Continuous Electrocoagulation Processes for Industrial Inorganic Pollutants Removal: A Critical Review of Performance and Applications
Abstract
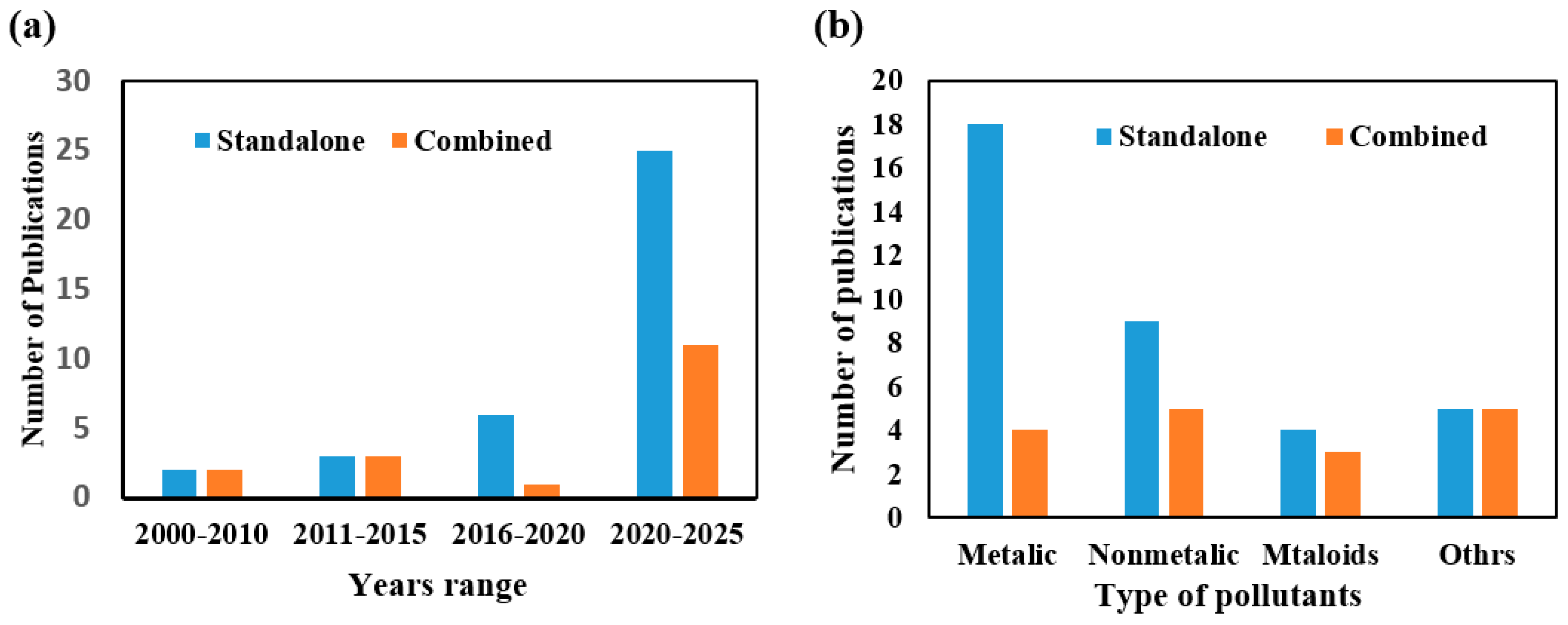
1. Introduction
2. Methodology
3. Standalone and Combined CEPs for Removing Inorganic Pollutants
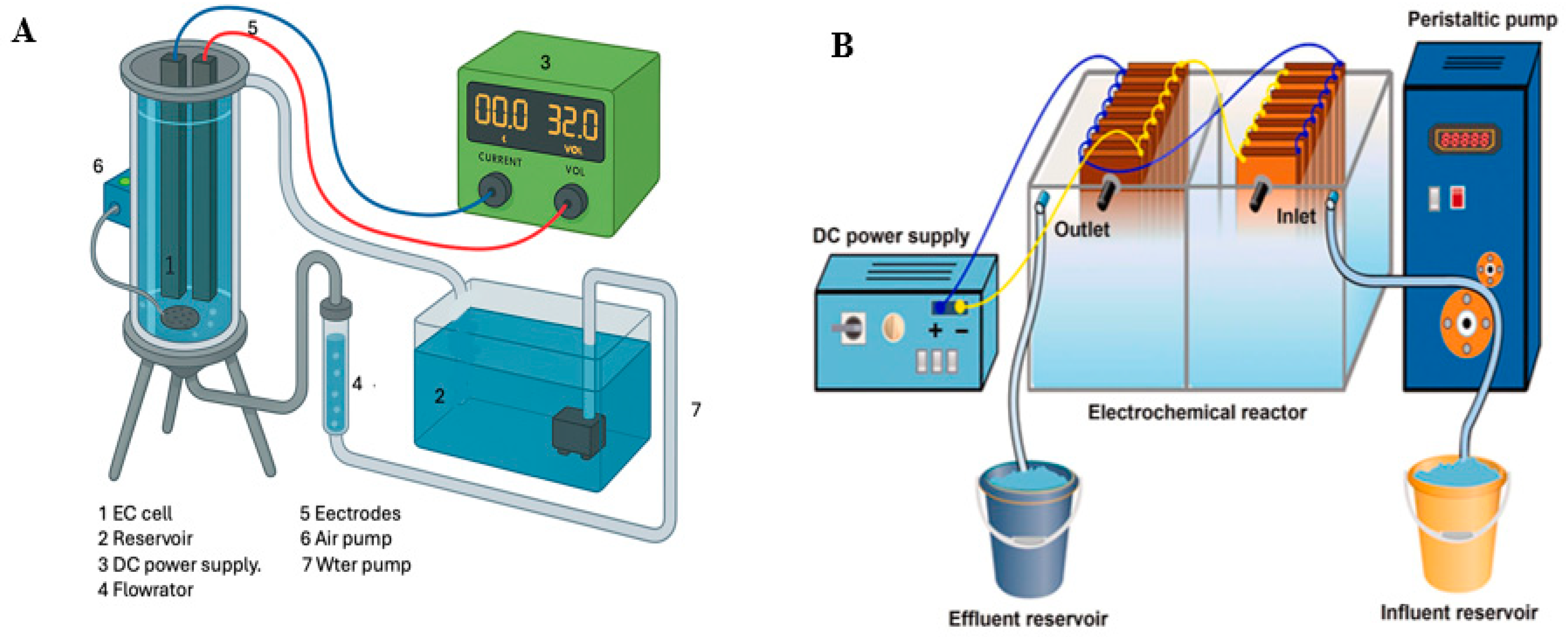
3.1. Standalone EC Treatment Processes
3.2. Combined Continuous Electrocoagulation Processes (CEPs)
4. Mathematical Modeling of CEPs for Inorganic Pollutant Removal
4.1. Kinetic and Isotherm Modeling of Continuous Electrocoagulation Processes
4.1.1. Standalone Processes
4.1.2. Combined Processes
4.2. Operational Parameter Optimization by Statistical and AI Methods
4.2.1. Standalone Continuous Electrocoagulation Processes
4.2.2. Combined Continuous Electrocoagulation Processes
5. Design Innovations in Electrocoagulation Processes
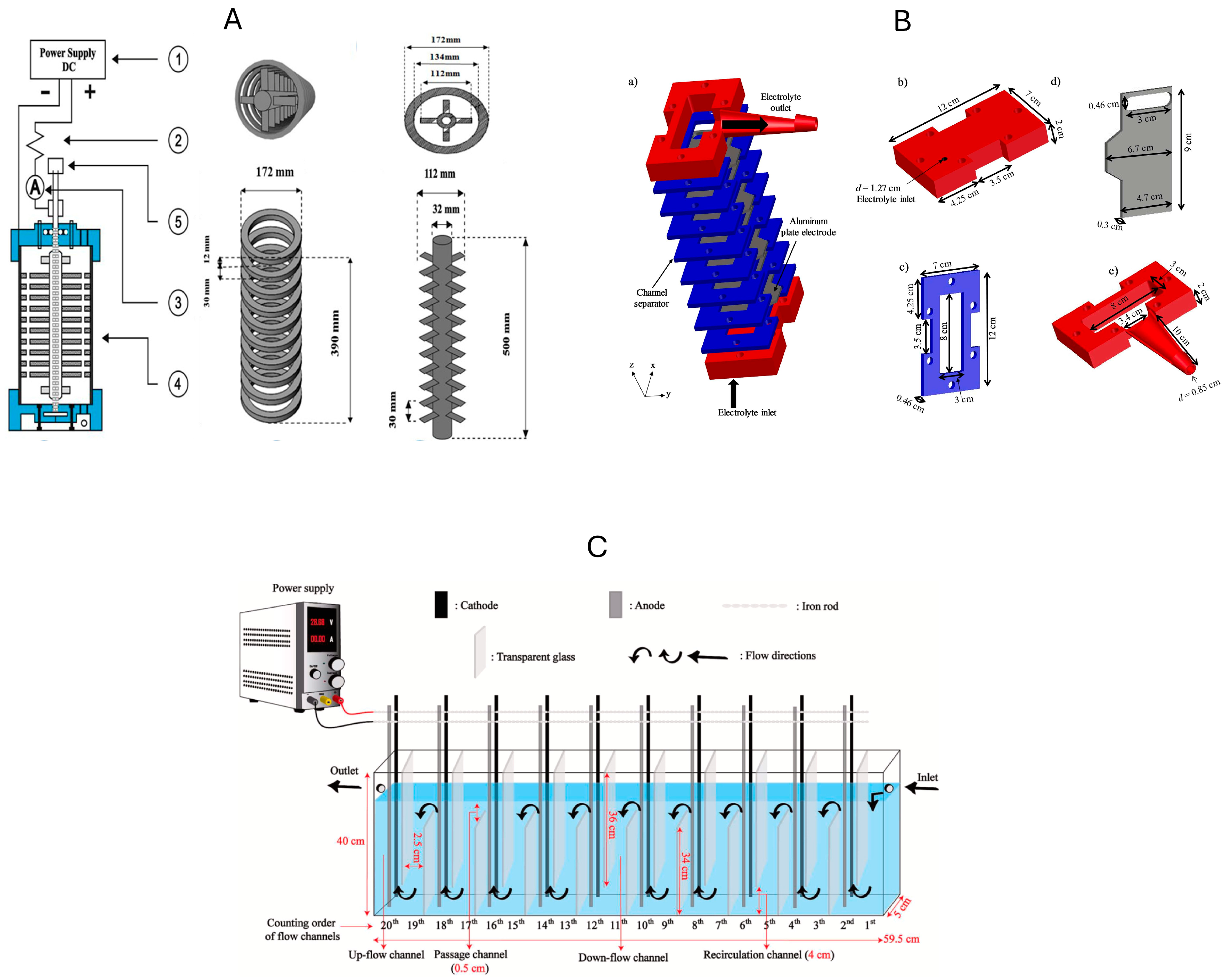
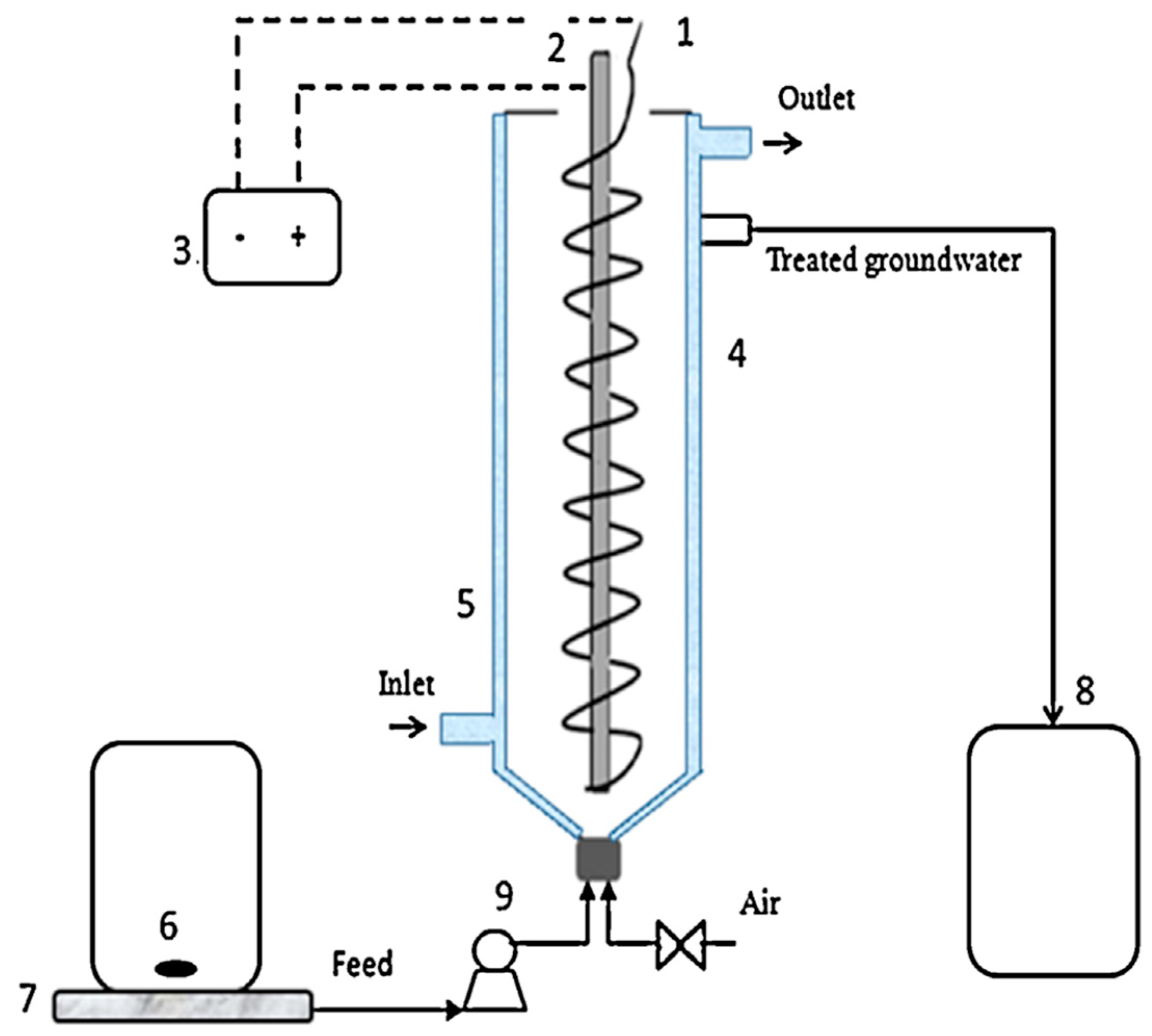
5.1. Standalone Continuous EC Processes
5.2. Combined Continuous EC Processes
6. Scale-Up of Continuous EC Processes
6.1. Technical Challenges in Scaling up EC
- Electrode consumption and maintenance: Electrode consumption rates can increase non-linearly with scale if the current distribution is uneven. Furthermore, cathode passivation is exacerbated during long-term continuous operation, leading to decreased efficiency over time [89]. This requires routine cleaning or operational strategies like polarity reversal or surface agitation to maintain performance [89]. These maintenance demands pose downtime and cost challenges in large systems.
