1. Introduction
The presence of heavy metals in drinking waters poses an increasing risk to human health; therefore, water purification through a filtration process has become a research objective focused on new adsorbent materials. Anthropogenic activities generate diverse toxic pollutants responsible for water contamination, and some of these pollutants include heavy metals and organic materials, among others [
1,
2,
3]. After iron and aluminum, copper is the most widely used metal; for example, copper is extensively used for electrical wiring in construction and pipes for gas and water, as well as for electronic devices. Copper alloys, bronze (90% Cu, 10% Sn) and brass (65% Cu, 35% Zn), are widely used for multiple purposes. Old American pennies, USD0.01, made of 88% copper and 12% nickel, are constantly discarded into the environment, where they corrode and are washed away by runoff that ends up in water bodies. Copper can enter the drinking water through pipe corrosion. Copper sulfate is used as an antibacterial and fungicide in swimming pool maintenance [
2,
3,
4].
The copper concentrations in drinking water vary widely depending on water characteristics such as the pH, hardness, and copper availability within the distribution system. Typical copper levels in drinking water range from ≤0.005 mg/L to values exceeding 30 mg/L. According to the United States Environmental Protection Agency (EPA), copper concentrations above 1.3 mg/L in drinking water pose a risk to human health [
3,
4,
5]. In urban areas with water treatment plants, the copper content can meet the standard; however, when copper pipes are used, the standard can be significantly exceeded, even more for aggressive waters. In rural zones, with little or no treatment, near mining activities or geologic mineral deposits, this limit can be easily exceeded. Underground wells are especially vulnerable due to the percolation process, especially in sandy soils. This situation is common in rural media throughout Latin America. In both cases, a previous filtration process is required to reduce the health risks.
There are various conventional physical, chemical, and biological methodologies that can be applied to remove metal pollutants from the water [
1,
5,
6,
7,
8]. These technologies include adsorption, chemical precipitation, flotation, coagulation–flocculation, and ion exchange. However, most of these technologies present serious disadvantages due to the high cost, and some of them produce dangerous waste products with high disposal costs; in addition to that, technical assistance is often required. Advanced technologies such as membrane technologies, ultrafiltration, nanofiltration, and reverse osmosis, although efficient, have an application cost that is high and justified only at medium and large scales. Notwithstanding, adsorption processes are being considered more efficient, versatile, and cheap methods due to their versatility and ease of operation compared to other techniques. There are a great variety of adsorbent materials that can be used [
5,
6,
7,
8,
9,
10,
11,
12]. However, not all adsorbent materials are easily available.
Synthetic activated carbon is widely employed as an adsorbent due to its high surface area and strong affinity for heavy metal ions. However, its feasibility for large-scale application is limited by production and activation costs [
8,
10,
11,
12]. Red mud (bauxite residue) has been proposed as an effective adsorbent [
11,
13]; however, its caustic and saline nature classifies it as hazardous waste. Zeolites (both natural and synthetic) are effective cation exchangers and have been extensively studied for the removal of Pb
2+, Cd
2+, Cu
2+, and other heavy metals due to their ion-exchange capacity and selectivity; however, the limited availability of certain natural zeolites and the high synthesis costs of high-purity synthetic variants constrain their economic viability in large-scale applications [
5,
8,
9,
10]. A new kind of adsorbent substrate based on natural oxidic and refractory lithologic materials has begun to be used for the purpose of exploring heavy metal adsorption from aqueous solutions [
6,
10,
11,
14,
15,
16,
17]. The chemical and physical characteristics of three of these natural geological materials have been reported in the literature [
18]. The thermal and mechanical properties, along with the chemical composition, make them suitable for pottery activities but also offer advantages for the preparation of a granular adsorbent substrate, through thermal treatment, with metal-binding properties able to retain contaminants from drinking waters. Two of these three substrates have been tested for metal adsorption studies [
14], using copper as an example of a transitional metal.
