Abstract
The proportion of neutral and weakly alkaline high-sulfate mine water in China is over 50%, resulting in the problem of high treatment costs. Low-cost, sustainable, and non-secondary pollution remediation technologies for in situ application in underground coal mines have rarely been reported. Here, the mixed packed and layered packed SRB-PRB (sulfate-reducing bacteria-permeable reactive barrier) column experiments at a flow speed of 300 mL/d using low-cost corncob as a carbon source were conducted to simulate sulfate in situ remediation in goafs. The column experiments utilized the simulated weakly alkaline mine water, with an initial sulfate concentration of 1027.45 mg/L. The results showed that during the 40 d operation, the SO42− removal kinetics included three stages: rapid reduction (0–6 d), stable reduction (6–16 d), and reduction attenuation (16–40 d). Corncob could provide a relatively long-term carbon source supply, with the maximum average removal efficiency of 65.5% for the mixed packed column and 56.6% for the layered packed column. A large number of complex organic-degrading bacteria were detected in both the effluent water samples and the solid packed media, while SRB became dominant only in the solid packed media. However, the low-abundance SRB could still maintain a high-efficiency SO42− reduction, due to the supply of readily utilizable carbon sources provided by hydrolytic and fermentative bacteria. This indicated that the synergistic effect between SRB and these organic matter-degrading bacteria was the critical limiting factor for SO42− removal. The microscopic characterizations of SEM-EDS (scanning electron microscopy and energy-dispersive spectroscopy) and FTIR (Fourier transform infrared spectroscopy) confirmed the damage of functional groups in corncobs and the generation of SO42− removal products (i.e., FeS). The engineering application schemes of the SRB-PRB under both in-production and abandoned mining scenarios were proposed. Additionally, the material cost estimate results showed that the SRB-PRB could achieve in situ low-cost remediation (0.2–1.55 USD/m3) of the characteristic pollutant SO42−. These findings would benefit the engineering application of in situ microbial remediation technology for high-sulfate mine water.
1. Introduction
China’s coal output accounts for half of the world’s output, and coal resource exploitation is dominated by underground coal mines. Mining activities inevitably disturb the roof and floor aquifers of the coal seam. To ensure safe production, a large amount of mine water must be discharged, with approximately 7 billion m3/a output in recent years [1]. Currently, high-sulfate mine water (SO42− concentration > 250 mg/L) is widespread in coal mines. Sun et al. (2025) [2] reported that among 314 coal mines in China, 210 discharge high-sulfate mine water, accounting for 67%. It is worth noting that well-known acid mine drainage (AMD, pH < 6) only accounts for 20% of high-sulfate mine water. Therefore, the remediation of neutral and weakly alkaline high-sulfate mine water, which has a relatively high proportion, deserves more attention. The formation mechanisms of high-sulfate mine water primarily include physical processes (leaching and dissolution of sulfur-bearing minerals, evaporation, and concentration), chemical processes (chemical oxidation/reduction and hydrolysis of metal sulfide minerals associated with coal), and microbial processes (actions of sulfur-oxidizing bacteria and sulfate-reducing bacteria) [3,4,5]. High-sulfate mine water could corrode metal mining equipment and backfill materials underground, while its discharge could lead to ecological damage such as increased salinity in surface water and soil salinization [6]. Moreover, after mine abandonment, the water level rises and establishes hydraulic connections with adjacent aquifers, resulting in long-term contamination of regional groundwater. Therefore, the low-cost and high-efficiency treatment of characteristic pollutants in mine water is an urgent requirement for achieving sustainable mining development.
For the treatment of high-sulfate mine water, various treatment technologies have been developed, mainly including physical, chemical, and biological methods [7]. Most physical and chemical treatment technologies are ex situ treatments, mainly including reverse osmosis, nanofiltration, ion exchange, electrodialysis, etc. [8]. Although these technologies are well-studied, they require continuous membrane regeneration or chemical dosing, resulting in high capital investments (generally exceeding 14 million USD) and operational costs (~3 USD/m3), referring to actual data statistics of typical coal mines in China. In contrast, biological treatment technologies leverage the continuous proliferation and metabolic activity of microorganisms to transform and remove pollutants, offering significant advantages such as low operational costs, environmental friendliness, and strong process adaptability. Consequently, it has become a research hotspot in mine water treatment [9,10,11]. Sulfate-reducing bacteria (SRB) are widely used for the reductive removal of sulfate, which can directly reduce sulfates to sulfides, significantly reducing the sulfate load. Moreover, the hydrogen sulfide produced by SRB can react with various heavy metal ions to form stable metal sulfide precipitates (e.g., CuS, PbS, etc.), realizing the immobilization of metal pollutants [12]. In recent years, the application of SRB-loaded permeable reactive barriers (PRBs) for in situ remediation of sulfate-contaminated mine water has gained increasing attention [13,14,15,16]. For the application of in situ treatment of SO42− pollution in mine water of underground coal mines, two scenarios could be distinguished: in-production coal mines and abandoned coal mines. For in-production coal mines, single-stage or multi-stage SRB-PRB systems could be installed at the drainage outlets of goafs. For abandoned coal mines, underground mine spaces (such as goafs and abandoned roadways) could serve as natural SRB-PRB reaction zones. Pre-filling these zones with adsorbents and carbon sources before closure can stimulate indigenous SRB to metabolically reduce and degrade SO42−, thereby preventing AMD formation at the source and controlling groundwater SO42− pollution in mining regions. In situ SRB-PRBs in underground mines also have potential advantages, including long hydraulic retention time, reduced pumping energy consumption, saved surface space, and utilization of the stable temperature underground environment. However, current research has mainly focused on the screening, development, and optimization of SRB-PRB fillers in surface or shallow groundwater. In contrast, studies on the in situ SRB-PRB application in underground coal mine goafs are rarely reported.
In mine water treatment, SRB, as a kind of heterotrophic microorganism, are constrained by a carbon source. Coal mine water with a high SO42− concentration generally contains relatively low dissolved organic matter (DOM), resulting in the availability of additional carbon sources, which is the most critical limiting factor for SO42− reduction by SRB [17]. Numerous reported studies prefer short-acting and expensive carbon sources such as sodium lactate, ethanol, and methanol. Although these materials demonstrate high treatment efficiency at increased dosages, they are economically unfeasible [18]. Biomass waste serves as a superior alternative carbon source, as it not only eliminates pollutant loads but also concurrently treats biomass waste, achieving dual benefits. Sato et al. (2018) [19] used rice bran as the carbon source for SRB in a sulfate-reducing bioreactor to treat mine wastewater. With the initial SO42− concentration ranging from 245 to 380 mg/L, the maximum removal efficiency reached 77.8%. Rodrigues et al. (2019) [20] utilized chitin from shrimp shell waste as the substrate for SRB, demonstrating 99.75% removal efficiency after 30 days of treating mine water containing 400 mg/L SO42−. Dovorogwa et al. (2022) [21] used tobacco waste as both a metal cation adsorbent and a carbon source for SRB to treat AMD. During the remediation process, the pH value of AMD increased by 2.05, and the removal efficiencies of SO42−, Fe, Ni, Cu, and Zn were 70%, 95%, 97%, 70%, and 93%, respectively. Di et al. (2022) [22] prepared immobilized materials using SRB (10%), R. spheroids (10%), and lignite (3%) as the main components and conducted dynamic column experiments for AMD treatment. The experimental results indicated that the combination of R. spheroids and lignite could continuously provide carbon sources for SRB, with the removal efficiencies of SO42−, Cu2+, and Zn2+ being 93.97%, 98.52%, and 94.42%, respectively. However, it also caused secondary pollution of chemical oxygen demand (COD) with an average concentration of 428.20 mg/L. As mentioned above, most laboratory experiments have achieved high removal efficiencies within a short time through supplemental bacterial inoculum and carbon sources without considering cost or secondary pollution. However, there remains a lack of research simulating practical engineering applications in goafs. In particular, once the underground in situ remediation project in the goaf is completed, it is difficult to replenish or replace carbon sources and SRB inoculum. Moreover, improper addition of carbon sources may also lead to secondary pollution problems such as increased some poisonous organic compounds in the treated water, which may exacerbate water pollution. Therefore, it is essential to stimulate the native microbial habitat and identify low-cost, slow-release, and highly bioavailable carbon sources to maintain sustained SRB activity.
This study focuses on the high-sulfate mine water from the goaf of the MKQ coal mine in Ordos, a typical mining region in China. On the basis of indigenous SRB identification, PRB media selection, and slow-release carbon source screening, an SRB-PRB column experiment was constructed and operated to simulate sulfate remediation in the goaf. The treatment efficiency of two packed modes (mixed packed and layered packed) was compared, and the reduction products of SO42− were verified. Through 16S rRNA high-throughput sequencing of microbial communities in the reaction system, combined with microscopic characterization of functional groups, morphology, and elemental composition of slow-release carbon sources, the mechanism of sulfate removal by SRB-PRB systems was elucidated. Furthermore, we preliminarily designed an engineering application scheme tailored to actual site conditions. The findings provide novel insights for cost-effective in situ treatment of high-sulfate mine water.
2. Materials and Methods
2.1. Materials
2.1.1. Inoculum
This study targeted the high-sulfate mine water from the goaf of the MKQ coal mine in a typical mining area of Ordos. The coal mine water from the 3103 goaf was selected as the source for the enrichment and cultivation of SRB. The liquid postgate medium used in the enrichment process is as follows: KH2PO4 0.5 g/L, NH4Cl 1.0 g/L, Na2SO4 1.0 g/L, CaCl2 0.05 g/L, MgCl2·6H2O 2.0 g/L, yeast extract fermentation 1.0 g/L, Vitamin C 0.1 g/L, C2H5NaO2S 0.1 g/L, FeSO4·7H2O 0.5 g/L, and D-C3H5O3Na 1.1 g/L. During the separation and purification process, the solid postgate medium was used and prepared by additionally adding 15 g of agar to 1 L of liquid postgate medium. A strain of SRB, designated as Desulfovibrio sp. strain menkeqing (NCBI GenBank accession number: PP869104), was isolated using the methods of dilution plating-streak plating-liquid culture. The optimal growth conditions for this strain were determined as follows: SO42− concentration = 500 mg/L, temperature = 30 °C, and pH = 6–7. The inoculum obtained from the final purification step was used as the original inoculum for the SRB-PRB system, with Desulfovibrio accounting for 82.34% of the microbial community.
2.1.2. Adsorbents Packed in PRB
Three economically low-cost and easily available materials (i.e., zeolite, volcanic rock, and ceramic pellets) were selected as potential packed media. Batch adsorption experiments revealed that zeolite exhibited the highest adsorption capacity, with an adsorption capacity of 2.28 mg/g (with an initial SO42− concentration of 1000 mg/L). Microbial immobilization tests demonstrated maximum fixation efficiencies of 20.45%, 34.85%, and 16.67% for volcanic rock, zeolite, and ceramic pellets, respectively. More detailed information about the batch adsorption experiments conducted is shown in the Supplementary Materials. Based on the comprehensive performance evaluation, zeolite with a particle size range of 2–4 mm was selected as the optimal packed medium.
2.1.3. Low-Cost and Slow-Release Carbon Source Packed in PRB
Bagasse, corncob, and sawdust, as typical agricultural wastes, are rich in cellulose and hemicellulose, exhibiting potential as slow-release carbon sources for SRB. Additionally, these materials are easily accessible and cost-effective. The results from carbon release kinetics through soaking and SRB utilization experiments indicated that the cumulative carbon release amounts of bagasse, corncob, and sawdust within 120 h were 218.44, 77.72, and 39.87 mg/(g·L), respectively. The DOM released from bagasse mainly consisted of fulvic-like (III zone) and humic-like (V zone) with relatively large molecular weights, whereas those from corncob and sawdust were predominantly tryptophan-like (II zone), tyrosine-like (I zone), and soluble microbial products (IV zone), which showed better microbial availability. The enzymatic reaction rates of SO42− reduction by SRB utilizing bagasse, corncob, and sawdust were 3.88, 3.21, and 2.37 mmol/(L·d), respectively. Corncob, with moderate carbon release capacity and a favorable enzymatic reaction rate, was determined to be the most suitable slow-release carbon source material. Consequently, corncob with a particle size of 2–4 mm was selected as the carbon source for the SRB-PRB system.
2.2. Construction of the SRB-PRB Column Experiments
2.2.1. Simulated Mine Water
Simulated mine water was prepared according to the hydrochemical characteristics of the 3103 goaf water, and SRB-PRB remediation simulation experiments were carried out. The chemical reagents and their dosages are detailed in Table S3. The hydrochemical components and their concentrations of the simulated mine water are shown in Table 1, among which the concentration of the characteristic pollutant SO42− is 1027.45 mg/L.

Table 1.
Concentrations of hydrochemical components in the simulated mine water.
2.2.2. Design of Column Experiments
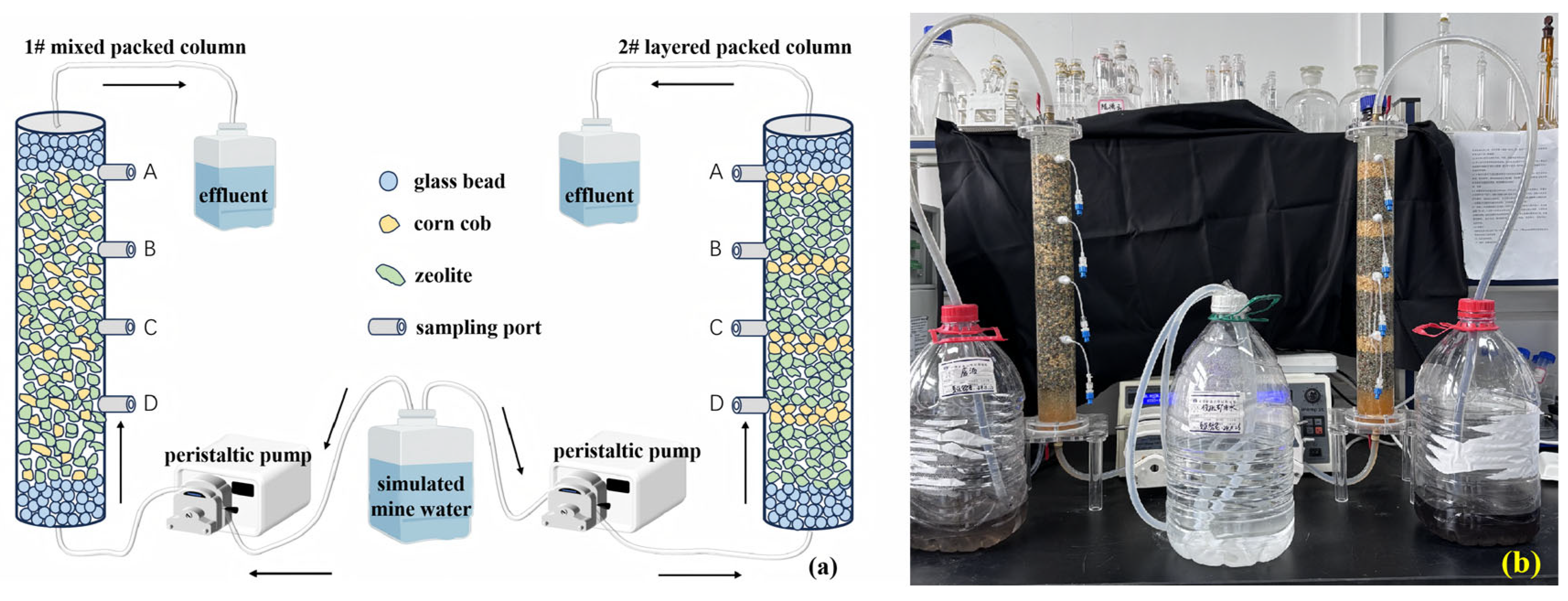
Two sets of SRB-PRB columns were established according to two common modes of practical packed media for PRBs. Column 1# was mixed packed with zeolite and corncob, and column 2# was layered packed with zeolite and corncob (Figure 1). To prevent excessive carbon sources, the packed ratio of zeolite to corncob was 3:1 (V:V).
