Abstract
Candida albicans, listed by WHO as a priority fungal (yeast) pathogen, can cause invasive infections resistant to drugs, thus demanding novel strategies of disinfection. This study examines the inactivation, reactivation in darkness, and susceptibility to fluconazole of an antifungal-resistant C. albicans strain through UVC photolysis, chemical oxidation, and photooxidation using hydrogen peroxide (H2O2), peroxydisulfate (PDS), or peroxymonosulfate (PMS). Tests were performed in deionized water over very short treatment times (0–80 s). Also, standardized CLSI methods for antifungal sensitivity studies and morphological microscopic views were carried out. The fungus disinfection order was UVC/H2O2 > UVC/PDS > UVC/PMS > UVC. The photooxidation processes followed pseudo-first-order kinetics, with the highest rate constant for the UVC/H2O2 process. Direct oxidation, photoinactivation, and attacks of radical species were responsible for the inactivation of the antifungal-resistant microorganism. The fluconazole susceptibility of yeasts was significantly decreased (from 64 to 8 µg mL−1) by the action of UVC/H2O2. A low reactivation in the dark and strong changes in the yeast morphology were found, indicating that the use of UVC light and radical-based processes is an effective alternative for fluconazole-resistant yeasts and could be promising to deal with hospital wastewater loaded with resistant fungi.
1. Introduction
Yeasts are unicellular, heterotrophic, facultative fungi that can be found in several aquatic environments (e.g., surface water, drinking water, and groundwater) [1,2,3,4,5,6]. Although interest in yeast contamination of water has been increasing in recent years, this topic is still unexplored. One possible reason is that consumption of yeast-contaminated water rarely causes overt clinical symptoms, despite many fungi being opportunistic and posing a significant health risk to immunocompromised patients [7,8,9]. For instance, some Candida species pose a growing threat to public health due to their association with high levels of morbidity and mortality, as well as the limited number of antifungal drugs available to treat invasive fungal infections. (IFI) [10,11,12,13]. Each year, fungal pathogens are responsible for an estimated 13 million illnesses and 1.5 million deaths worldwide [14]. Annually, about 1.9 million people are affected by acute IFIs, while another 3 million suffer from severe chronic fungal infections. [15]. Although these infections have been linked to immunocompromised individuals, immunocompetent individuals are affected every day, with high mortality rates [16].
Among yeasts, Candida species, especially Candida albicans, represent the main target in freshwater environments polluted with agricultural and human antifungals, which can activate genetic mechanisms that generate resistance to antifungals [17]. Recent research has examined the susceptibility of yeasts isolated from freshwater samples, identifying strains resistant to at least one of the antifungal drugs tested in the trials, with these strains standing out for their high rate of recovery and resistance to antifungal drugs, especially fluconazole [18,19].
In recognition of the problem linked to antifungal resistance, the World Health Organization (WHO) published a priority list of pathogenic fungi in October 2022, underlining the urgent need to find solutions to this critical situation. Depending on their threat levels, C. albicans and Candida auris (now Candidozyma auris) were classified as critical priority pathogens and often show varying degrees of resistance to antifungal agents [20]. Candida albicans is one of the 10 most common pathogens in intensive care units, accounting for approximately 10% of nosocomial infections. It can cause invasive candidiasis, an opportunistic mycosis with mortality rates between 40 and 55%. Worldwide prevalence ranges from 250,000 to 700,000 cases per year, with incidence rates of 2–14 per 100,000 individuals. The WHO has highlighted the growing threat posed by these genera [21], due to their increasing resistance to antifungal agents and their ability to form biofilms [22].
In recent years, the use of antifungals in clinical therapy and the agricultural and industrial sectors has increased, as well as the number of species with intrinsic resistance, dose-dependent susceptibility, and acquired resistance to these products [23,24,25]. The challenges and implications of this resistance highlight the pressing need for solutions, as fungal decontamination systems for aquatic and surface systems remain precarious in developing countries [23].
To fight antifungal resistance, new treatments and infection control strategies have been developed toward contaminated clinical areas (water, air, and surfaces) [24]. Currently, environmental infection control procedures focus almost entirely on manual cleaning and chemical disinfection. Meanwhile, disinfection of aquatic systems has involved conventional methods, such as the use of strong oxidants, which can directly alter the structure and metabolism of fungi [25]. Alternative approaches include combining two or more physical and/or chemical processes, producing very reactive species such as the hydroxyl radical (•OH) [26].
In this context, advanced oxidation processes (AOPs) generate reactive oxygen species (ROS) such as hydroxyl radicals. These species formed in AOPs have high oxidative capacity, and they can degrade a wide range of pollutants, including pathogenic microorganisms. Some AOPs involve the photolysis of inorganic peroxides by ultraviolet light-C (UVC) to generate radical species, which are able to damage cellular components of diverse microorganisms [27,28].
It must be mentioned that the use of UVC radiation at 254 nm is itself a disinfection process. Commercial UVC lamps have a broad spectrum (200–280 nm) and have been demonstrated both individually and in combination with other oxidizing agents to have strong disinfecting action [29,30,31]. Moreover, the efficacy of UVC light has been used on different yeast species such as Candida sp., Cryptococcus sp., Metschnikowia sp., Rhodosporidium sp., and Rhodotorula spp. [32], reaching inactivation levels of up to 99% effectiveness. However, it has been found that a percentage of reactivation may occur in the dark and/or photo-reactivation when using this technique [33]. Moreover, to our best knowledge, no previous work about the inactivation of an antifungal-resistant C. albicans in aqueous media by the combination of UVC with different inorganic peroxides (i.e., photooxidations) has been published yet. Hence, in this research, the main objectives were as follows: (i) to establish the inactivation efficiency of the photolysis, chemical oxidation, and photooxidation (using UVC with H2O2, PDS, or PMS) toward the target C. albicans; and (ii) to determine the reactivation in darkness, and changes in the susceptibility of a fluconazole-resistant C. albicans strain.
2. Materials and Methods
2.1. Reagents
The culture media used were YPD agar (Sigma-Aldrich, Darmstadt, Germany) and Sabouraud agar (Merck, Rahway, NJ, USA). The inorganic peroxides were peroxydisulfate (PDS, 99.0% purity) and peroxymonosulfate (PMS, as Oxone®, in analytical grade), which were purchased from Sigma-Aldrich, and hydrogen peroxide (H2O2, 35% v/v), sodium chloride (99%, purity), fluconazole 99% purity (Sigma-Aldrich) and ethanol (96%, purity) were provided by Merck. All reagents were prepared with deionized water obtained from a Merck Milli-Q water system. All dilutions of the fungus for the different assays were performed with physiological solution (NaCl, 0.85% m/v). The peroxide solutions were prepared on the same day as they were assayed. The concentrations of the oxidants were 50 and 500 µmol L−1, which were obtained by dilution of concentrated PMS (0.1537 g in 10 mL), PDS (0.1190 g in 10 mL), and H2O2 (57 μL in 10 mL) solutions.
2.2. Fungal Strain and Inoculum
A fluconazole-resistant Candida albicans strain supplied by the Secretaría de Salud Municipal de la ciudad de Cali (Colombia) was used for the assays. The strain was preserved in YPD agar and then transferred to YPD broth, where it was incubated for 12 h with constant agitation at 28 °C. The concentration of the inoculum under study was measured in a spectrophotometer at a wavelength of 530 nm to obtain an optical density equal to 0.5 (OD530 = 0.5), which corresponds to 1 × 108 CFU mL−1. The OD measurements for the fungi were performed in a Spectronic spectrophotometer (Rochester, NY, USA).
2.3. Reaction System
Assays were performed in a homemade aluminum reflector box equipped with a UVC lamp (OSRAM HNS®, Munich, Germany) of 8 W and maximum emission of 254 nm, which has an actual intensity of 2.3 μW cm−1 (measured by hydrogen peroxide actinometry based on Kuhn et al. [34]). For the disinfection tests, 5 mL of the yeast inoculum with 45 mL of sterile water (pH 7.2 ± 0.2) and constant agitation (200 rpm) at 25 ± 2 °C were mixed, according to the protocol of Serna-Galvis et al. [35,36]. This mixture was left for 30 min in darkness, and three serial dilutions were made in physiological saline (10−1 to 10−4) to check the initial concentration of the inoculum. Then, it was exposed to UVC light, and sampling was performed at 15, 30, 45, 60, and 80 s of treatment, and the corresponding serial dilutions (10−1 to 10−4) were made in duplicate. For testing the photooxidation, the same procedure was repeated, but added PMS, PDS, and H2O2 individually before turning on the UVC lamp. The chemical oxidation control experiments (i.e., the peroxides without the UVC light) were set up by adding PMS, PDS, and H2O2 individually. The inorganic peroxides were used at a concentration of 500 µmol L−1; this concentration was selected based on previous works [35,36]. Also, it must be mentioned that each disinfection experiment was carried out in triplicate.
