Comparative Assessment of Different Electrode Combinations for Phosphate Removal from Onsite Wastewater via Electrocoagulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Matrices
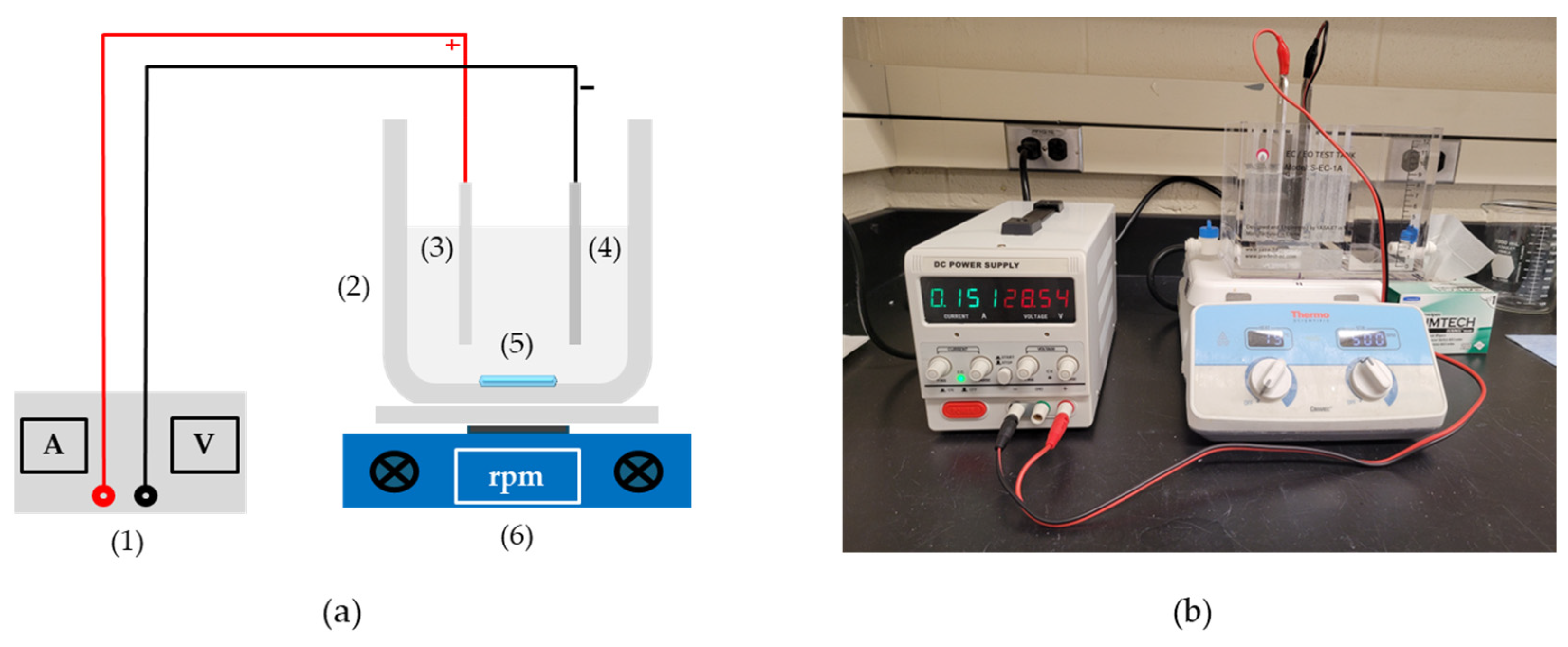
2.2. Experimental Setup and Reactor Configuration
2.3. Experimental Design and Procedure
2.4. Analytical Methods
3. Results and Discussion
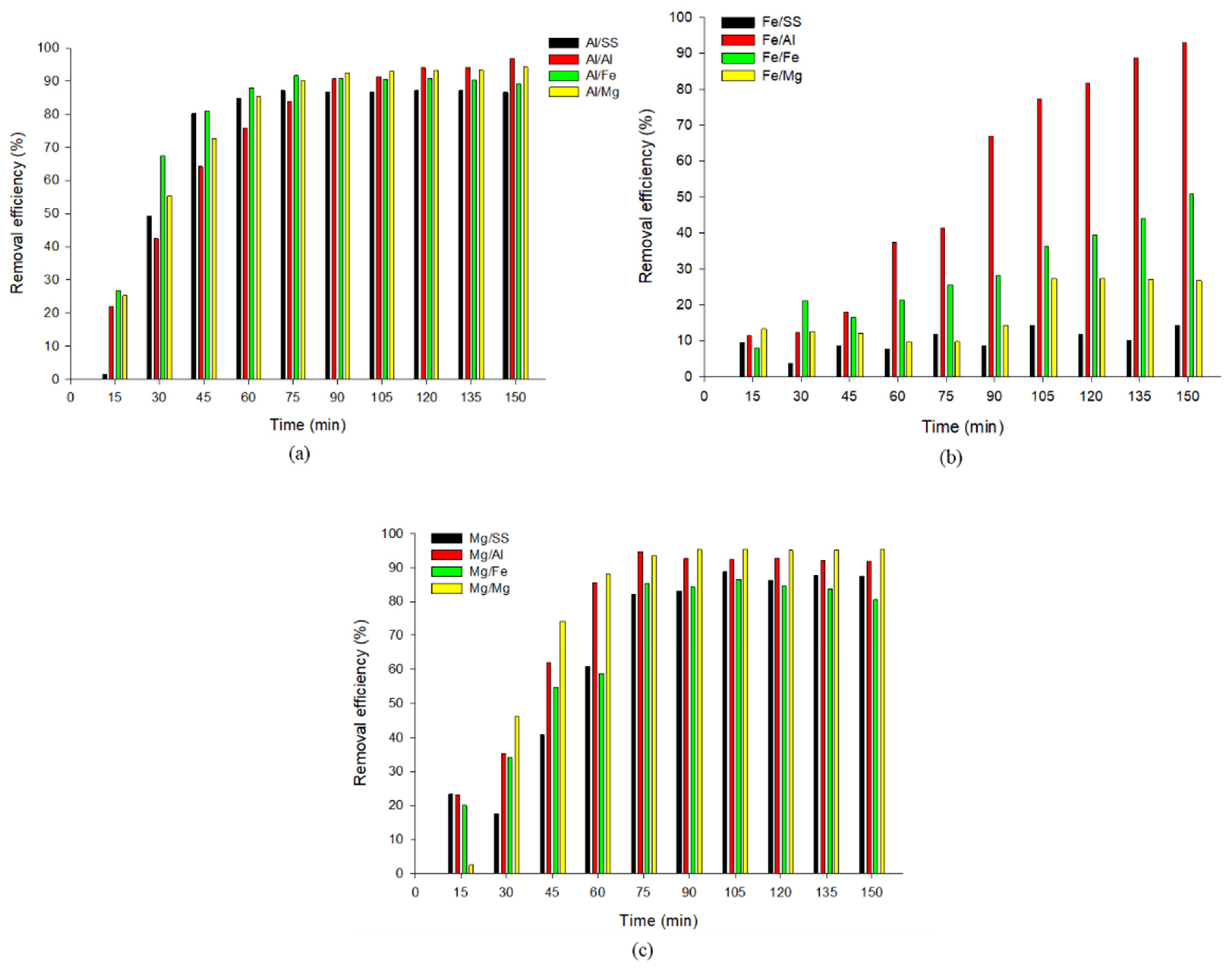
3.1. Effect of Treatment Time on Phosphate Removal
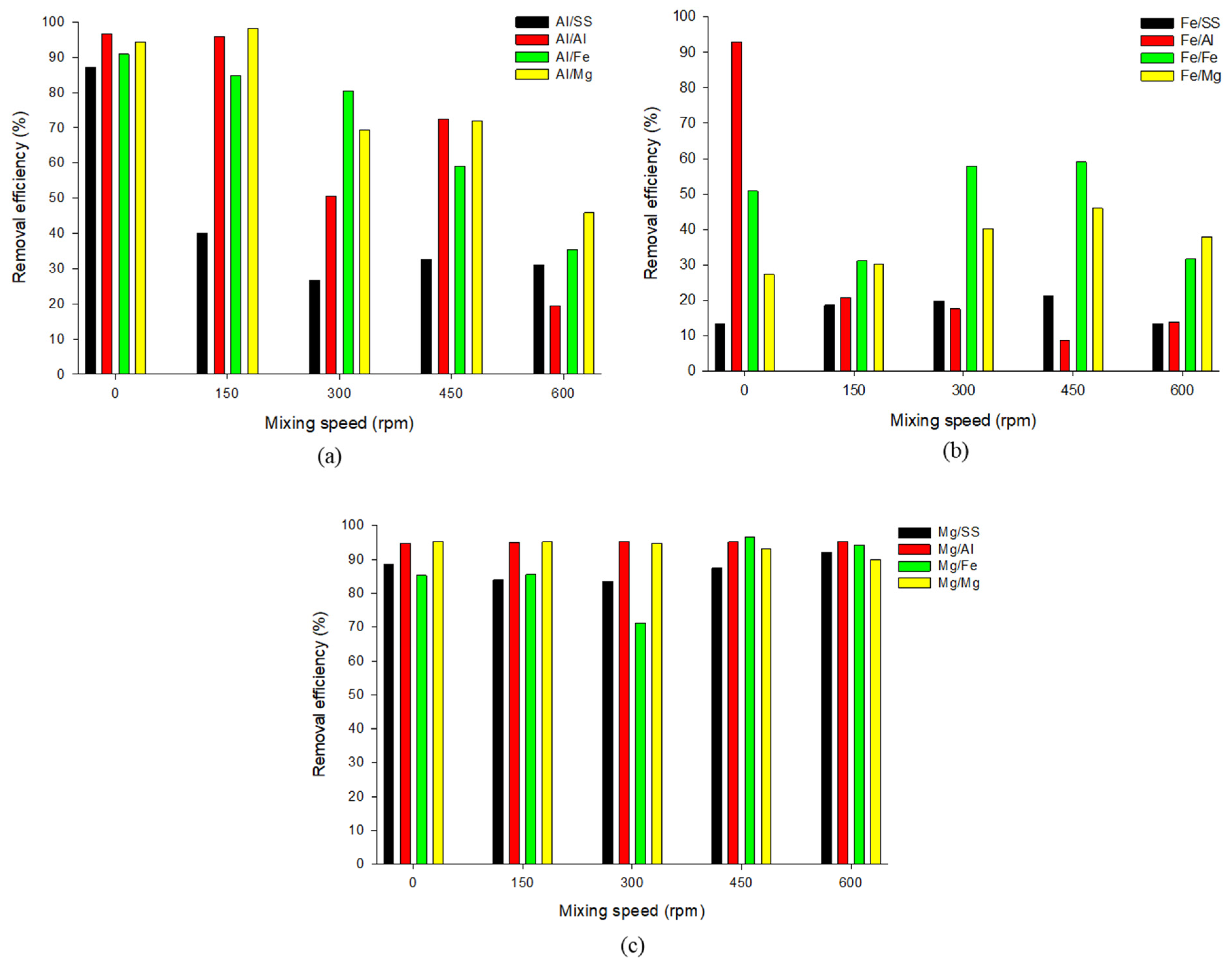
3.2. Effect of Mixing Speed on Phosphate Removal
3.3. Effect of Current Density on Phosphate Removal
3.4. Effect of pH on Phosphate Removal
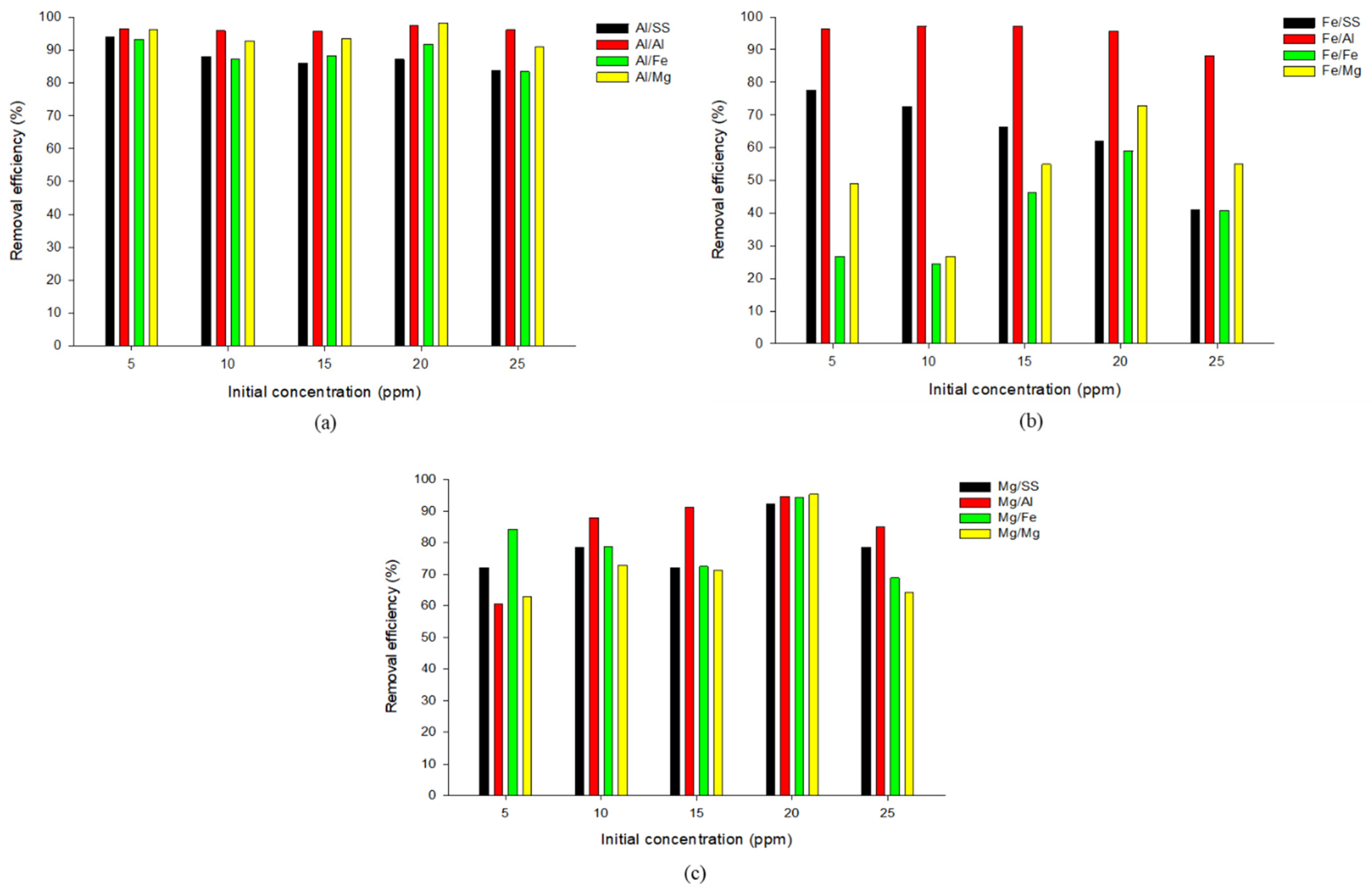
3.5. Effect of Initial Concentration on Phosphate Removal
3.6. Effect of the Extended Operational Period on Phosphate Removal
4. Comparative Evaluation of Phosphate Removal Across Wastewater Matrices Using Electrocoagulation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schellenger, F.L.; Hellweger, F.L. Phosphorus Loading from Onsite Wastewater Systems to a Lake (at Long Time Scales). Lake Reserv. Manag. 2019, 35, 90–101. [Google Scholar] [CrossRef]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human Impact on Erodable Phosphorus and Eutrophication: A Global Perspective. Bioscience 2001, 51, 227. [Google Scholar] [CrossRef]
- Vaccari, D.A. Phosphorus: A Looming Crisis. Sci. Am. 2009, 300, 54–59. [Google Scholar] [CrossRef]
- McGill, S.M. ‘Peak’Phosphorus? The Implications of Phosphate Scarcity for Sustainable Investors. J. Sustain. Financ. Invest. 2012, 2, 222–239. [Google Scholar]
- Doyle, S.; McCray, J.E.; Lowe, K.S.; Thyne, G.D. Modeling Phosphorus Reaction and Transport at an Experimental Onsite Wastewater Site. In Proceedings of the NOWRA 2005 Annual Technical Education Conference, Cleveland, OH, USA, 10–13 October 2005; Available online: https://www.researchgate.net/publication/337772225 (accessed on 15 July 2025).
- Efroymson, R.A.; Jones, D.S.; Gold, A.J. An Ecological Risk Assessment Framework for Effects of Onsite Wastewater Treatment Systems and Other Localized Sources of Nutrients on Aquatic Ecosystems. Hum. Ecol. Risk Assess. 2007, 13, 574–614. [Google Scholar] [CrossRef]