- Current distribution and scale of electrolysis: In a large reactor, ensuring uniform current density across all electrodes and throughout the reactor volume is difficult [93]. In addition, poor current distribution can cause localized under-treatment (in zones with lower current) or excessive electrode corrosion (in zones with higher current).
- Cost-effectiveness vs. maintenance: While EC can achieve high pollutant removal efficiencies, the increased maintenance demands at larger scales, such as frequent electrode replacement, cleaning, and system downtime, can offset its perceived cost-effectiveness. In practice, the operational expenses for electrode maintenance, energy consumption, and labor must be carefully considered when evaluating the economic feasibility of scaled-up EC systems. This highlights a critical trade-off between high removal performance and long-term operational costs [89,93].
- Mass transfer and mixing: Achieving adequate mixing and mass transfer in large-volume reactors is a challenge. Hydrodynamics in scaled systems must ensure that coagulant species and contaminants effectively collide and aggregate. Inadequate mixing in a large tank can lead to stratification, with portions of the flow under-treated. Many industrial EC designs incorporate baffling, stirring, or pumped recirculation to promote uniform contact [89,93].
- Solid handling and sludge management: A successful EC process generates flocculated solids (sludge) containing the removed inorganic pollutants (e.g., metal hydroxide sludge). At larger scales, the volume of sludge produced is significant, necessitating robust separation (sedimentation or filtration) and handling systems [89,93].
- Process stability and control: Industrial wastewaters can have variable compositions and flow rates, which tests the adaptability of an EC system. Fluctuations in pollutant concentrations, pH, or conductivity can alter EC performance and may require dynamic adjustment of current or retention time. At the lab scale, conditions are easily controlled; at scale, robust monitoring and control systems are needed to maintain treatment efficiency under varying influent conditions [89,93].
6.2. Case Studies: Successes and Lessons from Scale-Up
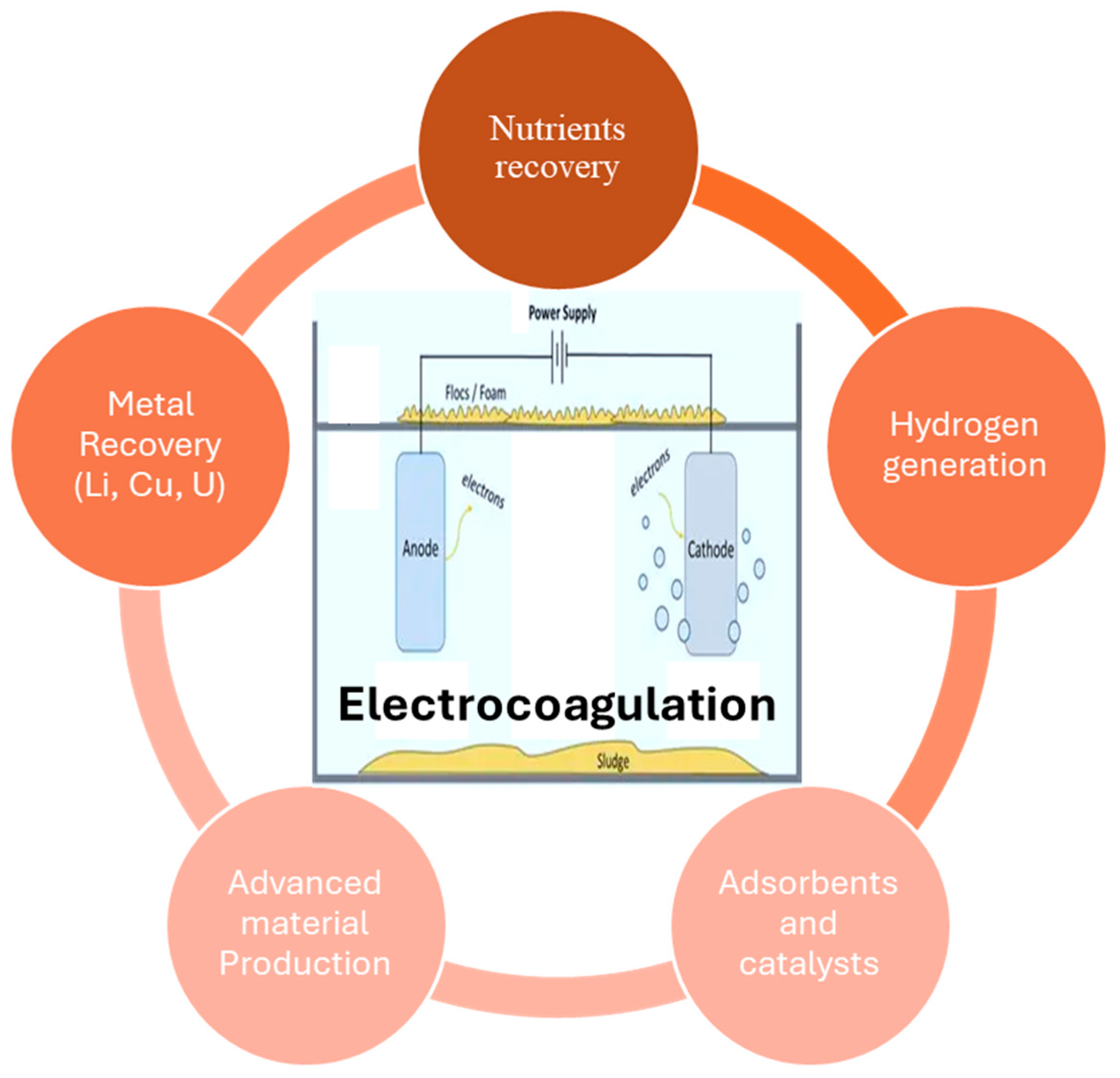
7. Circular Economy
7.1. Nutrient Recovery
7.2. Adsorbents and Catalysts
7.3. Advanced Material Production
7.4. Metal Recovery from Wastewater
7.5. Hydrogen Generation for Energy Recovery
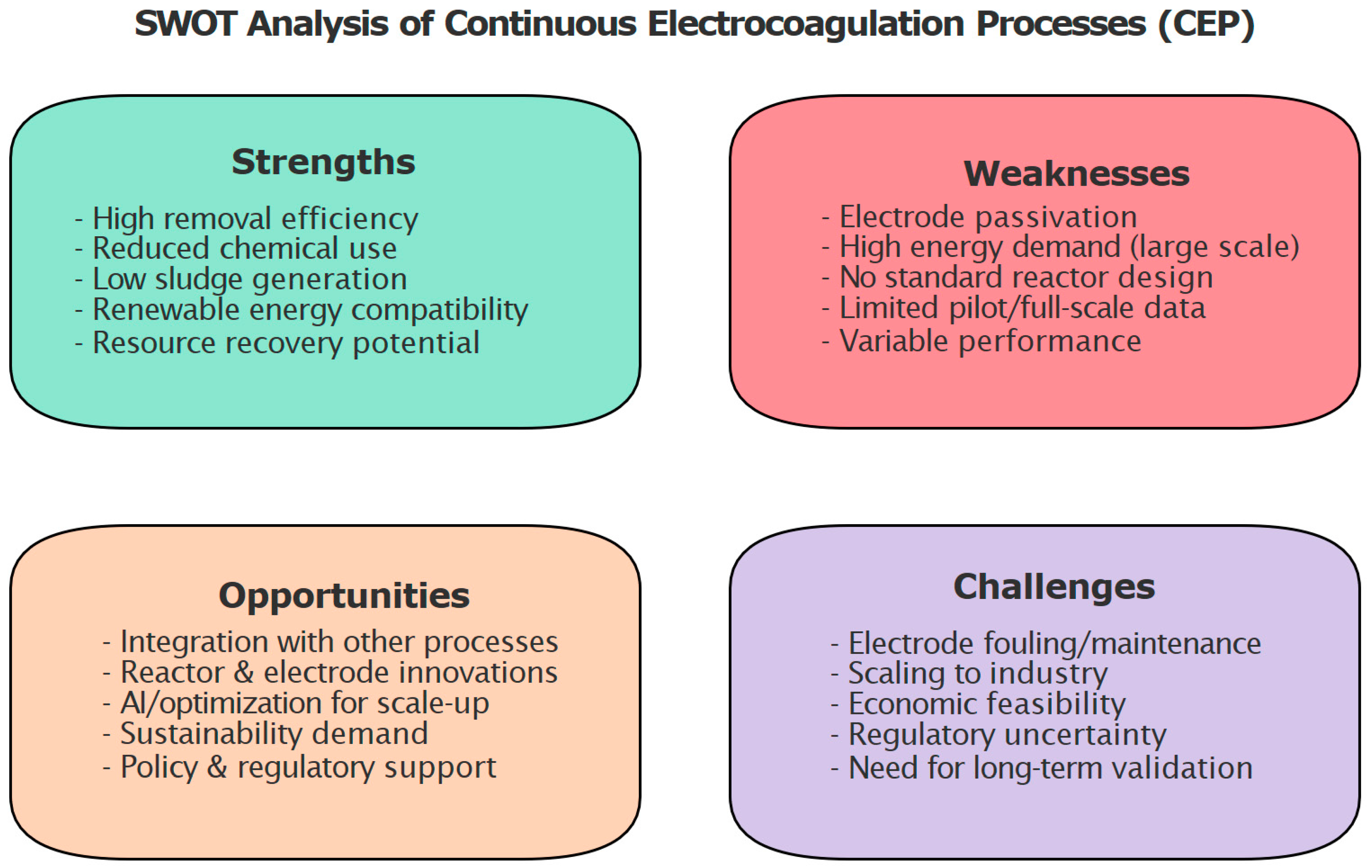
8. SWOT Analysis of CEPs
- Strengths: CEPs achieve high removal efficiencies for a wide range of inorganic contaminants, with advantages such as lower sludge generation, reduced chemical consumption, and compatibility with renewable energy sources. It also offers potential for resource recovery, supporting circular economy strategies.
- Weaknesses: Limitations include electrode passivation, relatively high energy demand at large scale, lack of standardized reactor designs, and performance variability across wastewater matrices. Furthermore, most applications remain at laboratory scale, with limited pilot or industrial demonstrations.
- Opportunities: CEPs can be integrated with complementary treatment technologies (e.g., membrane processes, flotation, advanced oxidation) and benefit from ongoing innovations in electrode design, hydrodynamics, and automation. The growing demand for sustainable wastewater treatment solutions, coupled with advancements in modeling, optimization, and AI-driven control, creates further potential for industrial adoption.
- Challenges: Major barriers include electrode fouling and maintenance, scaling from laboratory to industrial scale, economic feasibility, and regulatory uncertainty in certain regions [113]. The lack of long-term operational data also poses a challenge for commercialization and standardization [114].
9. Conclusions
10. Recommendations for Future Work
- ○
- Develop and adopt standardized guidelines for CEPs reactor design to ensure comparability of performance data, facilitate scale-up, and reduce design-related uncertainties in full-scale applications.
- ○
- Conduct more pilot-scale and full-scale studies under real industrial conditions using complex wastewater matrices to validate laboratory findings, evaluate long-term stability, and assess maintenance requirements.
- ○
- Expand the use of dynamic modeling (e.g., CFD, AI/ML, process simulation) to capture the coupled electrochemical, hydraulic, and mass transfer phenomena in CEPs. Experimental validation of these models will permit their use to design adaptive control strategies for real-time optimization.
- ○
- Explore the systematic integration of CEPs with other treatment technologies such as reverse osmosis, membrane bioreactors, ion exchange, and advanced oxidation processes. This includes evaluating synergies in performance, energy use, and cost reduction.
- ○
- Investigate novel and sustainable electrode materials (e.g., coated, composite, or recycled electrodes) that can reduce passivation, enhance electrochemical activity, and extend electrode lifespan.
- ○
- Emphasize CEPs’ potential for resource recovery—such as phosphorus, metals, and reusable water—within a circular economy framework. Life-cycle assessments (LCA) and techno-economic analyses (TEA) should be performed to support such implementations.
- ○
- Focus on reducing operational costs by optimizing current density, residence time, and flow rates, and by employing energy recovery systems or solar-powered configurations for decentralized applications.
- ○
- Undertake detailed environmental impact studies and collaborate with regulatory agencies to develop safety and compliance standards that encourage the adoption of CEPs in industrial practice.
