Machine Learning-Based Spatiotemporal Acid Mine Drainage Prediction Using Geological, Climate History, and Associated Water Quality Parameters
Abstract
1. Introduction
2. Materials and Methods
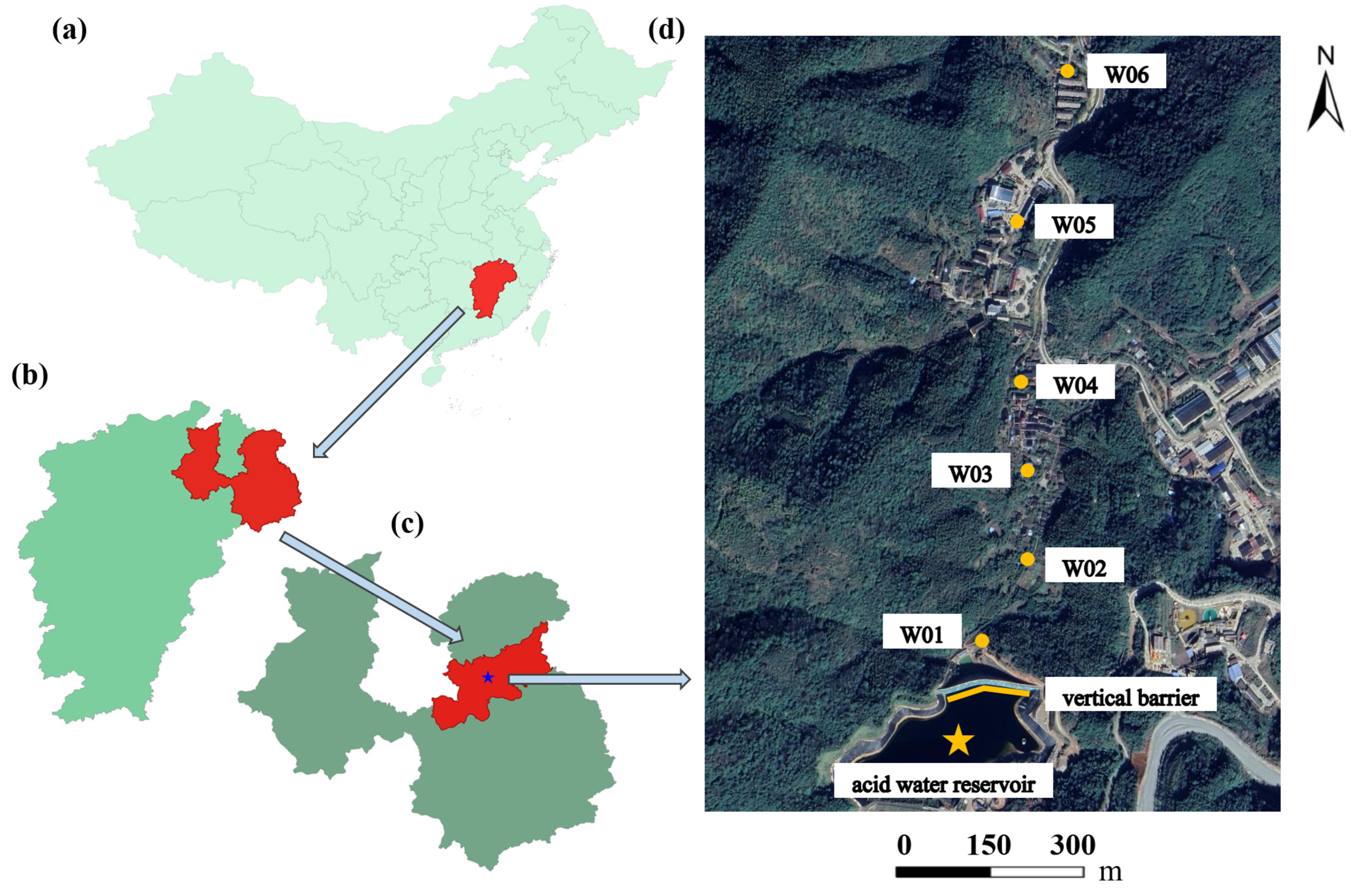
2.1. Study Area
2.2. Data Collection
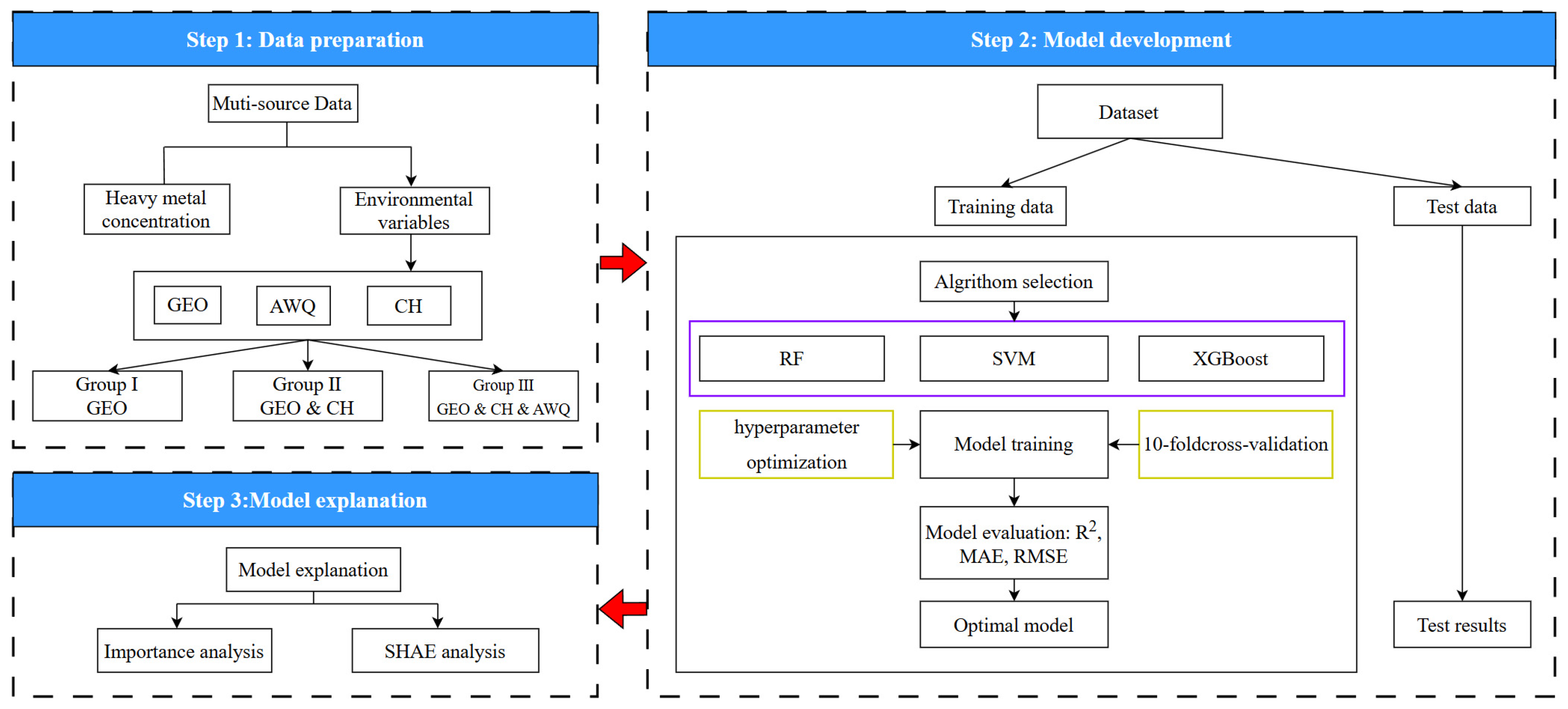
2.3. Workflow for Heavy Metal Concentration Prediction
2.4. Methods
2.4.1. Data Segmentation
2.4.2. Model Selection
2.4.3. Hyperparameter Optimization
2.4.4. SHAP
2.4.5. Model Evaluation
3. Results and Discussion
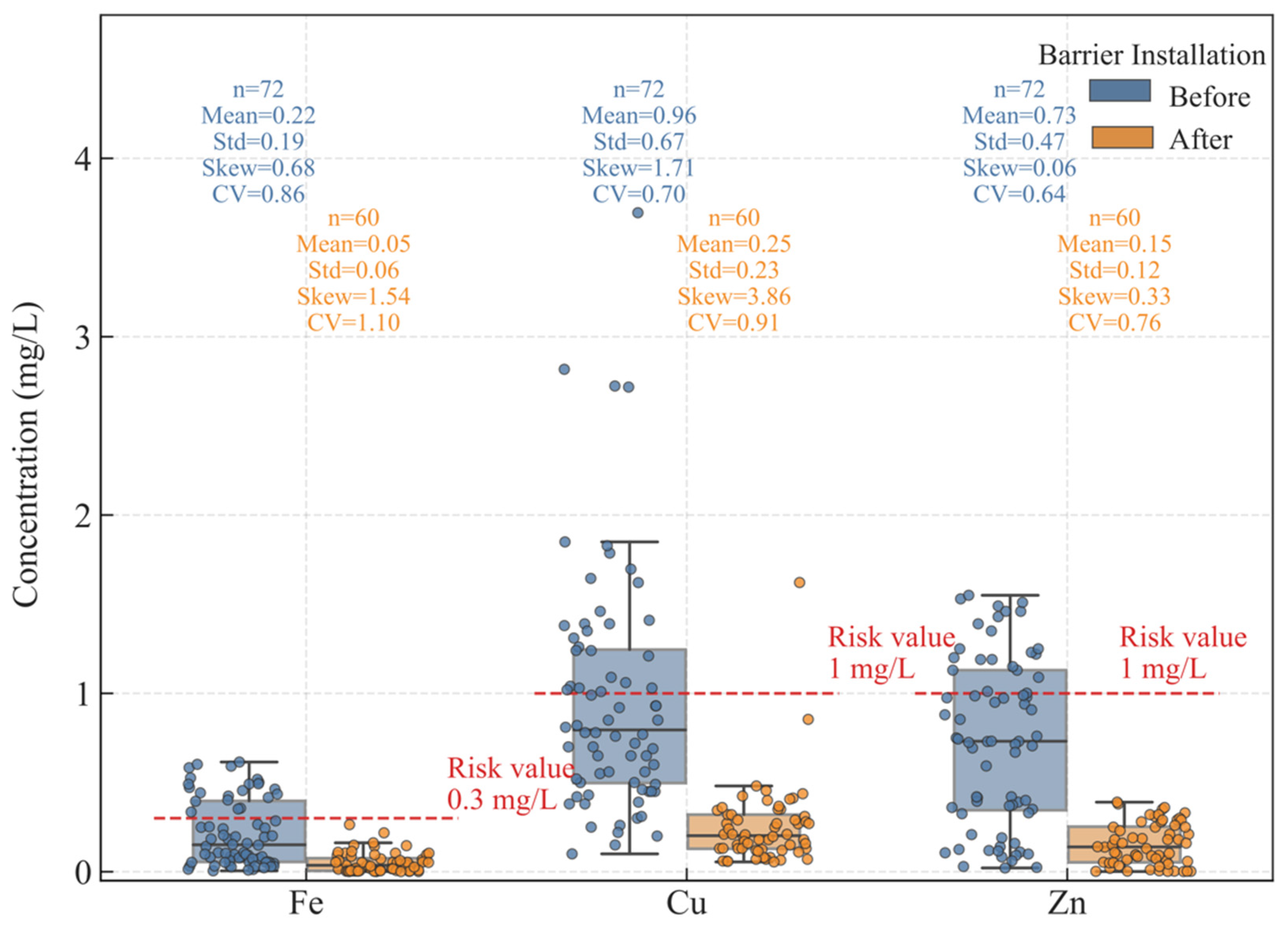
3.1. Heavy Metal Concentration
3.2. Overall Prediction Performance
3.3. Accuracy of Fe, Cu, and Zn Concentration Predictions
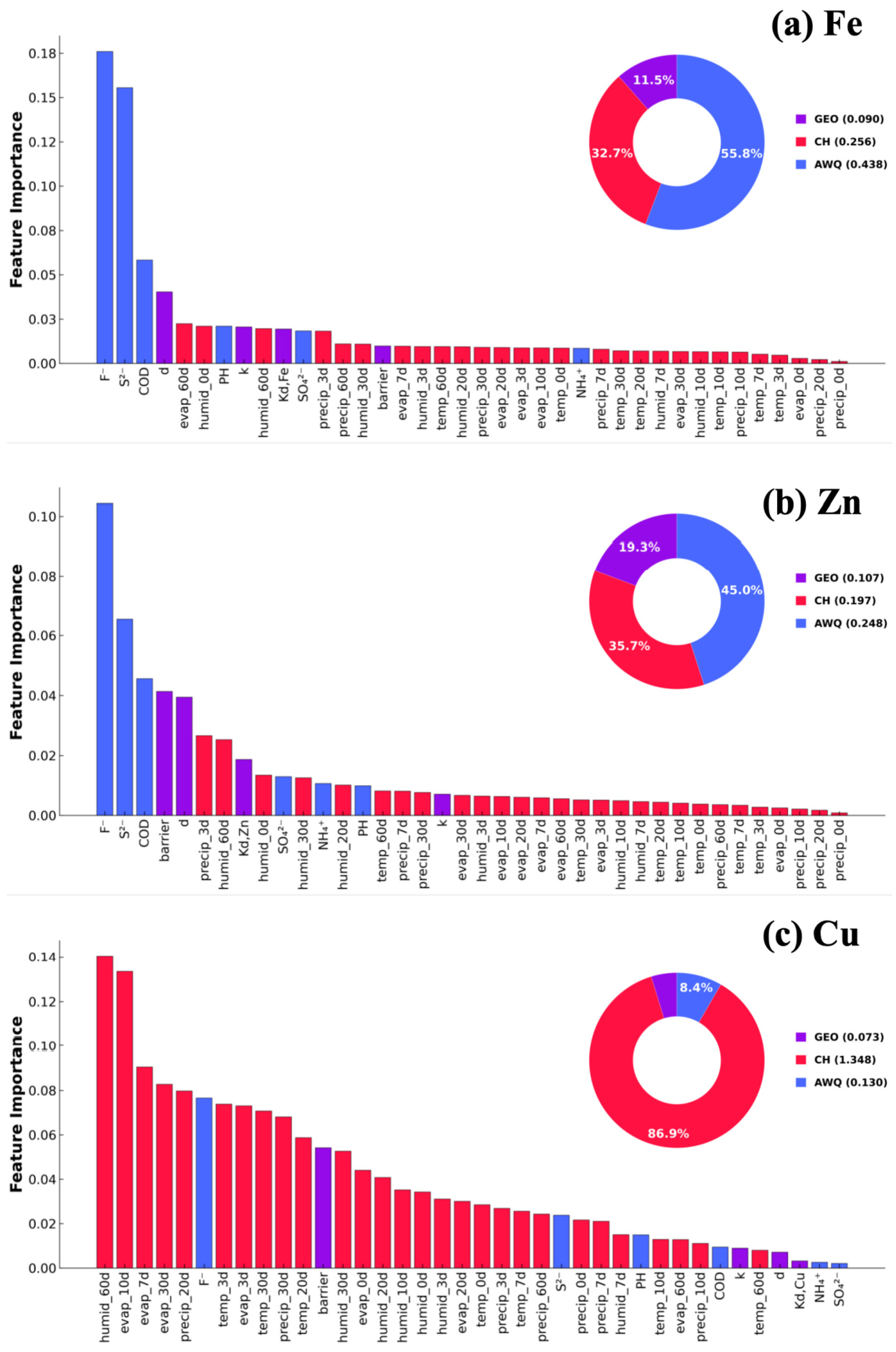
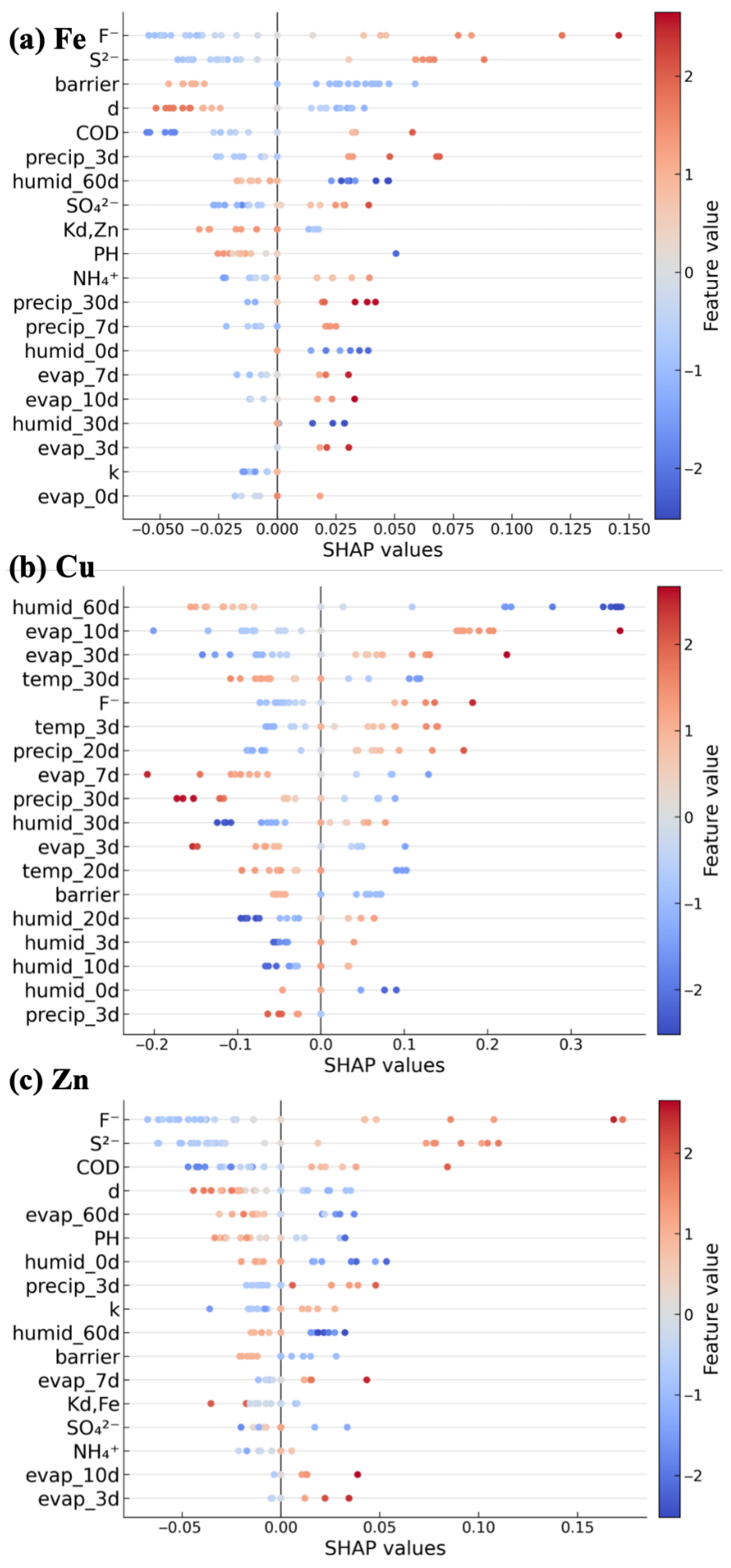
