Evaluation of Ultraviolet Light-Based Oxidative Systems for the Inactivation and Change in Susceptibility of a Fluconazole-Resistant Candida albicans Strain
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Fungal Strain and Inoculum
2.3. Reaction System
2.4. Yeast Counting
2.5. Dark Reactivation of Yeasts
2.6. Susceptibility of C. albicans to Fluconazole Using the Macrodilution Technique
2.7. Statistical Analysis
3. Results
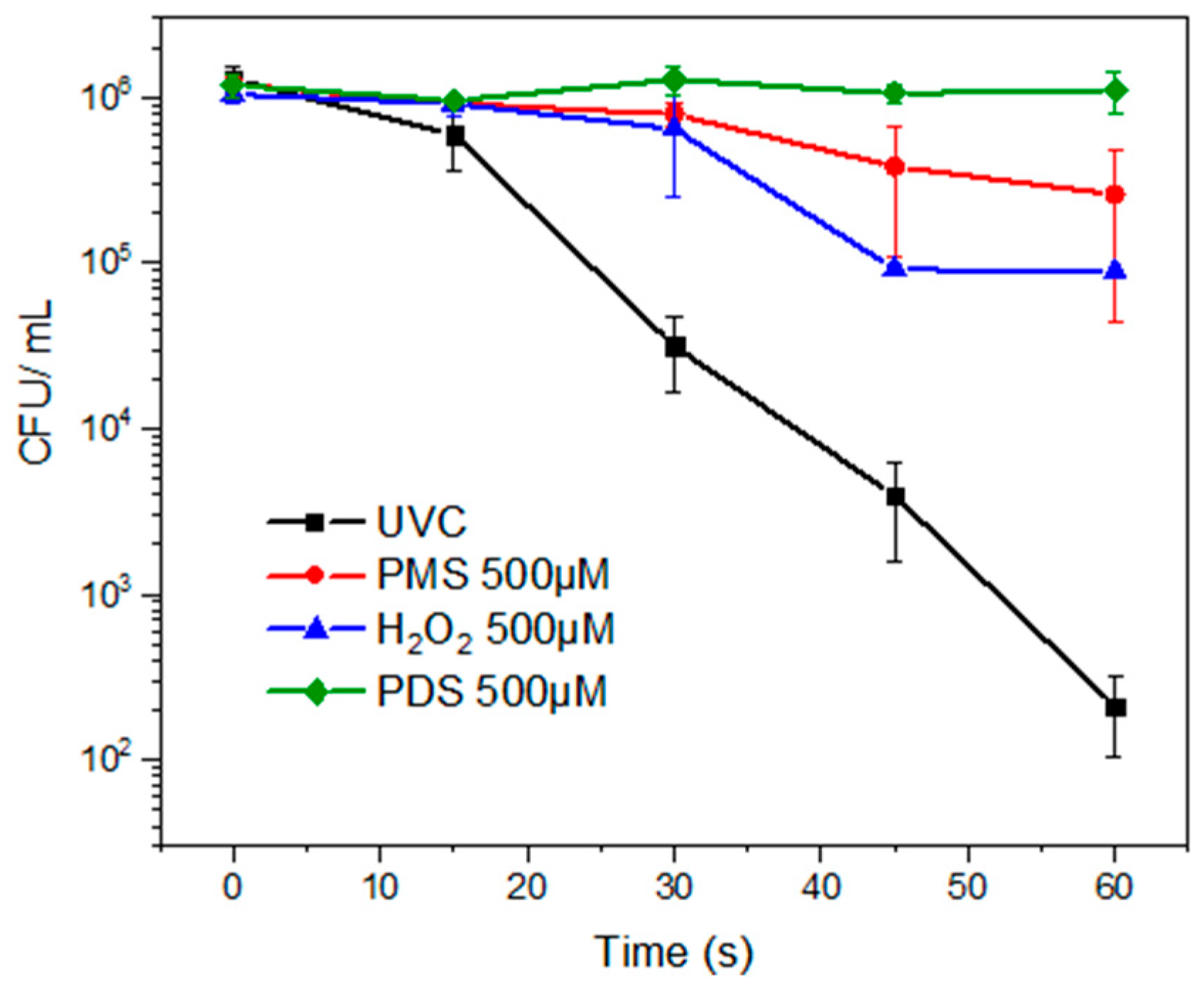
3.1. Inactivation of Fluconazole-Resistant C. albicans with Inorganic Peroxides in the Dark and UVC Control
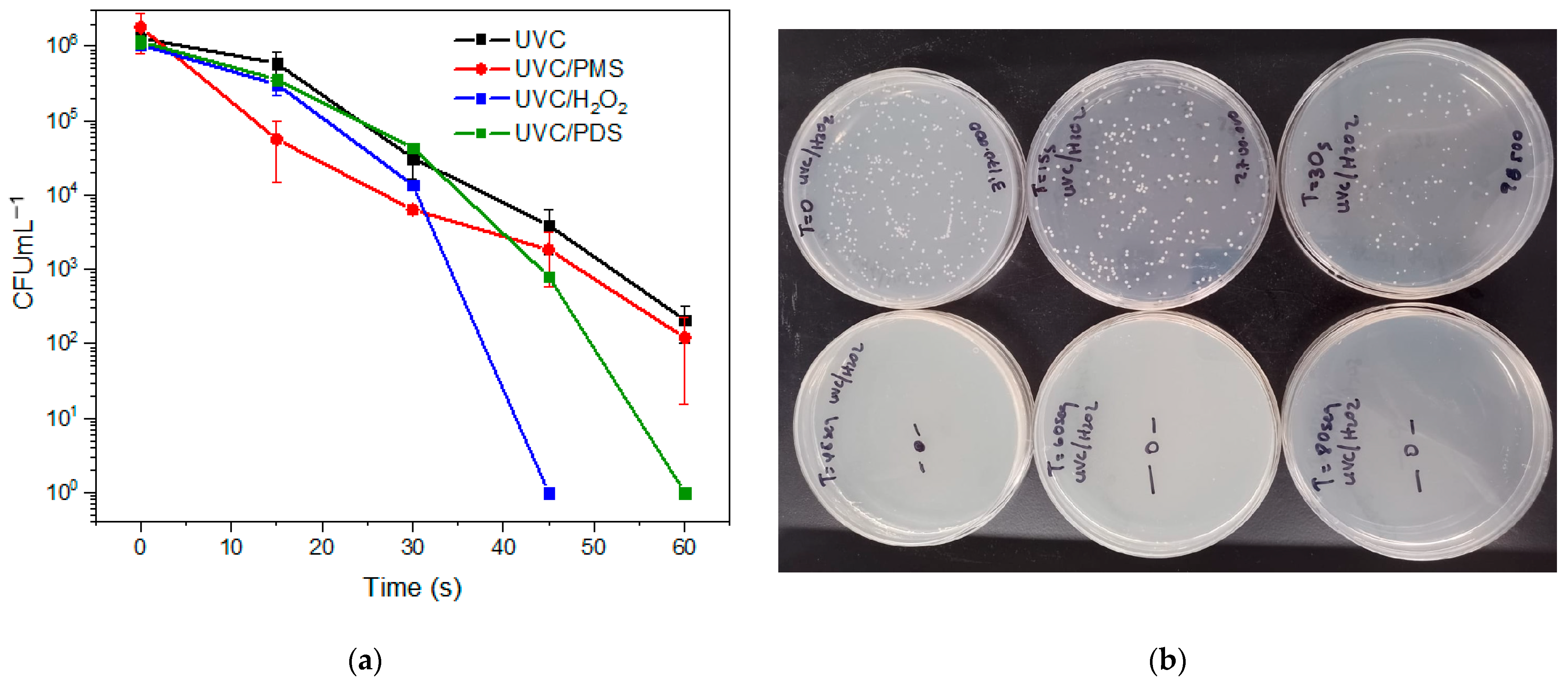
3.2. Inactivation of Fluconazole-Resistant C. albicans by Photooxidation with the Different Peroxides
3.3. Kinetic Results of the Inactivation of Fluconazole-Resistant C. albicans by Photooxidation with Different Peroxides
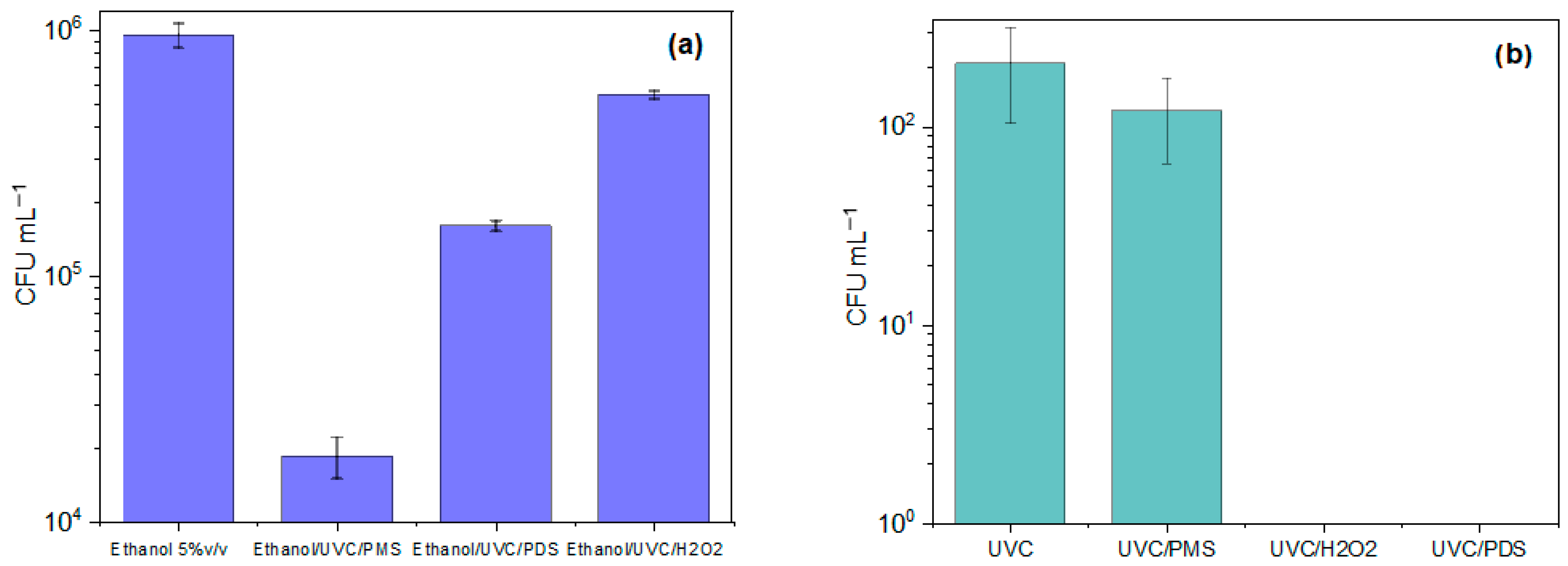
3.4. Elucidation of the Routes Involved in the Inactivation of Fluconazole-Resistant C. albicans by Photooxidation Processes
3.5. Reactivation of C. albicans in Darkness
3.6. Susceptibility to Fluconazole of the Treated C. albicans
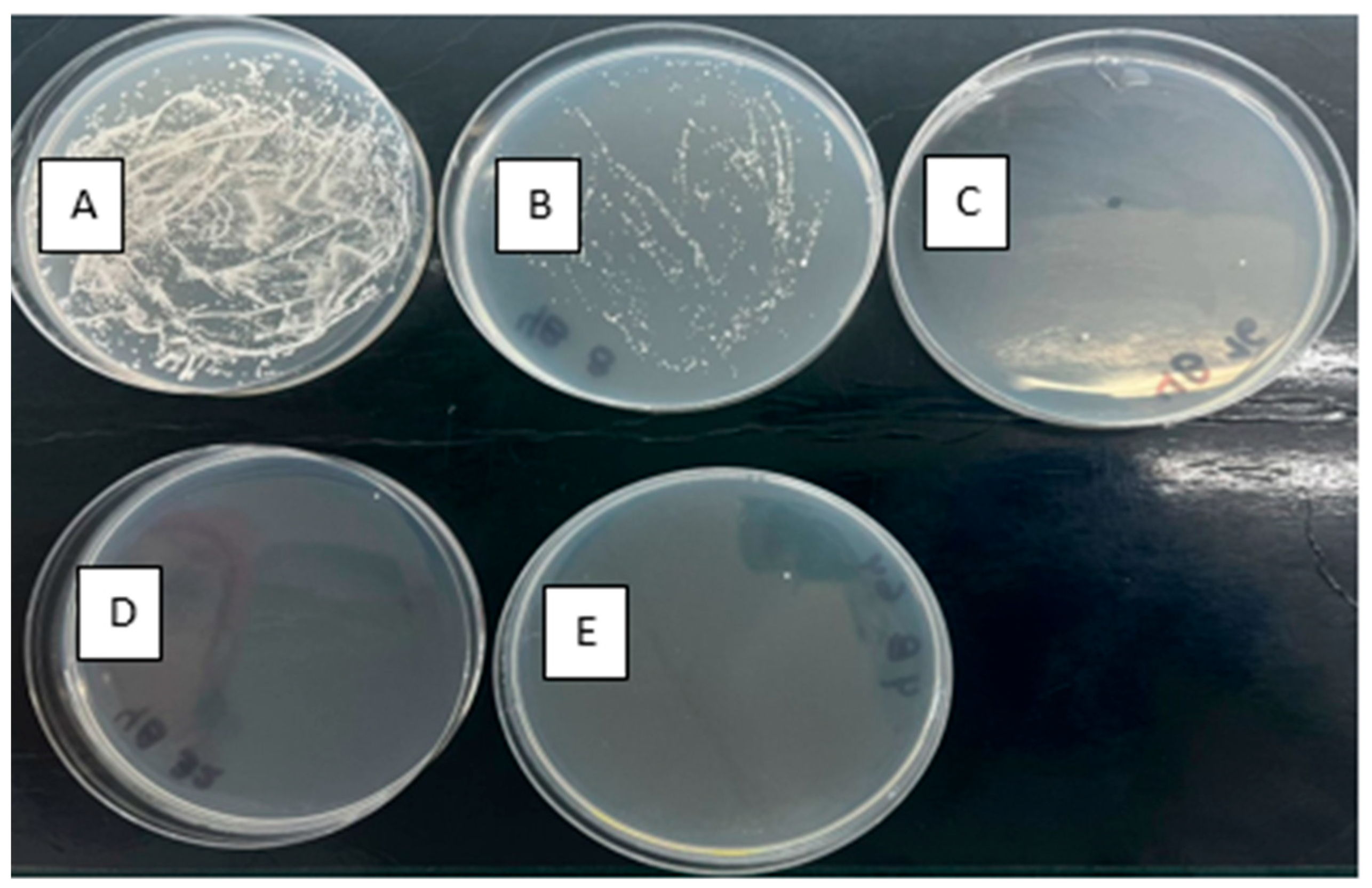
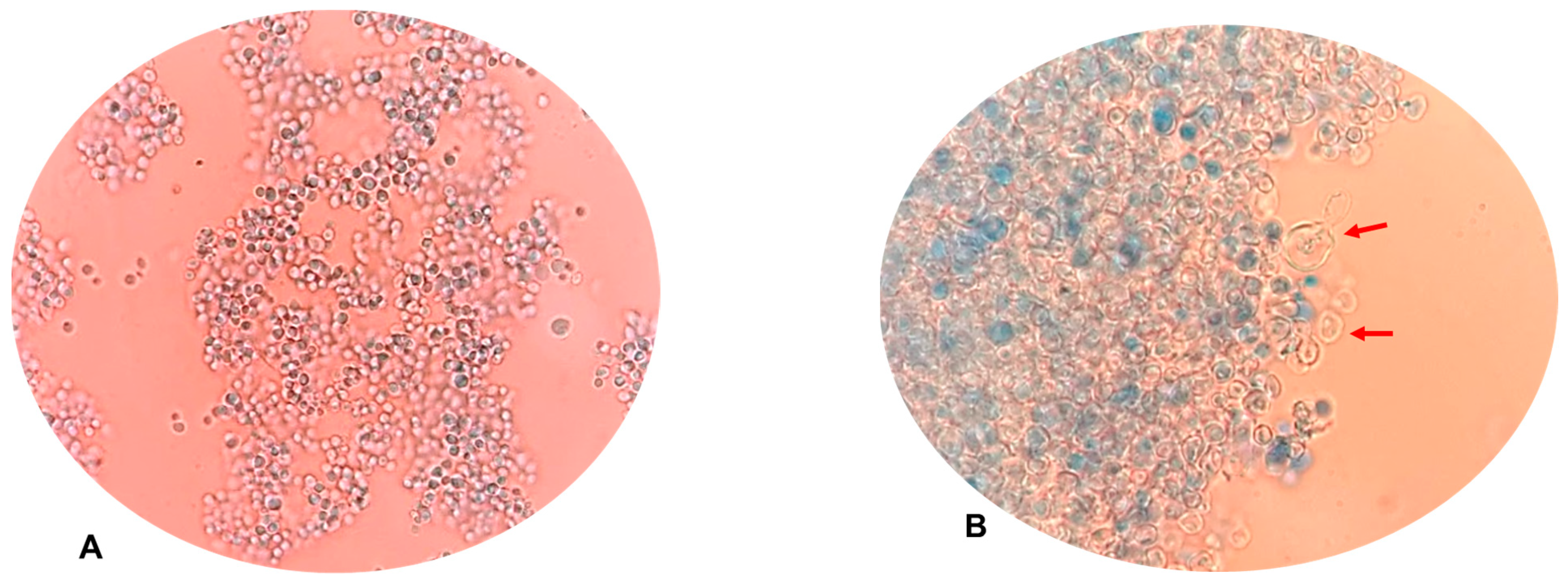
3.7. Changes in Yeast Morphology After the UVC/H2O2 Treatment
4. Discussion
4.1. Direct Yeast Inactivation by the Inorganic Peroxides
4.2. Inactivation of the Target Microorganism by the UVC Light Alone
4.3. Yeast Treatment by the Photooxidation Systems—Disinfection, Kinetics, and Routes
4.4. Extent of the Photooxidation Treatments—Reactivation and Susceptibility to Fluconazole of the Treated Yeast
4.5. Morphology Changes by the Action of the UVC/H2O2 Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOPs | Advanced oxidation processes |
| WHO | World Health Organization |
| PDS | Peroxydisulfate ion |
| PMS | Peroxymonosulfate ion |
| ROS | Reactive oxygen species |
| S | Synergy value |
| UVC | Radiation at 254 nm |
References
- Musie, W.; Gonfa, G. Heliyon Fresh Water Resource, Scarcity, Water Salinity Challenges and Possible Remedies: A Review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef]
- Caicedo-Bejarano, L.D.; Osorio-vanegas, L.S.; Ramirez-Castrillón, M.; Castillo, J.E.; Andrés, M.-G.C. Water Quality, Heavy Metals, and Antifungal Susceptibility to Fluconazole of Yeasts from Water Systems. Int. J. Environ. Res. Public Health 2023, 20, 3428. [Google Scholar] [CrossRef]
- Babič, M.N.; Gunde-Cimerman, N.; Vargha, M.; Tischner, Z.; Magyar, D.; Veríssimo, C.; Sabino, R.; Viegas, C.; Meyer, W.; Brandão, J. Fungal Contaminants in Drinking Water Regulation? A Tale of Ecology, Exposure, Purification and Clinical Relevance. Int. J. Environ. Res. Public Health 2017, 14, 636. [Google Scholar] [CrossRef]
