Optimization of the Wastewater Treatment Process Using Kinetic Equations for Nitrification Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Wastewater Treatment Plant Under Study and Monitored Parameters
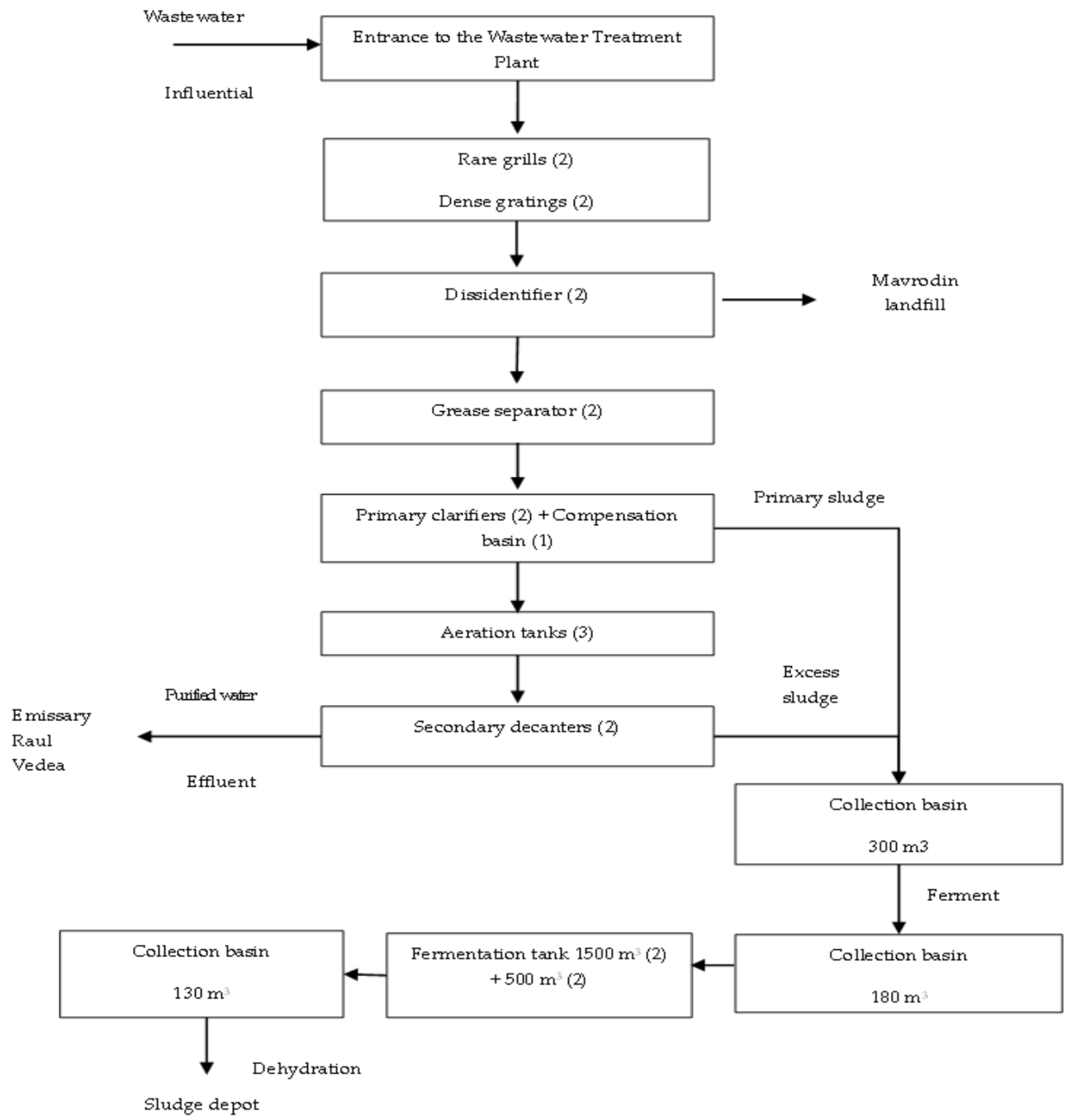
2.2. Description of the Operational Flow of the Wastewater Treatment Plant Under Study
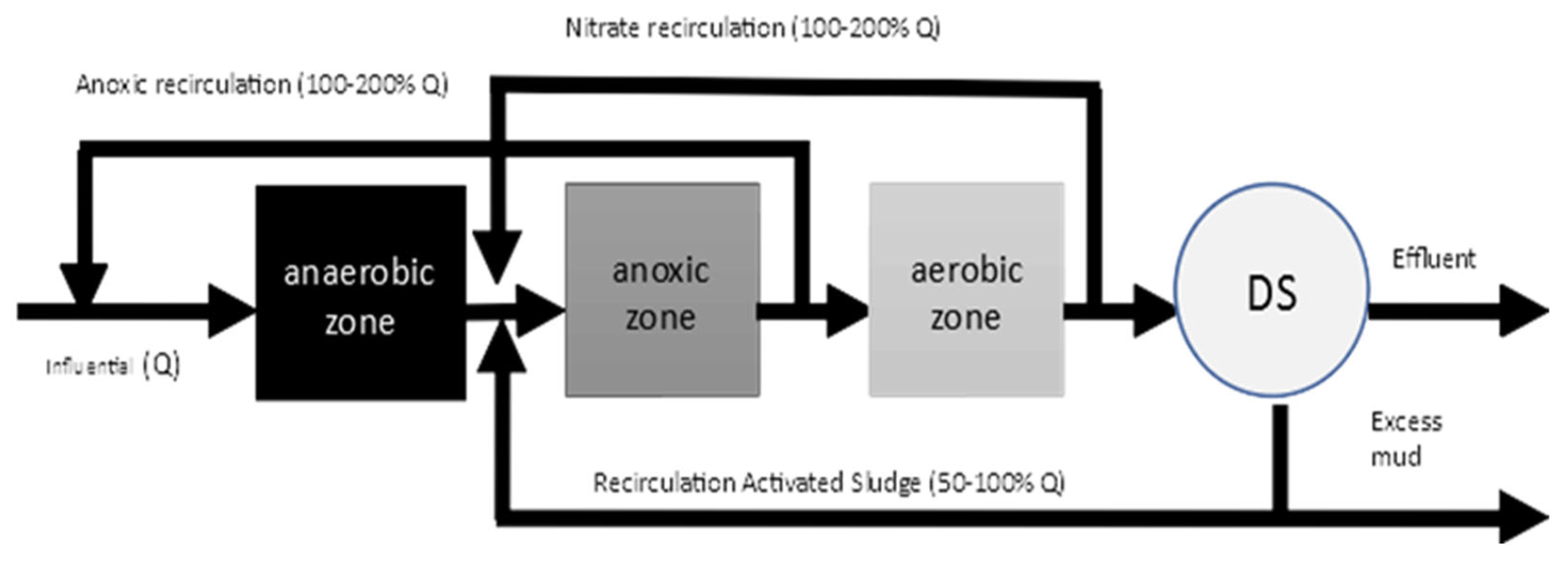
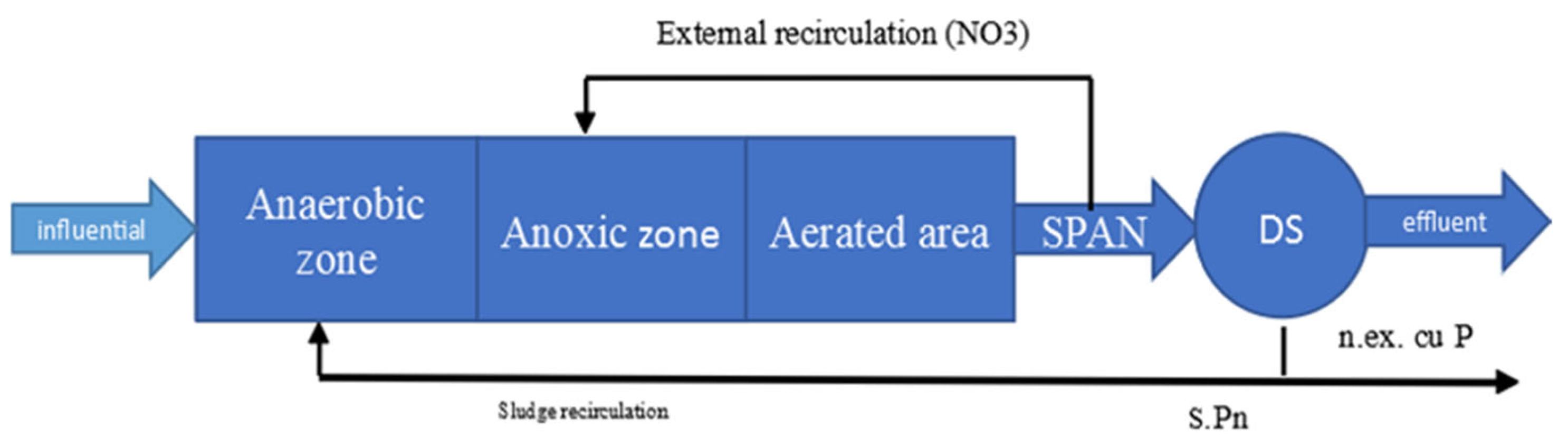
2.3. Description of the Nitrification/Denitrification Processes of the Wastewater Treatment Plant Under Study
2.4. The Effectiveness of Wastewater Treatment Process Optimization Using Kinetic Modeling
- ✓
- represents the oxidation potential of ammonium in the optimized technological flow;
- ✓
- is an indicator of the rate at which biomass increases for ammonium-oxidizing bacteria;
- ✓
- indicates the maximum specific growth rate. It has been determined that the substance in question is effective for ammonium-oxidizing bacteria;
- ✓
- shows the amount of N-NH4 in the water that has been treated at the plant;
- ✓
- represents indicates the saturation constant. This constant is equivalent to the N-NH4 concentration. At this concentration, the specific velocity is reduced to half its maximum value in Aeration Tank 1;
- ✓
- indicates the level of nitrated nitrogen present within the aerated sections;
- ✓
- is the saturation constant, which is the same as the NO2 concentration where the specific velocity is half of the max value in Aeration Tank 2;
- ✓
- is the amount of dissolved oxygen present in the aerated sections;
- ✓
- represents the O2 saturation constant for autotrophic nitrifying bacteria, which is numerically equal to the value at which saturation is half the maximum value Aeration Tank 2;
- ✓
- is the pH constant;
- ✓
- is the numerical value representing the saturation constant, which is equivalent to the concentration value + ;
- ✓
- represents the mortality rate of the autotrophic bacterial mass;
- ✓
- represents the active biomass.
