Spectroscopy-Based Methods for Water Quality Assessment: A Comprehensive Review and Potential Applications in Livestock Farming
Abstract
1. Introduction
2. Spectroscopy Principles
2.1. Spectroscopy
2.2. Electromagnetic Spectrum
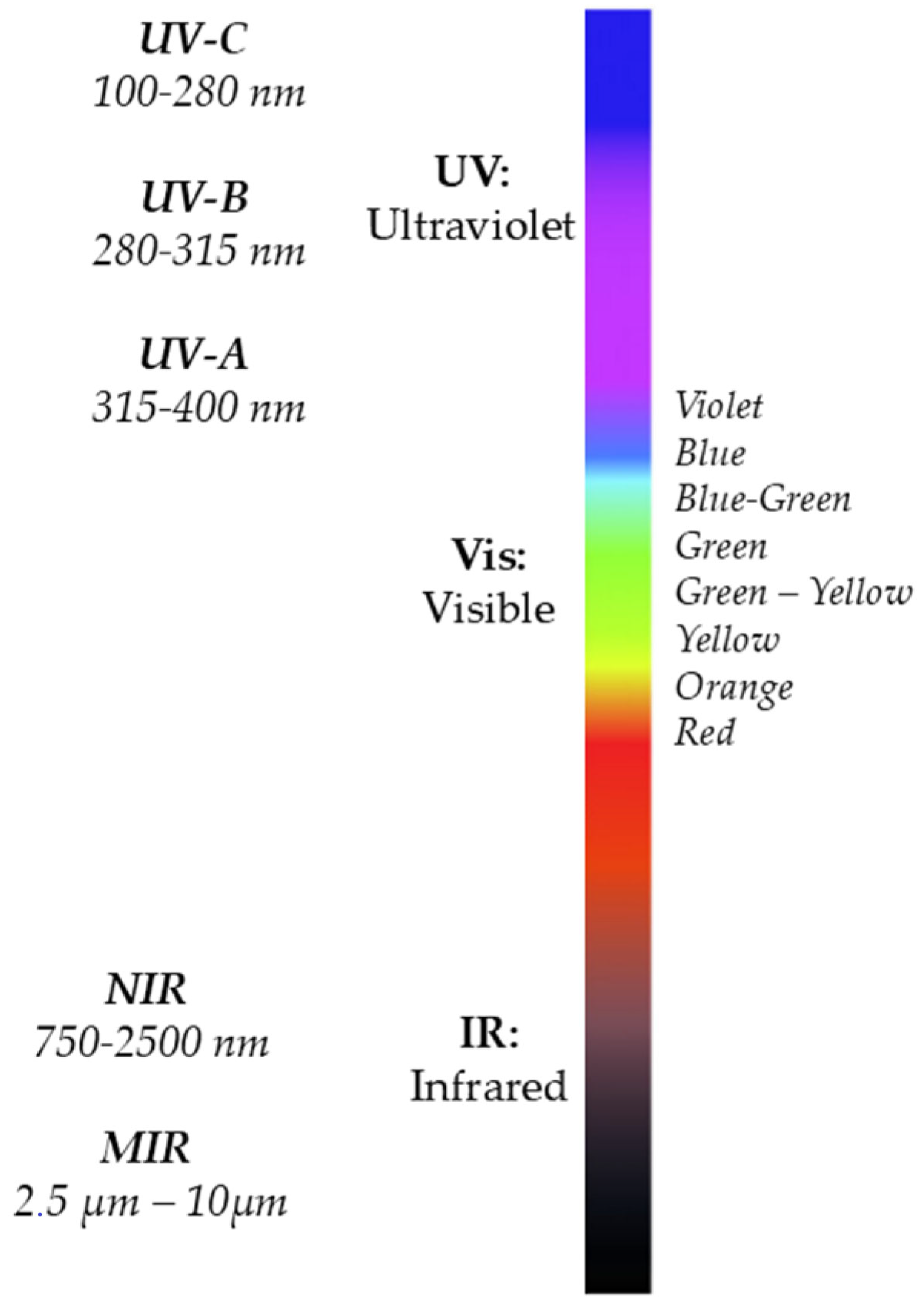
2.3. Lambert–Beer Law
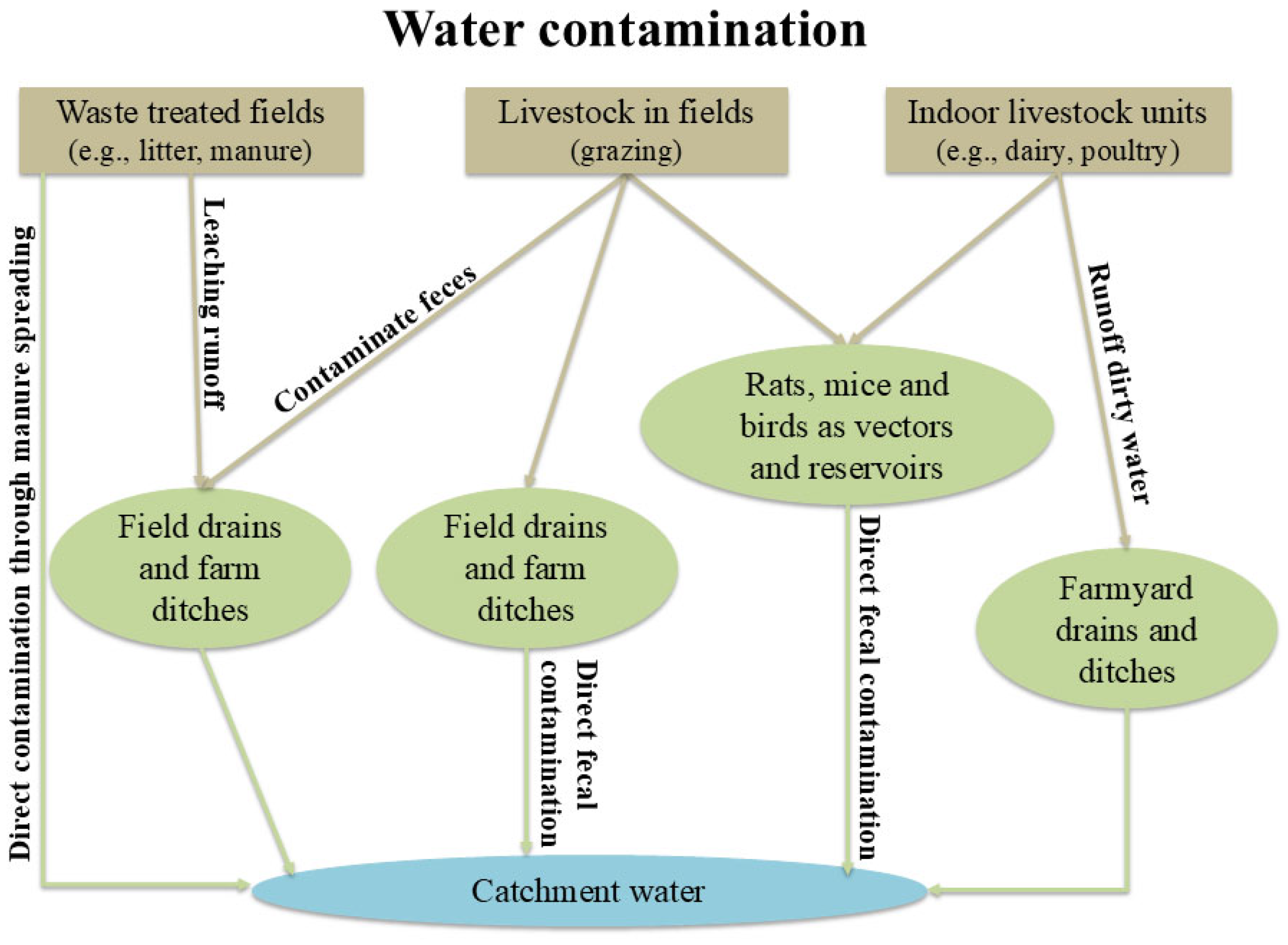
3. Water Quality and Its Importance in Livestock Farming
3.1. Water and Livestock Farming
| Elements | Threshold for Animal Watering | Threshold for Crop Irrigation |
|---|---|---|
| Chlorine (ppm) | 300 | |
| Chromium (ppm) | 1.0 | 1.0 |
| Copper (ppm) | 0.5 | 5.0 |
| Lead (ppm) | 0.1 | 10.0 |
| Mercury (ppm) | 0.01 | |
| Nickel (ppm) | 1.0 | 2.0 |
| Nitrate nitrogen (ppm) | 100 | |
| pH | 8.5 | |
| Phosphorus (ppm) | 0.7 | |
| Sulfates (ppm) | 300 | |
| Total bacteria (n/100 mL) | 1000 | |
| Total dissolved solids (ppm) | 5000 |

3.2. Water Quality Parameters and Standards
| Physical | Chemical | Biological | Radiological |
|---|---|---|---|
| Turbidity | pH | Bacteria | Radioactive substances |
| Temperature | Acidity | Algae | |
| Color | Alkalinity | Viruses | |
| Taste and odor | Chloride | Protozoa | |
| Solids | Chloride residual | ||
| Electrical conductivity (EC) | Sulfate | ||
| Fluoride | |||
| Iron and manganese | |||
| Copper and zinc | |||
| Hardness | |||
| dissolved oxygen | |||
| Biochemical oxygen demand (BOD) | |||
| Chemical oxygen demand (COD) | |||
| Toxic inorganic substances | |||
| Toxic organic substances |
- Chemical oxygen demand (COD) is defined as the quantity of oxidants consumed by the reducing substances in an one-liter oxidized water sample under specific conditions, expressed in milligrams per liter. It demonstrates the level of contamination brought on by introducing reducing agents into water [19].
- Dissolved organic carbon (DOC) represents a universal element of the freshwater carbon cycle. It is a water disinfection byproduct that affects human health and can impair the effectiveness of aquatic ecosystems and weaken ultraviolet radiation [40].