- Lusk, M.G.; Toor, G.S.; Yang, Y.-Y.; Mechtensimer, S.; De, M.; Obreza, T.A. A Review of the Fate and Transport of Nitrogen, Phosphorus, Pathogens, and Trace Organic Chemicals in Septic Systems. Crit. Rev. Environ. Sci. Technol. 2017, 47, 455–541. [Google Scholar] [CrossRef]
- Haque, S.E. How Effective Are Existing Phosphorus Management Strategies in Mitigating Surface Water Quality Problems in the US? Sustainability 2021, 13, 6565. [Google Scholar] [CrossRef]
- Joosse, P.J.; Baker, D.B. Context for Re-Evaluating Agricultural Source Phosphorus Loadings to the Great Lakes. Can. J. Soil. Sci. 2011, 91, 317–327. [Google Scholar] [CrossRef]
- Maccoux, M.J.; Dove, A.; Backus, S.M.; Dolan, D.M. Total and Soluble Reactive Phosphorus Loadings to Lake Erie. J. Great Lakes Res. 2016, 42, 1151–1165. [Google Scholar] [CrossRef]
- McComas, C.; McKinley, D. Reduction of Phosphorus and Other Pollutants from Industrial Dischargers Using Pollution Prevention. J. Clean. Prod. 2008, 16, 727–733. [Google Scholar] [CrossRef]
- Sharma, M.K.; Tyagi, V.K.; Singh, N.K.; Singh, S.P.; Kazmi, A.A. Sustainable Technologies for On-Site Domestic Wastewater Treatment: A Review with Technical Approach. Environ. Dev. Sustain. 2022, 24, 3039–3090. [Google Scholar] [CrossRef]
- Shaikh, I.; Ahammed, M.M. Coagulation Followed by Continuous Sand Filtration for Treatment of Graywater. J. Hazard. Toxic. Radioact. Waste 2021, 25, 04021032. [Google Scholar] [CrossRef]
- Hamisi, R.; Renman, A.; Renman, G.; Wörman, A.; Thunvik, R. Long-Term Phosphorus Sorption and Leaching in Sand Filters for Onsite Treatment Systems. Sci Total Environ. 2022, 833, 155254. [Google Scholar] [CrossRef] [PubMed]
- Werkneh, A.A. Decentralized Constructed Wetlands for Domestic Wastewater Treatment in Developing Countries: Field-Scale Case Studies, Overall Performance and Removal Mechanisms. J. Water Process Eng. 2024, 57, 104710. [Google Scholar] [CrossRef]
- Mechtensimer, S.; Toor, G.S. Septic Systems Contribution to Phosphorus in Shallow Groundwater: Field-Scale Studies Using Conventional Drainfield Designs. PLoS ONE 2017, 12, e0170304. [Google Scholar] [CrossRef]
- Arias, C.A.; Brix, H. Phosphorus Removal in Constructed Wetlands: Can Suitable Alternative Media Be Identified? Water Sci. Technol. 2005, 51, 267–273. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, F.; Chen, S.; Wehrmann, L.M.; Waugh, S.; Brownawell, B.J.; Gobler, C.J.; Mao, X. Phosphorus Attenuation and Mobilization in Sand Filters Treating Onsite Wastewater. Chemosphere 2024, 364, 143042. [Google Scholar] [CrossRef]