The present paper shows the results of the copper adsorption study on the third geologic material, not tested before. According to the chemical characterization [
18], the geologic material contains important amounts of iron and aluminum, as well as titanium and manganese, basically in the oxides form. The soil science literature explains the behavior of such kinds of oxides as amphoteric [
19,
20,
21], that is to say, they present variable electric surface charges, which are pH-dependent, according to Equation (1) [
6,
14,
21,
22]. In alkaline media, neutral oxide deprotonation creates an electric negative charge density on the oxide surface; on the other hand, in acid media, neutral oxide protonation creates an electric positive charge density [
21,
22,
23]. Therefore, with an appropriate chemical treatment, it is possible to achieve not only cation adsorption but also anion adsorption from aqueous media through the chemical modification of the surface charges.
In this model, M could be Fe, Al, Ti or Mn. These kinds of oxides are known as “Amphoteric oxides”. Therefore, by treating the oxide substrate through an alkaline attack, it is possible to promote cationic adsorption by increasing the negative charge density on the oxide surface. Anionic adsorption is then possible by increasing the positive charge density on the oxide surface through an acid attack on the substrate.
The literature also suggests a mechanism for the adsorption of transitional metals on these kinds of surfaces through the formation of a mono- or bidentate inner sphere complex between the metal ion and the oxidic surface, which bonds covalently [
6,
12,
23,
24,
25]. However, the main characteristic of such a kind of reaction is the production of hydronium ions, according to Equation (2), which means that the pH must decrease during the adsorption reaction:
The reaction is also characterized by high specificity for trace metals and the tendency toward irreversibility.
Similar substrates, prepared with different geologic materials, have been tested for different objectives like water softening [
26], showing that alkaline and alkaline earth participate in ionic exchange reactions or non-specific adsorption. Copper adsorption [
10,
11,
14,
27] and lead adsorption [
6] have been tested, showing that these transitional metals participate in a covalent bonding reaction. On the other hand, by treating the substrate in acid media, the adsorption of phosphate [
28,
29], arsenate and arsenite [
11,
30,
31,
32] has also been studied. Furthermore, mixtures of these substrates have been used for wastewater treatment; using fixed column experiments showed a 98% reduction of BOD, 94% reduction of COD, and 60% reduction of turbidity compared with reductions of 86%, 83% and 44%, respectively, using sand filtration [
33].
In the same direction, the general purpose of this work is to investigate the potentialities of this new substrate for cation retention from aqueous solutions and compare its effectiveness against other similar substrates prepared with different geologic materials of the same nature. Experiments are expected to reveal the characteristic of the variable electric charge of the material by comparing the differential behavior on the treated and non-treated substrates, according to Equation (1), during the adsorption reaction. If this is correct, then the adsorption on the treated substrate will be greater. By monitoring the pH during the adsorption reaction, it will be possible to infer the chemisorption explained by Equation (2), shedding light on the nature of the adsorption along with the isotherm type. Finally, the classical models of Freundlich and Langmuir for adsorption will support the adsorption data. Copper is selected as a target metal as an example of a transitional metal and because of its ease for chemical analysis, and because it is expected that any other transitional metal will behave in the same way as copper does.
2. Materials and Methods
2.1. Raw Material
The original oxidic geologic material called V material cannot be classified as a soil but as a lithologic material from the Earth’s crust. It has a certain resemblance to arid soil, mainly composed of aluminum and iron oxides, along with other minerals and crystalline phases such as quartz, clays, and scarce organic matter content. The raw material was sampled from a geological deposit at the following coordinates: 8°31′6′′ N and 71°6′58′′ W.
Table 1 shows the results of routine analysis [
18].
V material is a yellowish–brown sandy loam material, with a relatively low pH, cationic exchange capacity, CEC, electric conductivity, EC, and organic matter, OM. A low EC means low solubility and low content of soluble ionic compounds. However, there is an important metallic content, with Al, Fe, Ti and Mn present as the major metals, most likely in their oxide form. Alkaline and alkaline earth metals are below 0.1% and transitional metals are at trace levels. The presence of zirconium and albite provides mechanical and thermal resistance, characteristic of ceramic materials [
18]. These properties are necessary to prepare the solid substrate via thermal treatment.