- ✓
- is the “hydraulic reaction time,” which is a quantitative metric used to assess the efficiency of the aeration system
3. Results
3.1. Calculation of the Ammonium Oxidation Potential in the Optimized Technological Flow
3.2. Calculation of Simultaneous Nitrification Efficiency and Denitrification Efficiency Determined Using Kinetic Equations
- ✓
- N-NH4 inf is an indicator of the ammoniacal nitrogen concentration present in the influent, which is measured and represented here;
- ✓
- N-NH4 efl is the ammoniacal nitrogen in the effluent, which serves as a proxy for the concentration of this element in the water. The determination of this measurement is crucial for evaluating the quality of the effluent and for determining the necessary treatment processes;
- ✓
- N-NO3 efl is the nitrate nitrogen present in the effluent expressed as a concentration;
- ✓
- N-NO2 efl represents the concentration the effluent of nitrate nitrogen;
- ✓
- N-NH4 inf calc. is the concentration calculated according to Formula (4) in the influent of ammonium nitrogen;
- ✓
- ∆N-NH4 ex. shows the amount of ammonium nitrogen that was removed through the boosted nitrification process by the activity of the microorganisms in the activated sludge;
- ✓
- ∆N-NH4 calc. is the amount of ammonium nitrogen removed by activated sludge, which represents the nitrification efficiency of the system;
- ✓
- ∆N-NH4 indicates the quantity of ammonium nitrogen that has been extracted from the given sample or system.
3.3. Calculation of Hydraulic Retention Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mąkinia, J.; Zaborowska, E. Mathematical Modelling and Computer Simulation of Activated Sludge Systems; IWA Publishing: London, UK, 2020; Volume 4, pp. 19–63. [Google Scholar] [CrossRef]
- Hanhan, O.; Insel, G.; Yagci, N.O.; Artan, N.; Orhon, D. Mechanism and design of intermittent aeration activated sludge process for nitrogen removal. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2010, 46, 9–16. [Google Scholar] [CrossRef]
- Bertanza, G. Simultaneous nitrification–denitrification process in extended aeration plants: Pilot and real scale experiences. Water Sci. Technol. 1997, 35, 53–61. [Google Scholar] [CrossRef]
- Batchelor, B. Kinetic analysis of alternative configurations for single–sludge nitrification/denitrification. J. Water Pollut. Control F 1982, 54, 1493–1504. [Google Scholar]
- Ip, S.; Bridger, J.S.Y.; Mills, N.F. Effect of alternating aerobic and anaerobic conditions on the economics of the activated sludge system. Water Sci. Technol. 1987, 19, 911–918. [Google Scholar] [CrossRef]
- Andersen, P.H. Sustainable Operations Management (SOM) Strategy and Management: An Introduction to Part I: New Research Perspectives. In Operations Management and Sustainability: New Research Perspectives; Palgrave Macmillan: London, UK, 2019; pp. 15–25. ISBN 978-3-319-93211-8. [Google Scholar]
- Sarkis, J.A. Boundaries and Flows Perspective of Green Supply Chain Management. Supply Chain Manag. Int. J. 2012, 17, 202–216. [Google Scholar] [CrossRef]
- Corominas, L.; Foley, J.; Guest, J.S.; Hospido, A.; Larsen, H.F.; Morera, S. Shaw A Life cycle assessment applied to wastewater treatment: State of the art. Water Res. 2013, 47, 5480–5492. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Henderson, A.K.; Reissman, D.B. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci. Total Environ. 2004, 329, 99–113. [Google Scholar] [CrossRef]
- Bumbiere, K.; Barisa, A.; Pubule, J.; Blumberga, D.; Gomez-Navarro, T. Transition to Climate Neutrality at University Campus. Case Study in Europe, Riga. Environ. Clim. Technol. 2022, 26, 941–954. [Google Scholar] [CrossRef]
- Cantu, J.; Beruvides, M.; Fedler, C. An Economic Framework for Adaptive Wastewater Reuse for Future Crop Production Sustainability. In Proceedings of the IIE Annual Conference, Pittsburgh, PA, USA, 20–23 May 2017; pp. 1566–1570. [Google Scholar]
- Panagopoulos, Y.; Karpouzos, D.; Georgiou, P.; Papamichail, D. Ecosystem Services Evaluation from Sustainable Water Management in Agriculture: An Example from An Intensely Irrigated Area in Central Greece. Environ. Sci. Proc. 2023, 25, 4. [Google Scholar]
- Salgot, M.; Folch, M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Liu, A.; Wu, H.; Naeem, A.; Du, Q.; Ni, B.; Liu, H.; Li, Z.; Ming, L. Cellulose Nanocrystalline from Biomass Wastes: An Overview of Extraction, Functionalization and Applications in Drug Delivery. Int. J. Biol. Macromol. 2023, 241, 124557. [Google Scholar] [CrossRef] [PubMed]
- Sardi, C.; Skanavis, C. Training a New Generation of Environmental Stewards in Greece. In Educating the Sustainability Leaders of the Future; Leal Filho, W., Lange Salvia, A., Pallant, E., Choate, B., Pearce, K., Eds.; World Sustainability Series; Springer Nature: Cham, Switzerland, 2023; pp. 125–145. [Google Scholar]