- Cupozak-pinheiro, W.J.; De Almeida-apolonio, A.A.; Hatsue, M.; Halimy, N.; Pires, R.; Araújo, D.; Beraldo, D.; Chang, M.R.; Mari, K.; Oliveira, P. De Candida Species Contamination in Drinking Groundwater from Residence Wells in Three Municipalities of Midwestern Brazil and the Potential Human Health Risks. Microb. Pathog. 2022, 169, 105660. [Google Scholar] [CrossRef] [PubMed]
- Assress, H.A.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Occurrence and Risk Assessment of Azole Antifungal Drugs in Water and Wastewater. Ecotoxicol. Environ. Saf. 2020, 187, 109868. [Google Scholar] [CrossRef] [PubMed]
- Novak Babič, M.; Marolt, G.; Imperl, J.; Breskvar, M.; Džeroski, S.; Gunde-Cimerman, N. Effect of Location, Disinfection, and Building Materials on the Presence and Richness of Culturable Mycobiota through Oligotrophic Drinking Water Systems. J. Fungi 2023, 9, 111086. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.; Paulo, J.; Fernandes, A.; Rowan, N. Pulsed Light for the Inactivation of Fungal Bio Fi Lms of Clinically Important Pathogenic Candida Species. Yeast 2015, 32, 533–540. [Google Scholar] [CrossRef]
- Baker, T.; Sebolai, O. Yeast-Contaminated Water as a Potential Emerging Health Concern: A Review. Water SA 2024, 50, 404–410. [Google Scholar] [CrossRef]
- Corrêa-moreira, D.; Lara, G.; Pinto, T.N.; Araujo, J.; Amorim, M.C.; Martins, L.B.; Zahner, V. Detection and Taxonomic Identification of Emerging Pathogenic Yeast in Surface Waters from Lagoon Systems in Rio de Janeiro, Brasil. Environ. Monit. Assess. 2025, 197, 596. [Google Scholar] [CrossRef]
- Klimas, F.; Zatłoka-mazur, D.; Rusiński, K.; Zając, P.; Pawłowski, B.; Sienkiewicz, M.; Potoczek, A.; Zięba, Z.; Pudełko, I.; Klimas, F. Fungal Infections: Epidemiology, Clinical Challenges, and Advances in Diagnosis and Treatment—A Review. Qual. Sport 2025, 38, 57928. [Google Scholar] [CrossRef]
- Corrêa-Moreira, D.; Baptista, B.d.O.; Giosa, D.; Oliveira, M.M.E. Editorial: Emerging Fungal Pathogens: Perspectives. Front. Fungal Biol. 2024, 5, 10–12. [Google Scholar] [CrossRef]
- Paveley, N.; van den Bosch, F.; Grimmer, M. Assessing the Potential Antifungal Resistance Risk from Dual Use of a Mode of Action in Agriculture and Medical Treatment of Human Pathogens. bioRxiv 2024. [Google Scholar] [CrossRef]
- Fischer, M.; Gurr, S.; Cuomo, C.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; Gow, N.A.R.; Klein, B.S.; Kronstad, J.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef]
- Rayens, E.; Norris, K.A. Prevalence and Healthcare Burden of Fungal Infections in the United States, 2018. Open Forum Infect. Dis. 2022, 9, ofab593. [Google Scholar] [CrossRef]
