Spatial Distribution, Key Influencing Factors, and Ecological Risk of Microplastics in Pearl River Estuary Water and Sediments
Abstract
1. Introduction
2. Study Area and Data Source
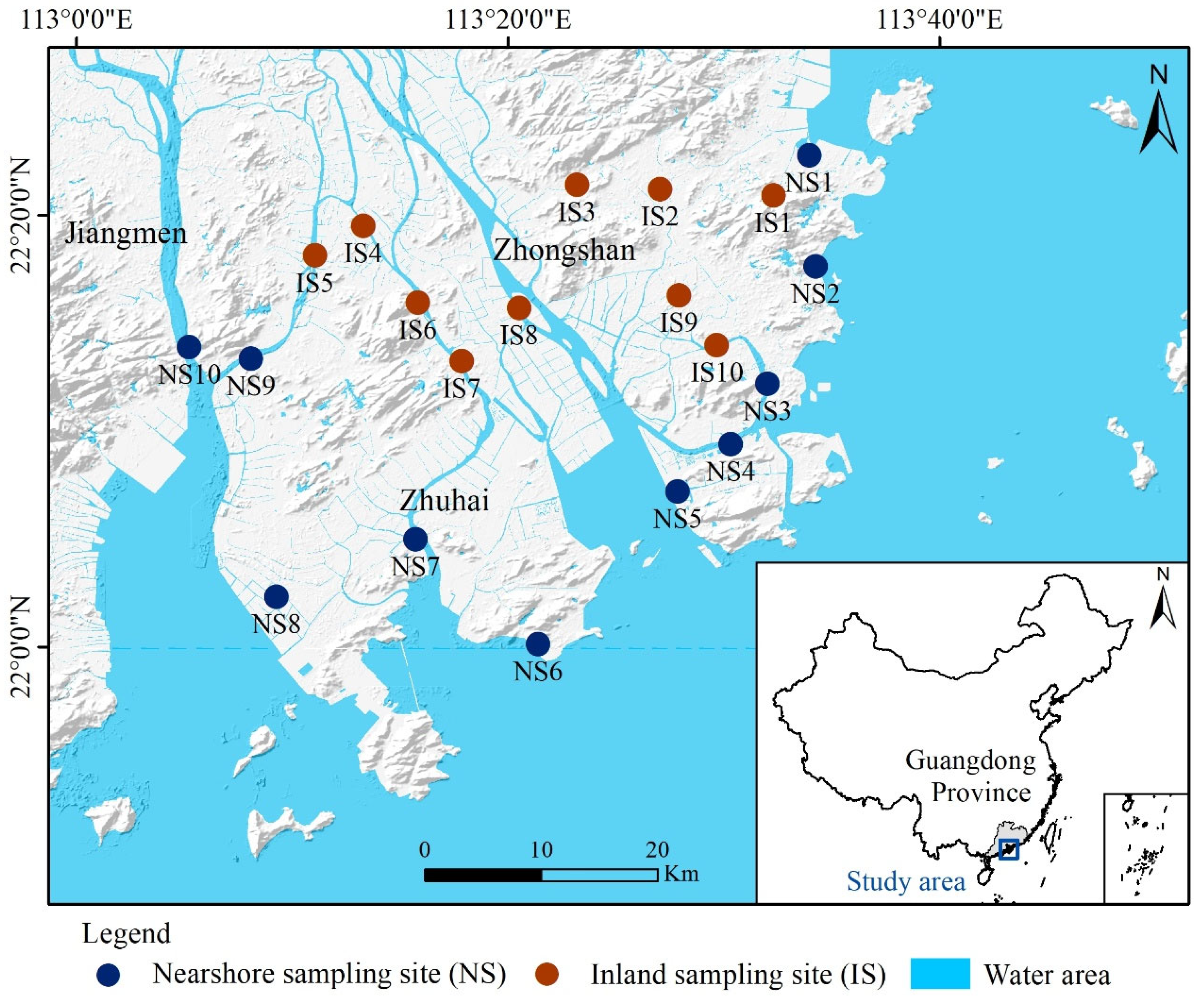
2.1. Study Area
2.2. MPs Sampling and Testing
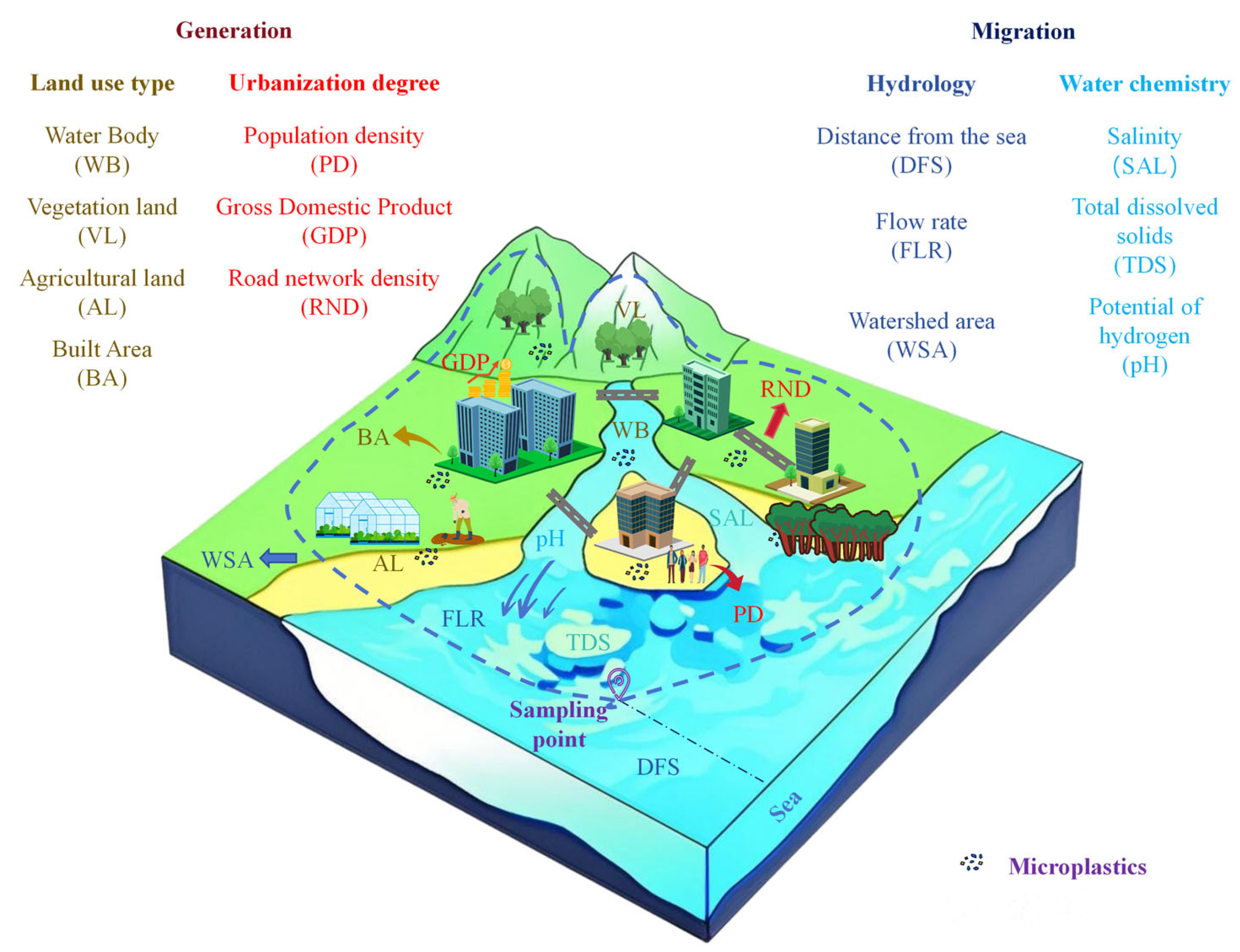
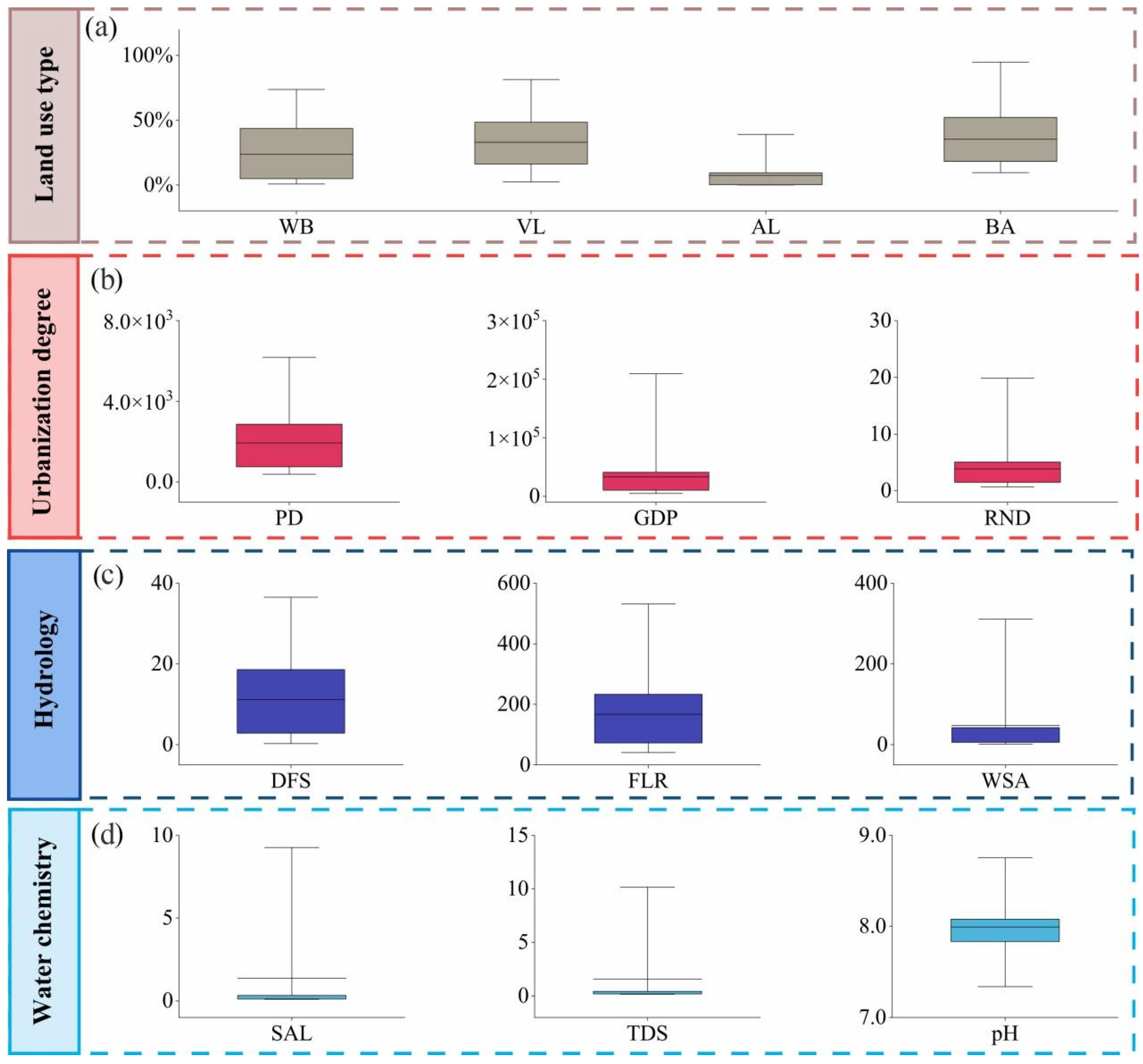
2.3. IFs Data and Characteristics
3. Analytical Methods
3.1. Microplastic Diversity Integrated Index (MDII)
3.2. Multivariate Statistical Analysis Methods
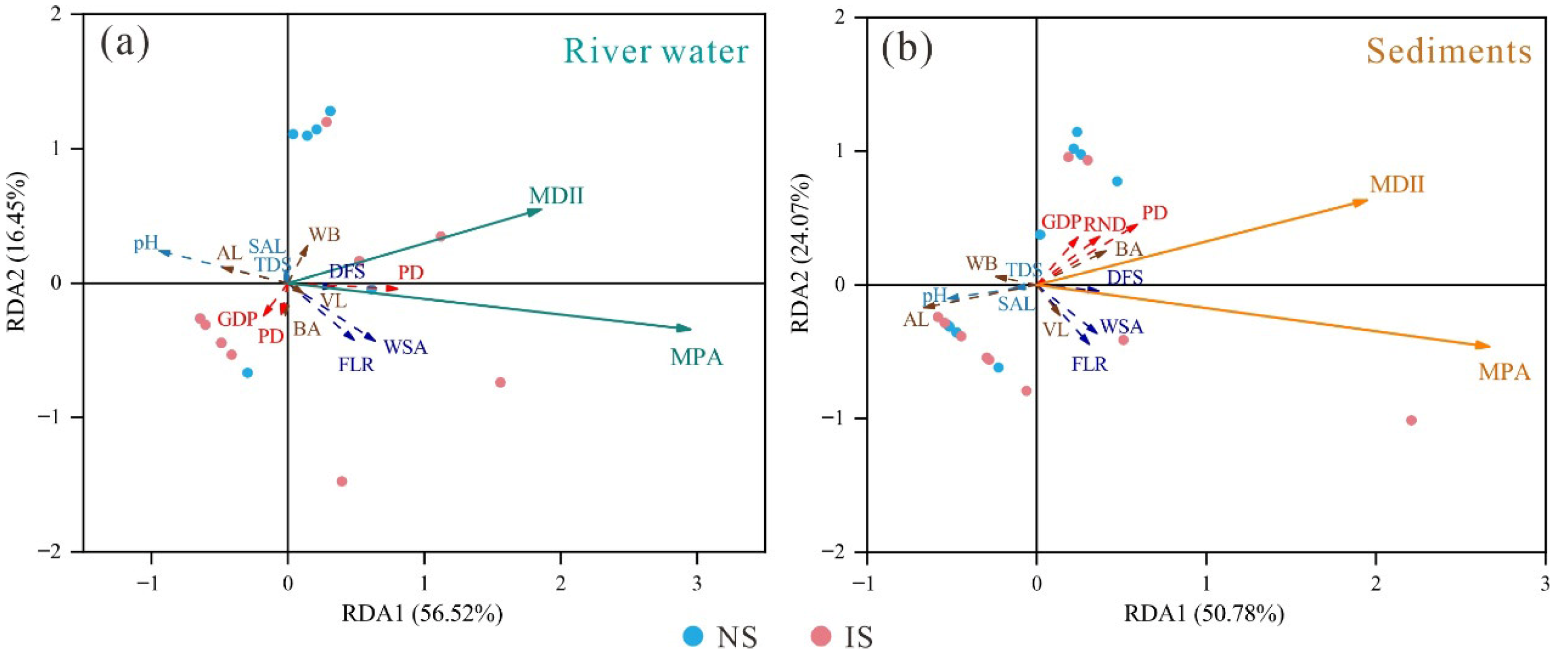
3.2.1. Redundancy Analysis (RDA)
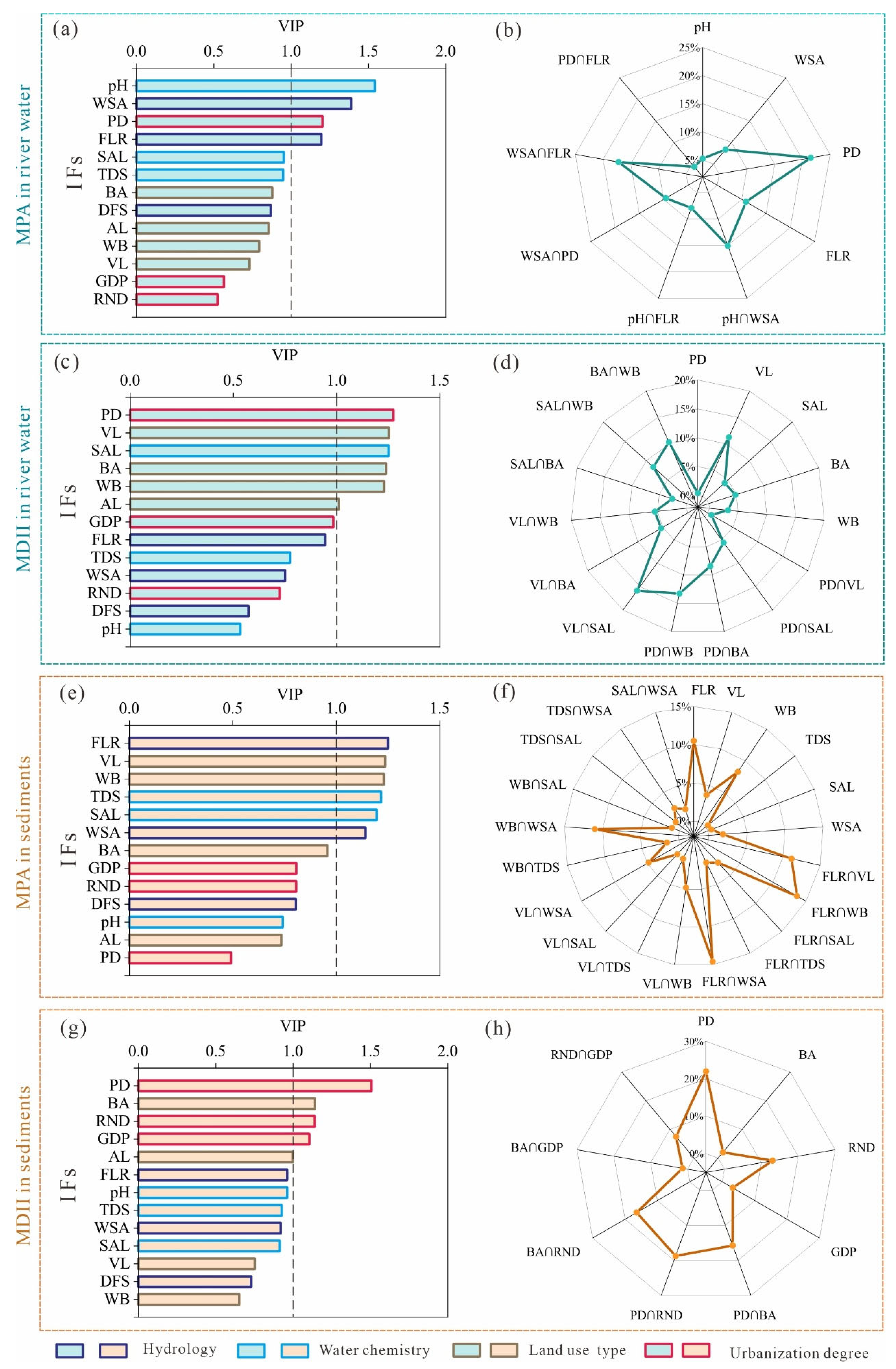
3.2.2. Partial Least Squares Regression (PLSR)
3.2.3. Ridge Regression (RR)
3.3. Ecological Risk Assessment
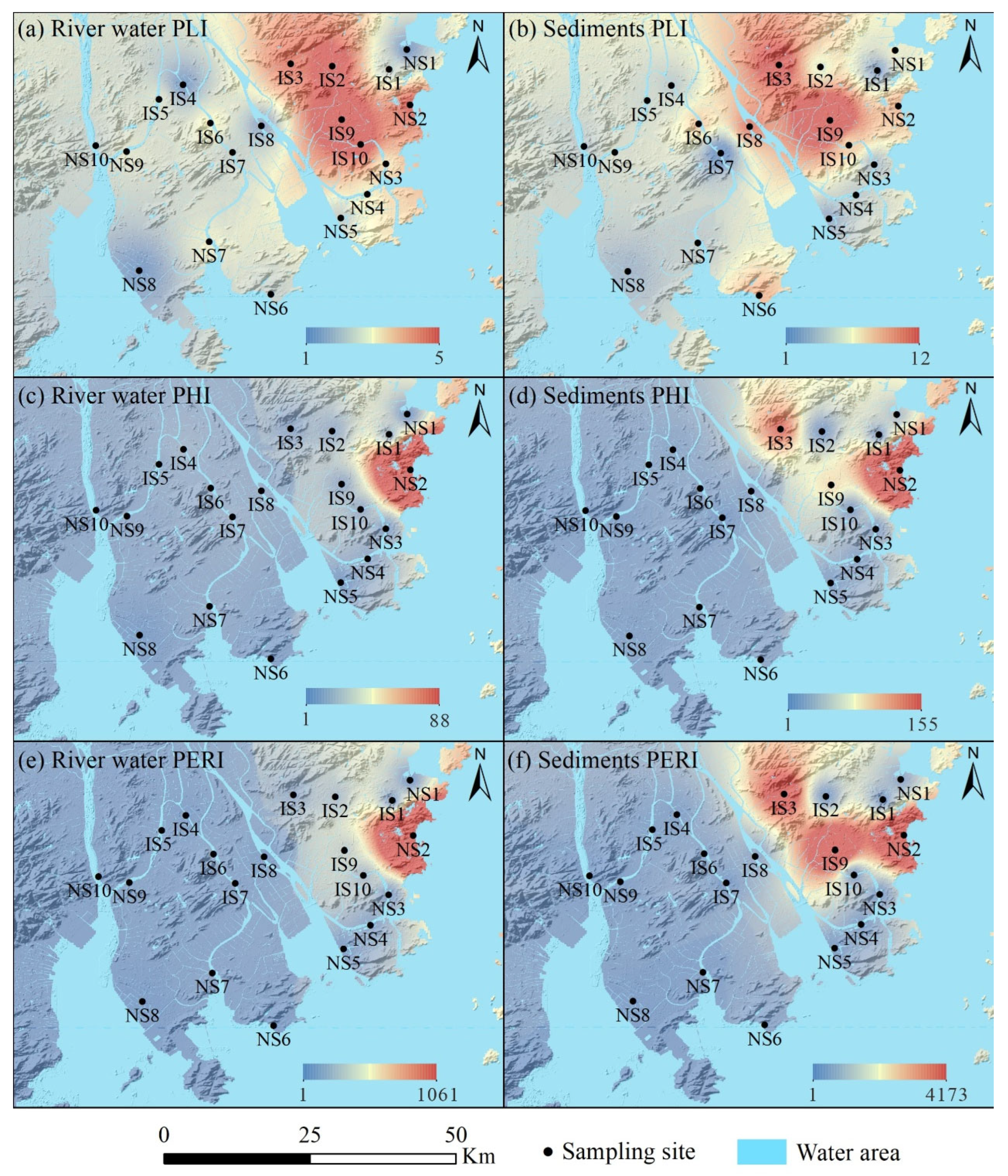
3.3.1. Pollution Load Index (PLI)
3.3.2. Polymer Hazardous Index (PHI)
3.3.3. Polymer Ecological Risk Index (PERI)
4. Results and Discussion
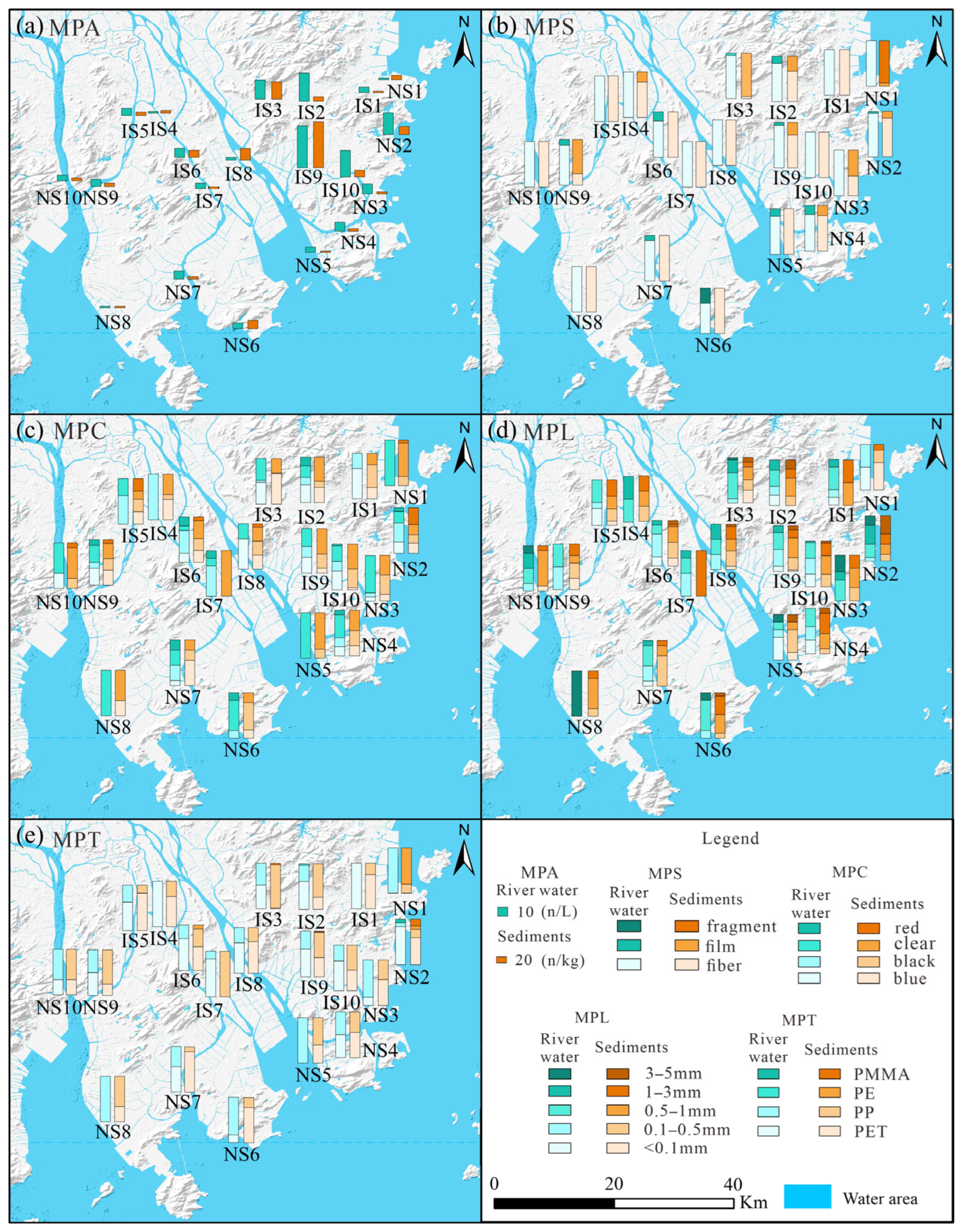
4.1. Spatial Distribution of MPs in the River Water and Sediments
4.2. Dominant IFs for MPA and MDII Variation
4.3. Ecological Risk Assessment for MPs Pollution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Alam, F.C.; Sembiring, E.; Muntalif, B.S.; Suendo, V. Microplastic Distribution in Surface Water and Sediment River around Slum and Industrial Area (Case Study: Ciwalengke River, Majalaya District, Indonesia). Chemosphere 2019, 224, 637–645. [Google Scholar] [CrossRef]
- Fu, L.; Guo, Y.; Tang, J.; Yang, M.; Huang, L.; Xiao, J.; Fan, Z.; Wang, Z.; Zhi, J.; Huang, Z.; et al. Comparative Analysis of Microplastic and Microbial Communities in Varied Aquatic Environments: Disparities in Occurrence, Interconnections, and Ecological Implications. J. Hazard. Mater. 2025, 495, 138795. [Google Scholar] [CrossRef]
- Hoang, V.-H.; Nguyen, M.-K.; Hoang, T.-D.; Nguyen, H.-L.; Lin, C.; Yousaf, B.; Ha, M.C.; Bui, V.K.H.; Pham, M.-T.; Chang, S.W.; et al. Global Occurrence and Environmental Fate of Microplastics in Stormwater Runoff: Unlock the In-Depth Knowledge on Nature-Based Removal Strategies. Rev. Environ. Contam. Toxicol. 2025, 263, 5. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Cha, J.; Ha, K.; Viaroli, S. Microplastic Pollution in Groundwater: A Systematic Review. Environ. Pollut. Bioavailab. 2024, 36, 2299545. [Google Scholar] [CrossRef]
- Buteler, M.; Fasanella, M.; Alma, A.M.; Silva, L.I.; Langenheim, M.; Tomba, J.P. Lakes with or without Urbanization along Their Coasts Had Similar Level of Microplastic Contamination, but Significant Differences Were Seen between Sampling Methods. Sci. Total Environ. 2023, 866, 161254. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Haider, A.; Ahmad, H.M.; Mohyuddin, A.; Umer Aslam, H.M.; Nadeem, S.; Javed, M.; Othman, M.H.D.; Goh, H.H.; Chew, K.W. Source, Occurrence, Distribution, Fate, and Implications of Microplastic Pollutants in Freshwater on Environment: A Critical Review and Way Forward. Chemosphere 2023, 325, 138367. [Google Scholar] [CrossRef]
- Ren, S.-Y.; Kong, S.-F.; Ni, H.-G. Contribution of Mulch Film to Microplastics in Agricultural Soil and Surface Water in China. Environ. Pollut. 2021, 291, 118227. [Google Scholar] [CrossRef]