2.4. Yeast Counting
During the assays, 1.0 mL of the reactor content was transferred to screw-capped tubes containing 10 mL of sterile physiological solution (0.85% NaCl) at a 10−1 dilution. This was followed by subsequent serial dilutions from 10−2 to 10−4. Finally, 100 μL of the dilution tubes were seeded on a YPD plate and incubated (24–48 h at 25 °C) for yeast enumeration. During the different assays, aliquots were taken for yeast observation with a microscope (Olympus CX21, Tokyo, Japan).
2.5. Dark Reactivation of Yeasts
The reactivation study was conducted using the UVC, UVC/PMS, UVC/PDS, and UVC/H2O2 treatments, performed at 30, 45, 60, 80, and 120 s. For such tests, 100 µL of each sample was taken and inoculated in 900 mL of Sabouraud broth, left in the dark for five days, and observed for the presence of turbidity. Then, 100 µL of the incubated broth was inoculated into Petri dishes containing Sabouraud agar, then incubated in the dark, and fungal growth was observed at 48 h. Tubes with turbidity were considered positive, and yeast growth was checked in the agar cultures. Possible fungistatic action was determined in the tubes without turbidity. The tests were performed in triplicate.
2.6. Susceptibility of C. albicans to Fluconazole Using the Macrodilution Technique
The methods standardized by CLSI for the study of antifungal sensitivity (Documents M27-A3, M38-A, and M44-A) [37] were followed. Sterile tubes of 11 × 70 mm and a final volume of 1 mL in each tube were used. The culture medium used was synthetic RPMI 1640 medium with glutamine and without sodium bicarbonate (Gibco, Carlsbad, CA, USA), 10.40 g buffered with 0.164 µmol L−1 morpholino propane sulfonic acid (MOPDS) (Sigma), 34.53 g adjusted to pH 7.0 ± 0.1, and containing 0.2% glucose. Preparation of the culture medium and antifungal stock solution was consistent with the microdilution method [38]. The indicated amounts were dissolved in 900 mL of distilled water, with stirring until complete dissolution. The pH was adjusted to 6.9–7.1, using 1 N NaOH, and distilled water was added up to 1 L. A 0.22 µm Millipore filter was used, and the sample was filtered under sterile conditions. The solution was kept refrigerated (4–8 °C).
Fluconazole standard was weighed to obtain a concentration at least 10 times the maximum concentration of the antifungal to be tested (1.28 mg mL−1 sterile distilled water). It was divided into 1.1-mL aliquots and frozen at −40 °C. Dilutions were made starting from the concentration of 640 μg mL−1 up to dilution 1.25 μg mL−1. On the day of testing, the tubes were thawed and diluted 1/10 by adding 0.9 mL of inoculated RPMI to each tube, thus obtaining the dilution 640 μg mL−1.
Before sensitivity testing, two passages were performed on 20% Sabouraud dextrose agar (Merck 103873). An inoculum for Candida albicans was prepared by probing with the culture loop five colonies ≥ 1 mm and 24 h of growth on an SDA plate, and then suspended in a tube of physiological solution (NaCl 0.85%). The initial inoculum was shaken and adjusted to an optical density of 0.5 McFarland at a wavelength of 530 nm, corresponding to an approximate concentration of 1 × 106–5 × 106 CFU mL−1. Subsequently, a 1:2000 dilution was performed with RPMI medium. The final concentration of yeast on the plates was 0.5 × 103–2.5 × 103.
A control strain was included in each assay to detect any abnormalities or inactivation of the antifungal. The strain used was C. parapsilosis ATCC 22019. This test was performed in triplicate. The reading was performed visually at 48 h of incubation by comparing the turbidity of the tubes with that of the diluted control 1/20 (0.2 mL of the control tube plus 0.8 mL of RPMI). The MIC of the azoles was the lowest antifungal concentration that produced 80% growth inactivation. The MIC range of the antifungals for quality control strains of C. parapsilosis 22019, determined by the M27-A3 macrodilution method, was 2–8 μg mL−1.
2.7. Statistical Analysis
All experiments were performed in triplicate. Data are shown as mean ± standard error. Analysis of variance (ANOVA) was used to examine whether there were significant differences between the inactivation of C. albicans, represented by the number of CFUmL−1 resulting from the different treatments. Infostat version 2022.06.01 and OriginPro version 10.1.0.0.0 were utilized for the statistical analyses.
3. Results
3.1. Inactivation of Fluconazole-Resistant C. albicans with Inorganic Peroxides in the Dark and UVC Control
Initially, the treatment of the fluconazole-resistant C. albicans under the individual action of UVC light and three different inorganic peroxides (i.e., PDS, PMS, and H2O2) at 500 μmol L−1 was carried out. The evolution of the C. albicans strain under such systems can be observed in Figure 1. After 60 s of treatment, the percentage of inactivation with UVC light was 99.94% and a decrease of 3.25 log units was found. The percentage inactivation using PDS, PMS, and H2O2 was 6.76%, 82.30%, and 85.83%, and a decrease of 0.85, 1.04, and 1.08 log units, respectively. Figure S1 (in the Supplementary Materials) displays the percentages of yeast inactivation with different treatment times. When comparing the three oxidants, it was found to be statistically significant with a p < 0.0001 (the significance level for correlation was set at p < 0.05), with effectiveness for the microorganism inactivation following the order of H2O2 > PMS > PDS.
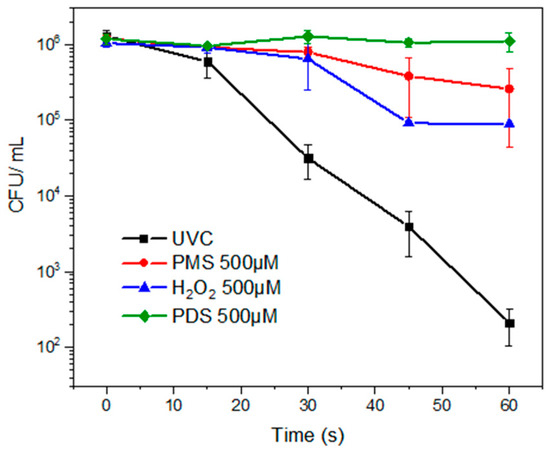
Figure 1.
Inactivation of the fluconazole-resistant C. albicans by photolysis and direct oxidation with the peroxides. Experimental conditions: deionized water. V: 50 mL, P: 8 W, [PMS], [H2O2], [PDS]: 500 μmol L−1, pH: 7.4. UVC lamp (254 nm).
3.2. Inactivation of Fluconazole-Resistant C. albicans by Photooxidation with the Different Peroxides
To enhance the yeast inactivation, the UVC light was combined with the peroxides (generating photooxidation processes). Figure 2 depicts the evolution of the C. albicans yeast by photooxidation processes, i.e., PDS, PMS, or H2O2 combined with the UVC irradiation. After 60 s of photooxidation, the percentage of disinfection by UVC/PDS, UVC/PMS, and UVC/H2O2 was 99.58%, 99.90%, and 99.99%, and the corresponding inactivation was of 2.39, 3.02, and 6.16 log units, respectively (see Figure 2a). Also, Figure S2 displays the percentages of yeast inactivation with different treatment times for the photooxidation systems. In turn, Figure 2b shows the growth of resistant C. albicans yeast in the culture medium at different reaction times for the UVC/H2O2 treatment. No growth was observed after 45 s; these results evidenced the superior behavior of the UVC/H2O2 system under the tested conditions.
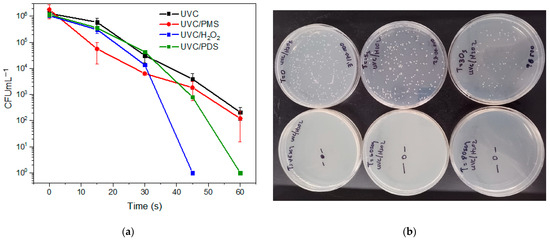
Figure 2.
Evolution of the fluconazole-resistant C. albicans under the diverse photooxidation systems. (a) Comparison of the photo-oxidative methods. Experimental conditions: deionized water. V: 50 mL, [PMS], [H2O2], [PDS]: 500 µmol L−1, pH: 7.4. UVC lamp (254 nm). (b) Growth of the resistant Candida albicans in the culture media during the treatment by UVC/H2O2 (time: 0, 15, 30, 45, 60, and 80 s).