- Gaffar, N.R.; Valand, N.; Girija, U.V. Candidiasis: Insights into Virulence Factors, Complement Evasion and Antifungal Drug Resistance. Microorganism 2025, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.K.; Padmavathi, A.R.; Nancharaiah, Y.V. Fungal Infections: Pathogenesis, Antifungals and Alternate Treatment Approaches. Curr. Res. Microb. Sci. 2022, 3, 100137. [Google Scholar] [CrossRef] [PubMed]
- Adenike, M.; Precious, T.; Oyebamiji, O.; Kelvin, K. Antifungal Resistance Profile and Genetic Relatedness of Moulds from Rural Groundwater Sources. Discov. Public Health 2024, 21, 109. [Google Scholar] [CrossRef]
- Milanezi, A.C.M.; Witusk, J.P.D.; van der Sand, S.T. Antifungal Susceptibility of Yeasts Isolated from Anthropogenic Watershed. An. Acad. Bras. Cienc. 2019, 91, e20170369. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Paiva, M.A.N.; Sampaio, C.M.S.; Castelo-Branco, D.S.C.M.; Teixeira, C.E.C.; de Alencar, L.P.; Bandeira, T.J.P.G.; Monteiro, A.J.; Cordeiro, R.A.; Pereira-Neto, W.A.; et al. Azole Resistance in Candida Spp. Isolated from Catú Lake, Ceará, Brazil: An Efflux-Pump-Mediated Mechanism. Braz. J. Microbiol. 2016, 47, 33–38. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Nosanchuk, J.D. Fungal Diseases as Neglected Pathogens: A Wake-Up Call to Public Health Officials. PLOS Neglected Trop. Dis. 2020, 14, e0007964. [Google Scholar] [CrossRef]
- Casalini, G.; Giacomelli, A.; Antinori, S. Personal View The WHO Fungal Priority Pathogens List: A Crucial Reappraisal to Review the Prioritisation. Lancet Microbe 2024, 5, 717–724. [Google Scholar] [CrossRef]
- Amann, V.; Kissmann, A.; Firacative, C. Biofilm-Associated Candidiasis: Pathogenesis, Prevalence, Challenges and Therapeutic Options. Pharmaceuticals 2025, 18, 460. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (OH/O−) in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Mancipe-Bohorquez, J.E. Apoyo En El Desarrollo de Actividades Para El Mejoramiento e Implementación Del Plan de Gestión Integral de Residuos de Atención En Salud y Otras Actividades (PGIRASA) En La E.S.E. Hospital San Vicente Del Municipio de Ramiriqui, Boyaca, Colombia. Bachelor’s Thesis, Universidad Distrital Francisco José de Caldas, Bogotá, Colombia, 2023. [Google Scholar]
- Caicedo-Bejarano, L.D.; Morante-caicedo, A.; Castro-Narváez, S.P.; Serna-galvis, E.A. Alternative and Classical Processes for Disinfection of Water Polluted by Fungi: A Systematic Review. Water 2024, 16, 936. [Google Scholar] [CrossRef]
- Kim, J.Y. Human Fungal Pathogens: Why Should We Learn? J. Microbiol. 2016, 54, 145–148. [Google Scholar] [CrossRef]