- Mohammed, A.S.; Kapri, A.; Goel, R. Heavy metal pollution: Source, impact, and remedies. In Biomanagement of Metal-Contaminated Soils; Environmental Pollution; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–28. [Google Scholar]
- Hussain, M.M.; Wang, J.; Bibi, I.; Shahid, M.; Niazi, N.K.; Iqbal, J.; Mian, I.A.; Shaheen, S.M.; Bashir, S.; Shah, N.S. Arsenic speciation and biotransformation pathways in the aquatic ecosystem: The significance of algae. J. Hazard. Mater. 2020, 403, 124027. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S. Water Encyclopedia: Domestic, Municipal, and Industrial Water Supply and Waste Disposal. J. Environ. Qual. 2008, 37, 1299. [Google Scholar] [CrossRef]
- Lehr, J.H.; Keeley, J.; Lehr, J. Domestic, Municipal, and Industrial Water Supply and Waste Disposal; Wiley Interscience: Hoboken, NJ, USA, 2005; pp. 623–681. [Google Scholar]
- Caicedo, C.; Rosenwinkel, K.-H.; Exner, M.; Verstraete, W.; Suchenwirth, R.; Hartemann, P.; Nogueira, R. Legionella occurrence in municipal and industrial wastewater treatment plants and risks of reclaimed wastewater reuse. Water Res. 2019, 149, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Peter, C.; Shukla, S.K.; Govender, P.P.; Joshi, G.M.; Wang, R. Environmental issues: A challenge for wastewater treatment. In Green Materials for Wastewater Treatment; Environmental Chemistry for Sustainable World; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–12. [Google Scholar]
- MGIMO University, Moscow, Russia; Financial University under the Government of the Russian Federation, Moscow, Russia; Platonova, I.N.; Maksakova, M.A.; MGIMO University, Moscow, Russia; Institute of Economics of the Russian Academy of Sciences, Moscow, Russia. Promoting Small and Medium-Sized Businesses in Europe for Sustainable Development in the Digitalization Era. In Proceedings of the Sustainable and Innovative Development in the Global Digital Age, Rostov-on-Don, Russia, 19–21 May 2022; Dela Press Publishing House: Laragh, Ireland, 2022; Volume 3, pp. 1–4. Available online: https://dpcsebm.delapress.com/index.php/dpcsebm/article/view/198/188 (accessed on 1 December 2024).
- European Union. Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment; European Union: Brussels, Belgium, 1991; Volume 135. [Google Scholar]
- Hong, Z.; Zhang, H.; Gong, Y.; Yu, Y. Towards a Multi-Party Interaction Framework: State-of-the-Art Review in Sustainable Operations Management. Int. J. Prod. Res. 2022, 60, 2625–2661. [Google Scholar] [CrossRef]
- Angelakis, A.N.; Capodaglio, A.G.; Dialynas, E.G. Wastewater Management: From Ancient Greece to Modern Times and Future. Water 2022, 15, 43. [Google Scholar] [CrossRef]
- Borah, P.; Kumar, M.; Devi, P. Types of inorganic pollutants: Metals/metalloids, acids, and organic forms. In Inorganic Pollutants in Water; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–31. [Google Scholar]
- Viktorovna, F.I.; Yurievich, N.V.; Viktorovna, P.I.; Mikhailovna, O.L. Impact of renewable energy sources consumption on economic growth in Europe and Asia-Pacific Region. Int. J. Energy Econ. Policy 2021, 11, 270–278. [Google Scholar] [CrossRef]
- Integrated Urban Water Management. Available online: www.gwp.org (accessed on 22 December 2024).
- Carey, R.O.; Migliaccio, K.W. Contribution of Wastewater Treatment Plant Effluents to Nutrient Dynamics in Aquatic Systems: A Review. Environ. Manag. 2009, 44, 205–217. [Google Scholar] [CrossRef]
- Muga, H.E.; Mihelcic, J.R. Sustainability of wastewater treatment technologies. J. Environ. Manag. 2008, 88, 437–447. [Google Scholar] [CrossRef]
- Falås, P.; Wick, A.; Castronovo, S.; Habermacher, J.; Ternes, T.; Joss, A. Tracing the limits of organic micropollutant removal in biological wastewater treatment. Water Res. 2016, 95, 240–249. [Google Scholar] [CrossRef]
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-Din, W.; Ashir Hameed, M.; Mohsin Abrar, M.; Maitlo, A.A.; Noreen, S.; et al. Current and Emerging Adsorbent Technologies for Wastewater Treatment: Trends, Limitations, and Environmental Implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- Whitton, R.; Ometto, F.; Pidou, M.; Jarvis, P.; Villa, R.; Jefferson, B. Microalgae for municipal wastewater nutrient remediation: Mechanisms, reactors and outlook for tertiary treatment. Environ. Technol. Rev. 2015, 4, 133–148. [Google Scholar] [CrossRef]
- Zheng, C.; Zhao, L.; Zhou, X.; Fu, Z.; Li, A. Treatment technologies for organic wastewater. Water Treat. 2013, 11, 250–286. [Google Scholar]
- Marques, R. Regulation of Water and Wastewater Services. Available online: https://books.google.com/ (accessed on 1 December 2024).