2.2. Preparation of the Substrate
After grinding, three granulometric fractions were separated: fine fraction, with a particles smaller than 250 μm and labeled as VFF, medium fraction, with a particle size between 425 and 250 μm and labeled as VMF and the gross fraction, with a particle size between 1200–425 μm and labeled as VGF.
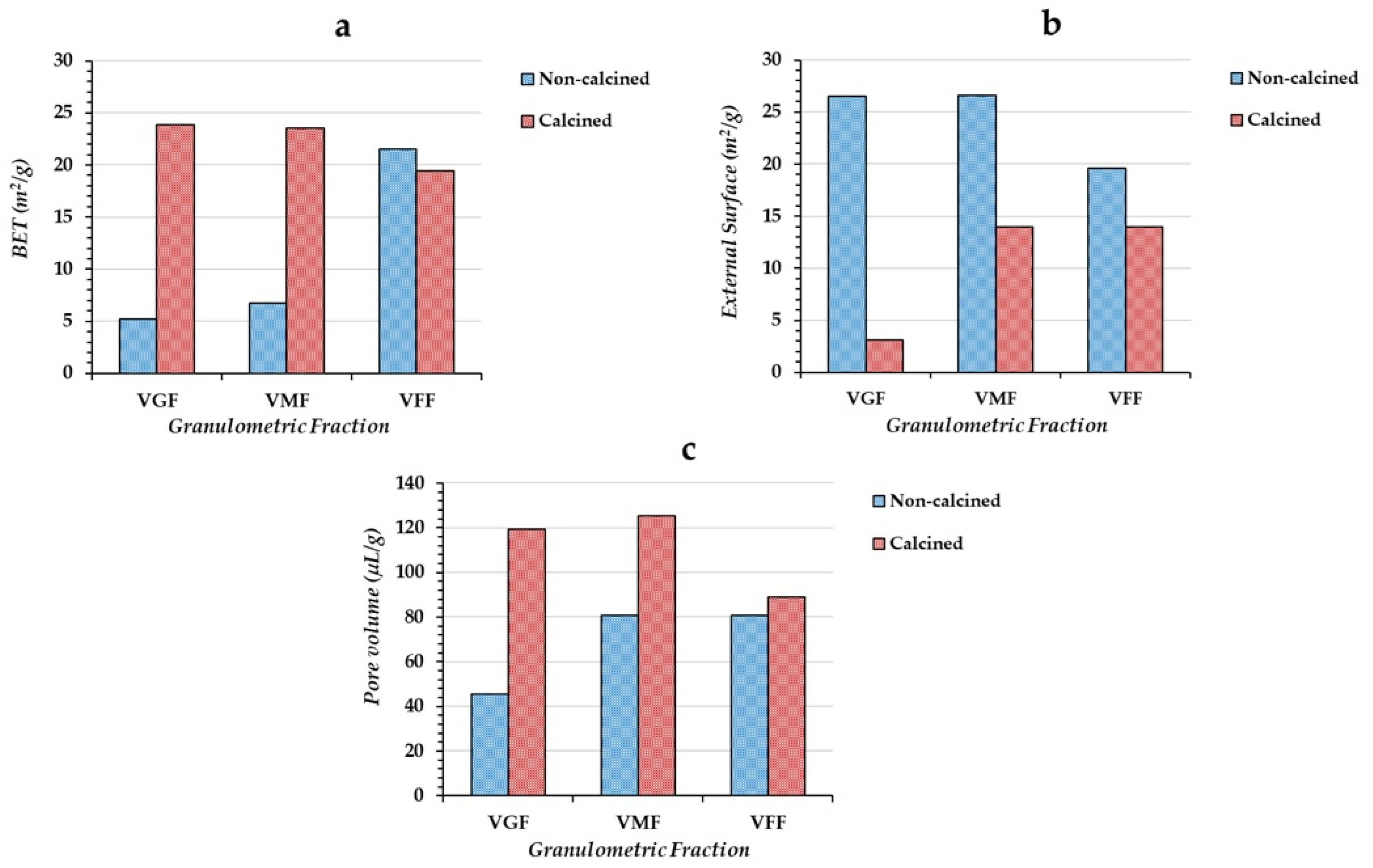
Granulometric separation was performed using an ASTM Laboratory Test Sieve (Endecotts Ltd., London, UK) with an automatic vibrator Octagon Digital CE for 15 min. The substrate was prepared with the three granulometric fractions. After grinding, sieving, and separating the granulometric fractions, a soft mud or saturated paste was prepared with distilled water. Then, using a 60 mL syringe, cylindrical strips of 3 mm diameter were extruded and cut into 5–6 mm long pellets, then air dried for 24 h, and oven dried up to 120 °C, for another 24 h. The dry substrate was thermally treated up to 750 °C for 4 h in a muffle furnace (Thermolyne F30428C, Thermo Fisher Scientific, Waltham, MA, USA). The furnace was allowed to cool until 20 °C for 12 h before taking the substrate out from the muffle. Thermal treatment achieves three targets. First, the organic matter is eliminated, so it does not interfere with the mineral phase in the adsorption reaction. Second, high temperatures promote oxide formation through the reaction with oxygen. Last, but no less important, the thermal treatment hardens the pellets, avoiding their solubilization or dispersion in the solution.
The specific surface of the substrate was measured by N2 adsorption. The analysis was performed through a Micromeritics ASAP 2420 Surface Area and Porosity Analyzer (Micromeritics Instrument Corporation, Norcross, GA, USA). The specific surface area and average pore volume of the calcined materials were determined using the N2 adsorption method at −196 °C. The samples were pre-treated at 400 °C under a vacuum for 12 h using a Micromeritics ASAP 2420 Analyzer (Norcross, GA, USA). The IR spectra were recorded through an FT-IR Spectrum BX Perkin Elmer (Perkin Elemer, Waltham, MA, USA) with an MIR source and DTGS detector on 5% sample–KBr pellets.
2.3. Reagents