- Bhagat, J.; Singh, N.; Nishimura, N.; Shimada, Y. A Comprehensive Review on Environmental Toxicity of Azole Compounds to Fish. Chemosphere 2021, 262, 128335. [Google Scholar] [CrossRef]
- Almirante, B.; Rodrı, D.; Park, B.J.; Cuenca-estrella, M.; Planes, A.M.; Almela, M.; Mensa, J.; Sanchez, F.; Ayats, J.; Saballs, P.; et al. Epidemiology and Predictors of Mortality in Cases of Candida Bloodstream Infection: Results from Population-Based Surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 2005, 43, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.; Simons, R.; Beck, S.E.; Adeli, B. UV 101: Desinfección Ultravioleta, Una Perspectiva General; International Ultraviolet Association (IUVA): Chevy Chase, MD, USA, 2020. [Google Scholar]
- Reedy, J.M.; Pousty, D.; Waliaula, B.W.; Maniga, J.; Mamane, H.; Mariita, R.M. Global Health Economics and Sustainability Enhancing Quality of Life, Public Health, and Economic Development in the Global South through Waterborne Disease Prevention with Ultraviolet C Light-Emitting Diode Technology. Glob. Health Econ. Sustain. 2024, 2, 1984. [Google Scholar] [CrossRef]
- Rozanska, A.; Walkowicz, M.; Bulanda, M.; Kasperski, T.; Synowiec, E.; Osuch, P.C.; Chmielarczyk, A. Evaluation of the Efficacy of UV-C Radiation in Eliminating Microorganisms of Special Epidemiological Importance from Touch Surfaces under Laboratory Conditions and in the Hospital Environment. Healthcare 2023, 11, 3096. [Google Scholar] [CrossRef]
- Pereira, V.J.; Ricardo, J.; Galinha, R.; Benoliel, M.J.; Barreto Crespo, M.T. Occurrence and Low Pressure Ultraviolet Inactivation of Yeasts in Real Water Sources. Photochem. Photobiol. Sci. 2013, 12, 626–630. [Google Scholar] [CrossRef]
- Wen, G.; Chen, Z.; Wan, Q.; Zhao, D.; Xu, X.; Wang, J.; Li, K.; Huang, T. Activation of PMS by Pipe Corrosion Products for Fungi Disinfection in Water: Performance and Mechanisms. Chem. Eng. J. 2020, 382, 123003. [Google Scholar] [CrossRef]
- Kuhn, H.J.; Braslavskyl, S.E.; Schmidt, R. Chemical Actinometry. Pure Appl. Chem. 1989, 61, 187–210. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Salazar-Ospina, L.; Jiménez, J.N.; Pino, N.J.; Torres-Palma, R.A. Elimination of Carbapenem Resistant Klebsiella Pneumoniae in Water by UV-C, UV-C/Persulfate and UV-C/H2O2. Evaluation of Response to Antibiotic, Residual Effect of the Processes and Removal of Resistance Gene. J. Environ. Chem. Eng. 2020, 8, 102196. [Google Scholar] [CrossRef]
- Verbel-Olarte, M.I.; Serna-Galvis, E.A.; Salazar-Ospina, L.; Jiménez, J.N.; Porras, J.; Pulgarin, C.; Torres-Palma, R.A. Irreversible Inactivation of Carbapenem-Resistant Klebsiella Pneumoniae and Its Genes in Water by Photo-Electro-Oxidation and Photo-Electro-Fenton—Processes Action Modes. Sci. Total Environ. 2021, 792, 148360. [Google Scholar] [CrossRef] [PubMed]