3.3. Kinetic Results of the Inactivation of Fluconazole-Resistant C. albicans by Photooxidation with Different Peroxides
The shape of the curves (i.e., no linear change in the plot of CFU mL−1 vs. time) in Figure 2a suggests that the inactivation of the target microorganism can follow pseudo-first-order kinetics. Then, the fitting to such kinetics was tested. It is important to remember that pseudo-first-order kinetics obey the mathematical expression presented in Equation (1), where [N]t represents the microorganism population at any time, [N]0 is the initial fungus population, t is the time, and k is the pseudo-first-order rate constant.
The fitting to such kinetics of the disinfection tests presented in Figure 1 and Figure 2a was assessed. Table 1 provides the kinetic results obtained during photolysis, oxidation, and the photooxidation process of the fluconazole-resistant C. albicans using various oxidizing agents (PMS, PDS, and H2O2) with and without UVC radiation exposure.

Table 1.
Pseudo-first-order kinetic results for photolysis, oxidation, and photooxidation of the fluconazole-resistant strain of C. albicans.
3.4. Elucidation of the Routes Involved in the Inactivation of Fluconazole-Resistant C. albicans by Photooxidation Processes
The combination of UVC light with inorganic peroxides (i.e., the photooxidation processes) can produce strong disinfecting species such as hydroxyl radical and/or sulfate anion radical. To demonstrate the participation of these species in the inactivation of the target yeast by the photooxidation systems, experiments using a well-known scavenger (i.e., ethanol, k with hydroxyl radical: 2.8 × 109 M−1 s−1 and k with sulfate radical: 7.7 × 107 M−1 s−1, [39]) were performed. Firstly, the interaction of the resistant C. albicans strain with ethanol was evaluated. Then, the treatment of the microorganism in the presence of the scavenger by the three photooxidation processes was performed.
Figure 3a shows the growing response of the resistant C. albicans strain during the treatments in the presence of ethanol. The alcohol in the dark did not induce a significant inactivating action on the microorganism (0.24 log reduction in the yeast population). Moreover, after 60 s of treatment, the inactivation percentages were as follows: 99.95% (equivalent to 3.29 log reduction) for ethanol/UVC/PMS, 9.19% (1.04 log reduction) for ethanol/UVC/PDS, and 46.58% (0.33 log reduction) for ethanol/UVC/H2O2. It can be noted that for all the photooxidation systems (Figure 3a), a greater growth of the strain (i.e., a lower disinfection) was observed in the presence of ethanol compared to its absence (Figure 2a), thus confirming that radical species take part in the action routes of the three photooxidation processes.
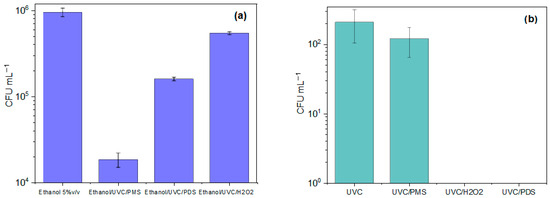
Figure 3.
Inactivation kinetics of fluconazole-resistant C. albicans by the diverse disinfection methods in the presence of ethanol. Experimental conditions: deionized water. V: 50 mL, P: 8 W, pH: 7.4. UVC lamp (254 nm). (a) Remaining fungus population after 60 s of treatment in the presence of the scavenger in the dark and during the photooxidation treatments (Experimental conditions: ethanol (5% v/v). [PMS]: [H2O2]: [PDS]: 500 µmol L−1. Treatment time: 60 s. (b) Remaining fungus population after 60 s of treatment at low concentration of the peroxide by the photooxidation treatments (Experimental conditions: [PMS], [H2O2], [PDS]: 50 µmol L−1).
To evaluate the relevant role of the peroxide precursor of radicals, the effect of the inorganic peroxide concentration in the photooxidation systems was also tested. Then, the concentrations of PDS, PMS, and H2O2 were changed from 500 μmol L−1 to 50 μmol L−1. Figure 3b presents the remaining population of the resistant yeast by UVC/PDS, UVC/PMS, and UVC/H2O2 (with the oxidant concentration of 50 μmol L−1), which was 99.99%, 99.64%, and 99.99%, and the corresponding inactivation of 5.00, 2.45, and 5.30 log units’ reduction, respectively.
3.5. Reactivation of C. albicans in Darkness
To better understand if the surviving strains could recover or adapt under these specific conditions, the reactivation of the treated yeast was evaluated. It is important to remember that the detection limit for the culture of microorganisms on plates is 100 CFU mL−1. The reactivation in darkness (at the detection limits) was applied after exposures to the different treatments (i.e., UVC and UVC plus oxidants) at 30, 45, 60, 80, and 120 s (Table 2). At 80 s of action of the UVC-only system, no yeast regrowth was observed for this system. The combination of UVC with the inorganic peroxides showed no reactivation in the dark when the photooxidation treatment was applied for 60 s. We must remark that the treatment with the best performance was the UVC/H2O2, which showed no reactivation when the microorganism was treated for 45 s (Table 2).

Table 2.
Fluconazole-resistant Candida albicans strain inhibition and reactivation in darkness.
3.6. Susceptibility to Fluconazole of the Treated C. albicans
For those yeast strains that were able to recover themselves after the treatments (see the previous section), tests of susceptibility to fluconazole were carried out. Table 3 and Figure 4 summarize the changes given for the fluconazole-resistant C. albicans strain (which had an original cut-off of 64 μg mL−1) after photolysis and photooxidation with different peroxides. For the yeast treated by using UVC alone, there was a reduction in the cut-off from 64 to 16 μg mL−1; thus, such a yeast strain is considered still resistant. In turn, the processes UVC/PMS and UVC/PDS showed no significant changes in the susceptibility to the antifungal pharmaceutical. During the UVC/H2O2 treatment, the yeast went from a cut-off of 64 to 8 μg mL−1 (Figure 4), considered, according to CLSI, as a drug-sensitive yeast. The standard error for each experiment measuring the percentage inhibition of fluconazole-resistant C. albicans by different advanced oxidation processes is included in the Supplementary Material (Table S1 and Figure S3).

Table 3.
Percentage inhibition of fluconazole-resistant C. albicans against different advanced oxidation processes.
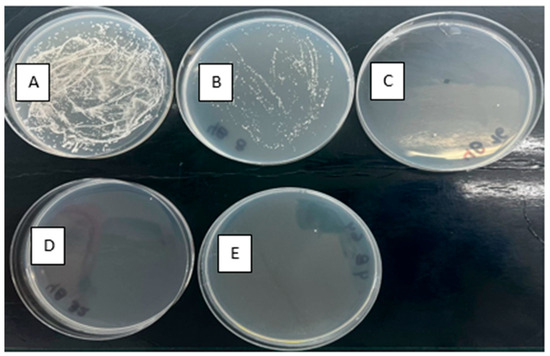
Figure 4.
Susceptibility to fluconazole of the resistant Candida albicans treated by UVC/H2O2. (A) without antifungal, (B) 8 µg mL−1, (C) 16 µg mL−1, (D) 32 µg mL−1, and (E) 64 µg mL−1.
3.7. Changes in Yeast Morphology After the UVC/H2O2 Treatment
As shown in the previous subsections, under the tested conditions, the UVC/H2O2 system exhibited the best disinfecting action on the fluconazole-resistant C. albicans (see Figure 2a and Figure 3b). Then, the physiological action of such a photooxidation process on the yeast was considered. Alterations in yeast morphology were observed using microscopy. Hence, the morphology of C. albicans before and after the treatment was compared (Figure 5).
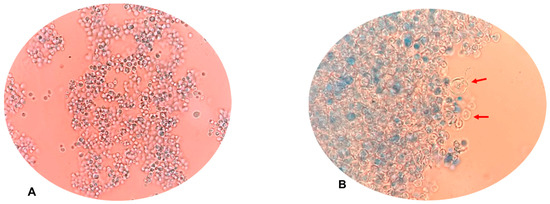
Figure 5.
Changes in yeast morphology after the UVC/H2O2 treatment. (A) Untreated fluconazole-resistant C. albicans. (B) Fluconazole-resistant C. albicans treated with the UVC/H2O2 process. The red arrows in the figure remark big changes in the morphology of the yeast by the process action (e.g., enlarged size, deformation, and cell emptying).