- Humphrey, C.P.; Anderson-Evans, E.; O’Driscoll, M.; Manda, A.; Iverson, G. Comparison of Phosphorus Concentrations in Coastal Plain Watersheds Served by Onsite Wastewater Treatment Systems and a Municipal Sewer Treatment System. Water Air Soil. Pollut. 2015, 226, 2259. [Google Scholar] [CrossRef]
- Lowe, K.S.; Tucholke, M.B.; Tomaras, J.M.B.; Conn, K.; Hoppe, C.; Drewes, J.E.; McCray, J.E.; Munakata-Marr, J. Influent Constituent Characteristics of the Modern Waste Stream from Single Sources; Water Environment Research Foundation: Alexandria, VA, USA, 2009; ISBN 1843393514. [Google Scholar]
- Mechtensimer, S.; Toor, G.S. Fate, Mass Balance, and Transport of Phosphorus in the Septic System Drainfields. Chemosphere 2016, 159, 153–158. [Google Scholar] [CrossRef]
- Correll, D.L. The Role of Phosphorus in the Eutrophication of Receiving Waters: A Review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef]
- Lu, C.; Chen, Y.; Shuang, C.; Wang, Z.; Tian, Y.; Song, H.; Li, A.; Chen, D.; Li, X. Simultaneous Removal of Nitrate Nitrogen and Orthophosphate by Electroreduction and Electrochemical Precipitation. Water Res. 2024, 250, 121000. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varshney, G.; Varshney, V.; Hejase, C.A. Recent Advances in Technologies for Phosphate Removal and Recovery: A Review. ACS Environ. Au 2024, 4, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zheng, W.; Duan, X.; Goswami, N.; Liu, Y. Recent Advances in Electrochemical Removal and Recovery of Phosphorus from Water: A Review. Environ. Funct. Mater. 2022, 1, 10–20. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—Science and Applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [PubMed]
- Chen, G. Electrochemical Technologies in Wastewater Treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Harif, T.; Khai, M.; Adin, A. Electrocoagulation versus Chemical Coagulation: Coagulation/Flocculation Mechanisms and Resulting Floc Characteristics. Water Res. 2012, 46, 3177–3188. [Google Scholar] [CrossRef]
- Tegladza, I.D.; Xu, Q.; Xu, K.; Lv, G.; Lu, J. Electrocoagulation Processes: A General Review about Role of Electro-Generated Flocs in Pollutant Removal. Process Saf. Environ. Prot. 2021, 146, 169–189. [Google Scholar] [CrossRef]