- Cantón, E.; Martín-Mazuelos, E.; Espinel-ingroff, A. Métodos Estandarizados Por El CLSI Para El Estudio de La Sensibilidad a Los Antifúngicos (Documentos M27-A3, M38-A y M44-A); Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2007; ISBN 978-84-611-8776-8. [Google Scholar]
- Espinel-ingroff, A. Commercial Methods for Antifungal Susceptibility Testing of Yeasts: Strengths and Limitations as Predictors of Resistance. J. Fungi 2022, 8, 309. [Google Scholar] [CrossRef]
- Gao, L.; Guo, Y.; Zhan, J.; Yu, G.; Wang, Y. Assessment of the Validity of the Quenching Method for Evaluating the Role of Reactive Species in Pollutant Abatement during the Persulfate-Based Process. Water Res. 2022, 221, 118730. [Google Scholar] [CrossRef]
- Al-salihi, S.S.; Jumaah, I.A.M. Activity of Some Disinfectants, Detergents and Essentials Oils Pm Growth of the Yeast Candida albicans. Al-Mustansiriyah J. Sci. 2017, 28, 25–34. [Google Scholar] [CrossRef]
- Larsen, B.; White, S. Antifungal Effect of Hydrogen Peroxide on Catalase-Producing Strains of Candida Spp. Infect. Dis. Obstet. Gynecol. 1995, 78, 73–78. [Google Scholar] [CrossRef]
- Swenson, K.A.; Min, K.; Konopka, J.B. Candida albicans Pathways That Protect against Organic Peroxides and Lipid Peroxidation. PLoS Genet. 2024, 20, e1011455. [Google Scholar] [CrossRef]
- Abegg, M.A.; Lucietto, R.; Alabarse, P.V.G.; Mendes, M.F.A.; Benfato, M.S. Differential Resistance to Oxidants and Production of Hydrolytic Enzymes in Candida albicans. Mycopathologia 2011, 171, 35–41. [Google Scholar] [CrossRef]
- Miramon, P.; Dunker, C.; Kasper, L.; Jacobsen, I.D.; Miram, P.; Barz, D.; Kurzai, O.; Hube, B. A Family of Glutathione Peroxidases Contributes to Oxidative Stress Resistance in Candida albicans. Med. Mycol. 2014, 52, 223–239. [Google Scholar] [CrossRef]
- Chen, N.; Lee, D.; Kang, H.; Cha, D.; Lee, J. Catalytic Persulfate Activation for Oxidation of Organic Pollutants: A Critical Review on Mechanisms and Controversies. J. Environ. Chem. Eng. 2022, 10, 107654. [Google Scholar] [CrossRef]
- Khan, M.; Mcdonald, M.; Mundada, K. Efficacy of Ultraviolet Radiations against Coronavirus, Bacteria, Fungi, Fungal Spores and Biofilm. Hygiene 2022, 2, 120–131. [Google Scholar] [CrossRef]
- Lee, M.M.; O’Neil, C.A.; Vogt, L.; Kwon, J.H. Environmental Hygiene Strategies to Combat Antimicrobial Resistance in Healthcare Settings. Antimicrob. Steward. Healthc. Epidemiol. 2025, 5, e71. [Google Scholar] [CrossRef] [PubMed]
- Braga, G.U.L.; Rangel, D.E.N.; Fernandes, É.K.K.; Flint, S.D.; Roberts, D.W. Molecular and Physiological Effects of Environmental UV Radiation on Fungal Conidia. Curr. Genet. 2015, 61, 405–425. [Google Scholar] [CrossRef]