3.4. Parametric Analysis of Fe Concentration Predictions
3.5. Parametric Analysis of Cu Concentration Predictions
3.6. Parametric Analysis of Zn Concentration Predictions
4. Conclusions
- (1)
- Groundwater monitoring before and after the barrier installation revealed a significant reduction in Fe, Zn, and Cu concentrations, demonstrating the barrier’s effectiveness in limiting heavy metal transport. However, the high CV (>50%) and increased skewness of the heavy metal concentration, concentrations indicated substantial spatial-temporal variability and potential extreme values. These fluctuations are largely influenced by the regional hydrological cycle, especially during the wet season, which enhances leaching and pollutant mobility.
- (2)
- The combined use of all three input parameter sets (GEO, CH and AWQ) generally yielded the best predictions. The XGBoost predictions exhibited the highest R2 values for Fe (0.805) and Cu (0.773) concentrations, while SVM predictions provided the best predictive performance for Zn concentrations (R2 of 0.94). The use of two sets of input parameters (GEO and CH) generally yielded better prediction than the use of geological parameters alone. Given the general availability and affordability of climate history data, as well as the physical relationships between climate history and downstream contaminant concentrations, the combined use of these two sets of input parameters are recommended.
- (3)
- Associated water quality parameters, and especially F− and S2− concentrations, are the most relevant for predicting both Fe and Zn concentrations, also ranking second most important for Cu concentration predictions. The shared upstream reservoir origin and downstream transportation pathways are the likely reasons these AWQs are good predictors for Fe and Zn concentrations, wherein the high solubility of FeF3 promotes the release or fixation of both Fe3+ and F−, emphasizing their physical linkage.
- (4)
- Climate history was the most important factor for Cu concentration prediction and the second most important factor for Fe and Zn concentration predictions. Accumulated precipitation and evaporation up to 60 days are related to metal concentrations via dynamic water rebalance and solute redistribution mechanisms.
- (5)
- Installation of a vertical contaminant-containing barrier was the most effective geological parameter, followed by distance and the partition coefficient. All three metal contaminant concentrations were drastically reduced (73–80%) following barrier installation.
- (6)
- This study’s scope is confined to observations from a singular AMD reservoir site, potentially restricting the generalizability of the results to other settings. Enhancing the analysis by incorporating data from multiple copper mine AMD reservoir sites could bolster the reliability and wider relevance of the conclusions. Additionally, the utilization of remote sensing data offers valuable prospects for capturing spatial and temporal fluctuations in environmental factors. Subsequent research endeavors could investigate the integration of such data into the modeling framework to potentially elevate predictive precision and utility [22].