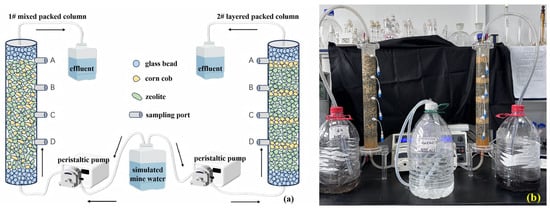
Figure 1.
The schematic diagram (a) and physical picture (b) of the SRB-PRB column experiment. Note: the capital letters in the (a) represent the sampling ports of the columns; the black arrows indicate the direction of the water flow.
The SRB-PRB experimental system consisted of a transparent acrylic glass column (50 cm height × 6 cm outer diameter × 5 cm inner diameter), with 5 cm layers of glass beads packed at both the bottom and top, resulting in an effective treatment length of 40 cm. Four sampling ports (A, B, C and D in Figure 1a) in the device were installed along the column height at 15 cm, 25 cm, 35 cm, and 45 cm. The sampler was a specially purchased soil solution sampler (CSS-5cm, Rhizosphere, Wageningen, The Netherlands), which was inserted into the center of the column. The system operated in upflow mode, with simulated mine water pumped through the column using a peristaltic pump (iPUMP 2L, Baoding Signal Fluid Technology, Baoding, China) connected by PTFE tubing. This experiment was run for 40 d, during which ORP (ORP30, Clean, San Leandro, CA, USA), pH (PB-10, Sartorius, Göttingen, Germany), and SO42− (UV-1900i, Shimadzu, Kyoto, Japan) were monitored at different sampling times.
After constructing the mixed packed column and layered packed column, the porosity, effective porosity, and unit PV (the time required for a complete displacement of fluid in the pores) of the experimental columns were measured. The dry weight was recorded after packing, followed by filling the columns with water to determine the wet weight. Once fully filled with water, the bottom valve was opened to allow gravitational water drainage, and the mass after draining gravitational water was measured. Total porosity reflects the total water storage capacity of the SRB-PRB columns but includes dead pores and micro-capillary pores. In contrast, effective porosity characterizes the pore volume that can actually participate in fluid flow, which directly affects the migration rate and the hydraulic retention time of simulated mine water in the SRB-PRB columns. After determining the effective porosity of the SRB-PRB columns, the unit PV time was calculated according to the influent flow rate. The related pore parameters of the two SRB-PRB columns are presented in Table S4.
2.3. Operation of the SRB-PRB Column Experiments
2.3.1. Microbial Biofilm Colonization Startup Experiment for SRB-PRB Columns
The preserved glycerol inoculum was revived and subjected to liquid culture. The SRB inoculum in the logarithmic growth stage was inoculated into fresh sterilized medium at a ratio of 10%. After 48 h of cultivation in the anaerobic environment, it was used as the biofilm colonization inoculum. Then, a peristaltic pump was employed to pump the inoculum into the mixed packed column and layered packed column at a flow rate of 7 mL/min, establishing dynamic biofilm colonization via rapid recirculation. The pH, ORP, and SO42−of the effluent were monitored by sampling daily. The biofilm colonization was considered successful when the SO42− removal efficiency of the effluent reached 85%.
2.3.2. Continuous Operation of SRB-PRB Column Experiments
The prepared simulated mine water was used as the influent for the experiments. A peristaltic pump was employed to pump the mine water into both the mixed packed column and layered packed column in an upward direction at a flow speed of 300 mL/d, with continuous operation maintained for 40 d. The hydraulic retention time (i.e., PV) of column 1# was 0.97 d, and that of column 2# was 0.9 d (Table S4). Samples were collected on days 1, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, 33, 36, and 40. The pH, ORP, and SO42− concentrations at each effluent outlet were monitored.
2.3.3. Analysis of the Sulfate Removal Mechanism for SRB-PRB Column Experiments
During the continuous operation of SRB-PRB, effluent samples were collected every 5 d for three-dimensional excitation–emission matrix (3D-EEM) fluorescence analysis (F-7000, Hitachi, Tokyo, Japan) and 16S rRNA gene sequencing. In order to collect biomass, these water samples (~1 L) were filtered through a 0.22 μm pre-sterilized PES membrane immediately. The filter membranes with biomass were transported to the molecular biology laboratory promptly under dry ice storage and stored at −80 °C until DNA extraction. After 40 d operation, the columns were disassembled, and the zeolite and corncob materials inside the columns were taken out. A portion of the zeolite and corncob, similar to the aforementioned microbial filter membrane, was transported under dry ice storage to the molecular biology laboratory within 24 h for microbiological analysis. The DNA extraction and 16S rRNA gene sequencing methods were similar to our previous studies [23,24]. Another portion was freeze-dried for scanning electron microscopy and energy-dispersive spectroscopy analysis (SEM-EDS, Quattro S, Thermo Fisher Scientific, Waltham, MA, USA). After being ground to 60 mesh, the samples were characterized by Fourier transform infrared spectroscopy (FTIR, Nicolet iS20, Thermo Fisher Scientific, Waltham, MA, USA).
3. Results and Discussion
3.1. Performance of SRB-PRB Column Experiments Operation
3.1.1. Hydrochemical Evolution Characteristics During Biofilm Colonization in SRB-PRB Columns
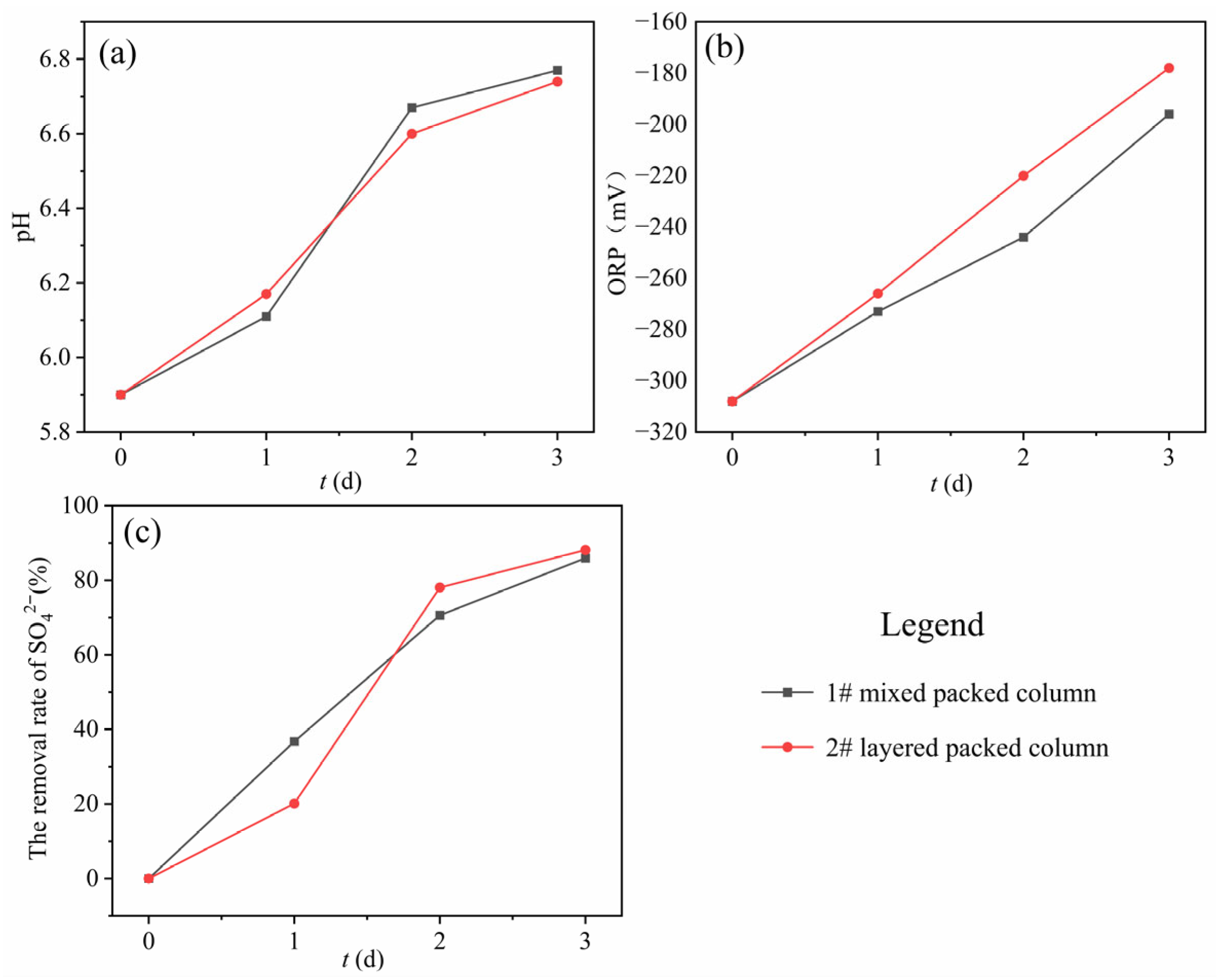
The dynamic biofilm colonization was achieved by continuous recirculation of SRB inoculum (flow rate: 7 mL/min) through both #1 mixed packed column and #2 layered packed column, with pH, ORP, and SO42− monitored throughout the process (Figure 2). The initial pH value of the inoculum was 5.9. The pH increased continuously during biofilm colonization in both columns. This was attributed to two factors: (1) the metabolic activities of SRB in the inoculum, which utilized the slow-release carbon source for growth and (2) certain alkaline minerals occurring in zeolite, which exerted a buffering effect on the pH of the system. Regarding ORP, the initial value was −308 mV, and it increased continuously during biofilm formation in both columns. This was because the inoculum after 48 h of anaerobic cultivation was in a strongly reducing environment. However, upon introduction into the experiment columns, it came into contact with oxygen, and the packed media and carbon source released oxidized chemical components. Thus, the ORP continued to increase. Nevertheless, the system remained in a relatively strong reducing environment, which was suitable for SRB growth. In terms of SO42− removal efficiency, the removal efficiencies of both columns continuously increased during the biofilm colonization process, reaching 85% by 3 d, which indicated the successful SRB biofilm colonization. During the biofilm colonization period, corncob underwent hydrolysis to release a large amount of low-molecular-weight organic substances, enabling SRB to proliferate significantly and become immobilized in the SRB-PRB columns. The cyclic flow for biofilm colonization facilitated this process. After successful biofilm colonization in the SRB-PRB columns, a 40 d continuous experiment was initiated.

Figure 2.
Changes in hydrochemical components during the microbial biofilm colonization process ((a) pH; (b) ORP; (c) SO42− removal efficiency).
3.1.2. pH Variation During Continuous Operation
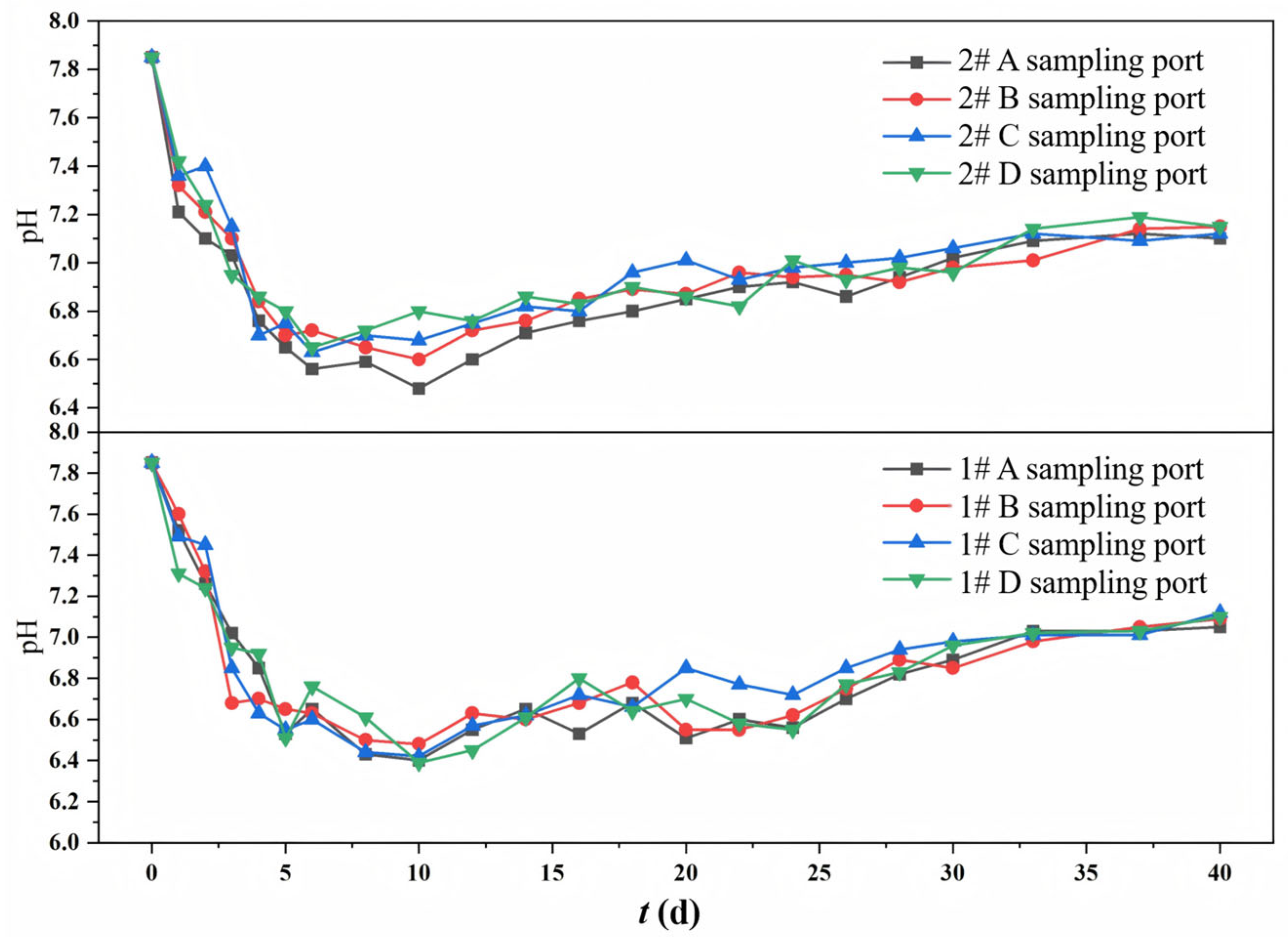
The variation trends of pH values at different sampling ports in the 1# mixed packed column and the 2# layered packed column during the continuous operation of the column experiments are shown in Figure 3. The initial pH of the simulated mine water was 7.85. The pH values at different sampling ports in both columns were relatively close during operation, generally showing a trend of rapid decrease first and then gentle increase. The operation process can be divided into the pH rapid decrease stage (0–10 d) and the pH slow increase stage (10–40 d). During 0–10 d, organic-degrading bacteria preferentially utilized the easily degradable organics released by corncob, and also decomposed high-molecular-weight organic matter (e.g., lignin and cellulose), producing acidic substances (e.g., volatile fatty acids (VFAs) and lactic acid), which rapidly decreased the pH. SRB require an anaerobic environment and have a relatively slow metabolic rate. In the pH rapid decrease stage, SRB may be in an inferior position due to residual DO or limited electron donor availability, and the SO42− reduction metabolism did not dominate. In the pH slow increase stage, with the release of carbon sources and the decomposition of high-molecular-weight organic matter by hydrolytic and fermentative bacteria, bioavailable carbon sources accumulated, and the environment tended to be reducing in the system, enhancing SRB-mediated sulfate reduction. SRB utilized low-molecular-weight organic matter (e.g., VFAs and lactic acid) as electron donors to reduce SO42− to S2−, while generating HCO3− (an alkaline substance). Meanwhile, the zeolite with a porous structure could adsorb H+, buffering the pH.
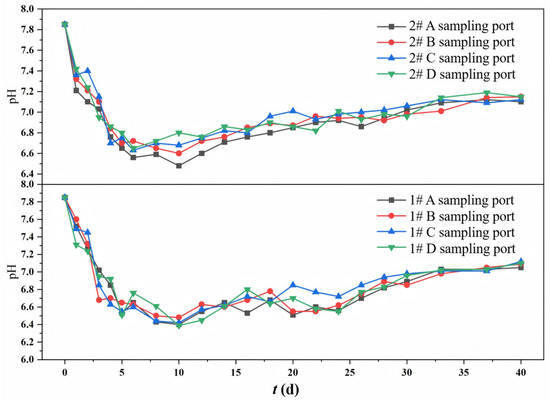
Figure 3.