When comparing the yeasts’ morphologies without treatment (no UVC or oxidants) with those treated by the UVC/H2O2 process, appreciable cellular clustering and apparent damage to the cell wall and membrane were evidenced (see Figure 5B). The yeasts exhibited ruptures, swelling, and appeared translucent, increased in size, with the appearance of empty shells; others had rough and wrinkled cell surfaces, indicating oxidative stress and structural damage (Figure 5B).
4. Discussion
4.1. Direct Yeast Inactivation by the Inorganic Peroxides
The results in Figure 1 revealed that among the three inorganic peroxides (acting alone), H2O2 had the highest inactivation percentage of 85.83%, corresponding to a loss of 1.08 log of the initial yeast population. The disinfecting action of H2O2 toward our target yeast (Figure 1) is consistent with the previous work by Al-Salihi et al., who found that hydrogen peroxide shows complete antifungal activity against non-resistant Candida albicans [40]. Also, it should be mentioned that H2O2 itself has shown an antifungal effect on Candida spp. strains, inhibiting their growth at low concentrations (in the mmol L−1 range) [41].
The hydrogen peroxide is able to penetrate the yeast. Inside the yeast cell, the H2O2 can generate reactive oxygen species (ROS), which can alter the internal balance, leading to oxidative stress and strong damage [42,43]. It has also been shown to cause cellular damage and oxidation of the cellular components, leading to alteration of DNA and protein structure and function, thus resulting in mutations and loss of enzymatic activity [42]. Unsaturated lipids in cell membranes can also undergo peroxidation due to oxidative stress by hydrogen peroxide, compromising the integrity of cell membranes. In the same sense, another previous study on the response of Candida albicans to oxidative stress induced by H2O2 reported changes in the protein abundance and phosphorylation events. The ROS (coming from H2O2 inside the cell) can cause cell damage, in addition to alterations in antioxidant mechanisms, regulation of protein phosphorylation, and signaling pathways [44].
Concerning the other two peroxides (which were less efficient than H2O2 to inactivate the yeast; see Figure 1), at the experimental level, PMS showed a higher inactivation percentage (82.30%) than PDS (6.36%, Figure 1). To understand the differences among the three peroxides, we should consider the electric charge on them. H2O2 is neutral, while PMS is negatively charged, and PDS is doubly charged. Due to the negative charges on PMS and PDS, they have limited penetration into the cell of yeast. For instance, electrostatic repulsion can occur between the persulfates and the lipid cell membrane of the microorganism, thus making them less efficient than H2O2 for fungus inactivation.
4.2. Inactivation of the Target Microorganism by the UVC Light Alone
According to the results in Figure 1, UVC radiation demonstrated high effectiveness in inactivating our target Candida albicans. To interpret this result, we must consider that the cell wall and membrane of yeast can allow the UVC light to enter, which facilitates damage to genetic material and other internal components. In addition, yeasts have a larger size compared to bacteria, increasing the surface area through which UVC radiation can enter. Although yeasts possess mechanisms to repair the damage, they may not be able to counteract the formation of pyrimidine dimers and other effects of UVC light when sufficiently high doses of radiation are applied [45].
The mechanism of the UVC action is based on the induction of photochemical reactions that damage their RNA and DNA through the formation of pyrimidine dimers (thymine and cytosine dimers) cyclobutane (CPDs) and pyrimidine (6-4)-pyrimidone photoproducts (6-4PPs), which block the function of DNA polymerase, the enzyme responsible for copying DNA during replication. These dimers, which are abnormal covalent bonds between adjacent bases in DNA, prevent cells from dividing correctly by altering the action of RNA polymerase, which transcribes DNA into messenger RNA. This affects the production of proteins essential for cell survival, preventing the yeast from reproducing and functioning normally, leading to cell death or inactivation as the yeast cannot repair the accumulated damage [46,47]. Furthermore, the UVC radiation can also cause damage to the yeast cell membrane, affecting lipids and proteins essential for its structure and function. In some cases, ROS are generated, which induce additional oxidative damage to lipids, proteins, and DNA, amplifying the germicidal effect [47,48].
Our results, for the UVC acting alone, observed in Figure 1, agree with data obtained by Pereira and collaborators [49], who also found that UVC light significantly reduced this type of microorganism. In addition, we should mention that some commercial devices, such as the MUVi-UVC, developed by Mobile UV Innovations Pty Ltd. for the disinfection of mobile medical equipment, have confirmed inactivation for Candida auris, a multidrug-resistant yeast, within 5 min of exposure with an efficacy of 99.999%. Our results, joined with the previous literature, suggest that UVC technology is a useful tool for some fungal species [49,50,51]. However, some fungi species could develop resistance to UVC action. Although Candida is not resistant to UVC radiation, it can activate DNA repair mechanisms such as nucleotide excision repair, a mechanism that removes DNA segments containing lesions such as cyclobutane and pyrimidine dimers and 6-4 photoproducts (6-4PPs), or make incisions on both sides of the lesion and replace the damaged segment with new nucleotides via DNA polymerase and DNA ligase [47,48]. Hence, stronger systems, e.g., photooxidations, should be applied. In such systems, the synergy between radical species and UVC light is an alternative to inhibit repair mechanisms.
4.3. Yeast Treatment by the Photooxidation Systems—Disinfection, Kinetics, and Routes
Figure 2 presents the results of the yeast inactivation under the three photooxidation systems (i.e., UVC/H2O2, UVC/PDS, and UVC/PMS). For such disinfection curves, the kinetics fitting was tested, showing that the direct oxidation and photooxidation followed a pseudo-first-order pattern (Table 1). The adjustment was obtained from an improved linearization when the variations in the natural logarithm of the number of CFU mL−1 regarding the initial population were plotted versus the treatment time (we should mention that for the photooxidation systems, only the points after the lag phase were considered). Then, the values of the reaction rate constant (k) were determined from the slope of the linear form (Equation (1)), and, to calculate the half-life time (t1/2), we used Equation (2). In this study, k is linked to the speed of microorganism inactivation in the process, and t1/2 indicates the time required to halve the yeast population by the tested treatments. Also, the coefficient of determination (R2) reflects how well the model fits the experimental data obtained [52]:
Results in Table 1 reveal that the treatment with the greatest rate constant was UVC/H2O2 (k: 0.3164 s−1), followed by UVC/PDS (k: 0.2824 s−1), and UVC/PMS (k: 0.1510 s−1). Additionally, the data indicated that the control assay with only UVC light yielded a rate constant of 0.1728 s−1, which is higher than the rate constants observed in the assays performed in darkness with the peroxides. It is relevant to note that combining UVC radiation with peroxides, specifically UVC/H2O2 and UVC/PDS, showed a significant increase in the inactivation kinetics constants (and consequently diminishes the t1/2 values) concerning the oxidation by the peroxide or the UVC acting independently. Moreover, we should mention that the UVC/H2O2 combination achieved the shortest half-life time, recorded at 2.191 s. The control assay with UVC light showed a half-life time of 4.011 s, lower than the peroxides under dark conditions, where the half-life times ranged from 11.61 to 2310 s.
To demonstrate the usefulness of the combination of UVC with the inorganic peroxides, the synergy was calculated (S = kUVC/peroxide/(kUVC + kperoxide)). Table 4 shows the synergy values for the different treatments (photooxidations). When the result of this operation is a value that exceeds 1.0 (which translates into a positive synergy), it can be concluded that the combination of the peroxide and UVC-based treatment significantly increases the inactivation efficiency. On the contrary, values below 1.0 establish that the treatment shows antagonist effects. The photooxidation treatments with H2O2 showed good synergy, having an S value of 1.4. The UVC/PDS system was also synergistic (S: 1.6). PDS in the dark experienced slow kinetics; however, the application of UVC to PDS increased its performance, achieving synergy. On the other hand, the combination of UVC with PMS was antagonistic (Table 4); indeed, this photooxidation system had the worst performance for the yeast inactivation (see Figure 2a).

Table 4.
Synergy for the inactivation of a fluconazole-resistant strain of C. albicans by photooxidation treatments.
The origin of the synergy in the photooxidation system can be linked to the formation of radical species (e.g., HO• and SO4•−) coming from the homolytic cleavage of the O–O bond on H2O2, PDS, and PMS. Then, to verify the participation of the radical routes in the photooxidation processes, experiments using a scavenger of radicals (ethanol) and decreasing the peroxide concentration were performed (see Figure 3a). At the used concentration of the scavenger (which is low) in the dark and without peroxides, this did not induce significant inactivation. However, the addition of ethanol into the photooxidative treatments for C. albicans induced a drastic decrease in the inactivation (high remaining fungus population), compared to those carried out in water without the scavenger (Figure 2).