- ○
- Establish a centralized, open access database that compiles performance data, cost metrics, energy consumption, and modeling results of CEP systems across different applications to support meta-analysis and informed decision-making.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Anode |
| AC | Alternating current |
| ACA | Activated Carbon Adsorption |
| AI | Artificial Intelligence |
| Al | Aluminum |
| ARE | Average relative error |
| AUD | Australian dollars |
| BBD | Box–Behnken Design |
| BOD5 | Biochemical Oxygen Demand after 5 Days |
| Br- | Bromide ion |
| C | Crystallization, Cathode |
| CAD | Canadian dollars |
| CC | Chemical Coagulation |
| CCD | Central Composite Design |
| CD | Current Density |
| CE | Circular economy |
| CEPs | Continuous Electrocoagulation Processes |
| CFD | Computational fluid dynamics |
| Cl- | Chloride ion |
| Co | The initial concentration of the pollutants before treatment |
| Cr | Chromium |
| Ct | The concentration of the pollutants after treatment |
| CTV | Conversion Time Kinetics |
| Cu | Copper |
| D | Diameter |
| DC | Direct current |
| E | Energy Consumption |
| EC | Electrocoagulation |
| ECF | Electrocoagulation/Flotation |
| ECT | Electrocoagulation time |
| EDI | Electrochemical Oxidation Index |
| ED-EC | Electro-Disinfection–Electrocoagulation |
| EFlo | Electro-flotation |
| El | Electrolysis |
| EO | Electrooxidation |
| EUR | Euro |
| F | Filtration |
| Fe | Iron |
| Flo | Floatation |
| Flu | Fluctuation |
| FP | Filter Press |
| GBM | Gradient Boosting Machine |
| Ge | Graphite |
| I | The applied current |
| IE | Ion Exchange |
| LCA | Life Cycle Assessment |
| Li | Lithium |
| m | Amount of dissolved anode |
| MAMs | Mesoporous alumina microcapsules |
| MD | Membrane Distillation |
| MF | Microfiltration |
| Mg | Magnesium |
| ML | Machine learning |
| Mn | Manganese |
| Mw | The molecular weight of electrode material |
| n | Electrode Number |
| NF | Nano Filtration |
| NH3-N | Ammonia Nitrogen |
| Ni | Nickel |
| NTU | Nephelometric Turbidity Unit |
| OO | Ozone Oxidation |
| R | Removal efficiency |
| R2 | Regression correlation coefficient |
| RO | Reverse osmosis |
| RSM | Response Surface Methodology |
| RTD | Residence Time Distribution |
| S | Sedimentation |
| SEEC | Specific Electrical Energy Consumption |
| SF | Sand Filtration |
| SMBR | Submerged Membrane Bioreactor |
| SS | Stainless Steel |
| St | Steel |
| SWOT | Strengths, Weaknesses, Opportunities, and Challenges |
| T | Temperature |
| t | Electrocoagulation time |
| Ti | Titanium |
| TDS | Total Dissolved Solids |
References
- Al-Ananzeh, N.; Bani-Melhem, K.; Khasawneh, H.E.; Tawalbeh, M.; Al-Qodah, Z.; Al-Bodour, A. Investigating the potential of using solid waste generated from stone cutting factories for phenol removal from wastewater: A study of adsor ption kinetics and isotherms. Results Eng. 2023, 20, 101404. [Google Scholar] [CrossRef]
- Zeghioud, H.; Fryda, L.; Djelal, H.; Assadi, A.; Kane, A. A comprehensive review of biochar in removal of organic pollutants from wastewater: Characterization, toxicity, activation/functionalization and influencing treatment factors. J. Water Process Eng. 2022, 47, 102801. [Google Scholar] [CrossRef]
- Idris, A.O.; Orimolade, B.; Dennany, L.; Mamba, B.; Azizi, S.; Kaviyarasu, K.; Maaza, M. A Review on Monitoring of Organic Pollutants in Wastewater Using Elect rochemical Approach. Electrocatalysis 2023, 14, 659–687. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, M.; Sondhi, S.; Kaur, K.; Sharma, D.; Sharma, S.; Utreja, D. Removal of Inorganic Pollutants from Wastewater: Innovative Technologies and Toxicity Assessment. Sustainability 2023, 15, 16376. [Google Scholar] [CrossRef]
- Gomaa, H.E.; Alotaibi, A.A.; Gomaa, F.A.; Bajuayfir, E.; Ahmad, A.; Alotaibi, K.M. Integrated ion exchange-based system for nitrate and sulfate removal f rom water of different matrices: Analysis and optimization using respo nse surface methodology and Taguchi experimental design techniques. Process Saf. Environ. Prot. 2021, 153, 500–517. [Google Scholar] [CrossRef]
- Banu, H.A.T.; Karthikeyan, P.; Meenakshi, S. Synthesis and characterization of Ce(III) decorated Duolite resin and its removal performance of toxic anions from aqueous solutions. Environ. Chem. Ecotoxicol. 2021, 3, 8–16. [Google Scholar] [CrossRef]
- Xiang, H.; Min, X.; Tang, C.-J.; Sillanpää, M.; Zhao, F. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.; Rodríguez-Díaz, J.; Montenegro, M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- El-Baz, A.; Hendy, I.; Dohdoh, A.; Srour, M. Adsorption technique for pollutants removal; current new trends and fu ture challenges—A Review Egypt. Int. J. Eng. Sci. Technol. Ogy 2020, 32, 1–24. [Google Scholar]
- Al-Qodah, Z.; Dweiri, R.; Khader, M.; Al-Sabbagh, S.; Al-Shannag, M.; Qasrawi, S.; Al-Halawani, M. Processing and characterization of magnetic composites of activated carbon, fly ash, and beach sand as adsorbents for Cr(VI) removal. Case Stud. Chem. Environ. Eng. 2023, 7, 100333. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Nawasreh, M.; Al-Hamamreh, Z.; Bani-Melhem, K.; Alkasrawi, M. On the performance of Ballota undulata biomass for the removal of cadm ium(II) ions from water. Desalin. WATER Treat. 2017, 67, 223–230. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.M. A comprehensive review on conventional and biological-driven heavy met als removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Guleria, A.; Kumari, G.; Lima, E.C.; Ashish, D.K.; Thakur, V.; Singh, K. Removal of inorganic toxic contaminants from wastewater using sustaina ble biomass: A review. Sci. Total Environ. 2022, 823, 153689. [Google Scholar] [CrossRef]
- Dawam, M.; Gobara, M.; Oraby, H.; Zorainy, M.Y.; Nabil, I.M. Advances in Membrane Technologies for Heavy Metal Removal from Polluted Water: A Comprehensive Review. Water Air Soil Pollut. 2025, 236, 461. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Le, V.G.; Badruddin, I.A.; Khan, T.M.Y.; Ong, H.C. Progress and challenges of contaminate removal from wastewater using m icroalgae biomass. Chemosphere 2022, 286, 131656. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Tseng, T.-W. Electrochemical synthesis of magnetite nanoparticles for decentralized arsenic removal and recovery from synthetic groundwater. Sep. Purif. Technol. 2025, 376, 133976. [Google Scholar] [CrossRef]
- Nee Kew, S.Y.; Lau, S.Y. Review of electrochemical reactors for the efficient removal of heavy metals from wastewater. J. Ind. Eng. Chem. 2025, S1226086X25003107. [Google Scholar] [CrossRef]
- Lee, J.; Antonini, G.; Al-Omari, A.; Muller, C.; Mathew, J.; Bell, K.; Pearce, J.M.; Santoro, D. Electrochemical Methods for Nutrient Removal in Wastewater: A Review o f Advanced Electrode Materials, Processes, and Applications. Sustainability 2024, 16, 9764. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M.; Bani-Melhem, K.; Assirey, E.; Yahya, M.A.; Al-Shawabkeh, A. Free radical-assisted electrocoagulation processes for wastewater treatment. Environ. Chem. Lett. 2018, 16, 695–714. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Al-Shannag, M.; Alrousan, D.; Al-Kofahi, S.; Al-Qodah, Z.; Al-Kilani, M.R. Impact of soluble COD on grey water treatment by electrocoagulation te chnique. Desalin. Water Treat. 2017, 89, 101–110. [Google Scholar] [CrossRef]
- Merzouk, B.; Gourich, B.; Sekki, A.; Madani, K.; Vial, C.; Barkaoui, M. Studies on the decolorization of textile dye wastewater by continuous electrocoagulation process. Chem. Eng. J. 2009, 149, 207–214. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Alnaief, M.; Al-Qodah, Z.; Al-Shannag, M.; Elnakar, H.; AlJbour, N.; Alu’dAtt, M.; Alrosan, M.; Ezelden, E. On the performance of electrocoagulation treatment of high-loaded gray water: Kinetic modeling and parameters optimization via response surf ace methodology. Appl. Water Sci. 2025, 15, 114. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Kadier, A.; Al-Qodah, Z.; Akkaya, G.K.; Song, D.; Peralta-Hernández, J.M.; Wang, J.-Y.; Phalakornkule, C.; Bajpai, M.; Muhammad Niza, N.; Gilhotra, V.; et al. A state-of-the-art review on electrocoagulation (EC): An efficient, em erging, and green technology for oil elimination from oil and gas indu strial wastewater streams. Case Stud. Chem. Environ. Eng. 2022, 6, 100274. [Google Scholar] [CrossRef]
- Oktiawan, W.; Sarminingsih, A.; Hadiwidodo, M.; Purwono, P. Electrocoagulation process for phosphate recovery of agricultural wastewater: Effect of calcium adding, voltage, and time. Environ. Monit. Assess. 2024, 196, 842. [Google Scholar] [CrossRef]
- Rakhmania; Kamyab, H.; Yuzir, M.A.; Abdullah, N.; Quan, L.M.; Riyadi, F.A.; Marzouki, R. Recent applications of the electrocoagulation process on agro-based in dustrial wastewater: A review. Sustainability 2022, 14, 1985. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Al Bsoul, A.; Al-Qodah, Z.; Al-Ananzeh, N.; Al-Kilani, M.R.; Al-Shannag, M.; Bani-Salameh, W. Impact of a Sand Filtration Pretreatment Step on High-Loaded Greywater Treatment by an Electrocoagulation Technique. Water 2023, 15, 990. [Google Scholar] [CrossRef]
- Jamrah, A.; Al-Zghoul, T.M.; Al-Qodah, Z.; Al-Karablieh, E. Performance of Combined Olive Mills Wastewater Treatment System: Electrocoagulation-Assisted Adsorption as a Post Polishing Sustainable Process. Water 2025, 17, 1697. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M.; Hudaib, B.; Bani-Salameh, W.; Shawaqfeh, A.T.; Assirey, E. Synergy and enhanced performance of combined continuous treatment processes of pre-chemical coagulation (CC), solar-powered electrocoagulation (SAEC), and post-adsorption for Dairy wastewater. Case Stud. Chem. Environ. Eng. 2025, 11, 101183. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Elektorowicz, M. Development of a novel submerged membrane electro-bioreactor (SMEBR): Performance for fouling reduction. Environ. Sci. Technol. 2010, 44, 3298–3304. [Google Scholar] [CrossRef] [PubMed]