Changes in pH at different sampling ports of 1# mixed packed column and 2# layered packed column.
The effect of packing mode on pH was reflected in the more significant pH decrease in the 1# column, with a minimum pH approximately 0.2 lower than that in the 2# column. This phenomenon suggested that the mixed packed mode facilitated a more uniform and stable release of carbon sources, enabling microorganisms to utilize and decompose organic matter more efficiently, thereby generating and accumulating more acidic metabolites. In addition, the close contact between zeolite and corncob in the mixed packed system may result in the occupation of adsorption sites by organic matter, thereby weakening the alkaline buffering capacity of zeolite.
3.1.3. ORP Variation During Continuous Operation
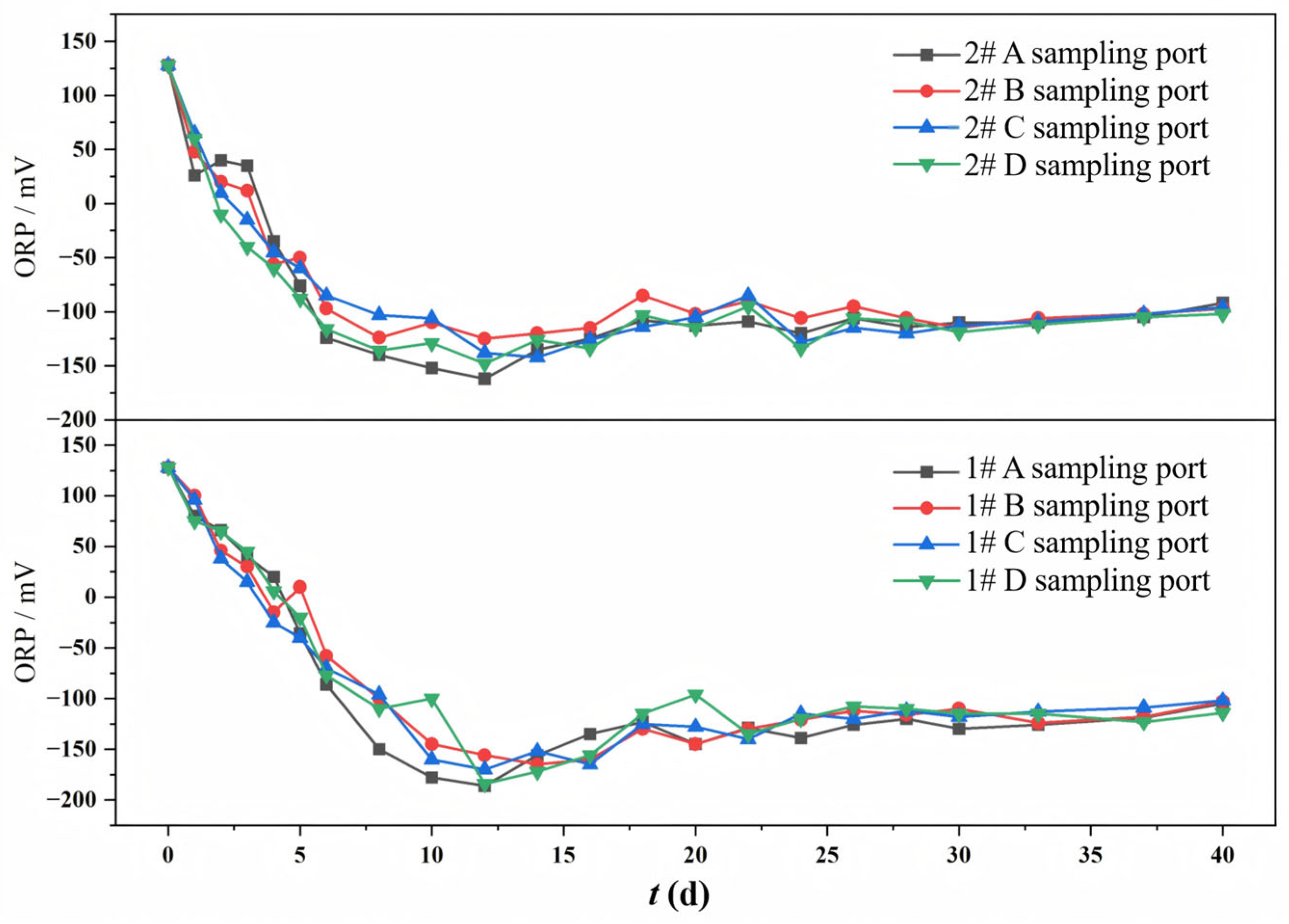
The variation in ORP at different sampling ports in both columns during the continuous operation is shown in Figure 4. Both experiment columns exhibited a consistent trend in ORP evolution, characterized by a rapid initial decrease, followed by a slow recovery, and finally a tendency to stabilize. The initial ORP of the simulated mine water was +128 mV. During the rapid decrease stage, the sufficient carbon sources stimulated vigorous microbial growth, leading to the reduction in oxidized species (i.e., O2, NO3−, SO42−) and consequent generation of reduced substances (i.e., sulfides). In the subsequent slow recovery stage, as the environment became strongly reducing, SRB emerged as the dominant bacterial community. During this stage, the carbon source became limited, with high-molecular-weight organic compounds (lignin and cellulose from corncob) serving as the primary reducing organics through microbial degradation. This constrained the SRB-mediated sulfate reduction process, resulting in decreased production of reduced substances and consequent ORP recovery. Ultimately, the experiment system maintained a relatively stable reductive state, where SRB, aerobic microorganisms, and the generation and oxidation of sulfides tended to reach a dynamic equilibrium.
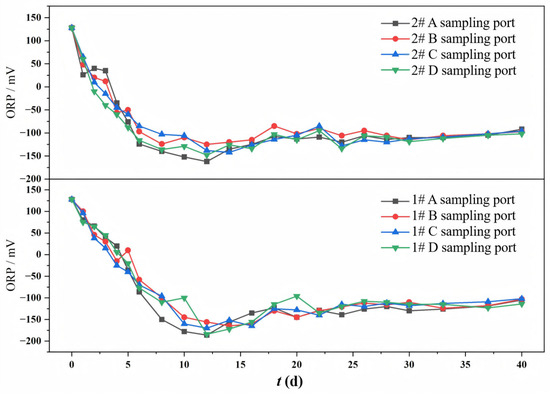
Figure 4.
Changes in ORP at different sampling ports of 1# mixed packed column and 2# layered packed column.
The influence of the packing mode on ORP was as follows: the #1 mixed packed column achieved a minimum ORP of −186 mV and stabilized at −120 mV, while the #2 layered packed column reached −160 mV and maintained −100 mV. Both the minimum and final ORP values of the mixed packed column were lower than those of the layered packed column, indicating that more reductive substances were generated in the mixed packed column. The homogeneous distribution of corncobs in the mixed packed column facilitated uniform carbon release, promoting vigorous metabolic activity of heterotrophic bacteria. This resulted in the increased production of VFAs and other low-molecular-weight carbon sources, thereby enhancing the SO42− reduction by SRB. In contrast, in the layered packed column, the release of carbon sources was relatively concentrated, and the microbial growth on the zeolite surface and the decomposition of corncob exhibited hysteretic behavior, making the SO42− reduction less effective than in the mixed packed column. The final ORP value reflects the overall reduction degree of the system. In the later stage of the experiment, both the mixed packed column and the layered packed column maintained a strong reducing environment, which was suitable for the SRB growth.
3.1.4. SO42− Variation During Continuous Operation
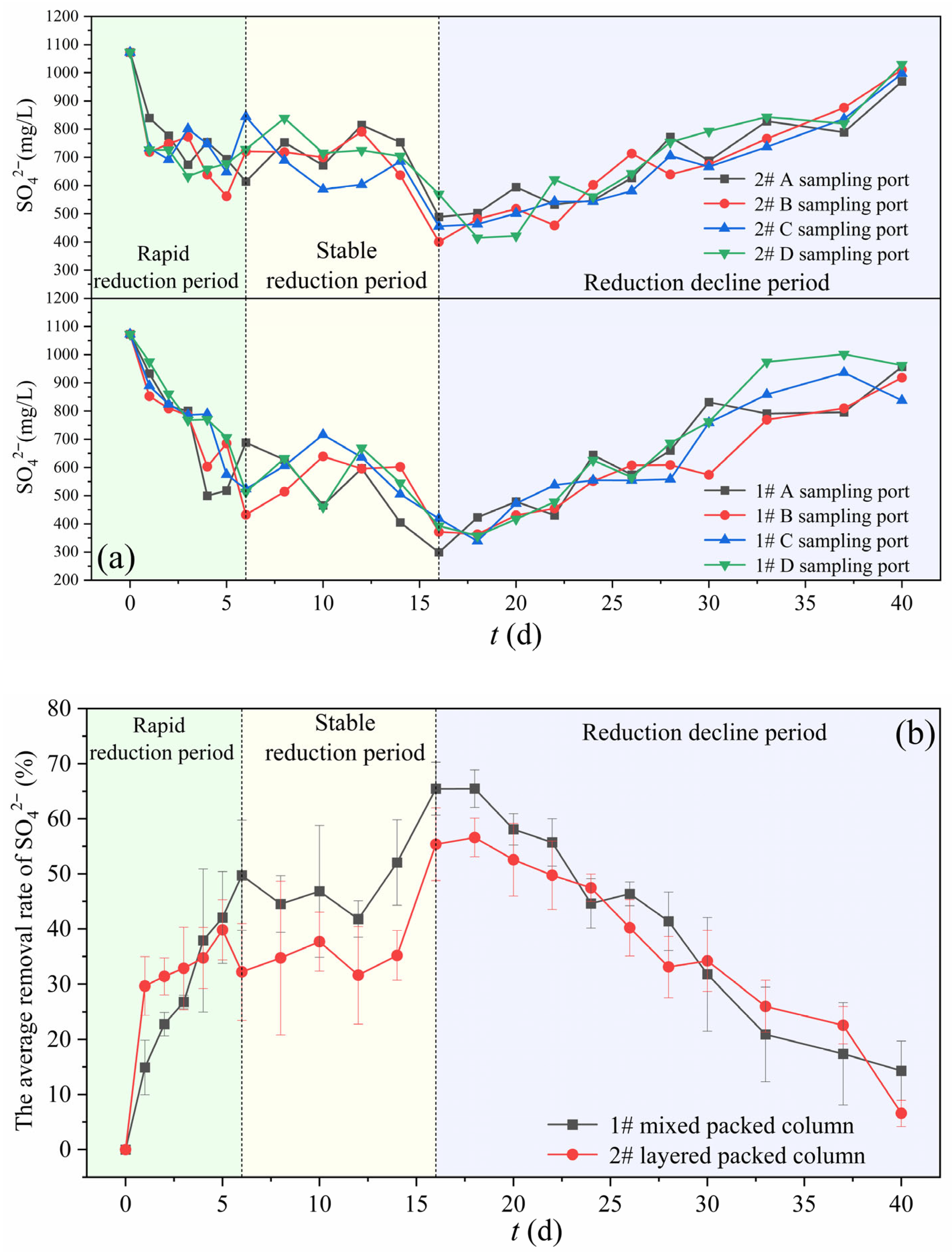
As the most critical parameter in this experiment, SO42− concentration was used to evaluate the carbon source suitability and microbial treatment efficiency. During the column experiments operation, the changes in SO42− concentration and removal efficiency at different sampling ports of the 1# and 2# columns are shown in Figure 5. There were differences in SO42− concentration at different sampling ports between the two experiment columns, but the average relative standard deviation (RSD) was only 9.3%, and the overall change trend showed good consistency. This indicated that within 1 PV hydraulic retention time (~1 d), the effect of SRB reducing SO42− in the reaction column has a certain degree of uniformity. The variation in SO42− concentration can be roughly divided into three stages: the rapid reduction period (0–6 d), the stable reduction period (6–16 d), and the reduction attenuation period (16–40 d).
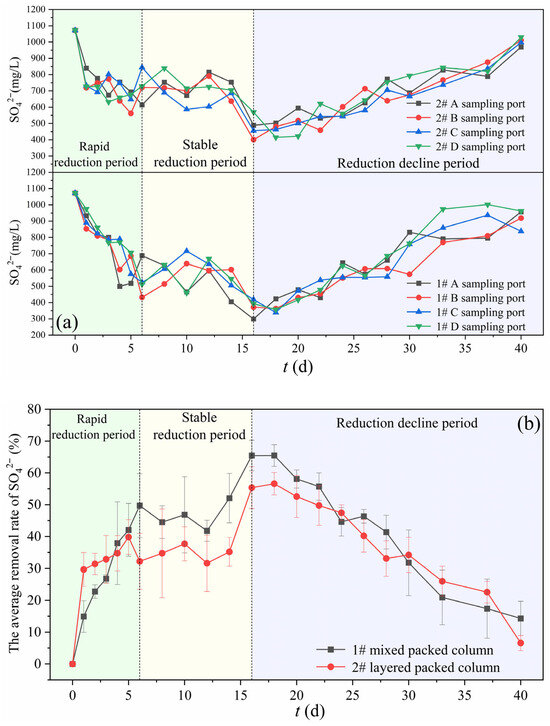
Figure 5.
Changes in SO42− at different sampling ports (a) and their average removal efficiencies (b) of 1# mixed packed column and 2# layered packed column.
During the rapid reduction period (0–6 d), corncob initially released large amounts of low-molecular-weight organic matter, while high-molecular-weight organics (e.g., lignin and cellulose) were decomposed by hydrolytic and fermentative bacteria into low-molecular-weight organic carbon sources. During this stage, SRB proliferated extensively with active metabolic activity, reducing and degrading substantial amounts of SO42−, while zeolite simultaneously adsorbed SO42−. In comparison, the removal efficiency of the 1# column increased relatively uniformly, while the average removal efficiency of the 2# column reached 29.6% on the first day, followed by a slower increase during 2–6 d. This phenomenon may be attributed to the more concentrated carbon source distribution in the layered packed mode, which led to the faster initiation of SRB-mediated SO42− reduction.
During the stable reduction period (6–16 d), the changes in the removal efficiencies of the 1# and 2# columns were relatively consistent. But the maximum average removal efficiency of the 1# column was higher, reaching 65.5%, while that of the 2# column was only 56.6%. SRB tended to adhere more easily to zeolite. In the mixed packed column, zeolite and corncob were in closer contact, and meanwhile, corncob, as the carbon source, released more uniformly. Therefore, the sulfate removal efficiency of the mixed packed column was higher than that of the layered packed column. In this stage, the release amount of carbon source was the highest, and low-molecular-weight organic carbon sources were sufficient. The system remained in a strong reducing environment. SRB were the dominant bacterial community, and stably utilized organic carbon sources to degrade SO42−. In addition, considering pH and ORP reached their troughs on the 10–12 d, sulfate removal appeared to be delayed relative to the pH and ORP changes. For pH, it was subject to the dual effects of acidic organic substances produced by carbon source hydrolysis and alkali generated from sulfate reduction. Therefore, when the sulfate removal efficiency exceeded the carbon source hydrolysis efficiency, pH tended to increase. For ORP, it was influenced by various redox substances (i.e., O2, NO3−, SO42−, sulfides). As the SO42− reductive removal efficiency increased, sulfides accumulated and gradually formed metal sulfide precipitates, and the lack of deoxygenation treatment for the influent water resulted in a gradual increase in ORP after 12 d.
During the reduction attenuation period (16–40 d), both the mixed and layered packed columns exhibited a gradual decline in sulfate removal efficiency. This phenomenon can be attributed to two primary reasons: (1) Prolonged operation led to carbon source limitation and metabolic product inhibition, resulting in decreased SRB metabolic activity and growth. The carbon release capacity of corncob progressively diminished, reducing the bioavailable carbon supply for SRB. (2) Continuous inflow with oxygen disturbed the reducing environment, while microbial competition further impacted SRB viability, as evidenced by the gradual ORP increase in both columns. However, both columns maintained the ~30% removal efficiency at 30 d. To enhance long-term sustainability, methods such as backwashing and aerating nitrogen into the influent to remove oxygen could be adopted.
