Enteropathogenic Bacteria in Water Sources Associated with Faecal Waste from Open Defecation and Animals in Rural Communities of Vhembe District, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Clearance
2.3. Study Area
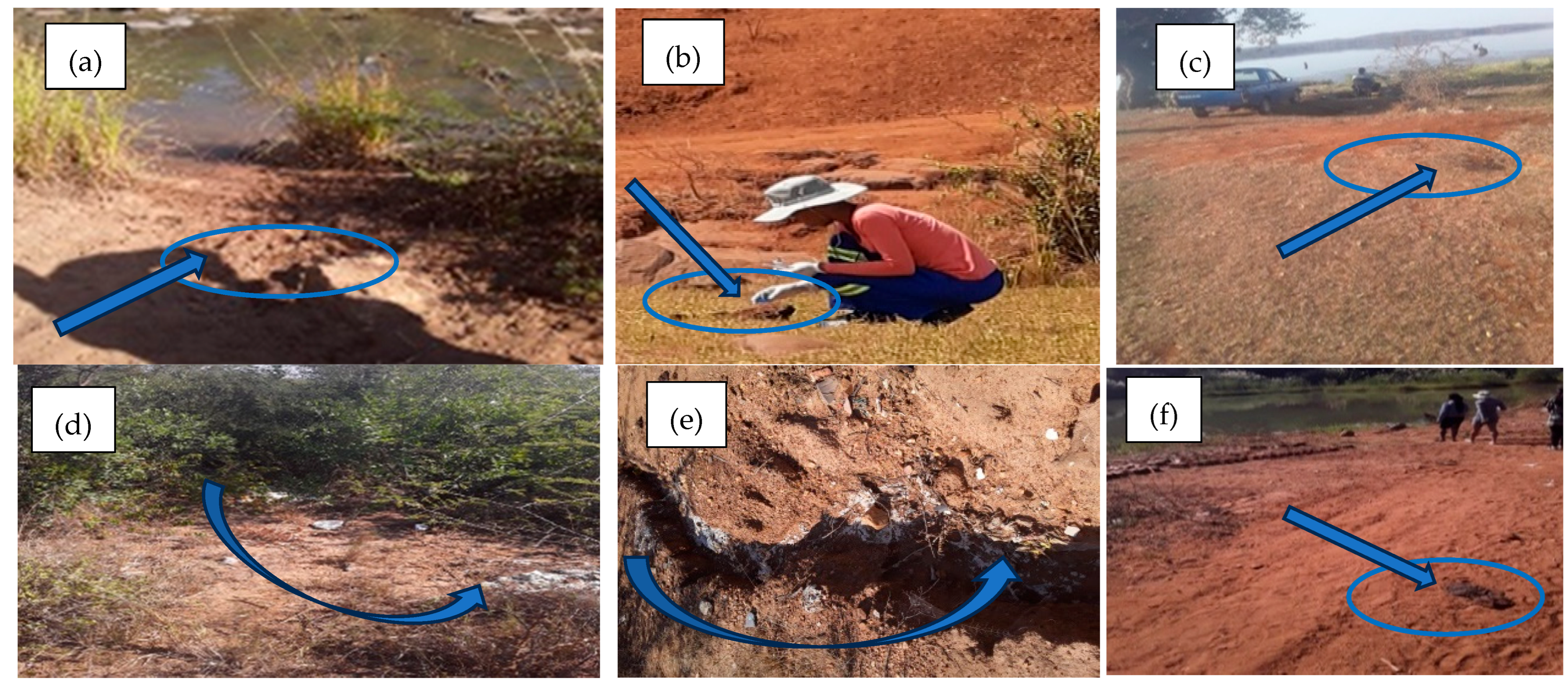
2.4. Collection of Samples
2.4.1. Faecal Sample Collection
2.4.2. Drinking Water Samples
2.5. Processing of Samples
2.5.1. Faecal Samples
2.5.2. Water Samples
2.6. Molecular Identification of Target Enteropathogenic Bacteria
2.6.1. DNA Extraction
2.6.2. Determination of qPCR Efficiency and Lower Limit of Quantification of Primers
2.6.3. Enteropathogenic Bacteria Identification
2.7. Statistical Analysis
3. Results
3.1. Parameters Used for Detection and Quantification of Enteropathogenic Bacteria
3.2. Prevalence of Enteropathogenic Bacteria in Faecal Samples Collected in the Vicinities of Various Water Sources
3.3. Prevalence of Enteropathogenic Bacteria in Surface Water Sources and Household Water (End Users)
3.3.1. Households Using Treated Surface Water (Rivers and Dams)
3.3.2. Households only Depending on Untreated River Water Sources
3.3.3. Only Depending on Untreated Spring Water and Household Container-Stored Water
3.3.4. Households only Depending on Untreated Groundwater such as Hand-Dug Wells and Household Container-Stored Water
3.4. Associations Between Target Enteropathogenic Bacteria in Faecal Samples and in Water Sources
3.5. Associations Between Target Enteropathogenic Bacteria in Water Sources and in Household Container-Stored Water
4. Discussion
5. Conclusions
6. Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization) Drinking Water. 2022. Available online: https://www.who.int (accessed on 10 November 2022).