All the reagents used in the experiments were analytical grade: Merck NaOH (Merck, Darmstadt, Germany), CuSO4, and Sigma-Aldrich EDTA (Sigma-Aldrich, St. Louis, MO, USA). Moreover, a 0.1 M mother solution of CuSO4 and 1 M NaOH working solutions were obtained by dilution. All the solutions were prepared with distilled water. Copper was analyzed through complexometric titration using EDTA mM, murexide as the metallochromic indicator, and a buffer solution of pH 10. A buffer solution was prepared by a mixture of sodium tetraborate and sodium hydroxide (Na2B4O7/NaOH). The indicator murexide was prepared in a mixture of 1:100 with NaCl, solid and dry.
2.4. Activation of the Substrate
Activation of the substrate was performed through chemical surface modification by treating the calcined substrate with an alkaline solution (0.1 N NaOH) overnight. The alkaline medium caused the oxides deprotonation reaction to take place, increasing the negative charge density on the substrate surface, according to Equation (1), so cation adsorption reaction could occur. Next, the substrate was washed out with distilled water until neutral pH and oven dried for 12 h. The chemically treated sample was labeled as Activated substrate, while the non-treated substrate was labeled as Non-activated substrate.
2.5. Adsorption Experiments
Adsorption experiments were performed in triplicate trials using a batch equilibration procedure with activated and non-activated substrates. Seven samples of two grams of substrate were placed in seven 100 mL beakers, along with 5, 10, 15, 20, 30, 40 and 50 mL of 10
−3 M Cu
2+ solution, respectively. Suspensions were kept under isothermal conditions (20 ± 2 °C) for 24 h and periodically shaken. The equilibrium concentration was determined through complexometric titration with a 10
−3 M EDTA standard solution. The adsorbed amount corresponded to the difference between the initial and the equilibrium concentrations. By plotting the adsorbed amount (mmol/g substrate) against the equilibrium concentration (mmol), adsorption isotherms were obtained. Then, the adsorption data were checked out against the linear form of the Freundlich models (3) and (4) [
34,
35,
36,
37,
38]:
Equation (4) is the equation for a straight line, whose intercept is equal to log K and slope is equal to 1/
n. The Langmuir model (5) was also applied to the adsorption data and
K2 and
K1 are given by the intercept and the slope of the straight line (Equation (6)) [
36,
37,
39].