In conclusion, the SRB-PRB column utilizing corncob as the slow-release carbon source demonstrated effective sulfate removal. Compared to conventional carbon sources (e.g., sodium lactate, ethanol, and methanol), corncob can offer distinct advantages, including local availability, low cost, and sustained carbon release without excess. The maximum average removal efficiency of the mixed packed column was 65.5%, which was higher than 56.6% of the layered packed column. Therefore, the mixed packed mode should be selected in the subsequent SRB-PRB design.
3.2. DOM Variation Characteristics
3.2.1. Variation Trend of DOM in the Effluent During Operation
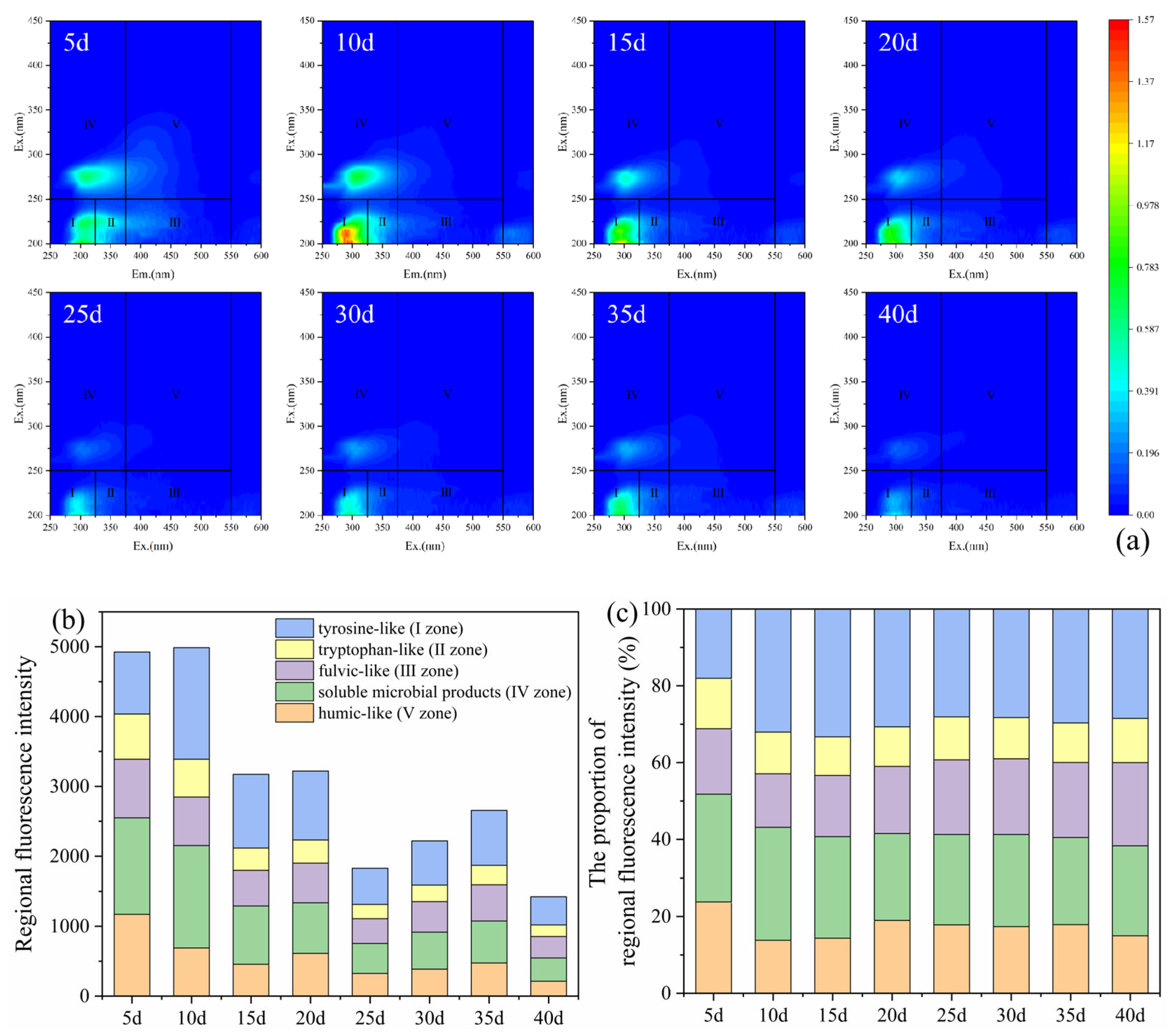
During the column experiments operation, effluent samples from the two columns were collected every 5 d for 3D-EEM fluorescence analysis. The DOM composition directly reflected the dynamic release of slow-release carbon sources from corncob and their microbial utilization processes. The 3D-EEM spectra and fluorescence regional integral intensity results of effluent from the layered packed column at different time points are shown in Figure 6. The results indicated that the DOM in the effluent primarily consisted of tyrosine-like (I zone), tryptophan-like (II zone), and soluble microbial products (IV zone). The fluorescence intensity was relatively higher in the first 10 d, reaching the maximum at 10 d, and then subsequently decreased. However, a slight rebound occurred between 25 and 35 d. During the experiment operation, the proportion of tyrosine-like (I zone) ranged from 18.9 to 33.3%, and that of soluble microbial products (IV zone) ranged from 22.6 to 29.3% of the total DOM. These two components accounted for the majority of the DOM, indicating that the DOM in the column experiment was mainly low-molecular-weight substances with good bioavailability (Figure 6c) [25,26]. In the initial 10 d, a large amount of low-molecular-weight and highly bioavailable organic matter was released from corncob driven by heterotrophic bacteria. Subsequent intensity decrease resulted from the DOM consumption of SRB and adsorption on zeolite. During 25–35 d, the recalcitrant components in corncob underwent slow degradation during long-term operation, and the accumulated microbial metabolites could increase the DOM concentration. The composition proportion of DOM was relatively stable in the later stage, indicating that the microbial communities’ succession and the decomposition/consumption of carbon sources were in a relative equilibrium state.

Figure 6.
Three-dimensional fluorescence spectra diagram (a), regional fluorescence integral intensity (b), and proportion of fluorescence intensity (c) of effluent in the 2# layered packed column.
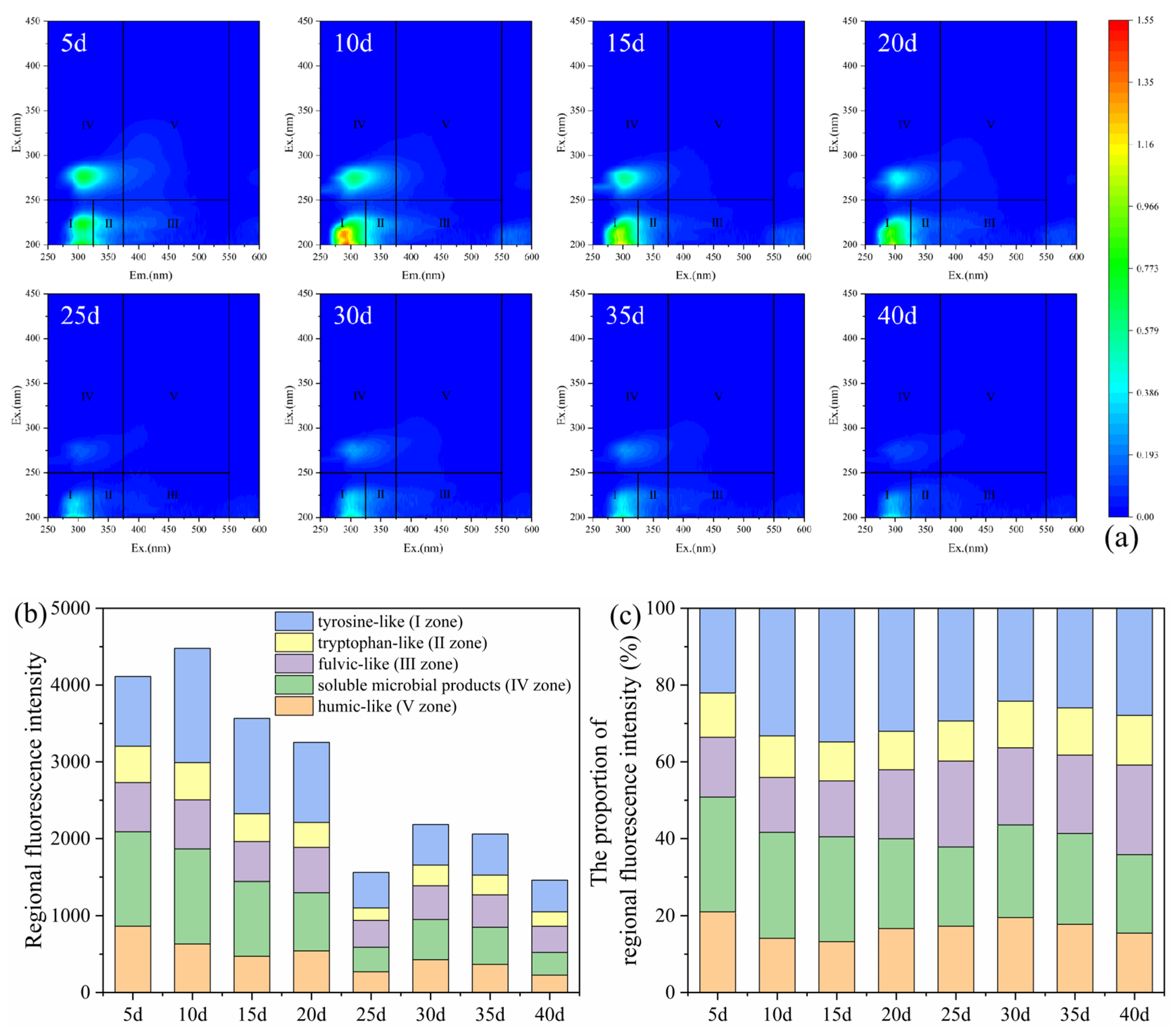
The 3D-EEM spectra and regional fluorescence integral intensity plots of the effluent from the mixed packed column at different time points are presented in Figure 7. Similar to the layered packed column, the DOM in the effluent was also predominantly composed of tyrosine-like (I zone), tryptophan-like (II zone), and soluble microbial products (IV zone). Throughout the experimental period, tyrosine-like (I zone) accounted for 22.1–33.2%, and soluble microbial products (IV zone) represented 20.5–29.8%; both together constituted the majority of DOM (Figure 7c). Unlike the layered packed column, although the DOM fluorescence intensity of the effluent from the mixed column was lower than that in the layered column within the first 10 d, its DOM fluorescence intensity was higher during the period of 10–20 d. This was because the packed mode affected the decomposition and utilization of corncob by microorganisms. The layered pack promoted concentrated and rapid carbon release from corncob, whereas the mixed pack facilitated more uniform and sustained decomposition. The overall variation trend in the DOM paralleled that observed in the layered packed column, with analogous explanatory mechanisms. The DOM dynamics in the SRB-PRB effluent exhibited a strong correlation with sulfate removal efficiency, where higher DOM concentrations led to higher SO42− removal. These findings also suggested that the mixed packed mode offered superior advantages for sustained carbon release.

Figure 7.
Three-dimensional fluorescence spectra diagram (a), regional fluorescence integral intensity (b), and proportion of fluorescence intensity (c) of effluent in the 1# mixed packed column.
3.2.2. Comparison of DOM Compositions Leached from the Corncobs Before and After Experiments
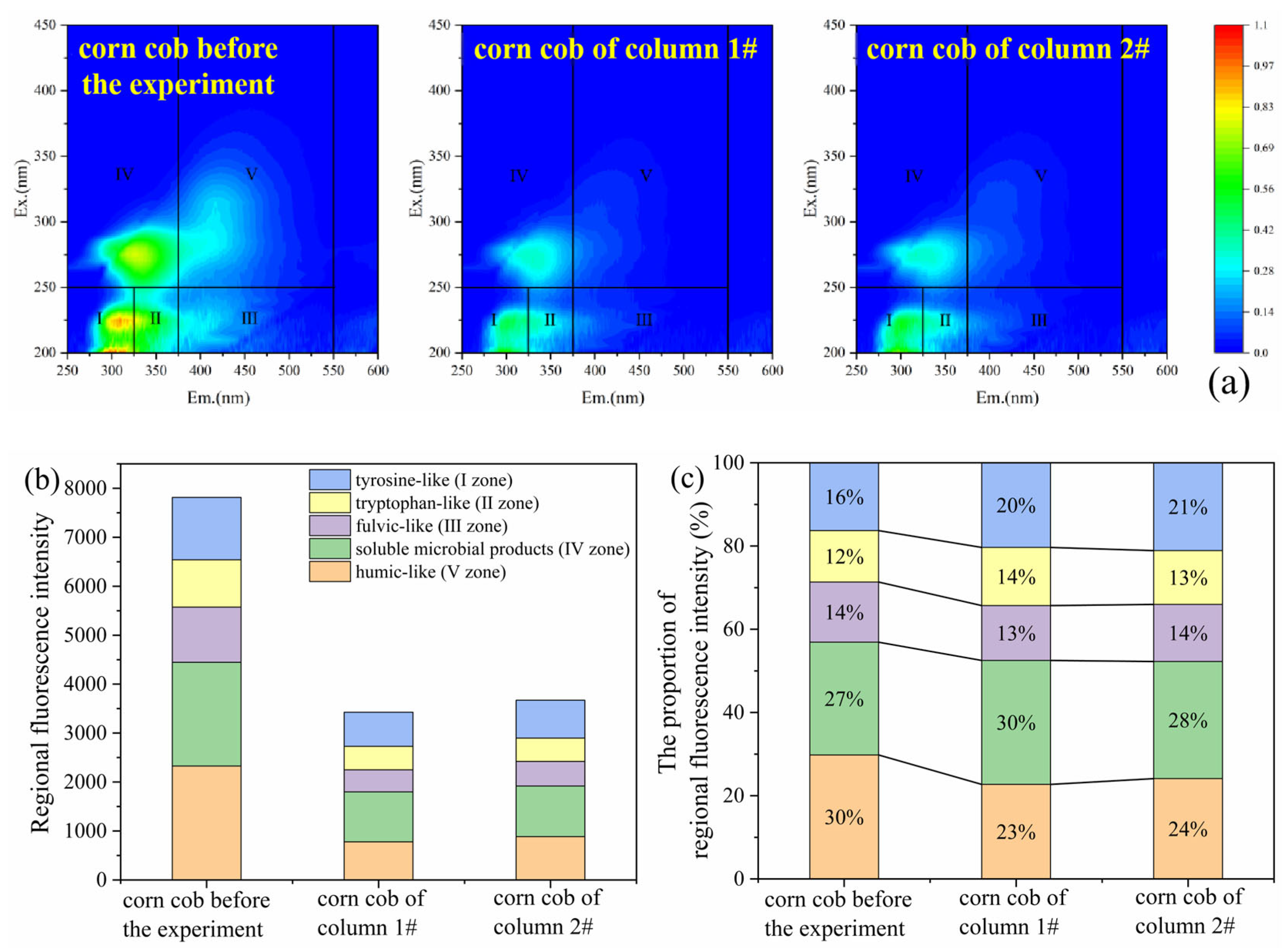
To compare the changes in the composition of DOM leached from corncobs before and after microbial utilization, and to evaluate its residual carbon release potential, corncob samples were collected from both columns after the 40 d experiments. These samples, along with untreated corncob, were subjected to a 24 h immersion, followed by 3D-EEM analysis of the leaching solutions. The 3D-EEM spectra and regional integration results (Figure 8) indicated that the dominant DOM components in corncob leachates remained consistent before and after the reaction, primarily consisting of tyrosine-like (I zone), tryptophan-like (II zone), and soluble microbial products (IV zone). These three types of substances had low molecular weights and were readily utilizable by microorganisms. After 40 d operation, the regional fluorescence intensity decreased to approximately half of the initial value, confirming the occurrence of carbon release from corncob and its decomposition and utilization by microorganisms during the experiment. Notably, compared to the layered packed column, the corncob from the mixed packed column exhibited a more pronounced decline in fluorescence intensity, suggesting its higher microbial utilization efficiency. Both retained residual carbon release potential (Figure 8b). As shown in Figure 8c, the proportion of protein-like substance zones (I, II, and IV zones) in corncobs increased, while the proportion of humic-like substance zones (III and V zones) decreased after the reaction. This indicated that after microbial utilization, the high-molecular-weight substances in corncob were gradually decomposed and transformed by microorganisms into low-molecular-weight substances with better availability. These findings further validated the feasibility of corncob as a slow-release carbon source in SRB-PRB.