The decrease in the photooxidative capacity toward the yeast in the presence of ethanol was from 6.16 to 0.33 log reduction for UVC/H2O2, from 2.39 to 1.04 log reduction for UVC/PDS, and from 3.02 to 3.29 log reduction for UVC/PMS. These results are in agreement with those reported by Wang and collaborators, who established that the presence of alcohols demonstrates the generation of radicals in the processes [53]. It is important to mention that the scavenging of hydroxyl radical with ethanol can produce H2O2. Thereby, it is proposed for future work on fungi disinfection to develop other experiments using tert-butanol or iso-butanol (as scavengers), which have a lower potential for hydrogen peroxide generation. Moreover, it should be considered that the results with ethanol (or another alcohol) as a scavenger indirectly indicate the formation of radicals. Thus, to better and directly demonstrate the formation of radicals, future analyses of electron paramagnetic resonance (EPR) could be performed.
Regarding the diminution of the peroxide concentration (Figure 3b), it can be noted that when the amount of PDS, PMS, and H2O2 was changed from 500 μmol L−1 to 50 μmol L−1, the inactivation efficiencies were decreased. Thus, if the concentration of the peroxide (i.e., the radical precursor) is lower, the production of radicals is diminished. Therefore, the results using the scavenger and decreasing the peroxide concentration support the relevant participation of radical species in the inactivation of the fluconazole-resistant C. albicans by photooxidation processes.
After analyzing the participation of radicals on the yeast disinfection, it must be mentioned that the UVC irradiation combined with H2O2 had a remarkable ability to inhibit C. albicans yeasts with an experimentally decreased 6.16 log of the yeast population, improving the values obtained with H2O2 alone (1.08 log reduction) and UVC alone (3.25 log reduction, Figure 1). The UV irradiation at a wavelength of 254 nm breaks the chemical bond in H2O2 and generates HO• (Equation (3)) [54]. The UV/H2O2 process is an attractive option for producing a non-selective and highly reactive radical (•OH, E0 = 1.8–2.7 V) [55], which is mainly responsible for the target yeast inactivation in this photooxidation system, in addition to the direct actions of H2O2 and UVC.
Figure 2a also shows that photooxidation with peroxydisulfate (PDS) and peroxymonosulfate (PMS) and UV radiation allowed obtaining of the yeast inactivation associated with the action of radicals (as demonstrated previously, Figure 3). It must be taken into account that the peroxydisulfate anion (S2O82−) acts as a strong oxidant in water treatment processes, especially when activated to generate highly reactive radicals. In a pH range of 7.0 to 7.4 (neutral or slightly alkaline conditions), activation of S2O82− with UV light can generate both SO4•− radicals (Equation (4)). Additionally, the sulfate radical can react with water to produce •OH (Equation (5)). In addition, the activation of PMS by UVC radiation (λ: 254 nm) generates radicals SO4•− and •OH through homolytic cleavage of the peroxide bond in PMS (see Equation (6)) [56]. Then, in the UVC/PMS process, both radicals could contribute to the yeast inactivation (Figure 2 and Figure 3), and the photolysis of PMS under UVC irradiation is a key factor in the production of radicals [56]. However, the antagonistic behavior (S value: 0.8, Table 4) for this last system was unexpected. This could be associated with an excess of PMS that induces radical competition and the formation of less reactive species (Equation (7)), but this aspect should be deeply investigated in future works.
It should be taken into account that the cell wall of C. albicans is mainly composed of β-glucans (β-1,3 and β-1,6), chitin (β-1,4-N-acetylglucosamine), and mannoproteins (protein-bound mannans) [57]. Previous studies have shown that •OH can interact with mannans and glucans, causing their degradation and reaching between 82% and 91.5% in the absence of salicylate. Indeed, yeast mannans and glucans such as laminarin, lichenan, curdlan, and CM-glucan show high susceptibility to degradation by •OH [58]. This suggests that the UVC/H2O2 process, through the generation of •OH, impacts the structure of these polysaccharides, promoting their fragmentation and structural alteration (this aspect is discussed below in Section 4.5). In the case of the UVC/persulfate systems, we can consider that the generated sulfate and hydroxyl radicals can also interact with the organic compounds in the yeast cell structures, causing their degradation [56]. The sulfate radical is more selective than the hydroxyl radical [59], allowing for the oxidation of specific sites on the yeast cell components, thus explaining the slower inactivation of the target microorganism by the UVC/persulfate systems regarding the UVC/H2O2 system, as observed in Figure 2.
4.4. Extent of the Photooxidation Treatments—Reactivation and Susceptibility to Fluconazole of the Treated Yeast
To go beyond the measurement of the yeast population decrease, the reactivation and susceptibility to fluconazole of the treated C. albicans were assessed (Table 2 and Table 3). The results of this study showed that with up to 80 s of using the system with UVC alone, yeast regrowth was observed. Interestingly, the UVC/H2O2 system limited the yeast reactivation in the dark at shorter treatment times (45 and 60 s) than the UVC alone and the other photooxidation systems. The non-reactivation of the microorganism denotes that the action of the photooxidation process induced very strong and irreparable damage to the yeast cell.
Our results are consistent with the studies by Wen and co-workers [60], who have also evaluated the reactivation in darkness of fungal spores of Trichoderma harzianum, Aspergillus niger, and Penicillium polonicum, indicating that the repair mechanism in darkness is too weak. This reinforces the conclusion that dark repair of fungi does not occur significantly, contrasting with photoreactivation, which does show higher levels of recovery under UVA light [60]. Also, it is noted in the literature that darkness attenuates photoreactivation, and a prolonged dark delay may be an effective strategy to reduce fungal photoreactivation [60]. In the same context, another work reports that the dark reactivation of Trichoderma harzianum, Aspergillus niger, and Penicillium polonicum spores after 2-log10 inactivation by UV irradiation is negligible. In fact, the concentration of surviving fungi decreased slightly in the dark. This suggests that DNA repair mechanisms in the absence of light, such as the nucleotide excision repair system for fungi, are almost blocked [61].
On the other hand, the application of UVC light alone and the UVC/persulfates systems to C. albicans showed a reduction in resistance at two cut-offs (16 μg mL−1), with the fungal strain still resistant. However, with the UVC/H2O2 system, the yeast went from being resistant to being sensitive to fluconazole, with a cut-off of 8 μg mL−1 (Table 3). Again, these results indicate the best performance of the UVC/H2O2 system. It is important to consider that the action of this photooxidation process transforms the microorganism into a sensitive yeast. This highlights a beneficial/positive aspect of the disinfection of water loaded with antifungal-resistant microorganisms because it can contribute to limiting the proliferation of resistance. This agrees with the studies conducted by Nowrozi et al., who evaluated the efficacy of UV on the sensitivity of 12 strains of Candida spp. to the pharmaceuticals itraconazole, fluconazole, and amphotericin B, where the authors determined that after subjecting the strains to UV light, MICs decreased steadily for all drugs studied [62]. They similarly evaluated the effect of UV radiation on 12 clinical strains of Rhizopus spp., and after irradiation with UV, the MICs decreased [63]. Contrary to the report by Lotfali et al., who found that prolonged exposure to UVC of fungi such as Aspergillus spp., Verticillium spp., and Alternaria spp., increases resistance to itraconazole, voriconazole, and fluconazole [64]. The exposure to UV light modifies to defense mechanisms of the fungus (e.g., changes in the expression of certain genes related to resistance and pathogenicity) [65]. In our case, the concomitant action of UVC and radicals (as in UVC/H2O2) could lead to intense alterations of the defense mechanisms of the fungus, making it sensitive to fluconazole.
4.5. Morphology Changes by the Action of the UVC/H2O2 Process
From the pictures in Figure 5, it can be noted that the photooxidation with H2O2 caused damage to the surface structures of the yeast cell wall and cell membrane; the cells became clumped, increased in size, and looked empty inside. These results are expected because, as shown in the previous sections, the UVC/H2O2 process involves the attack of hydroxyl radicals in addition to the direct action of H2O2 and UVC light on the C. albicans.