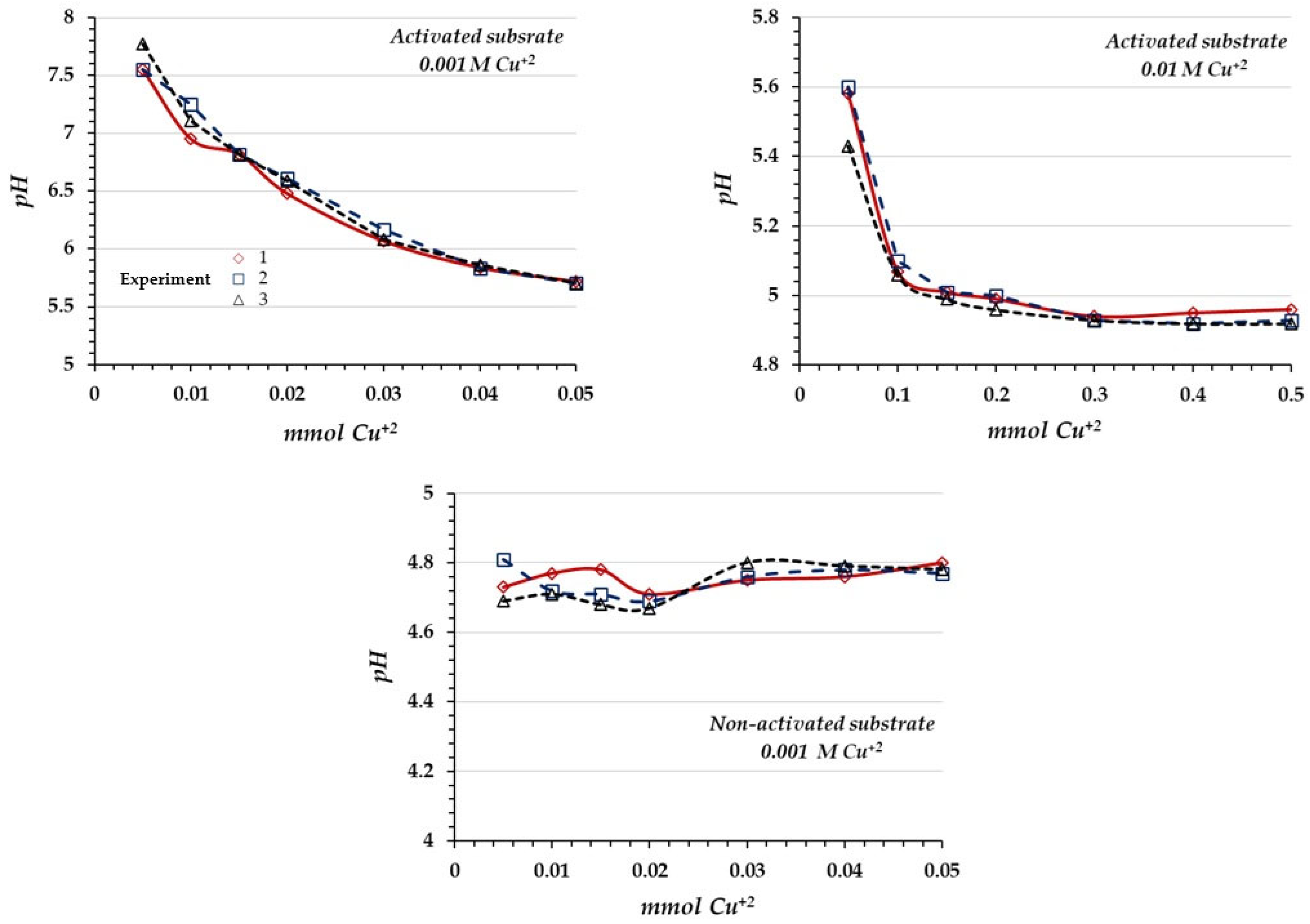
The pH study was performed in triplicate trials, using the batch equilibration procedure, by treating 2 g of activated and non-activated substrate with increasing volumes of 10−3 and 10−2 M Cu2+ solutions. The pH was measured using a Hanna HI2210-01 pH meter (HANNA® Instruments, San José, Costa Rica), calibrated with pH 4 and 7 buffer solutions.
