Ultrasonic Inactivation of Escherichia coli with Multi-Walled Carbon Nanotubes: Effects of Solution Chemistry †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Cultivation of E. coli
2.3. Procedures of Inactivating E. coli
2.4. Sample Analyses
3. Results
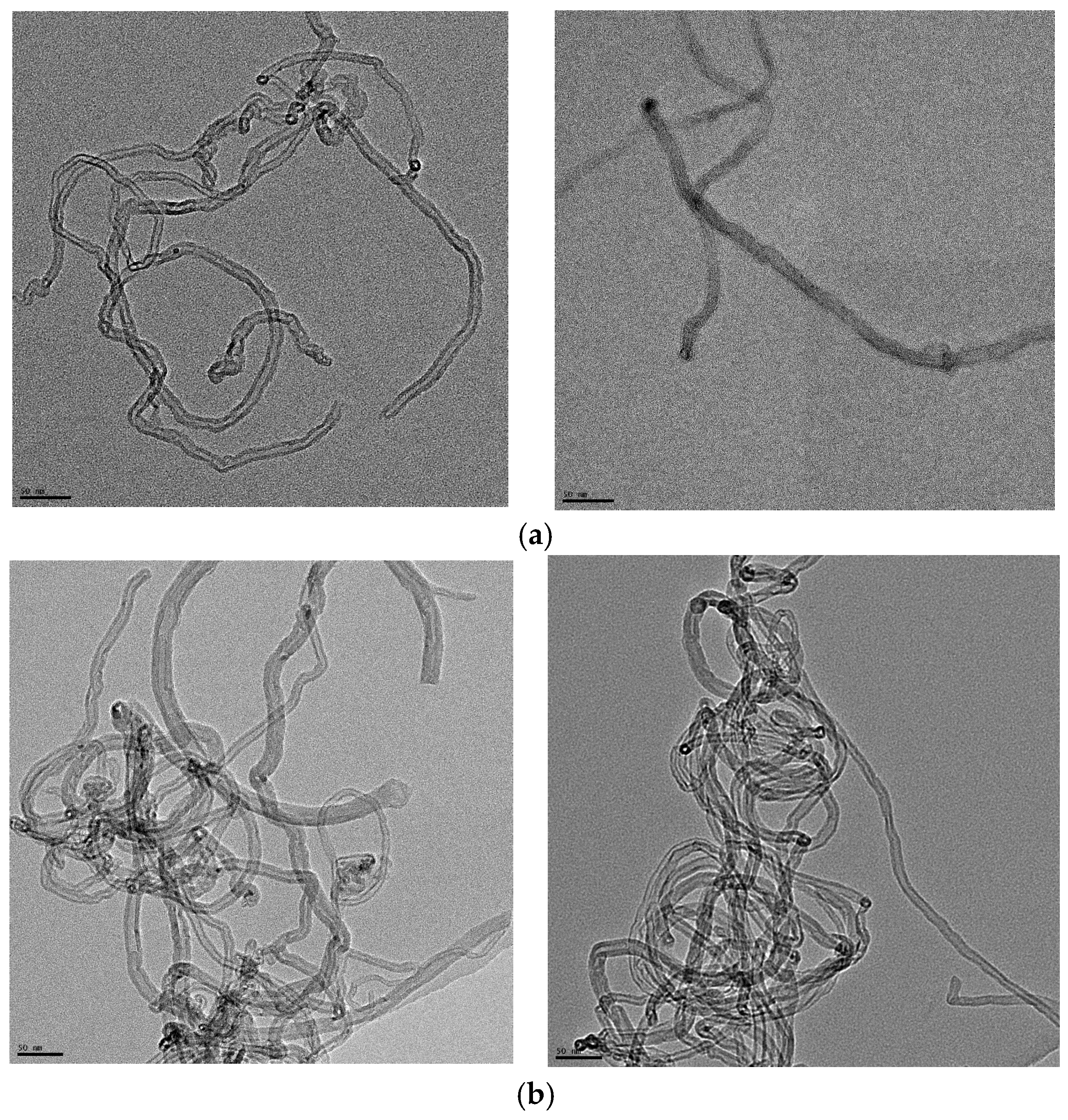
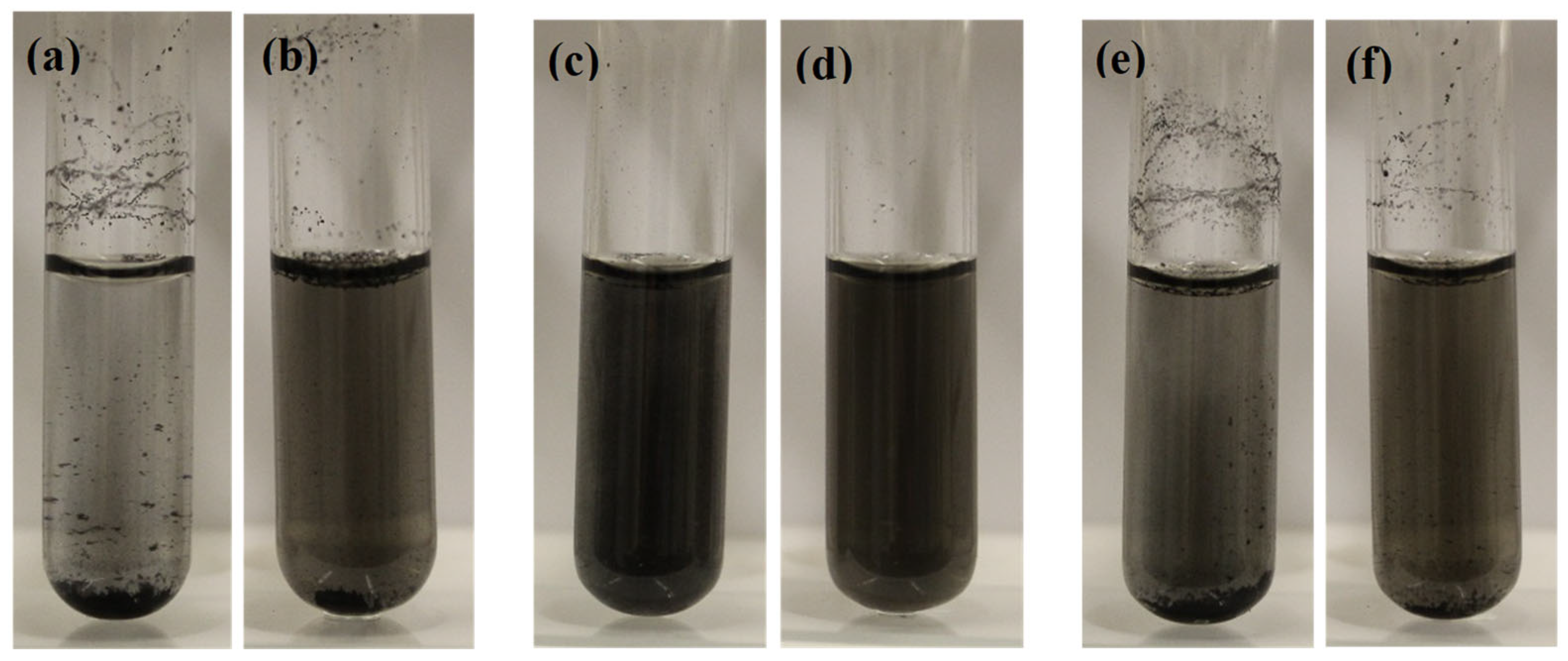
3.1. Morphology and Dispersion of MWCNTs in Different Solutions
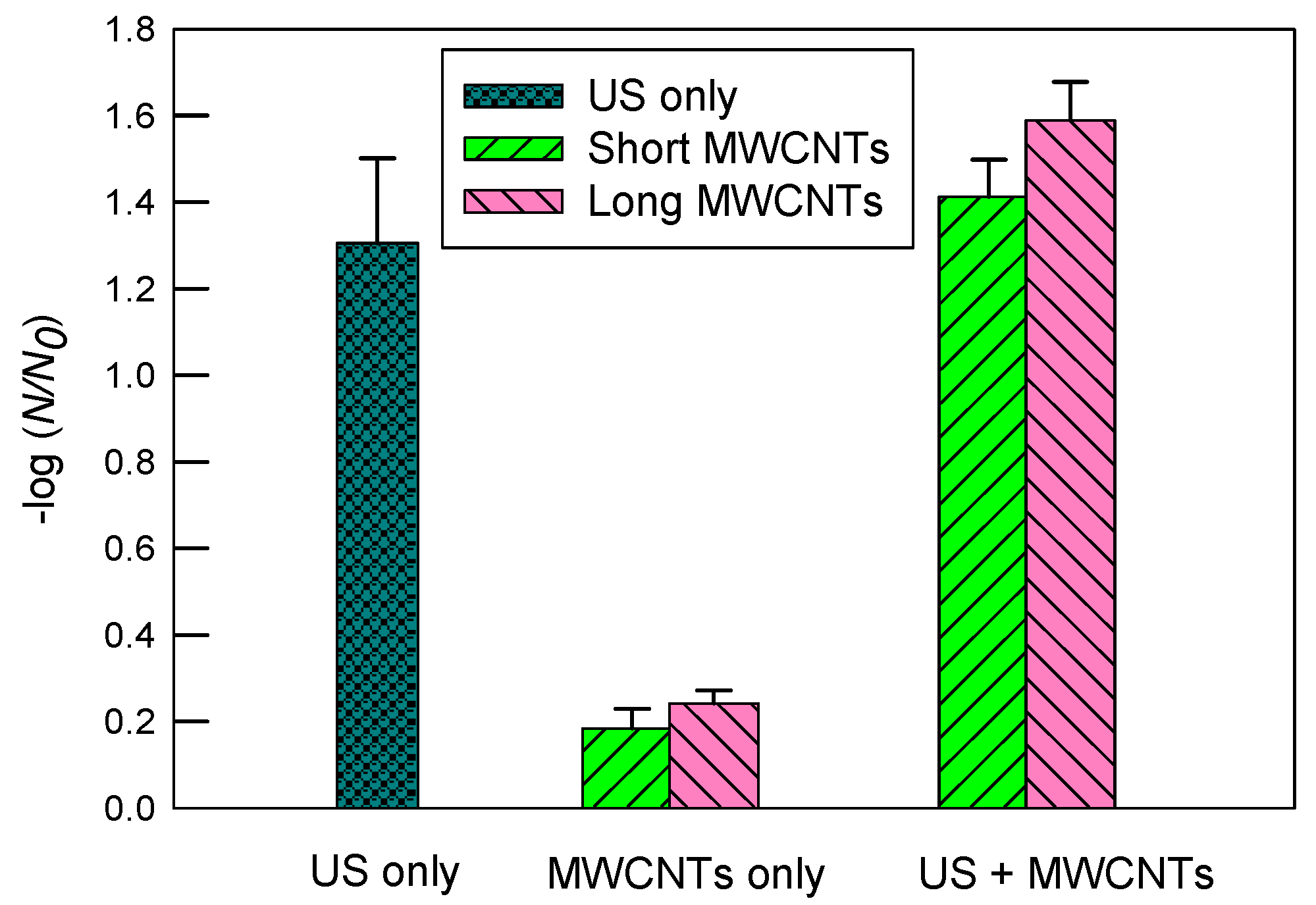