- Siegfried, M.; Koelmans, A.A.; Besseling, E.; Kroeze, C. Export of Microplastics from Land to Sea. A Modelling Approach. Water Research 2017, 127, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Trindade, L.d.S.; Gloaguen, T.V.; Benevides, T.d.S.F.; Valentim, A.C.S.; Bomfim, M.R.; Gonzaga Santos, J.A. Microplastics in Surface Waters of Tropical Estuaries around a Densely Populated Brazilian Bay. Environ. Pollut. 2023, 323, 121224. [Google Scholar] [CrossRef]
- Barceló, D.; Picó, Y.; Alfarhan, A.H. Microplastics: Detection in Human Samples, Cell Line Studies, and Health Impacts. Environ. Toxicol. Pharmacol. 2023, 101, 104204. [Google Scholar] [CrossRef]
- Çıtar Dazıroğlu, M.E.; Bilici, S. The Hidden Threat to Food Safety and Human Health: Microplastics. Environ. Dev. Sustain. 2024, 26, 21913–21935. [Google Scholar] [CrossRef]
- Eze, C.G.; Nwankwo, C.E.; Dey, S.; Sundaramurthy, S.; Okeke, E.S. Food Chain Microplastics Contamination and Impact on Human Health: A Review. Environ. Chem. Lett. 2024, 22, 1889–1927. [Google Scholar] [CrossRef]
- Lu, L.; Luo, T.; Zhao, Y.; Cai, C.; Fu, Z.; Jin, Y. Interaction between Microplastics and Microorganism as Well as Gut Microbiota: A Consideration on Environmental Animal and Human Health. Sci. Total Environ. 2019, 667, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and Effects of Plastic Additives on Marine Environments and Organisms: A Review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Purwiyanto, A.I.S.; Suteja, Y.; Trisno; Ningrum, P.S.; Putri, W.A.E.; Rozirwan; Agustriani, F.; Fauziyah; Cordova, M.R.; Koropitan, A.F. Concentration and Adsorption of Pb and Cu in Microplastics: Case Study in Aquatic Environment. Mar. Pollut. Bull. 2020, 158, 111380. [Google Scholar] [CrossRef]
- Han, X.; Wu, H.; Li, Q.; Cai, W.; Hu, S. Assessment of Heavy Metal Accumulation and Potential Risks in Surface Sediment of Estuary Area: A Case Study of Dagu River. Mar. Environ. Res. 2024, 196, 106416. [Google Scholar] [CrossRef]
- Makhinov, A.N. Mouth Areas of Rivers on the Southwestern Coast of the Sea of Okhotsk and Role of Ice in the Formation of Their Channels under Conditions of High Tides. Water Resour. 2022, 49, 860–868. [Google Scholar] [CrossRef]
- Preston-Whyte, F.; Silburn, B.; Meakins, B.; Bakir, A.; Pillay, K.; Worship, M.; Paruk, S.; Mdazuka, Y.; Mooi, G.; Harmer, R.; et al. Meso- and Microplastics Monitoring in Harbour Environments: A Case Study for the Port of Durban, South Africa. Mar. Pollut. Bull. 2021, 163, 111948. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.C.; deCastro, M.; Gago, J.; Ribeiro, A.S.; Des, M.; Gómez-Gesteira, J.L.; Dias, J.M.; Gomez-Gesteira, M. Modelling the Distribution of Microplastics Released by Wastewater Treatment Plants in Ria de Vigo (NW Iberian Peninsula). Mar. Pollut. Bull. 2021, 166, 112227. [Google Scholar] [CrossRef]
- Soltani, N.; Keshavarzi, B.; Moore, F.; Busquets, R.; Nematollahi, M.J.; Javid, R.; Gobert, S. Effect of Land Use on Microplastic Pollution in a Major Boundary Waterway: The Arvand River. Sci. Total Environ. 2022, 830, 154728. [Google Scholar] [CrossRef]