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
References
- Lefebvre, R.; Hockley, D.; Smolensky, J.; Gélinas, P. Multiphase transfer processes in waste rock piles producing acid mine drainage: 1: Conceptual model and system characterization. J. Contam. Hydrol. 2001, 52, 137–164. [Google Scholar] [CrossRef]
- Gao, T.; Wu, A.; Wang, S.; Ruan, Z.; Chen, C.; Sun, W. Compression behavior and microscopic damage mechanism of waste rock-tailings matrix composites: Experiments and models. Constr. Build. Mater. 2024, 425, 136076. [Google Scholar] [CrossRef]
- Cánovas, C.; Olías, M.; Macias, F.; Torres, E.; San Miguel, E.; Galván, L.; Ayora, C.; Nieto, J. Water acidification trends in a reservoir of the Iberian Pyrite Belt (SW Spain). Sci. Total Environ. 2016, 541, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yang, Y.-X.; Cao, J.-J.; Meng, L.-L.; Cao, J.-N.; Zhang, C.; Zhang, S.; Bate, B. Monitoring of copper adsorption on biochar using spectral induced polarization method. Environ. Res. 2024, 251, 118778. [Google Scholar] [CrossRef] [PubMed]
- Kalin, M.; Fyson, A.; Wheeler, W.N. The chemistry of conventional and alternative treatment systems for the neutralization of acid mine drainage. Sci. Total Environ. 2006, 366, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ye, Y.; Yao, N.; Hu, N.; Zhang, J.; Huang, Y. A critical review of prevention, treatment, reuse, and resource recovery from acid mine drainage. J. Clean. Prod. 2021, 329, 129666. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Z.; Lin, H. Hydrophobic modification of pyrite with a composite of sodium oleate and SiO2 nanoparticles to inhibit its oxidation for controlling acid mine drainage. J. Environ. Chem. Eng. 2023, 11, 109571. [Google Scholar] [CrossRef]
- Lin, H.; Zhi, T.; Zhang, L.; Liu, C.; Dong, Y. Effects of acid/alkali-pretreated peanut shells as a cheap carbon source for the bio-reduction of sulfate. J. Clean. Prod. 2023, 385, 135753. [Google Scholar] [CrossRef]
- Yuan, S.; Han, G.; Liang, Y. Groundwater control in open-pit mine with grout curtain using modified lake mud: A case study in East China. Arab. J. Geosci. 2021, 14, 1148. [Google Scholar] [CrossRef]
- Cacciuttolo, C.; Pastor, A.; Valderrama, P.; Atencio, E. Process water management and seepage control in tailings storage facilities: Engineered environmental solutions applied in Chile and Peru. Water 2023, 15, 196. [Google Scholar] [CrossRef]
- Wu, Q.; Li, X.; Feng, Q.; Li, X. Source reduction and end treatment of acid mine drainage in closed coal mines of the Yudong River Basin. Water Sci. Technol. 2024, 89, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Sun, B.; Han, G.; Duan, W.; Wang, Z. Application and prospect of curtain grouting technology in mine water safety management in China: A review. Water 2022, 14, 4093. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Liang, M.; Hu, H.; Chen, S.; Duan, L.; Chen, Z.; Yang, X.; Cai, J. Experimental study on the stabilization and anti-seepage treatment of lead and zinc elements in heavy metal tailings pond using cement slurry containing heavy metal stabilizing agent. Constr. Build. Mater. 2024, 425, 135964. [Google Scholar] [CrossRef]
- Li, X.; Ren, H.; Xu, Z.; Chen, G.; Zhang, S.; Zhang, L.; Sun, Y. Practical application for legacy acid mine drainage (AMD) prevention and treatment technologies in karst-dominated regions: A case study. J. Contam. Hydrol. 2023, 258, 104238. [Google Scholar] [CrossRef] [PubMed]
- Cacciuttolo, C.; Cano, D. Environmental impact assessment of mine tailings spill considering metallurgical processes of gold and copper mining: Case studies in the Andean countries of Chile and Peru. Water 2022, 14, 3057. [Google Scholar] [CrossRef]