- Bain, R.; Cronk, R.; Wright, J.; Yang, H.; Slaymaker, T.; Bartram, J. Fecal contamination of drinking-water in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001644. [Google Scholar] [CrossRef]
- NDoH (National Department of Health). Annual Health Report: Burden of Diarrhoeal Disease in South Africa; Goverment of South Africa: Pretoria, South Africa, 2022. Available online: https://www.health.gov.za (accessed on 10 September 2024).
- Mure, A.; Kamika, I.; Samie, A.; Momba, M.N.B. Assessment of the water sources for potential channels of faecal contamination within Vhembe District Municipality using sanitary inspections and hydrogen sulphide test. Sci. Rep. 2023, 13, 6250. [Google Scholar] [CrossRef]
- Galan, D.I.; Kim, S.S.; Graham, J.P. Exploring changes in open defecation prevalence in sub-Saharan Africa based on national level indices. BMC Public Health 2013, 13, 527. [Google Scholar] [CrossRef]
- WHO (World Health Organisation). Progress on Household Drinking Water, Sanitation and Hygiene 2000–2020: Five Years into the SDGs; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/publications/i/item/9789240030848 (accessed on 3 November 2022).
- FAO (Food and Agriculture Organization of the United Nations). Livestock and Water: Managing Water for Sustainable Food and Agriculture. 2018. Available online: http://www.fao.org/3/I6602EN/i6602en.pdf (accessed on 1 September 2024).
- Sharma, S.; Sobsey, M. Pathogen and Indicator Microbial Contamination and Disinfection of Drinking Water in Developing Countries. Water Res. 2017, 125, 587–605. [Google Scholar] [CrossRef]
- Stats SA (Statistics South Africa). General Household Survey 2021. Pretoria 2021. Available online: https://www.statssa.gov.za/publications/P0318/P03182021.pdf (accessed on 10 September 2024).
- Shaheed, A.; Orgill, J.; Montgomery, M.A.; Jeuland, M.A.; Brown, J. Why improved water sources are not always safe. Bull. World Health Organ. 2014, 92, 283–289. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). World Report on Disability; WHO: Geneva, Switzerland, 2011. Available online: https://www.who.int/publications/i/item/9789241564182 (accessed on 2 August 2025).
- DWS (Department of Water and Sanitation). National Water and Sanitation Master Plan: Volume 1–Call to Action; Government of South Africa: Pretoria, South Africa, 2020. Available online: https://www.dws.gov.za (accessed on 6 September 2024).