- Trusler, M.M.; Moss-Hayes, V.L.; Cook, S.; Lomax, B.H.; Vane, C.H. Microplastics Pollution in Sediments of the Thames and Medway Estuaries, UK: Organic Matter Associations and Predominance of Polyethylene. Mar. Pollut. Bull. 2024, 208, 116971. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, P.; Verma, A.; Jha, P.K.; Singh, P.; Gupta, P.K.; Chandra, R.; Prasad, P.V.V. Effect of Physical Characteristics and Hydrodynamic Conditions on Transport and Deposition of Microplastics in Riverine Ecosystem. Water 2021, 13, 2710. [Google Scholar] [CrossRef]
- Wu, J.; Ye, Q.; Sun, L.; Liu, J.; Huang, M.; Wang, T.; Wu, P.; Zhu, N. Impact of Persistent Rain on Microplastics Distribution and Plastisphere Community: A Field Study in the Pearl River, China. Sci. Total Environ. 2023, 879, 163066. [Google Scholar] [CrossRef]
- Malli, A.; Corella-Puertas, E.; Hajjar, C.; Boulay, A.-M. Transport Mechanisms and Fate of Microplastics in Estuarine Compartments: A Review. Mar. Pollut. Bull. 2022, 177, 113553. [Google Scholar] [CrossRef]
- Ta, A.T.; Babel, S. Occurrence and Spatial Distribution of Microplastic Contaminated with Heavy Metals in a Tropical River: Effect of Land Use and Population Density. Mar. Pollut. Bull. 2023, 191, 114919. [Google Scholar] [CrossRef]
- Zuo, L.; Sun, Y.; Li, H.; Hu, Y.; Lin, L.; Peng, J.; Xu, X. Microplastics in Mangrove Sediments of the Pearl River Estuary, South China: Correlation with Halogenated Flame Retardants’ Levels. Sci. Total Environ. 2020, 725, 138344. [Google Scholar] [CrossRef] [PubMed]
- Defontaine, S.; Sous, D.; Tesan, J.; Monperrus, M.; Lenoble, V.; Lanceleur, L. Microplastics in a Salt-Wedge Estuary: Vertical Structure and Tidal Dynamics. Mar. Pollut. Bull. 2020, 160, 111688. [Google Scholar] [CrossRef] [PubMed]
- Ta, A.T.; Babel, S. Microplastics Pollution with Heavy Metals in the Aquaculture Zone of the Chao Phraya River Estuary, Thailand. Mar. Pollut. Bull. 2020, 161, 111747. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended Microplastics in the Surface Water of the Yangtze Estuary System, China: First Observations on Occurrence, Distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Chau, H.S.; Xu, S.; Ma, Y.; Wang, Q.; Cao, Y.; Huang, G.; Ruan, Y.; Yan, M.; Liu, M.; Zhang, K.; et al. Microplastic Occurrence and Ecological Risk Assessment in the Eight Outlets of the Pearl River Estuary, a New Insight into the Riverine Microplastic Input to the Northern South China Sea. Mar. Pollut. Bull. 2023, 189, 114719. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, L.; Wei, N.; Bai, M.; Liu, K.; Wang, X.; Jiang, C.; Zong, C.; Zhang, F.; Li, C.; et al. Transport Mechanism of Microplastics from a Still Water System to a Dynamic Estuarine System: A Case Study in Macao SAR. Mar. Pollut. Bull. 2025, 218, 118223. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Sun, D.; Pan, Z.; Zou, J. Investigation of Microplastics in Surface Water and Estuarine Mullet Mugil Cephalus from 23 Estuary Areas, South China. Sustainability 2023, 15, 4193. [Google Scholar] [CrossRef]
- van der Mheen, M.; van Sebille, E.; Pattiaratchi, C. Beaching Patterns of Plastic Debris along the Indian Ocean Rim. Ocean Science 2020, 16, 1317–1336. [Google Scholar] [CrossRef]
- Gao, W.; Xiong, F.; Lu, Y.; Qu, X.; Xin, W.; Chen, Y. Development of a Phytoplankton-Based Index of Biotic Integrity for Ecological Health Assessment in the Yangtze River. Ecol. Process. 2023, 12, 41. [Google Scholar] [CrossRef]