- Koyuncu, S.; Arıman, S. Domestic Wastewater Treatment by Real-Scale Electrocoagulation Process. Water Sci. Technol. 2020, 81, 656–667. [Google Scholar] [CrossRef]
- Hayden, J.; Abbassi, B. Continuous Flow Electrocoagulation System for Enhanced Phosphorous Removal in Decentralized Wastewater Treatment Systems. Water 2025, 17, 202. [Google Scholar] [CrossRef]
- Akhtar, N.A.; Kobya, M.; Gengec, E. Chemical and Electrochemical Coagulation Processes as a Tertiary Treatment for Residual Phosphate Removal from Domestic Wastewater: Effect of Operating Parameters and Calculation of Operating Cost. Environ. Sci. 2025, 11, 1554–1567. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy Metals Removal from Aqueous Environments by Electrocoagulation Process–a Systematic Review. J. Environ. Health Sci. Eng. 2015, 13, 74. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Review of Pollutants Removed by Electrocoagulation and Electrocoagulation/Flotation Processes. J. Environ. Manag. 2009, 90, 1663–1679. [Google Scholar] [CrossRef]
- Reza, A.; Chen, L. Electrochemical Treatment of Livestock Waste Streams. A Review. Environ. Chem. Lett. 2022, 20, 1863–1895. [Google Scholar] [CrossRef]
- Omwene, P.I.; Kobya, M. Treatment of Domestic Wastewater Phosphate by Electrocoagulation Using Fe and Al Electrodes: A Comparative Study. Process Saf. Environ. Prot. 2018, 116, 34–51. [Google Scholar] [CrossRef]
- Reza, A.; Haller, S.; Mao, X. Electrocoagulation as a Remedial Approach for Phosphorus Removal from Onsite Wastewater: A Review. Water 2024, 16, 3206. [Google Scholar] [CrossRef]
- İrdemez, Ş.; Demircioğlu, N.; Yildiz, Y.Ş. The Effects of PH on Phosphate Removal from Wastewater by Electrocoagulation with Iron Plate Electrodes. J. Hazard. Mater. 2006, 137, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- İrdemez, Ş.; Yildiz, Y.Ş.; Tosunoğlu, V. Optimization of Phosphate Removal from Wastewater by Electrocoagulation with Aluminum Plate Electrodes. Sep. Purif. Technol. 2006, 52, 394–401. [Google Scholar] [CrossRef]