3.2. Inactivation of E. coli in DI Water
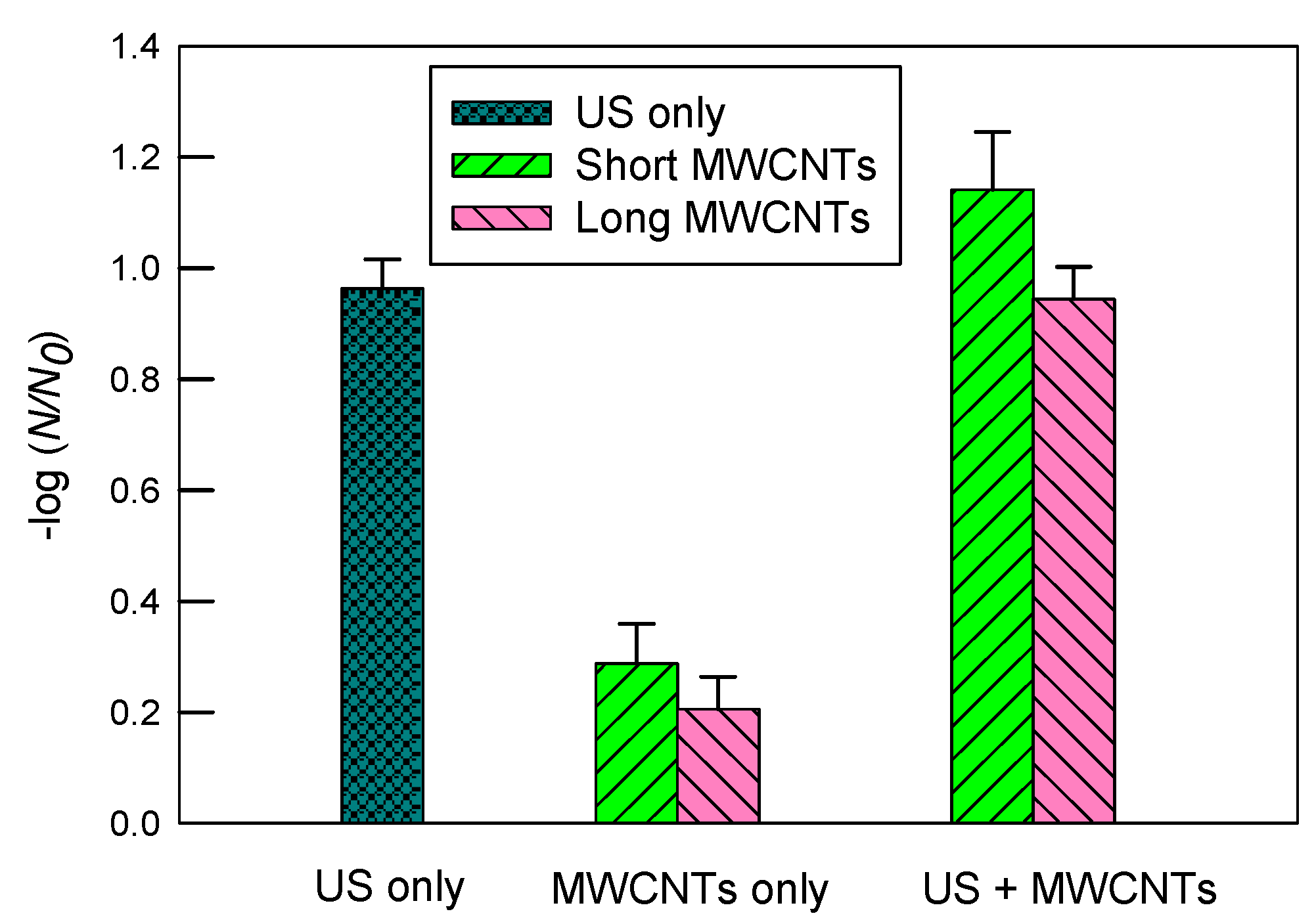
3.3. Inactivation of E. coli in 10 mgC/mL SRNOM
3.4. Inactivation of E. coli in 2 mM SDS Solution
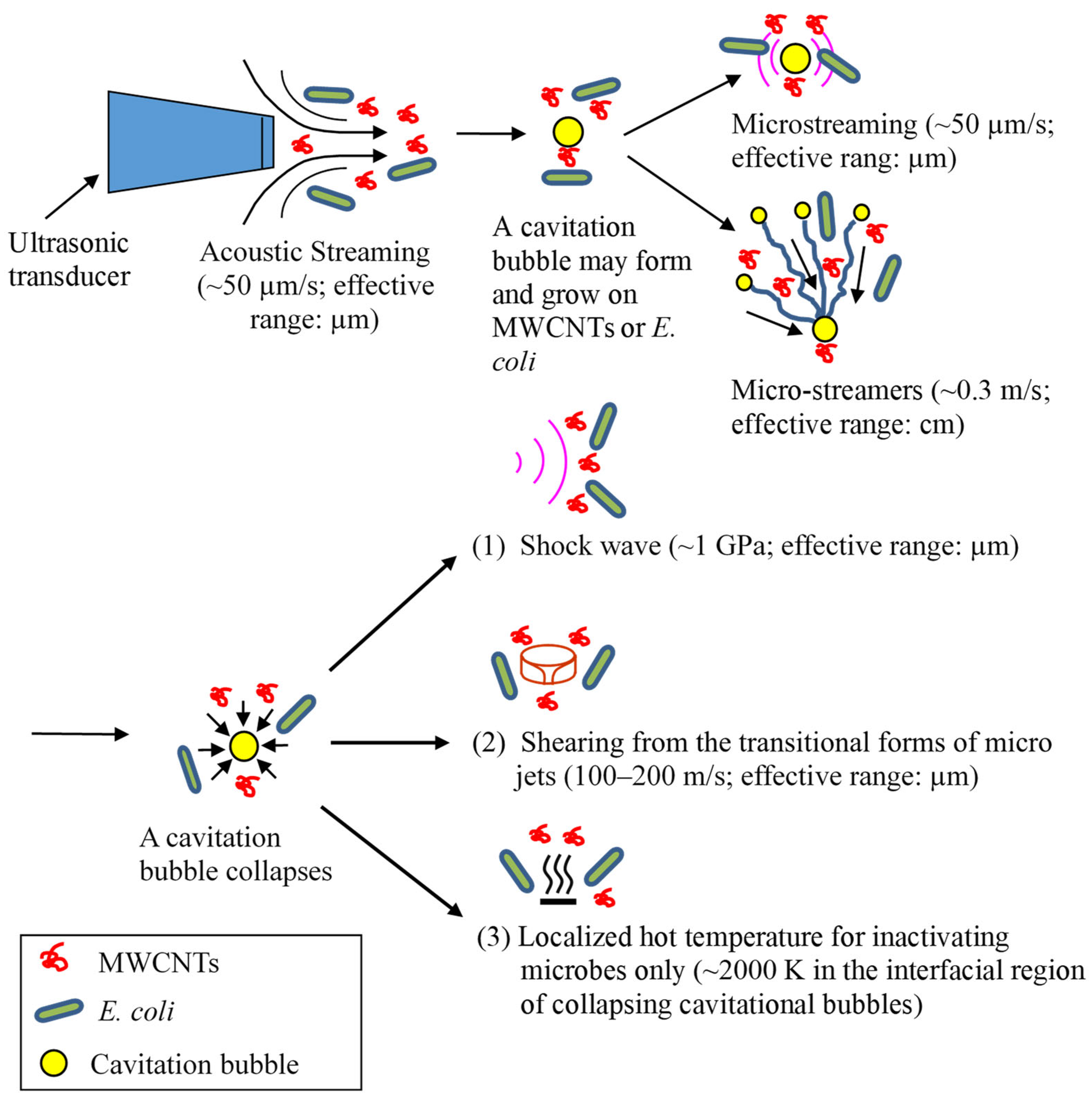
3.5. The Antimicrobial Mechanisms of Ultrasound and MWCNTs
4. Conclusions
- (1)
- The results showed that the inactivation rate of E. coli by MWCNTs was determined primarily by their dispersity in water solutions.