- Al-Qodah, Z.; Tawalbeh, M.; Al-Shannag, M.; Al-Anber, Z.; Bani-Melhem, K. Combined electrocoagulation processes as a novel approach for enhanced pollutants removal: A state-of-the-art review. Sci. Total Environ. 2020, 744, 140806. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M.; Hudaib, B.; Bani-Salameh, W. Enhancement of dairy wastewater treatment efficiency in batch chemical-assisted solar-powered electrocoagulation-adsorption system. Case Stud. Chem. Environ. Eng. 2024, 9, 100760. [Google Scholar] [CrossRef]
- Lin, X.; Gong, J.; Li, H.; Zhang, H.; Yu, Y.; Tan, W. Solar-powered electrocoagulation treatment of wet flue gas desulfurization wastewater using dimensionally stable anode and induced electrode. Environ. Eng. Res. 2022, 28, 210590–210596. [Google Scholar] [CrossRef]
- Achagri, G.; Ismail, R.; Kadier, A.; Ma, P.-C. A solar-powered electrocoagulation process with a novel CNT/silver nanowire coated basalt fabric cathode for effective oil/water separation: From fundamentals to application. J. Environ. Manag. 2025, 375, 124289. [Google Scholar] [CrossRef]
- Boulanouar, L.; Louhichi, B.; Hamdi, W.; Jellali, S.; L’taief, B.; Hamdi, N.; Rebouh, N.; Houas, A. Parametric study of cadmium and nickel removal from synthetic and actu al industrial wastewater industry by electrocoagulation using solar en ergy. J. Water Process Eng. 2025, 71, 107261. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Al-Kilani, M.R.; Tawalbeh, M. Evaluation of scrap metallic waste electrode materials for the application in electrocoagulation treatment of wastewater. Chemosphere 2023, 310, 136668. [Google Scholar] [CrossRef]
- Nippatla, N.; Philip, L. Electrochemical process employing scrap metal waste as electrodes for dye removal. J. Environ. Manag. 2020, 273, 111039. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Ma, L. A continuous flow-through system with integration of electrosorption and peroxi-coagulation for efficient removal of organics. Chemosphere 2021, 274, 129983. [Google Scholar] [CrossRef]
- Ammar, M.; Yousef, E.; Ashraf, S.; Baltrusaitis, J. Removal of Inorganic Pollutants and Recovery of Nutrients from Wastewa ter Using Electrocoagulation: A Review. Separations 2024, 11, 320. [Google Scholar] [CrossRef]
- Salinas-Echeverría, D.D.; Sánchez-De La Cruz, L.C.; Zambrano-Intriago, L.A.; Rodríguez-Díaz, J.M.; Sanoja-Lopez, K.A.; Luque, R.; Fernández-Andrade, K.J.; Salcedo, Y.G.; Crespo, R.J.B. Evaluation of a continuous flow electrocoagulation reactor for turbidi ty removal from surface water. Chem. Eng. Res. Des. 2023, 198, 478–488. [Google Scholar] [CrossRef]
- Hayden, J.; Abbassi, B. Continuous Flow Electrocoagulation System for Enhanced Phosphorous Rem oval in Decentralized Wastewater Treatment Systems. Water 2025, 17, 202. [Google Scholar] [CrossRef]
- Abdulhadi, B.; Kot, P.; Hashim, K.; Shaw, A.; Muradov, M.; Al-Khaddar, R. Continuous-flow electrocoagulation (EC) process for iron removal from water: Experimental, statistical and economic study. Sci. Total Environ. 2021, 760, 143417. [Google Scholar] [CrossRef] [PubMed]
- Emamjomeh, M.M.; Sivakumar, M. Fluoride removal by a continuous flow electrocoagulation reactor. J. Environ. Manag. 2009, 90, 1204–1212. [Google Scholar] [CrossRef]
- Tenodi, K.Z.; Tenodi, S.; Nikić, J.; Mohora, E.; Agbaba, J.; Rončević, S. Optimizing arsenic removal from groundwater using continuous flow electrocoagulation with iron and aluminum electrodes: An experimental and modeling approach. J. Water Process Eng. 2024, 66, 106082. [Google Scholar] [CrossRef]
- Genawi, N.M.; Mahmud, N.; Hassan, E.A.; El-Naas, M.H. Continuous electrocoagulation treatment of chromium from tannery waste water in a cylindrical column. Next Sustain. 2025, 6, 100113. [Google Scholar] [CrossRef]
- Mehralian, M.; Ehrampoush, M.H.; Ebrahimi, A.A.; Dalvand, A. Development of electrocoagulation-based continuous-flow reactor for leachate treatment: Performance evaluation, energy consumption, modeling, and optimization. Appl. Water Sci. 2023, 13, 162. [Google Scholar] [CrossRef]
- Hamdan, S.S.; El-Naas, M.H. An electrocoagulation column (ECC) for groundwater purification. J. Water Process Eng. 2014, 4, 25–30. [Google Scholar] [CrossRef]
- Kobya, M.; Akyol, A.; Demirbas, E.; Oncel, M.S. Removal of arsenic from drinking water by batch and continuous electrocoagulation processes using hybrid Al-Fe plate electrodes. Environ. Prog. Sustain. Energy 2014, 33, 131–140. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Yin, M.; Ma, X.; Lin, S. Removing heavy metal ions with continuous aluminum electrocoagulation: A study on back mixing and utilization rate of electro-generated Al ions. Chem. Eng. J. 2015, 267, 86–92. [Google Scholar] [CrossRef]
- Karamati Niaragh, E.; Alavi Moghaddam, M.R.; Emamjomeh, M.M. Techno-economical evaluation of nitrate removal using continuous flow electro-coagulation process: Optimization by Taguchi model. Water Supply 2017, 17, 1703–1711. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.; Cao, G.; Liu, S.; Duan, Z.; Song, S.; Song, M.; Zhang, M. Optimization and assessment of Fe–electrocoagulation for the removal o f potentially toxic metals from real smelting wastewater. J. Environ. Manag. 2018, 218, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Mohora, E.; Rončević, S.; Agbaba, J.; Zrnić, K.; Tubić, A.; Adžemović, M.; Dalmacija, B. Effects of combined Fe-Al electrodes and groundwater temperature on arsenic removal by electrocoagulation. Environ. Prot. Eng. 2019, 45. [Google Scholar] [CrossRef]
- Karamati-Niaragh, E.; Alavi Moghaddam, M.R.; Emamjomeh, M.M.; Nazlabadi, E. Evaluation of direct and alternating current on nitrate removal using a continuous electrocoagulation process: Economical and environmental approaches through RSM. J. Environ. Manag. 2019, 230, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Betancor-Abreu, A.; Mena, V.F.; González, S.; Delgado, S.; Souto, R.M.; Santana, J.J. Design and optimization of an electrocoagulation reactor for fluoride remediation in underground water sources for human consumption. J. Water Process Eng. 2019, 31, 100865. [Google Scholar] [CrossRef]
- Castañeda, L.F.; Coreño, O.; Nava, J.L.; Carreño, G. Removal of fluoride and hydrated silica from underground water by electrocoagulation in a flow channel reactor. Chemosphere 2020, 244, 125417. [Google Scholar] [CrossRef]
- Syam Babu, D.; Vijay, K.; Nidheesh, P.V.; Suresh Kumar, M. Performance of continuous aerated iron electrocoagulation process for arsenite removal from simulated groundwater and management of arsenic-iron sludge. Sustain. Energy Technol. Assess. 2021, 47, 101476. [Google Scholar] [CrossRef]
- Kuokkanen, V.; Kuokkanen, M.; Hynynen, I.; Kuokkanen, T. Electrocoagulation treatment of metallurgical industry wastewater—A laboratory scale batch and pilot scale continuous study. Hydrometallurgy 2021, 202, 105596. [Google Scholar] [CrossRef]
- Graça, N.S.; Ribeiro, A.M.; Rodrigues, A.E. Modeling and optimization of a continuous electrocoagulation process u sing an artificial intelligence approach. Water Supply 2021, 22, 643–658. [Google Scholar] [CrossRef]
- Al-Raad, A.A.; Hanafiah, M.M. SO42−, Cl−, Br−, and TDS removal by semi continuous electrocoagula tion reactor using rotating anode. Environ. Technol. Innov. 2022, 28, 102917. [Google Scholar] [CrossRef]
- Jasim, M.A.; AlJaberi, F.Y.; Salman, A.D.; Alardhi, S.M.; Le, P.-C.; Kulcsár, G.; Jakab, M. Studying the effect of reactor design on the electrocoagulation treatment performance of oily wastewater. Heliyon 2023, 9, e17794. [Google Scholar] [CrossRef]
- Tello, M.V.C.; Mendoza, G.E.R.; Taranco, O.J.R.; Chavez, C.M.L.; Pisfil, J.A.M.; Ramos, M.E.B.; Meneses, P.A.; Collana, J.T.M. Turbidity Removal from a Model Solution by Continuous Mode Electrocoagulation and Evaluation of Energy Consumption. Int. J. Membr. Sci. Technol. 2023, 10, 1304–1317. [Google Scholar] [CrossRef]
- Vargas, A.R.; Guillen, C.S.; Magaña Haynes, M.E.; AlJaberi, F.Y. Nickel removal from an industrial effluent by electrocoagulation in semi-continuous operation: Hydrodynamic, kinetic and cost analysis. Results Eng. 2023, 17, 100961. [Google Scholar] [CrossRef]
- Amusa, A.A.; Taib, M.R.; Xian, W.Z. Continuous Flow Electrochemical Process for Sanitary Landfill Leachate Treatment: Role of Inlet Flow Rate and Current Density. Water Air Soil Pollut. 2023, 234, 488. [Google Scholar] [CrossRef]
- Krystynik, P.; Kluson, P.; Masin, P.; Syc, M.; Jadrny, J.; Krusinova, Z. Implementation of electrocoagulation for reduction of Zn in an outlet stream from waste incineration plant. Chem. Eng. Process.—Process Intensif. 2023, 188, 109368. [Google Scholar] [CrossRef]
- Abdul Rahman, N.; Jose Jol, C.; Albania Linus, A.; Wan Borhan, W.W.S.; Abdul Jalal, N.S.; Baharudin, N.; Samsul, S.N.A.; Mutalip, N.A.; Jitai, A.A.; Hamid, D.F.A.A.A. Continuous electrocoagulation treatment system for partial desalinatio n of tropical brackish peat water in Sarawak coastal peatlands. Sci. Total Environ. 2023, 880, 163517. [Google Scholar] [CrossRef]
- Soto-Uribe, J.C.; Valenzuela-Garcia, J.L.; Salazar-Campoy, M.M.; Parga-Torres, J.R.; Vazquez-Vazquez, V.M.; Encinas-Romero, M.A.; Martinez-Ballesteros, G. Electrocoagulation Process for Recovery of Precious Metals from Cyanid e Leachates Using a Low Voltage. ACS Eng. Au 2024, 4, 139–144. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, P.; Lu, J.; Xu, H.; Zhang, W.; Yu, W.; Jiang, G. Fe electrocoagulation technology for effective removal of molybdate from water: Main influencing factors, response surface optimization, and mechanistic analysis. J. Environ. Chem. Eng. 2024, 12, 112127. [Google Scholar] [CrossRef]
- García-Colindres, M.A.; Requena-Alvarez, B.L.; Castillo-Suárez, L.A.; Linares-Hernández, I.; Martínez-Miranda, V. Removal of Nitrates in Drinking Water Polluted with Landfill Leachate by an Electrocoagulation System with Mg-Zn. Water Air Soil Pollut. 2024, 235, 269. [Google Scholar] [CrossRef]
- Ardhianto, R.; Anggrainy, A.D.; Samudro, G.; Triyawan, A.; Bagastyo, A.Y. A study of continuous-flow electrocoagulation process to minimize chemicals dosing in the full-scale treatment of plastic plating industry wastewater. J. Water Process Eng. 2024, 60, 105217. [Google Scholar] [CrossRef]
- Harahap, M.G.; Abfertiawan, M.S.; Syafila, M.; Handajani, M.; Gultom, T.H. Electrocoagulation for nickel, chromium, and iron removal from mine water using aluminum electrodes. Heliyon 2024, 10, e40784. [Google Scholar] [CrossRef]