- Ionescu, G.C.; Ionescu, I.L. Judicious operation of wastewater treatment plants. In Drinking Water Treatment Technologies, Wastewater Treatment, Sludge Management, Equipment: Conference “Technical-Scientific Performance in Water-Sewer Services”; ARA Publishing House: Bucharest, Romania, 2014; pp. 3–7. [Google Scholar]
- Wastewater Treatment. Available online: www.consilium.europa.eu/ro/policies/wastewater-treatment/ (accessed on 22 December 2024).
- Collivignarelli, M.C.; Abbà, A.; Bertanza, G. Why use a thermophilic aerobic membrane reactor for the treatment of industrial wastewater/liquid waste. Environ. Technol. 2015, 36, 2115–2124. [Google Scholar] [CrossRef]
- Cao, G.; Yang, G.; Sheng, M.; Wang, Y. Chemical industrial wastewater treated by combined biological and chemical oxidation process. Water Sci. Technol. 2009, 59, 1019–1024. [Google Scholar] [CrossRef]
- Rubalcaba, A.; Suárez-Ojeda, M.E.; Stüber, F.; Fortuny, A.; Bengoa, C.; Metcalfe, I.; Font, J.; Carrera, J.; Fabregat, A. Phenol wastewater remediation: Advanced oxidation processes coupled to a biological treatment. Water Sci. Technol. 2007, 55, 221–227. [Google Scholar] [CrossRef]
- Sarria, V.S.; Parra, N.; Adler, P.; Pulgarín, P.C. Recent developments in the coupling of photoassisted and aerobic biological processes for the treatment of biorecalcitrant compounds. Catal. Today 2002, 76, 301–315. [Google Scholar] [CrossRef]
- Scott, J.P.; Ollis, D.F. Engineering models of combined chemical and biological processes. J. Environ. Eng. 1996, 122, 1110–1114. [Google Scholar] [CrossRef]
- Tabrizi, G.B.; Mehrvar, M. Integration of advanced oxidation technologies and biological processes: Recent developments, trends, and advances. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2004, 39, 3029–3081. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.P.; Ollis, D.F. Integration of chemical and biological oxidation processes for water treatment: Review and recommendations. Environ. Prog. 1995, 14, 88–103. [Google Scholar] [CrossRef]
- Marin, E.; Rusănescu, C.O. Agricultural Use of Urban Sewage Sludge from the Wastewater Station in the Municipality of Alexandria in Romania. Water 2023, 15, 458. [Google Scholar] [CrossRef]
- Integrated Biotechnologies with Physico-Chemical Processes for Urban Wastewater Treatment and for the Treatment of Residual Sludge for Reuse. Available online: www.researchgate.net (accessed on 6 January 2025).
- Maass, O.; Grundmann, P. Added-value from linking the value chains of wastewater treatment, crop production and bioenergy production: A case study on reusing wastewater and sludge in crop production in Braunschweig (Germany). Resour. Conserv. Recycl. 2016, 107, 195–211. [Google Scholar] [CrossRef]
- Esplugas, S.; Ollis, D. Economic aspects of integrated (chemical + biological) processes for water treatment. J. Adv. Oxid. Technol. 1997, 2, 197–202. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sanchez-Perez, J.A. Combination of advanced oxidation processes and biological treatments for wastewater decontamination: A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- OECD. Revised Introduction to the OECD Guidelines for Testing of Chemicals; Section 3; OECD Publishing: Paris, France, 2006. [Google Scholar]
- Abedinzadeh, N.; Shariat, M.; Monavari, S.M.; Pendashteh, A. Evaluation of color and COD removal by Fenton from biologically (SBR) pre-treated pulp and paper wastewater. Process Saf. Env. Prot. 2018, 116, 82–91. [Google Scholar] [CrossRef]
- Biological Wastewater Treatment Stage. Available online: www.rasfoiesc.com (accessed on 6 January 2025).
- Adishkumar, S.; Kanmani, S. Treatment of phenolic wastewaters in single baffle reactor by solar/TiO2/H2O2 process. Desalin. Water Treat. 2010, 24, 67–73. [Google Scholar] [CrossRef]
- Ammonia and Ammonium. 2025. Available online: https://ro.hach.com/parameters/ammonia (accessed on 6 January 2025).
- Arslan-Alaton, I.; Gursoy, B.H.; Schmidt, J.E. Advanced oxidation of acid and reactive dyes: Effect of Fenton treatment on aerobic, anoxic and anaerobic processes. Dye. Pigment 2008, 78, 117–130. [Google Scholar] [CrossRef]
- Castillo-Suárez, L.A.; Lugo-Lugo, V.; Linares-Hernández, I.; Martínez-Miranda, V.; Esparza-Soto, M.; Mier-Quiroga, M.d.l.Á. Biodegradability index enhancement of landfill leachates using a Solar Galvanic-Fenton and Galvanic-Fenton system coupled to an anaerobic–aerobic bioreactor. Sol. Energy 2019, 188, 989–1001. [Google Scholar] [CrossRef]
- De Morais, J.L.; Zamora, P.P. Use of advanced oxidation processes to improve the biodegradability of mature landfill leachates. J. Hazard Mater. 2005, 123, 181–186. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Marin, E.; Rusănescu, C.O.; Paraschiv, G.; Ciuca, C.V. Research on wastewater treatment using activated sludge technology IN the anaerobic-anoxic-aerobic configuration. UPB Sci. Bull. Series B 2023, 85, 331–342. [Google Scholar]
- Gogate, P.R.; Thanekar, P.D.; Oke, A.P. Strategies to improve biological oxidation of real wastewater using cavitation based pre-treatment approaches. Ultrason. Sonochem. 2020, 64, 105016. [Google Scholar] [CrossRef] [PubMed]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Biotechnology Compendium. Available online: https://pdfcoffee.com/compendiu-biotehnologii-pdf-free.html (accessed on 6 January 2025).