- Maurya, P.K.; Ali, S.A.; Alharbi, R.S.; Yadav, K.K.; Alfaisal, F.M.; Ahmad, A.; Ditthakit, P.; Prasad, S.; Jung, Y.-K.; Jeon, B.-H. Impacts of Land Use Change on Water Quality Index in the Upper Ganges River near Haridwar, Uttarakhand: A GIS-Based Analysis. Water 2021, 13, 3572. [Google Scholar] [CrossRef]
- Raduła, M.W.; Szymura, T.H.; Szymura, M.; Swacha, G.; Kącki, Z. Effect of Environmental Gradients, Habitat Continuity and Spatial Structure on Vascular Plant Species Richness in Semi-Natural Grasslands. Agric. Ecosyst. Environ. 2020, 300, 106974. [Google Scholar] [CrossRef]
- Yao, S.; Chen, C.; He, M.; Cui, Z.; Mo, K.; Pang, R.; Chen, Q. Land Use as an Important Indicator for Water Quality Prediction in a Region under Rapid Urbanization. Ecol. Indic. 2023, 146, 109768. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C.; Herring, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Marine Debris Program; National Oceanic and Atmospheric Administration, U.S. Department of Commerce: Washington, DC, USA, 2015; pp. 1–29. [Google Scholar]
- Gong, X.; Tian, L.; Wang, P.; Wang, Z.; Zeng, L.; Hu, J. Microplastic Pollution in the Groundwater under a Bedrock Island in the South China Sea. Environ. Res. 2023, 239, 117277. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L.; Shi, G.; Cao, S.; Li, Y. Characteristics and Sources of Microplastic Pollution in the Water and Sediments of the Jinjiang River Basin, Fujian Province, China. China Geol. 2022, 5, 429–438. [Google Scholar] [CrossRef]
- Shu, X.; Xu, L.; Yang, M.; Qin, Z.; Zhang, Q.; Zhang, L. Spatial Distribution Characteristics and Migration of Microplastics in Surface Water, Groundwater and Sediment in Karst Areas: The Case of Yulong River in Guilin, Southwest China. Sci. Total Environ. 2023, 868, 161578. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.R.; Garcia, F.; Riem-Galliano, L.; Tudesque, L.; Albignac, M.; ter Halle, A.; Cucherousset, J. Urbanization and Hydrological Conditions Drive the Spatial and Temporal Variability of Microplastic Pollution in the Garonne River. Sci. Total Environ. 2021, 769, 144479. [Google Scholar] [CrossRef] [PubMed]
- Grbić, J.; Helm, P.; Athey, S.; Rochman, C.M. Microplastics Entering Northwestern Lake Ontario Are Diverse and Linked to Urban Sources. Water Res. 2020, 174, 115623. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; He, Y.; Liang, D.; Shen, Y.; Gu, Q.; Zeng, Y. How Microplastic Loads Relate to Natural Conditions and Anthropogenic Activities in the Yangtze River Basin. Chemosphere 2023, 342, 140146. [Google Scholar] [CrossRef]
- D’Hont, A.; Gittenberger, A.; Leuven, R.S.E.W.; Hendriks, A.J. Dropping the Microbead: Source and Sink Related Microplastic Distribution in the Black Sea and Caspian Sea Basins. Mar. Pollut. Bull. 2021, 173, 112982. [Google Scholar] [CrossRef]
- Mani, T.; Burkhardt-Holm, P. Seasonal Microplastics Variation in Nival and Pluvial Stretches of the Rhine River—From the Swiss Catchment towards the North Sea. Sci. Total Environ. 2020, 707, 135579. [Google Scholar] [CrossRef]
- Sang, W.; Chen, Z.; Mei, L.; Hao, S.; Zhan, C.; Zhang, W.b.; Li, M.; Liu, J. The Abundance and Characteristics of Microplastics in Rainwater Pipelines in Wuhan, China. Sci. Total Environ. 2021, 755, 142606. [Google Scholar] [CrossRef]