Figure 8.
Three-dimensional fluorescence spectra diagram (a), regional fluorescence integral intensity (b), and proportion of fluorescence intensity (c) of the leaching solutions from corncobs before and after the experiment.
3.3. Microscopic Mechanism of SO42− Reductive Removal by SRB
During the SRB-PRB column operation, the amount of black suspended solids in the effluent from both columns gradually increased (Figure S5a). A distinct smell of rotten eggs (hydrogen sulfide) could be detected when approaching the columns, and a large amount of black substances was observed on the inner walls of the effluent pipes (Figure S5b). Additionally, black precipitates were observed adhering to the surface of glass beads inside the columns (Figure S5c), preliminarily identified as metal sulfides. To further validate and characterize the changes in corncob and zeolite in the SRB-PRB before and after the reaction, the fillers in the columns were taken out in an anaerobic glove box after 40 d operation. The changes in the microbial communities, functional groups, morphology, and elemental composition of the fillers were clarified as follows. Based on the above results, the microscopic mechanism of SO42− reduction and removal by SRB in the SRB-PRB columns was revealed.
3.3.1. Microbial Community Variation Mechanism
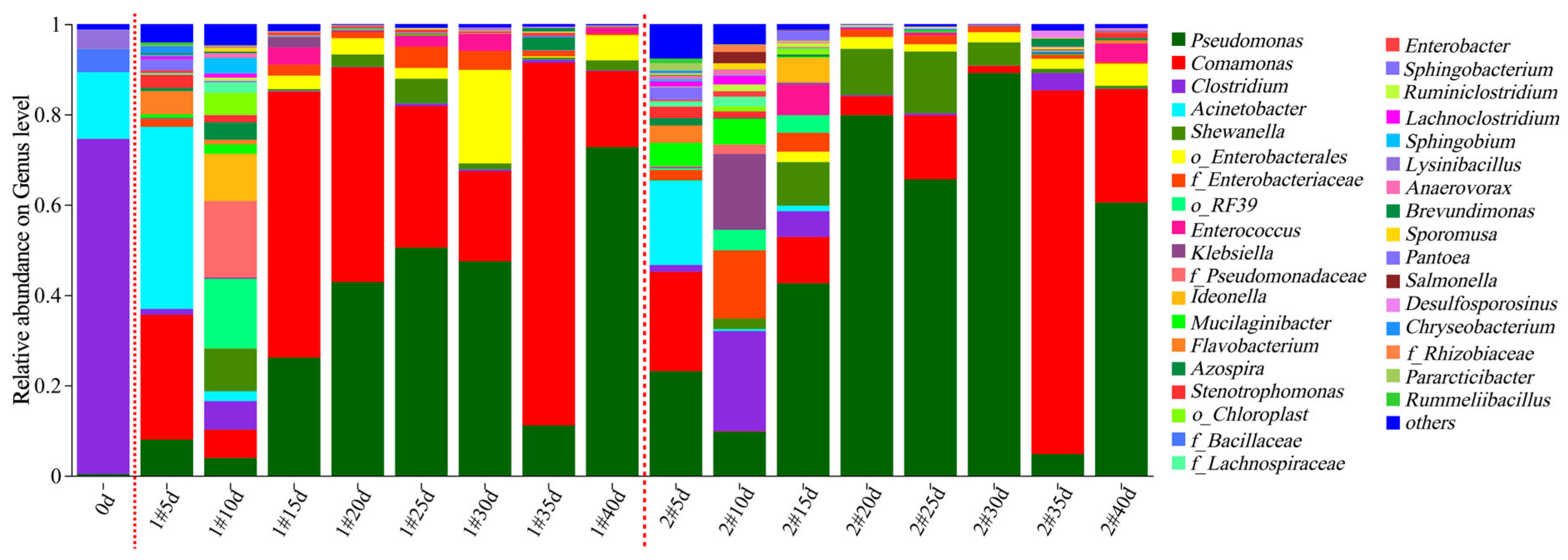
Microbial community composition of the column experiment samples was analyzed based on the MiSeq sequencing results. The microbial community compositions on the genus level in the effluent water and the solid media samples are shown in Figure 9 and Figure 10, respectively. There was a significant difference in the microbial community compositions between the effluent water and the solid media (p < 0.05). During the 40 d column operation, 35 bacterial genera with relative abundances exceeding 1% were identified in the effluent of the two columns. The dominant genera included Pseudomonas (0.37–89.17%), Comamonas (0.00–80.51%), Clostridium (0.06–74.22%), Acinetobacter (0.00–40.37%), Shewanella (0.00–13.70%), o_Enterobacterales (0.00–20.76%), f_Enterobacteriaceae (0.00–15.11%), o_RF39 (0.00–15.40%), and Klebsiella (0.00–16.81%). Most of these genera were bacteria capable of organic matter degradation and driving the nitrogen cycle [27,28,29,30,31]. The inter-group species differences between the two experimental columns were not significant (p > 0.1); however, for each column, the microbial species composition varied significantly with the reaction time (p < 0.05). Following SRB biofilm colonization, the microbial composition in the effluent on 0 d was dominated by Clostridium (74.22%, capable of cellulose degradation under anaerobic conditions) [32,33,34], Acinetobacter (14.75%, degrading complex organics like alkanes and PAHs) [35,36,37], f_Bacillaceae (5.12%, secreting hydrolytic enzymes like amylase, cellulase, protease and lipase for polysaccharide/protein/lipid degradation) [38,39,40], and Lysinibacillus (4.30%, capable of heavy metal reduction and organic degradation) [41,42,43]. During the 0–10 d operation period, the richness and diversity of the microbial community increased (Table S5). The number of dominant genera expanded, most of which had the functions of degrading complex organic matter, denitrification, and iron reduction, including Comamonas, Acinetobacter, Clostridium, Pseudomonas, Shewanella, f_Enterobacteriaceae, o_RF39, Klebsiella, f_Pseudomonadaceae, Ideonella, etc. [28,44,45]. It was mainly because after biofilm colonization, simulated mine water was introduced into the reaction column, and a large amount of easily biodegradable organic matter on the surface of corncob provided substrates for these microorganisms, which were fully hydrolyzed to release low-molecular-weight organic carbon sources. Therefore, sufficient carbon sources were supplied for SRB to efficiently reduce SO42−, corresponding to the rapid and stable SO42− removal. During the 10–40 d operation period, the richness and diversity of the microbial community decreased (Table S5), with a reduction in the number of dominant genera. These genera included aerobic or facultatively anaerobic Comamonas (1.59–80.51%) and Pseudomonas (4.79–89.17%), which were capable of degrading complex organic substances (i.e., cellulose and lignin) and performing denitrification [26,46], and Shewanella (0.34–13.70%), a facultatively anaerobic genus that could degrade organic carbon compounds and carry out dissimilatory metal reduction. The sum of the relative abundances of Comamonas and Pseudomonas exceeded 50%. This indicated that after 10 d reaction, as easily degradable cellulose and lignin were hydrolyzed and consumed, the proportion of remaining refractory high-molecular-weight organic carbon compounds gradually increased. These required degradation by versatile complex organic matter-degrading bacteria such as Comamonas and Pseudomonas to release small-molecular-weight organic carbon sources for SRB. This stage corresponded to the SO42− reduction attenuation stage. The gradual decrease in carbon source release directly led to a reduction in the efficiency of SO42− reduction by SRB. Notably, during the 10–25 d reaction, the sum of the relative abundances of Comamonas and Pseudomonas was higher in the 1# mixed packed column (Figure 9), which was consistent with the higher DOM fluorescence intensity in the 1# column (Figure 6 and Figure 7). This suggested that the mixed packed mode was more conducive to the growth of bacteria degrading high-molecular-weight organic matter such as cellulose and lignin, thereby continuously providing more low-molecular-weight organic carbon sources for SRB, and resulting in a higher SO42− removal efficiency.
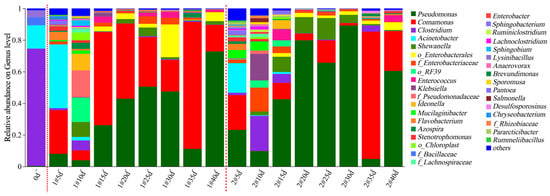
Figure 9.
Relative abundance of the dominant lineages on genus levels for column experiment effluent samples. The relative abundance of microbial genera > 1% was plotted, and the remaining microorganisms were classified as others. Note: the first red dashed line separates the initial data before column experiments operation from the data during column experiments operation; the second red dashed line separates the data of columns 1# and 2#.
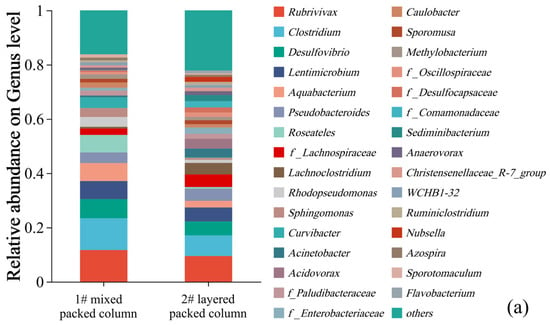
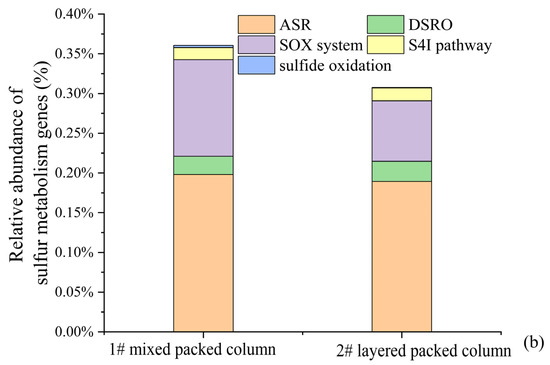
Figure 10.
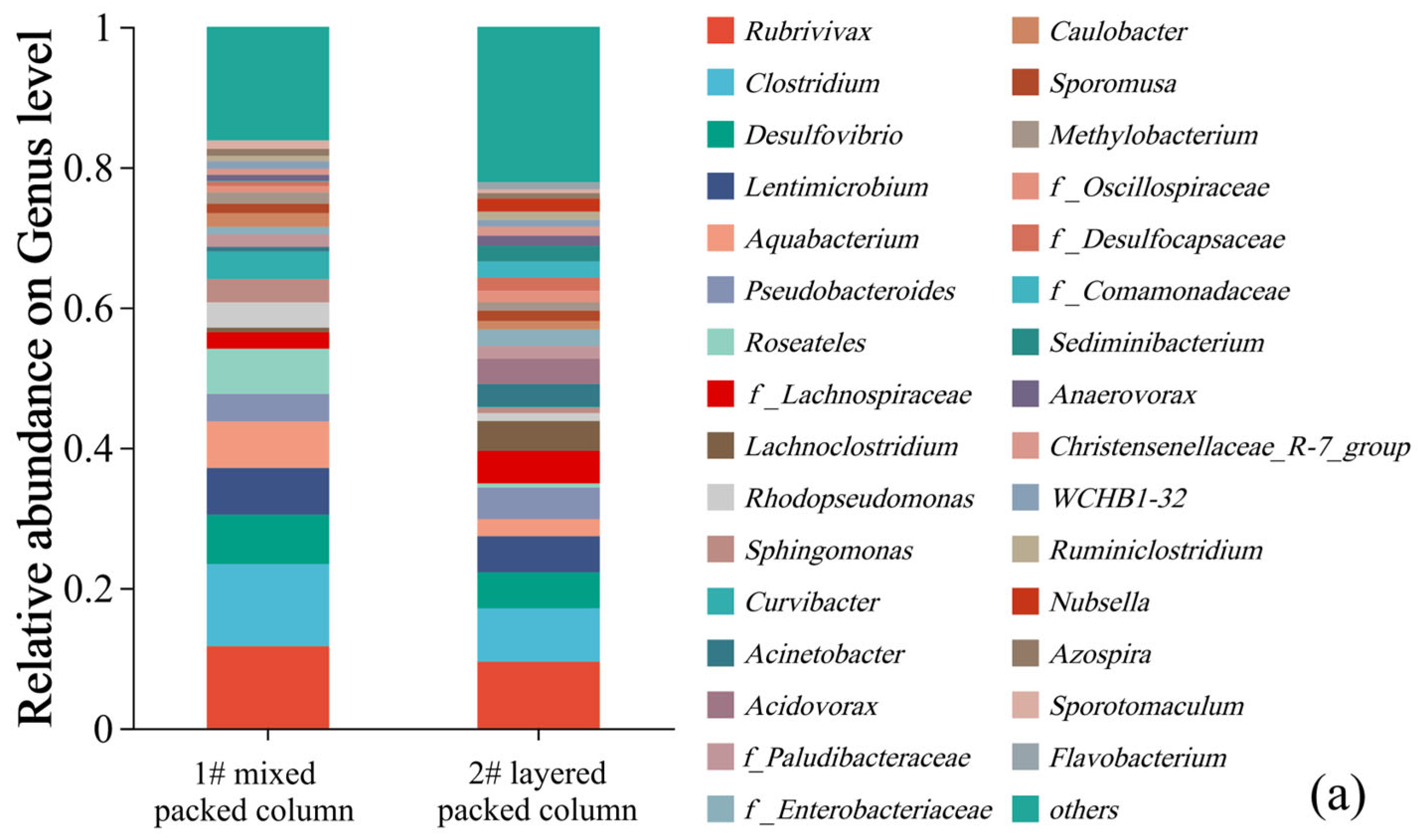
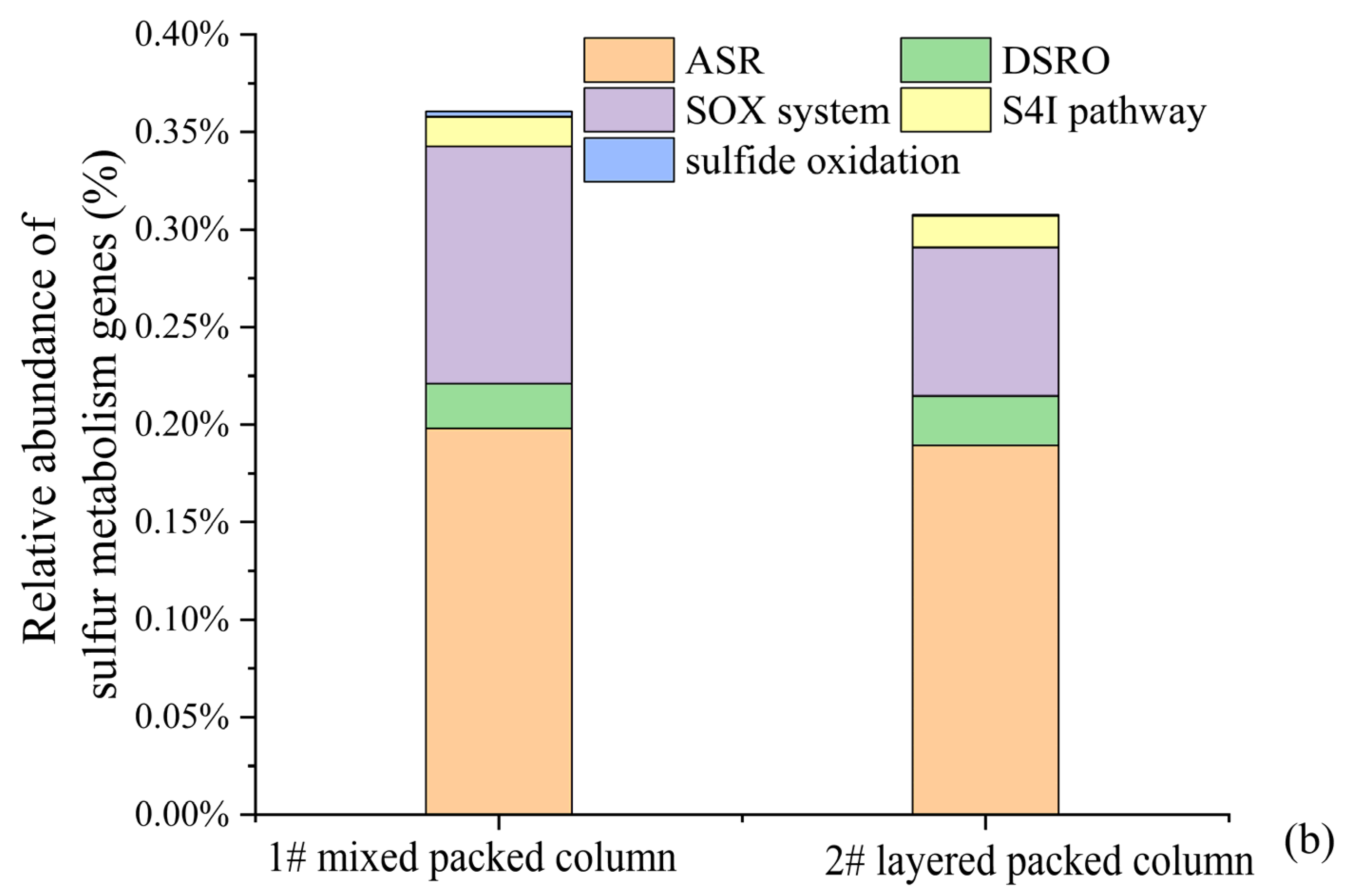
Relative abundance of the dominant lineages on genus levels for solid packed medium in column experiments (a). Relative abundance of different sulfur metabolism genes for solid packed medium in columns 1# and 2# (b).