- Li, G.; Zheng, B.; Zhang, W.; Liu, Q.; Li, M.; Zhang, H. Phosphate Removal Efficiency and Life Cycle Assessment of Different Anode Materials in Electrocoagulation Treatment of Wastewater. Sustainability 2024, 16, 3836. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of Wastewater by Electrocoagulation: A Review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Y.; Wang, W.; Li, Z.; Gao, J.; Bao, K.; Han, R.; Zhang, R. Phosphorus Removal from Continuous Phosphate-Contaminated Water by Electrocoagulation Using Aluminum and Iron Plates Alternately as Electrodes. Sep. Sci. Technol. 2014, 49, 939–945. [Google Scholar] [CrossRef]
- Kuokkanen, V.; Kuokkanen, T.; Rämö, J.; Lassi, U.; Roininen, J. Removal of Phosphate from Wastewaters for Further Utilization Using Electrocoagulation with Hybrid Electrodes—Techno-Economic Studies. J. Water Process Eng. 2015, 8, e50–e57. [Google Scholar] [CrossRef]
- Lee, B.C.Y.; Mahtab, M.S.; Neo, T.H.; Farooqi, I.H.; Khursheed, A. A Comprehensive Review of Design of Experiment (DOE) for Water and Wastewater Treatment Application—Key Concepts, Methodology and Contextualized Application. J. Water Process Eng. 2022, 47, 102673. [Google Scholar] [CrossRef]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C.K.M. Strategies for Fermentation Medium Optimization: An In-Depth Review. Front. Microbiol. 2017, 7, 2087. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Morkovsky, P.; Gomes, J.A.G.; Kesmez, M.; Parga, J.; Cocke, D.L. Fundamentals, Present and Future Perspectives of Electrocoagulation. J. Hazard. Mater. 2004, 114, 199–210. [Google Scholar] [CrossRef]
- Bender, S.; Goellner, J.; Heyn, A.; Schmigalla, S. A New Theory for the Negative Difference Effect in Magnesium Corrosion. Mater. Corros. 2012, 63, 707–712. [Google Scholar] [CrossRef]
- Yang, Y.; Scenini, F.; Curioni, M. A Study on Magnesium Corrosion by Real-Time Imaging and Electrochemical Methods: Relationship between Local Processes and Hydrogen Evolution. Electrochim. Acta 2016, 198, 174–184. [Google Scholar] [CrossRef]
- Holt, P.K.; Barton, G.W.; Mitchell, C.A. The Future for Electrocoagulation as a Localised Water Treatment Technology. Chemosphere 2005, 59, 355–367. [Google Scholar] [CrossRef]
- Grundl, T.; Delwiche, J. Kinetics of Ferric Oxyhydroxide Precipitation. J. Contam. Hydrol. 1993, 14, 71–87. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation Process in Water Treatment: A Review of Electrocoagulation Modeling Approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Attour, A.; Touati, M.; Tlili, M.; Ben Amor, M.; Lapicque, F.; Leclerc, J.-P. Influence of Operating Parameters on Phosphate Removal from Water by Electrocoagulation Using Aluminum Electrodes. Sep. Purif. Technol. 2014, 123, 124–129. [Google Scholar] [CrossRef]