- An, X.; Li, W.; Lan, J.; Adnan, M. Preliminary Study on the Distribution, Source, and Ecological Risk of Typical Microplastics in Karst Groundwater in Guizhou Province, China. Int. J. Environ. Res. Public Health 2022, 19, 14751. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, K.; Song, W.; Song, G.; Chen, D.; Hayat, T.; Alharbi, N.S.; Chen, C.; Sun, Y. Impact of Water Chemistry on Surface Charge and Aggregation of Polystyrene Microspheres Suspensions. Sci. Total Environ. 2018, 630, 951–959. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Q.; Yang, Z. Identifying the Spatial Range of the Pearl River Delta Urban Agglomeration from a Differentiated Perspective of Population Distribution and Population Mobility. Appl. Sci. 2025, 15, 945. [Google Scholar] [CrossRef]
- He, J.; Yang, S.; Deng, S.; Ye, J.; Chen, H. Research on the Decoupling Relationship between Transportation Land and Population Growth: A Case of Guangdong Province in China. Land 2024, 13, 484. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, B.; Shen, C.; Wang, R.; Yin, S.; Li, F.; Xu, C. Are We Underestimating the Driving Factors and Potential Risks of Freshwater Microplastics from in Situ and in Silico Perspective? Water Res. 2025, 281, 123568. [Google Scholar] [CrossRef]
- Fang, Q.; Niu, S.; Yu, J. Characterising Microplastic Pollution in Sediments from Urban Water Systems Using the Diversity Index. J. Clean. Prod. 2021, 318, 128537. [Google Scholar] [CrossRef]
- Guo, S.; Xiao, G.; Chen, Y.; Zhang, J.; Zhang, B.; Ru, S.; Zhao, M. Unraveling the Characteristics of Microplastics in Agricultural Soils upon Long-Term Organic Fertilizer Application: A Comprehensive Study Using Diversity Indices. Chemosphere 2024, 364, 143235. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gan, Y.; Zhang, C.; He, H.; Fang, J.; Wang, L.; Wang, Y.; Liu, J. “Microplastic Communities” in Different Environments: Differences, Links, and Role of Diversity Index in Source Analysis. Water Res. 2021, 188, 116574. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; He, L.; Liu, J.; Liu, X.; Huang, J.; Rong, L. Experimental Study of Interception Effect by Submerged Dam on Microplastics. J. Hazard. Mater. 2024, 480, 135924. [Google Scholar] [CrossRef]
- Maity, S.; Maiti, R.; Mukherjee, S. Catchment Scale Land Use and Hydrogeomorphology Relationship in Assessing River Water Quality Using SWAT and RDA Modelling in the Damodar River Basin, West Bengal, India. Adv. Space Res. 2025, 75, 7889–7902. [Google Scholar] [CrossRef]
- Nakar, A.; Schmilovitch, Z.; Vaizel-Ohayon, D.; Kroupitski, Y.; Borisover, M.; Sela, S. Quantification of Bacteria in Water Using PLS Analysis of Emission Spectra of Fluorescence and Excitation-Emission Matrices. Water Res. 2020, 169, 115197. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, C.; Li, Z.; Huang, J. Modeling Risk Assessment of Soil Heavy Metal Pollution Using Partial Least Squares and Fuzzy Logic: A Case Study of a Gully Type Coal-Based Solid Waste Dumpsite. Environ. Pollut. 2024, 352, 124147. [Google Scholar] [CrossRef]