- Nitrogen- and phosphorus-containing compounds (e.g., detergents, fertilizers) are being released into the aquifer in mass quantities, disrupting the nitrogen and phosphorus equilibrium in aquatic ecosystems and causing eutrophication, the rapid and excessive growth of algae and other microorganisms, and the aquatic ecosystem degradation due to hypoxia. Consequently, water quality will eventually deteriorate, and aquatic life will be challenged, which will have a remarkable impact on marine ecosystems and human production activities. [19].
| Parameter | European Union [41] | WHO [39] |
|---|---|---|
| pH | 6.5–9.5 | - |
| Electrical conductivity (μS cm−1 at 20 °C) | 2500 | - |
| Ammonia/ammonium (mg/L) | 0.5 | 0.2 |
| Chloride (mg/L) | 250 | 250 |
| Chromium (μg/L) | 25 | 50 |
| Copper (mg/L) | 2.0 | 2.0 |
| Fluoride (mg/L) | 1.5 | 1.5 |
| Lead (μg/l) | 5.0 | 10 |
| Nitrate (mg/L) | 50 | 50 |
| Nitrite (mg/L) | 0.5 | 3.0 |
| Sulfate (mg/L) | 250 | - |
| Pesticides (total) (μg/L) | 0.5 | - |
4. Machine Learning and Chemometrics
- Partial Least Squares (PLS): It is a regression model that predicts a group of dependent variables from a group of independent variables (predictors). By projecting both input and output variables into a new space that maximizes the covariance allowing to model the interactions between them, it is considered efficient when there are more predictors than observations or in cases of multicollinearity [47].
- Support Vector Machines (SVM): They are supervised learning models, considered as binary linear classifiers that classify observations by finding the optimal boundary which maximizes the distance (margin) between different classes [48]. Using various kernel functions, they are effective in high-dimensional issues and perform well on both linear and non-linear classification tasks.