- Kobya, M.; Can, O.T.; Bayramoglu, M. Treatment of Textile Wastewaters by Electrocoagulation Using Iron and Aluminum Electrodes. J. Hazard. Mater. 2003, 100, 163–178. [Google Scholar] [CrossRef]
- Kuokkanen, V.; Kuokkanen, T.; Rämö, J.; Lassi, U. Recent Applications of Electrocoagulation in Treatment of Water and Wastewater—A Review. Green. Sustain. Chem. 2013, 3, 89–121. [Google Scholar] [CrossRef]
- Kruk, D.J.; Elektorowicz, M.; Oleszkiewicz, J.A. Struvite Precipitation and Phosphorus Removal Using Magnesium Sacrificial Anode. Chemosphere 2014, 101, 28–33. [Google Scholar] [CrossRef] [PubMed]
- İrdemez, Ş.; Demircioğlu, N.; Yıldız, Y.Ş.; Bingül, Z. The Effects of Current Density and Phosphate Concentration on Phosphate Removal from Wastewater by Electrocoagulation Using Aluminum and Iron Plate Electrodes. Sep. Purif. Technol. 2006, 52, 218–223. [Google Scholar] [CrossRef]
- Vasudevan, S.; Sozhan, G.; Ravichandran, S.; Jayaraj, J.; Lakshmi, J.; Sheela, M. Studies on the Removal of Phosphate from Drinking Water by Electrocoagulation Process. Ind. Eng. Chem. Res. 2008, 47, 2018–2023. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Jayaraj, J.; Sozhan, G. Remediation of Phosphate-Contaminated Water by Electrocoagulation with Aluminium, Aluminium Alloy and Mild Steel Anodes. J. Hazard. Mater. 2009, 164, 1480–1486. [Google Scholar] [CrossRef]
- Hu, C.; Liu, H.; Chen, G.; Qu, J. Effect of Aluminum Speciation on Arsenic Removal during Coagulation Process. Sep. Purif. Technol. 2012, 86, 35–40. [Google Scholar] [CrossRef]
- Lin, J.-L.; Huang, C.; Dempsey, B.; Hu, J.-Y. Fate of Hydrolyzed Al Species in Humic Acid Coagulation. Water Res. 2014, 56, 314–324. [Google Scholar] [CrossRef]
- Han, X.; Qi, H.; Qu, Y.; Feng, Y.; Zhao, X. Simultaneous Phosphate Removal and Power Generation by the Aluminum–Air Fuel Cell for Energy Self-Sufficient Electrocoagulation. Appl. Sci. 2023, 13, 4628. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A Comprehensive Review of Electrocoagulation for Water Treatment: Potentials and Challenges. J Environ. Manag. 2017, 186, 24–41. [Google Scholar]