- Dhifallah, S.; Attour, A.; Vial, C.; Zagrouba, F.; Audonnet, F. Continuous Electrocoagulation for a Sustainable Water Treatment: Effec ts of Electrode Configuration, Electrical Connection Mode, and Polarit y Reversal on Fluoride Removal. Sustainability 2024, 16, 5765. [Google Scholar] [CrossRef]
- AlRubaye, S.F.; Al Haboubi, N.A.; Al-Amili, H.A. Removal of Sulfate and Iron from a Water Solution Using a New Flow Pattern in an Electrocoagulation Reactor. Tikrit J. Eng. Sci. 2024, 31, 205–218. [Google Scholar] [CrossRef]
- Costigan, E.; Wu, S.; Eckelman, M.; Fernandez, L.; Mueller, A.; Alshawabkeh, A.; Larese-Casanova, P. Chromium removal from concentrated ammonium-nitrate solution: Electrocoagulation with iron in a plug-flow reactor. Sep. Purif. Technol. 2025, 354, 129353. [Google Scholar] [CrossRef]
- Hojabri, S.; Rajic, L.; Zhao, Y.; Alshawabkeh, A.N. Simulation of hexavalent chromium removal by electrocoagulation using iron anode in flow-through reactor. J. Hazard. Mater. 2024, 476, 135195. [Google Scholar] [CrossRef]
- Al Anbari, R.H.; Albaidani, J.; Alfatlawi, S.M.; Al-Hamdani, T.A. Removal of Heavy Metals From Industrial Water Using Electro-Coagulation Technique. In Proceedings of the Twelfth International Water Technology Conference, Alexandria, Egypt, 27–30 March 2008. [Google Scholar]
- Mavrov, V.; Stamenov, S.; Todorova, E.; Chmiel, H.; Erwe, T. New hybrid electrocoagulation membrane process for removing selenium from industrial wastewater. Desalination 2006, 201, 290–296. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Elektorowicz, M. Performance of the submerged membrane electro-bioreactor (SMEBR) with iron electrodes for wastewater treatment and fouling reduction. J. Membr. Sci. 2011, 379, 434–439. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Smith, E. Grey water treatment by a continuous process of an electrocoagulation unit and a submerged membrane bioreactor system. Chem. Eng. J. 2012, 198–199, 201–210. [Google Scholar] [CrossRef]
- Flores, O.J.; Nava, J.L.; Carreño, G.; Elorza, E.; Martínez, F. Arsenic removal from groundwater by electrocoagulation in a pre-pilot-scale continuous filter press reactor. Chem. Eng. Sci. 2013, 97, 1–6. [Google Scholar] [CrossRef]
- Sandoval, M.A.; Fuentes, R.; Nava, J.L.; Coreño, O.; Li, Y.; Hernández, J.H. Simultaneous removal of fluoride and arsenic from groundwater by elect rocoagulation using a filter-press flow reactor with a three-cell stack. Adv. Electrochem. Technol. Environ. Appl. 2019, 208, 208–216. [Google Scholar]
- Jebur, M.; Bachynska, Y.; Hao, X.; Wickramasinghe, S.R. Integrated Electrocoagulation, Ultrafiltration, Membrane Distillation, and Crystallization for Treating Produced Water. Membranes 2023, 13, 597. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Elektorowicz, M.; Tawalbeh, M.; Al Bsoul, A.; El Gendy, A.; Kamyab, H.; Yusuf, M. Integrating of electrocoagulation process with submerged membrane bioreactor for wastewater treatment under low voltage gradients. Chemosphere 2023, 339, 139693. [Google Scholar] [CrossRef]
- Lee, J.; So, Y.; Kim, S.; Yoon, Y.; Rho, H.; Park, C. Efficient integration of electro-coagulation and ceramic membranes for the treatment of real chemical mechanical polishing (CMP) slurry wastewater from the semiconductor industry. J. Water Process Eng. 2024, 61, 105326. [Google Scholar] [CrossRef]
- Dong, P.; Parmentier, D.; Picavet, E.; D’Haese, A.; Wu, Y.; Zhu, H.; Van Hulle, S.W. Optimized removal of silica during manure treatment by electrocoagulation-flotation (EC-F) in view of fouling prevention of reverse osmosis membranes. Desalination 2024, 591, 118031. [Google Scholar] [CrossRef]
- Waghe, P.; Ansari, K.; Dehghani, M.H.; Gupta, T.; Pathade, A.; Waghmare, C. Treatment of greywater by Electrocoagulation process coupled with sand bed filter and activated carbon adsorption process in continuous mode. AIMS Environ. Sci. 2024, 11, 57–74. [Google Scholar] [CrossRef]
- Channa, N.; Gadhi, T.A.; Mahar, R.B.; Ali, I.; Sajjad, S.; Freyria, F.S.; Bonelli, B.; Widderich, S.; Frechen, F.-B. Efficient and Rapid Combined Electrocoagulation–Filtration of Arsenic in Drinking Water. Water 2024, 16, 1684. [Google Scholar] [CrossRef]
- Isawi, H.; Sadik, M.A.; Nasr, F.A. Combined electrocoagulation/flotation technique and membrane desalinat ion for textile wastewater reuse. J. Environ. Chem. Eng. 2024, 12, 113661. [Google Scholar] [CrossRef]
- García, I.; Castañeda, L.F.; Nava, J.L.; Coreño, O. Continuous electrocoagulation-flocculation-sedimentation process to remove arsenic, fluoride, and hydrated silica from drinking water. J. Water Process Eng. 2025, 69, 106571. [Google Scholar] [CrossRef]
- Shahedi, A.; Jamshidi-Zanjani, A.; Darban, A.K.; Homaee, M.; Taghipour, F. Nickel, cyanide, zinc, and copper removal from the effluent using photo-electrocoagulation-oxidation. J. Hazard. Mater. Adv. 2025, 17, 100550. [Google Scholar] [CrossRef]
- Liu, B.; Cui, X.; Lu, X.; Zhu, X.; Wang, L.; Zhu, J. Sustainable and exceptional Li+ /Mg2+selectivity through electrocoagulation enhanced triamino guanidine modified membrane. J. Membr. Sci. 2025, 722, 123884. [Google Scholar] [CrossRef]
- Basha, C.A.; Selvi, S.J.; Ramasamy, E.; Chellammal, S. Removal of arsenic and sulphate from the copper smelting industrial ef fluent. Chem. Eng. J. 2008, 141, 89–98. [Google Scholar] [CrossRef]
- Meng, X.; Zeng, P.; Lin, S.; Wu, M.; Yang, L.; Bao, H.; Kang, J.; Han, H.; Zhang, C.; Sun, W. Deep removal of fluoride from tungsten smelting wastewater by combined chemical coagulation-electrocoagulation treatment: From laboratory te st to pilot test. J. Clean. Prod. 2023, 416, 137914. [Google Scholar] [CrossRef]
- Meena, R.R.; Singh, R.M.; Soni, P.; Kumar, R.; Kumar, S. Fluoride removal using a rotating anode electro-coagulation reactor: Parametric optimization using response surface methodology, isotherms and kinetic studies, economic analysis and sludge characterization. J. Environ. Manag. 2024, 370, 122600. [Google Scholar] [CrossRef] [PubMed]
- Nariyan, E.; Wolkersdorfer, C.; Sillanpää, M. Sulfate removal from acid mine water from the deepest active European mine by precipitation and various electrocoagulation configurations. J. Environ. Manag. 2018, 227, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Bennajah, M.; Maalmi, M.; Darmane, Y.; Touhami, M. Defluoridation Of Drinking Water By Electrocoagulation/Electroflotation—Kinetic Study. J. Urban Environ. Eng. 2010, 4, 37–45. [Google Scholar] [CrossRef]
- Ghahrchi, M.; Rezaee, A.; Adibzadeh, A. Study of kinetic models of olive oil mill wastewater treatment using electrocoagulation process. Desalin. Water Treat. 2021, 211, 123–130. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; AL-Rajabi, M.M.; Al Amayreh, H.H.; Assirey, E.; Bani-Melhem, K.; Al-Shannag, M. Performance of Continuous Electrocoagulation Processes (CEPs) as an Efficient Approach for the Treatment of Industrial Organic Pollutants: A Comprehensive Review. Water 2025, 17, 2351. [Google Scholar] [CrossRef]
- Ezechi, E.H.; Isa, M.H.; Kutty, S.R.M.; Yaqub, A. Boron removal from produced water using electrocoagulation. Process Saf. Environ. Prot. 2014, 92, 509–514. [Google Scholar] [CrossRef]
- Valentín-Reyes, J.; Nava, J.L. Modeling of a novel cascade-type electrocoagulation reactor using iron electrodes. Characterization of bubbling flow and coagulant dosage. Chem. Eng. Process.—Process Intensif. 2023, 189, 109411. [Google Scholar] [CrossRef]
- Peñafiel, R.; Flores Tapia, N.E.; Mayacela Rojas, C.M.; Lema Chicaiza, F.R.L.; Pérez, L. Electrocoagulation Coupled with TiO2 Photocatalysis: An Advanced Strategy for Treating Leachates from the Degradation of Green Waste and Domestic WWTP Biosolids in Biocells. Processes 2025, 13, 1746. [Google Scholar] [CrossRef]
- Pinedo-Hernández, J.; Marrugo-Negrete, J.; Pérez-Espitia, M.; Durango-Hernández, J.; Enamorado-Montes, G.; Navarro-Frómeta, A. A pilot-scale electrocoagulation-treatment wetland system for the trea tment of landfill leachate. J. Environ. Manag. 2024, 351, 119681. [Google Scholar] [CrossRef]
- Odongo, I.E.; McFarland, M.J. Electrocoagulation treatment of metal finishing wastewater. Water Environ. Res. 2014, 86, 579–583. [Google Scholar] [CrossRef]
- Uludag-Demirer, S.; Olson, N.; Ives, R.; Nshimyimana, J.P.; Rusinek, C.A.; Rose, J.B.; Liao, W. Techno-economic analysis of electrocoagulation on water reclamation an d bacterial/viral indicator reductions of a high-strength organic wast ewater—Anaerobic digestion effluent. Sustainability 2020, 12, 2697. [Google Scholar] [CrossRef]
- Murrieta, M.F.; Cornejo, O.M.; Rivera, F.F.; Nava, J.L. Electrochemical recovery of inorganic value-added products from wastewater: Toward a circular economy model. Curr. Opin. Electrochem. 2024, 46, 101498. [Google Scholar] [CrossRef]
- Isik, Z.; Dizge, N. Recovery of ferrous ammonium phosphate fertilizer from source-separated urine by electrocoagulation using response surface methodology (RSM). Process Saf. Environ. Prot. 2025, 194, 985–996. [Google Scholar] [CrossRef]
- Sharma, L.; Rohilla, J.; Ingole, P.P.; Halder, A. Utilization of Electrocoagulated Sewage as a Photoelectrocatalyst for Water Splitting. ACS Mater. Au 2024, 4, 459–467. [Google Scholar] [CrossRef]
- Santana, J.J.; Mena, V.F.; Betancor-Abreu, A.; Rodríguez-Raposo, R.; Izquierdo, J.; Souto, R.M. Use of alumina sludge arising from an electrocoagulation process as functional mesoporous microcapsules for active corrosion protection of aluminum. Prog. Org. Coat. 2021, 151, 106044. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, R.; Sun, W.; Wang, L.; Tang, H. Li extraction from model brine via electrocoagulation: Processing, kinetics, and mechanism. Sep. Purif. Technol. 2020, 250, 117234. [Google Scholar] [CrossRef]
- Li, P.; Chen, P.; Wang, G.; Wang, L.; Wang, X.; Li, Y.; Zhang, W.; Jiang, H.; Chen, H. Uranium elimination and recovery from wastewater with ligand chelation-enhanced electrocoagulation. Chem. Eng. J. 2020, 393, 124819. [Google Scholar] [CrossRef]
- Mehdipoor, M.A.; Moosavirad, S.M. Effect of Holed Ferrum electrodes (HFE) on the efficiency of the electrocoagulation process for copper recovery and optimization of parameters, using RSM. Hydrometallurgy 2020, 194, 105313. [Google Scholar] [CrossRef]