- Artificial Neural Networks (ANNs): ANNs are computational models based on a network that consists of several connected nodes inspired by the human brain and are known as artificial neurons, with the simplest one being perceptron (used for binary classification of linearly separable data) [49]. There are many types of NNs specialized in different scientific areas and applications, including spatial data and image-like pattern analyses (convolutional NNs) [50].
- Decision Trees (DT): It is a tree-like non-parametric method that can be used to solve classification and regression problems. The leaves of the trees represent different labels or outcomes (classes). They are easy to implement; however, in the case of complex datasets, they are sensitive to overfitting [51].
- Random Forest (RF): RF is considered an ensemble learning method for which multiple DTs are trained. For classification problems, the model outputs the class chosen by the majority of trees, while for regression problems, it returns the average of the trees’ outputs [52].
5. Applications of Spectroscopy in Water Quality Assessment
5.1. Ultraviolet–Visible (UV–Vis) Spectroscopy
| Wavelength | No of Samples | Origin of Sample | Chemometrics | Application | RMSE | Reference | |
|---|---|---|---|---|---|---|---|
| 200–900 | 11 | Cultivated bacteria | PCA-MC | Bacteria detection | 0.9954 | NA | [63] |
| 220–750 | 66 | Fabricated | PLS | COD, turbidity | 0.99 | 2.42 mg/L | [67] |
| 270, 350 | 252 | Tropical peatlands | NLR, LR | DOC quantification | 0.86–0.98 | 1.51–6.89 mg/L | [69] |
| 200–800 | 183, 142 | Catchment water | MSR | DOC, Fe | 0.973, 0.989 | 2.599 mg/L, 108.905 μg/L | [68] |
| 193.91–1121.69 | 144 | Lake water | siPLS | COD | 0.8334 | 2.63 1 | [65] |
| 200–650 | 98 | Wastewater | FiPLS | COD | 0.936 | 122 mg/L 2 | [64] |
| 200–1100 | 48 | Fabricated | PLS | COD, turbidity | 0.69, 0.95 | 35%, 21% 3 | [66] |
| 220–700 * | 192 | Different sewer networks | FNN, CNN | Drainage type recognition | NA | NA | [70] |
| 225–260 260–320 320–700 | 144 | Fabricated | PLS | Nitrate, COD, turbidity | 0.993, 0.982, 0.998 | 1.29 mg/L, 2.337 mg/L, 0.696 mg/L | [75] |
| 250–600 | ND | Fabricated | EKF-DM | Copper, cobalt, nickel | 0.9958, 0.9976, 0.9915 | NA | [76] |
| 520/610 ** | ND | Fabricated | ND | metal ions | NA | NA | [77] |
5.2. Infrared Spectroscopy
| Wavelength | No of Samples | Origin of Sample | Chemometrics | Application | RMSE | Reference | |
|---|---|---|---|---|---|---|---|
| 200–14000 cm−1 | 276 | Sludge wastewater treatment | PCA, PLS | IBU, SMX, E2, EE2, CRB | 0.943, 0.948, 0.951, 0.858, 0.963 | 5.47%, 4.91%, 6.16%, 10.12%, 5.10% | [84] |
| 4000–650 cm−1 | 94 | River and lake water | PCA, PLSR | Nitrate monitoring | 0.8868–0.9720, 0.7836–0.9938 | NA | [85] |
| 4000–800 cm−1 | 100 | River and lake water | PCA, SA-PLS | Phosphorus monitoring | 0.973 | 0.015 mg/L | [86] |
| 390–1000 nm | ND | Lake water | NA | Polluted/ non-polluted | NA | NA | [32] |
| 780–2500 nm | 83 | Industrial wastewater | CNN | Pollution level | 0.914 | 25.47 1 | [80] |
| 780–2500 nm | 83 | Wastewater | LSSVM | COD | 0.912 | 20.19 mg/L * | [60] |
| 700–900 nm * | 418 | Cultivated bacteria | PCA, PLS2-DA, SIMCA | Bacterial identification | NA | NA | [82] |
| 1100–2500 nm | 140 | Cultivated bacteria | PCA, PLS | Bacterial identification | 0.983–0.99 | 0.09–0.28 log cfu/mL | [83] |
| 700–2500 nm | 32 | Dairy process | PLS | Urea, lactose | NA | 12.1 ppm | [81] |
5.3. Limitations and Challenges in UV–Vis and IR Spectroscopy for Water Quality Assessment
5.4. Other Spectroscopy Methods
5.5. Comparative Assessment of Spectral Techniques
6. Future Research
7. Conclusions
Author Contributions
Funding

Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Three-Quarters of the Earth Has Gotten Permanently Drier|TIME. Available online: https://time.com/7201214/three-quarters-of-the-earth-has-gotten-permanently-drier/ (accessed on 1 August 2025).
- Body Water—Wikipedia. Available online: https://en.wikipedia.org/wiki/Body_water (accessed on 3 January 2025).
- Musie, W.; Gonfa, G. Fresh Water Resource, Scarcity, Water Salinity Challenges and Possible Remedies: A Review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef]
- One Health. Available online: https://www.who.int/health-topics/one-health?#tab=tab_1 (accessed on 25 February 2025).
- About One Health|One Health|CDC. Available online: https://www.cdc.gov/one-health/about/index.html? (accessed on 25 February 2025).
- One Health—WOAH—World Organisation for Animal Health. Available online: https://www.woah.org/en/what-we-do/global-initiatives/one-health/ (accessed on 25 February 2025).
- Silva, G.M.E.; Campos, D.F.; Brasil, J.A.T.; Tremblay, M.; Mendiondo, E.M.; Ghiglieno, F. Advances in Technological Research for Online and In Situ Water Quality Monitoring—A Review. Sustainability 2022, 14, 5059. [Google Scholar] [CrossRef]
- Shi, Z.; Chow, C.W.K.; Fabris, R.; Liu, J.; Jin, B. Applications of Online UV-Vis Spectrophotometer for Drinking Water Quality Monitoring and Process Control: A Review. Sensors 2022, 22, 2987. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Cumberland, S.A.; Bradley, C.; Buckley, C.; Bridgeman, J. To What Extent Can Portable Fluorescence Spectroscopy Be Used in the Real-Time Assessment of Microbial Water Quality? Sci. Total Environ. 2015, 532, 14–19. [Google Scholar] [CrossRef]
- Spectroscopy|Definition, Types, & Facts|Britannica. Available online: https://www.britannica.com/science/spectroscopy (accessed on 8 January 2025).