- Omwene, P.I.; Kobya, M.; Can, O.T. Phosphorus Removal from Domestic Wastewater in Electrocoagulation Reactor Using Aluminium and Iron Plate Hybrid Anodes. Ecol. Eng. 2018, 123, 65–73. [Google Scholar] [CrossRef]
- Devlin, T.R.; Kowalski, M.S.; Pagaduan, E.; Zhang, X.; Wei, V.; Oleszkiewicz, J.A. Electrocoagulation of Wastewater Using Aluminum, Iron, and Magnesium Electrodes. J. Hazard. Mater. 2019, 368, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Kabdaşlı, I.; Arslan-Alaton, I.; Ölmez-Hancı, T.; Tünay, O. Electrocoagulation Applications for Industrial Wastewaters: A Critical Review. Environ. Technol. Rev. 2012, 1, 2–45. [Google Scholar] [CrossRef]
- Zheng, X.; Kong, H.-N.; Wu, D.; Wang, C.; Li, Y.; Ye, H. Phosphate Removal from Source Separated Urine by Electrocoagulation Using Iron Plate Electrodes. Water Sci. Technol. 2009, 60, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, M.; ALAVI, M.M.R.; Arami, M. A Comparison between Aluminum and Iron Electrodes on Removal of Phosphate from Aqueous Solutions by Electrocoagulation Process. Int. J. Environ. Res. 2011, 5, 403–412. [Google Scholar]
- Ozyonar, F.; Karagozoglu, B. Operating Cost Analysis and Treatment of Domestic Wastewater by Electrocoagulation Using Aluminum Electrodes. Pol. J. Environ. Stud. 2011, 20, 173. [Google Scholar]
- Behbahani, M.; Alavi Moghaddam, M.R.; Arami, M. Phosphate Removal by Electrocoagulation Process: Optimization by Response Surface Methodology Method. Environ. Eng. Manag. J. 2013, 12, 2397–2405. [Google Scholar] [CrossRef]
- Đuričić, T.; Malinović, B.N.; Bijelić, D. The Phosphate Removal Efficiency Electrocoagulation Wastewater Using Iron and Aluminum Electrodes. Bull. Chem. Technol. Bosnia Herzeg. 2016, 47, 33–38. [Google Scholar]
- Arambarri, J.; Abbassi, B.; Zytner, P. Enhanced Removal of Phosphorus from Wastewater Using Sequential Electrocoagulation and Chemical Coagulation. Water Air Soil. Pollut. 2019, 230, 312. [Google Scholar] [CrossRef]
- Tibebe, D.; Kassa, Y.; Bhaskarwar, A.N. Treatment and Characterization of Phosphorus from Synthetic Wastewater Using Aluminum Plate Electrodes in the Electrocoagulation Process. BMC Chem. 2019, 13, 107. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Y.; Zhang, C.; Li, X. Study on Influencing Parameters and Long-Term Operation of Electrocoagulation Phosphorus Removal from Small Rural Domestic Sewage. Water Sci. Technol. 2023, 87, 1866–1878. [Google Scholar] [CrossRef]