To explain the morphology changes in the yeast (results in Figure 5), we should consider that the hydroxyl radical interacts with mannans and glucans (main components of the outer part of the target yeast), causing their degradation. In fact, yeast mannans and glucans such as laminarin, lichenan, curdlan, and CM-glucan are very susceptible to degradation by •OH [58]. Consequently, the surface structures of the yeast cell are strongly affected by the UVC/H2O2 process. Also, it must be recognized that hydrogen peroxide can directly cause significant damage to Candida albicans cell wall components, including glucans. This damage occurs primarily through oxidation processes that affect the structure and function of cell wall polymers, weakening the cell wall and making the cell more susceptible to lysis.
H2O2 and •OH can also oxidize amino acid residues in cell wall proteins, causing misfolding, loss of function, and fragmentation. Although lipids are not the major component of the C. albicans cell wall, ROS induces lipid peroxidation, affecting cell wall structure. Cumulative damage to glucans, proteins, and other cell wall components can compromise their fluidity, permeability, and integrity, facilitating the entry of the damaging agents [66]. Furthermore, when hydrogen peroxide penetrates the yeast cell, proteins with iron–sulfur groups are susceptible to oxidation, leading to the Fenton reaction to produce •OH radicals (Equation (8)), which cause significant cellular internal damage [43]. Also, it is well-known that exposure to high concentrations of H2O2 can analogously cause cell death as apoptosis [42].
On the other hand, we should recognize that our experiments were performed in deionized water, as an initial approach, which allows us to understand some fundamental aspects of the fungi inactivation, but such water lacks the complexity of environmental or clinical wastewater. For instance, wastewater contains organic matter and inorganic ions that absorb part of the UV-C light. Therefore, these matrix components can reduce the action of light on microorganisms and the generation of radicals [67]. Also, it must be taken into account that the presence of phosphate, chloride, and carbonate ions can generate positive synergies or antagonisms since these ions can generate radical species with lower reactivity than hydroxyl or sulfate radicals [68]. Moreover, cations in water, such as Fe2+ or Cu+, could participate in external photo-Fenton processes [69]. Therefore, although this study did not consider the inactivation of fluconazole-resistant Candida albicans in complex matrices such as environmental or clinical wastewater, future studies developing these topics are necessary.
5. Conclusions
The considered Candida albicans strain was prone to inactivation by the photolytic method using UVC light and by the direct action of the peroxides such as H2O2 and PMS. The combination of UVC and chemical oxidants (photooxidations) significantly improved the inactivation of C. albicans, being mainly synergistic in the cases of UVC/H2O2 and UVC/PDS. The photooxidations followed pseudo-first-order kinetics with half-lives of less than 10 s. The experiments in the presence of ethanol as a scavenger confirmed the relevant role of radical species in the photooxidative processes. The tests of reactivation in darkness evidenced the strong damage to yeast caused by the UVC/H2O2 system, revealing its suitability for the application and making it relevant for controlling C. albicans in aquatic systems. The surface morphological changes and yeast clustering caused by UVC/H2O2 demonstrated the alterations of the yeast cell structure. Furthermore, colonies at the final action of the photooxidative processes with H2O2 showed high susceptibility to fluconazole, being changed from resistant to sensitive. Our results suggest that the photooxidation methods can be tested for future applications in environmental water, wastewater, and hospital wastewater samples loaded with antifungal-resistant microorganisms. Finally, it is important to know the possible damage at the level of the yeast genetic material (in further work) to corroborate the elimination of the genes involved in the resistance to fluconazole.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17162448/s1, Table S1. Summary of the susceptibility to fluconazole of a fluconazole-resistant strain of C. albicans against different advanced oxidation processes. Figure S1. Percentage of inactivation of fluconazole-resistant C. albicans by photolysis and direct oxidation with peroxides. Figure S2. Percentage of inactivation of the fluconazole-resistant C. albicans under the diverse photooxidation systems. Figure S3. The susceptibility of a fluconazole-resistant strain of C. albicans to fluconazole under different advanced oxidation processes.
Author Contributions
Conceptualization, L.D.C.-B. and E.A.S.-G.; methodology, all authors; software, L.D.C.-B. and S.P.C.-N.; validation, L.D.C.-B., A.M.C.-B. and E.A.S.-G.; formal analysis, all authors; data curation, all authors; writing—original draft preparation, L.D.C.-B., A.M.C.-B. and E.A.S.-G.; writing—review and editing, L.D.C.-B., S.P.C.-N. and E.A.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the Dirección General de Investigaciones of Universidad Santiago de Cali, Call No. 10-2022. The APC was supported by the Dirección General de Investigaciones of the Universidad Santiago de Cali under Call No. DGI-01-2025.
Data Availability Statement
All data used for the analyses carried out in this review are included in the article and Supplementary Materials. Any additional data will be available upon request to the authors.
Acknowledgments
This research has been funded by the Dirección General de Investigaciones of Universidad Santiago de Cali under call No. DGI-01-2025.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AOPs | Advanced oxidation processes |
| WHO | World Health Organization |
| PDS | Peroxydisulfate ion |
| PMS | Peroxymonosulfate ion |
| ROS | Reactive oxygen species |
| S | Synergy value |
| UVC | Radiation at 254 nm |
References
- Musie, W.; Gonfa, G. Heliyon Fresh Water Resource, Scarcity, Water Salinity Challenges and Possible Remedies: A Review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef]
- Caicedo-Bejarano, L.D.; Osorio-vanegas, L.S.; Ramirez-Castrillón, M.; Castillo, J.E.; Andrés, M.-G.C. Water Quality, Heavy Metals, and Antifungal Susceptibility to Fluconazole of Yeasts from Water Systems. Int. J. Environ. Res. Public Health 2023, 20, 3428. [Google Scholar] [CrossRef]
- Babič, M.N.; Gunde-Cimerman, N.; Vargha, M.; Tischner, Z.; Magyar, D.; Veríssimo, C.; Sabino, R.; Viegas, C.; Meyer, W.; Brandão, J. Fungal Contaminants in Drinking Water Regulation? A Tale of Ecology, Exposure, Purification and Clinical Relevance. Int. J. Environ. Res. Public Health 2017, 14, 636. [Google Scholar] [CrossRef]
- Cupozak-pinheiro, W.J.; De Almeida-apolonio, A.A.; Hatsue, M.; Halimy, N.; Pires, R.; Araújo, D.; Beraldo, D.; Chang, M.R.; Mari, K.; Oliveira, P. De Candida Species Contamination in Drinking Groundwater from Residence Wells in Three Municipalities of Midwestern Brazil and the Potential Human Health Risks. Microb. Pathog. 2022, 169, 105660. [Google Scholar] [CrossRef] [PubMed]
- Assress, H.A.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Occurrence and Risk Assessment of Azole Antifungal Drugs in Water and Wastewater. Ecotoxicol. Environ. Saf. 2020, 187, 109868. [Google Scholar] [CrossRef] [PubMed]