- Diouf, K.; Tabatabai, P.; Rudolph, J.; Marx, M. Diarrhoea prevalence in children under five years of age in rural Burundi: An assessment of social and behavioural factors at the household level. Glob. Health Action 2014, 7, 24895. [Google Scholar] [CrossRef]
- Ezeh, O.K.; Agho, K.E.; Dibley, M.J.; Hall, J.; Page, A.N. The impact of water and sanitation on childhood mortality in Nigeria: Evidence from demographic and health surveys, 2003–2013. Int. J. Environ. Res. Public Health 2014, 11, 9256–9272. [Google Scholar] [CrossRef]
- Obi, C.L.; Green, E.; Bessong, P.; De Villiers, B.; Hoosen, A.A.; Igumbor, E.O.; Potgieter, N. Gene encoding virulence markers among Escherichia coli isolates from diarrhoeic stool samples and river sources in rural Venda communities of South Africa. Water SA 2004, 30, 37–42. [Google Scholar] [CrossRef]
- Gumbo, J.R.; Dzaga, R.A.; Nethengwe, N.S. Impact on water quality of Nandoni water reservoir downstream of municipal sewage plants in Vhembe district, South Africa. Sustainability 2016, 8, 597. [Google Scholar] [CrossRef]
- Howard, G.; Schmoll, O. Water Safety Plans: Risk management approaches for the delivery of safe drinking-water from groundwater sources. In Protecting Groundwater for Health–Managing the Quality of Drinking-Water Sources; Schmoll, O., Howard, G., Chilton, J., Chorus, I., Eds.; IWA Publishing: London, UK, 2006. [Google Scholar] [CrossRef]
- UNICEF (United Nations Children’s Fund); World Health Organization (WHO). Diarrhoea: Why Children Are Still Dying and What Can Be Done. 2009. Available online: https://data.unicef.org/resources/diarrhoea-why-are-children-dying_UNICEF_WHO.pdf (accessed on 2 August 2025).
- Mogane, B.; Kachienga, L.O.; Kamika, I.; Ngobeni-Nyambi, R.; Momba, M.N.B. Distribution of host-specific Bacteriodales marker genes in water sources of selected rural areas of Vhembe District, South Africa. Sci Rep. 2024, 14, 19758, Erratum in Sci. Rep. 2024, 14, 23325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association (APHA): Washington DC, USA; Water Environment Federation (WEF): Cologny, Switzerland; American Water Works Association (AWWA): Denver, CO, USA, 2001; pp. 254–278. [Google Scholar]
- Saingam, P.; Li, B.; Yan, T. Fecal indicator bacteria, direct pathogen detection, and microbial community analysis provide different microbiological water quality assessment of a tropical urban marine estuary. Water Res. 2020, 185, 116280. [Google Scholar] [CrossRef]
- Baudart, J.; Grabulos, J.; Barusseau, J.P.; Lebaron, P. Salmonella spp. and fecal coliform loads in coastal waters from a point vs. nonpoint source of pollution. J. Environ. Qual. 2000, 29, 241–250. [Google Scholar] [CrossRef]
- Brown, P.E.; Christensen, O.F.; Clough, H.E.; Diggle, P.J.; Hart, C.A.; Hazel, S.; Kemp, R.; Leatherbarrow, A.J.H.; Moore, A.; Sutherst, J.; et al. Frequency and spatial distribution of environmental Campylobacter spp. Appl. Environ. Microbiol. 2004, 70, 6501–6511. [Google Scholar] [CrossRef] [PubMed]
- Hancock, D.; Besser, T.; Lejeune, J.; Davis, M.; Rice, D. The control of VTEC in the animal reservoir. Int. J. Food Microbiol. 2001, 66, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Obi, C.L.; Potgieter, N.; Bessong, P.O.; Matsaung, G. Assessment of the microbial quality of river water sources in rural Venda communities in South Africa. Water SA 2002, 28, 287–292. [Google Scholar] [CrossRef]