- Oturan, M.A.; Aaron, J.-J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Safta, V.V. Course Notes—Advanced Water and Air Depollution Procedures; Faculty of Biotechnical Systems Engineering, “Politehnică” University: Bucharest, Romania, 2020. [Google Scholar]
- Djafer, A.; Djafer, L.; Maimoun, B.; Iddou, A.; Mostefai, S.K.; Ayral, A. Reuse of waste activated sludge for textile dyeing wastewater treatment by biosorption: Performance optimization and comparison. Water Environ. J. 2017, 31, 105–112. [Google Scholar] [CrossRef]
- Henze, M.; Gujer, W.; Mino, T.; Van Loosedrecht, M. Activated Sludge Models ASM1, ASM2, ASM2d and ASM3; IWA Publishing: London, UK, 2006; ISBN 9781780402369. [Google Scholar] [CrossRef]
- Regional Water and Wastewater Infrastructure Development Project from Teleorman County in the Period 2014–2020. Available online: https://www.anpm.ro/documents/27905/38872680/MP+Apa+Serv.pdf (accessed on 6 January 2025).
- Azevedo, C.S.; Correa, C.Z.; Lopes, D.D.; Pescim, R.R.; Prates, K.V.M.C.; Barana, A.C. Aeration and non-aeration cycles (AE/NA) time: Influence in combined organic matter and nitrogen removal and features of biofilm. Environ. Tehnol. 2021, 43, 2443–2456. [Google Scholar] [CrossRef]
- Thalla, A.K.; Bhargava, R.; Kumar, P. Nitrification kinetics of activated sludge-biofilm system: A mathematical model. Bioresour. Technol. 2010, 101, 5822–5835. [Google Scholar] [CrossRef]
- Randall, C.W.; Dipankar, S. Full-scale evaluation of an integrated fixed film-media in activated sludge (IFAS) process for nitrogen removal. Water Sci. Technol. 1996, 33, 152–162. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Fan, Y.; Meng, J. A quantified nitrogen metabolic network by reaction kinetics and mathematical model in a single-stage microaerobic system treating low COD/TN wastewater. J. Water Res. 2022, 225, 119112. [Google Scholar] [CrossRef]
- Gernaey, K.V.; van Loosdrecht, M.C.M.; Henze, M.; Lind, M.; Jørgensen, S.B. Activated sludge wastewater treatment plant modelling and simulation. Water Res. 2004, 19, 763–783. [Google Scholar] [CrossRef]
- Council Directive of 21 May 1991 Concerning Urban Waste-Water Treatment. 1991. Available online: http://data.europa.eu/eli/dir/1991/271/oj (accessed on 1 December 2024).


| Stoichiometric Constants and Coefficients Are Important in Chemistry Because They Help to Determine the Amounts of Reacting Substances Needed for a Chemical Reaction to Occur. | Symbol | Unit of Measurement | Value |
|---|---|---|---|
| Ammonium-oxidizing bacteria clearly demonstrate a high biomass growth rate. | |||
| YNH4,max | g MLSS/gN | 0.24 | |
| The maximum specific growth rate of ammonium-oxidizing bacteria is a critical metric in microbiology. | µNH4,20C,max | Zi−1 | 0.8 |