- Pu, Y.-Y.; O’Donnell, C.; Tobin, J.T.; O’Shea, N. Review of Near-Infrared Spectroscopy as a Process Analytical Technology for Real-Time Product Monitoring in Dairy Processing. Int. Dairy J. 2020, 103, 104623. [Google Scholar] [CrossRef]
- Spectroscopy—Wikipedia. Available online: https://en.wikipedia.org/wiki/Spectroscopy (accessed on 8 January 2025).
- Electromagnetic Spectrum|Definition, Diagram, & Uses|Britannica. Available online: https://www.britannica.com/science/electromagnetic-spectrum (accessed on 8 January 2025).
- Newton, I. A New Theory about Light and Colors. Am. J. Phys. 1993, 61, 108–112. [Google Scholar] [CrossRef]
- Herschel, W. Investigation of the Powers of the Prismatic Colours to Heat and Illuminate Objects; with Remarks, That Prove the Different Refrangibility of Radiant Heat. To Which Is Added, an Inquiry into the Method of Viewing the Sun Advantageously, with Telescopes of Large Apertures and High Magnifying Powers. Philos. Trans. R. Soc. Lond. 1800, 90, 255–283. [Google Scholar] [CrossRef]
- Frercks, J.; Weber, H.; Wiesenfeldt, G. Reception and Discovery: The Nature of Johann Wilhelm Ritter’s Invisible Rays. Stud. Hist. Philos. Sci. Part A 2009, 40, 143–156. [Google Scholar] [CrossRef]
- Agiomavriti, A.-A.; Nikolopoulou, M.P.; Bartzanas, T.; Chorianopoulos, N.; Demestichas, K.; Gelasakis, A.I. Spectroscopy-Based Methods and Supervised Machine Learning Applications for Milk Chemical Analysis in Dairy Ruminants. Chemosensors 2024, 12, 263. [Google Scholar] [CrossRef]
- Qi, X.; Lian, Y.; Xie, L.; Wang, Y.; Lu, Z. Water Quality Detection Based on UV-Vis and NIR Spectroscopy: A Review. Appl. Spectrosc. Rev. 2024, 59, 1036–1060. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on Water Quality Detection by UV-Vis Spectroscopy. Appl. Sci. 2020, 10, 6874. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Omer, N.H. Water Quality Parameters; Water Quality-Science, Assessments and Policy; IntechOpen: London, UK, 2019; Volume 18. [Google Scholar]
- Mekonnen, M.M.; Hoekstra, A.Y. A Global Assessment of the Water Footprint of Farm Animal Products. Ecosystems 2012, 15, 401–415. [Google Scholar] [CrossRef]
- Minasyan, K. Water Use in Livestock Production Systems and Supply Chains. FAO. ISBN: 978-92-5-131713-6. Available online: https://www.researchgate.net/publication/337363415_Water_use_in_livestock_production_systems_and_supply_chains_Guidelines_for_assessment_Water_use_in_livestock_production_systems_and_supply_chains_Guidelines_for_assessment_FOOD_AND_AGRICULTURE_ORGANIZ (accessed on 9 July 2025).
- Schlink, A.C.; Nguyen, M.L.; Viljoen, G.J. Water Requirements for Livestock Production: A Global Perspective: -EN- -FR- L’utilisation de l’eau Dans Le Secteur de l’élevage: Une Perspective Mondiale -ES- Necesidades de Agua Para La Producción Pecuaria Desde Una Perspectiva Mundial. Rev. Sci. Tech. OIE 2010, 29, 603–619. [Google Scholar] [CrossRef]
- Tullo, E.; Finzi, A.; Guarino, M. Review: Environmental Impact of Livestock Farming and Precision Livestock Farming as a Mitigation Strategy. Sci. Total Environ. 2019, 650, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Rosegrant, M.; Steinfeld, H.; Ehui, S.; Courbois, C. Livestock to 2020: The Next Food Revolution. Outlook Agric 2001, 30, 27–29. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Santos, F.; Ferreira, A.C.F.; Lourinha, I.; Basto, M.C.P.; Mucha, A.P. Can Veterinary Antibiotics Affect Constructed Wetlands Performance during Treatment of Livestock Wastewater? Ecol. Eng. 2017, 102, 583–588. [Google Scholar] [CrossRef]
- Hooda, P.S.; Edwards, A.C.; Anderson, H.A.; Miller, A. A Review of Water Quality Concerns in Livestock Farming Areas. Sci. Total Environ. 2000, 250, 143–167. [Google Scholar] [CrossRef]
- Wilkinson, J.; Garnsworthy, P. Impact of Diet and Fertility on Greenhouse Gas Emissions and Nitrogen Efficiency of Milk Production. Livestock 2017, 22, 140–144. [Google Scholar] [CrossRef]
- Mantovi, P.; Bonazzi, G.; Maestri, E.; Marmiroli, N. Accumulation of Copper and Zinc from Liquid Manure in Agricultural Soils and Crop Plants. Plant Soil 2003, 250, 249–257. [Google Scholar] [CrossRef]
- Xu, P. Research and Application of Near-Infrared Spectroscopy in Rapid Detection of Water Pollution. Desalination Water Treat. 2018, 122, 1–4. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Chen, Y.; Huang, H.; Ren, T. Antibiotic Residues in Liquid Manure from Swine Feedlot and Their Effects on Nearby Groundwater in Regions of North China. Environ. Sci. Pollut. Res. 2018, 25, 11565–11575. [Google Scholar] [CrossRef]
- Robles-Jimenez, L.E.; Aranda-Aguirre, E.; Castelan-Ortega, O.A.; Shettino-Bermudez, B.S.; Ortiz-Salinas, R.; Miranda, M.; Li, X.; Angeles-Hernandez, J.C.; Vargas-Bello-Pérez, E.; Gonzalez-Ronquillo, M. Worldwide Traceability of Antibiotic Residues from Livestock in Wastewater and Soil: A Systematic Review. Animals 2021, 12, 60. [Google Scholar] [CrossRef]
- Boyd, C.E. Water Quality: An Introduction, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-17446-4. [Google Scholar]
- Kruse, P. Review on Water Quality Sensors. J. Phys. D: Appl. Phys. 2018, 51, 203002. [Google Scholar] [CrossRef]
- Definition of “Contaminant”|US EPA. Available online: https://www.epa.gov/ccl/definition-contaminant (accessed on 19 May 2025).