- Traoré, A.N.; Mulaudzi, K.; Chari, G.J.E.; Foord, S.H.; Mudau, L.S.; Barnard, T.G.; Potgieter, N. The impact of human activities on microbial quality of rivers in the Vhembe District, South Africa. Int. J. Environ. Res. Public Health 2016, 13, 817. [Google Scholar] [CrossRef]
- Mudau, M.; Ngobeni-Nyambi, R.; Momba, M.N.B. The fascinating cross-paths of pathogenic bacteria, human and animal faecal sources in water-stressed communities of Vhembe District, South Africa. Pathogens 2023, 12, 1085. [Google Scholar] [CrossRef]
- Maheux, A.F.; Bissonnette, L.; Boissinot, M.; Bernier, J.L.T.; Huppé, V.; Picard, F.J.; Bérubé, È.; Bergeron, M.G. Rapid concentration and molecular enrichment approach for sensitive detection of Escherichia coli and Shigella species in potable water samples. Appl. Environ. Microbiol. 2011, 77, 6199–6207. [Google Scholar] [CrossRef]
- Khan, I.U.H.; Gannon, V.; Kent, R.; Koning, W.; Lapen, D.R.; Miller, J.; Neumann, N.; Phillips, R.; Robertson, W.; Topp, E.; et al. Development of a rapid quantitative PCR assay for direct detection and quantification of culturable and non-culturable Escherichia coli from agriculture watersheds. J. Microbiol. Methods 2007, 69, 480–488. [Google Scholar] [CrossRef]
- Ståhlberg, A.; Kubista, M. The workflow of single-cell expression profiling using quantitative real-time PCR. Expert Rev. Mol. Diagn. 2014, 14, 323–331. [Google Scholar] [CrossRef]
- Kagambèga, A.; Lienemann, T.; Aulu, L.; Traoré, A.S.; Barro, N.; Siitonen, A.; Haukka, K. Prevalence and characterization of Salmonella enterica from the feces of cattle, poultry, swine and hedgehogs in Burkina Faso and their comparison to human Salmonella isolates. BMC Microbiol. 2013, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.; Singh, S.P.; Gill, J.P.S.; Kumar, R.; Kumar, D. Isolation and identification of Shigella spp. from human fecal samples collected from Pantnagar, India. Vet. World 2013, 6, 376–379. [Google Scholar] [CrossRef]
- Kusiluka, L.J.M.; Karimuribo, E.D.; Mdegela, R.H.; Luoga, E.J.; Munishi, P.K.T.; Mlozi, M.R.S.; Kambarage, D.M. Prevalence and impact of water-borne zoonotic pathogens in water, cattle and humans in selected villages in Dodoma Rural and Bagamoyo districts, Tanzania. Phys. Chem. Earth 2005, 30, 818–825. [Google Scholar] [CrossRef]
- Laukkanen-Ninios, R.; Fredriksson-Ahomaa, M.; Korkeala, H. Enteropathogenic Yersinia in the pork production chain: Challenges for control. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1165–1191. [Google Scholar] [CrossRef]
- Ostroff, S. Yersinia as an emerging infection: Epidemiologic aspects of Yersiniosis. Contrib. Microbiol. Immunol. 1995, 13, 5–10. [Google Scholar] [PubMed]
- Singh, I.; Bhatnagar, S.; Virdi, J.S. Isolation and characterization of Yersinia enterocolitica from diarrhoeic human subjects and other sources. Curr. Sci. 2003, 84, 1353–1355. [Google Scholar]