- Cui, S.; Adamowski, J.F.; Wu, M.; Zhang, P.; Yue, Q.; Cao, X. An Integrated Framework for Improving Green Agricultural Production Sustainability in Human-Natural Systems. Sci. Total Environ. 2024, 945, 174153. [Google Scholar] [CrossRef]
- Zhu, L.; Xiong, Q. Key Influencing Factor and Future Scenario Simulation of China’s CO2 Emissions from Road Freight Transportation. Sustain. Prod. Consum. 2023, 37, 11–25. [Google Scholar] [CrossRef]
- Gélinas, A.; Profili, J.; Fotouhiardakani, F.; Caceres Ferreira, W.M.; Laurent, M.; Ravichandran, S.; Laroche, G. Toward a Better Interpretation of the Partial Least Squares Regression Models for Fluoropolymers Treated by Dielectric Barrier Discharges at Atmospheric Pressure. Plasma Process. Polym. 2024, 21, 2300098. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Luo, D.; Kang, M.; Li, Y.; Huang, Y.; Bai, X. Interventions of River Network Structures on Urban Aquatic Microplastic Footprint from a Connectivity Perspective. Water Res. 2023, 243, 120418. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, B.; Qannari, E.M.; Jaillais, B. Extension and Significance Testing of Variable Importance in Projection (VIP) Indices in Partial Least Squares Regression and Principal Components Analysis. Chemom. Intell. Lab. Syst. 2023, 242, 104986. [Google Scholar] [CrossRef]
- Ahn, J.J.; Kim, Y.M.; Yoo, K.; Park, J.; Oh, K.J. Using GA-Ridge Regression to Select Hydro-Geological Parameters Influencing Groundwater Pollution Vulnerability. Environ. Monit. Assess. 2012, 184, 6637–6645. [Google Scholar] [CrossRef]
- Wen, L.; Shao, H. Analysis of Influencing Factors of the Carbon Dioxide Emissions in China’s Commercial Department Based on the STIRPAT Model and Ridge Regression. Environ. Sci. Pollut. Res. 2019, 26, 27138–27147. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, F.; Li, Y.; Tan, W.; Yuan, Y.; Jiang, Y. Influence of Landfill Leachate Microenvironment on the Occurrence of Microplastics: TOC Changes Are the Main Driving Factor. J. Hazard. Mater. 2025, 492, 138080. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and Health Hazard Ranking and Assessment of Plastic Polymers Based on Chemical Composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Dong, Y.; Qiao, Y.; Lin, H. Insights into Microplastics in the Soil Environment: Migration, Biodegradation, Toxicity and Risk Assessment. Process Saf. Environ. Prot. 2024, 189, 1450–1463. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.P.; Li, H.; Lin, L.; Xu, X.; Yuan, X.; Koongolla, J.B.; Li, H. Microplastic Contamination in Coral Reef Fishes and Its Potential Risks in the Remote Xisha Areas of the South China Sea. Mar. Pollut. Bull. 2023, 186, 114399. [Google Scholar] [CrossRef]
- Wu, L.; Li, C.; Huang, C.; Luo, Y.; Huang, J.; Chen, F.; Huang, X.; Sun, R. Ocean Current Modulation of the Spatial Distribution of Microplastics in the Surface Sediments of the Beibu Gulf, China. J. Hazard. Mater. 2025, 488, 137332. [Google Scholar] [CrossRef]
- Corlett, W.B.; Geyer, W.R. Frontogenesis at Estuarine Junctions. Estuaries Coasts 2020, 43, 722–738. [Google Scholar] [CrossRef]