- Water Pollution—Wikipedia. Available online: https://en.wikipedia.org/wiki/Water_pollution?utm_source=chatgpt.com (accessed on 19 May 2025).
- World Health Organization (Ed.) Guidelines for Drinking-Water Quality, 4th ed.; incorporating the first and second addenda; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004506-4. [Google Scholar]
- Karlsson, J.; Byström, P.; Ask, J.; Ask, P.; Persson, L.; Jansson, M. Light Limitation of Nutrient-Poor Lake Ecosystems. Nature 2009, 460, 506–509. [Google Scholar] [CrossRef]
- 98/83/EC; The Council of the European Union 2020 European Union Council Directive 98/83/EC of 23 December 2020 on the Quality of Water Intended for Human Consumption Official Journal of the European Communities. European Union: Brussels, Belgium, 2023; Volume 41, pp. 34–61.
- Forstinus, N.; Ikechukwu, N.; Emenike, M.; Christiana, A. Water and Waterborne Diseases: A Review. Int. J. Trop. Dis. Health 2016, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhao, N.; Yin, G.; Gan, T.; Yang, R.; Chen, X.; Chen, M.; Duan, J. Artificial Neural Networks Combined Multi-Wavelength Transmission Spectrum Feature Extraction for Sensitive Identification of Waterborne Bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 251, 119423. [Google Scholar] [CrossRef]
- Onyeaka, H.; Akinsemolu, A.; Miri, T.; Nnaji, N.D.; Emeka, C.; Tamasiga, P.; Pang, G.; Al-sharify, Z. Advancing Food Security: The Role of Machine Learning in Pathogen Detection. Appl. Food Res. 2024, 4, 100532. [Google Scholar] [CrossRef]
- Dhapre, M.; Jadhav, S.; Das, D.; Khan, J.; Kim, Y.; Chiao, S.; Danielson, T. A Systematic Review of Machine Learning in Groundwater Monitoring. Environ. Model. Softw. 2025, 192, 106549. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal Component Analysis. WIREs Comput. Stats 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Abdi, H. Partial Least Squares Regression and Projection on Latent Structure Regression (PLS Regression). WIREs Comput. Stats 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-Vector Networks. Mach Learn 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Neural Network (Machine Learning)—Wikipedia. Available online: https://en.wikipedia.org/wiki/Neural_network_(machine_learning) (accessed on 14 April 2025).
- Convolutional Neural Network—Wikipedia. Available online: https://en.wikipedia.org/wiki/Convolutional_neural_network (accessed on 14 April 2025).
- Quinlan, J.R. Induction of Decision Trees. Mach Learn 1986, 1, 81–106. [Google Scholar] [CrossRef]
- Random Forest—Wikipedia. Available online: https://en.wikipedia.org/wiki/Random_forest (accessed on 14 April 2025).
- Wang, H.; Cao, H.; Yang, L. Machine Learning-Driven Multidomain Nanomaterial Design: From Bibliometric Analysis to Applications. ACS Appl. Nano Mater. 2024, 7, 26579–26600. [Google Scholar] [CrossRef]
- Chu, H.-J.; He, Y.-C. Remote Sensing Water Quality Inversion Using Sparse Representation: Chlorophyll-a Retrieval from Sentinel-2 MSI Data. Remote Sens. Appl. Soc. Environ. 2023, 31, 101006. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Yang, X.; Zhang, Y.; Zhang, L.; Ren, H.; Wu, B.; Ye, L. A Review of the Application of Machine Learning in Water Quality Evaluation. Eco-Environ. Health 2022, 1, 107–116. [Google Scholar] [CrossRef]
- Nasir, N.; Kansal, A.; Alshaltone, O.; Barneih, F.; Sameer, M.; Shanableh, A.; Al-Shamma’a, A. Water Quality Classification Using Machine Learning Algorithms. J. Water Process Eng. 2022, 48, 102920. [Google Scholar] [CrossRef]
- Kaddoura, S. Evaluation of Machine Learning Algorithm on Drinking Water Quality for Better Sustainability. Sustainability 2022, 14, 11478. [Google Scholar] [CrossRef]
- Najah Ahmed, A.; Binti Othman, F.; Abdulmohsin Afan, H.; Khaleel Ibrahim, R.; Ming Fai, C.; Shabbir Hossain, M.; Ehteram, M.; Elshafie, A. Machine Learning Methods for Better Water Quality Prediction. J. Hydrol. 2019, 578, 124084. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhou, A.; Zhang, L. Identification of Groundwater Pollution from Livestock Farming Using Fluorescence Spectroscopy Coupled with Multivariate Statistical Methods. Water Res. 2021, 206, 117754. [Google Scholar] [CrossRef]
- Chen, H.; Xu, L.; Ai, W.; Lin, B.; Feng, Q.; Cai, K. Kernel Functions Embedded in Support Vector Machine Learning Models for Rapid Water Pollution Assessment via Near-Infrared Spectroscopy. Sci. Total Environ. 2020, 714, 136765. [Google Scholar] [CrossRef]
- Figueiró, C.S.M.; Bastos De Oliveira, D.; Russo, M.R.; Caires, A.R.L.; Rojas, S.S. Fish Farming Water Quality Monitored by Optical Analysis: The Potential Application of UV–Vis Absorption and Fluorescence Spectroscopy. Aquaculture 2018, 490, 91–97. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Karoui, R.; De Baerdemaeker, J.; Ramon, H. Characterization of Soil Water Content Using Measured Visible and Near Infrared Spectra. Soil Sci. Soc Amer J 2006, 70, 1295–1302. [Google Scholar] [CrossRef]