- Okwori, A.E.; Agina, S.E.; Olabode, A.O.; Fadera, M.A.K.; Ibu, J.; Odugbo, M. Faecal carriage of Yersinia species in pigs, sheep and poultry on display for sale in Vom and Bukuru areas of Jos South Local Government Area (LGA). Plateau state, Nigeria. J. Microbiol. 2005, 19, 1–2. [Google Scholar]
- Arvanitidou, M.; Kanellou, K.; Vagiona, D.G. Diversity of Salmonella spp. and fungi in northern Greek rivers and their correlation to fecal pollution indicators. Environ. Res. 2005, 99, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.P.S. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef]
- Potgieter, N.; Obi, C.L.; Bessong, P.O.; Igumbor, E.O.; Samie, A.; Nengobela, R. Bacterial contamination of Vhuswa: A local weaning food and stored drinking-water in impoverished households in the Venda region of South Africa. JHPN 2005, 23, 150–155. [Google Scholar] [PubMed]
- Plym-Forshell, L.; Ekesbo, I. Survival of Salmonella in urine and dry faeces from cattle—An experimental study. Acta Vet. Scand. 1996, 37, 127–131. [Google Scholar] [CrossRef]
- Schriewer, A.; Miller, W.A.; Byrne, B.A.; Miller, M.A.; Oates, S.; Conrad, P.A.; Hardin, D.; Yang, H.; Chouicha, N.; Melli, A.; et al. Presence of Bacteroidales as a predictor of pathogens in surface waters of the Central California Coast. Appl. Environ. Microbiol. 2010, 76, 5802–5814. [Google Scholar] [CrossRef]
- Li, A.; Chen, L.; Zhang, Y.; Tao, Y.; Xie, H.; Li, S.; Sun, W.; Pan, J.; He, Z.; Mai, C.; et al. Occurrence and distribution of antibiotic resistance genes in the sediments of drinking water sources, urban rivers, and coastal areas in Zhuhai, China. Environ. Sci. Pollut. Res. 2018, 25, 26209–26217. [Google Scholar] [CrossRef]
- Mahagamage, M.G.Y.L.; Pathirage, M.V.S.C.; Manage, P.M. Contamination status of Salmonella spp., Shigella spp. and Campylobacter spp. in surface and groundwater of the Kelani River Basin, Sri Lanka. Water 2020, 12, 2187. [Google Scholar] [CrossRef]
- Jenkins, M.B.; Endale, D.M.; Fisher, D.S. Most probable number methodology for quantifying dilute concentrations and fluxes of Salmonella in surface waters. J. Appl. Microbiol. 2008, 104, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Soupir, M.L.; Haddad, M.; Rothwell, J.J. Assessing the impacts of watershed indexes and precipitation on spatial in-stream E. coli concentrations. Ecol. Indic. 2012, 23, 641–652. [Google Scholar] [CrossRef]
- Stats SA GHS (General Household Survey). GHS 2019. Available online: https://www.statssa.gov.za/publications/P0318/P03182019.pdf (accessed on 13 October 2022).
- Slavik, I.; Oliveira, K.R.; Cheung, P.B.; Uhl, W. Water quality aspects related to domestic drinking water storage tanks and consideration in current standards and guidelines throughout the world–A review. J. Water Health 2021, 18, 439–463. [Google Scholar] [CrossRef]
- Momba, M.N.B.; Kaleni, P. Regrowth and survival of indicator microorganisms on the surfaces of household containers used for the storage of drinking water in rural communities of South Africa. Water Res. 2002, 36, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Momba, M.N.B.; Notshe, T.L. The microbiological quality of groundwater-derived drinking water after long storage in household containers in a rural community of South Africa. J. Water Supply Res. Technol. Aqua 2003, 52, 67–77. [Google Scholar] [CrossRef]