- Pereira, A.R.; Braga, D.F.O.; Vassal, M.; Gomes, I.B.; Simões, M. Ultraviolet C Irradiation: A Promising Approach for the Disinfection of Public Spaces? Sci. Total Environ. 2023, 879, 163007. [Google Scholar] [CrossRef]
- Lin, C.; He, J.; Liu, Z.; Liang, Q. Effectiveness, Safety, and Challenges of UVC Irradiation in Indoor Environments: A Decade of Review and Prospects. Build. Environ. 2025, 276, 112868. [Google Scholar] [CrossRef]
- de Souza, S.O.; Cardoso, A.A., Jr.; Sales, A.; Sarmento, C.; Errico, F. Effectiveness of a UVC Air Disinfection System for the HVAC of an ICU. Eur. Phys. J. Plus 2022, 137, 37. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Moreira, S.I.; Lucas, M.S.; Fernandes, J.R.; Tavares, P.B.; Sampaio, A.; Peres, J.A. Disinfection of Simulated and Real Winery Wastewater Using Sulphate Radicals: Peroxymonosulphate/Transition Metal/UV-A LED Oxidation. J. Clean. Prod. 2017, 149, 805–817. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Dionysiou, D.D.; Chen, B.; Yang, J.; Li, J. Overlooked Formation of H2O2 during the Hydroxyl Radical- Scavenging Process When Using Alcohols as Scavengers. Environ. Sci. Technol. 2022, 56, 3386–3396. [Google Scholar] [CrossRef]
- Ashrafivala, M.; Borhan, S.; Zeinali, S.; Heidari, M.; Mohammadpourfard, M.; Aslani, H. Investigation of H2O2/UV Advanced Oxidation Process on the Removal Rate of Coliforms from the Industrial Effluent: A Pilot-Scale Study. Int. J. Hydrogen Energy 2022, 47, 33530–33540. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, M.; Dionysiou, D.D. What Is the Role of Light in Persulfate-Based Advanced Oxidation for Water Treatment? Water Res. 2021, 189, 116627. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y. Science of the Total Environment A Comprehensive Review on Persulfate Activation Treatment of Wastewater. Sci. Total Environ. 2022, 831, 154906. [Google Scholar] [CrossRef]
- Kapteyn, J.; Hoyer, L.L.; Hecht, J.E.; Müller, W.H.; Andel, A.; Verkleij, A.J.; Makarow, M.; Ende, H.V.D.; Klis, F.M. The Cell Wall Architecture of Candida albicans Wild—Type Cells and Cell Wall-Defective Mutants. Mol. Microbiol. 2000, 35, 601–611. [Google Scholar] [CrossRef]
- Machová, E.; Bystrický, S. Antioxidant Capacities of Mannans and Glucans Are Related to Their Susceptibility of Free Radical Degradation. Int. J. Biol. Macromol. 2013, 61, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Xu, X.; Zhu, H.; Huang, T.; Ma, J. Inactivation of Four Genera of Dominant Fungal Spores in Groundwater Using UV and UV/PMS: Efficiency and Mechanisms. Chem. Eng. J. 2017, 328, 619–628. [Google Scholar] [CrossRef]