- Sharma, L.; Prabhakar, S.; Tiwari, V.; Dhar, A.; Halder, A. Optimization of EC parameters using Fe and Al electrodes for hydrogen production and wastewater treatment. Environ. Adv. 2021, 3, 100029. [Google Scholar] [CrossRef]
- Phu, T.K.C.; Nguyen, P.L.; Phung, T.V.B. Recent Progress in Highly Effective Electrocoagulation-Coupled Systems for Advanced Wastewater Treatment. iScience 2025, 28, 111965. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.K.; Kumar, S. Intensification of Electrocoagulation Treatment of Simulated Tannery Wastewater Using Rotating Electrodes: Parametric, Isotherms, Kinetic and Techno-Economic Studies. Chem. Eng. Process.—Process Intensif. 2025, 209, 110150. [Google Scholar] [CrossRef]



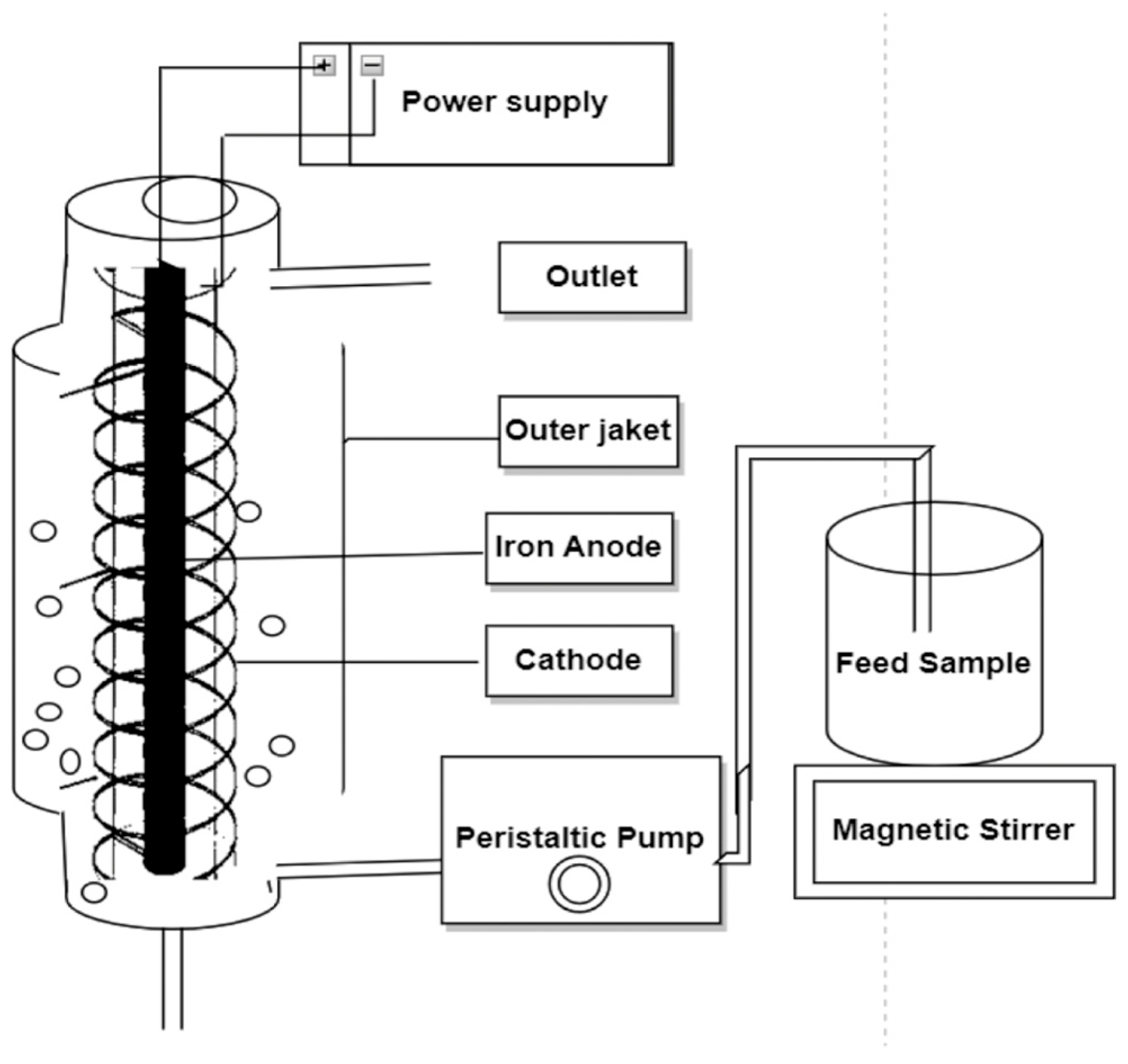

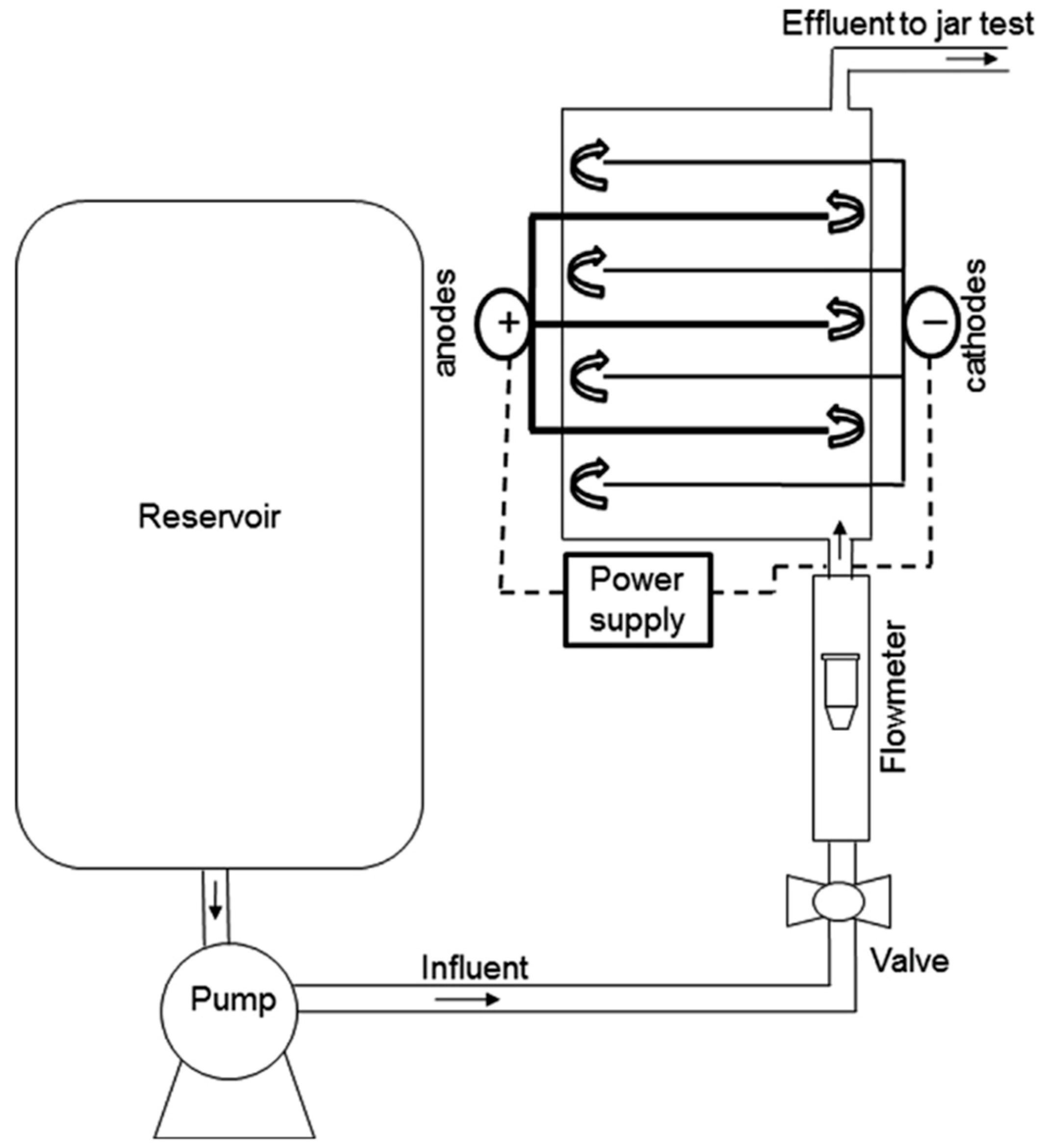

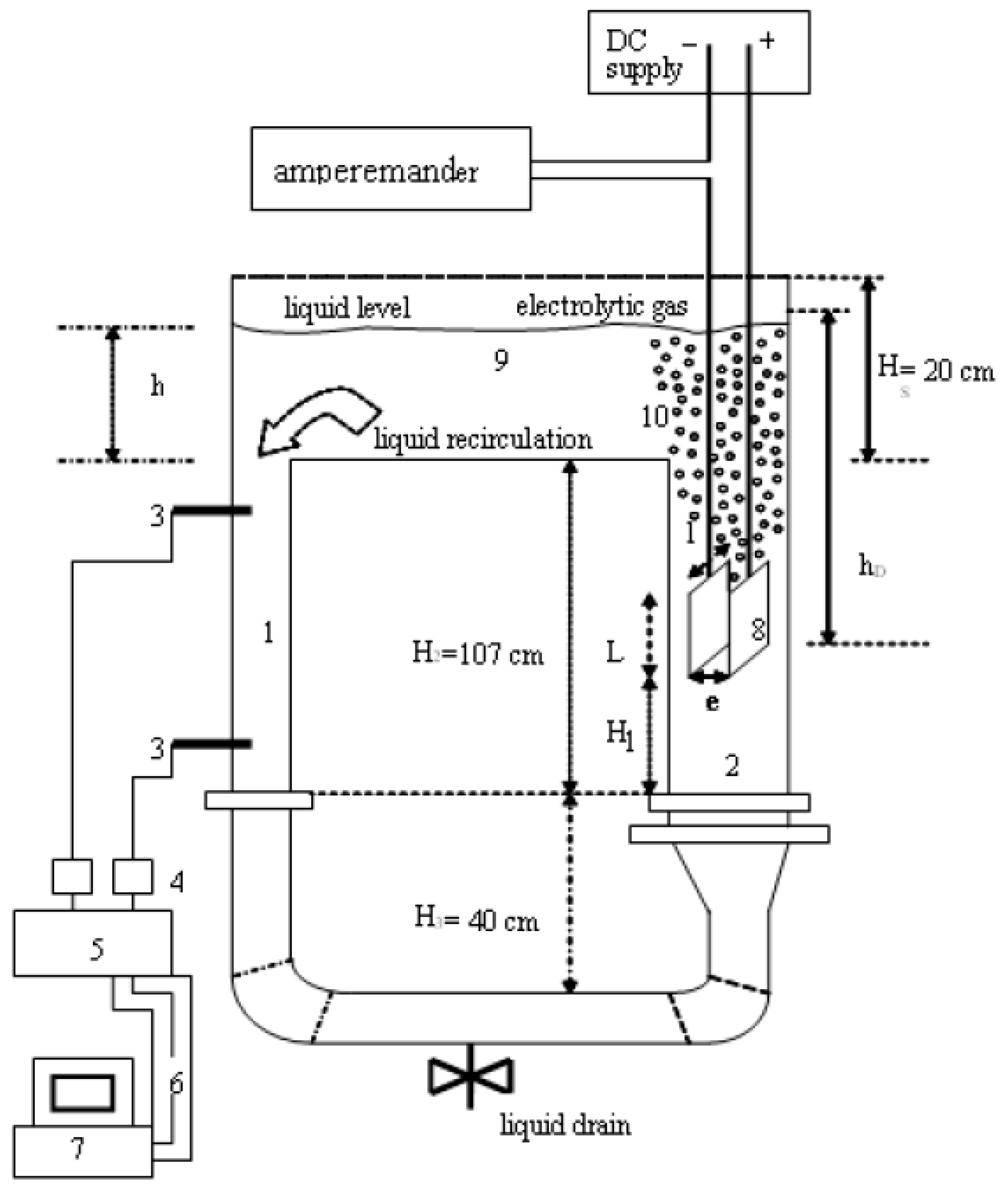

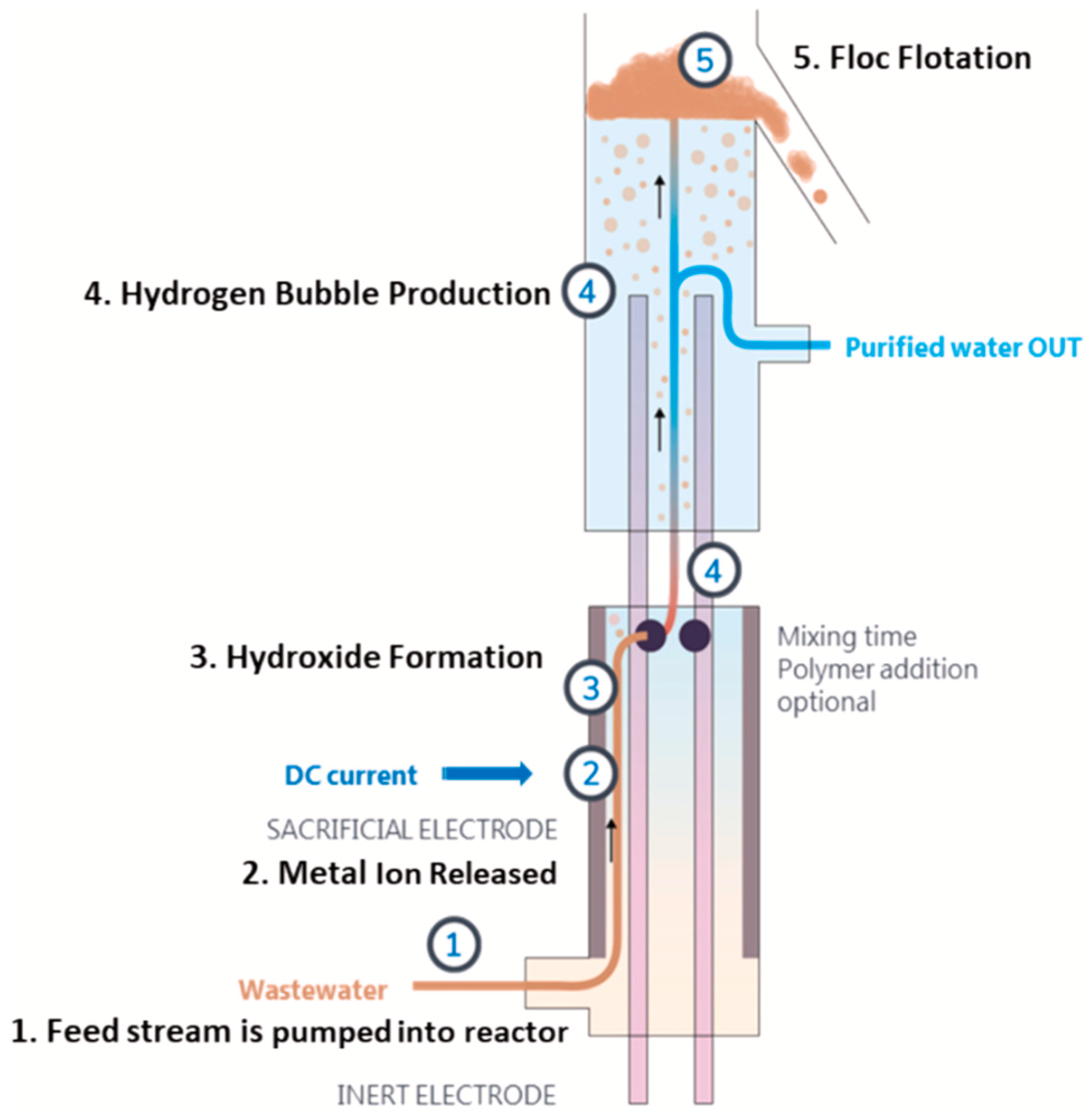
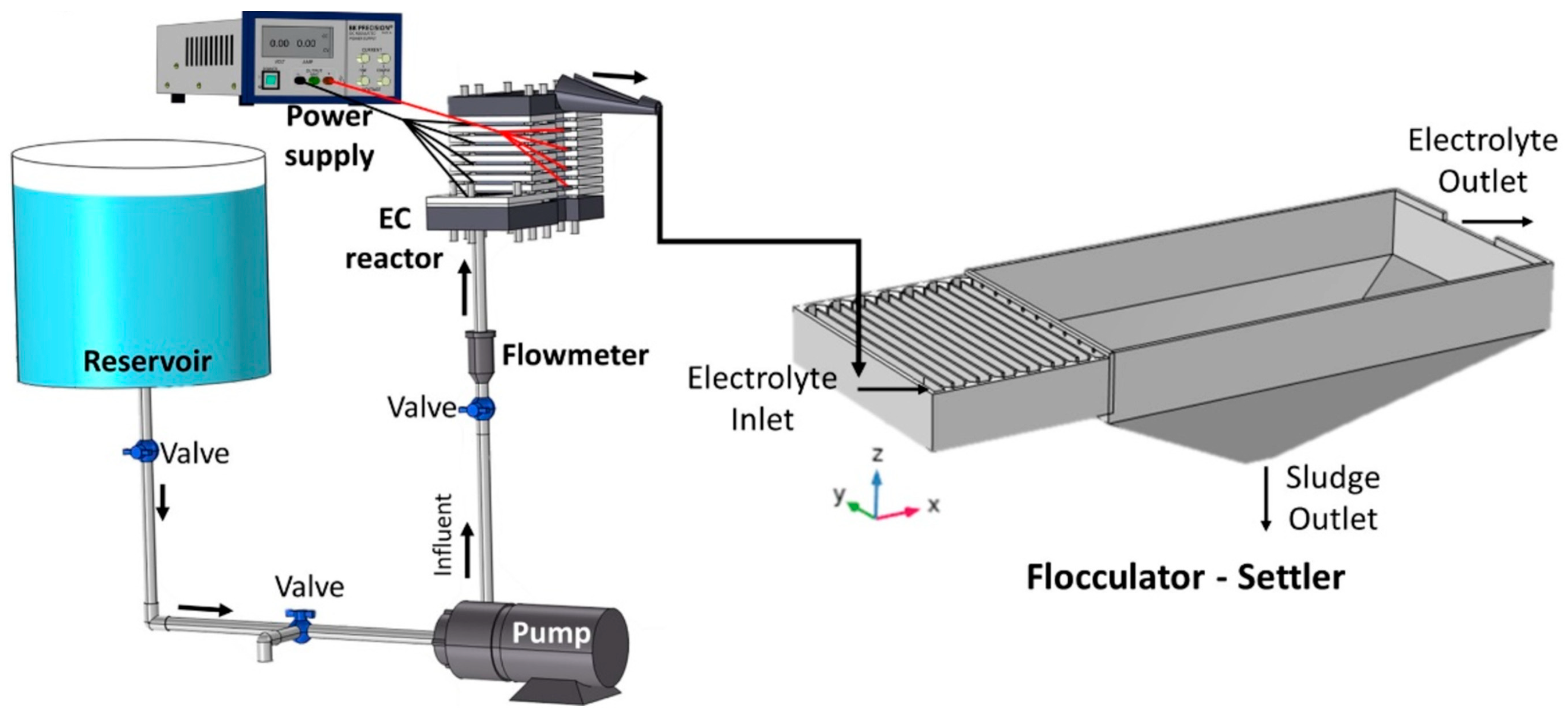
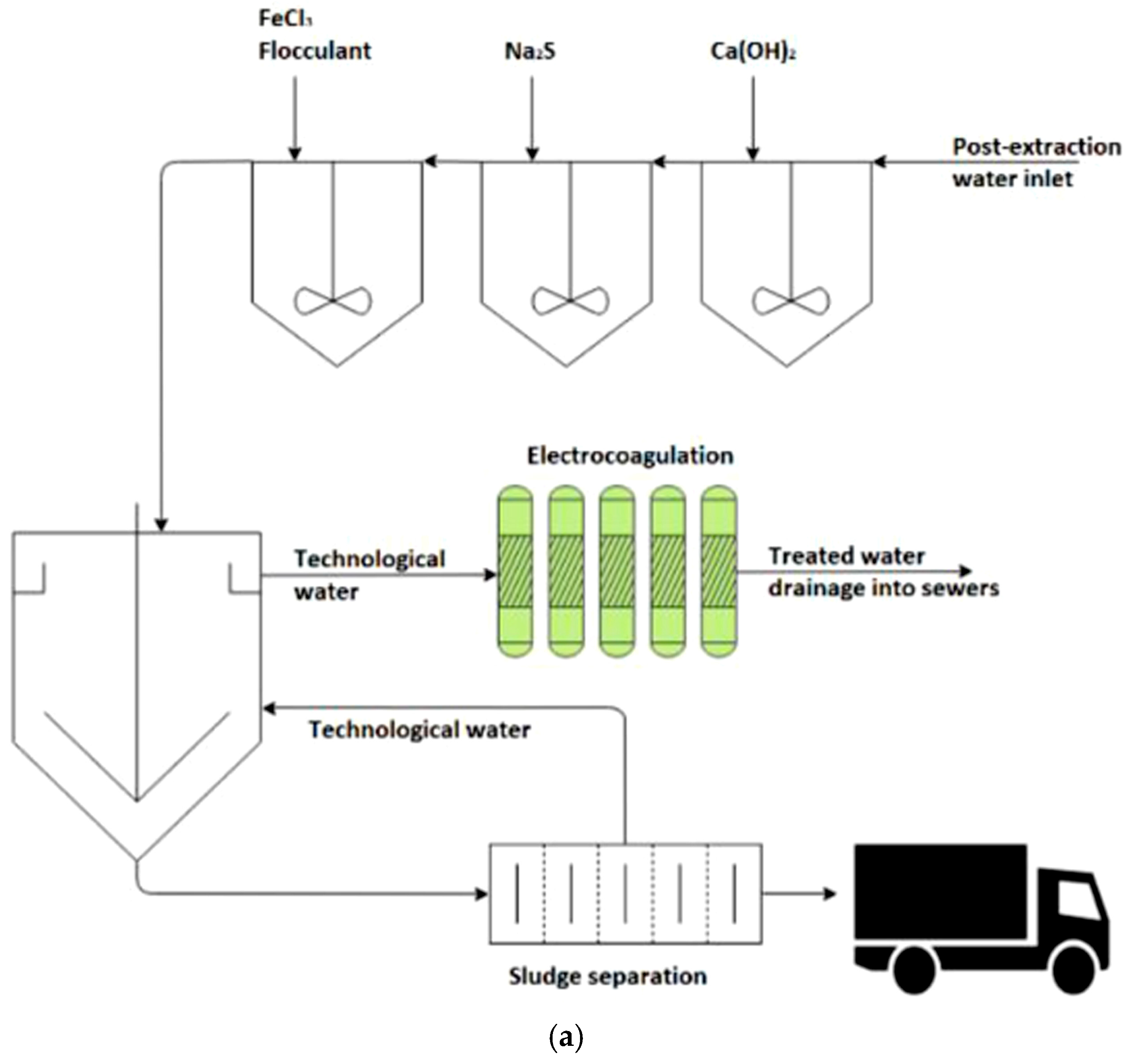

| Waste Water/Pollutants | Design and Operational Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Electrodes | Reactor Design/Electrodes’ Geometry | Applied Current Density (2) (mA/cm2) | pH, T (°C), ECT (min) | Flow Rate (3) (mL/min) | R (%) | E (kWh/m3) m (mg/L) (4) | Operational Cost (5) | Ref. | |
| Turbidity from surface water | Al–Al | A glass container of 8.7 L volume and 20 Al electrodes with 99.5% purity and dimensions of 50 cm × 2 cm × 0.2 cm. | V = 30 V | 30 min | 300 | 97.26 | - | 0.087 $/m3 | [41] |
| Dissolved phosphorus from wastewater | Al–Al | Three columns connected with horizontal PVC tubing. The effective volume is 2.78 L. Two Al plate electrodes were used with an effective area of 132.1 cm2. | 2 | 10 min | 4–600 | Orthophosphate = 99.9 Total-phosphate = 88.1 | m: 1.0 mg/L | 0.056 CAD/m3 | [42] |
| Iron from drinking water | Al–Al | The EC contains drilled plates (4 electrodes) to mix the solution without using external mixers. | 1.5–4.5 | pH: 4–10 10–50 min | - | 99.9 | - | 0.78 $/m3 | [43] |
| Fluoride from water | Al–Al | Two Al anode and cathode (950 mm × 200 mm × 3 mm) in a Perspex reactor (1000 mm× 210 mm × 48 mm). | 12.5–50 | pH: 4–8 | 150–400 | 99 | - | 0.36 AUD/m3 | [44] |
| Arsenic removal from groundwater | Al–Fe | 2 or 4 Al and Fe electrodes (14 cm× 13 cm× 0.3 cm) with an active surface area of each electrode of 136 cm2. | Al: 9.2–56.5 Fe: 12.0–57.8 | 60 min | 208 | Al: 52–89 Fe: 46–96 | - | 0.012–0.15 €/m3 | [45] |
| Chromium from tannery wastewater | Fe–Fe | Plexiglas cylindrical column (ID = 6.0 cm and H = 53 cm) and 1700 mL volume. Fe rod anode (D = 1.15 cm and L = 8.3 cm) of surface area of 30 cm2. Fe cathode (4.0 m) was wrapped around the anode at a distance of 3.0 cm. | 10 | pH: 8 34 min | 50 | 99.94 | - | - | [46] |
| Removal of heavy metals from leachate wastewater | Al–Fe | Continuous-flow EC reactor. | 1.1 | pH: 11 50 min | 40 | Mn3+: 99 | - | 0.21 $/m3 | [47] |
| Removal of Cr(VI) from Brackish groundwater | Fe–Fe | A novel EC column with an iron rod anode and helical cathode. | 3.04–15.21 | T: 25 °C. | 30–90 | 30–100 | E: 0.75 m: 0.185 mg/L | 0.03 US$/m3 | [48] |
| Removal of arsenic from drinking water | Fe–Al–Al–Fe | A cylindrical EC reactor with 2 anodes and 2 cathodes with different combinations of hybrid plate’s using monopolar series mode. | 0.25 | 3–20 min | 50–200 | ≥99.7 | - | 0.009–0.060 €/m3 | [49] |
| Removal of nickel from synthetic wastewater | Al–Al | The EC reactor is made of Pyrex glass with two Al plates (305 mm × 46 mm × 2 mm) of 120 cm2 effective surface area and 10 mm gap. | 0.25–2.75 | pH: 3–8 10–30 mL/min | - | 95–98 | - | - | [50] |
| Nitrate removal from water | - | 8 | pH: 8 | 50 | 61.7 | - | 1.278 US$/g | [51] | |
| Removal of toxic metals from real smelting wastewater | Fe–Fe | Batch with recirculation EC cell. | 10–20 | 120 min | 100 | Zn2+: 99.93 | E: 14.76 m: 2.09 | 2.2 US$/m3 | [52] |
| Arsenic from ground water | Fe–Al | A horizontal-flow continuous EC reactor with a capacity of 300 dm3/day. The hybrid Fe–Al electrodes were positioned normally to the flow. | 0.198 | T: 16.1 °C 25 min | 82 | 0.0339 kg Fe/m3 0.0145 kg Al/m3. | 0.182 €/m3 | [53] | |
| Nitrate removal from water | 2.4 L Plexiglas with 8 Al electrodes (100 mm × 70 mm × 1 mm) connected in a monopolar parallel mode with 10 mm gaps. | 4.5–10.5 | pH:2–10 | 40–120 | ≥60 | DC: m: 19.96 g Al/g nitrate removed AC: 4.96 g Al/g nitrate removed- | DC: 54 US$/kg AC: 9 US$/kg | [54] | |
| Fluoride remediation from groundwater | Al–Al | 14 Al electrodes with 5 mm gap and perpendicular to the direction of flow. The total electrode surface was 840 cm2. | 5,7.5–10 | pH: 8.2 | - | 70.1–81.0 | E: 0.92–1.95 | 0.12–0.26 €/m3 | [55] |
| Fluoride and hydrated silica from groundwater | Al–Al | An up-flow EC reactor with 6-cell stack in a serpentine array, opened at the top of the cell to favor gas release. | 4–7 | 1.2 ≤ u ≤ 4.8 cm s−1 | 96 | E: 2.48 | 0.441 US$/m3 | [56] | |
| Arsenic in simulated groundwater | Fe–Fe | 10 L EC reactor divided into two cells (26.0 cm × 13.0 cm × 18.0 cm) and 14 electrodes (7A and 7C) arranged in a vertical manner and parallel to one another with 1 cm gap. | 2 V | pH: 6 | 5–10 L/h | - | - | - | [57] |
| Metallurgical industry wastewater | Al–Fe | Two parallel horizontal electrodes (three active electrode pairs were used). The first electrode pair has a hole at bottom, through which water flows between the plates, over the second plate, which is set to a lower height than the first plate. | 6–18 A | pH: 8.9 | 135 L/h | 90–95 | 0.2–0.5 kWh/m3 | 0.04–0.09 €/m3 | [58] |
| Removal of fluoride from water | Al–Al | Four compartments: The first contains the electrodes and is connected to the inlet; a second receives the water from the first and is connected to a third with a 1.5 cm × 12.5 cm gap in the bottom of the reactor to avoid the floating solids from the second compartment passing directly to the reactor outlet. Four Al electrodes, 15 cm × 10 cm × 0.2 cm, with a DC power supply. | 88.3 mA | 73.6 | 85 | - | 0.05 €/L | [59] | |
| Removal of different ions from saline lake water | Al–Al | EC continuous reactor with rotating impeller plate anode. | 2 | pH: 8 40 min | 0.25 | SO: 90.2 Cl−: 93.4 Br−:90.8 TDS: 92.3 | - | US$0.1766 | [60] |
| TDS from Oily wastewater | Al–Al | 2.5 L plastic EC reactor with 3 concentric electrode tubes. The cathode was located in between the outer and inner anode. | 0.63–5.0 | 4–60 | 50–150 | - | [61] | ||
| Turbidity Removal from a Model Solution | Al–Fe Fe–Fe | 6 cells in series, coupled to a flocculator and a clarifier, each cell composed of a cylindrical Al or Fe anode and a solid SS rod as cathode with a DC power supply. | 3–9 V | - | - | 82.29 | 0.7142 kWh/m3 | [62] | |
| Nickel from industrial wastewater | Al–Al | Four equal-sized semi-continuous stirred-tank reactors of 200 L volume. Each reactor is equipped with two pairs of parallel flat electrodes: 125 mm × 190 mm arranged transversally to the main flow, with a useful area of 0.095 m2. | 6.26 | pH: 8.40 T: 50 °C 92 min | 2 | 97.8 | - | $1.008/kg | [63] |
| Sanitary landfill leachate | Al–Al | The EC cell consisted of 12 Al plates (100 mm × 18 mm × 2 mm), arranged in a monopolar parallel plate configuration, including 6A and 6C. | 10–50 | 30 min | 50–400 | NH3-N: 13.25–22.11 | - | - | [64] |
| Zn in an outlet stream from waste incineration plant | mild steel | 5 cells in parallel made of PVC tube (D = 200 mm, L = 1200 mm) with conical bottom and inlet of water. The 7 anodes and 7 cathodes are made of mild SS with dimensions of 960 mm × 100 mm × 6 mm. | 1.4–2 V | - | 1200–1400 L/h | ≥90 | 0.75–1.1 kWh/m3 | 1.6 EUR/m3 | [65] |
| Partial desalination of brackish peat water | Al–Al | An EC unit with reactor (36 cm × 20 cm × 20 cm) segmented into four main chambers for (i) brackish peat water, (ii) EC reaction, (iii) filtration, and (iv) treated water outlet. 10 Al electrodes were used (20 cm × 15 cm × 0.1 cm). | 1–5 A | - | 0.4–2 | Salinity: 91.78 | - | USD 0.06/m3 | [66] |
| Recovery of metals from cyanide leachates | Al–Al | A 500 mL acrylic and Al electrodes with an effective area of 30 cm2 (3 A and 3 C). The cyanide solution is recirculated through the cell a by peristaltic pump. | 15.2 | pH: 11 56 min | 40 | Gold: 66.3 Silver: 85.8 Copper:45.3 | - | - | [67] |
| Removal of molybdate | Fe–Fe | A 2.26 L baffled continuous-flow reactor with 8 Fe electrodes with 0.9 cm gap. | 0.25 | pH: 7.3–9.2 1.5 h | - | 83.4 | - | 0.172 US$/m3 | [68] |
| Removal of nitrates in drinking water | Mg-Zn | The EC cell was a 365 cm3 volume with 5 mm homogenization tabs for water. It used cylindrical Mg anode/Zn cathode electrodes (Mg: 1.8 cm × 15.2 cm, Zn: 1.1 cm × 15 cm) placed with a 0.5 cm gap and an effective anode area of 81.7 cm2. | 0.6 A | 86.81 min | 2.9 mL/min | 93.15 | - | 2.65 US$/m3 | [69] |
| Heavy metals from plastic plating wastewater | Iron-SS | The EC unit equipped with multi-rod helical systems made of an iron anode and a SS cathode. | 1000–1100 A | - | 18 m3/h | >99 | 7200 kWh/month | 2.87 US$/m3 | [70] |
| Nickel, chromium, and iron removal from mine water | Al–Al | The EC reactor contained 12 Al plates (23 cm × 20 cm × 0.2 cm), arranged monopolar with an interdistance of 3 cm. The plates were immersed to a depth of 14.8 cm with a total area of 592 cm2. | 3.378–10.135 | 45 min | 0.3–1 L/min | Cr: 99.51 | - | - | [71] |
| Fluoride removal from tap water | 2.5 L EC reactor with up to 10 electrodes separated by 1.5 cm, and an active surface of 105 cm2. | 4.7 to 9.5 | T: 20 °C. | 20 L/h | 79 | 0.253 and 0.405 kWh·m−3 | EUR 0.154/m3 | [72] | |
| Iron and sulfate from rejected water from a reverse osmosis | Al–Al | Rectangular EC with 2–4 Al plates. | - | - | 600 and 1000 L/h | Sulfate: 47 Iron: 79 | - | - | [73] |
| Chromium from synthetic wastewater | Fe–Fe | The 16 cm EC column contains a central iron rod anode (D = 1.4 cm) surrounded by a cylinder of SS mesh as the cathode. | 50–200 mA | 5–60 min | 0.02–0.14 L/min | >99 | - | - | [74] |
| Cr(VI) contaminated water | Fe anode | Flow-through EC reactor: 77 cm tall 5 cm radius, sand medium (porosity 0.31), electrode distance 8 cm. | 0.036 | pH: 5 1–2 min | 0.375 | ~100 | - | - | [75] |
