-J. Salinisation of rivers: An urgent ecological issue. Environ. Pollut. 2013, 173, 157–167. [Google Scholar] [CrossRef]
- Aral, H.; Vecchio-Sadus, A. Toxicity of lithium to humans and the environment—A literature review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef]
- Eisler, R. Boron Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review; Patuxent Wildlife Research Center: Laurel, MD, USA, 1990; p. 39.
- Grynpas, M.D.; Marie, P.J. Effects of low doses of strontium on bone quality and quantity in rats. Bone 1990, 11, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-H.; Gopalakrishnan, A.; Dong, Q.; Schäfer, A.I. Removal of strontium by nanofiltration: Role of complexation and speciation of strontium with organic matter. Water Res. 2024, 253, 121241. [Google Scholar] [CrossRef] [PubMed]
- GB5084-2021; Standard for Irrigation Water Quality. Ministry of Ecological Environment, State Administration for Market Regulation: Beijing, China, 2021.
- EPA. Secondary Drinking Water Standards: Guidance for Nuisance Chemicals; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2023.
- European Union. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. Off. J. Eur. Union 2020, 2020, 435. [Google Scholar]
- Health Canada. Guidelines for Canadian Drinking Water Quality—Summary Tables; Health Canada: Ottawa, ON, Canada, 2024.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).