- (2)
- Overall, 2 mM SDS had the greatest improvement, while 10 mgC/L SRNOM had a moderate improvement in the dispersity of short MWCNTs in DI water. Correspondingly, the log inactivation of E. coli without ultrasound was 0.67 ± 0.12 in SDS, 0.29 ± 0.07 in SRNOM, and 0.18 ± 0.05 in DI.
- (3)
- However, long MWCNTs used alone had better dispersity in DI water and slightly greater log inactivation (0.24 ± 0.03) than short MWCNTs.
- (4)
- When combined with ultrasound, the greatest synergistic log inactivation of E. coli (1.80 ± 0.02) was observed with short MWCNTs in 2 mM of SDS, although SDS and MWCNTs could occupy the cavitational bubble–water interfacial regions and scavenge •OH radicals.
- (5)
- The results suggest that the sonophysical effects of ultrasound are more important than the sonochemical effects for the inactivation of E. coli. The sonophysical mechanisms for inactivating E. coli and/or energizing MWCNTs include acoustic streaming, microstreaming, microstreamers, shock waves, transitional forms of microjets, and localized hot temperatures from the transient collapse of cavitation bubbles. The results of this study are in agreement with the toxicity mechanisms of CNTs, i.e., impinging bacterial cells and/or direct contact with the bacteria.
- (6)
- This study aims to understand the mechanisms of key chemicals present in background solutions and their influence on disinfection using ultrasound and MWCNTs. In the future, we plan to expand the scope of our research to include chloride, soluble extracellular polymeric substances, and different microbial species in water, as well as to investigate the effects of different ultrasonic frequencies, power levels, and MWCNT concentrations.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mlosek, R.K.; Migda, B.; Migda, M. High-frequency ultrasound in the 21st century. J. Ultrason. 2021, 20, e233–e241. [Google Scholar] [CrossRef]
- Thompson, L.; Doraiswamy, L. Sonochemistry: Science and engineering. Ind. Eng. Chem. Res. 1999, 38, 1215–1249. [Google Scholar] [CrossRef]
- Chen, D. Applications of ultrasound in water and wastewater treatment. In Handbook on Applications of Ultrasound: Sonochemistry for Sustainability; Chen, D., Sharma, S.K., Mudhoo, A., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Pflieger, R.; Lejeune, M.; Noel, C.; Belmonte, T.; Nikitenko, S.I.; Draye, M. Diagnosing the plasma formed during acoustic cavitation in [BEPip][NTf2] ionic liquid. Phys. Chem. Chem. Phys. 2019, 21, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Merouani, S.; Dehane, A.; Hamdaoui, O.; Yasui, K.; Ashokkumar, M. Review on the impacts of external pressure on sonochemistry. Ultrason. Sonochem. 2024, 106, 106893. [Google Scholar] [CrossRef]
- Engler, C.; Robinson, C. Effects of organism type and growth conditions on cell disruption by impingement. Biotechnol. Lett. 1981, 3, 83–88. [Google Scholar] [CrossRef]
- Keshavarz, T.; Eglin, R.; Walker, E.; Bucke, C.; Holt, G.; Bull, A.; Lilly, M. The large-scale immobilization of Penicillium chrysogenum: Batch and continuous operation in an air-lift reactor. Biotechnol. Bioeng. 1990, 36, 763–770. [Google Scholar] [CrossRef]
- Sundaram, J.; Mellein, B.R.; Mitragotri, S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys. J. 2003, 84, 3087–3101. [Google Scholar] [CrossRef]
- Furuta, M.; Yamaguchi, M.; Tsukamoto, T.; Yim, B.; Stavarache, C.; Hasiba, K.; Maeda, Y. Inactivation of Escherichia coli by ultrasonic irradiation. Ultrason. Sonochem. 2004, 11, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Oyane, I.; Furuta, M.; Stavarache, C.; Hashiba, K.; Mukai, S.; Nakanishi, M.; Kimata, I.; Maeda, Y. Inactivation of Cryptosporidium parvum by ultrasonic irradiation. Environ. Sci. Technol. 2005, 39, 7294–7298. [Google Scholar] [CrossRef]