Only one type of traditional SRB with very low relative abundance (0–1.53%), namely Desulfosporosinus, was detected in the microbial community of the effluent. Then, where did all the SRB reside? The microbial community compositions of the solid packed medium in both columns are shown in Figure 10a. Thirty-one bacterial genera had a relative abundance exceeding 1%, among which only two genera belonged to the traditional SRB group. It is noteworthy that the SRB genus with the highest relative abundance was also Desulfosporosinus (1#: 7.05%; 2#: 5.12%), rather than Desulfovibrio (82.34%), which was the dominant genus during the biofilm colonization stage at the initiation of the column experiment. The reason may be related to the adaptability of different SRB genera. Although Desulfovibrio was enriched under laboratory conditions, it might not adapt to the environment of the column experiment. In contrast, Desulfosporosinus was more adaptable to the column experimental environment and thus proliferated in large numbers, becoming the dominant SRB genus. This finding indicated that in practical engineering applications of SRB-PRB, it was more important to regulate the growth conditions of functional strains (i.e., carbon sources, DO, and redox conditions) to enhance the proliferation and functional metabolism of indigenous microorganisms than to directly inject functional strains. Based on the KEGG database (Kyoto Encyclopedia of Genes and Genomes), metabolic pathways and gene distributions related to sulfur metabolism were searched. Emphasis was placed on statistics of sulfide oxidation, tetrathionate intermediate (S4I) pathway, sulfur-oxidizing X protein (SOX) system, assimilatory sulfate reduction (ASR), and dissimilatory sulfate reduction/oxidation genes (DSRO). The results are shown in Figure 10b. The abundance of sulfate reduction genes (ASR + DSRO) in the 1# mixed packed column (1#: 0.2211%) was slightly higher than that in the 2# layered packed column (2#: 0.2144%), which was consistent with the higher SO42− reduction intensity of the 1# column. Assimilatory sulfate reduction dominated this process. Meanwhile, there was a certain abundance of sulfur oxidation genes in the packed media of the column experiment. In particular, the SOX system exhibited the highest abundance, with that in column 1# (0.1220%) being higher than in column 2# (0.0761%). This may be related to the stronger SO42− reduction in column 1#, which resulted in the generation of reduced sulfur compounds.
In addition, similar to the effluent samples, bacterial genera that were capable of organic matter degradation and denitrification were detected in the solid packed media of the columns with relatively high relative abundances. Particularly, more types of bacterial genera were detected in the packed media, with a more uniform distribution. These genera mainly included Rubrivivax (9.48–11.69%), Clostridium (7.61–11.69%), Lentimicrobium (5.16–6.66%), Aquabacterium (2.43–6.62%), Pseudobacteroides (3.96–4.48%), Roseateles (0.60–6.42%), f_Lachnospiraceae (2.37–4.65%), Lachnoclostridium (0.64–4.26%), etc. The above-mentioned genera could degrade complex organic substances in corncobs into low-molecular-weight organic substances, which could be utilized by SRB. Thus, these bacterial genera played a key role in the microbial removal of SO42−. It was reported that SRB cannot decompose complex organic substances. Therefore, the observed diverse bacterial community structure and the functional roles of their corresponding coexisting species may be the reason for the effectiveness of this bioremediation process [20]. In summary, although the relative abundance of SRB genera detected in both the effluent and solid packed medium of the column experiment was low, these SRB could still sustain a high efficiency of sulfate reduction. This process was more correlated with the sustained supply of readily available carbon sources provided by hydrolytic and fermentative bacteria [47,48,49]. This study confirmed the existence of a synergistic effect between SRB and organic matter-degrading bacteria (i.e., bacteria that hydrolyze complex organic substances such as cellulose and lignin), which was the critical limiting factor for sulfate removal.
3.3.2. Organic Functional Groups Variation in Corncobs
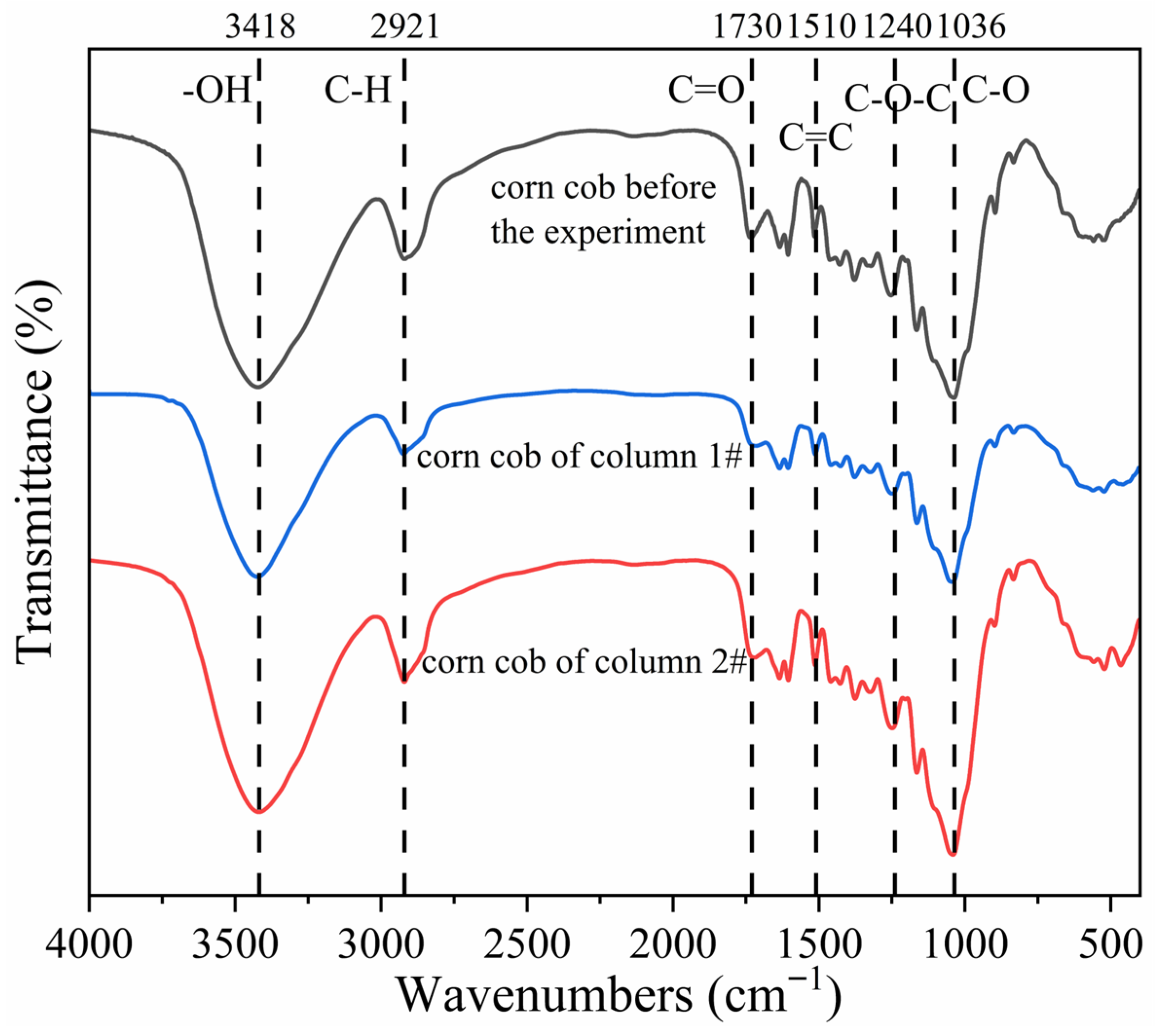
The main components of corncob are cellulose, hemicellulose, and lignin. During the operation of the column experiment, cellulose and hemicellulose were first degraded by organic-degrading bacteria, followed by the gradual decomposition of lignin in later stages. FTIR spectroscopy was employed to identify the functional groups in corncob. Unreacted corncobs and corncobs in the column after 40 d reaction were collected for microscopic characterization to detect changes in the main functional groups after microbial utilization. The results are shown in Figure 11. According to the wavenumbers corresponding to the representative functional groups in cellulose, hemicellulose, and lignin reported by Kostryukov et al. (2023) [50], the transmittance (%) of the three samples at wavenumbers corresponding to key functional groups was quantified, and the data are shown in Table 2. A FTIR spectra comparison of corncob before and after the reaction revealed that the positions and trends of major functional groups remained largely consistent, but the corresponding transmittance was different. In FTIR spectra, higher transmittance indicated lower absorption peaks, corresponding to lower content of the functional groups. As shown in Table 2, after 40 d reaction, the transmittance at the –OH, C–H, C=O, C–O–C, C=C, and C–O functional groups in the corncobs of the 1# mixed packed column increased significantly, suggesting substantial disruption of the functional group structures of cellulose, hemicellulose, and lignin, accompanied by decreased absorption peaks. In contrast, the corncob from the 2# layered packed column exhibited only minor increases in transmittance at the –OH, C–H, C=O, C–O–C, and C=C functional groups, indicating structural degradation but to a lesser extent than in the mixed packed column. In conclusion, after 40 d reaction, the primary components of corncob were decomposed and utilized by hydrolytic and fermentative bacteria and SRB. The mixed packed mode facilitated more pronounced structural changes in corncob, suggesting that SRB-PRB interactions under mixed packed conditions enhanced microbial utilization efficiency and improved carbon source release.
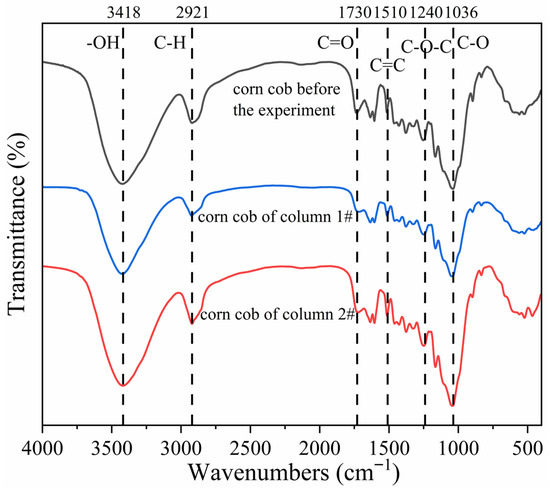
Figure 11.
Fourier infrared spectra of corncob before and after experiment reaction.

Table 2.
Changes in transmittance of characteristic functional groups of corncob.
3.3.3. Morphology and Elemental Composition Variation in the Fillers
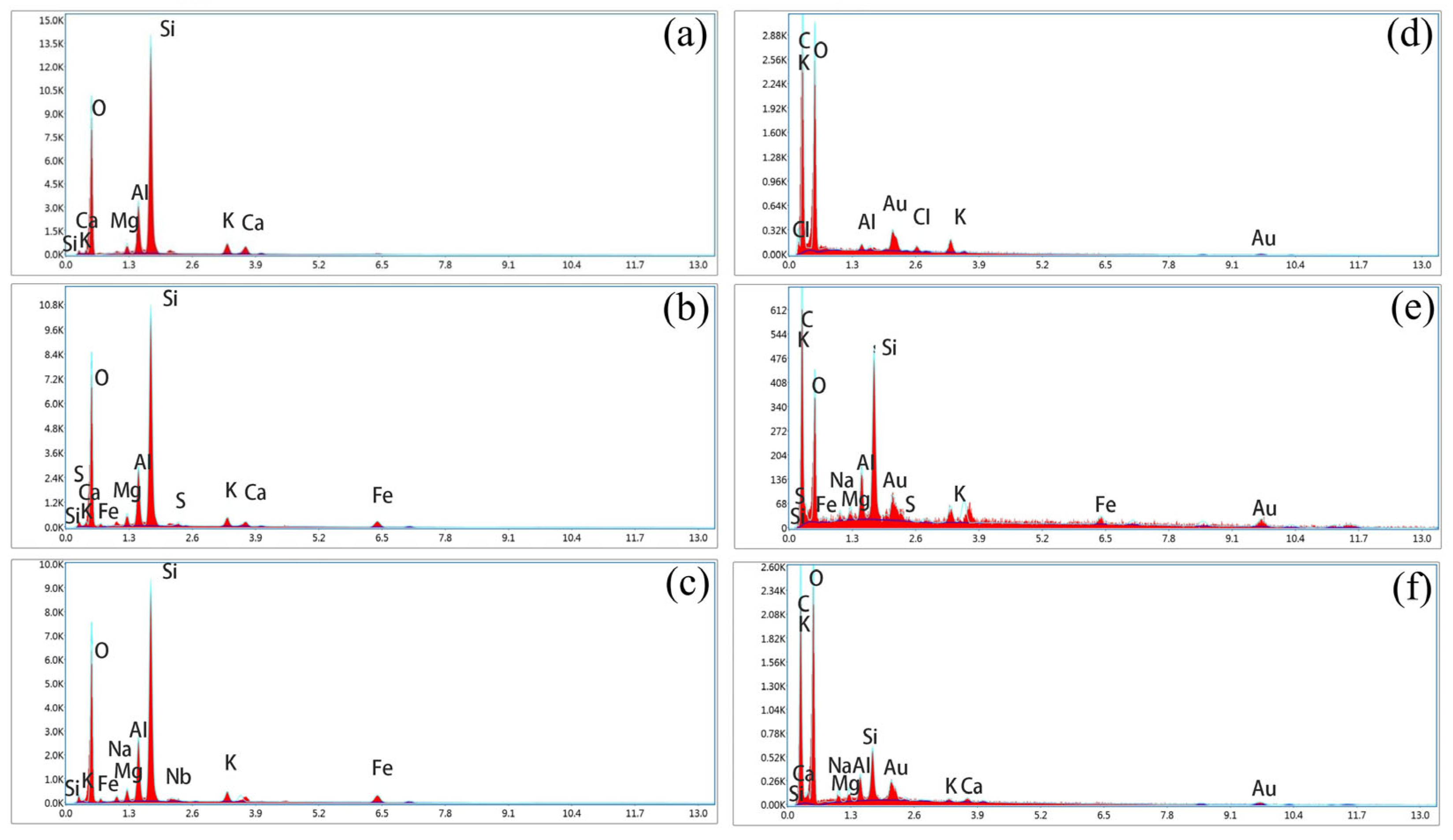
Zeolite and corncob samples (4–5 particles) were collected before the reaction and after 40 d operation in the SRB-PRB columns, and then characterized using SEM. As shown in Figure 12a, the surface of unreacted zeolite appeared relatively smooth, with fewer particulate deposits and larger particle sizes. In contrast, the zeolites from the mixed packed column and layered packed column exhibited rough and uneven structures after the reaction, with partial pore blockage and localized fine mineral deposits (Figure 12b,c). These deposits were likely reduction products of SRB metabolism. To further verify the elemental composition of the deposits and the presence of sulfides or sulfates, EDS elemental mapping was performed to obtain elemental spectra (Figure 13). The results indicated that the main metal elements present on the surface of unreacted zeolite were Mg, Al, Ca, and K (Figure 13a). After the reaction, the peak of Fe on the zeolite surface increased in both the mixed packed column and the layered packed column, and the zeolite from the mixed packed column also showed the presence of S (Figure 13b,c). This confirms that SO42− reduction by SRB occurred in the SRB-PRB column, with concurrent enrichment of Fe elements within the zeolite. Due to the more complete SO42− reduction in the mixed packed column, Fe elements combined with S2− to form FeS precipitation, which was extensively attached to the surface, resulting in a higher S peak. Conversely, in the layered packed column, insufficient SO42− reduction allowed more S to escape as H2S, reducing its deposition on the zeolite.