- Novak Babič, M.; Marolt, G.; Imperl, J.; Breskvar, M.; Džeroski, S.; Gunde-Cimerman, N. Effect of Location, Disinfection, and Building Materials on the Presence and Richness of Culturable Mycobiota through Oligotrophic Drinking Water Systems. J. Fungi 2023, 9, 111086. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.; Paulo, J.; Fernandes, A.; Rowan, N. Pulsed Light for the Inactivation of Fungal Bio Fi Lms of Clinically Important Pathogenic Candida Species. Yeast 2015, 32, 533–540. [Google Scholar] [CrossRef]
- Baker, T.; Sebolai, O. Yeast-Contaminated Water as a Potential Emerging Health Concern: A Review. Water SA 2024, 50, 404–410. [Google Scholar] [CrossRef]
- Corrêa-moreira, D.; Lara, G.; Pinto, T.N.; Araujo, J.; Amorim, M.C.; Martins, L.B.; Zahner, V. Detection and Taxonomic Identification of Emerging Pathogenic Yeast in Surface Waters from Lagoon Systems in Rio de Janeiro, Brasil. Environ. Monit. Assess. 2025, 197, 596. [Google Scholar] [CrossRef]
- Klimas, F.; Zatłoka-mazur, D.; Rusiński, K.; Zając, P.; Pawłowski, B.; Sienkiewicz, M.; Potoczek, A.; Zięba, Z.; Pudełko, I.; Klimas, F. Fungal Infections: Epidemiology, Clinical Challenges, and Advances in Diagnosis and Treatment—A Review. Qual. Sport 2025, 38, 57928. [Google Scholar] [CrossRef]
- Corrêa-Moreira, D.; Baptista, B.d.O.; Giosa, D.; Oliveira, M.M.E. Editorial: Emerging Fungal Pathogens: Perspectives. Front. Fungal Biol. 2024, 5, 10–12. [Google Scholar] [CrossRef]
- Paveley, N.; van den Bosch, F.; Grimmer, M. Assessing the Potential Antifungal Resistance Risk from Dual Use of a Mode of Action in Agriculture and Medical Treatment of Human Pathogens. bioRxiv 2024. [Google Scholar] [CrossRef]
- Fischer, M.; Gurr, S.; Cuomo, C.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; Gow, N.A.R.; Klein, B.S.; Kronstad, J.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef]
- Rayens, E.; Norris, K.A. Prevalence and Healthcare Burden of Fungal Infections in the United States, 2018. Open Forum Infect. Dis. 2022, 9, ofab593. [Google Scholar] [CrossRef]
- Gaffar, N.R.; Valand, N.; Girija, U.V. Candidiasis: Insights into Virulence Factors, Complement Evasion and Antifungal Drug Resistance. Microorganism 2025, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.K.; Padmavathi, A.R.; Nancharaiah, Y.V. Fungal Infections: Pathogenesis, Antifungals and Alternate Treatment Approaches. Curr. Res. Microb. Sci. 2022, 3, 100137. [Google Scholar] [CrossRef] [PubMed]
- Adenike, M.; Precious, T.; Oyebamiji, O.; Kelvin, K. Antifungal Resistance Profile and Genetic Relatedness of Moulds from Rural Groundwater Sources. Discov. Public Health 2024, 21, 109. [Google Scholar] [CrossRef]
- Milanezi, A.C.M.; Witusk, J.P.D.; van der Sand, S.T. Antifungal Susceptibility of Yeasts Isolated from Anthropogenic Watershed. An. Acad. Bras. Cienc. 2019, 91, e20170369. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Paiva, M.A.N.; Sampaio, C.M.S.; Castelo-Branco, D.S.C.M.; Teixeira, C.E.C.; de Alencar, L.P.; Bandeira, T.J.P.G.; Monteiro, A.J.; Cordeiro, R.A.; Pereira-Neto, W.A.; et al. Azole Resistance in Candida Spp. Isolated from Catú Lake, Ceará, Brazil: An Efflux-Pump-Mediated Mechanism. Braz. J. Microbiol. 2016, 47, 33–38. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Nosanchuk, J.D. Fungal Diseases as Neglected Pathogens: A Wake-Up Call to Public Health Officials. PLOS Neglected Trop. Dis. 2020, 14, e0007964. [Google Scholar] [CrossRef]
- Casalini, G.; Giacomelli, A.; Antinori, S. Personal View The WHO Fungal Priority Pathogens List: A Crucial Reappraisal to Review the Prioritisation. Lancet Microbe 2024, 5, 717–724. [Google Scholar] [CrossRef]
- Amann, V.; Kissmann, A.; Firacative, C. Biofilm-Associated Candidiasis: Pathogenesis, Prevalence, Challenges and Therapeutic Options. Pharmaceuticals 2025, 18, 460. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (OH/O−) in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Mancipe-Bohorquez, J.E. Apoyo En El Desarrollo de Actividades Para El Mejoramiento e Implementación Del Plan de Gestión Integral de Residuos de Atención En Salud y Otras Actividades (PGIRASA) En La E.S.E. Hospital San Vicente Del Municipio de Ramiriqui, Boyaca, Colombia. Bachelor’s Thesis, Universidad Distrital Francisco José de Caldas, Bogotá, Colombia, 2023. [Google Scholar]
- Caicedo-Bejarano, L.D.; Morante-caicedo, A.; Castro-Narváez, S.P.; Serna-galvis, E.A. Alternative and Classical Processes for Disinfection of Water Polluted by Fungi: A Systematic Review. Water 2024, 16, 936. [Google Scholar] [CrossRef]
- Kim, J.Y. Human Fungal Pathogens: Why Should We Learn? J. Microbiol. 2016, 54, 145–148. [Google Scholar] [CrossRef]
- Bhagat, J.; Singh, N.; Nishimura, N.; Shimada, Y. A Comprehensive Review on Environmental Toxicity of Azole Compounds to Fish. Chemosphere 2021, 262, 128335. [Google Scholar] [CrossRef]
- Almirante, B.; Rodrı, D.; Park, B.J.; Cuenca-estrella, M.; Planes, A.M.; Almela, M.; Mensa, J.; Sanchez, F.; Ayats, J.; Saballs, P.; et al. Epidemiology and Predictors of Mortality in Cases of Candida Bloodstream Infection: Results from Population-Based Surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 2005, 43, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.; Simons, R.; Beck, S.E.; Adeli, B. UV 101: Desinfección Ultravioleta, Una Perspectiva General; International Ultraviolet Association (IUVA): Chevy Chase, MD, USA, 2020. [Google Scholar]
- Reedy, J.M.; Pousty, D.; Waliaula, B.W.; Maniga, J.; Mamane, H.; Mariita, R.M. Global Health Economics and Sustainability Enhancing Quality of Life, Public Health, and Economic Development in the Global South through Waterborne Disease Prevention with Ultraviolet C Light-Emitting Diode Technology. Glob. Health Econ. Sustain. 2024, 2, 1984. [Google Scholar] [CrossRef]
- Rozanska, A.; Walkowicz, M.; Bulanda, M.; Kasperski, T.; Synowiec, E.; Osuch, P.C.; Chmielarczyk, A. Evaluation of the Efficacy of UV-C Radiation in Eliminating Microorganisms of Special Epidemiological Importance from Touch Surfaces under Laboratory Conditions and in the Hospital Environment. Healthcare 2023, 11, 3096. [Google Scholar] [CrossRef]
- Pereira, V.J.; Ricardo, J.; Galinha, R.; Benoliel, M.J.; Barreto Crespo, M.T. Occurrence and Low Pressure Ultraviolet Inactivation of Yeasts in Real Water Sources. Photochem. Photobiol. Sci. 2013, 12, 626–630. [Google Scholar] [CrossRef]
- Wen, G.; Chen, Z.; Wan, Q.; Zhao, D.; Xu, X.; Wang, J.; Li, K.; Huang, T. Activation of PMS by Pipe Corrosion Products for Fungi Disinfection in Water: Performance and Mechanisms. Chem. Eng. J. 2020, 382, 123003. [Google Scholar] [CrossRef]
- Kuhn, H.J.; Braslavskyl, S.E.; Schmidt, R. Chemical Actinometry. Pure Appl. Chem. 1989, 61, 187–210. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Salazar-Ospina, L.; Jiménez, J.N.; Pino, N.J.; Torres-Palma, R.A. Elimination of Carbapenem Resistant Klebsiella Pneumoniae in Water by UV-C, UV-C/Persulfate and UV-C/H2O2. Evaluation of Response to Antibiotic, Residual Effect of the Processes and Removal of Resistance Gene. J. Environ. Chem. Eng. 2020, 8, 102196. [Google Scholar] [CrossRef]
- Verbel-Olarte, M.I.; Serna-Galvis, E.A.; Salazar-Ospina, L.; Jiménez, J.N.; Porras, J.; Pulgarin, C.; Torres-Palma, R.A. Irreversible Inactivation of Carbapenem-Resistant Klebsiella Pneumoniae and Its Genes in Water by Photo-Electro-Oxidation and Photo-Electro-Fenton—Processes Action Modes. Sci. Total Environ. 2021, 792, 148360. [Google Scholar] [CrossRef] [PubMed]
- Cantón, E.; Martín-Mazuelos, E.; Espinel-ingroff, A. Métodos Estandarizados Por El CLSI Para El Estudio de La Sensibilidad a Los Antifúngicos (Documentos M27-A3, M38-A y M44-A); Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2007; ISBN 978-84-611-8776-8. [Google Scholar]