| Synthetic wastewater with Zn2+, Cu2+, Ni2+, Cr3+, Cd2+, Co2+ | Fe anode, SS cathode | 12 cells in ladder series; concentric design with 2 mm gap; 154 cm2 surface area/cell; total cell volume ≈ 4.5 L each | 1.0–5.5 A; 6.5–35.7 | pH: 7 6.4–30 min | 1.9 m3/h | Zn, Cu, Cr, Ni: 99 | - | - | [76] |
| Waste Water Type | Combined Process | EC Process | Removal (%) | Energy Consumption/Cost | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Electrodes Information ** | Operational Parameters | |||||||||
| Type | No. | Arrangement | Current Density (mA/cm2) | ECT (min) | Flow Rate mL/min | |||||
| EC as a pretreatment step * | ||||||||||
| Industrial wastewater | EC/MF | Fe–Fe (SS) | 4A–4C | Bench-scale. | 5–10 | 5–20 | 3–10 L/h | Se: 98.7 As: 99.9 Cu, Pb ≥ 98 Zn, Cd ≥ 99 | - | [77] |
| Synthetic municipal wastewater | EC/UF | Fe–Fe | 1A–1C | EC/UF integrated in a single reactor. | 1 V/cm | 15 On 45 Off | - | PO4-P: 98 | - | [78] |
| Real gray water | EC/UF | Al–Al | 1A–1C | EC reactor with Al sheet electrodes. | 12 V | 15 On 15 Off | - | TSS:100 NH3-N:77.8 PO4−3: 94.3 | - | [79] |
| Groundwater | EC/FP | Al–Al | 3A–4C | Monopolar configuration of electrodes with propylene separator. | 4–6 | - | 0.91–4.55 cm/s | As: 100 | 3.9 kWh/m3 | [80] |
| Groundwater | EC/FP | Al–Al | 2 plates | Two polyethylene plates, two SS plates with four Al electrodes separated by polypropylene. | 6 | - | 0.23 cm /s | - | 6.7 kWh m−3 0.14 USD m−3 | [81] |
| Produced water | EC/UF/MD/C | Al–Fe | 5 electrodes | Bipolar series configuration. | 1–3 A | 5 | 1.5 | [82] | ||
| Gray water | EC/UF | Al–Al | EC followed by SMBR system. | 0.64, 1.64 V/cm | 15 On 15 Off | - | NH3–N: 86.1 TP: 80–96.5 | - | [83] | |
| Semiconductor industry wastewater | EC/MF/NF | Al–Al | 2 electrodes | EC with ceramic microfiltration and nanofiltration. | 50–400 C/L, pH = 4.3 | - | - | Nitrate: 95.8 | - | [84] |
| Manure wastewater | EC/Flo/RO | Al–Fe | 1A–1C | Tubular electrolytic cell composed of two concentric tubular electrodes positioned vertically. The outer is Al anode, and the inner is SS cathode. | 16 pH = 8.2 | 23 Sec | 1–20 L/h | Silica: 88 | 1.75 €/m3 | [85] |
| Gray water | EC/SF/ACA | Al–Fe | 2A–2C | Continuous modes and different electrode arrangements of (Al–Fe–Al–Fe), (Fe–Al–Fe–Al), (Al–Al–Al–Al) and (Fe–Fe–Fe–Fe). | 12 V | - | 0.05–0.1 | 85–90 | 0.5 to 4.75 KWh/m3 | [86] |
| Drinking water | EC/F | Al–Fe | 1A–1C | Pilot-scale system using Al and Fe electrodes. | 12 | 5 | 1 | As: 99 | 0.795$/200 L (Al electrodes) 0.223$/200 L (Fe electrodes) | [87] |
| Textile wastewater | EC/F/RO | Fe–Fe | 2A–2C | 4 Fe electrodes connected as monopolar in serial connections. | 50–600 mA | - | - | Color: 99 | - | [88] |
| Drinking water | EC/Flu/S | Al–Fe | 5Al/C 2Fe/A 2Al/A | The EC reactor includes Al–Fe hybrid plate electrodes as sacrificial anodes. | 3–5 | Flu: 15 S: 160 | 1.31 < u < 5.26 cm s−1 | As: 100 F-: 82.8 SiO2: 98.6 | 0.41 USD m−3 | [89] |
| Gold processing wastewater | EC/UV/OO | Gr–Al–Fe–SS | 2A–2C | Fe–SS sacrificial anodes and Gr–Al cathodes. | 15 pH = 10 | 60 | 30 | CN: 100 Ni:73 Cu: 100 Zn: 78.8 | - | [90] |
| Salt-lake brines | EC/NF | Al–Fe | 1A–1C | The EC with metal sheets with a reaction area of 40 cm2. | 10 | - | - | - | [91] | |
| EC as a post-treatment step * | ||||||||||
| Copper smelting wastewater | IE/El/EC | Fe–Fe | 1A–1C | The EC reactor has a mild steel anode and stainless cathode positioned vertically and parallel to each other. | 15 | 30 | - | Ar: 91.4 SO4−2: 37.1 Using EI, Ar: 58.2 SO4−2: 72.7 | 0.82–3.13 kWh/kg | [92] |
| Tungsten smelting wastewater | CC/EC | Al–Fe | 6A–7C | The cathode and the anode plates were inserted vertically into the wastewater. | 15–50 | - | 250–500 L/h | F− from 66 to 128 mg/L to <10 mg/L | 0.99–1.51 USD/m3 | [93] |
| Waste Water Type | Pollutant | Process Type | Model Used | Key Parameters | R2 or ARE | Ref. |
|---|---|---|---|---|---|---|
| Underground volcanic spring water | F− | Standalone | Variable-Order Kinetic | Power-law relationship between concentration and residence time | R2 > 0.99 | [55] |
| Nickel production plant effluent | Standalone | Multi-branch tanks-in-series RTD model and Conversion Time Kinetic (CTV) | RTD: mean residence time = 92 min CTV: time to complete conversion = 23.3 min chemical reaction control | R2 = 0.98.1 ARECTV = 2.23% | [63] | |
| Industrial wastewater | Fe+3 | Standalone | CFD (two-phase H2O–H2 flow, Fe3+ generation, RTD) | Mean linear inflow velocity 2.56–2.63 cm/s Current density 4–8 mA/cm2 | ARERTD < 4% | [100] |
| Mine water | Ni+2 | Standalone | First-order kinetic | K = 0.0211 min−1 | R2 = 0.8901 | [71] |
| Cr+3 | Second-order kinetic | K = 0.0799 min−1 | R2 = 0.4921 | |||
| Fe+3 | Second-order kinetic | K = 0.0001 min−1 | R2 = 0.9898 | |||
| Synthetic with high ammonium nitrate | Cr+6 | Standalone | Advection–Dispersion–Reaction | Flow rate (mL/min), Current (mA), Cr concentration (mg/L), Reaction rate constant k (min−1) | K: 0.11–23.2; E: 1.3 × 10−6–2.4 × 10−1 | [74] |
| Drinking water (synthetic) | F− | Combined (EC/EF) | Langmuir–Freundlich | qmax = 0.75 ± 13 mmol/g, kLF = 1600 ± 9.8 (L mol−1)−n, N = 1.15 ± 0.03 | 0.998 | [96] |
| Waste Water Type | Process Type | Model Used | Optimum Values of the Operating Parameters | Predicted Responses at Optimum Conditions | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Nitrate-contaminated water | Standalone | Taguchi design (L27 orthogonal array) | 100 mg/L | 50 mL/min | 80 A/m2 | pH 8 | 61.70% nitrate removal efficiency; 1.278 US$/g NO3− removed | [51] |
| Synthetic water (iron) | Standalone | BBD (RSM) | 10 mg/L | 3 mA/cm2 | Fe concentration | pH 7 | 50 min | [43] |
| Oily wastewater (petroleum effluent) | Standalone | BBD (RSM) | 60 min | 5 mA/cm2 | 50 mL/min | Final TDS 1842.54 mg/L (reduction of 307.46 mg/L); R2 = 97.99% | [61] | |
| Model water (high turbidity) | Standalone | Two-factor factorial design | 9 V | Aluminum anode | 82.29% turbidity removal; Energy consumption 0.7142 kWh/m3 | [62] | ||
| Simulated groundwater | Standalone | BBD (RSM) | pH 5.5–7.0 | 3.5–4.0 A/m2 | Electrode distance 0.5–0.9 cm | 83.4% Mo(VI) removal efficiency | [68] | |
| Groundwater (arsenic) | Standalone | Gradient Boosting Machine (GBM) | 4 Al electrodes | 9.14 V | 140 min | 9.73 μg/L As | [45] | |
| Acid | 7.5 V | 40 min | 9.97 μg/L As | |||||
| Drinking water well near landfill | Standalone | Factorial design (32) | 0.6 A | 2.9 mL/min | 86.81 min | 93.15% nitrate removal | [69] | |
| Water with Cr(VI) | Standalone | Simulation model (PHREEQC–MATLAB coupling) | 0.05–0.3 mA/cm2 | Design criterion for Cr(VI) removal (10–50 mg/L) | [75] | |||
| Manure treatment effluent | Combined/flotation | CCD (RSM) | 104 mg/L | 160 A/m2 | 23 s | pH 8.2 | 88% silica removal; fouling potential reduced by 28% | [85] |
| Design Innovation | Impact of the Innovation | Ref. |
|---|---|---|
| Larger working volume with enhanced mixing and drain positioned opposite to the inlet and bipolar configuration. | Real-scale application with improved contact between coagulants and contaminants. The drain position promotes uniform flow and sediment separation. Uniform electrode wear and simplified maintenance. | [55] |
| Modular continuous mode, effective aeration control, and adaptability to real, complex wastewater. | High potential for industrial-scale application in toxic metal remediation from real complex wastewater. | [52] |
| Drilled aluminum electrodes with opposite hole distribution. | Improved mixing efficiency, no need for external mixers, simple, more efficient, scalable, and cost-effective solution. | [43] |
| Rotating anode. | High removal efficiencies with significantly reduced energy consumption and aluminum usage. | [60] |
| A ladder series of 12 electrolytic cells, each with a narrow concentric gap between a low-carbon steel (iron) anode and a stainless steel cathode. | Enhances EC by improving mass transfer, reducing energy losses, and achieving high removal efficiencies. | [76] |
| Different Fe–Al hybrid electrode combinations. | Fe–Al–Al–Fe hybrid plate electrodes arrangement achieved 96% removal efficiency of arsenic. | [49] |
| EC column with a helical iron cathode wrapped around an anode rod. Air provided good mixing and enhanced the EC process. | Significantly improve mass transfer, mixing, and coagulant dispersion, resulting in higher contaminant removal efficiency and reducing energy consumption and operational instabilities. | [48] |
| Horizontal-continuous EC using a combined Fe–Al electrode in a monopolar–bipolar configuration. | Efficient arsenic removal under realistic conditions with the shortest run time (25 min) and lowest charge loading for the Fe–Fe–Al–Fe formation. | [53] |
| Flow-channel EC reactor with a six-cell herringbone array of aluminum electrodes and open to enable rapid release of hydrogen gas. | Simultaneous and efficient removal of fluoride and hydrated silica from groundwater with low operating costs and energy consumption. | [56] |
| CSTR EC reactor equipped with two pairs of parallel flat electrodes arranged transversely to the main flow and a dual propeller. | High nickel removal efficiency of 97.8% and an effective operating cost. | [63] |
| Heating-free continuous-flow CE process. | Higher pollutant removal efficiency with lower operating cost. | [64] |
| Continuous EC cell equipped with aluminum electrodes to overcome the significant challenge of short circuiting. | Prevent short circuiting, thus the reactor can achieve longer reaction times and increase metal recovery efficiency. | [67] |
| Continuous EC reactor with 20 interconnected channels in series and 20 aluminum electrode plates in a monopolar parallel configuration and internal glass plates acting as flow reversers. | Improves hydrodynamic conditions, especially at high flow rates, resulting in a flow pattern similar to plug-flow reactor, effectively reducing back-mixing and dead zones. | [41] |
| Continuous, full-scale system with five cells connected in parallel, two operating simultaneously, two as backup, and one additional spare. | Reduces zinc concentration to less than 0.5 mg/L. | [65] |
| A continuous cascade-type EC reactor using seven iron plates arranged horizontally with a serpentine flow path and is open at the top. | Facilitate the release of hydrogen bubbles. | [100] |