- Mahulkar, A.; Riedel, C.; Gogate, P.; Neis, U.; Pandit, A. Effect of dissolved gas on efficacy of sonochemical reactors for microbial cell disruption: Experimental and numerical analysis. Ultrason. Sonochem. 2009, 16, 635–643. [Google Scholar] [CrossRef]
- Scherba, G.; Weigel, R.; O’brien, W. Quantitative assessment of the germicidal efficacy of ultrasonic energy. Appl. Environ. Microbiol. 1991, 57, 2079–2084. [Google Scholar] [CrossRef]
- Bakhtiari, A.; Schumm, B.; Schönfelder, M.; Kähler, C.J. Investigating cell viability under shear stress in complex microstreaming flows generated by ultrasound-driven actuated microbubbles. Flow 2025, 5, E7. [Google Scholar] [CrossRef]
- Yang, Y.; Nie, G.; Bi, J.; Zhang, P.; Huo, P. Agitation-intensified sonochemistry for water disinfection. Environ. Surf. Interfaces 2025, 3, 176–182. [Google Scholar] [CrossRef]
- Hua, I.; Thompson, J.E. Inactivation of Escherichia coli by sonication at discrete ultrasonic frequencies. Water Res. 2000, 34, 3888–3893. [Google Scholar] [CrossRef]
- Joyce, E.; Phull, S.; Lorimer, J.; Mason, T. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrason. Sonochem. 2003, 10, 315–318. [Google Scholar] [CrossRef]
- Gogate, P.R. Application of cavitational reactors for water disinfection: Current status and path forward. J. Environ. Manag. 2007, 85, 801–815. [Google Scholar] [CrossRef]
- Blume, T.; Neis, U. Improved wastewater disinfection by ultrasonic pre-treatment. Ultrason. Sonochem. 2004, 11, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Thacker, J. An approach to the mechanism of killing of cells in suspension by ultrasound. BBA-Gen. Subj. 1973, 304, 240–248. [Google Scholar] [CrossRef]
- Broekman, S.; Pohlmann, O.; Beardwood, E.; de Meulenaer, E.C. Ultrasonic treatment for microbiological control of water systems. Ultrason. Sonochem. 2010, 17, 1041–1048. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef]
- Brady-Estevez, A.S.; Nguyen, T.H.; Gutierrez, L.; Elimelech, M. Impact of solution chemistry on viral removal by a single-walled carbon nanotube filter. Water Res. 2010, 44, 3773–3780. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and faster “nano darts” kill more bacteria: A study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef]
- Li, A.; Yao, J.; Li, N.; Shi, C.; Bai, M.; Wang, Z.; Hrynsphan, D.; Savitskayac, T.; Chen, J. Effect of biochar, graphene, carbon nanotubes, and nanoparticles on microbial denitrification: A review. Crit. Rev. Environ. Sci. Technol. 2024, 55, 99–122. [Google Scholar] [CrossRef]
- Yang, C.; Mamouni, J.; Tang, Y.; Yang, L. Antimicrobial activity of single-walled carbon nanotubes: Length effect. Langmuir 2010, 26, 16013–16019. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Maksimova, Y.; Zorina, A.; Nesterova, L. Oxidative stress response and E. coli biofilm formation under the effect of pristine and modified carbon nanotubes. Microorganisms 2023, 11, 1221. [Google Scholar] [CrossRef]
- Moskvitina, E.; Kuznetsov, V.; Moseenkov, S.; Serkova, A.; Zavorin, A. Antibacterial effect of carbon nanomaterials: Nanotubes, carbon nanofibers, nanodiamonds, and onion-like carbon. Materials 2023, 16, 957. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Microbial cytotoxicity of carbon-based nanomaterials: Implications for river water and wastewater effluent. Environ. Sci. Technol. 2009, 43, 2648–2653. [Google Scholar] [CrossRef]
- Fenoglio, I.; Tomatis, M.; Lison, D.; Muller, J.; Fonseca, A.; Nagy, J.B.; Fubini, B. Reactivity of carbon nanotubes: Free radical generation or scavenging activity? Free Radic. Biol. Med. 2006, 40, 1227–1233. [Google Scholar] [CrossRef]