- Rooki, R.; Doulati Ardejani, F.; Aryafar, A.; Bani Asadi, A. Prediction of heavy metals in acid mine drainage using artificial neural network from the Shur River of the Sarcheshmeh porphyry copper mine, Southeast Iran. Environ. Earth Sci. 2011, 64, 1303–1316. [Google Scholar] [CrossRef]
- Kabuba, J.; Maliehe, A.V. Application of neural network techniques to predict the heavy metals in acid mine drainage from South African mines. Water Sci. Technol. 2021, 84, 3489–3507. [Google Scholar] [CrossRef]
- Trifi, M.; Gasmi, A.; Carbone, C.; Majzlan, J.; Nasri, N.; Dermech, M.; Charef, A.; Elfil, H. Machine learning-based prediction of toxic metals concentration in an acid mine drainage environment, northern Tunisia. Environ. Sci. Pollut. Res. 2022, 29, 87490–87508. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Fu, Y.; Liu, Z.; Wang, P.; Yin, J.; Kou, J.; Sun, C.; Liu, W. Enhanced leaching of copper from refractory oxidized copper ore by calcium fluoride: Behavior and mechanism. Green Smart Min. Eng. 2024, 1, 85–95. [Google Scholar] [CrossRef]
- Hasrod, T.; Nuapia, Y.B.; Tutu, H. Comparison of individual and ensemble machine learning models for prediction of sulphate levels in untreated and treated Acid Mine Drainage. Environ. Monit. Assess. 2024, 196, 332. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, K.; Xiong, B.; Guo, C.; Dang, Z. Prediction of adsorption of metal cations by clay minerals using machine learning. Sci. Total Environ. 2024, 924, 171733. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Acid rock drainage and climate change. J. Geochem. Explor. 2009, 100, 97–104. [Google Scholar] [CrossRef]
- Anawar, H.M. Impact of climate change on acid mine drainage generation and contaminant transport in water ecosystems of semi-arid and arid mining areas. Phys. Chem. Earth Parts A/B/C 2013, 58, 13–21. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Y.; Chen, J.; Azam, M.; Asubonteng, D.; Ngoie, M.; Lin, S.; Sun, W. Advancements in Removing Fluorine from Copper Concentrate. Min. Metall. Explor. 2023, 40, 1487–1497. [Google Scholar] [CrossRef]
- Solid Waste—Determination of Metals—Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Ministry of Ecology and Environment of the People’s Republic of China (MEE). 2015. Available online: https://std.samr.gov.cn/search/std?tid=&q=HJ766-2015 (accessed on 10 May 2025).
- Li, K.; Guo, G.; Zhang, D.; Lei, M.; Wang, Y. Accurate prediction of spatial distribution of soil potentially toxic elements using machine learning and associated key influencing factors identification: A case study in mining and smelting area in southwestern China. J. Hazard. Mater. 2024, 478, 135454. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pang, Y.; Ding, Y.; Fan, Z.; Xu, Y.; Liu, W. Data-driven machine learning modeling reveals the impact of micro/nanoplastics on microalgae and their key underlying mechanisms. J. Hazard. Mater. 2025, 496, 139338. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Parmar, A.; Katariya, R.; Patel, V. A review on random forest: An ensemble classifier. In Proceedings of the International Conference on Intelligent Data Communication Technologies and Internet of Things, Coimbatore, India, 7–8 August 2018; pp. 758–763. [Google Scholar]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K.; Mitchell, R.; Cano, I.; Zhou, T. Xgboost: Extreme Gradient Boosting, R Package Version 0.4-2; Scientific Research Publishing Inc.: Wuhan, China, 2015. [CrossRef]
- Jakkula, V. Tutorial on support vector machine (svm). Sch. EECS Wash. State Univ. 2006, 37, 3. [Google Scholar] [CrossRef]
- Joachims, T. Making Large-Scale SVM Learning Practical; Technical Report No. 1998,28; Universität Dortmund: Dortmund, Germany, 1998. [Google Scholar]
- Standard for Groundwater Quality. Standardization Administration of China (SAC). 2017. Available online: https://std.samr.gov.cn/search/std?q=GB/T14848-2017 (accessed on 10 May 2025).