| A single-sided aluminum cathode with 27 fins strategically positioned between a pair of cylindrical aluminum anodes. | Increased the cathode surface and improved pollutant removal efficiency. | [61] |
| Continuous-flow EC reactor consists of nine aluminum electrodes arranged in a parallel, single-pole junction. | A turbidity removal efficiency of 82% and low electrical energy consumption of 0.7142 kWh/m3. | [62] |
| Continuous-flow EC reactor with flat-surfaced electrodes made of aluminum and iron, with reversing electrode polarity every 30 min. | Higher arsenic removal efficiencies with iron electrodes with pretreatment of the electrodes. | [45] |
| Reactor equipped with Al plates with strategically placed holes. | Facilitating the distribution of coagulants in water samples. | [73] |
| Concentric cylindrical design combined with enhanced mixing via bottom aeration. | Maximizes surface area, enhances EC efficiency, allows uniform electric field distribution, ensuring effective mixing and circulation of wastewater. | [46] |
| Compact, vertical EC reactor featuring a central iron rod anode and a surrounding stainless steel mesh cathode, arranged concentrically. | Ensure even electric field distribution and efficient ionic migration. Increases surface area, allowing for improved solution flow and mass transfer around the electrodes. | [74] |
| Multi-column system. | Enhances scalability, flow uniformity, and treatment efficiency. | [42] |
| The Combined System | The Design Innovation | Impact of the Innovation | Ref. |
|---|---|---|---|
| EC/FP | Tortuous flow path with multiple narrow channels. | Improves the efficiency and stability of the EC process and successfully overcomes electrode passivation and non-uniform coagulant dispersion. | [80] |
| EC/membrane | Integrating EC cell with microfiltration membranes. | Complete removal of fine particles and acts as a secondary adsorbent barrier with 98.7% removal of selenium during continuous operation. | [77] |
| IE/El/EC | The reactor is integrated with electrolysis (ED) and anion exchange membranes. | Complete arsenic removal, reduced chemical additives, lowered total dissolved solids (TDS), and minimized alkali needed to adjust pH in the electrolysis step, thus reducing sludge production. | [92] |
| EC/Eflo | External air-transfer reactor. | Eliminates the need for a mechanical system or external gas inputs. Thus, increasing the overall separation performance at a lower cost. | [96] |
| EC/FP | Combined filtration and flow reactor with three-cell stack and Al electrodes. | Effective removal of fluoride and arsenic simultaneously from real groundwater with low energy consumption. | [81] |
| CC/EC | Combining CC with EC in pilot plant. | The successful transition from laboratory experiments to a pilot plant demonstrates the practicality and industrial scalability of this innovative combined treatment. | [93] |
| EC/UF/MD/C | A novel multi-stage integrated treatment system combines EC, UD, and crystallization. | EC and ultrafiltration as pretreatment steps significantly reduce membrane fouling in the membrane distillation stage, thus increasing efficiency and minimizing cost. | [82] |
| EC/membrane | EC process as a pretreatment step with a submerged membrane bioreactor. | Minimizes energy use, reduces membrane fouling, improves system stability and efficiency, lowers coagulant chemical usage, extends membrane lifespan, reduces maintenance and replacement costs while achieving high removal efficiency. | [79] |
| EC/membrane | Efficient integration of EC with ceramic membranes. | Effective way to treat one of the most challenging wastewater streams. | [84] |
| EC/Flo | Axial cylindrical electrocoagulation-flotation reactor for improved compost processing. | 88% silica removal, reactor extended the life of the reverse osmosis membrane by reducing the likelihood of fouling. Thus, reduces costs. | [85] |
| EC/Filtration/ACA | Integration of EC with filtration and adsorption processes with different electrode configurations. | The integrated EC/filtration/adsorption system with Al–Fe–Al–Fe electrode demonstrated superior cost-effectiveness. | [86] |
| EC/Flo/membrane | Combined EC with flotation and membrane processes. | Incorporation of EC as a pretreatment step proved significant in improving the overall performance and sustainability of the membrane-based systems. | [88] |
| EC/Flu/S | A laboratory-scale EC–flocculation–sedimentation flow plant. | Efficient coagulant generation, improved mixing and turbulence, and effective coagulant formation and sedimentation, resulting in improved contaminant removal, uniform flow distribution, continuous and scalable treatment performance with improved retention times for both coagulation and sedimentation. | [89] |
| EC/F | EC unit with ultrafiltration membrane filter. Two pilot-scale versions using iron and aluminum electrodes. | 99% arsenic removal in just 5 min. | [87] |
| EC/UV/OO | Synergy between EC, UV, and ozone oxidation. | Almost complete removal of cyanide and copper, which is difficult to achieve with conventional methods alone. Reduced cost and environmental impact. | [90] |
| EC/photocatalysis | EC with titanium dioxide-based photocatalysis. | Provides a more robust and effective treatment strategy for treating both organic and inorganic simultaneously. | [101] |
| EC/NF | Hybrid system combining EC and novel bilayer NF membranes for the separation of lithium (Li+) and magnesium (Mg+) with high selectivity in complex salt-lake brines. | Improves separation selectivity and reduces fouling on the membrane, enabling more efficient and stable operation. | [91] |
| Pollutant/Wastewater | Scale and Reactor Type | EC Performance Achieved | Energy Consumption and Cost | Notes on Scalability | Ref. |
|---|---|---|---|---|---|
| Landfill Leachate (mixed inorganics) | Pilot-scale, EC + wetland hybrid (Fe electrodes) | 79% COD, ~90% BOD, high TSS removal in leachate; significant ammonia and metal reduction | EC stage energy: on the order of ~10 kWh/m3; Treatment cost shared with passive wetland. | Scale-up successful in continuous mode; required electrode cleaning due to scaling. | [102] |
| Cadmium-heavy Wastewater (synthetic) | Lab scale vs. optimized bench (Fe anode) | 100% Cd2+ removal achieved in lab with optimized design; operating cost ~$0.06 USD/~1 L (vs. $2.1 USD via CC) | Energy: low per L in lab (high efficiency); Cost: ~$1.7/m3 for EC vs. $3.5/m3 chemical in a study. At small scale: ~$3 per 1000 L observed. | EC could be more cost effective than CC for heavy metal removal. Extrapolation suggests ~$1.7–3/m3 for scaled operations, assuming power and electrode costs optimized. | [104] |
| Colored Agro-Wastewater (Olive mill) | Lab vs. Pilot (Al electrodes, batch vs. continuous) | Lab: 50% COD removal, 100% decolorization; Pilot: 42.5% COD, 85.3% color removal | Lab energy: ~55–62 kWh/m3 for ~1 h treatment (Fe vs. Al electrodes); Pilot energy: slightly higher due to longer treatment needed for similar removal. | There is a need for better mixing/retention at pilot. Higher energy per removal noted at pilot (less efficient kinetics). Underscores importance of reactor design in bridging lab-to-pilot gap. | [27] |
| Plating/Electroplating Waste (multi-metal) | Full-scale in-plant unit (Fe plates) | Metals (Cr, Ni, Zn) reduced from tens of mg/L to <1 mg/L (>95–99% removal, meeting discharge limits) | Energy: ~5–15 kWh/m3 (estimated, high conductivity lowers cell voltage); Electrode consumption: significant (regular replacement of Fe anodes). | Industrial deployment achieved for heavy metals. Required robust design (corrosion-resistant reactor, H2 venting) and automatic pH and current control. Demonstrated reliable continuous operation with modular plate reactors; integration allowed water reuse. | [103] |
| Fluoride-contaminated water (synthetic) | Pilot-scale continuous-flow reactor with monopolar Al electrodes | 89–99% fluoride removal depending on current density and initial concentration | Specific Electrical Energy Consumption (SEEC) evaluated; current densities 12.5–50 A/m2; operational voltage 0–18 V. | Demonstrated continuous-flow effectiveness; fluoride removal is efficient with optimal current density and pH (6–8); sludge characterized by Al(OH)3 formation. | [44] |
| Nickel-rich industrial effluent | Semi-continuous 4 stirred-tank EC reactors | Maximum 97.8% Ni removal; typical 99.7% at 11.0 mA/cm2 | SEC = 0.5–4.4 kWh/kg Ni; operating cost ~$1/kg Ni; current 18–24 A. | Extensively studied scale-up with RTD and kinetic modeling (CTV); high reproducibility and industrial applicability. | [63] |
| Post-extraction water from waste incineration plant | Full-scale, 5 EC cells with mild steel electrodes (monopolar, parallel) | Zn consistently reduced to <0.5 mg/L (below regulatory limit) | 0.75–1.1 kWh/m3; ~1.6 EUR/m3 including electrode replacement. | Full-scale implementation successful; continuous operation with periodic electrode regeneration. | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qodah, Z.; AL-Rajabi, M.M.; Da’na, E.; Al-Shannag, M.; Bani-Melhem, K.; Assirey, E. Continuous Electrocoagulation Processes for Industrial Inorganic Pollutants Removal: A Critical Review of Performance and Applications. Water 2025, 17, 2639. https://doi.org/10.3390/w17172639
Al-Qodah Z, AL-Rajabi MM, Da’na E, Al-Shannag M, Bani-Melhem K, Assirey E. Continuous Electrocoagulation Processes for Industrial Inorganic Pollutants Removal: A Critical Review of Performance and Applications. Water. 2025; 17(17):2639. https://doi.org/10.3390/w17172639
Chicago/Turabian StyleAl-Qodah, Zakaria, Maha Mohammad AL-Rajabi, Enshirah Da’na, Mohammad Al-Shannag, Khalid Bani-Melhem, and Eman Assirey. 2025. "Continuous Electrocoagulation Processes for Industrial Inorganic Pollutants Removal: A Critical Review of Performance and Applications" Water 17, no. 17: 2639. https://doi.org/10.3390/w17172639
APA StyleAl-Qodah, Z., AL-Rajabi, M. M., Da’na, E., Al-Shannag, M., Bani-Melhem, K., & Assirey, E. (2025). Continuous Electrocoagulation Processes for Industrial Inorganic Pollutants Removal: A Critical Review of Performance and Applications. Water, 17(17), 2639. https://doi.org/10.3390/w17172639