4. Discussion
According to the objectives set at the beginning, copper adsorption on an oxidic variable charge substrate was studied by comparing the alkaline-treated and non-treated substrates. The alkaline treatment seeks to activate, homogenize and widen the negative charge density on the oxide surface. The results showed that the adsorption reaction on the activated substrate is much more intense than on the non-activated substrate because there is more affinity between Cu
2+ ions and the charged surface. The adsorption capacities, represented by
K values in the Freundlich model and
K2 values in the Langmuir model, are higher for the adsorption reaction on the activated substrates than on the non-activated substrates. These results can be interpreted in terms of the oxide deprotonation reaction through the alkaline attack, which increases the negative charge density on the substrate surface. As a consequence, the adsorption of copper ions (or any other cationic species) looks very favored on the activated substrate. The behavior of the variable charge surface replicates using other substrates prepared with different tested materials, as reported in the literature [
14,
15,
26,
31].
The isotherm profile might suggest what kind of interaction between Cu2+ ions and the substrate surface takes place; however, it is not conclusive evidence about the nature of the interaction. The adsorption process on the activated substrate is defined by an L-type isotherm, which is indicative of great affinity between the adsorbate and the adsorbent. Contrarily, isotherms associated with the adsorption reaction on the non-activated substrate show much less or no affinity for the Cu2+ ions, especially on the substrates prepared with the medium and fine fraction.
In the case of the activated substrate, the alkaline treatment creates a homogeneous negative charge density on the adsorbent substrate surface, because the OH
− groups from amphoteric oxides deprotonate during the alkaline reaction, increasing the number of active sites where the adsorption can be promoted. The alkaline treatment takes place accordingly to Equation (7) [
20,
21,
22,
31].
Therefore, the new negative charges that are promoted by the alkaline treatment are responsible for the highest affinity between the activated substrate and the Cu2+ ions. Consequently, the greater negative surface charge density allows a more extensive adsorption reaction on the activated substrate.
The shape of the isotherms associated with the adsorption reaction on the activated substrate suggests that they finally reach the flat zone where the saturated Cu2+ monolayer is located on the surface. It likely forms according to the Freundlich model, and chemisorption takes place on an ionic monolayer.
Similar isotherm profiles have been reported in the literature for Cu
2+ adsorption on goethite and γ–Al
2O
3, confirming specific adsorption or chemisorption between Cu
2+ ions and the oxide surface [
14,
25,
45]. Likewise, a comparable mechanism has been proposed for the adsorption of Cu
2+ ions on TiO
2 oxide [
25,
46].
However, the isotherm profile is not enough to infer the nature of the adsorption, which could be specific or non-specific. Equation (8) represents the theoretical model that explains the chemisorption of copper ions through the formation of a complex bonding between the oxidic surface and Cu
2+ ions [
14,
21,
25,
45]. M represents the metals as Al, Fe, Mn and Ti, which can form amphoteric metallic oxides characterized by pH-variable surface charges.
The former model also predicts the solution acidification due to the formation of H
3O
+ ions during the adsorption reaction, which, in turn, has been confirmed by the pH measurements along the Cu
2+ adsorption reaction. This acidification can be interpreted in terms of chemisorption between Cu
2+ ions and the oxidic surface, according to Equation (8), resulting in the formation of the monodentate inner sphere complex between Cu
2+ ions and the deprotonated oxides on the substrate surface [
21,
25,
45]. This kind of chemisorption reaction is irreversible; therefore, to desorb the adsorbate, the covalent bond must be broken, which is difficult to achieve.