- Crum, L.A. Comments on the evolving field of sonochemistry by a cavitation physicist. Ultrason. Sonochem. 1995, 2, S147–S152. [Google Scholar] [CrossRef]
- Chen, D.; He, Z.; Weavers, L.K.; Chin, Y.-P.; Walker, H.W.; Hatcher, P.G. Sonochemical reactions of dissolved organic matter. Res. Chem. Intermed. 2004, 30, 735–753. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Biodegradation of the anionic surfactant sodium dodecyl sulfate at low temperatures. Int. Biodeterior. Biodegrad. 1998, 41, 139–143. [Google Scholar] [CrossRef]
- Deschenes, L.; Lafrance, P.; Villeneuve, J.-P.; Samson, R. Adding sodium dodecyl sulfate and Pseudomonas aeruginosa UG2 biosurfactants inhibits polycyclic aromatic hydrocarbon biodegradation in a weathered creosote-contaminated soil. Appl. Microbiol. Biotechnol. 1996, 46, 638–646. [Google Scholar] [CrossRef]
- Rahman, A.; Brown, C. Effect of pH on the critical micelle concentration of sodium dodecyl sulphate. J. Appl. Polym. Sci. 1983, 28, 1331–1334. [Google Scholar] [CrossRef]
- Klassen, N.V.; Marchington, D.; McGowan, H.C. H2O2 determination by the I3-method and by KMnO4 titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for The Examination of Water and Wastewater, 24th ed.; American Public Health Association (APHA): Washington, DC, USA, 2023. [Google Scholar]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128, 37–46. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Wang, R.; Lu, H.; Li, L.; Kong, Y.; Qi, K.; Xin, J. Artificial lotus leaf structures from assembling carbon nanotubes and their applications in hydrophobic textiles. J. Mater. Chem. 2007, 17, 1071–1078. [Google Scholar] [CrossRef]
- Rastogi, R.; Kaushal, R.; Tripathi, S.; Sharma, A.L.; Kaur, I.; Bharadwaj, L.M. Comparative study of carbon nanotube dispersion using surfactants. J. Colloid Interface Sci. 2008, 328, 421–428. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Edwards, M.; Benjamin, M.M.; Ryan, J.N. Role of organic acidity in sorption of natural organic matter (NOM) to oxide surfaces. Colloids Surf. A 1996, 107, 297–307. [Google Scholar] [CrossRef]
- Conte, P.; Agretto, A.; Spaccini, R.; Piccolo, A. Soil remediation: Humic acids as natural surfactants in the washings of highly contaminated soils. Environ. Pollut. 2005, 135, 515–522. [Google Scholar] [CrossRef]
- Hyung, H.; Fortner, J.D.; Hughes, J.B.; Kim, J.-H. Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ. Sci. Technol. 2007, 41, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Yang, J.; Bitter, J.L.; Ball, W.P.; Fairbrother, D.H. Influence of surface oxygen on the interactions of carbon nanotubes with natural organic matter. Environ. Sci. Technol. 2012, 46, 12839–12847. [Google Scholar] [CrossRef]
- Neppiras, E.A. Acoustic cavitation. Phys. Rep. 1980, 61, 159–251. [Google Scholar] [CrossRef]
- Verraes, T.; Lepoint-Mullie, F.; Lepoint, T.; Longuet-Higgins, M.S. Experimental study of the liquid flow near a single sonoluminescent bubble. J. Acoust. Soc. Am. 2000, 108, 117–125. [Google Scholar] [CrossRef]
- Matthess, G.; Bedbur, E.; Gundermann, K.-O.; Loof, M.; Peters, D. Vergleichende Untersuchung zum Filtrationsverhalten von Bakterien und organischen Partikeln in Porengrundwasserleitern. I, Grundlagen und Methoden. Zentralblatt Hyg. Umweltmed. 1991, 191, 53–97. [Google Scholar]
- Cornel, P.K.; Summers, R.S.; Roberts, P.V. Diffusion of humic acid in dilute aqueous solution. J. Colloid Interface Sci. 1986, 110, 149–164. [Google Scholar] [CrossRef]
- Hua, I.; Hoffmann, M.R. Optimization of ultrasonic irradiation as an advanced oxidation technology. Environ. Sci. Technol. 1997, 31, 2237–2243. [Google Scholar] [CrossRef]