| The saturation constant is numerically equivalent to the N-NH4 concentration when the specific velocity is half of the maximum value, as is the case for the nitrification process. | |||
| Ks,NH4,A | gN-NH4/m3 | 0.5 | |
| The O2 saturation constant for autotrophic nitrifying bacteria is numerically equal to the value at which saturation is half of the maximum value. | Ks,O2,A | gO2/m3 | 1.00 |
| Constant pH | KpH | - | 200 |
| Temperature constant | χ | °C−1 | 0.08 |
| Fraction of autotrophic bacteria in the active bacterial mass | ηa | - | 0.05 |
| The mass fraction of active bacteria in activated sludge is equal to the ratio VSS (volatile suspended solids)/MLSS (the concentration of suspended solids with mixed liquids). | δb | - | 0.65 |
| Mortality rate of bacterial population that is autonomous from its food | β | zi−1 | 0.05 |
| Parameter | Symbol | Unit of Measurement | Value |
|---|---|---|---|
| Volume of aerated compartments | |||
| V | m3 | 1300 | |
| Influent wastewater flow rate in the treatment plant | Qi | m3/zi | 1900 |
| N-NH4 concentration in wastewater treatment plant effluent | |||
| SNH4,e | g/m3 | variable | |
| Dissolved oxygen concentration in aerated compartments | SO2 | gO2/m3 | variable |
| The pH concentration in wastewater from the treatment plant’s influent is measured. | pH | - | variable |
| Activated sludge is added in suspended matter in aerated compartments. | MLSS | g/m3 | 1850–2150 |
| Temperature of wastewater undergoing treatment | t | °C | 10–26 |
| DO/mg/L | t, °C | |||||||
| 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| 1 | 19.10 | 20.64 | 21.32 | 22.13 | 23.09 | 25.22 | 27.53 | 29.02 |
| 2 | 26.23 | 28.37 | 30.68 | 32.19 | 34.91 | 37.86 | 40.05 | 44.50 |
| 3 | 29.79 | 31.23 | 33.87 | 35.72 | 38.82 | 42.17 | 45.81 | 48.74 |
| 4 | 31.93 | 34.54 | 37.37 | 40.44 | 43.76 | 47.36 | 50.26 | 53.49 |
| 5 | 33.36 | 36.09 | 39.05 | 43.25 | 46.73 | 49.49 | 552.57 | 56.98 |
| DO/mg/L | t, °C | |||||||
| 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | |
| 1 | 32.73 | 35.66 | 38.83 | 41.10 | 43.27 | 45.99 | 48.03 | 53.40 |
| 2 | 49.25 | 53.30 | 57.80 | 62.46 | 67.61 | 73.20 | 79.25 | 85.80 |
| 3 | 56.01 | 60.62 | 65.63 | 71.05 | 76.92 | 83.28 | 90.17 | 97.64 |
| 4 | 60.06 | 65.02 | 70.39 | 76.20 | 82.51 | 89.33 | 96.73 | 104.74 |
| 5 | 62.77 | 67.95 | 73.56 | 79.64 | 86.23 | 93.36 | 5101.09 | 109.47 |
| No. | Temp. | CODCr Influent | CODCr Effluent. | N-NO3 Effluent | N-NO2 Effluent | N-NH4 Influent | N-NH4 Effluent | Eff N-NH4 | Eff N |
|---|---|---|---|---|---|---|---|---|---|
| °C | gO2/m3 | gO2/m3 | g/m3 | g/m3 | g/m3 | g/m3 | % | % | |
| 1 | 9.01 | 460 | 109.03 | 42.09 | 6.08 | 82.05 | 16.05 | 80.01 | 19.08 |
| 2 | 10.02 | 535.07 | 118.02 | 36.05 | 4.06 | 65.07 | 2.25 | 96.06 | 33.9 |