In a similar manner, any other cationic species may suffer specific or non-specific adsorption reactions on the activated substrate. Unlike the cations from alkaline and alkaline earth, which can suffer nonspecific adsorption through a cation exchange reaction mechanism, so they are easily desorbed, the cations from transitional metals, like Cu2+ ions, can suffer specific adsorption through covalent bonding with the oxide on the surface, so they do not desorb easily.
Although the presence of other cations was not studied in this work, a previous publication reported results from the adsorption of copper and zinc [
27] from aqueous solution using fixed column experiments with three similar materials, including the V material. The results showed more affinity for copper ions than zinc ions. Similar results for copper and iron were reported in the literature [
16], using different oxidic geologic materials. In all cases, there is more affinity for the copper ion than for the iron ion because of its larger electronegativity and smaller size. It could be said that in the presence of competing ions, the electronegativity and hydration radius become the decisive factors in which cation adsorbs first. The cation with the largest electronegativity should bond first. On the other hand, the larger the ionic radius, the smaller the hydration radius, and then the smaller the cation should be adsorbed faster and in greater quantities.
Because transitional metal bonds to the oxidic surface through a covalent bond, the most electronegative should bond first; the electronegativity values for copper and zinc are 1.9 and 1.6, respectively. The strongest bond should be formed with the metal with the largest charge/radius ratio; the covalent radius are 1.28 and 1.39 for copper and zinc, respectively, so the charge/radius ratios are 1.56 and 1.45, respectively. Therefore, copper will form a stronger covalent bond and should be preferentially adsorbed. However, if there is a greater difference in the concentration of the competing cation, then the more concentrated cation could be adsorbed first [
47].
The acidification due to copper [
14] and lead [
6] adsorption on a calcined substrate prepared with different oxidic lithological materials was previously reported in the literature. For copper adsorption was reported acidification up to 0.03, and 1.2 mmol H
+ was reported when the substrate was treated with the 10
−2 M and 10
−1 M Cu
2+ solutions, respectively. In the case of lead adsorption, the production of 3.45 × 10
−3 mmol H
+ was reported when a 1 mM lead solution was used, and 0.0722 mmol H
+ when a 10 mM lead solution was used. This acidification can be interpreted in terms of Equation (2), and the
L-type isotherm, suggesting a covalence metal surface.
Fitted data using the Freundlich and Langmuir models show better linear correlation for activated substrates than for non-activated substrates; however, the differences between the calculated and experimental data are higher when the Freundlich model is applied. The Freundlich model describes multilayer or heterogeneous surface adsorption, while the Langmuir model describes adsorption on active sites in a monolayer adsorbent process. Actually, both models are applied to explain the same type of isotherm; however, the Freundlich equation is an empirical model, and the Langmuir model is based on theoretical considerations and is more restrictive about the surface conditions [
36,
38,
41].
A solid surface can be considered a homogeneous and regular structure as is required by Langmuir models; however, this kind of surface exists only as a theoretical element. In reality, the adsorbent substrate surface is very irregular, and the active sites for the adsorption reaction are not equivalent, as is demanded by the Langmuir model. The irregularities and the lack of homogeneity of the calcined substrate surfaces cause the differences and variability between the experimental and calculated data [
36,
38,
39]. Therefore, the Freundlich model fits better in the case of the adsorption reaction on the adsorbent substrate.
Compared with other similar substrates reported in the literature, such as materials L and G [
14], although all of them show similar tendencies, that is to say, the adsorption process is very favored on the activated substrate, the substrate prepared with the V material showed better performance, exceeding by up to ten times what is adsorbed by the other substrates.
Table 6 shows the ratios between the adsorbed amount by the activated substrate vs. the non-activated substrate for all three substrates reported in the former literature [
14] and the three granulometric fractions used in the preparation of the substrates. According to the Freundlich model, the ratios associated with the new substrate tested in this work are, by far, larger than other tested substrates (G and L), especially the substrates prepared with the gross and fine granulometric fractions. This could be interpreted in terms of the specific surface. Previous results showed that for the substrate prepared with V material, the thermal treatment favors the specific surface, while for G and L materials, thermal treatment causes a decrease in the specific surface, which in turn reduces the number of active sites suitable for the adsorption.