- Henglein, A. Contributions to various aspects of cavitation chemistry. In Advances in Sonochemistry; Mason, T., Ed.; JAI Press Ltd.: Bingley, UK, 1993; pp. 17–83. [Google Scholar]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms, hydroxyl radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Meier, M.; Namjesnik-Dejanovic, K.; Maurice, P.A.; Chin, Y.-P.; Aiken, G.R. Fractionation of aquatic natural organic matter upon sorption to goethite and kaolinite. Chem. Geol. 1999, 157, 275–284. [Google Scholar] [CrossRef]
- Wagoner, D.B.; Christman, R.F.; Cauchon, G.; Paulson, R. Molar mass and size of Suwannee River natural organic matter using multi-angle laser light scattering. Environ. Sci. Technol. 1997, 31, 937–941. [Google Scholar] [CrossRef]
- Leighton, T. The Acoustic Bubble; Academic Press, Inc.: London, UK, 1994. [Google Scholar]
- Roy, R.A. Cavitation sonophysics. In Sonochemisty and Sonolymninescence; Crum, L.A., Mason, T.J., Reisse, J.L., Suslick, K.S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1999; pp. 25–38. [Google Scholar]
- Luther, S.; Mettin, R.; Koch, P.; Lauterborn, W. Observation of acoustic cavitation bubbles at 2250 frames per second. Ultrason. Sonochem. 2001, 8, 159–162. [Google Scholar] [CrossRef]
- Holzfuss, J.; Rüggeberg, M.; Billo, A. Shock wave emissions of a sonoluminescing bubble. Phys. Rev. Lett. 1998, 81, 5434. [Google Scholar] [CrossRef]
- Pecha, R.; Gompf, B. Microimplosions: Cavitation collapse and shock wave emission on a nanosecond time scale. Phys. Rev. Lett. 2000, 84, 1328. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Ince, N.H.; Belen, R. Aqueous phase disinfection with power ultrasound: Process kinetics and effect of solid catalysts. Environ. Sci. Technol. 2001, 35, 1885–1888. [Google Scholar] [CrossRef]
- Price, G.J.; Smith, P.F. Ultrasonic degradation of polymer solutions: 2. The effect of temperature, ultrasound intensity and dissolved gases on polystyrene in toluene. Polymer 1993, 34, 4111–4117. [Google Scholar] [CrossRef]
- Chen, D.; Weavers, L.K.; Walker, H.W. Ultrasonic control of ceramic membrane fouling: Effect of particle characteristics. Water Res. 2006, 40, 840–850. [Google Scholar] [CrossRef]
- Chen, D. Ultrasonic inactivation of Escherichia coli with multi-walled carbon nanotubes: The effect of solution chemistry. In Proceedings of the World Environmental and Water Resources Congress, Anchorage, AK, USA, 18–21 May 2025; pp. 1218–1229. [Google Scholar]


| DI | 10 mgC/L SRNOM | 2 mM SDS | |
|---|---|---|---|
| N0 (CFU/mL) | (3.64 ± 0.90) × 108 | (7.77 ± 1.53) × 108 | (2.08 ± 0.04) × 108 |
| pH | 5.4 ± 0.2 | 5.8 ± 0.3 | 5.6 ± 0.2 |
| Chemical Solutions | DI | 10 mgC/L SRNOM | 2 mM SDS | |
|---|---|---|---|---|
| Ultrasound alone | 1.31 ± 0.20 | 0.96 ± 0.05 | 0.75 ± 0.04 | |
| Short MWCNTs | Alone | 0.18 ± 0.05 | 0.29 ± 0.07 | 0.67 ± 0.12 |
| With ultrasound | 1.41 ± 0.09 | 1.14 ± 0.10 | 1.80 ± 0.02 | |
| Long MWCNTs | Alone | 0.24 ± 0.03 | 0.21 ± 0.06 | 0.24 ± 0.01 |
| With ultrasound | 1.59 ± 0.09 | 0.94 ± 0.06 | 1.56 ± 0.06 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Chen, E.I. Ultrasonic Inactivation of Escherichia coli with Multi-Walled Carbon Nanotubes: Effects of Solution Chemistry. Water 2025, 17, 2472. https://doi.org/10.3390/w17162472
Chen D, Chen EI. Ultrasonic Inactivation of Escherichia coli with Multi-Walled Carbon Nanotubes: Effects of Solution Chemistry. Water. 2025; 17(16):2472. https://doi.org/10.3390/w17162472
Chicago/Turabian StyleChen, Dong, and Elisa I. Chen. 2025. "Ultrasonic Inactivation of Escherichia coli with Multi-Walled Carbon Nanotubes: Effects of Solution Chemistry" Water 17, no. 16: 2472. https://doi.org/10.3390/w17162472
APA StyleChen, D., & Chen, E. I. (2025). Ultrasonic Inactivation of Escherichia coli with Multi-Walled Carbon Nanotubes: Effects of Solution Chemistry. Water, 17(16), 2472. https://doi.org/10.3390/w17162472