- Budeli, P.; Moropeng, R.C.; Mpenyana-Monyatsi, L.; Momba, M.N.B. Inhibition of biofilm formation on the surface of water storage containers using biosand zeolite silver-impregnated clay granular and silver impregnated porous pot filtration systems. PLoS ONE 2018, 13, e0194715. [Google Scholar] [CrossRef] [PubMed]

| CLM | ||||||
|---|---|---|---|---|---|---|
| Wet Season | ||||||
| Frequency (No. of Positive Samples/No. of Tested Samples) | ||||||
| Enteropathogenic Bacteria | ||||||
| Sampling Site | Faecal Samples | Number of Samples | S. Typhimurium | C. jejuni | Y. enterocolitica | S. flexneri |
| Luvuvhu River downstream | Cow | 4 | 50% | 25% | 0% | 0% |
| Luvuvhu River upstream | Human | 5 | 40% | 20% | 0% | 60% |
| Total | 9 | 44% | 22% | 0% | 33% | |
| Dry season | ||||||
| Luvuvhu River downstream | Cow | 2 | 0% | 0% | 0% | 0% |
| Human | 4 | 50% | 0% | 0% | 25% | |
| Luvuvhu River upstream | Human | 4 | 25% | 0% | 0% | 25% |
| Total | 10 | 30% | 0% | 0% | 20% | |
| TLM | ||||||
| Wet season | ||||||
| Mvudi River downstream | Cow | 3 | 33% | 0% | 0% | 67% |
| Human | 3 | 0% | 0% | 0% | 33% | |
| Mvudi River upstream | Cow | 2 | 50% | 0% | 0% | 0% |
| Luvuvhu River downstream | Human | 4 | 25% | 0% | 0% | 0% |
| Luvuvhu River upstream | Cow | 2 | 0% | 0% | 0% | 50% |
| Human | 3 | 33% | 0% | 0% | 33% | |
| Mutshindudi River downstream | Human | 2 | 50% | 0% | 0% | 0% |
| Mutshindudi River upstream | Human | 3 | 67% | 0% | 0% | 67% |
| Nandoni Dam | Cow | 3 | 0% | 0% | 0% | 33% |
| Human | 2 | 50% | 0% | 0% | 0% | |
| Total | 27 | 27% | 0% | 0% | 27% | |
| Dry season | ||||||
| Mvudi River downstream | Human | 4 | 0% | 0% | 0% | 0% |
| Mvudi River upstream | Cow | 4 | 25% | 0% | 0% | 25% |
| Human | 2 | 0% | 0% | 0% | 0% | |
| Luvuvhu River downstream | Cow | 2 | 0% | 0% | 0% | 0% |
| Luvuvhu River upstream | Human | 3 | 67% | 0% | 0% | 0% |
| Mutshindudi River downstream | Cow | 3 | 0% | 0% | 0% | 0% |
| Mutshindudi River upstream | Dog Human Cow | 3 2 2 | 33% 50% 0% | 0% 0% 0% | 0% 0% 0% | 0% 0% 50% |
| Nandoni Dam | Cow | 2 | 0% | 0% | 0% | 50% |
| Total | 27 | 19% | 0% | 0% | 11% | |
| CLM | ||||||
|---|---|---|---|---|---|---|
| Wet Season | ||||||
| Frequency (No. of Positive Samples/No. of Tested Samples) Enteropathogenic Bacteria | ||||||
| Sampling Site | Faecal Samples | Number of Samples | S. Typhimurium | C. jejuni | Y. enterocolitica | S. flexneri |
| Dididi spring | Pig | 3 | 67% | 0% | 33% | 67% |
| Dog | 2 | 50% | 0% | 50% | 0% | |
| Total | 5 | 60% | 0% | 40% | 40% | |
| Dry season | ||||||
| Dididi spring | Cow | 3 | 67% | 0% | 0% | 0% |
| Pig | 2 | 0% | 0% | 50% | 33% | |
| Total | 5 | 40% | 0 | 20% | 20% | |
| TLM | ||||||
| Wet season | ||||||
| Tshivulani spring | Cow | 3 | 33% | 0% | 0% | 0% |
| Tshilapfene spring | Cow | 4 | 0% | 0% | 0% | 75% |
| Human | 2 | 50% | 0% | 0% | 50% | |
| Total | 9 | 22% | 0% | 0% | 44% | |
| Dry season | ||||||
| Tshivulani spring | Cow | 3 | 0% | 0% | 0% | 0% |
| Human | 2 | 50% | 0% | 0% | 0% | |
| Tshilapfene spring | Cow | 4 | 0% | 0% | 0% | 0% |
| Human | 2 | 50% | 0% | 0% | 50% | |
| Total | 11 | 18% | 0% | 0% | 9% | |
| TLM+ | ||||||
|---|---|---|---|---|---|---|