- Feng, C.; Zhao, N.; Yin, G.; Gan, T.; Yang, R.; Chen, M.; Duan, J.; Hu, Y. A New Method for Detecting Mixed Bacteria Based on Multi-Wavelength Transmission Spectroscopy Technology. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 270, 120852. [Google Scholar] [CrossRef]
- Chen, B.; Wu, H.; Li, S.F.Y. Development of Variable Pathlength UV–Vis Spectroscopy Combined with Partial-Least-Squares Regression for Wastewater Chemical Oxygen Demand (COD) Monitoring. Talanta 2014, 120, 325–330. [Google Scholar] [CrossRef]
- Li, J.; Tong, Y.; Guan, L.; Wu, S.; Li, D. Optimization of COD Determination by UV–Vis Spectroscopy Using PLS Chemometrics Algorithms. Optik 2018, 174, 591–599. [Google Scholar] [CrossRef]
- Agustsson, J.; Akermann, O.; Barry, D.A.; Rossi, L. Non-Contact Assessment of COD and Turbidity Concentrations in Water Using Diffuse Reflectance UV-Vis Spectroscopy. Environ. Sci. Process. Impacts 2014, 16, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wen, Y.; Wang, X. Novel Method of Turbidity Compensation for Chemical Oxygen Demand Measurements by Using UV–Vis Spectrometry. Sens. Actuators B Chem. 2016, 227, 393–398. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Pumpanen, J.; Keinänen, M.; Laudon, H.; Ojala, A.; Palviainen, M.; Kiirikki, M.; Neitola, K.; Berninger, F. Assessment of a Portable UV–Vis Spectrophotometer’s Performance for Stream Water DOC and Fe Content Monitoring in Remote Areas. Talanta 2021, 224, 121919. [Google Scholar] [CrossRef]
- Cook, S.; Peacock, M.; Evans, C.D.; Page, S.E.; Whelan, M.J.; Gauci, V.; Kho, L.K. Quantifying Tropical Peatland Dissolved Organic Carbon (DOC) Using UV-Visible Spectroscopy. Water Res. 2017, 115, 229–235. [Google Scholar] [CrossRef]
- Zhu, Q.; Gu, A.; Li, D.; Zhang, T.; Xiang, L.; He, M. Online Recognition of Drainage Type Based on UV-Vis Spectra and Derivative Neural Network Algorithm. Front. Environ. Sci. Eng. 2021, 15, 136. [Google Scholar] [CrossRef]
- Wang, K.; Yu, J.; Hou, D.; Yin, H.; Yu, Q.; Huang, P.; Zhang, G. Optical Detection of Contamination Event in Water Distribution System Using Online Bayesian Method with UV–Vis Spectrometry. Chemom. Intell. Lab. Syst. 2019, 191, 168–174. [Google Scholar] [CrossRef]
- Etheridge, J.R.; Birgand, F.; Osborne, J.A.; Osburn, C.L.; Burchell, M.R.; Irving, J. Using in Situ Ultraviolet-visual Spectroscopy to Measure Nitrogen, Carbon, Phosphorus, and Suspended Solids Concentrations at a High Frequency in a Brackish Tidal Marsh. Limnol. Ocean Methods 2014, 12, 10–22. [Google Scholar] [CrossRef]
- Mason, A.; Soprani, M.; Korostynska, O.; Amirthalingam, A.; Cullen, J.; Muradov, M.; Carmona, E.N.; Sberveglieri, G.; Sberveglieri, V.; Al-Shamma’a, A. Real-Time Microwave, Dielectric, and Optical Sensing of Lincomycin and Tylosin Antibiotics in Water: Sensor Fusion for Environmental Safety. J. Sens. 2018, 2018, 7976105. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Yang, M.; Zhu, M.; Chen, W.; Li, Q.; Sun, D.; Bi, X.; Maletskyi, Z.; Ratnaweera, H. Detection Limits of Antibiotics in Wastewater by Real-Time UV–VIS Spectrometry at Different Optical Path Length. Processes 2022, 10, 2614. [Google Scholar] [CrossRef]
- Chen, X.; Yin, G.; Zhao, N.; Gan, T.; Yang, R.; Xia, M.; Feng, C.; Chen, Y.; Huang, Y. Simultaneous Determination of Nitrate, Chemical Oxygen Demand and Turbidity in Water Based on UV–Vis Absorption Spectrometry Combined with Interval Analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 244, 118827. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, C.; Yang, C.; Zhu, H.; Li, Y. A Spectrophotometric Method for Simultaneous Determination of Trace Ions of Copper, Cobalt, and Nickel in the Zinc Sulfate Solution by Ultraviolet-Visible Spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117370. [Google Scholar] [CrossRef]
- Zhao, C.; Zhong, G.; Kim, D.-E.; Liu, J.; Liu, X. A Portable Lab-on-a-Chip System for Gold-Nanoparticle-Based Colorimetric Detection of Metal Ions in Water. Biomicrofluidics 2014, 8, 052107. [Google Scholar] [CrossRef]
- Steyer, J.P.; Bouvier, J.C.; Conte, T.; Gras, P.; Harmand, J.; Delgenes, J.P. On-Line Measurements of COD, TOC, VFA, Total and Partial Alkalinity in Anaerobic Digestion Processes Using Infra-Red Spectrometry. Water Sci. Technol. 2002, 45, 133–138. [Google Scholar] [CrossRef]
- Chandrasoma, A.; Hamid, A.A.A.; Bruce, A.E.; Bruce, M.R.M.; Tripp, C.P. An Infrared Spectroscopic Based Method for Mercury(II) Detection in Aqueous Solutions. Anal. Chim. Acta 2012, 728, 57–63. [Google Scholar] [CrossRef]
- Chen, H.; Chen, A.; Xu, L.; Xie, H.; Qiao, H.; Lin, Q.; Cai, K. A Deep Learning CNN Architecture Applied in Smart Near-Infrared Analysis of Water Pollution for Agricultural Irrigation Resources. Agric. Water Manag. 2020, 240, 106303. [Google Scholar] [CrossRef]