| Electrode (Anode/Cathode) | Wastewater | Initial PO43− Concentration (mg/L) | pH | Current Density (mA/cm2) | Mixing Speed (rpm) | Treatment Time (min) | PO43− Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Al/Al | Synthetic | 50–500 | 4–7 | 0.25–1 | 150 | 4–80 | 54.6–98.1 | [39] |
| Fe/Fe | Synthetic | 100 | 7 | 0.5 | 150 | 20 | 93 | [38] |
| Fe/Fe | Urine | 26.1 | 8.2–9 | 40 | 150 | 20 | 98 | [66] |
| Al/Al | Synthetic | 25–400 | 3 | 8.3 | – | 5–40 | 27.7–100 | [67] |
| Fe/Fe | 14.3–100 | |||||||
| Al/Al | Domestic | 12.9 | 7.8 | 10 | 10 | 98 | [68] | |
| Al/Al | Synthetic | 400 | 3 | 16.6 | – | 11.72 | 85.8 | [69] |
| Al/Al | Synthetic | 50 | 3 | 1 | – | 40 | 98.9 | [70] |
| Fe/Fe | 93.5 | |||||||
| Al-Mg/SS | Synthetic | 20–150 | 5 | 11 | – | 10–60 | 42–100 | [71] |
| Al/Al | Synthetic | 50 | 7 | 10 | 200 | 120 | 97.6 | [72] |
| Al/Ti | Synthetic | 52 | 4 | 2 | – | 50 | ~100 | [36] |
| Fe/Ti | 100 | |||||||
| Al-Fe/Ti | Synthetic | 52.1 | 4 | 2 | – | 80 | 99.9 | [63] |
| Fe/Fe | Domestic | 4.2–4.8 | 7 | 1 | – | 30 | 93.9 | [73] |
| Al/Al | Synthetic | 5–20 | 6 | 1.25 | – | 150 | 95.9–97.6 | This study |
| Al/Mg | 7 | 1 | – | 92.7–98.2 | ||||
| Fe/Al | 9 | 1.25 | – | 95.6–97.1 | ||||
| Mg/Mg | 7 | 1 | – | 90 | 62.9–97.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reza, A.; Jian, X.; Zeng, F.; Mao, X. Comparative Assessment of Different Electrode Combinations for Phosphate Removal from Onsite Wastewater via Electrocoagulation. Water 2025, 17, 2764. https://doi.org/10.3390/w17182764
Reza A, Jian X, Zeng F, Mao X. Comparative Assessment of Different Electrode Combinations for Phosphate Removal from Onsite Wastewater via Electrocoagulation. Water. 2025; 17(18):2764. https://doi.org/10.3390/w17182764
Chicago/Turabian StyleReza, Arif, Xiumei Jian, Fanjian Zeng, and Xinwei Mao. 2025. "Comparative Assessment of Different Electrode Combinations for Phosphate Removal from Onsite Wastewater via Electrocoagulation" Water 17, no. 18: 2764. https://doi.org/10.3390/w17182764
APA StyleReza, A., Jian, X., Zeng, F., & Mao, X. (2025). Comparative Assessment of Different Electrode Combinations for Phosphate Removal from Onsite Wastewater via Electrocoagulation. Water, 17(18), 2764. https://doi.org/10.3390/w17182764