- Espinel-ingroff, A. Commercial Methods for Antifungal Susceptibility Testing of Yeasts: Strengths and Limitations as Predictors of Resistance. J. Fungi 2022, 8, 309. [Google Scholar] [CrossRef]
- Gao, L.; Guo, Y.; Zhan, J.; Yu, G.; Wang, Y. Assessment of the Validity of the Quenching Method for Evaluating the Role of Reactive Species in Pollutant Abatement during the Persulfate-Based Process. Water Res. 2022, 221, 118730. [Google Scholar] [CrossRef]
- Al-salihi, S.S.; Jumaah, I.A.M. Activity of Some Disinfectants, Detergents and Essentials Oils Pm Growth of the Yeast Candida albicans. Al-Mustansiriyah J. Sci. 2017, 28, 25–34. [Google Scholar] [CrossRef]
- Larsen, B.; White, S. Antifungal Effect of Hydrogen Peroxide on Catalase-Producing Strains of Candida Spp. Infect. Dis. Obstet. Gynecol. 1995, 78, 73–78. [Google Scholar] [CrossRef]
- Swenson, K.A.; Min, K.; Konopka, J.B. Candida albicans Pathways That Protect against Organic Peroxides and Lipid Peroxidation. PLoS Genet. 2024, 20, e1011455. [Google Scholar] [CrossRef]
- Abegg, M.A.; Lucietto, R.; Alabarse, P.V.G.; Mendes, M.F.A.; Benfato, M.S. Differential Resistance to Oxidants and Production of Hydrolytic Enzymes in Candida albicans. Mycopathologia 2011, 171, 35–41. [Google Scholar] [CrossRef]
- Miramon, P.; Dunker, C.; Kasper, L.; Jacobsen, I.D.; Miram, P.; Barz, D.; Kurzai, O.; Hube, B. A Family of Glutathione Peroxidases Contributes to Oxidative Stress Resistance in Candida albicans. Med. Mycol. 2014, 52, 223–239. [Google Scholar] [CrossRef]
- Chen, N.; Lee, D.; Kang, H.; Cha, D.; Lee, J. Catalytic Persulfate Activation for Oxidation of Organic Pollutants: A Critical Review on Mechanisms and Controversies. J. Environ. Chem. Eng. 2022, 10, 107654. [Google Scholar] [CrossRef]
- Khan, M.; Mcdonald, M.; Mundada, K. Efficacy of Ultraviolet Radiations against Coronavirus, Bacteria, Fungi, Fungal Spores and Biofilm. Hygiene 2022, 2, 120–131. [Google Scholar] [CrossRef]
- Lee, M.M.; O’Neil, C.A.; Vogt, L.; Kwon, J.H. Environmental Hygiene Strategies to Combat Antimicrobial Resistance in Healthcare Settings. Antimicrob. Steward. Healthc. Epidemiol. 2025, 5, e71. [Google Scholar] [CrossRef] [PubMed]
- Braga, G.U.L.; Rangel, D.E.N.; Fernandes, É.K.K.; Flint, S.D.; Roberts, D.W. Molecular and Physiological Effects of Environmental UV Radiation on Fungal Conidia. Curr. Genet. 2015, 61, 405–425. [Google Scholar] [CrossRef]
- Pereira, A.R.; Braga, D.F.O.; Vassal, M.; Gomes, I.B.; Simões, M. Ultraviolet C Irradiation: A Promising Approach for the Disinfection of Public Spaces? Sci. Total Environ. 2023, 879, 163007. [Google Scholar] [CrossRef]
- Lin, C.; He, J.; Liu, Z.; Liang, Q. Effectiveness, Safety, and Challenges of UVC Irradiation in Indoor Environments: A Decade of Review and Prospects. Build. Environ. 2025, 276, 112868. [Google Scholar] [CrossRef]
- de Souza, S.O.; Cardoso, A.A., Jr.; Sales, A.; Sarmento, C.; Errico, F. Effectiveness of a UVC Air Disinfection System for the HVAC of an ICU. Eur. Phys. J. Plus 2022, 137, 37. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Moreira, S.I.; Lucas, M.S.; Fernandes, J.R.; Tavares, P.B.; Sampaio, A.; Peres, J.A. Disinfection of Simulated and Real Winery Wastewater Using Sulphate Radicals: Peroxymonosulphate/Transition Metal/UV-A LED Oxidation. J. Clean. Prod. 2017, 149, 805–817. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Dionysiou, D.D.; Chen, B.; Yang, J.; Li, J. Overlooked Formation of H2O2 during the Hydroxyl Radical- Scavenging Process When Using Alcohols as Scavengers. Environ. Sci. Technol. 2022, 56, 3386–3396. [Google Scholar] [CrossRef]
- Ashrafivala, M.; Borhan, S.; Zeinali, S.; Heidari, M.; Mohammadpourfard, M.; Aslani, H. Investigation of H2O2/UV Advanced Oxidation Process on the Removal Rate of Coliforms from the Industrial Effluent: A Pilot-Scale Study. Int. J. Hydrogen Energy 2022, 47, 33530–33540. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, M.; Dionysiou, D.D. What Is the Role of Light in Persulfate-Based Advanced Oxidation for Water Treatment? Water Res. 2021, 189, 116627. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y. Science of the Total Environment A Comprehensive Review on Persulfate Activation Treatment of Wastewater. Sci. Total Environ. 2022, 831, 154906. [Google Scholar] [CrossRef]
- Kapteyn, J.; Hoyer, L.L.; Hecht, J.E.; Müller, W.H.; Andel, A.; Verkleij, A.J.; Makarow, M.; Ende, H.V.D.; Klis, F.M. The Cell Wall Architecture of Candida albicans Wild—Type Cells and Cell Wall-Defective Mutants. Mol. Microbiol. 2000, 35, 601–611. [Google Scholar] [CrossRef]
- Machová, E.; Bystrický, S. Antioxidant Capacities of Mannans and Glucans Are Related to Their Susceptibility of Free Radical Degradation. Int. J. Biol. Macromol. 2013, 61, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Xu, X.; Zhu, H.; Huang, T.; Ma, J. Inactivation of Four Genera of Dominant Fungal Spores in Groundwater Using UV and UV/PMS: Efficiency and Mechanisms. Chem. Eng. J. 2017, 328, 619–628. [Google Scholar] [CrossRef]
- Wen, G.; Wan, Q.; Deng, X.; Cao, R.; Xu, X.; Chen, Z.; Wang, J.; Huang, T. Reactivation of Fungal Spores in Water Following UV Disinfection: Effect of Temperature, Dark Delay, and Real Water Matrices. Chemosphere 2019, 237, 124490. [Google Scholar] [CrossRef]
- Wen, G.; Deng, X.; Wan, Q.; Xu, X.; Huang, T. Photoreactivation of Fungal Spores in Water Following UV Disinfection and Their Control Using UV-Based Advanced Oxidation Processes. Water Res. 2019, 148, 1–9. [Google Scholar] [CrossRef]
- Nowrozi, H.; Kazemi, A.; Teshfam, M.; Temorian, S.H.; Adimi, P.; Bashashati, M. Efficacy of Ultraviolet Radiation on Drug Susceptibility of Candida Spp. to Itraconazole, Fluconazole and Amphotericin B. J. Gorgan Univ. Med. Sci. 2014, 15, 53–58. [Google Scholar]
- Nowrosi, H.K.A. The Effect of Ultraviolet (Uv) Radiation Duration on Drug Susceptibility Testing of Rhizopus Spp. to Amphotericin B, Itraconazole and Fluconazole. SSU J. 2014, 22, 850–857. [Google Scholar]
- Lotfali, E.; Valizadeh, B.; Ghasemi, R.; Feghhi, S.A.H. Does Prolonged Exposure of Environmental Fungi to Ultraviolet Irradiation Change the Pattern of Drug Resistance? Jundishapur J. Microbiol. 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Aghaei Gharehbolagh, S.; Salehi, Z.; Mahmoudi, S.; Noorbakhsh, F.; Rezaie, S. Effect of Various Ultraviolet Radiation on Antifungal Susceptibility Pattern and Related Genes Expression in Malassezia Sympodialis. Gene Rep. 2019, 17, 100506. [Google Scholar] [CrossRef]
- Arribas, V.; Gil, C.; Molero, G. Deciphering the Oxidative Stress Response in Candida albicans. Fungal Biol. Rev. 2025, 52, 100427. [Google Scholar] [CrossRef]
- Rubio, D.; Nebot, E.; Casanueva, J.F.; Pulgarin, C. Comparative Effect of Simulated Solar Light, UV, UV/H2O2 and Photo-Fenton Treatment (UV–Vis/H2O2/Fe2+,3+) in the Escherichia Coli Inactivation in Artificial Seawater. Water Res. 2013, 47, 6367–6379. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Liu, W. Degradation of Sulfamethoxazole by Medium Pressure UV and Oxidants: Peroxymonosulfate, Persulfate, and Hydrogen Peroxide. Chem. Eng. J. 2017, 313, 629–637. [Google Scholar] [CrossRef]
- Rommozzi, E.; Giannakis, S.; Giovannetti, R.; Vione, D.; Pulgarin, C. Detrimental vs. Beneficial Influence of Ions during Solar (SODIS) and Photo-Fenton Disinfection of E. Coli in Water: (Bi)Carbonate, Chloride, Nitrate and Nitrite Effects. Appl. Catal. B Environ. 2020, 270, 118877. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).