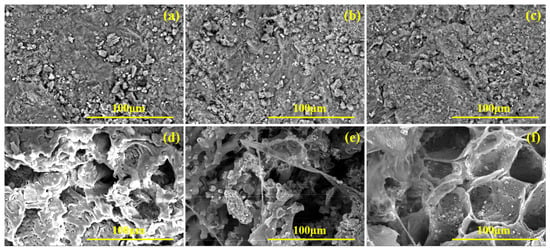
Figure 12.
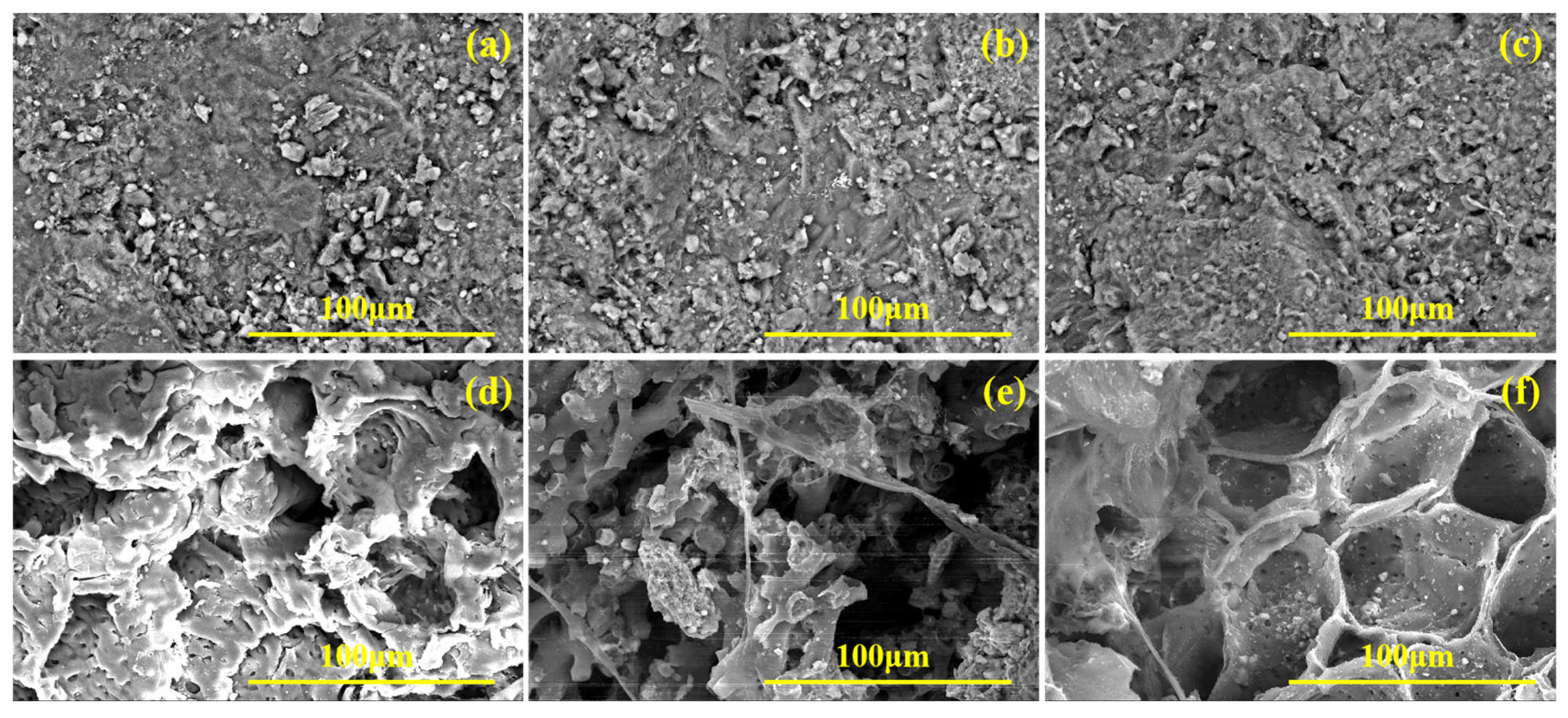
SEM of zeolites and corncobs before and after reaction ((a) zeolite before the experiment, (b) zeolite of column 1#, (c) zeolite of column 2#, (d) corncob before the experiment, (e) corncob of column 1#, (f) corncob of column 2#).

Figure 13.
EDS of zeolites and corncobs before and after reaction ((a) zeolite before the experiment, (b) zeolite of column 1#, (c) zeolite of column 2#, (d) corncob before the experiment, (e) corncob of column 1#, (f) corncob of column 2#). Note: the blue line represents the peak curves of different elements; the red filled area represents the peak area corresponding to different elements.
As shown in Figure 12d, the surface of unreacted corncobs appeared relatively flat with few and uniformly distributed pores. In contrast, after the reaction, the pore structure of corncobs increases significantly, displaying fractures, cavities, or local collapses (Figure 12e,f). In addition, microscopic analysis revealed the presence of needle-like, spherical, and flaky crystal structures adhering to both the surface and internal corncobs. These morphological changes resulted from the main components of corncob being decomposed by microorganisms, and the crystals were zeolite debris, metal precipitates, and biofilms. EDS results (Figure 13) confirmed substantial accumulation of metal elements on post-reaction corncob surfaces, indicating the occurrence of adsorption and metal precipitation. Notably, Fe and S were exclusively detected on corncob from the mixed packed column (Figure 13e), while absent in the layered packed column (Figure 13f), which further confirmed that the mixed packed mode was more conducive to SO42− reduction.
4. Application Prospect of SRB-PRB for In Situ Treatment in Coal Mine Goaf
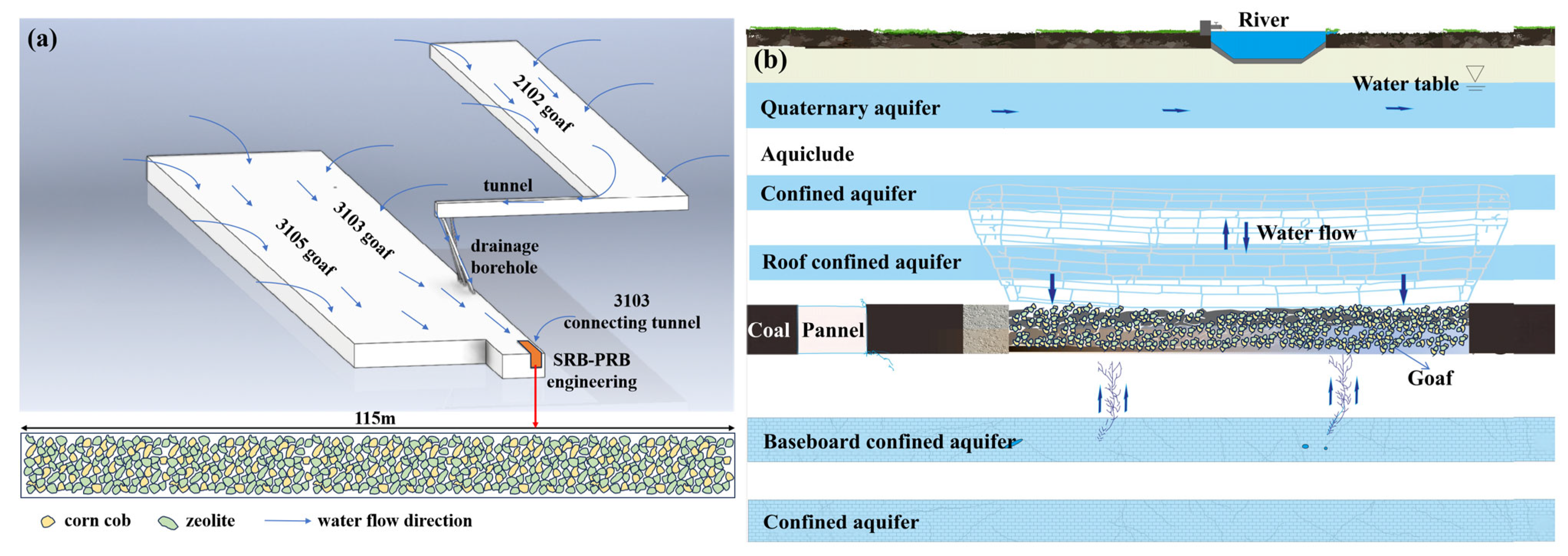
The results of the column experiment indicated the SRB-PRB system could effectively remove SO42−, a characteristic pollutant in mine water, and thus its engineering application could be considered in combination with the actual condition of the goaf. This study proposed an engineering design based on the MKQ coal mine in Ordos as a case study. The construction plan of the SRB-PRB project during the production stage is shown in Figure 14a. The project is located at the 3103 connecting roadway. Mine water from the 2102 goaf flows into the 3103 goaf through diversion boreholes, and mine water from the 3105 goaf can also be directed into this project for in situ underground pretreatment. The treated mine water is ultimately discharged into the sump. In addition, for closed coal mines, corncob and zeolite can be pre-embedded in the goaf before closure according to the SO42− concentration and the content of associated pyrite. This approach can transform the goaf into an in situ reaction zone, significantly reducing construction costs (Figure 14b). To ensure uniform material dispersion and enhance reaction efficiency, the materials can be packed in spherical cages with a density slightly higher than that of water before deployment. The microbial source in the SRB-PRB project is the indigenous microorganisms in the mine. Corncob serves as the slow-release carbon source in the PRB project, and zeolite can not only adsorb SO42− but also immobilize microorganisms. According to the column experiment results, corncob can provide a long-term carbon source supply. The SRB-PRB system offers a low-cost approach for mine water treatment. It can also serve as a complementary measure to other remediation technologies, providing an eco-friendly and economically viable strategy for mine water management.

Figure 14.
Engineering design for in situ application scenarios of SRB-PRB in operating coal (a) mines and abandoned coal (b) mines.
Cost estimation was conducted based on the SO42− removal effect in the column experiments. Firstly, the treatment capacity was deduced. Through integral calculation, the total SO42− removal over 40 d reaction was determined to be 4865.45 mg in the mixed packed column and 4410.03 mg in the layered packed column. The column volume was 490 cm3, with 367.5 cm3 of zeolite and 122.5 cm3 of corncob. The mass of zeolite was 0.897 kg, and that of corncobs was 0.077 kg, resulting in a total mass of 0.974 kg. After calculation, the treatment capacity of the mixed column was 4.97 mg/g, and that of the layered column was 4.52 mg/g. The concentration of SO42− as the characteristic pollutant in the 3103 goaf water was 1027.45 mg/L. The material cost of two operational scenarios was calculated: (1) Treatment to Class III water standard (GB/T 14848-2017) [51], reducing SO42− to 250 mg/L; (2) Treatment of incremental pollution: addressing a 10% increase in SO42− due to pyrite oxidation during mining, restoring concentrations to background groundwater levels to prevent contamination. The cost of zeolite was 9.5 USD/t, and that of corncob was 14 USD/t. The material cost of treating per cubic meter of water using the mixed packed method under different scenarios is shown in Table S6. The result indicated that the material costs for treating to the Class III water standard and treating the increased portion were only 1.55 USD/m3 and 0.20 USD/m3, respectively, which were much lower than the current cost (2.8 USD/m3) of mine water treatment in surface water treatment plants (excluding fixed equipment investments). Although the space requirement was quite substantial, renovating existing underground spaces or directly utilizing them as biological reaction zones could significantly reduce construction costs. Therefore, the SRB-PRB technology in this study can achieve in situ and low-cost removal of SO42− in coal mines.
5. Conclusions
Based on the research findings, the main conclusions are as follows:
- (1)
- Two SRB-PRB columns (1# mixed packed column and #2 layered packed column) were constructed. After 3 d of biofilm colonization, the sulfate removal efficiency reached 85%, indicating successful biofilm establishment. During the 40 d operation, the pH variation could be divided into a rapid decrease stage (0–10 d) and a slow increase stage (10–40 d), with a more significant pH decrease observed in the mixed packed column. The ORP variation showed the following trend: first rapidly decreasing, then rising slowly, and finally stabilizing. Eventually, ORP stabilized at ~−120 mV in the mixed packed column, and ~−100 mV in the layered packed column, both sustaining a strong reducing environment. According to the variation trend of SO42− removal efficiency, the experiment process was divided into three stages: rapid reduction (0–6 d), stable reduction (6–16 d), and reduction attenuation (16–40 d). Corncob could provide a relatively long-term carbon source supply, with the maximum average removal efficiency of 65.5% for the mixed packed column and 56.6% for the layered packed column.
- (2)
- The DOM in the effluents of both the mixed packed column and the layered packed column mainly consisted of tyrosine-like (I zone), tryptophan-like (II zone), and soluble microbial products (IV zone), which served as the main carbon sources for SRB. A large number of complex organic-degrading bacteria were detected in both the effluent water samples and the solid packed media, while SRB became dominant only in the solid packed media, with the relative abundances in 1# and 2# being merely 7.05% and 5.12%, respectively. However, the low-abundance SRB could still maintain a high-efficiency sulfate reduction. This process was closely related to the supply of readily utilizable carbon sources provided by hydrolytic and fermentative bacteria. This study confirmed the existence of a synergistic effect between SRB and these organic-degrading bacteria.
- (3)
- The SEM results of corncob and zeolite showed that the structures of both were damaged with fine mineral deposits, and EDS indicated that Fe and S were enriched on the surfaces of zeolite and corncobs in the mixed packed column. FTIR results revealed that the absorption intensity of functional groups in corncobs decreased after the reaction, with their structures damaged, and this phenomenon was more obvious in the corncob from the mixed packed column. All the above microscopic characterizations confirm that sulfate reduction of SO42− occurred in the SRB-PRB column.
- (4)
- Engineering applicability was evaluated based on 3103 goaf in the MKQ coal mine. The engineering application schemes of SRB-PRB under both in-production and abandoned mining scenarios were proposed. Additionally, a material cost estimate was carried out, and the results showed that SRB-PRB could achieve in situ and low-cost remediation (0.2–1.55 USD/m3) of the characteristic pollutant SO42−.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/w17182684/s1, Figure S1. Fitting results of Lagergren pseudo-first-order and Lagergren pseudo-second-order kinetic models for three adsorbent materials; Figure S2. Fitting results of Elovich kinetic model (a) and intraparticle diffusion model (b) for three adsorbent materials; Figure S3. Adsorption isotherm of SO42− on the three adsorbent materials (a) and Freundlich adsorption isothermal model fitting results (b); Figure S4. Changes in OD600 during SRB fixation in three adsorbent materials; Figure S5. Record of phenomena during SRB-PRB operation (a: experimental effluent; b: effluent pipe; c: glass bead matting); Table S1. Fitting parameters of the four adsorption kinetic models; Table S2. Langmuir and Freundlich adsorption isothermal model fitting parameters; Table S3. Chemical reagents used for preparing the simulated mine water; Table S4. Pore-related parameters of SRB-PRB columns; Table S5. Alpha diversity indexes of microbial community in two experimental columns; Table S6. Material cost of treating per cubic meter of mine water under different remediation targets.