- Wen, G.; Wan, Q.; Deng, X.; Cao, R.; Xu, X.; Chen, Z.; Wang, J.; Huang, T. Reactivation of Fungal Spores in Water Following UV Disinfection: Effect of Temperature, Dark Delay, and Real Water Matrices. Chemosphere 2019, 237, 124490. [Google Scholar] [CrossRef]
- Wen, G.; Deng, X.; Wan, Q.; Xu, X.; Huang, T. Photoreactivation of Fungal Spores in Water Following UV Disinfection and Their Control Using UV-Based Advanced Oxidation Processes. Water Res. 2019, 148, 1–9. [Google Scholar] [CrossRef]
- Nowrozi, H.; Kazemi, A.; Teshfam, M.; Temorian, S.H.; Adimi, P.; Bashashati, M. Efficacy of Ultraviolet Radiation on Drug Susceptibility of Candida Spp. to Itraconazole, Fluconazole and Amphotericin B. J. Gorgan Univ. Med. Sci. 2014, 15, 53–58. [Google Scholar]
- Nowrosi, H.K.A. The Effect of Ultraviolet (Uv) Radiation Duration on Drug Susceptibility Testing of Rhizopus Spp. to Amphotericin B, Itraconazole and Fluconazole. SSU J. 2014, 22, 850–857. [Google Scholar]
- Lotfali, E.; Valizadeh, B.; Ghasemi, R.; Feghhi, S.A.H. Does Prolonged Exposure of Environmental Fungi to Ultraviolet Irradiation Change the Pattern of Drug Resistance? Jundishapur J. Microbiol. 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Aghaei Gharehbolagh, S.; Salehi, Z.; Mahmoudi, S.; Noorbakhsh, F.; Rezaie, S. Effect of Various Ultraviolet Radiation on Antifungal Susceptibility Pattern and Related Genes Expression in Malassezia Sympodialis. Gene Rep. 2019, 17, 100506. [Google Scholar] [CrossRef]
- Arribas, V.; Gil, C.; Molero, G. Deciphering the Oxidative Stress Response in Candida albicans. Fungal Biol. Rev. 2025, 52, 100427. [Google Scholar] [CrossRef]
- Rubio, D.; Nebot, E.; Casanueva, J.F.; Pulgarin, C. Comparative Effect of Simulated Solar Light, UV, UV/H2O2 and Photo-Fenton Treatment (UV–Vis/H2O2/Fe2+,3+) in the Escherichia Coli Inactivation in Artificial Seawater. Water Res. 2013, 47, 6367–6379. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Liu, W. Degradation of Sulfamethoxazole by Medium Pressure UV and Oxidants: Peroxymonosulfate, Persulfate, and Hydrogen Peroxide. Chem. Eng. J. 2017, 313, 629–637. [Google Scholar] [CrossRef]
- Rommozzi, E.; Giannakis, S.; Giovannetti, R.; Vione, D.; Pulgarin, C. Detrimental vs. Beneficial Influence of Ions during Solar (SODIS) and Photo-Fenton Disinfection of E. Coli in Water: (Bi)Carbonate, Chloride, Nitrate and Nitrite Effects. Appl. Catal. B Environ. 2020, 270, 118877. [Google Scholar] [CrossRef]
| Treatment | k (s−1) | t1/2 (s) | Regression Equation | R2 |
|---|---|---|---|---|
| UVC | 0.1728 | 4.011 | y = −0.1728x + 0.8607 | 0.9957 |
| PMS | 0.0263 | 26.36 | y = −0.0263x + 0.1167 | 0.9449 |
| UVC/PMS | 0.1510 | 4.590 | y = −0.1510x + 0.595 | 0.9761 |