Various adsorbent materials, including clays, zeolites, industrial by-products, biochars, and oxide-modified substrates, demonstrate effective Cu
2+ adsorption capacities. Aluminosilicate-based and natural zeolite materials retain between 0.5 and 57.8 mg/g, depending on the surface area, adsorbent dosage, cation exchange capacity, and activation method [
5,
7,
10,
45]. Iron-oxide-coated sands and doped clays reach 6–83.3 mg/g under optimized synthesis conditions [
5,
10], although their preparation often involves multi-step procedures that hinder scalability. Industrial by-products, such as fly ash and iron-making by-products, exhibit capacities ranging from 1.18 to 40.0 mg/g, depending on the chemical treatment [
8,
10]. Modified biochars and activated carbons achieve higher values (typically between 10 and 130 mg/g) but require energy-intensive activation and incur elevated production costs [
9,
10,
11,
37]. In general, the adsorption capacity for copper ions depends on the physicochemical properties of the adsorbent, including the surface area, porosity, and functional groups, as well as on experimental conditions such as the pH, initial Cu
2+ concentration, contact time, and adsorbent dosage.
The results have demonstrated first that the substrate surface charge density can be changed and widened according to an acid or alkaline treatment. In a second place, as was predicted, the activated substrates efficiently retain metal ions, especially at low concentrations, through specific chemisorption mechanisms. The low affinity of the non-activated substrate for Cu2+ is obviously related to the lack of active sites for the reaction to occur. This behavior replicates when substrates have been used for anion retention, when an acid treatment on the substrate creates a larger positive charge density on the oxidic surface. In these cases, adsorption is also favored by the activated substrate.
The high affinity for Cu2+, the consistency across particle size fractions, and the strong fit to the Langmuir and Freundlich models validate their performance as functional adsorbents. The mineral composition, thermal and chemical stability, and low toxicity allow this geologic material to be used in the preparation of an adsorbent substrate to be applied in treatment systems for contaminated drinking water with heavy metals. These properties make them viable, low-cost, and high-performance solutions, especially useful in rural areas and regions affected by mining activities. With the substrate being environmentally friendly, once the filtering unit is saturated, the used substrate can be incorporated into the soil to improve soil structure and drainability. However, this issue must be investigated in future research.
5. Conclusions
In the search for new natural materials suitable for the preparation of adsorbent substrates that can be used in different technological applications, such as water treatment, the adsorbent substrate tested in these experiments has shown adsorbent properties that deserve to be studied for water treatment and its application in metal retention from polluted waters.
Because of its pH-dependent surface charges, after previous alkaline attack, amphoteric oxides deprotonate, creating a negative charge density on the substrate surface that allows cationic adsorption from aqueous media. The alkaline activation reaction of the substrate surface promotes the formation of new negative charges that can participate in the adsorption phenomena, improving the efficiency of the adsorbent substrate for cationic retention.
The results showed in the first place the variable charge nature of the oxidic surface and in the second place the good affinity between Cu2+ ions and the activated substrate. The associated L-type isotherm suggests a chemisorption reaction between the oxidic surface and Cu2+ ions; however, according to the literature, the Cu2+ chemisorption reaction on this kind of oxidic surface should produce H+ ions during the adsorption reaction, which has been evidenced by the pH measurements.
The isotherms’ shape and pH measurements support the hypothesis of a chemisorption reaction between Cu2+ ions and the activated oxidic surface. As well as copper ions, any other transition metal should behave similarly. These adsorbent substrates can be applied in water treatment to retain heavy metals as well as other contaminants present in polluted waters.
According to the investigation results, the oxidic substrate presents potential applicability in drinking water treatment, being able to act as an ionic adsorbent capable of retaining undesirable metallic species, as well as anionic contaminant species, from polluted drinkable water at low cost. The substrate is environmentally friendly and has a very low toxicity, and it is available for rural media and mining-impacted regions because the raw material is cheap and readily available in nature.