- Vibhatabandhu, P.; Srithongouthai, S. Influence of Seasonal Variations on the Distribution Characteristics of Microplastics in the Surface Water of the Inner Gulf of Thailand. Mar. Pollut. Bull. 2022, 180, 113747. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic—An Emerging Contaminant of Potential Concern? Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef]
- da Costa, J.P.; Avellan, A.; Mouneyrac, C.; Duarte, A.; Rocha-Santos, T. Plastic Additives and Microplastics as Emerging Contaminants: Mechanisms and Analytical Assessment. TrAC Trends Anal. Chem. 2023, 158, 116898. [Google Scholar] [CrossRef]
- Naidu, B.C.; Xavier, K.A.M.; Shukla, S.P.; Jaiswar, A.K.; Nayak, B.B. Comparative Study on the Microplastics Abundance, Characteristics, and Possible Sources in Yellow Clams of Different Demographic Regions of the Northwest Coast of India. J. Hazard. Mater. Lett. 2022, 3, 100051. [Google Scholar] [CrossRef]
- Nizzetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P.G. A Theoretical Assessment of Microplastic Transport in River Catchments and Their Retention by Soils and River Sediments. Environ. Sci. Process. Impacts 2016, 18, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Lipi, J.A.; Rima, N.N.; Riya, K.K.; Hossain, M.K.; Paray, B.A.; Arai, T.; Yu, J.; Hossain, M.B. Microplastic Contamination and Ecological Risk Assessment in Sediments and Waters of Ship-Dismantling Yards along the Bay of Bengal. Mar. Pollut. Bull. 2025, 217, 118032. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in Freshwater Systems: A Review on Occurrence, Environmental Effects, and Methods for Microplastics Detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoellein, T. The Global Odyssey of Plastic Pollution. Science 2020, 368, 1184–1185. [Google Scholar] [CrossRef]
- Groß, J. Variance Inflation Factors. R News 2003, 3, 13–15. [Google Scholar]
- Yang, T.; Zeng, Y.; Kang, Z.; Cai, M.; Chen, K.; Zhao, Q.; Lin, J.; Liu, R.; Xu, G. Enrichment and Ecological Risks of Microplastics in Mangroves of Southern Hainan Island, China. Sci. Total Environ. 2023, 889, 164160. [Google Scholar] [CrossRef]
- Pinheiro, L.M.; Agostini, V.O.; Lima, A.R.A.; Ward, R.D.; Pinho, G.L.L. The Fate of Plastic Litter within Estuarine Compartments: An Overview of Current Knowledge for the Transboundary Issue to Guide Future Assessments. Environ. Pollut. 2021, 279, 116908. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, P.; Muñoz, C.C.; Ikejima, K. Sources and Sinks of Plastic Debris in Estuaries: A Conceptual Model Integrating Biological, Physical and Chemical Distribution Mechanisms. Mar. Pollut. Bull. 2016, 113, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.K.; Gahrau, T.; Tamminga, M. Identification and Assessment of Potential Microplastic Emissions within the Lake Tollense Catchment Area, Mecklenburg-Western Pomerania, Germany. Sustainability 2023, 15, 15048. [Google Scholar] [CrossRef]
- Han, N.; Zhao, Q.; Wu, C. Threshold Migration Conditions of (Micro) Plastics under the Action of Overland Flow. Water Res. 2023, 242, 120253. [Google Scholar] [CrossRef]
- Yin, Z.; Zhao, Y. Microplastics Pollution in Freshwater Sediments: The Pollution Status Assessment and Sustainable Management Measures. Chemosphere 2023, 314, 137727. [Google Scholar] [CrossRef]