| H2O2 | 0.0597 | 11.61 | y = −0.0597x − 0.033 | 0.8619 |
| UVC/H2O2 | 0.3164 | 2.191 | y = −0.3164x − 1.1922 | 0.8815 |
| PDS | 0.0003 | 2310 | y = −0.0003x − 0.055 | 0.0038 |
| UVC/PDS | 0.2824 | 2.454 | y = −0.2824x + 0.0635 | 0.9449 |
| Treatment | 30 s | 45 s | 60 s | 80 s | 120 s | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I * | R | I | R | I | R | I | R | I | R | |
| UVC | + ** | + | + | + | + | + | − | + | − | − |
| UVC/H2O2 | + | + | − | + | − | − | − | − | − | − |
| UVC/PMS | + | + | + | + | + | + | − | − | − | − |
| UVC/PDS | + | + | + | + | − | + | − | − | − | − |
| Method | Control | 64 μg mL−1 (CFU mL−1) | % I * | 32 μg mL−1 (CFU mL−1) | % I * | 16 μg mL−1 (CFU mL−1) | % * | 8 μg mL−1 (CFUmL−1) | % I * |
|---|---|---|---|---|---|---|---|---|---|
| Without treatment | 6170 | 4043 | 34.47 | 5280 | 14.42 | 6360 | −3.08 | 6233 | −1.02 |
| UVC | 7253 | 112 | 98.46 | 17 | 99.77 | 1970 | 72.84 | 1907 | 73.71 |
| UVC/H2O2 | 8013 | 0.33 | 100.00 | 2 | 99.98 | 2 | 99.98 | 382 | 95.23 |
| UVC/PMS | 3943 | 2036 | 48.36 | 2607 | 33.88 | 2290 | 41.92 | 1990 | 49.53 |
| UVC/PDS | 6103 | 4833 | 20.81 | 3967 | 35.00 | 3747 | 38.60 | 3713 | 39.16 |
| C. parapsilosis ATCC 22019 | 6980 | 524 | 92.49 | 602 | 91.38 | 687 | 90.16 | 1580 | 77.36 |
| Treatment | UVC/H2O2 | UVC/PDS | UVC/PMS |
|---|---|---|---|
| S value * | 1.4 | 1.6 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caicedo-Bejarano, L.D.; Correa-Bermúdez, A.M.; Castro-Narváez, S.P.; Serna-Galvis, E.A. Evaluation of Ultraviolet Light-Based Oxidative Systems for the Inactivation and Change in Susceptibility of a Fluconazole-Resistant Candida albicans Strain. Water 2025, 17, 2448. https://doi.org/10.3390/w17162448
Caicedo-Bejarano LD, Correa-Bermúdez AM, Castro-Narváez SP, Serna-Galvis EA. Evaluation of Ultraviolet Light-Based Oxidative Systems for the Inactivation and Change in Susceptibility of a Fluconazole-Resistant Candida albicans Strain. Water. 2025; 17(16):2448. https://doi.org/10.3390/w17162448
Chicago/Turabian StyleCaicedo-Bejarano, Luz Dary, Adriana María Correa-Bermúdez, Sandra Patricia Castro-Narváez, and Efraím A. Serna-Galvis. 2025. "Evaluation of Ultraviolet Light-Based Oxidative Systems for the Inactivation and Change in Susceptibility of a Fluconazole-Resistant Candida albicans Strain" Water 17, no. 16: 2448. https://doi.org/10.3390/w17162448
APA StyleCaicedo-Bejarano, L. D., Correa-Bermúdez, A. M., Castro-Narváez, S. P., & Serna-Galvis, E. A. (2025). Evaluation of Ultraviolet Light-Based Oxidative Systems for the Inactivation and Change in Susceptibility of a Fluconazole-Resistant Candida albicans Strain. Water, 17(16), 2448. https://doi.org/10.3390/w17162448