| 3 | 15.01 | 522.06 | 116.04 | 42.05 | 0.049 | 70.04 | 0.659 | 99.01 | 38.06 |
| 4 | 15.09 | 556.08 | 93.01 | 12.01 | 0.199 | 49.05 | 22.419 | 95.02 | 70.02 |
| 5 | 17.03 | 616.02 | 99.01 | 1.657 | 8.12 | 103.03 | 7.91 | 92.04 | 82.09 |
| 6 | 17.06 | 488.03 | 73.02 | 16.07 | 0.109 | 68.98 | 0.722 | 98.09 | 74.01 |
| 7 | 20.08 | 493.07 | 96.06 | 5.324 | 0.038 | 65.03 | 0.437 | 99.03 | 91.01 |
| 8 | 20.04 | 341.01 | 29.05 | 6.03 | 0.109 | 61.02 | 0.625 | 98.08 | 88.06 |
| 9 | 25.04 | 622.05 | 63.9 | 13.04 | 0.455 | 79.08 | 0.935 | 98.08 | 81.05 |
| No. | ∆N-NH4exp., g/m3 | N-NH4 Influent calc. g/m3 | N-NH4 calc. g/m3 | N-NH4 g/m3 |
|---|---|---|---|---|
| 1 | 66.01 | 59.54 | 43.04 | 22.98 |
| 2 | 63.45 | 35.79 | 33.53 | 29.93 |
| 3 | 69.75 | 36.49 | 35.83 | 33.93 |
| 4* | 47.10 | 45.67 | 43.26 | 3.85 |
| 5 | 95.41 | 75.30 | 67.40 | 28.02 |
| 6 | 68.29 | 40.81 | 40.09 | 28.21 |
| 7 | 64.88 | 32.64 | 32.3 | 32.68 |
| 8 | 60.59 | 41.05 | 40.43 | 20.17 |
| 9 | 78.87 | 70.9 | 69.87 | 9.01 |
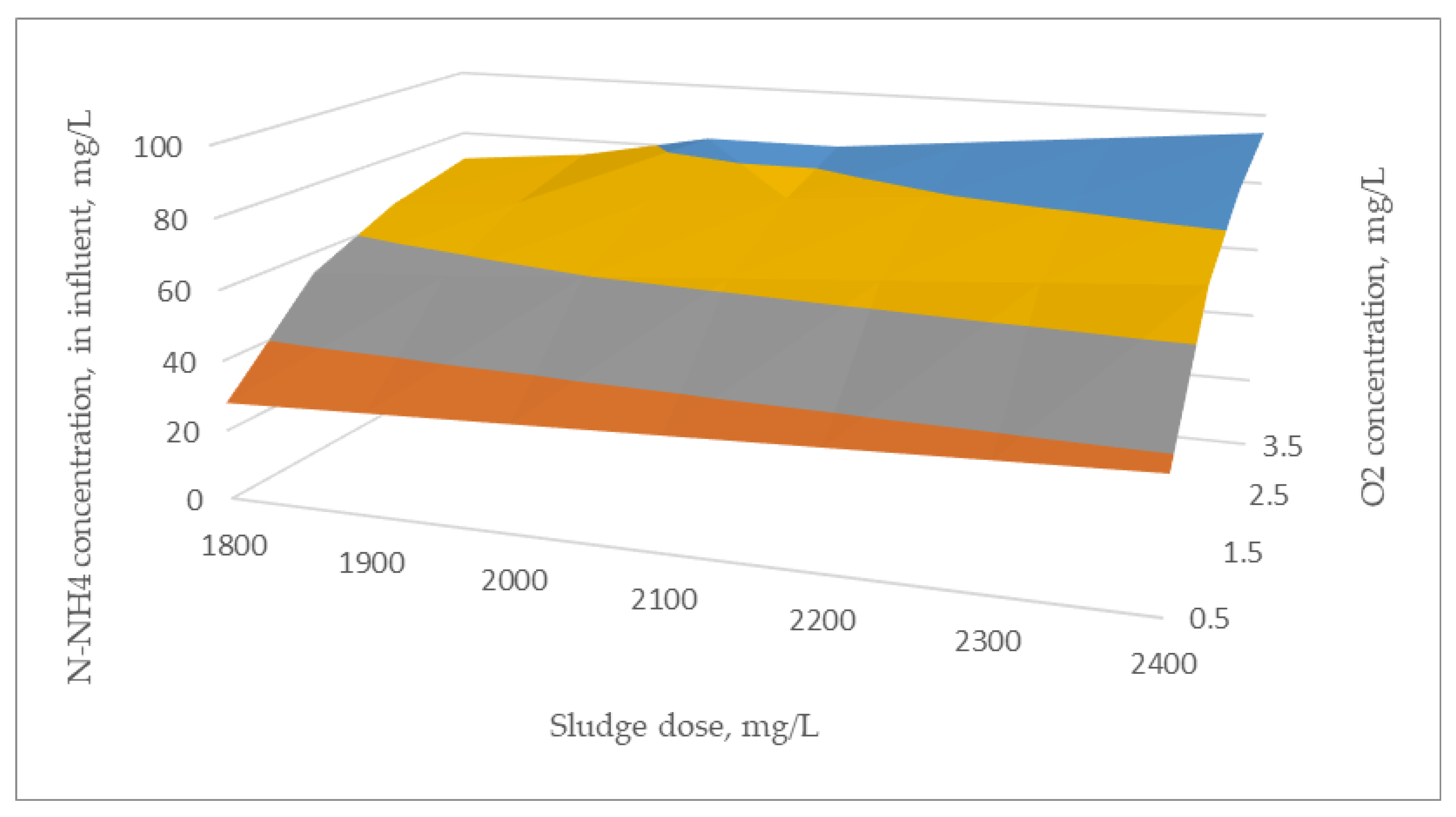
| Parameter OD/Dose/mg/L | Sludge Dose mg/L | ||||||
|---|---|---|---|---|---|---|---|
| 1800 | 1900 | 2000 | 2100 | 2200 | 2300 | 2400 | |
| 0.50 | 27.82 | 29.28 | 30.74 | 32.20 | 33.66 | 35.12 | 36.58 |
| 1.00 | 44.21 | 46.58 | 48.94 | 51.31 | 53.68 | 56.05 | 58.42 |
| 1.50 | 54.04 | 56.95 | 59.87 | 62.78 | 65.70 | 68.61 | 71.53 |
| 2.00 | 60.59 | 63.87 | 67.15 | 70.43 | 73.71 | 76.99 | 80.26 |
| 2.50 | 65.27 | 68.81 | 72.35 | 75.89 | 79.43 | 82.97 | 86.51 |
| 3.00 | 68.78 | 72.51 | 76.25 | 79.98 | 83.72 | 87.45 | 91.19 |
| 3.50 | 71.51 | 75.40 | 79.28 | 83.17 | 87.05 | 90.94 | 994.83 |
| C/N Ratio | Autotrophic Bacteria Fraction ηa | C/N Ratio | Autotrophic Bacteria Fraction ηa |
|---|---|---|---|
| 0.5 | 0.35 | 5 | 0.054 |
| 1 | 0.21 | 6 | 0.043 |
| 2 | 0.12 | 7 | 0.037 |
| 3 | 0.083 | 8 | 0.033 |
| 4 | 0.064 | 9 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, E.; Rusănescu, C.O. Optimization of the Wastewater Treatment Process Using Kinetic Equations for Nitrification Processes. Water 2025, 17, 2440. https://doi.org/10.3390/w17162440
Marin E, Rusănescu CO. Optimization of the Wastewater Treatment Process Using Kinetic Equations for Nitrification Processes. Water. 2025; 17(16):2440. https://doi.org/10.3390/w17162440
Chicago/Turabian StyleMarin, Eugen, and Carmen Otilia Rusănescu. 2025. "Optimization of the Wastewater Treatment Process Using Kinetic Equations for Nitrification Processes" Water 17, no. 16: 2440. https://doi.org/10.3390/w17162440
APA StyleMarin, E., & Rusănescu, C. O. (2025). Optimization of the Wastewater Treatment Process Using Kinetic Equations for Nitrification Processes. Water, 17(16), 2440. https://doi.org/10.3390/w17162440