- Skou, P.B.; Berg, T.A.; Aunsbjerg, S.D.; Thaysen, D.; Rasmussen, M.A.; Van Den Berg, F. Monitoring Process Water Quality Using Near Infrared Spectroscopy and Partial Least Squares Regression with Prediction Uncertainty Estimation. Appl Spectrosc 2017, 71, 410–421. [Google Scholar] [CrossRef]
- Alexandrakis, D.; Downey, G.; Scannell, A.G.M. Detection and Identification of Bacteria in an Isolated System with Near-Infrared Spectroscopy and Multivariate Analysis. J. Agric. Food Chem. 2008, 56, 3431–3437. [Google Scholar] [CrossRef]
- Cámara-Martos, F.; Zurera-Cosano, G.; Moreno-Rojas, R.; García-Gimeno, R.M.; Pérez-Rodríguez, F. Identification and Quantification of Lactic Acid Bacteria in a Water-Based Matrix with Near-Infrared Spectroscopy and Multivariate Regression Modeling. Food Anal. Methods 2012, 5, 19–28. [Google Scholar] [CrossRef]
- Quintelas, C.; Mesquita, D.P.; Ferreira, E.C.; Amaral, A.L. Quantification of Pharmaceutical Compounds in Wastewater Samples by near Infrared Spectroscopy (NIR). Talanta 2019, 194, 507–513. [Google Scholar] [CrossRef]
- Wu, K.; Ma, F.; Li, Z.; Wei, C.; Gan, F.; Du, C. In-Situ Rapid Monitoring of Nitrate in Urban Water Bodies Using Fourier Transform Infrared Attenuated Total Reflectance Spectroscopy (FTIR-ATR) Coupled with Deconvolution Algorithm. J. Environ. Manag. 2022, 317, 115452. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Ma, F.; Zhou, J.; Du, C. Monitoring of Total Phosphorus in Urban Water Bodies Using Silicon Crystal-Based FTIR-ATR Coupled with Different Machine Learning Approaches. Water 2024, 16, 2479. [Google Scholar] [CrossRef]
- Pampoukis, G.; Lytou, A.E.; Argyri, A.A.; Panagou, E.Z.; Nychas, G.-J.E. Recent Advances and Applications of Rapid Microbial Assessment from a Food Safety Perspective. Sensors 2022, 22, 2800. [Google Scholar] [CrossRef] [PubMed]
- Sampling Techniques for FTIR Spectroscopy—JASCO. Available online: https://jascoinc.com/learning-center/theory/spectroscopy/fundamentals-ftir-spectroscopy/sampling/ (accessed on 9 April 2025).
- Attenuated Total Reflectance—Wikipedia. Available online: https://en.wikipedia.org/wiki/Attenuated_total_reflectance (accessed on 9 April 2025).
- Dong, J.; Tang, J.; Wu, G.; Li, R. A Turbidity-Compensation Method for Nitrate Measurement Based on Ultraviolet Difference Spectroscopy. Molecules 2022, 28, 250. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, Y.; Lu, Y.; Liu, J.; Zhou, C.; Shao, Z. The Influence and Compensation of Environmental Factors (pH, Temperature, and Conductivity) on the Detection of Chemical Oxygen Demand in Water by UV-Vis Spectroscopy. Appl. Sci. 2025, 15, 1694. [Google Scholar] [CrossRef]
- Maguire, T.J.; Dominato, K.R.; Weidman, R.P.; Mundle, S.O.C. Ultraviolet-visual Spectroscopy Estimation of Nitrate Concentrations in Surface Waters via Machine Learning. Limnol. Ocean Methods 2022, 20, 26–33. [Google Scholar] [CrossRef]
- Gan, F.; Wu, K.; Ma, F.; Du, C. In Situ Determination of Nitrate in Water Using Fourier Transform Mid-Infrared Attenuated Total Reflectance Spectroscopy Coupled with Deconvolution Algorithm. Molecules 2020, 25, 5838. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhou, J.; Ma, F.; Du, C. A Self-Adaptive Model for Sensing Total Phosphorus in Natural Water Bodies Using Fourier Transform Mid-Infrared Attenuated Total Reflectance Spectroscopy. Sens. Actuators Rep. 2024, 8, 100230. [Google Scholar] [CrossRef]
- Li, Z.; Deen, M.; Kumar, S.; Selvaganapathy, P. Raman Spectroscopy for In-Line Water Quality Monitoring—Instrumentation and Potential. Sensors 2014, 14, 17275–17303. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Gu, X.; Bao, J.; Yang, H.; Sun, L. Laser-Induced Breakdown Spectroscopy Application in Environmental Monitoring of Water Quality: A Review. Env. Monit Assess 2014, 186, 8969–8980. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, Y.; Yu, K. LIBS in Agriculture: A Review Focusing on Revealing Nutritional and Toxic Elements in Soil, Water, and Crops. Comput. Electron. Agric. 2022, 197, 106986. [Google Scholar] [CrossRef]
- Aloisi, A.; Della Torre, A.; De Benedetto, A.; Rinaldi, R. Bio-Recognition in Spectroscopy-Based Biosensors for *Heavy Metals-Water and Waterborne Contamination Analysis. Biosensors 2019, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lin, Q.; Xie, S.; He, Y.; Tian, Y.; Duan, Y. A Novel Strategy for Rapid Detection of Bacteria in Water by the Combination of Three-Dimensional Surface-Enhanced Raman Scattering (3D SERS) and Laser Induced Breakdown Spectroscopy (LIBS). Anal. Chim. Acta 2018, 1043, 64–71. [Google Scholar] [CrossRef]
- Meng, D.; Zhao, N.; Wang, Y.; Ma, M.; Fang, L.; Gu, Y.; Jia, Y.; Liu, J. On-Line/on-Site Analysis of Heavy Metals in Water and Soils by Laser Induced Breakdown Spectroscopy. Spectrochim. Acta Part B: At. Spectrosc. 2017, 137, 39–45. [Google Scholar] [CrossRef]