Author Contributions
Conceptualization, L.Z. and Z.X.; methodology, M.X., L.G. and Y.G.; validation, Z.X., C.L. and J.G.; formal analysis, J.G.; investigation, L.Z. and M.X.; resources, L.Z. and L.G.; data curation, L.Z. and L.G.; writing—original draft preparation, L.Z. and M.X.; writing—review and editing, Z.X., Y.G. and C.L.; visualization, J.G.; supervision, M.X.; project administration, Z.X., L.G. and C.L.; funding acquisition, Z.X. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Yunlong Lake Laboratory of Deep Underground Science and Engineering Project 104024002, the National Natural Science Foundation of China 42172272, and the National Key Research and Development Program of China 2023YFC3012103.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge Zhong Tian He Chuang Energy Co., Ltd. (Ordos, China) for sampling support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gu, D.; Li, J.; Cao, Z.; Wu, B.; Jiang, B.; Yang, Y.; Yang, J.; Chen, Y. Technology and engineering development strategy of water protection and utilization of coal mine in China. J. China Coal Soc. 2021, 46, 3079–3089. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, J.; Xu, Z.; Zhang, L.; Chen, G.; Xiong, X.; Hua, J.; Mu, L.; Wu, W. Spatial distribution characteristics of mine water quality in coal mining areas of China and technological approaches for mine water treatment. J. China Coal Soc. 2025, 50, 584–599. [Google Scholar] [CrossRef]
- Li, X.; Ren, H.; Xu, Z.; Chen, G.; Zhang, S.; Zhang, L.; Sun, Y. Practical application for legacy acid mine drainage (AMD) prevention and treatment technologies in karst-dominated regions: A case study. J. Contam. Hydrol. 2023, 258, 104238. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, H.; Zhang, F.; Han, Z.; Shi, H.; Meng, J.; Feng, Q.; Chen, D. Study on the Impact of Closed Coal Mines on Groundwater in the Panlong River Basin (Shangdong Province, China) Based on Sulfur and Oxygen Isotopes. Water 2024, 16, 1634. [Google Scholar] [CrossRef]
- Banks, D.; Boyce, A.J.; Burnside, N.M.; Janson, E.; Roqueñi Gutierrez, N. On the common occurrence of sulphate with elevated δ34S in European mine waters: Sulphides, evaporites or seawater? Int. J. Coal Geol. 2020, 232, 103619. [Google Scholar] [CrossRef]
- Sun, W.; Ren, S.; Wu, Q.; Dong, D.; Gan, X. Waterpollution’s prevention and comprehensive utilization of abandoned coal mines in China under the new normal life. J. China Coal Soc. 2022, 47, 2161–2169. [Google Scholar] [CrossRef]
- Mosai, A.K.; Ndlovu, G.; Tutu, H. Improving acid mine drainage treatment by combining treatment technologies: A review. Sci. Total Environ. 2024, 919, 170806. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, B.; Zhang, H.; Li, J.; He, A.; Hao, J. Progress and optimization strategies for the application of sulfate reducing bacteria in mine water treatment. J. China Coal Soc. 2025, 50, 1–16. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Zhang, Z.; Zhang, Y. Review: Acid Mine Drainage (AMD) in Abandoned Coal Mines of Shanxi, China. Water 2021, 13, 8. [Google Scholar] [CrossRef]
- Virpiranta, H.; Sotaniemi, V.; Leiviskä, T.; Taskila, S.; Rämö, J.; Johnson, D.B.; Tanskanen, J. Continuous removal of sulfate and metals from acidic mining-impacted waters at low temperature using a sulfate-reducing bacterial consortium. Chem. Eng. J. 2022, 427, 132050. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Olanrewaju, O.S.; Babalola, O.O. Sulfate-Reducing Bacteria as an Effective Tool for Sustainable Acid Mine Bioremediation. Front. Microbiol. 2018, 9, 1986. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.K.; Jho, E.H. Heavy metal and sulfate removal from sulfate-rich synthetic mine drainages using sulfate reducing bacteria. Sci. Total Environ. 2018, 635, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Hayashi, K.; Masaki, Y.; Hamai, T.; Fuchida, S.; Takaya, Y.; Tokoro, C. Geochemical Modeling of Heavy Metal Removal from Acid Mine Drainage in an Ethanol-Supplemented Sulfate-Reducing Column Test. Materials 2023, 16, 928. [Google Scholar] [CrossRef] [PubMed]
- Miranda, E.M.; Severson, C.; Reep, J.K.; Hood, D.; Hansen, S.; Santisteban, L.; Hamdan, N.; Delgado, A.G. Continuous-mode acclimation and operation of lignocellulosic sulfate-reducing bioreactors for enhanced metal immobilization from acidic mining-influenced water. J. Hazard. Mater. 2022, 425, 128054. [Google Scholar] [CrossRef]
- Marques, J.P.; Rodrigues, V.G.S. A Systematic Literature Review of Treatment Approaches with Sulfate-Reducing Bacteria for Acid Mine Drainage. Water Air Soil Pollut. 2025, 236, 271. [Google Scholar] [CrossRef]
- Logan, M.V.; Reardon, K.F.; Figueroa, L.A.; Mclain, J.E.T.; Ahmann, D.M. Microbial community activities during establishment, performance, and decline of bench-scale passive treatment systems for mine drainage. Water Res. 2005, 39, 4537–4551. [Google Scholar] [CrossRef]
- Gibert, O.; de Pablo, J.; Luis Cortina, J.; Ayora, C. Chemical characterisation of natural organic substrates for biological mitigation of acid mine drainage. Water Res. 2004, 38, 4186–4196. [Google Scholar] [CrossRef]
- Magowo, W.E.; Sheridan, C.; Rumbold, K. Global Co-occurrence of Acid Mine Drainage and Organic Rich Industrial and Domestic Effluent: Biological sulfate reduction as a co-treatment-option. J. Water Process. Eng. 2020, 38, 101650. [Google Scholar] [CrossRef]
- Sato, Y.; Hamai, T.; Hori, T.; Habe, H.; Kobayashi, M.; Sakata, T. Year-Round Performance of a Passive Sulfate-Reducing Bioreactor that Uses Rice Bran as an Organic Carbon Source to Treat Acid Mine Drainage. Mine Water Environ. 2018, 37, 586–594. [Google Scholar] [CrossRef]
- Rodrigues, C.; Núñez-Gómez, D.; Silveira, D.D.; Lapolli, F.R.; Lobo-Recio, M.A. Chitin as a substrate for the biostimulation of sulfate-reducing bacteria in the treatment of mine-impacted water (MIW). J. Hazard. Mater. 2019, 375, 330–338. [Google Scholar] [CrossRef]
- Dovorogwa, H.; Harding, K. Exploring the Use of Tobacco Waste as a Metal Ion Adsorbent and Substrate for Sulphate-Reducing Bacteria during the Treatment of Acid Mine Drainage. Sustainability 2022, 14, 14333. [Google Scholar] [CrossRef]
- Di, J.; Ma, Y.; Wang, M.; Gao, Z.; Xu, X.; Dong, Y.; Fu, S.; Li, H. Dynamic experiments of acid mine drainage with Rhodopseudomonas spheroides activated lignite immobilized sulfate-reducing bacteria particles treatment. Sci. Rep. 2022, 12, 8783. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Gao, Y.; Tan, X.; Sun, Y.; Chen, W. Effects of Coal Mining Activities on the Changes in Microbial Community and Geochemical Characteristics in Different Functional Zones of a Deep Underground Coal Mine. Water 2024, 16, 1836. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Sun, Y.; Gao, Y.; Zhu, L. Coal Mining Activities Driving the Changes in Microbial Community and Hydrochemical Characteristics of Underground Mine Water. Int. J. Environ. Res. Public Health 2022, 19, 13359. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yao, B.; Jin, L.; Zheng, X.; Ma, J.; Benedetti, M.F.; Li, Y.; Ren, Z. Characterizing Soil Dissolved Organic Matter in Typical Soils from China Using Fluorescence EEM–PARAFAC and UV–Visible Absorption. Aquat. Geochem. 2020, 26, 71–88. [Google Scholar] [CrossRef]
- Taubert, M.; Overholt, W.A.; Heinze, B.M.; Matanfack, G.A.; Houhou, R.; Jehmlich, N.; von Bergen, M.; Rösch, P.; Popp, J.; Küsel, K. Bolstering fitness via CO2 fixation and organic carbon uptake: Mixotrophs in modern groundwater. ISME J. 2022, 16, 1153–1162. [Google Scholar] [CrossRef]
- Yang, Y.; Abdelfattah, A.; Jia, H.; Kumar, V.; Jiang, Y.; Cheng, L. Enhanced nitrogen removal by Comamonas 110 colonization and bioaugmentation in sequencing batch activated sludge bioreactor. Bioresour. Technol. 2025, 433, 132759. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zaiden, N.; Cao, B. The Core- and Pan-Genomic Analyses of the Genus Comamonas: From Environmental Adaptation to Potential Virulence. Front. Microbiol. 2018, 9, 3096. [Google Scholar] [CrossRef]
- Silva, I.; Tacão, M.; Henriques, I. Hidden threats in the plastisphere: Carbapenemase-producing Enterobacterales colonizing microplastics in river water. Sci. Total Environ. 2024, 922, 171268. [Google Scholar] [CrossRef]
- Xiao, X.; Li, C.; Peng, J.; Fan, Y.; Li, W. Dynamic roles of inner membrane electron-transfer hub of Shewanella oneidensis MR-1 in response to extracellular reduction kinetics. Chem. Eng. J. 2023, 451, 138717. [Google Scholar] [CrossRef]
- Tao, X.; Morgan, J.S.; Liu, J.; Kempher, M.L.; Xu, T.; Zhou, J. Target integration of an exogenous β-glucosidase enhances cellulose degradation and ethanol production in Clostridium cellulolyticum. Bioresour. Technol. 2023, 376, 128849. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, J.E.; Houghton, J.N.I.; Rooks, D.J.; Allison, H.E.; Mccarthy, A.J. The microbial ecology of anaerobic cellulose degradation in municipal waste landfill sites: Evidence of a role for fibrobacters. Environ. Microbiol. 2012, 14, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Kurosaki, M.; Nihei, S.; Hasegawa, H.; Shinoda, S.; Haruki, M.; Hirano, N. Enzymatic diversity of the Clostridium thermocellum cellulosome is crucial for the degradation of crystalline cellulose and plant biomass. Sci. Rep. 2016, 6, 35709. [Google Scholar] [CrossRef]
- Wang, X.; Shen, S.; Wu, H.; Wang, H.; Wang, L.; Lu, Z. Acinetobacter tandoii ZM06 Assists Glutamicibacter nicotianae ZM05 in Resisting Cadmium Pressure to Preserve Dipropyl Phthalate Biodegradation. Microorganisms 2021, 9, 1417. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Wu, X.; Xu, X.; Kong, S.; Tong, L.; Jiang, Z.; Li, B. Biodegradation of PAHs by Acinetobacter isolated from karst groundwater in a coal-mining area. Environ. Earth Sci. 2015, 73, 7479–7488. [Google Scholar] [CrossRef]
- Cui, J.; Chen, H.; Sun, M.; Wen, J. Comparison of bacterial community structure and function under different petroleum hydrocarbon degradation conditions. Bioprocess Biosyst. Eng. 2020, 43, 303–313. [Google Scholar] [CrossRef]
- El-Messiry, H.M.; Hamdan, A.M.; Ghanem, N.B.; Hagar, M. Exopolysaccharide produced from Lactiplantibacillus plantarum HAN99 and its nanoparticle formulations in agricultural applications. Sci. Rep. 2025, 15, 19188. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, S.Y. Sorption of Cellulases in Biofilm Enhances Cellulose Degradation by Bacillus subtilis. Microorganisms 2022, 10, 1505. [Google Scholar] [CrossRef]
- Bian, X.; Miao, W.; Zhao, M.; Zhao, Y.; Xiao, Y.; Li, N.; Wu, J. Microbiota drive insoluble polysaccharides utilization via microbiome-metabolome interplay during Pu-erh tea fermentation. Food Chem. 2022, 377, 132007. [Google Scholar] [CrossRef]
- Maharaja, P.; Boopathy, R.; Anushree, V.V.; Mahesh, M.; Swarnalatha, S.; Ravindran, B.; Chang, S.W.; Sekaran, G. Bio removal of proteins, lipids and mucopolysaccharides in tannery hyper saline wastewater using halophilic bacteria. J. Water Process. Eng. 2020, 38, 101674. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, R.; Li, X.; Yan, H.; Zheng, J.; Peng, N.; Zhao, S. Effect of simplified inoculum agent on performance and microbiome during cow manure-composting at industrial-scale. Bioresour. Technol. 2024, 393, 130097. [Google Scholar] [CrossRef]
- Cherian, T.; Maity, D.; Rajendra Kumar, R.T.; Balasubramani, G.; Ragavendran, C.; Yalla, S.; Mohanraju, R.; Peijnenburg, W.J.G.M. Green Chemistry Based Gold Nanoparticles Synthesis Using the Marine Bacterium Lysinibacillus odysseyi PBCW2 and Their Multitudinous Activities. Nanomaterials 2022, 12, 2940. [Google Scholar] [CrossRef]
- Walter, A.; Sopracolle, L.; Mutschlechner, M.; Spruck, M.; Griesbeck, C. Biodegradation of different PET variants from food containers by Ideonella sakaiensis. Arch. Microbiol. 2022, 204, 711. [Google Scholar] [CrossRef]
- Tanasupawat, S.; Takehana, T.; Yoshida, S.; Hiraga, K.; Oda, K. Ideonella sakaiensis sp. nov., isolated from a microbial consortium that degrades poly(ethylene terephthalate). Int. J. Syst. Evol. Microbiol. 2016, 66, 2813–2818. [Google Scholar] [CrossRef]
- Gao, P.; Sun, X.; Xiao, E.; Xu, Z.; Li, B.; Sun, W. Characterization of iron-metabolizing communities in soils contaminated by acid mine drainage from an abandoned coal mine in Southwest China. Environ. Sci. Pollut. Res. 2019, 26, 9585–9598. [Google Scholar] [CrossRef]
- Sato, Y.; Hamai, T.; Hori, T.; Aoyagi, T.; Inaba, T.; Kobayashi, M.; Habe, H.; Sakata, T. Desulfosporosinus spp. were the most predominant sulfate-reducing bacteria in pilot- and laboratory-scale passive bioreactors for acid mine drainage treatment. Appl. Microbiol. Biotechnol. 2019, 103, 7783–7793. [Google Scholar] [CrossRef] [PubMed]
- Mirjafari, P.; Baldwin, S.A. Decline in Performance of Biochemical Reactors for Sulphate Removal from Mine-Influenced Water is Accompanied by Changes in Organic Matter Characteristics and Microbial Population Composition. Water 2016, 8, 124. [Google Scholar] [CrossRef]
- Rodrigues, C.; Núñez-Gómez, D.; Follmann, H.V.D.M.; Silveira, D.D.; Nagel-Hassemer, M.E.; Lapolli, F.R.; Lobo-Recio, M.Á. Biostimulation of sulfate-reducing bacteria and metallic ions removal from coal mine-impacted water (MIW) using shrimp shell as treatment agent. J. Hazard. Mater. 2020, 398, 122893. [Google Scholar] [CrossRef] [PubMed]
- Kostryukov, S.G.; Matyakubov, H.B.; Masterova, Y.Y.; Kozlov, A.S.; Pryanichnikova, M.K.; Pynenkov, A.A.; Khluchina, N.A. Determination of Lignin, Cellulose, and Hemicellulose in Plant Materials by FTIR Spectroscopy. J. Anal. Chem. 2023, 78, 718–727. [Google Scholar] [CrossRef]
- GB/T 14848-2017; Standard for Groundwater Quality. Standards Press of China: Beijing, China, 2017.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).