| Wet Season | ||||||
| Frequency (No. of Positive Samples/No. of Tested Samples) | ||||||
| Enteropathogenic Bacteria | ||||||
| Sampling Site | Faecal Samples | Number of Samples | S. Typhimurium | C. jejuni | Y. enterocolitica | S. flexneri |
| Tshivulani hand-dug wells | Chicken | 3 | 0% | 0% | 0% | 67% |
| Dog | 2 | 50% | 0% | 50% | 0% | |
| Total | 5 | 20% | 0% | 20% | 40% | |
| Dry season | ||||||
| Tshivulani hand-dug wells | Cow | 3 | 33% | 0% | 0 | 33% |
| Total | 3 | 33 | 0% | 0% | 33% | |
| CLM | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wet Season | Dry Season | ||||||||
| Frequency (No. of Positive Samples/No. of Tested Samples | |||||||||
| Enteropathogenic Bacteria | |||||||||
| Sampling Site | Number of Samples | S. Typhimurium | C. jejuni | Y. enterocolitica | S. flexneri | S. Typhimurium | C. jejuni | Y. enterocolitica | S. flexneri |
| n = 176 | n = 176 | ||||||||
| Luvuvhu River downstream | 4 | 100% | 0% | 0% | 100% | 75% | 0% | 0% | 100% |
| Luvuvhu River upstream | 4 | 100% | 0% | 0% | 100% | 50% | 0% | 0% | 100% |
| WTP abstraction point | 4 | 100% | 0% | 0% | 50% | 100% | 0% | 0% | 50% |
| WTP—treated water at point of treatment | 4 | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| HH *—standpipe | 80 | 15% | 0% | 0% | 5% | 6% | 0% | 0% | 3% |
| HH—container-stored water | 80 | 9% | 0% | 0% | 20% | 6% | 0% | 0% | 5% |
| TLM | |||||||||
| n = 188 | n = 188 | ||||||||
| Mvudi River downstream | 4 | 100% | 0% | 0% | 100% | 75% | 0% | 0% | 75% |
| 0% | |||||||||
| Mvudi River upstream | 4 | 100% | 0% | 100% | 50% | 0% | 0% | 75% | 100% |
| Luvuvhu River downstream | 4 | 100% | 0% | 0% | 100% | 75% | 0% | 0% | 100% |
| Luvuvhu River upstream | 4 | 100% | 0% | 0% | 100% | 75% | 100% | 0% | 0% |
| Nandoni Dam | 4 | 100% | 0% | 0% | 75% | 75% | 0% | 0% | 100% |
| WTP abstraction point | 4 | 100% | 0% | 0% | 100% | 100% | 100% | 0% | 0% |
| WTP—treated water at point of treatment | 4 | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| HH *—standpipe | 80 | 9% | 0% | 0% | 5% | 4% | 0% | 0% | 3% |
| HH—container-stored water | 80 | 14% | 0% | 0% | 5% | 6% | 0% | 0% | 4% |
| TLM | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wet Season | Dry Season | ||||||||
| Frequency (No. of Positive Samples/No. of Tested Samples) | |||||||||
| Enteropathogenic Bacteria | |||||||||
| n = 44 | n = 44 | ||||||||
| Sampling Site | Number of Samples | S. Typhimurium | C. jejuni | Y. enterocolitica | S. flexneri | S. Typhimurium | C. jejuni | Y. enterocolitica | S. flexneri |
| Mutshindudi River downstream | 4 | 100% | 0% | 0% | 100% | 50% | 0% | 0% | 100% |
| Mutshindudi River upstream | 4 | 100% | 0% | 0% | 100% | 75% | 0% | 0% | 50% |
| HH *—container-stored water | 36 | 33% | 0% | 0% | 22% | 25% | 0% | 0% | 14% |
| CLM | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wet Season | Dry Season | ||||||||
| Sampling Site | Number of Samples | S. Typhimurium | C. jejuni | Y. enterocolitica | S. flexneri | S. Typhimurium | C. jejuni | Y. enterocolitica | S. flexneri |
| n = 36 | n = 36 | ||||||||
| Spring | 4 | 100% | 0% | 100% | 100% | 75% | 0% | 75% | 50% |
| HH *—container-stored water | 32 | 56% | 0% | 28% | 38% | 34% | 0% | 16% | 31% |