- Prarat, P.; Hongsawat, P.; Chouychai, B. Microplastic Occurrence in Surface Sediments from Coastal Mangroves in Eastern Thailand: Abundance, Characteristics, and Ecological Risk Implications. Reg. Stud. Mar. Sci. 2024, 71, 103389. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Wang, H.; Xing, S.; Yin, R.; Fu, W.; Rillig, M.C.; Chen, B.; Zhu, Y. Arbuscular Mycorrhizal Fungi Can Inhibit the Allocation of Microplastics from Crop Roots to Aboveground Edible Parts. J. Agric. Food Chem. 2023, 71, 18323–18332. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Hu, J.; Li, Y.; Wang, C.; Zhang, W.; Hu, Q.; Wang, L.; Zhang, H. Effects of Long-Term Exposure to Silver Nanoparticles on the Structure and Function of Microplastic Biofilms in Eutrophic Water. Environ. Res. 2022, 207, 112182. [Google Scholar] [CrossRef] [PubMed]
- Trifuoggi, M.; Pagano, G.; Oral, R.; Pavičić-Hamer, D.; Burić, P.; Kovačić, I.; Siciliano, A.; Toscanesi, M.; Thomas, P.J.; Paduano, L.; et al. Microplastic-Induced Damage in Early Embryonal Development of Sea Urchin Sphaerechinus granularis. Environ. Res. 2019, 179, 108815. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; He, L.; Zheng, S.; Liu, J.; Gong, L. A Review of Microplastic Transport in Coastal Zones. Mar. Environ. Res. 2024, 196, 106397. [Google Scholar] [CrossRef]
- Wu, F.; Reding, L.; Starkenburg, M.; Leistenschneider, C.; Primpke, S.; Vianello, A.; Zonneveld, K.A.F.; Huserbråten, M.B.O.; Versteegh, G.J.M.; Gerdts, G. Spatial Distribution of Small Microplastics in the Norwegian Coastal Current. Sci. Total Environ. 2024, 942, 173808. [Google Scholar] [CrossRef]
- Ducklow, H.W.; Doney, S.C.; Steinberg, D.K. Contributions of Long-Term Research and Time-Series Observations to Marine Ecology and Biogeochemistry. Annu. Rev. Mar. Sci. 2009, 1, 279–302. [Google Scholar] [CrossRef]
- Karra, K.; Kontgis, C.; Statman-Weil, Z.; Mazzariello, J.C.; Mathis, M.; Brumby, S.P. Global Land Use/Land Cover with Sentinel 2 and Deep Learning. In Proceedings of the 2021 IEEE International Geoscience and Remote Sensing Symposium IGARSS, Brussels, Belgium, 11–16 July 2021; pp. 4704–4707. [Google Scholar] [CrossRef]
- Xu, X. China GDP Spatial Distribution Kilometre Grid Dataset; Resource and Environmental Science Data Registration and Publishing System: Beijing, China, 2017. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Li, C.; Deng, L.; Yan, Z.; Gong, X. Spatial Distribution, Key Influencing Factors, and Ecological Risk of Microplastics in Pearl River Estuary Water and Sediments. Water 2025, 17, 2572. https://doi.org/10.3390/w17172572
Hu J, Li C, Deng L, Yan Z, Gong X. Spatial Distribution, Key Influencing Factors, and Ecological Risk of Microplastics in Pearl River Estuary Water and Sediments. Water. 2025; 17(17):2572. https://doi.org/10.3390/w17172572
Chicago/Turabian StyleHu, Jiyuan, Chengliang Li, Lichi Deng, Ziyan Yan, and Xing Gong. 2025. "Spatial Distribution, Key Influencing Factors, and Ecological Risk of Microplastics in Pearl River Estuary Water and Sediments" Water 17, no. 17: 2572. https://doi.org/10.3390/w17172572
APA StyleHu, J., Li, C., Deng, L., Yan, Z., & Gong, X. (2025). Spatial Distribution, Key Influencing Factors, and Ecological Risk of Microplastics in Pearl River Estuary Water and Sediments. Water, 17(17), 2572. https://doi.org/10.3390/w17172572