- Barroso, A.; Henriques, R.; Cerqueira, Â.; Gomes, P.; Antunes, I.M.H.R.; Reis, A.P.M.; Valente, T.M. Acid mine drainage and waste dispersion in legacy mining sites: An integrated approach using UAV photogrammetry and geospatial analysis. J. Hazard. Mater. 2025, 495, 138827. [Google Scholar] [CrossRef]
- Domingos, P. A few useful things to know about machine learning. Commun. ACM 2012, 55, 78–87. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z. A risk prediction model for type 2 diabetes based on weighted feature selection of random forest and xgboost ensemble classifier. In Proceedings of the 2019 Eleventh International Conference on Advanced Computational Intelligence (ICACI), Guilin, China, 7–9 June 2019; pp. 278–283. [Google Scholar]
- Dhaliwal, S.S.; Nahid, A.-A.; Abbas, R. Effective intrusion detection system using XGBoost. Information 2018, 9, 149. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Z.; Hu, Y. XGBoost algorithm-based prediction of safety assessment for pipelines. Int. J. Press. Vessel. Pip. 2022, 197, 104655. [Google Scholar] [CrossRef]
- Cevikalp, H. Best fitting hyperplanes for classification. IEEE Trans. Pattern Anal. Mach. Intell. 2016, 39, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Yin, W.; Jia, H.; Xu, J.; Li, N.; Chen, Q.; Zhong, Z.; Wang, J.; Shi, Z. Dynamics of dissolved heavy metals in reservoir bays under different hydrological regulation. J. Hydrol. 2021, 595, 126042. [Google Scholar] [CrossRef]
- Hutter, F.; Kotthoff, L.; Vanschoren, J. Automated Machine Learning: Methods, Systems, Challenges; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Gholami, R.; Kamkar-Rouhani, A.; Doulati Ardejani, F.; Maleki, S. Prediction of toxic metals concentration using artificial intelligence techniques. Appl. Water Sci. 2011, 1, 125–134. [Google Scholar] [CrossRef]
- Maulana, A.; Irfan, U.R. Characteristic of Alteration and Mineralization of Sulfide Deposits at Sasak area, Tana Toraja, Indonesia. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Surabaya, Indonesia, 25–26 October 2023; p. 012029. [Google Scholar] [CrossRef]
- Gálvez, J.L.; Dufour, J.; Negro, C.; López-Mateos, F. Determination of iron and chromium fluorides solubility for the treatment of wastes from stainless steel mills. Chem. Eng. J. 2008, 136, 116–125. [Google Scholar] [CrossRef]
- Gallagher, T.C.; Sandstrom, S.K.; Wu, C.-Y.; Stickle, W.; Fulkerson, C.R.; Hagglund, L.; Ji, X. Copper metal electrode reversibly hosts fluoride in a 16 m KF aqueous electrolyte. Chem. Commun. 2022, 58, 10218–10220. [Google Scholar] [CrossRef]
- Su, H.; Qian, X.; Gu, Z.; Xu, Z.; Lou, H.; Bian, X.; Zeng, T.; Lin, D.; Filser, J.; Li, L. Cu(OH)2 nanorods undergo sulfidation in water: In situ formation of CuO nanorods as intermediates and enhanced toxicity to Escherichia coli. Environ. Chem. Lett. 2020, 18, 1737–1744. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Zeng, D. Experimental measurement of the solid–liquid equilibrium of the systems MF2 + H2O (M = Mg, Ca, Zn) from 298.15 to 353.15 K. J. Chem. Eng. Data. 2018, 63, 1733–1736. [Google Scholar] [CrossRef]
- Yang, K.; Li, B.; Zeng, G. Effects of temperature on properties of ZnS thin films deposited by pulsed laser deposition. Superlattices Microstruct. 2019, 130, 409–415. [Google Scholar] [CrossRef]
| Well ID | d (m) | Kd,Cu | Kd,Fe | Kd,Zn | k |
|---|---|---|---|---|---|
| W01 | 299 | 48.4 | 12.7 | 7.7 | 0.091 |
| W02 | 432 | 94.1 | 340.9 | 134.5 | 0.091 |
| W03 | 575 | 77.6 | 627.2 | 15.8 | 0.091 |
| W04 | 855 | 64.7 | 2914.7 | 3.0 | 0.085 |
| W05 | 1219 | 53.9 | 740.5 | 64.3 | 0.081 |
| W06 | 1562 | 57.6 | 691.9 | 126.5 | 0.084 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Chen, Z.; Wang, B.; Luo, Y.; Du, A.; Wang, Q.; Bate, B. Machine Learning-Based Spatiotemporal Acid Mine Drainage Prediction Using Geological, Climate History, and Associated Water Quality Parameters. Water 2025, 17, 2661. https://doi.org/10.3390/w17182661
Wu X, Chen Z, Wang B, Luo Y, Du A, Wang Q, Bate B. Machine Learning-Based Spatiotemporal Acid Mine Drainage Prediction Using Geological, Climate History, and Associated Water Quality Parameters. Water. 2025; 17(18):2661. https://doi.org/10.3390/w17182661
Chicago/Turabian StyleWu, Xinyu, Zhitao Chen, Bin Wang, Yuanyuan Luo, Aifang Du, Qiong Wang, and Bate Bate. 2025. "Machine Learning-Based Spatiotemporal Acid Mine Drainage Prediction Using Geological, Climate History, and Associated Water Quality Parameters" Water 17, no. 18: 2661. https://doi.org/10.3390/w17182661
APA StyleWu, X., Chen, Z., Wang, B., Luo, Y., Du, A., Wang, Q., & Bate, B. (2025). Machine Learning-Based Spatiotemporal Acid Mine Drainage Prediction Using Geological, Climate History, and Associated Water Quality Parameters. Water, 17(18), 2661. https://doi.org/10.3390/w17182661