| TLM | |||||||||
| n = 40 | n = 40 | ||||||||
| Spring | 4 | 100% | 0% | 0% | 100% | 75% | 0% | 0% | 75% |
| HH *—container-stored water | 36 | 28% | 0% | 0% | 33% | 8% | 0% | 0% | 22% |
| TLM | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wet Season | Dry Season | ||||||||
| Sampling Site | Number of Samples (n) | S. Typhimurium | C. jejuni | Y. enterocolitica | S. flexneri | S. Typhimurium | C. jejuni | Y. enterocolitica | S. flexneri |
| n = 32 | n = 32 | ||||||||
| Hand-dug well | 4 | 100% | 0% | 100% | 100% | 50% | 0% | 50% | 75% |
| HH *—container-stored water | 28 | 18% | 0% | 7% | 43% | 14% | 0% | 7% | 32% |
| Wet Season | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. Typhimurium | S. flexneri | Y. enterocolitica | ||||||||||
| Water Sources and HH * Container-Stored Water | Coefficient | Standard Error | R2 | p Value | Coefficient | Standard Error | R2 | p Value | Coefficient | Standard Error | R2 | p Value |
| River water | 0.334 | 0.141 | 0.567 | 0.092 | 0.073 | 0.114 | 0.344 | 0.014 | 3.86 | 1.021 | 0.190 | 0.169 |
| Dam water | 0.282 | 0.064 | 0.184 | 0.066 | 1.265 | 0.172 | 0.227 | 0.028 | 1.285 | 0.015 | 0.147 | 0.187 |
| Treated water | 0.358 | 0.258 | 0.246 | 0.261 | 3.216 | 0.135 | 0.164 | 0.225 | 2.284 | 0.731 | 0.101 | 0.125 |
| Spring water | 0.418 | 0.361 | 0.346 | 0.041 | 0.447 | 0.095 | 0.358 | 0.057 | 1.135 | 0.074 | 0.141 | 0.211 |
| Hand-dug well water | 0.364 | 0.017 | 0.651 | 0.003 | 2.319 | 0.103 | 0.424 | 0.022 | 0.354 | 0.059 | 0.046 | 0.141 |
| Dry Season | ||||||||||||
| River water | 0.352 | 0.135 | 0.319 | 0.041 | 1.117 | 0.026 | 0.211 | 0.147 | 1.715 | 0.255 | 0.024 | 0.251 |
| Dam water | 0.213 | 0.510 | 0.140 | 0.242 | 2.465 | 0.324 | 0.105 | 0.028 | 0.386 | 0.024 | 0.165 | 0.138 |
| Treated water | 0.485 | 0.143 | 0.194 | 0.191 | 0.317 | 0.069 | 0.121 | 0.242 | 1.081 | 0.312 | 0.067 | 0.111 |
| Spring water | 0.418 | 0.263 | 0.117 | 0.032 | 1.693 | 0.096 | 0.138 | 0.001 | 0.187 | 0.151 | 0.125 | 0.313 |
| Hand-dug well water | 0.494 | 0.412 | 0.292 | 0.024 | 2.754 | 0.010 | 0.291 | 0.038 | 0.165 | 0.021 | 0.103 | 0.276 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogane, B.; Momba, M.N.B. Enteropathogenic Bacteria in Water Sources Associated with Faecal Waste from Open Defecation and Animals in Rural Communities of Vhembe District, South Africa. Water 2025, 17, 2410. https://doi.org/10.3390/w17162410
Mogane B, Momba MNB. Enteropathogenic Bacteria in Water Sources Associated with Faecal Waste from Open Defecation and Animals in Rural Communities of Vhembe District, South Africa. Water. 2025; 17(16):2410. https://doi.org/10.3390/w17162410
Chicago/Turabian StyleMogane, Barbara, and Maggy Ndombo Benteke Momba. 2025. "Enteropathogenic Bacteria in Water Sources Associated with Faecal Waste from Open Defecation and Animals in Rural Communities of Vhembe District, South Africa" Water 17, no. 16: 2410. https://doi.org/10.3390/w17162410
APA StyleMogane, B., & Momba, M. N. B. (2025). Enteropathogenic Bacteria in Water Sources Associated with Faecal Waste from Open Defecation and Animals in Rural Communities of Vhembe District, South Africa. Water, 17(16), 2410. https://doi.org/10.3390/w17162410