- Angelotti De Ponte Rodrigues, N.; Carmigniani, R.; Guillot-Le Goff, A.; Lucas, F.S.; Therial, C.; Naloufi, M.; Janne, A.; Piccioni, F.; Saad, M.; Dubois, P.; et al. Fluorescence Spectroscopy for Tracking Microbiological Contamination in Urban Waterbodies. Front. Water 2024, 6, 1358483. [Google Scholar] [CrossRef]
- Apostolakis, A.; Aoust, G.; Maisons, G.; Laurent, L.; Pereira, M.F. Photoacoustic Spectroscopy Using a Quantum Cascade Laser for Analysis of Ammonia in Water Solutions. ACS Omega 2024, 9, 19127–19135. [Google Scholar] [CrossRef]
- Wang, D.; Kannojia, H.K.; Jouy, P.; Giraud, E.; Suter, K.; Maulini, R.; Gachet, D.; Hetier, L.; Van Steenberge, G.; Kuyken, B. Innovative Integration of Dual Quantum Cascade Lasers on Silicon Photonics Platform. Micromachines 2024, 15, 1055. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, M.; Kienberger, R.; Spielmann, C.; Reider, G.A.; Milosevic, N.; Brabec, T.; Corkum, P.; Heinzmann, U.; Drescher, M.; Krausz, F. Attosecond Metrology. Nature 2001, 414, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Trubetskov, M.; Schweinberger, W.; Jacob, P.; Zigman, M.; Krausz, F.; Pupeza, I. Standardized Electric-Field-Resolved Molecular Fingerprinting. Anal. Chem. 2024, 96, 13110–13119. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, S.; Jiang, Y.; Chen, A.; Jin, M. Direct Analysis of Heavy Metal Elements in Liquid Water Using Femtosecond Laser-Induced Breakdown Spectroscopy for High-Sensitivity Detection. Talanta 2025, 286, 127512. [Google Scholar] [CrossRef]
- Sagan, V.; Peterson, K.T.; Maimaitijiang, M.; Sidike, P.; Sloan, J.; Greeling, B.A.; Maalouf, S.; Adams, C. Monitoring Inland Water Quality Using Remote Sensing: Potential and Limitations of Spectral Indices, Bio-Optical Simulations, Machine Learning, and Cloud Computing. Earth-Sci. Rev. 2020, 205, 103187. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Mukhtar, H.; Huang, K.-T.; Petway, J.R.; Lin, C.-M.; Chou, C.-F.; Liao, S.-W. Real-Time Identification of Irrigation Water Pollution Sources and Pathways with a Wireless Sensor Network and Blockchain Framework. Sensors 2020, 20, 3634. [Google Scholar] [CrossRef]
- Alharbi, N.; Althagafi, A.; Alshomrani, O.; Almotiry, A.; Alhazmi, S. A Blockchain Based Secure IoT Solution for Water Quality Management. In Proceedings of the 2021 International Congress of Advanced Technology and Engineering (ICOTEN), Virtual, 4–5 July 2021; IEEE: Taiz, Yemen, 2021; pp. 1–8. [Google Scholar]
- Samanta, S.; Sarkar, A. IoT and Blockchain for Smart Water Quality Management in Future Cities: A Hyperledger Fabric Framework for Smart Water Quality Management and Distribution. Res. Sq. 2023; preprint. [Google Scholar]
- Aira, J.; Olivares, T.; Delicado, F.M. SpectroGLY: A Low-Cost IoT-Based Ecosystem for the Detection of Glyphosate Residues in Waters. IEEE Trans. Instrum. Meas. 2022, 71, 1–10. [Google Scholar] [CrossRef]


| Algorithm | Accuracy | Computational Cost | Real-Time Suitability | Overfitting Risk | Interpretability |
|---|---|---|---|---|---|
| PLS | ++ | + | +++ | + | +++ |
| SVM | +++ | ++ | ++ | ++ | ++ |
| ANNs | ++++ | +++ | ++ | +++ | + |
| DT | ++ | + | +++ | +++ | +++ |
| RF | +++ | + | ++ | ++ | ++ |
| Spectral Technique | Sample Preparation | Sensitivity | Cost | Portability | Convenience |
|---|---|---|---|---|---|
| UV–Vis | + | ++ | + | +++ | +++ |
| NIRS | + | ++ | ++ | +++ | +++ |
| MIRS | ++ | +++ | +++ | + | + |
| FTIR | ++ | +++ | +++ | ++ | ++ |
| LIBS | + | +++ | ++ | ++ | ++ |
| Raman | + | ++ | +++ | ++ | ++ |
| Fluorescence | + | +++ | ++ | +++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agiomavriti, A.-A.; Bartzanas, T.; Chorianopoulos, N.; Gelasakis, A.I. Spectroscopy-Based Methods for Water Quality Assessment: A Comprehensive Review and Potential Applications in Livestock Farming. Water 2025, 17, 2488. https://doi.org/10.3390/w17162488
Agiomavriti A-A, Bartzanas T, Chorianopoulos N, Gelasakis AI. Spectroscopy-Based Methods for Water Quality Assessment: A Comprehensive Review and Potential Applications in Livestock Farming. Water. 2025; 17(16):2488. https://doi.org/10.3390/w17162488
Chicago/Turabian StyleAgiomavriti, Aikaterini-Artemis, Thomas Bartzanas, Nikos Chorianopoulos, and Athanasios I. Gelasakis. 2025. "Spectroscopy-Based Methods for Water Quality Assessment: A Comprehensive Review and Potential Applications in Livestock Farming" Water 17, no. 16: 2488. https://doi.org/10.3390/w17162488
APA StyleAgiomavriti, A.-A., Bartzanas, T., Chorianopoulos, N., & Gelasakis, A. I. (2025). Spectroscopy-Based Methods for Water Quality Assessment: A Comprehensive Review and Potential Applications in Livestock Farming. Water, 17(16), 2488. https://doi.org/10.3390/w17162488









